Abstract
Nucleic acid-based therapy is a growing field of drug delivery research. Although ultrasound has been suggested to enhance transfection decades ago, it took a combination of ultrasound with nucleic acid carrier systems (microbubbles, liposomes, polyplexes, viral carriers) to achieve reasonable nucleic acid delivery efficacy. Microbubbles serve as foci for local deposition of ultrasound energy near the target cell, and greatly enhance sonoporation. Major advantage of this approach is in the minimal transfection in the non-insonated non-target tissues. Microbubbles can be simply co-administered with the nucleic acid carrier or can be modified to carry nucleic acid themselves. Liposomes with embedded gas or gas precursor particles can also be used to carry nucleic acid, release and deliver it by the ultrasound trigger. Successful testing in a wide variety of animal models (myocardium, solid tumors, skeletal muscle, pancreas) proves the potential usefulness of this technique for nucleic acid drug delivery.
Keywords: Acoustic, transfection, siRNA, miRNA, plasmid, viral, focused ultrasound, gene delivery
1. Introduction
The use of ultrasound energy to enhance nucleic acid delivery into the target cells has been considered since 1980s [1]. While initial efforts were limited to the in vitro cell culture testing, ultrasound-assisted transfection studies eventually moved to the in vivo setting, with the ultimate aim to apply ultrasound-assisted transfection to clinical practice. The latter goal has not been achieved yet, even for a clinical trial stage. Certain attractive features of ultrasound transfection maintain the interest in this approach; research in this area continues to advance and is rapidly accelerating lately (e.g., [2-5]. In this review we provide examples of the approaches to ultrasound-enhanced nucleic acid delivery. At the initial part of the review (Section 2), we focus on the historical development of ultrasound utilization for transfection and general design logic of the sonosensitive nucleic acid delivery particles. We then look at the characteristic examples of the potential biomedical applications of ultrasound-assisted nucleic acid delivery in the specific animal models (Section 3).
2. Ultrasound delivery: history and nucleic acid carrier design
2.1. Ultrasound and plasmid co-application: the first acoustic transfection tool
As the early discovery step, ultrasound-assisted plasmid delivery to cultured mammalian cells was investigated as a purely mechanical method, in vitro, in a cell culture setting, using a laboratory 20 KHz probe-type sonicator unit [1]. Transfection efficacy was reasonable for its time (when calcium phosphate coprecipitate of the plasmid was common practice, and microinjection was state of the art), and comparable with mechanical scraping of the cells from the dish to achieve delivery of plasmid inside the cells. Co-loading of FITC-dextran in the ultrasound-treated cells was noted in that study. Interestingly, lipofection was developed at the same time [6], and the latter became a standard nucleic acid delivery tool in almost every in vitro biomedical lab setting, and reached multiple clinical trials [7]. Ultrasound-assisted delivery required a combination skill set, with a strong knowledge of medical physics, cell and molecular biology; thus, development proceeded much more slowly. Only a decade later medical ultrasound (MHz frequency, as used for physical therapy or medical imaging) was successfully used to enhance cell transfection in culture in vitro [8]. Acoustic pressure necessary to achieve transfection during short (20 s) insonation was at ∼300-400 KPa level. Use of cavitation nuclei (i.e., microbubbles) helped to reduce the amount of transmitted acoustic energy required for successful transfection, achieved with even shorter (1 s) ultrasound pulses [9]. A combination of ultrasound, dispersed microbubbles and an aqueous solution of a hydrophilic plasmid DNA encoding green fluorescent protein in the cell culture medium led to a successful intracellular delivery of plasmid and expression of the encoded protein. With that study, the interest in ultrasound-assisted transfection started to increase and is reflected in hundreds of published manuscripts at this point.
2.2. Ultrasound, microbubbles and plasmid co-administration: enhancement of intracellular delivery in vitro and in vivo
While microbubbles made their way into clinical practice as ultrasound contrast blood pool agents, they were also investigated as energy deposition foci to enhance drug delivery in general and nucleic acid in particular [10] .
The advantage of this combination is in its simplicity and an easier path of translation towards clinical practice (Figure 1): a plasmid (or a shorter nucleic acid fragment), either unprotected, or complexed to a lipofection gene delivery system, is added to cultured cells [9], injected intravenously, intraarterially or locally [11], along with microbubbles (those already approved for human use as ultrasound contrast agents), and ultrasound is applied in the area where the transfection is desired, using a clinical ultrasound imaging apparatus, or a wider insonation field ultrasound equipment as used in physical therapy, or even a recently approved MRI-guided focused ultrasound system. All types of ultrasound systems can provide efficient microbubble cavitation (stable cavitation, where bubbles vibrate continuously, or inertial cavitation, where bubbles rapidly expand, compress and collapse). The gas inside a microbubble is orders of magnitude more compressible than the surrounding biological tissue or blood. Passage of ultrasound waves through the media results in the rapid pressure variations within the tissue, leading to the rapid cyclic compression and expansion of the bubbles, with focal deposition of energy at the bubble surface, leading to microstreaming, cavitation and jetting. If this happens in close proximity to a target cell, formation of transient pores in the cell plasma membrane in response to such treatment takes place [12]. Pores, which cells can seal by energy- and calcium-dependent repair, are relatively small (from tens to hundreds of nanometers) and most of them do not stay open for a much longer than a minute or two [13-15], so most efficient transfer may happen during insonation: plasma membrane needs to seal rapidly to maintain cell viability. Despite this established fact, intracellular delivery of small molecules (e.g., fluorescent dyes) may be efficient hours after ultrasound treatment [16, 17]. It is generally assumed that nucleic acid can enter cells by diffusion through the transient submicron pores; transfection efficacy is thus expected to be lower, when compared with viral vectors. Yet up to 70% cells were transfected by sonoporation recently in an in vitro study in a glioblastoma model [18], which may point at some other mechanisms of nucleic acid delivery inside the target cells. An alternative mechanism to pore formation, a caveolin- and clathrin- dependent increase of cellular endocytosis in response to microbubble insonation by cell surface was suggested [19]. In vivo, following intravascular administration of the microbubbles and nucleic acid, the initial target of transfection is endothelium. However, endothelium barrier function may also be altered during microbubble insonation, and nucleic acid may get delivered beyond endothelial lining, either via fenestrations/pores or by transcytosis. Efficacy of all these processes needs to be taken into account.
Figure 1.
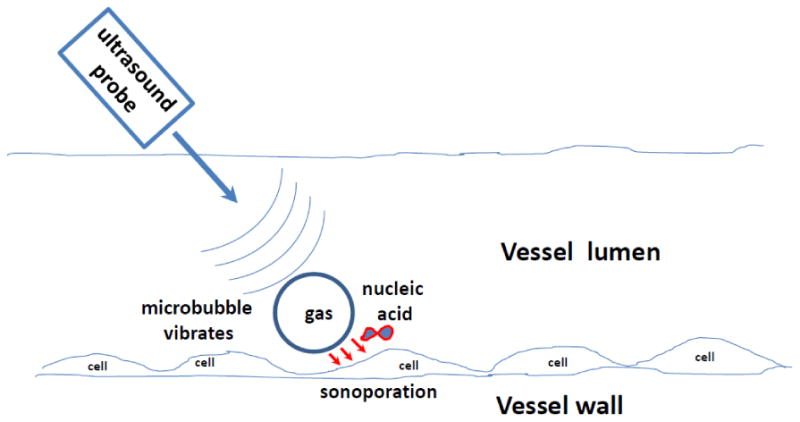
Microbubble vibration in the ultrasound field near the vessel wall results in sonoporation and intracellular delivery of co-injected nucleic acid.
With a wide availability of microbubbles for clinical use, and a developed manufacturing capability for sterile pyrogen-free nucleic acids, it is possible that the modest transfection efficacy might be the reason that clinical trials with this approach have not yet taken place. Ultrasound-based plasmid transfection cannot yet achieve the same nucleic acid transfer efficacy in vivo when compared with viral delivery systems, such as adenovirus, most of the time, but ultrasound can help with selectivity: transfection of non-insonated non-target tissues can be orders of magnitude lower than in the insonated target tissues. Co-administration of microbubbles and nucleic acid can be localized, e.g., intramuscular [20] or intraarterial [12] or systemic/intravenous [11, 12]. Targeting tumor nodes, skeletal muscles, kidney, pancreas and myocardium may be most appropriate for ultrasound-assisted transfection applications. In Section 3 of this review we address these specific targets in more detail.
2.3 Ultrasound, microbubbles and separate nucleic-acid carrier complex: transfection enhancement opportunities
Unprotected nucleic acid, such as a plasmid or an oligonucleotide, is rapidly degraded in biological milieu [21]; therefore, delivery efficacy can be improved by complexing nucleic acid with a protective delivery vehicle, and co-injecting this material with microbubbles. Since the initial lipofection studies [6], there has been a whole industry created for the delivery of nucleic acid materials [22], first with plasmids, and, lately, with shorter oligonucleotide, such as antisense oligonucleotides, interfering RNA, microRNA, mRNA [23] and minicircles [24]. These particles can be positively charged fusogenic liposomes, smaller-size lipoplexes, polyplexes, or polyethyleneimine derivatives [25], so taking advantage of these nucleic acid carriers is a logical step in improving sonoporation transfection.
Initial combination “ultrasound and microbubbles” studies [26] have confirmed the improved efficacy of this approach over simply co-injecting microbubbles with the unprotected plasmid. As expected, complexing and stabilizing the plasmid with a polymer (PEG-polyethyleneimine with a high degree of substitution) resulted in a very significant enhancement of transfection in a skeletal muscle in a rodent model, with intravenous co-injection of plasmid-polymer construct with microbubbles, followed by targeted ultrasound treatment [27]. One possible undesired feature of using transfection-enhancing particles as nucleic acid carriers could be a potential risk of nonspecific transfection in the non-insonated tissues, but the latter study did not report this problem: PEG-PEI/plasmid complex administration did not result in a significant transfection in the absence of microbubbles or insonation.
2.4 Microbubble nucleic acid carrier constructs
Performing sonoporation directly with a nucleic acid carrier microbubble offers a significant advantage: the material to be delivered to the cell is located in an immediate proximity to the surface of the target cell. Transient pores offer limited time for intracellular transfer [13]; thus, proximity should improve delivery efficiency. So, the plasmid or oligonucleotide should be placed on the microbubble surface, which is easily accomplished by electrostatic binding. Indeed, a direct comparison study has determined that microbubble-plasmid complexes provided significantly better transfection than co-administered plasmid and microbubbles incapable of complex formation [5, 28].
Placing positive charge on the surface of microbubbles is simple from the preparative standpoint: a lipid with a net positive charge is simply added to the lipid mixture composition prior to bubble formulation (Figure 2). It was first suggested by Unger et al [29], who have successfully modified preparation of MRX-115 microbubbles (lipid shell based on zwitterionic dipalmitoyl phosphatidylcholine), to include a mixture of positively charged dipalmitoylethylphosphocholine and fusogenic dioleoyl phosphatidylethanolamine. The resulting microbubbles were capable of tightly binding plasmid DNA encoding chloramphenicol acetyl transferase; upon ultrasound treatment transfection was observed in cell culture [29] and in the insonated dog heart [30]. Some nonspecific transfection in the lung, liver and other tissues that were not treated with ultrasound has also taken place [31]. MRX-115 (now named Definity, a clinical ultrasound diagnostic imaging contrast) microbubbles manufacturing is performed by the bedside, by rapid vibration of a sealed vial with the aqueous media containing lipids and perfluoropropane headspace, in a desktop amalgamator. So, adding a positively charged lipid to the aqueous phase and making it uniformly mixed with other lipid components does not add a significant technical complexity to the formulation.
Figure 2.
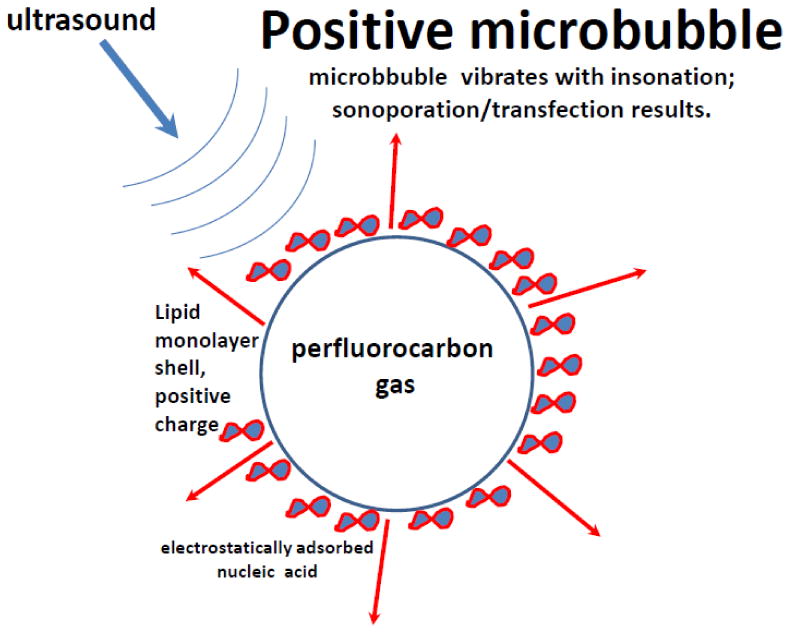
Nucleic acid (negative electrical charge) is adhered on the microbubble shell (positive charge) via an electrostatic interaction. In response to ultrasound, microbubble vibration releases the nucleic acid, possibly associated with attached shell fragments, and drives it into the cell.
In an effort to improve stability of the microbubble formulation, a switch to longer-chain lipids with higher phase transition temperature was suggested: microbubble shells were made of distearoyl phosphatidylcholine co-mixed with positive distearoyl trimetylammonium propane and a neutral PEG stearate [12]. Poorly water-soluble perfluorobutane gas improved microbubble stability and circulation time; DSTAP/DSPC/PEG-stearate microbubbles were pre-manufactured by sonication dispersion of decafluorobutane gas in the aqueous micellar lipid mixture. These microbubbles were stored refrigerated for many months in sealed vials under perfluorobutane atmosphere. They provided efficient transfection in the insonated skeletal muscle or myocardium in animal models, with minimal non-target expression of the reporter genes [12].
DSPC/DSTAP combination has become applicable generally, with a number of published studies from several laboratories [2, 5, 32-34]. In this formulation, microbubbles have to be purified from unincorporated lipid micelles by a short centrifugal flotation in a cell culture centrifuge (∼100-200 g, for several minutes); plasmid can be then added directly to the aqueous microbubble dispersion for electrostatic binding. Typically, several thousand plasmid molecules can be placed on each microbubble [12] (this is calculated from 4 ug plasmid bound to 108 microbubbles). For the small oligonucleotide fragments, the number of molecules bound per single bubble will be several orders of magnitude higher [35] due to much smaller molecular mass of an oligonucleotide. Electrostatic interaction between the bubbles and nucleic acid can be controlled by adjusting the ionic strength of the incubation media, selection of the appropriate concentrations of the reactants and their order of mixing, to avoid formation of large aggregates comprising a multiplicity of microbubbles glued by plasmid.
Instead of DSTAP, other positively charged lipids, such as a fully biocompatible phosphate ethyl ester of phosphatidylcholine [3] or lipofection liposomes [36] can be added to the lipid mixture in the aqueous media prior to bubble manufacturing. It has been confirmed that plasmid attached onto DSTAP/DSPC microbubbles that carry a grafted PEG brush is partially protected from nuclease degradation [5].
Some reports of oligonucleotide attachment to protein (human albumin) microbubbles have appeared, but the mechanism of the attachment of the bubble shell and nucleic acid is still not completely understood: albumin bubble shell surface should carry a net negative surface charge, and nucleic acid is highly negatively charged, so these materials should be electrostatically repelled from each other. Nucleic acid is also extremely hydrophilic, so hydrophobic interaction with denatured albumin is not likely. Yet microcalorimetry studies confirm binding of oligonucleotide to microbubbles, with a significant energy and affinity [35]
Additional protection of nucleic acid on the surface of the bubble can be achieved by proper formulation. Positively charged liposomes, lipoplexes or polyplexes, nanostructures that entrap and/or stabilize nucleic acid, can be placed on the surface of the microbubbles (Figure 3), either via streptavidin-biotin bonding [37], [38] or by covalent coupling [39], as has been done earlier for liposome attachment to microbubbles [40]; this approach may significantly enhance delivery efficacy.
Figure 3.
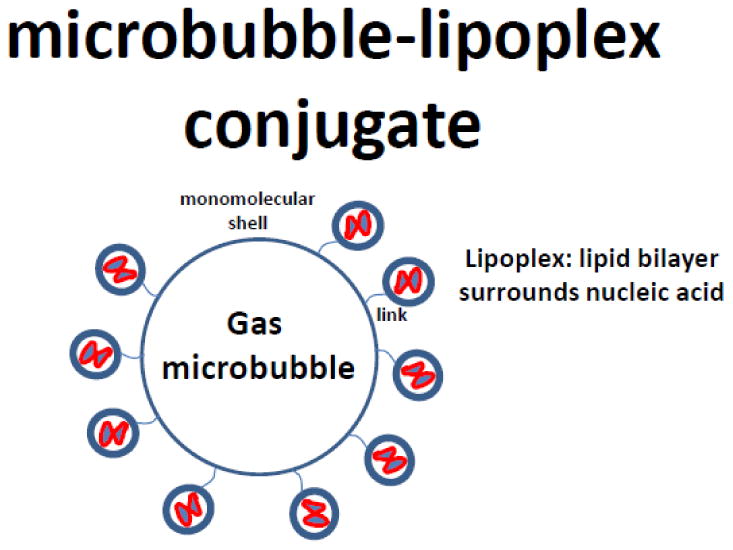
Micrometer-size bubble is decorated on the outside with a coat of nucleic acid carrier nanostructures, such as lipoplexes (lipid bilayer nanospheres with condensed nucleic acid as the inner cores).
Improvement of the nucleic acid load per microbubble can be also achieved by surface engineering: layer-by-layer (LBL) deposition of the opposite-charge polyelectrolytes on surfaces [41] can create multiple-layer sandwiches. This is a perfect tool to increase the plasmid or oligonucleotide load on the bubble surface: layered polyelectrolytes form extremely stable microcapsules, so nucleic acid layers can be embedded between the layers of a biocompatible polymer, e.g., polylysine [42]. Care should be taken to make a proper selection of polyelectrolyte to complex to nucleic acid, though: if not optimal, the complex might be too stable to accomplish effective release of nucleic acid in the cell cytoplasm and/or delivery into the nucleus, if necessary.
Viral particles placed on the microbubble shell may provide desired transfection efficacy, combined with acoustic activation specificity, if a virus cannot enter the cells by itself. One example of this approach was a study by Taylor et al., who took a deficient form of a retroviral vector incapable of self-replication and entry in the target cells, and placed it on the DSTAP/DSPC microbubbles by electrostatic coupling. Transfection with this system could only take place following ultrasound treatment [43]. Likewise, for cationic AAV virus attached to anionic microbubble shell, AAV6-microbubble constructs achieved an excellent transfection in insonated rat heart, an order of magnitude higher than plain virus - but in this case, the viral particle was fully functional and liver transfection was significant. Interestingly, when the same experiment was performed with AAV9-microbubbles, liver transfection was minimal, perhaps due to a difference in the binding affinity of the virus particle to certain cell types [44]. AAV9 serotype seems to be potentially important for ultrasound-microbubble gene delivery: recently, targeted transfection of brain in a murine model was accomplished by MR-guided focused ultrasound with a simple intravenous administration of Definity microbubbles, where ultrasound and microbubbles provided blood brain barrier permeability enhancement, 5 min later followed by AAV9 administration, so that the virus could enter the brain from the vasculature and provide transfection of neurons, astrocytes and oligodendrocytes with long-term expression of GFP [45] - we should note that in this case AAV was not attached to the microbubble surface.
2.5 Targeted microbubbles as nucleic acid delivery vehicles
Combination of positive electrostatic surface charge and negatively charged plasmid on the surface of the microbubbles does not preclude the use of targeting ligands for selective delivery of the particles to the tissue of interest. Phillips [46] tested this hypothesis in vitro and achieved selective targeting of activated smooth muscle cells by positively charged microbubbles carrying a eGFP-encoding plasmid, and decorated with anti-VCAM-1 antibodies; as compared with non-targeted bubbles, transfection efficacy increased by 5.5-fold, with just 200 KPa peak negative acoustic pressure. Xie and colleagues [2] compared transfection efficiencies between selectin-targeted and non-targeted positively charged microbubbles carrying a reporter transgene plasmid in a mouse model of hindlimb ischemia. It was found that targeting resulted in significantly higher transfection when sonoporation was performed at 0.6 MPa, although this difference was abrogated at higher acoustic pressures. Tlaxca and colleagues [47] examined inflammation marker-targeted plasmid-carrying microbubbles in a mouse model of inflammatory bowel disease. In this experiment, sonoporation of the abdomen was performed 10 minutes after anti-MAd-CAM-1 or anti-VCAM-1 antibody microbubble administration in order to allow circulating microbubbles to clear the vasculature (Figure 4). A potentially useful scenario justifying the use of these particles is the ability to minimize non-specific transfection, because targeted bubbles would preferably adhere to the target cells that express disease marker, e.g., VCAM-1. Even more important, there may be no need to use focused ultrasound to perform selective sonoporation of the tissue of interest under imaging guidance: wide unfocused “painting” with ultrasound probe will still allow specific transfection of the target, because circulating (or adherent) bubbles would not be present in other areas.
Figure 4.
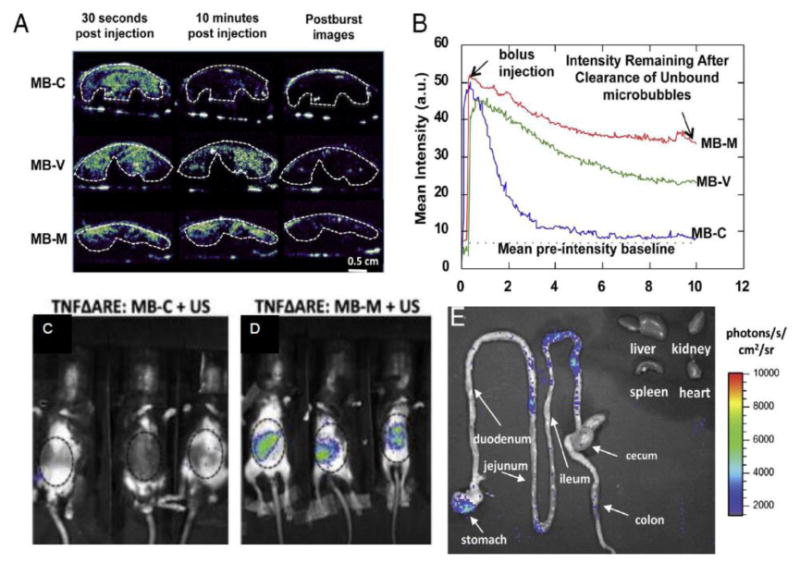
Delivery of plasmid to inflamed small bowel in mouse model of Crohn's disease. A) contrast ultrasound images showing behavior of plasmid-bearing microbubbles targeted with a control antibody (top), or with antibodies to VCAM-1 (mid) or MAdCAM-1 (bottom). B) Time-intensity curves corresponding to images in panel A. Sustained retention of MAdCAM-1 or VCAM-1 targeted agents is appreciated, while the control agents wash out of the bowel within 5 minutes. 24 hours after treatment, in vivo biolumenescence imaging shows C) minimal luciferase is detectible in mice treated with control targeted microbubbles, but (D) significant expression in mice receiving MAdCAM-1 targeted agent. E) Ex vivo biolumenescence imaging shows luciferase expression confined to small bowel, with undetectible off-target signal on heart, liver, kidney, and spleen. Reprinted with permission from [47] with permission, Copyright, 2013, Elsevier B.V.
2.6 Liposome constructs that entrap fluorocarbon nanoparticles, for ultrasound-assisted nucleic acid delivery
A lipid nanoparticle termed “bubble-liposome” was developed by Maruyama, Suzuki and colleagues at Teikyo University in Japan [48, 49]. This nanoparticle is a liposome which in addition to a payload encapsulates a perfluoropropane nanoparticle inside it (Figure 5). Initially tested for drug delivery, this particle is now widely investigated in animal models for nucleic acid delivery: a plasmid or an oligonucleotide can be entrapped inside the aqueous core of the liposome [50]. Alternatively, nucleic acid can be attached to the outside of the nanoparticles, with a standard positive lipid electrostatic interaction – in this case, positively charged lipid component is used for the liposome formulation [51, 52]. The advantage of the latter approach – an excellent yield of nucleic acid coupling to the particle and ease of use; disadvantage – in the potential nonspecific adhesion in non-target areas due to electrostatic interaction.
Figure 5.
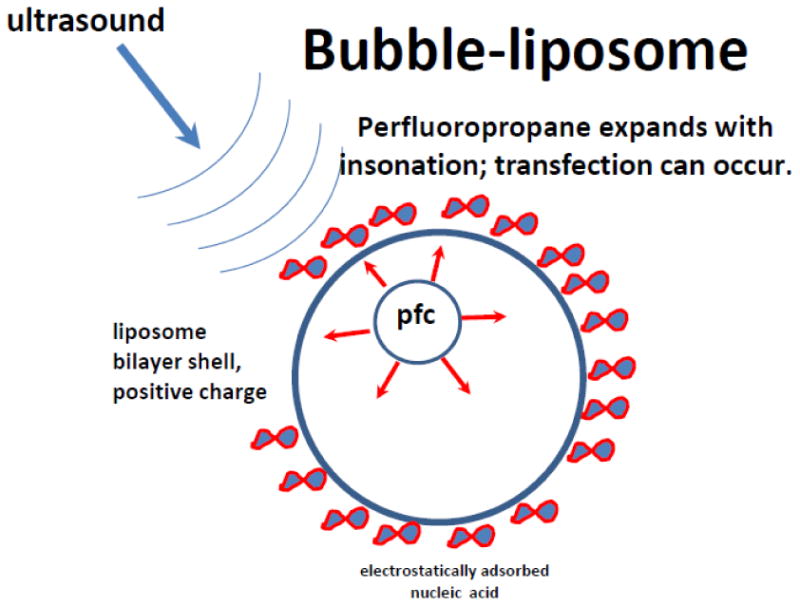
Bubble-liposome formulation [50]: a liposome nanoparticle, surrounded by a lipid bilayer that entraps even smaller perfluoropropane particle which can expand in response to ultrasound. Nucleic acid can be electrostatically attached to the outside of the liposome (as in traditional lipofection) or entrapped in the inner aqueous core.
Preparation of bubble-liposomes is quite simple: pre-formulated liposomes as an aqueous dispersion were placed in a vial with perfluoropropane atmosphere, further pressurized with perfluoropropane gas via a syringe and sealed, and subjected to bath sonication at 42 KHz for several minutes. Electron microscopy confirmed the presence of nanoparticle “droplets” inside the liposomes. For nucleic acid delivery, it was added to pre-formulated positively charged bubble-liposome preparations; successful in vitro and in vivo delivery [51, 52] and application of this approach for cancer vaccine has been reported [53]. It was noted by other investigators [54] that a perfluoropropane nanoparticle inside a liposome, due to its small size, under 60 nm, might be subjected to excessive Laplace pressure, calculated to reach tens of atmospheres. In their opinion, such gas particle would be unstable. Alternatively, one might hypothesize that perfluoropropane inside bubble-liposome may be in a liquid form. In that case, liquefied superheated perfluoropropane droplet, sequestered inside a liposome, would be triggered by ultrasound to become a much larger gas bubble, quite efficiently, despite the small initial size of the nanodroplet [55, 56]. It will expand and trigger the release of the liposome content in response to ultrasound treatment, which might explain stability and functionality of the “bubble liposome” formulation.
A formulation with a similar structure, termed “eliposome”, was manufactured by a different protocol and entraps not perfluoropropane, but liquid perfluoropentane gas precursor (Figure 6), which would expand to become gas at physiological temperature during ultrasound treatment [57]. A method of preparation of eliposomes is more complicated than for bubble liposomes: a lipid-stabilized liquid perfluoropentane nanoemulsion is prepared first, followed by co-entrapment of plasmid and nanoemulsion particles inside the carrier liposomes. The advantage of this formulation is in the lack of positive electrostatic charge on the external surface (thus, nonspecific adhesion to non-target cells could be reduced). To achieve selective adhesion, targeting ligands directed at the target cells receptors (e.g., folate) can be placed on the outer layer of eliposome [57].
Figure 6.
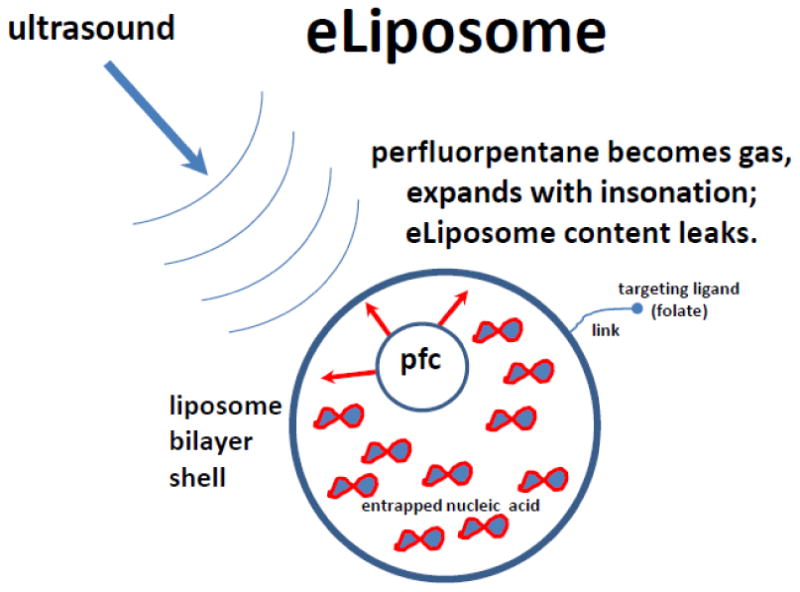
Eliposome formulation [54]: a liposome nanoparticle, consisting of a lipid bilayer that entraps inner aqueous core with perfluoropentane superheated droplets which can quickly expand to become gas at physiological temperatures in response to ultrasound. Triggered liposome contents release can thus take place. Nucleic acid can be entrapped and sequestered inside the liposome. For molecular targeting, selective ligands (e.g., folate) can be attached to the liposome surface.
A significant advantage of these nanoparticles when compared with gas-filled microbubbles comes from their smaller size: if liposomes are <250nm in diameter, with proper PEG coating they can generally circulate in the bloodstream for hours [58]. Presumably, encapsulated liquid fluorocarbon nanoparticle will stay inside the liposomes during recirculation, providing enough time to perform ultrasound treatment and improve delivery efficacy to the target by extending the time of ultrasound treatment. Smaller liposomes can also extravasate in the areas of disease, such as tumor (leaky neovasculature) or ischemic cardiac or skeletal muscle, where endothelial inflammation leads to permeabilization of the vessel lining [59]. Typical microbubble formulations used for gene delivery have the same size parameters as typical microbubbles approved for diagnostic ultrasound imaging, i.e., diameter range is ∼1-3um. Microbubbles will circulate only for minutes, with relatively rapid gas loss by exhalation via a lung route. Microbubbles stay intravascular, unless intense ultrasound treatment would result in the extravascular delivery of microbubble-associated plasmid, as was indeed detected by fluorescence microscopy of plasmid-associated dye in the interstitial space following plasmid-microbubble insonation in the cremaster muscle vasculature [12]. As a result of intense insonation, some RBC extravasation was also observed, which might not always be a desirable outcome. Thus, moderation of applied acoustic energy may result in directing transfection mostly to endothelial lining of the vessel wall [2, 60], which could be beneficial for certain research and clinical scenarios.
A multilamellar formulation, termed “echogenic liposome”, proposed almost two decades ago [61], was manufactured by hydration of the dry lyophilized precursor matrix. These particles have been tested for ultrasound-assisted plasmid delivery [62] and oligonucleotide delivery [63]. Particle size here is significantly larger than for eliposomes or bubble-liposomes. Acoustic response of these particles was found to be dependent on the pockets of entrapped air [64]: air core is postulated to be placed within the lipid bilayer of the liposome membrane [65]. Choice of air as an entrapped gas may reduce the lifetime in the bloodstream and in response to ultrasound treatment even if the gas pocket is inside the liposome; it is generally believed that low-solubility fluorocarbons, such as perfluorobutane or perfluoropropane, with water solubility orders of magnitude less than of air, provide much longer lifetime and slower gas exchange. Thus, further improvement of echogenic liposome formulations might be feasible.
3. In vivo nucleic acid delivery with sonoporation: tissues and diseases
Small animal studies have evaluated sonoporation based delivery using microbubbles in nearly every organ system, including heart [66], pancreas [36], liver [67], kidney [68], bowel [47], skin [24], eye [69], solid tumors [70] [71], joint synovium [72], dental pulp [73] and tendons [74]. While not sonoporation per se, opening of the blood brain barrier with microbubbles and focused ultrasound has also been demonstrated [75]. Although there are numerous applications for in vitro and ex vivo use in life science research and approaches towards clinical use, we will confine our discussion to key in vivo applications.
3.1 Myocardial ischemia
Early in vivo work in the field was concentrated on the heart, although this has shifted somewhat in recent years. Numerous studies have demonstrated delivery of reporters to myocardium. In general, most studies have confirmed that the ultrasound energy must be applied to the microbubble for payload delivery [76-78]; that is, delivery outside the ultrasound beam or mediated by ultrasound alone (without microbubbles) occurs at a very low rate. However, early work by [30] and [79] found transgene expression in distant (non-sonoporated organs), although this may be due to the use of a viral payload in the case of the latter. Delivery of functional luciferase-encoding plasmid to the myocardium in rats was reported [80], with little gene expression in the non-target organs, when microbubble composition was selected appropriately., Microbubble-assisted siRNA delivery to coronary endothelium was demonstrated [77].
Induction of therapeutic angiogenesis as a treatment for ischemic heart disease has been a significant focus in the cardiac sonoporation field. In one of the earliest studies, Mukherjee et al [81] demonstrated delivery of recombinant VEGF protein directly (not via gene delivery) and resultant endothelial proliferation in hypertensive rats. Similar findings of increased vascular density were reported by [78] and [82] following treatment with VEGF165 plasmid. Delivery of plasmid coding for stem cell factor (SCF) was explored by Fujii and colleagues [82, 83]. As was found with VEGF treatment, SCF delivery improved vascular density, cardiac function, and progenitor cell recruitment in a mouse model of myocardial ischemia. Repeated SCF treatment (up to 6 times over ten days) resulted in increased vascular density, improved cardiac function, and reduced scar size relative to a single treatment [83]. Delivery of hepatocyte growth factor to rodents, either in the form of recombinant protein [84] or (more appropriate for the topic of this review) a plasmid [66] also resulted in proliferation and improvement in cardiac function. These results demonstrate the feasibility of sonoporation to deliver functionally significant quantities of plasmid, siRNA, and even protein to the myocardium.
Cell-based therapy strategies have been examined in the context of ischemic cardiac disease, generally for stimulation/recruitment of progenitor cells. Delivery of plasmids coding for SDF-1 and SCF [83] or VEGF [82] were shown to increase the number of cardiac progenitor cells in the treated region. Sustained expression of TB4 over 12 weeks was shown to induce proliferation and differentiation of cardiac progenitor cells [70]. The use of sonoporation-based delivery strategies to induce the post-ischemic heart to create or recruit endogenous cell factors, either alone or with concomitant revascularization therapy, is a rapidly developing research topic.
3.2 Skeletal muscle and peripheral arterial disease
Skeletal muscle is a versatile tissue for sonoporation-based treatment, due both to its ease of access (typically hindlimb in experimental animals) and relevance to cardiovascular disease. Numerous studies have demonstrated transfection of muscle cells in mice, rats, and rabbits following intramuscular administration of microbubbles and reporter plasmids [20, 85-88]. It should be noted that skeletal muscle represents one of the few tissues in which contact-based delivery techniques (such as electroporation) can be readily performed without surgical access. However, the ability of intravascularly administered microbubbles to direct delivery specifically to the skeletal muscle vasculature represents a key advantage over competing systems.
Work by Christiansen [12] using cationic microbubbles showed successful transfection following either intra-arterial or intravenous administration. Intravital microscopy suggested that the vascular endothelium and associated cells appeared to be the dominant site of delivery, a finding supported by later studies [33]. Kobulnik [32] subsequently compared the efficacy of intravenously administered microbubble-nucleic acid complex with sonoporation to direct intramuscular injection of the same mass of transgene. It was found that direct intramuscular administration resulted in a significantly higher transfection, although this was confined to cells adjacent to the injection site. In contrast, sonoporation with intravenously administered microbubbles resulted in a more uniform treatment pattern. Interestingly, both methods resulted in similar therapeutic efficacy in a rat model of hindlimb ischemia.
Compelling work has been done using cationic microbubbles bearing pro-angiogenic transgenes in the context of chronic ischemic disease. Leong-Poi [33] demonstrated up-regulation of VEGF and other pro-angiogenic factors for up to two weeks following a single sonoporation treatment with a VEGF plasmid. Functional improvement in blood flow was observed at two weeks post treatment and persisted, although at a reduced level, for up to 6 weeks. Taniyama [88] used a protein shell microbubble and co-injected hepatocyte growth factor plasmid in a rabbit model of hind limb ischemia. Increased capillary density and arterial flow was demonstrated at 5 weeks after sonoporation treatment.
A recent study [34] explored treatment with an Ang-1 coding plasmid two weeks following VEGF-coding plasmid treatment, and demonstrated that this two-phase protocol produced a sustained improvement in functional blood flow over 6 weeks (Figure 7). This result was likely due to the formation of functionally mature vessels in response to the temporally separated delivery of two distinct pro-angiogenic therapies. The authors reported the predominant site of transfection to be arteriolar endothelium, with minimal capillary involvement.
Figure 7.

Sequential delivery of VEGF and Ang1 plasmids improves formation of functional vasculature in rat model of ischemic injury. A) Fluorescence microangiography images showing vascularity in injured tissue at 4 (top row) and 8 (bottom row) weeks after treatment. Untreated (control) animals exhibit low vessel density. Treatment with VEGF alone results in formation of immature vessels that largely regress within 8 weeks. Delivery of both VEGF and Ang1 together (V/A1 early) results in improved vessel density, while delivering Ang1 2 weeks after VEGF treatment (V+A1 late) yields sustained and functional vasculature. B) Non-invasive imaging of blood flow revealed that simultaneous or sequential treatment with VEGF and Ang1 results in a sustained improvement to vascular function relative to treatment with VEGF alone. Reprinted with permission from [34], Copyright, 2012 by the American College of Cardiology Foundation.
3.3 Nucleic acid delivery in pancreas and in diabetes treatment
Targeted gene delivery and interference strategies hold particular attraction for diabetes and other chronic metabolic disease, due to high incidence of the disease and difficulty in finding long-term treatment. A significant technical challenge here is confining the acoustic beam to the pancreas, owing to the close association of the pancreas to other abdominal organs and its complex anatomy in mice. The use of organ-specific promoters in the context of gene therapy appears to be a potential solution to this, with relatively low off-target transfection reported in the literature [36, 89].
In an elegant set of studies, Chen and colleagues [36] demonstrated specific delivery and expression of the plasmids that encoded human insulin and hexokinase to islet beta cells in rats (Figure 8). Use of a rat insulin promoter [36, 89] enabled specific transgene expression in the islets, with low to undetectable expression in the spleen, liver, and kidney. Transgene expression peaked at day 4 and then decayed steadily over 4 weeks following a single treatment, while improvement in functional parameters was demonstrated to persist for at least 10 days. Increased insulin secretion and commensurate reduction in plasma glucose was demonstrated in healthy rats following delivery of a hexokinase plasmid. Subsequent studies have focused on gene therapy approaches for beta cell regeneration.
Figure 8.

Delivery of plasmid to islet beta cells in rat. Top row are confocal micrographs of an islet from an animal treated with DsRed-coding plasmid. The location of the transferred gene (red) co-localizes with insulin-staining beta cells, demonstrating specific delivery. Bottom panel shows minimal payload deliver outside pancreas. Reprinted with permission from [36]. Copyright, 2006, National Academy of Sciences USA.
Sonoporation-based delivery of plasmid coding for both the mitogen betacellulin and pancreatic duodenal homeobox-1 (PDX-1) transcription factor (necessary for pancreatic development and β cell maturation in rodents) was shown to improve insulin production and glucose tolerance in a rat model of chemically induced beta cell destruction [90]. Interestingly, neither mature islets nor true beta cells were observed by traditional histological analyses. Yet a population of insulin producing cells that expressed certain beta cell markers were found in the exocrine pancreas of treated animals. Subsequent studies in which plasmid coding for transcription factors neuroD1 under an optimized [89] insulin promoter [91] or Nkx2.2 using a piggyBac transposon plasmid [92] were used, and initiated proliferation of adult pancreatic progenitor cells within islets to mature islets with β cells and α cells. A single ultrasound-microbubble-plasmid treatment demonstrated islet regeneration. Pretreatment with a JNK protein kinase inhibitor was shown to normalize blood glucose and insulin response for up to 90 days after a single treatment. Long term normalization (up to 6 months) was reported [93] following single treatment with a cocktail of plasmids designed to enable residual beta cell replication. A key aspect of these studies is that sustained transgene expression is not necessarily required to effect a clinically significant improvement in diabetes. Rather, transient expression (generally reported as 2-4 weeks) can be sufficient to initiate a sequence of events that alters the disease phenotype, and provides long term therapeutic effects.
3.4 Nucleic acid delivery to solid tumors
Solid tumors are an attractive target for many targeted delivery technologies, including sonoporation. At the current time, the volume of published work on tumors is significantly greater than that for any other organ system. A systematic assessment of the literature in this field is unfortunately beyond the scope of our current review; rather, we present experimental details and some key findings from the literature in Table 1 and discuss several most interesting examples below. We have concentrated on the studies in which therapeutically meaningful payloads have been reported.
Table 1.
Sonoporation for nucleic acid delivery in cancer: animal model studies.
| Tumor Model | Payload | Method | Findings | Reference |
|---|---|---|---|---|
| Pancreatic adenocarcinoma, mouse SubQ | p16 (plasmid) |
Polymer microbubbles with conjugated payload, single treatment, IV | Reduced tumor growth rate | Hauff et al, 2005 [71] |
| Hepatic adenocarcinoma, mouse SubQ | IFN-beta (plasmid) |
Lipid microbubbles, single treatment, intratumoral | Reduced tumor volume at 6 weeks | Sakakima et al, 2005 [99] |
| Cervical carcinoma, mouse SubQ | Human survivin (shRNA) |
Lipid microbubbles + PEG, single treatment, IV | ∼4-fold reduction in survivin expression at 3 weeks | Chen et al, 2010 [100] |
| Colon carcinoma, mouse intrahepatic | IFN-beta (plasmid) |
Cationic “liposome” microbubbles, single treatment, intratumoral | ∼2 fold increase in survival in microbubble treated animals, slight increase with addition of ultrasound | Hayashi et al, 2009 [101] |
| Gingival squamous carcinoma, mouse SubQ | Bleomycin (protein) or cytolethal descending toxin B (plasmid) |
Albminin microbubbles, 8 treatments, intratumoral | Tumor regression over 28 days, apoptosis | Iwanaga et al, 2007 [102] |
| Radiation induced fibrosarcoma (RIF-1), mouse SubQ | Luciferase (plasmid) |
Lipid microbubble, single treatment, intratumoral | Peak luciferase expression at day 4, sustained expression over 15 days | Li et al, 2009 [103] |
| Liver (BNL, SubQ or orthotopic), lung (LL/2, orthotopic), glioma (RT-2, orthotopic). All mouse. | Calreticulin or endostatin (plasmid) |
Lipid microbubble co-injected with plasmid, 4 treatments, intramuscular. | Plasmid delivery to skeletal muscle can treat distant tumors. | Liao et al, 2012 [104] |
| Breast carcinoma (MDA-MB-231), mouse orthotopic | Adenovirus coding for secreted tumor marker | Lyophilized lipid microbubble reconstituted with virus, targeting VEGFR2/integrin/P-selectin, single treatment, IV | Expression of reporter for 2 days after treatment. | Warram et al, 2012 [105] |
| Melanoma (C32), mouse subQ | IFN-beta (plasmid) |
Lipid microbubbles co-injected with plasmid, 4 treatments, intratumoral | Reduction in tumor growth rate during treatment | Yamaguchi et al, 2011 [106] |
| Squamous cell carcinoma, mouse SubQ | HSV thymidine kinase (plasmid) + daily ganciclovir |
Cationic lipid microbubble, single treatment, IV | Increased doubling time and apoptosis in treated tumors | Carson et al, 2011 [4] |
| Squamous cell carcinoma (SCC-VII), mouse SubQ | EGFR (siRNA) |
Lipid microbubble manufactured with siRNA, 2 treatments, IV | Decreased EGFR expression and increased doubling time | Carson et al, 2012 [3] |
| Prostatic carcinoma (DU-145), mouse SubQ | Adenovirus coding for MDA-7 (IL-24) | Lyophilized lipid microbubble reconstituted with virus, 5 treatments, IV | Eradication of both sonoporated and distant tumors | Greco et al, 2010 [107] |
| Prostatic carcinoma, orthotopic (Hi-Myc transgenic mouse) | Adenovirus coding for MDA-7 (IL-24) | Lyophilized lipid microbubble reconstituted with virus, 9 treatments, IV | Reduction in size of prostate and increased tumor apoptosis in treated animals | Dash et al, 2011 [94] |
Much of the work in this field has been done in subcutaneous tumor models in mice. Although this minimizes the technical challenges of performing the sonoporation procedure, it should be noted that subcutaneous models may not represent a realistic scenario for the tumors that would be treated in human disease. Several notable studies have utilized orthotopic models, e.g., of prostate cancer [94], providing compelling evidence of efficacy.
Of particular interest for cancer therapy is the ability to either express the genes encoded in plasmids or deliver small molecules such as siRNA with simple positively charged perfluorobutane microbubbles used as a carrier, combined with ultrasound to achieve delivery specificity. This approach was described by Villanueva for targeted ultrasound-triggered thymidine kinase gene expression, which resulted in tumor growth inhibition in a murine cancer model when combined with prodrug gancyclovir, activated by the enzyme [4]. Suppression of tumor growth was reported by Leong Poi [95], who also used decafluorobutane microbubbles with DSPC/PEG stearate shell with positively charged lipid for nucleic acid attachment. These microbubbles were carrying a short hairpin RNA-encoding plasmid to inhibit VEGFR2 in rodents. It was noted, that for the most efficient delivery, ultrasound application in a slow tumor vasculature blood flow scenario should be performed intermittently, with long intervals (up to 10-20 seconds between ultrasound pulses), to allow complete replenishment of the blood vessels with the microbubble-nucleic acid construct, followed by the subsequent ultrasound pulse. Likewise, ultrasound-triggered activation of microbubbles carrying siRNA directed to EGF receptor in a murine squamous cell cancer model allowed to decrease EGFR expression and inhibited tumor growth [3].
One of the interesting strategies in the development of cancer vaccines is to transfect the gene that encodes the desired antigen into dendritic cells for its expression and proper presentation, which would be then applied to obtain antitumor immune response. This transfection can be achieved with ultrasound activation of microbubble-lipoplex complexes, where a plasmid entrapped in lipoplex is encoding the antigen. Cell viability was retained, and transient protein expression was achieved. Authors propose to co-transfect the antigen and dendritic cell maturation signals to improve the efficacy [23].
Use of virus-based systems for tumor therapy should not be overlooked: virus-microbubble mixture can be injected intravenously, followed by focused ultrasound treatment [96], which resulted in the increase of luciferase expression in the insonated tumor up to 50-fold.
Recently, the first clinical trial utilizing sonoporation and microbubbles for local therapeutic delivery was published by Kotopoulis and colleagues [97]. In that study, five patients with inoperable pancreatic cancer were administered gemcitabine followed by sequential doses of microbubble contrast agents and ultrasound within a 30 min interval. Low-intensity ultrasound was administered with a diagnostic imaging ultrasound system, enabling simultaneous visualization and image-guided sonoporation. Compared with a historical control group of 80 patients treated with gemcitabine alone, treatment patients were able to tolerate a greater number of chemotherapy rounds and exhibited a reduction in tumor size and/or growth rate. No adverse effects relating to the sonoporation procedure were reported, although the treatment protocol used somewhat lower intensity than is typically applied in animal studies. This study establishes that a sonoporation protocol can be performed successfully in a clinical setting, and represents an important first step toward clinical development of this promising technique. While there was no nucleic acid delivery reported in this particular study, its general importance and relevance for the nucleic acid delivery field is clear, especially combined with the point that nucleic acid delivery may avoid systemic toxicity associated with regular chemotherapy. An example of such combination gene therapy is an animal study by Machluf et al [98], where Optison microbubbles were locally administered in the tumor along with a plasmid encoding anti-angiogenic hemopexin-like domain fragment (PEX) in mice bearing subcutaneous prostate cancer, and clinical ultrasound applied with a physical therapy apparatus. Local administration of microbubbles and the plasmid allowed efficient and direct transfection of the cells in the tumor mass. Thus, a single treatment resulted in a significant reduction of the tumor growth rate, for up to 28 days.
4. Conclusions
Application of ultrasound for nucleic acid delivery provides a unique ability to trigger the transfection specifically in the areas of disease. Ultrasound can be applied with a simplest physical therapy apparatus, or with diagnostic clinical equipment which also provide ultrasound imaging guidance, or even more precise and powerful MRI-guided focused ultrasound systems. While sonoporation transfection efficacy levels are generally not perfect, the ability to achieve nucleic acid delivery specifically in the ultrasound-treated areas, and not in the traditional nonspecific targets (e.g., liver or lung) convey a significant advantage to this approach. With the existing ultrasound contrast agents (microbubbles) already approved for clinical diagnostic imaging, and a wide array of nucleic acid materials and carriers available as GLP/GMP materials in clinical trials already, a combination approach that takes advantage of the localized energy deposition and nucleic acid carriers should reach clinical trials stage in the near future.
More complicated structures, which combine nucleic acid and ultrasound-triggered carrier into micro- or nanoparticles, realistically, should take a much longer time to bring to clinic (they will be regarded by FDA as drugs, and historically it takes many years). These combined particles might provide more efficient therapy, especially for the delivery of short nucleic acid fragments (antisense, siRNA, microRNA). Use of viral structures, such as adenovirus or AAV, regarded as therapeutic biological products by FDA, in combination with microbubbles and ultrasound, might also reach clinical testing within a few years, especially if viral transfection in the remote organs is negligible, and safety is assured. Overall, with the progress of technology, ultrasound-assisted nucleic acid delivery may provide much needed therapeutic breakthrough, especially for the scenarios where only palliative treatment is currently available.
Acknowledgments
Authors are grateful to collaborators and colleagues, especially Jonathan Lindner, Klaus Ley, John Hossack and many others, at University of Virginia in Charlottesville and other research institutions for guidance and advice. A. Klibanov was supported in part via NIH R21/33 CA102880 and R21 EB016752. J. Rychak was supported in part via NIH R44 DK083142.
Abbreviations
- AAV
Adeno-associated virus
- Ang1
Angiopoietin 1
- DSPC
distearoyl phosphatidylcholine
- DSTAP
distearoyl trimethylammonium propane
- EGF
epidermal growth factor
- eGFP
enhanced GFP
- EGFR
EGF receptor
- GFP
green fluorescent protein
- GLP/GMP
good laboratory practice / good manufacturing practice
- JNK
c-Jun N-terminal kinase
- MAdCAM-1
mucosal vascular addressin cell adhesion molecule 1
- neuroD1
neurogenic differentiation factor 1
- Nkx2.2
NK-type homeodomain transcription factor
- PDX-1
pancreatic duodenal homeobox-1 transcription factor
- PEG
poly(ethylene glycol)
- PiggyBac
piggyBac transposon
- RBC
red blood cell
- SDF-1
stromal cell-derived factor 1
- siRNA
small interfering RNA
- TB4
Thymosin beta-4
- VCAM-1
vascular cell adhesion molecule 1
- VEGF
vascular endothelium growth factor
- VEGFR2
VEGF Receptor 2
Footnotes
Chemical compounds: Perfluoropropane, CID: 6432. Perfluorobutane, CID: 9638. Perfluoropentane, CID: 12675. Distearoyl phosphatidylcholine, CID: 94190.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fechheimer M, Boylan JF, Parker S, Sisken JE, Patel GL, Zimmer SG. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:8463–8467. doi: 10.1073/pnas.84.23.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie A, Belcik T, Qi Y, Morgan TK, Champaneri SA, Taylor S, Davidson BP, Zhao Y, Klibanov AL, Kuliszewski MA, Leong-Poi H, Ammi A, Lindner JR. Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles. JACC Cardiovascular imaging. 2012;5:1253–1262. doi: 10.1016/j.jcmg.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson AR, McTiernan CF, Lavery L, Grata M, Leng X, Wang J, Chen X, Villanueva FS. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer research. 2012;72:6191–6199. doi: 10.1158/0008-5472.CAN-11-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson AR, McTiernan CF, Lavery L, Hodnick A, Grata M, Leng X, Wang J, Chen X, Modzelewski RA, Villanueva FS. Gene therapy of carcinoma using ultrasound-targeted microbubble destruction. Ultrasound in medicine & biology. 2011;37:393–402. doi: 10.1016/j.ultrasmedbio.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang DS, Panje C, Pysz MA, Paulmurugan R, Rosenberg J, Gambhir SS, Schneider M, Willmann JK. Cationic versus neutral microbubbles for ultrasound-mediated gene delivery in cancer. Radiology. 2012;264:721–732. doi: 10.1148/radiol.12112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. The journal of gene medicine. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Human gene therapy. 1996;7:1339–1346. doi: 10.1089/hum.1996.7.11-1339. [DOI] [PubMed] [Google Scholar]
- 9.Greenleaf WJ, Bolander ME, Sarkar G, Goldring MB, Greenleaf JF. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound in medicine & biology. 1998;24:587–595. doi: 10.1016/s0301-5629(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 10.Blomley M. Which US microbubble contrast agent is best for gene therapy? Radiology. 2003;229:297–298. doi: 10.1148/radiol.2292031048. [DOI] [PubMed] [Google Scholar]
- 11.Pislaru SV, Pislaru C, Kinnick RR, Singh R, Gulati R, Greenleaf JF, Simari RD. Optimization of ultrasound-mediated gene transfer: comparison of contrast agents and ultrasound modalities. European heart journal. 2003;24:1690–1698. doi: 10.1016/s0195-668x(03)00469-x. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen JP, French BA, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound in medicine & biology. 2003;29:1759–1767. doi: 10.1016/s0301-5629(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 13.Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound in medicine & biology. 2006;32:915–924. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- 14.Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound in medicine & biology. 2004;30:519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 15.van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, de Jong N. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. Journal of controlled release : official journal of the Controlled Release Society. 2006;112:149–155. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Yudina A, Lepetit-Coiffe M, Moonen CT. Evaluation of the temporal window for drug delivery following ultrasound-mediated membrane permeability enhancement. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2011;13:239–249. doi: 10.1007/s11307-010-0346-5. [DOI] [PubMed] [Google Scholar]
- 17.Yudina A, de Smet M, Lepetit-Coiffe M, Langereis S, Van Ruijssevelt L, Smirnov P, Bouchaud V, Voisin P, Grull H, Moonen CT. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. Journal of controlled release : official journal of the Controlled Release Society. 2011;155:442–448. doi: 10.1016/j.jconrel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Escoffre JM, Novell A, Piron J, Zeghimi A, Doinikov A, Bouakaz A. Microbubble attenuation and destruction: are they involved in sonoporation efficiency? IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2013;60:46–52. doi: 10.1109/TUFFC.2013.2536. [DOI] [PubMed] [Google Scholar]
- 19.Meijering BD, Juffermans LJ, van Wamel A, Henning RH, Zuhorn IS, Emmer M, Versteilen AM, Paulus WJ, van Gilst WH, Kooiman K, de Jong N, Musters RJ, Deelman LE, Kamp O. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circulation research. 2009;104:679–687. doi: 10.1161/CIRCRESAHA.108.183806. [DOI] [PubMed] [Google Scholar]
- 20.Lu QL, Liang HD, Partridge T, Blomley MJ. Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene therapy. 2003;10:396–405. doi: 10.1038/sj.gt.3301913. [DOI] [PubMed] [Google Scholar]
- 21.Houk BE, Martin R, Hochhaus G, Hughes JA. Pharmacokinetics of plasmid DNA in the rat. Pharmaceutical research. 2001;18:67–74. doi: 10.1023/a:1011078711008. [DOI] [PubMed] [Google Scholar]
- 22.Fenske DB, Cullis PR. Liposomal nanomedicines. Expert opinion on drug delivery. 2008;5:25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 23.De Temmerman ML, Dewitte H, Vandenbroucke RE, Lucas B, Libert C, Demeester J, De Smedt SC, Lentacker I, Rejman J. mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials. 2011;32:9128–9135. doi: 10.1016/j.biomaterials.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Yoon CS, Jung HS, Kwon MJ, Lee SH, Kim CW, Kim MK, Lee M, Park JH. Sonoporation of the minicircle-VEGF(165) for wound healing of diabetic mice. Pharmaceutical research. 2009;26:794–801. doi: 10.1007/s11095-008-9778-x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:1298–1304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene therapy. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 27.Burke CW, Suk JS, Kim AJ, Hsiang YH, Klibanov AL, Hanes J, Price RJ. Markedly enhanced skeletal muscle transfection achieved by the ultrasound-targeted delivery of non-viral gene nanocarriers with microbubbles. Journal of controlled release : official journal of the Controlled Release Society. 2012;162:414–421. doi: 10.1016/j.jconrel.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panje CM, Wang DS, Pysz MA, Paulmurugan R, Ren Y, Tranquart F, Tian L, Willmann JK. Ultrasound-mediated gene delivery with cationic versus neutral microbubbles: effect of DNA and microbubble dose on in vivo transfection efficiency. Theranostics. 2012;2:1078–1091. doi: 10.7150/thno.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unger EC, McCreery TP, Sweitzer RH. Ultrasound enhances gene expression of liposomal transfection. Investigative radiology. 1997;32:723–727. doi: 10.1097/00004424-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Vannan M, McCreery T, Li P, Han Z, Unger E, Kuersten B, Nabel E, Rajagopalan S. Ultrasound-mediated transfection of canine myocardium by intravenous administration of cationic microbubble-linked plasmid DNA. Journal of the American Society of Echocardiography. 2002;15:214–218. doi: 10.1067/mje.2002.119913. [DOI] [PubMed] [Google Scholar]
- 31.Anwer K, Kao G, Proctor B, Anscombe I, Florack V, Earls R, Wilson E, McCreery T, Unger E, Rolland A, Sullivan SM. Ultrasound enhancement of cationic lipid-mediated gene transfer to primary tumors following systemic administration. Gene therapy. 2000;7:1833–1839. doi: 10.1038/sj.gt.3301302. [DOI] [PubMed] [Google Scholar]
- 32.Kobulnik J, Kuliszewski MA, Stewart DJ, Lindner JR, Leong-Poi H. Comparison of gene delivery techniques for therapeutic angiogenesis ultrasound-mediated destruction of carrier microbubbles versus direct intramuscular injection. J Am Coll Cardiol. 2009;54:1735–1742. doi: 10.1016/j.jacc.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ, Lindner JR. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circulation research. 2007;101:295–303. doi: 10.1161/CIRCRESAHA.107.148676. [DOI] [PubMed] [Google Scholar]
- 34.Smith AH, Kuliszewski MA, Liao C, Rudenko D, Stewart DJ, Leong-Poi H. Sustained improvement in perfusion and flow reserve after temporally separated delivery of vascular endothelial growth factor and angiopoietin-1 plasmid deoxyribonucleic acid. J Am Coll Cardiol. 2012;59:1320–1328. doi: 10.1016/j.jacc.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Porter TR, Xie F, Knapp D, Iversen P, Marky LA, Tsutsui JM, Maiti S, Lof J, Radio SJ, Kipshidze N. Targeted vascular delivery of antisense molecules using intravenous microbubbles. Cardiovascular revascularization medicine : including molecular interventions. 2006;7:25–33. doi: 10.1016/j.carrev.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Ding JH, Bekeredjian R, Yang BZ, Shohet RV, Johnston SA, Hohmeier HE, Newgard CB, Grayburn PA. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8469–8474. doi: 10.1073/pnas.0602921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandenbroucke RE, Lentacker I, Demeester J, De Smedt SC, Sanders NN. Ultrasound assisted siRNA delivery using PEG-siPlex loaded microbubbles. Journal of controlled release : official journal of the Controlled Release Society. 2008;126:265–273. doi: 10.1016/j.jconrel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Yang D, Gao YH, Tan KB, Zuo ZX, Yang WX, Hua X, Li PJ, Zhang Y, Wang G. Inhibition of hepatic fibrosis with artificial microRNA using ultrasound and cationic liposome-bearing microbubbles. Gene therapy. 2013 doi: 10.1038/gt.2013.41. [DOI] [PubMed] [Google Scholar]
- 39.Sirsi SR, Hernandez SL, Zielinski L, Blomback H, Koubaa A, Synder M, Homma S, Kandel JJ, Yamashiro DJ, Borden MA. Polyplex-microbubble hybrids for ultrasound-guided plasmid DNA delivery to solid tumors. Journal of controlled release : official journal of the Controlled Release Society. 2012;157:224–234. doi: 10.1016/j.jconrel.2011.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geers B, Lentacker I, Sanders NN, Demeester J, Meairs S, De Smedt SC. Self-assembled liposome-loaded microbubbles: The missing link for safe and efficient ultrasound triggered drug-delivery. Journal of controlled release : official journal of the Controlled Release Society. 2011;152:249–256. doi: 10.1016/j.jconrel.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Antipov AA, Sukhorukov GB. Polyelectrolyte multilayer capsules as vehicles with tunable permeability. Advances in colloid and interface science. 2004;111:49–61. doi: 10.1016/j.cis.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Borden MA, Caskey CF, Little E, Gillies RJ, Ferrara KW. DNA and polylysine adsorption and multilayer construction onto cationic lipid-coated microbubbles. Langmuir : the ACS journal of surfaces and colloids. 2007;23:9401–9408. doi: 10.1021/la7009034. [DOI] [PubMed] [Google Scholar]
- 43.Taylor SL, Rahim AA, Bush NL, Bamber JC, Porter CD. Targeted retroviral gene delivery using ultrasound. The journal of gene medicine. 2007;9:77–87. doi: 10.1002/jgm.1003. [DOI] [PubMed] [Google Scholar]
- 44.Muller OJ, Schinkel S, Kleinschmidt JA, Katus HA, Bekeredjian R. Augmentation of AAV-mediated cardiac gene transfer after systemic administration in adult rats. Gene therapy. 2008;15:1558–1565. doi: 10.1038/gt.2008.111. [DOI] [PubMed] [Google Scholar]
- 45.Thevenot E, Jordao JF, O'Reilly MA, Markham K, Weng YQ, Foust KD, Kaspar BK, Hynynen K, Aubert I. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Human gene therapy. 2012;23:1144–1155. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips LC, Klibanov AL, Wamhoff BR, Hossack JA. Intravascular ultrasound detection and delivery of molecularly targeted microbubbles for gene delivery. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2012;59:1596–1601. doi: 10.1109/TUFFC.2012.2359. [DOI] [PubMed] [Google Scholar]
- 47.Tlaxca JL, Rychak JJ, Ernst PB, Konkalmatt PR, Shevchenko TI, Pizzaro TT, Rivera-Nieves J, Klibanov AL, Lawrence MB. Ultrasound-based molecular imaging and specific gene delivery to mesenteric vasculature by endothelial adhesion molecule targeted microbubbles in a mouse model of Crohn's disease. Journal of controlled release : official journal of the Controlled Release Society. 2013;165:216–225. doi: 10.1016/j.jconrel.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Negishi Y, Endo Y, Fukuyama T, Suzuki R, Takizawa T, Omata D, Maruyama K, Aramaki Y. Delivery of siRNA into the cytoplasm by liposomal bubbles and ultrasound. Journal of controlled release : official journal of the Controlled Release Society. 2008;132:124–130. doi: 10.1016/j.jconrel.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki R, Takizawa T, Negishi Y, Utoguchi N, Maruyama K. Effective gene delivery with novel liposomal bubbles and ultrasonic destruction technology. International journal of pharmaceutics. 2008;354:49–55. doi: 10.1016/j.ijpharm.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki R, Maruyama K. Effective in vitro and in vivo gene delivery by the combination of liposomal bubbles (bubble liposomes) and ultrasound exposure. Methods Mol Biol. 2010;605:473–486. doi: 10.1007/978-1-60327-360-2_33. [DOI] [PubMed] [Google Scholar]
- 51.Endo-Takahashi Y, Negishi Y, Nakamura A, Suzuki D, Ukai S, Sugimoto K, Moriyasu F, Takagi N, Suzuki R, Maruyama K, Aramaki Y. pDNA-loaded Bubble liposomes as potential ultrasound imaging and gene delivery agents. Biomaterials. 2013;34:2807–2813. doi: 10.1016/j.biomaterials.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 52.Endo-Takahashi Y, Negishi Y, Kato Y, Suzuki R, Maruyama K, Aramaki Y. Efficient siRNA delivery using novel siRNA-loaded Bubble liposomes and ultrasound. International journal of pharmaceutics. 2012;422:504–509. doi: 10.1016/j.ijpharm.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials. 2010;31:7813–7826. doi: 10.1016/j.biomaterials.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 54.Pitt WG, Husseini GA. On bubbles and liposomes (June 11, 2007) Journal of controlled release : official journal of the Controlled Release Society. 2008;125:174–175. doi: 10.1016/j.jconrel.2007.10.004. discussion 175-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsunaga TO, Sheeran PS, Luois S, Streeter JE, Mullin LB, Banerjee B, Dayton PA. Phase-change nanoparticles using highly volatile perfluorocarbons: toward a platform for extravascular ultrasound imaging. Theranostics. 2012;2:1185–1198. doi: 10.7150/thno.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheeran PS, Luois SH, Mullin LB, Matsunaga TO, Dayton PA. Design of ultrasonically-activatable nanoparticles using low boiling point perfluorocarbons. Biomaterials. 2012;33:3262–3269. doi: 10.1016/j.biomaterials.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Javadi M, Pitt WG, Tracy CM, Barrow JR, Willardson BM, Hartley JM, Tsosie NH. Ultrasonic gene and drug delivery using eLiposomes. Journal of controlled release : official journal of the Controlled Release Society. 2013;167:92–100. doi: 10.1016/j.jconrel.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Klibanov AL, Maruyama K, Beckerleg AM, Torchilin VP, Huang L. Activity of amphipathic poly(ethylene glycol) 5000 to prolong the circulation time of liposomes depends on the liposome size and is unfavorable for immunoliposome binding to target. Biochimica et biophysica acta. 1991;1062:142–148. doi: 10.1016/0005-2736(91)90385-l. [DOI] [PubMed] [Google Scholar]
- 59.Rodrigues SF, Granger DN. Role of blood cells in ischaemia-reperfusion induced endothelial barrier failure. Cardiovascular research. 2010;87:291–299. doi: 10.1093/cvr/cvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geis NA, Mayer CR, Kroll RD, Hardt SE, Katus HA, Bekeredjian R. Spatial distribution of ultrasound targeted microbubble destruction increases cardiac transgene expression but not capillary permeability. Ultrasound in medicine & biology. 2009;35:1119–1126. doi: 10.1016/j.ultrasmedbio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Alkan-Onyuksel H, Demos SM, Lanza GM, Vonesh MJ, Klegerman ME, Kane BJ, Kuszak J, McPherson DD. Development of inherently echogenic liposomes as an ultrasonic contrast agent. Journal of pharmaceutical sciences. 1996;85:486–490. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- 62.Tiukinhoy SD, Mahowald ME, Shively VP, Nagaraj A, Kane BJ, Klegerman ME, MacDonald RC, McPherson DD, Matsumura JS. Development of echogenic, plasmid-incorporated, tissue-targeted cationic liposomes that can be used for directed gene delivery. Investigative radiology. 2000;35:732–738. doi: 10.1097/00004424-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Buchanan KD, Huang SL, Kim H, McPherson DD, MacDonald RC. Encapsulation of NF-kappaB decoy oligonucleotides within echogenic liposomes and ultrasound-triggered release. Journal of controlled release : official journal of the Controlled Release Society. 2010;141:193–198. doi: 10.1016/j.jconrel.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang SL, Hamilton AJ, Pozharski E, Nagaraj A, Klegerman ME, McPherson DD, MacDonald RC. Physical correlates of the ultrasonic reflectivity of lipid dispersions suitable as diagnostic contrast agents. Ultrasound in medicine & biology. 2002;28:339–348. doi: 10.1016/s0301-5629(01)00512-9. [DOI] [PubMed] [Google Scholar]
- 65.Nahire R, Paul S, Scott MD, Singh RK, Muhonen WW, Shabb J, Gange KN, Srivastava DK, Sarkar K, Mallik S. Ultrasound enhanced matrix metalloproteinase-9 triggered release of contents from echogenic liposomes. Molecular pharmaceutics. 2012;9:2554–2564. doi: 10.1021/mp300165s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kondo I, Ohmori K, Oshita A, Takeuchi H, Fuke S, Shinomiya K, Noma T, Namba T, Kohno M. Treatment of acute myocardial infarction by hepatocyte growth factor gene transfer: the first demonstration of myocardial transfer of a “functional” gene using ultrasonic microbubble destruction. J Am Coll Cardiol. 2004;44:644–653. doi: 10.1016/j.jacc.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 67.Shen ZP, Brayman AA, Chen L, Miao CH. Ultrasound with microbubbles enhances gene expression of plasmid DNA in the liver via intraportal delivery. Gene therapy. 2008;15:1147–1155. doi: 10.1038/gt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. Journal of the American Society of Nephrology : JASN. 2003;14:1535–1548. doi: 10.1097/01.asn.0000067632.04658.b8. [DOI] [PubMed] [Google Scholar]
- 69.Sonoda S, Tachibana K, Uchino E, Okubo A, Yamamoto M, Sakoda K, Hisatomi T, Sonoda KH, Negishi Y, Izumi Y, Takao S, Sakamoto T. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Investigative ophthalmology & visual science. 2006;47:558–564. doi: 10.1167/iovs.05-0889. [DOI] [PubMed] [Google Scholar]
- 70.Chen S, Shimoda M, Chen J, Grayburn PA. Stimulation of adult resident cardiac progenitor cells by durable myocardial expression of thymosin beta 4 with ultrasound-targeted microbubble delivery. Gene therapy. 2013;20:225–233. doi: 10.1038/gt.2012.89. [DOI] [PubMed] [Google Scholar]
- 71.Hauff P, Seemann S, Reszka R, Schultze-Mosgau M, Reinhardt M, Buzasi T, Plath T, Rosewicz S, Schirner M. Evaluation of gas-filled microparticles and sonoporation as gene delivery system: feasibility study in rodent tumor models. Radiology. 2005;236:572–578. doi: 10.1148/radiol.2362040870. [DOI] [PubMed] [Google Scholar]
- 72.Saito M, Mazda O, Takahashi KA, Arai Y, Kishida T, Shin-Ya M, Inoue A, Tonomura H, Sakao K, Morihara T, Imanishi J, Kawata M, Kubo T. Sonoporation mediated transduction of pDNA/siRNA into joint synovium in vivo. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25:1308–1316. doi: 10.1002/jor.20392. [DOI] [PubMed] [Google Scholar]
- 73.Nakashima M, Tachibana K, Iohara K, Ito M, Ishikawa M, Akamine A. Induction of reparative dentin formation by ultrasound-mediated gene delivery of growth/differentiation factor 11. Human gene therapy. 2003;14:591–597. doi: 10.1089/104303403764539369. [DOI] [PubMed] [Google Scholar]
- 74.Delalande A, Bouakaz A, Renault G, Tabareau F, Kotopoulis S, Midoux P, Arbeille B, Uzbekov R, Chakravarti S, Postema M, Pichon C. Ultrasound and microbubble-assisted gene delivery in Achilles tendons: long lasting gene expression and restoration of fibromodulin KO phenotype. Journal of controlled release : official journal of the Controlled Release Society. 2011;156:223–230. doi: 10.1016/j.jconrel.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 75.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. Journal of neurosurgery. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 76.Chen Z, Xie M, Wang X, Lv Q, Ding S. Efficient gene delivery to myocardium with ultrasound targeted microbubble destruction and polyethylenimine. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2008;28:613–617. doi: 10.1007/s11596-008-0528-4. [DOI] [PubMed] [Google Scholar]
- 77.Tsunoda S, Mazda O, Oda Y, Iida Y, Akabame S, Kishida T, Shin-Ya M, Asada H, Gojo S, Imanishi J, Matsubara H, Yoshikawa T. Sonoporation using microbubble BR14 promotes pDNA/siRNA transduction to murine heart. Biochemical and biophysical research communications. 2005;336:118–127. doi: 10.1016/j.bbrc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 78.Korpanty G, Chen S, Shohet RV, Ding J, Yang B, Frenkel PA, Grayburn PA. Targeting of VEGF-mediated angiogenesis to rat myocardium using ultrasonic destruction of microbubbles. Gene therapy. 2005;12:1305–1312. doi: 10.1038/sj.gt.3302532. [DOI] [PubMed] [Google Scholar]
- 79.Chen S, Shohet RV, Bekeredjian R, Frenkel P, Grayburn PA. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by Ultrasound-Targeted microbubble destruction. Journal of the American College of Cardiology. 2003;42:301–308. doi: 10.1016/s0735-1097(03)00627-2. [DOI] [PubMed] [Google Scholar]
- 80.Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108:1022–1026. doi: 10.1161/01.CIR.0000084535.35435.AE. [DOI] [PubMed] [Google Scholar]
- 81.Mukherjee D, Wong J, Griffin B, Ellis SG, Porter T, Sen S, Thomas JD. Tenfold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. J Am Coll Cardiol. 2000;35:1678–1686. doi: 10.1016/s0735-1097(00)00575-1. [DOI] [PubMed] [Google Scholar]
- 82.Fujii H, Sun Z, Li SH, Wu J, Fazel S, Weisel RD, Rakowski H, Lindner J, Li RK. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovascular imaging. 2009;2:869–879. doi: 10.1016/j.jcmg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Fujii H, Li SH, Wu J, Miyagi Y, Yau TM, Rakowski H, Egashira K, Guo J, Weisel RD, Li RK. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. European heart journal. 2011;32:2075–2084. doi: 10.1093/eurheartj/ehq475. [DOI] [PubMed] [Google Scholar]
- 84.Iwasaki M, Adachi Y, Nishiue T, Minamino K, Suzuki Y, Zhang Y, Nakano K, Koike Y, Wang J, Mukaide H, Taketani S, Yuasa F, Tsubouchi H, Gohda E, Iwasaka T, Ikehara S. Hepatocyte growth factor delivered by ultrasound-mediated destruction of microbubbles induces proliferation of cardiomyocytes and amelioration of left ventricular contractile function in Doxorubicin-induced cardiomyopathy. Stem cells. 2005;23:1589–1597. doi: 10.1634/stemcells.2005-0049. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, Wang Z, Ran H, Fu X, Li X, Zheng Y, Peng M, Chen M, Schutt CE. Enhanced gene delivery into skeletal muscles with ultrasound and microbubble techniques. Academic radiology. 2006;13:363–367. doi: 10.1016/j.acra.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Li T, Tachibana K, Kuroki M. Gene transfer with echo-enhanced contrast agents: comparison between Albunex, Optison, and Levovist in mice--initial results. Radiology. 2003;229:423–428. doi: 10.1148/radiol.2292020500. [DOI] [PubMed] [Google Scholar]
- 87.Wang X, Liang HD, Dong B, Lu QL, Blomley MJ. Gene transfer with microbubble ultrasound and plasmid DNA into skeletal muscle of mice: comparison between commercially available microbubble contrast agents. Radiology. 2005;237:224–229. doi: 10.1148/radiol.2371040805. [DOI] [PubMed] [Google Scholar]
- 88.Taniyama Y, Tachibana K, Hiraoka K, Aoki M, Yamamoto S, Matsumoto K, Nakamura T, Ogihara T, Kaneda Y, Morishita R. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene therapy. 2002;9:372–380. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]
- 89.Chai R, Chen S, Ding J, Grayburn PA. Efficient, glucose responsive and islet-specific transgene expression by a modified rat insulin promoter. Gene therapy. 2009;16:1202–1209. doi: 10.1038/gt.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen S, Ding J, Yu C, Yang B, Wood DR, Grayburn PA. Reversal of streptozotocin-induced diabetes in rats by gene therapy with betacellulin and pancreatic duodenal homeobox-1. Gene therapy. 2007;14:1102–1110. doi: 10.1038/sj.gt.3302963. [DOI] [PubMed] [Google Scholar]
- 91.Chen S, Shimoda M, Wang MY, Ding J, Noguchi H, Matsumoto S, Grayburn PA. Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy. Gene therapy. 2010;17:1411–1420. doi: 10.1038/gt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S, Shimoda M, Chen J, Matsumoto S, Grayburn PA. Ectopic transgenic expression of NKX2.2 induces differentiation of adult pancreatic progenitors and mediates islet regeneration. Cell cycle. 2012;11:1544–1553. doi: 10.4161/cc.19928. [DOI] [PubMed] [Google Scholar]
- 93.Chen S, Shimoda M, Chen J, Matsumoto S, Grayburn PA. Transient overexpression of cyclin D2/CDK4/GLP1 genes induces proliferation and differentiation of adult pancreatic progenitors and mediates islet regeneration. Cell cycle. 2012;11:695–705. doi: 10.4161/cc.11.4.19120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, Rahmani M, Wei J, Hedvat M, Dent P, Dmitriev IP, Curiel DT, Grant S, Wu B, Stebbins JL, Pellecchia M, Reed JC, Sarkar D, Fisher PB. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujii H, Matkar P, Liao C, Rudenko D, Lee PJ, Kuliszewski MA, Prud'homme GJ, Leong-Poi H. Optimization of Ultrasound-mediated Anti-angiogenic Cancer Gene Therapy. Molecular therapy Nucleic acids. 2013;2:e94. doi: 10.1038/mtna.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bazan-Peregrino M, Rifai B, Carlisle RC, Choi J, Arvanitis CD, Seymour LW, Coussios CC. Cavitation-enhanced delivery of a replicating oncolytic adenovirus to tumors using focused ultrasound. Journal of controlled release : official journal of the Controlled Release Society. 2013;169:40–47. doi: 10.1016/j.jconrel.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 97.Kotopoulis S, Dimcevski G, Gilja OH, Hoem D, Postema M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Medical physics. 2013;40:072902. doi: 10.1118/1.4808149. [DOI] [PubMed] [Google Scholar]
- 98.Duvshani-Eshet M, Benny O, Morgenstern A, Machluf M. Therapeutic ultrasound facilitates antiangiogenic gene delivery and inhibits prostate tumor growth. Molecular cancer therapeutics. 2007;6:2371–2382. doi: 10.1158/1535-7163.MCT-07-0019. [DOI] [PubMed] [Google Scholar]
- 99.Sakakima Y, Hayashi S, Yagi Y, Hayakawa A, Tachibana K, Nakao A. Gene therapy for hepatocellular carcinoma using sonoporation enhanced by contrast agents. Cancer gene therapy. 2005;12:884–889. doi: 10.1038/sj.cgt.7700850. [DOI] [PubMed] [Google Scholar]
- 100.Chen ZY, Liang K, Qiu RX. Targeted gene delivery in tumor xenografts by the combination of ultrasound-targeted microbubble destruction and polyethylenimine to inhibit survivin gene expression and induce apoptosis. Journal of experimental & clinical cancer research : CR. 2010;29:152. doi: 10.1186/1756-9966-29-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hayashi S, Mizuno M, Yoshida J, Nakao A. Effect of sonoporation on cationic liposome-mediated IFNbeta gene therapy for metastatic hepatic tumors of murine colon cancer. Cancer gene therapy. 2009;16:638–643. doi: 10.1038/cgt.2008.1. [DOI] [PubMed] [Google Scholar]
- 102.Iwanaga K, Tominaga K, Yamamoto K, Habu M, Maeda H, Akifusa S, Tsujisawa T, Okinaga T, Fukuda J, Nishihara T. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer gene therapy. 2007;14:354–363. doi: 10.1038/sj.cgt.7701026. [DOI] [PubMed] [Google Scholar]
- 103.Li YS, Davidson E, Reid CN, McHale AP. Optimising ultrasound-mediated gene transfer (sonoporation) in vitro and prolonged expression of a transgene in vivo: potential applications for gene therapy of cancer. Cancer letters. 2009;273:62–69. doi: 10.1016/j.canlet.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 104.Liao ZK, Tsai KC, Wang HT, Tseng SH, Deng WP, Chen WS, Hwang LH. Sonoporation-mediated anti-angiogenic gene transfer into muscle effectively regresses distant orthotopic tumors. Cancer gene therapy. 2012;19:171–180. doi: 10.1038/cgt.2011.73. [DOI] [PubMed] [Google Scholar]
- 105.Warram JM, Sorace AG, Saini R, Borovjagin AV, Hoyt K, Zinn KR. Systemic delivery of a breast cancer-detecting adenovirus using targeted microbubbles. Cancer gene therapy. 2012;19:545–552. doi: 10.1038/cgt.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamaguchi K, Feril LB, Jr, Tachibana K, Takahashi A, Matsuo M, Endo H, Harada Y, Nakayama J. Ultrasound-mediated interferon beta gene transfection inhibits growth of malignant melanoma. Biochemical and biophysical research communications. 2011;411:137–142. doi: 10.1016/j.bbrc.2011.06.115. [DOI] [PubMed] [Google Scholar]
- 107.Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, Miranda M, Brunetti A, Salvatore M, Claudio L, Sarkar D, Dent P, Curiel DT, Fisher PB, Claudio PP. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]


