Abstract
Background
A particularly difficult challenge for community treatment of people with serious mental illnesses is the delivery of an acceptable level of care during the acute phases of severe mental illness. Crisis intervention models of care were developed as a possible solution.
Objectives
To review the effects of crisis intervention models for anyone with serious mental illness experiencing an acute episode, compared with ‘standard care’.
Search methods
We updated the 1998, 2003 and 2006 searches with a search of the Cochrane Schizophrenia Group’s Register of trials (2010) which is based on regular searches of CINAHL, EMBASE, MEDLINE, and PsycINFO.
Selection criteria
We included all randomised controlled trials of crisis intervention models versus standard care for people with severe mental illnesses.
Data collection and analysis
We independently extracted data from these trials and we estimated risk ratios (RR) or mean differences (MD), with 95% confidence intervals (CI). We assumed that people who left early from a trial had no improvement.
Main results
Three new studies have been found since the last review in 2006 to add to the five studies already included in this review. None of the previously included studies investigated crisis intervention alone; all used a form of home care for acutely ill people, which included elements of crisis intervention. However, one of the new studies focuses purely on crisis intervention as provided by Crisis Resolution Home Teams within the UK; the two other new studies investigated crisis houses i.e. residential alternatives to hospitalisation providing home-like environments.
Crisis intervention appears to reduce repeat admissions to hospital after the initial ‘index’ crises investigated in the included studies, this was particularly so for mobile crisis teams supporting patients in their own homes.
Crisis intervention reduces the number of people leaving the study early, reduces family burden, is a more satisfactory form of care for both patients and families and at three months after crisis, mental state is superior to standard care. We found no differences in death outcomes. Some studies found crisis interventions to be more cost effective than hospital care but all numerical data were either skewed or unusable. No data on staff satisfaction, carer input, complications with medication or number of relapses were available.
Authors’ conclusions
Care based on crisis intervention principles, with or without an ongoing home care package, appears to be a viable and acceptable way of treating people with serious mental illnesses. If this approach is to be widely implemented it would seem that more evaluative studies are still needed.
Medical Subject Headings (MeSH): Caregivers [psychology], Crisis Intervention [*methods], Mental Disorders [psychology; *therapy], Randomized Controlled Trials as Topic
MeSH check words: Humans
BACKGROUND
Description of the condition
Severe psychiatric illnesses are phasic. After initial treatment, people with schizophrenia or other similar disorders usually experience long periods of relative stability (Bleuler 1974). Relapses can, however, occur for reasons such as exposure to environmental stressors or difficulties with medication concordance. During a psychotic relapse sufferers experience a sudden exacerbation of acute symptoms such as delusions and hallucinations and consequently will have disturbed and difficult behaviour. Some people become aggressive, threatening harm to themselves or others. Intervention at this stage is crucial as it brings much needed relief for both the sufferer and their carers and can help prevent further deterioration (Weisman 1989).
During the last 40 years large-scale closure of psychiatric hospitals and reduction in the availability of bed spaces has facilitated a sharp rise in the number of people with serious mental illnesses being treated in the community. After an initial reduction in admissions however, there was a rise in the number of people requiring hospital readmission, suggesting that this policy of community care was perhaps failing some vulnerable people (Ellison 1974). Although research suggested there were many benefits to community care (Pasamanick 1967; Langsley 1968), in practice it was proving difficult to implement. A particularly difficult area was the delivery of an acceptable level of care during the acute phases of severe mental illness (Audit Comm 1986; WHO 1987). A major problem with early community care was that although it could care for people during their relatively stable periods, it was unable to cope with acute phases or relapses. This created a cyclic pattern whereby people were hospitalised for short periods during a crisis, then discharged into the community until a further crisis arose (Hoult 1986).
Description of the intervention
Breaking this cycle required the development of some form of community care that could adequately treat psychiatric crises in the home environment. Psychiatric services in Amsterdam were at the forefront of such treatment introducing a 24-hour ‘first-aid’ emergency home service just after the Second World War (Querido 1968). In the 1970’s more specific crisis intervention models were introduced. Like Amsterdam’s first-aid service, crisis intervention models aimed to treat psychiatric crises in the community and if possible avoid hospitalisation or, if this was unavoidable, reduce time spent in hospital (Weisman 1989). Crisis intervention models for people with serious mental illnesses were based on models originally developed to treat normally healthy individuals in psychological crisis. A crisis can be defined as a situation where a person experiencing overwhelming stress due to a life event such as bereavement, rape or major illness finds that their usual coping mechanisms for everyday life break down (Caplan 1964; Lindemann 1944). People with severe psychiatric illnesses may have fragile coping mechanisms. If exposed to excessive stress, these coping mechanisms can breakdown, leading to an exacerbation of their acute symptoms for which crisis intervention techniques may be used (Weisman 1989).
In keeping with the original ethos of earlier crisis intervention models, the models used for people with serious mental illnesses usually, but not always, require a multidisciplinary team of specifically trained staff. These teams may be available 24 hours a day. They advocate prompt detection of exacerbation of serious mental illness followed by swift, time-limited, intense treatment delivered in a community setting. There is immediate assessment and identification of problems followed by initial implementation of treatment. Treatment usually involves a combination of medication, counselling/therapy plus practical help with living skills and support for close family members. After the crisis has been stabilised, sufferers are carefully introduced to other models of care more suited for the chronic phases of psychiatric illnesses. The aim of crisis intervention models is to prevent, where possible, hospitalisation, further deterioration of symptoms and stress experienced by relatives/others involved in the crisis situation (Thomas 1970). Since their initial introduction several ‘crisis’ programmes have emerged, all designed to offer intensive crisis-oriented treatment to severely disturbed mentally ill people in a variety of community settings. These include programmes such as mobile crisis teams, crisis units in hospitals, crisis day treatment centres and crisis residential programs. This expansion of crisis intervention programs has been dramatic. In countries such as Australia and in North America it is now the central method of treatment used in community mental health programmes (Finch 1991; Weisman 1989). In the UK, government policy mandated that crisis resolution home teams (CRHTs) be established throughout England (Department of Health 2000).
How the intervention might work
The rapid dissemination of crisis intervention models suggests they have been successful methods of treatment for psychiatric crises. Supporting this is much research suggesting that crisis intervention models are beneficial in that they reduce hospital admissions by up to 50%, are more cost-effective, and reduce the stigma of institutionalisation for both the sufferer and their family (Hoult 1984a; Hoult 1984b; Hoult 1986; Lamb 1979; Schoenfeld 1986; Stein 1978; Test 1978). In addition, early intervention with immediate reduction of psychotic symptoms is said to be beneficial for the long-term prognoses of these illnesses (McGorry 1996). A survey, however, has suggested that the original claims for the efficacy of mobile crisis teams were not based on enough empirical evidence and it calls for more research into the effects of this intervention (Geller 1995).
Why it is important to do this review
The review was last updated in 2006, and after this update, the data relating to readmission, length of stay, general functioning and mental state remained inconclusive. The 2006 review is now somewhat out-of-date, and more recent studies have been published. This is a subject that has also been covered by other reviews within The Cochrane Collaboration. Crisis interventions for people with borderline personality as well as alternatives to inpatient mental health care for children and young people have been reviewed (see Table 1).
Table 1 .
Other relevant reviews
| Title | Stage | Reference | Cochrane Editorial Group |
|---|---|---|---|
| Crisis intervention for people with severe mental illnesses | Review | This review | Cochrane Schizophrenia Group |
| Crisis interventions for people with borderline personality disorder | Protocol | Borschmann 2011 | Cochrane Developmental, Psychosocial and Learning Problems Group |
| Crisis interventions for people with affective disorder | Proposed title | Cochrane Depression, Anxiety and Neurosis Group | |
| Alternatives to inpatient mental health care for children and young people | Review | Shepperd 2009 | Cochrane Effective Practice and Organisation of Care Group |
OBJECTIVES
To review the effects of crisis intervention models for anyone with serious mental illness experiencing an acute episode compared to the standard care they would normally receive. If possible, to compare the effects of mobile crisis teams visiting patients’ homes with crisis units based in home-like residential houses.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. If a trial had been described was ‘double-blind’ but only implied randomisation, we would have included it in a sensitivity analysis of all such trials. If there was no substantive difference within primary outcomes (see Types of outcome measures) when these ‘implied randomisation’ studies were added, then we would have included them in the final analysis. If there was a substantive difference, we would have only included clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi-randomised studies, such as those allocating by using alternate days of the week.
Types of participants
1. For previous versions
Adults, however defined, with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder and delusional disorder, again, by any means of diagnosis. We are interested in making sure that information is as relevant to the current care of people with schizophrenia as possible so propose to clearly highlight the current clinical state (acute, early post-acute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent) and as to whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment-resistant illnesses).
2. For 2010 update
In previous versions of this review we included studies such as Stein 1975 which did not describe clearly the illness from which people suffered. This, we feel was correct to do as it was in keeping with the title of this review and the desired focus of this work. However, on consideration, the definition regarding types of participants used in the older versions is not correct and we now wish to be clearer.
Adults, however defined, with either (a) severe mental illness as defined for the previous version of the review or (b) adults with severe mental health conditions except where the focus of the trial is one particular group of people only with a particular condition. For example, a study that includes adults with severe depression only would be excluded, but a mixed study including severe depression and other severe mental illnesses would be included.
Types of interventions
1. Crisis intervention
Any type of crisis-orientated treatment of an acute psychiatric episode by staff with a specific remit to deal with such situations, in and beyond ‘office hours’. This can include mobile teams caring for patients within their own homes, or non-mobile residential programmes based in a home-like houses within the community.
2. Standard care
The normal care given to those suffering from acute psychiatric episodes in the area concerned.
3. Different forms of crisis interventions
If data were available we would have assessed one delivery setting for crisis care with another (mobile versus non-mobile) in separate comparisons.
Types of outcome measures
We divided outcomes into very short-term (less than three months), short term (less than six months), medium term (seven to 12 months) and long term (over one year).
Primary outcomes
1. Service utilisation
1.1 Admission to hospital
1.2 Number of days in hospital
1.3 Number of staff/user contacts
Secondary outcomes
1. Satisfaction with treatment
1.1 Number of people leaving the study early
1.2 Patient satisfaction
1.3 Staff satisfaction
1.4 Carer satisfaction
2. Clinical outcome
2.1 Death/suicide
2.2 Improvement, general or specific
2.3 Medication Concordance
2.4 Antipsychotic medication
2.5 Relapses
3. Social outcome
3.1 Social functioning including life skills
3.2 Employed (paid/voluntary/attendance at school/college)
3.3 Able to live independently
3.4 Number of carers - professional or significant others - needed to maintain stable state
4. Cost of treatment
4.1 Total, mental health care or medical care costs
4.2 Staff input - hours worked
4.3 Carer input - change in lifestyle/no change in lifestyle/loss of income
We have selected outcome measures that provide global estimations of functioning. We did not report highly specific outcomes, such as, ‘sense of safety’. Such specific outcomes are rarely reported in more than one study and it is difficult to assess their relevance to the effectiveness of the treatment.
Search methods for identification of studies
Electronic searches
For previous electronic search terms please see Appendix 1
1.1 Update search (2010)
We searched the Cochrane Schizophrenia Group Trials Register (March 2010)
The register was searched using the phrase: [(acute* or cris?s* or emergenc* or intensiv* or mobile* or outreach* or (time* and limit*) or commun* or home*) and (* care* or interven* or treat* or therap* or managem* or model* or programm* or team* or service* or base*) * or hospital* and (diversion* or alternative*) in title and *acute* or *cris?s* or *emergenc* or *intensiv* or *mobile* or *outreach* or * (time and limit*) or *commun* or *home*) and (*care* or *interven* or *treat* or *therap* or *managem* or *model* or *programm* or *team* or *service* or *base*) * or *hospital* and (diversion* or *alternative*) in title, abstract or Index terms of REFERENCE) or (brief Hosp* OR community mental health service, I* OR community resid* OR crisis* OR critical time int* OR district psychiatric c* OR *brief intensive* in interventions of STUDY field)]
This register is compiled by systematic searches of major databases, handsearches and conference proceedings (see Group Module)
Searching other resources
1. Reference searching
We inspected references of all identified studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
Review author SM independently inspected citations from the searches and identified relevant abstracts. The protocol planned that a random 20% sample should be independently re-inspected by RD to ensure reliability, however, as only seven studies met the review criteria, all of these were checked by RD. Where disputes arose, the full report was acquired for more detailed scrutiny. Full reports of the abstracts meeting the review criteria were obtained and inspected by SM. Where it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
Review author SM extracted data from all included studies. The protocol stated that, to ensure reliability, RD would independently extract data from a random sample of these studies, comprising 10% of the total, however, there were actually only three new studies so all were checked. Disagreement on the data extracted were discussed, decisions documented and, if necessary, we contacted authors of studies for clarification. With remaining problems CI and CA helped clarify issues and these final decisions were documented. Data presented only in graphs and figures were extracted whenever possible, but included only if the two review authors independently had the same result. Attempts were made to contact authors through an open-ended request in order to obtain missing information or for clarification whenever necessary. If studies were multi-centre, where possible, we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale-derived data
We included continuous data from rating scales only if: a. the psychometric properties of the measuring instrument have been described in a peer-reviewed journal (Marshall 2000); and b. the measuring instrument has not been written or modified by one of the trialists for that particular trial.
Ideally the measuring instrument should either be i. a self-report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, in Description of studies we noted if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between-person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint) which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. Endpoint and change data were combined in the analysis as we used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011, Chapter 9.4.5.2).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non-parametric data, we aimed to apply the following standards to all data before inclusion: a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS) which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S-S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. When continuous data are presented on a scale that included a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We entered skewed data from studies of less than 200 participants in additional tables and marked the data as skewed rather than into an analysis. Skewed data pose less of a problem when looking at means if the sample size is large (over 200) and we entered such data into the syntheses.
2.5 Common measure
To facilitate comparison between trials, we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, efforts were made to convert outcome measures to dichotomous data. This can be done by identifying cut-off points on rating scales and dividing participants accordingly into ‘clinically improved’ or ‘not clinically improved’. It is generally assumed that if there is a 50% reduction in a scale-derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cutoff presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for crisis intervention. Where keeping to this makes it impossible to avoid outcome titles with clumsy double-negatives (e.g. ‘Not improved’), we reported data where the left of the line indicates an unfavourable outcome. This was noted in the relevant graphs.
2.8 Summary of findings table
We used the GRADE approach to interpret findings (Schünemann 2008) and used GRADE profiler (GRADE Profiler) to import data from RevMan 5 (RevMan) to create ‘Summary of findings’ tables. These tables provide outcome-specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient-care and decision making. We selected the following main outcomes for inclusion in the Summary of findings for the main comparison. Outcomes were selected using the following criteria, in priority order: endpoint versus change data, data where loss was below 30%, largest sample size for a particular outcome, the longest follow-up time available for a particular outcome.
1. Service utilisation outcomes
Hospital use
2. Quality of Life
As measured by the Manchester Short Assessment of quality of life (MANSA)
3. Clinical response in global state
As measured by the Global Assessment Scale (GAS)
4. Clinical response in general mental state
As Measured by the Brief Psychiatric Rating Scale (BPRS)
5. Burden on family
Overall burden on family by six months
Assessment of risk of bias in included studies
Again, SM and RD worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011 to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
Where the raters disagreed, the final rating was made by consensus, with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. Non-concurrence in quality assessment was reported.
The level of risk of bias was noted in both the text of the review and in the Summary of findings for the main comparison.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000).
2. Continuous data
For continuous outcomes, we estimated the mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference SMD). However, if scales of very considerable similarity were used, we would have presumed there was a small difference in measurement, and we would have calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ ‘cluster randomisation’ (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra-class correlation in clustered studies, leading to a ‘unit of analysis’ error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering was not accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra-class correlation coefficients for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non-cluster randomised study, but adjust for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a ‘design effect’. This is calculated using the mean number of participants per cluster (m) and the intra-class correlation coefficient (ICC) [Design effect = 1+ (m-1) *ICC] (Donner 2002). If the ICC is not reported it will be assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account intra-class correlation coefficients and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross-over trials
A major concern of cross-over trials is the carry-over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash-out phase. For the same reason cross-over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data from the first phase of cross-over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, the additional treatment arms were presented in comparisons. If data were binary, we simply added and combined the data within the two-by-two table. If data were continuous, we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where the additional treatment arms were not relevant, these data were not reproduced.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow-up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we marked such data with (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, data were presented on a ‘once-randomised-always-analyse’ basis (an intention-to-treat analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes the rate of those who stayed in the study - in that particular arm of the trial - were used for those who did not. A sensitivity analysis was undertaken testing how prone the primary outcomes are to change when ‘completer’ data only were compared to the intention-to-treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50% and completer-only data were reported, we reproduced these.
3.2 Standard deviations
If standard deviations (SDs) were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals (CIs) available for group means, and either a ‘P’ value or ‘t’ value available for differences in mean, we calculated them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): When only the SEwas reported, SDs were calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 (Higgins 2011) present detailed formula for estimating SDs from P values, t or F values, CIs, ranges or other statistics. If these formula did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’ s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data had been used in the trial, if less than 50% of the data had been assumed, we reproduced these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, these were fully discussed.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, these were fully discussed.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
Heterogeneity between studies was investigated by considering the I2 method alongside the Chi2 ‘P’ value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. ‘P’ value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, was interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 - Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small-study effects. We did not use funnel plots as there were less than 10 included studies. For future updates of this review, we will use the same methodology and not use funnel plots for outcomes where there are 10 or fewer studies, or where all studies are of similar sizes. In other cases, where funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed-effect or random-effects models. The random-effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random-effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random-effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose random-effects model for all analyses. The reader is, however, able to choose to inspect the data using the fixed-effect model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses - only primary outcomes
We anticipated subgroup analyses investigating mobile crisis teams versus non-mobile residential home-like programmes. In the event however, such analyses were not possible due to lack of data comparing these conditions directly against each other.
1.2 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of crisis intervention for people with severe mental illnesses. In addition, however, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, this was reported. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if heterogeneity was restored. For this review, we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, data would be presented. If not, data would not be pooled and issues would be discussed. We know of no supporting research for this 10% cut-off but are investigating the use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we simply stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes, we included these studies and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then all data were employed from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow-up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported the results and discussed them but continued to employ our assumption.
Where assumptions have to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with completer data only. A sensitivity analysis was undertaken testing how prone results changed when ‘completer’ data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available), allocation concealment, blinding and outcome reporting for the meta-analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analysis
4. Imputed values
We also undertook a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster randomised trials.
If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately
5. Fixed and random effects
All data were synthesised using a random-effects model, however, we also synthesised data for the primary outcome using a fixed-effect model to evaluate whether the greater weights assigned to larger trials with greater event rates, altered the significance of the results compared with the more evenly distributed weights in the random-effects model.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
1. 2010 search
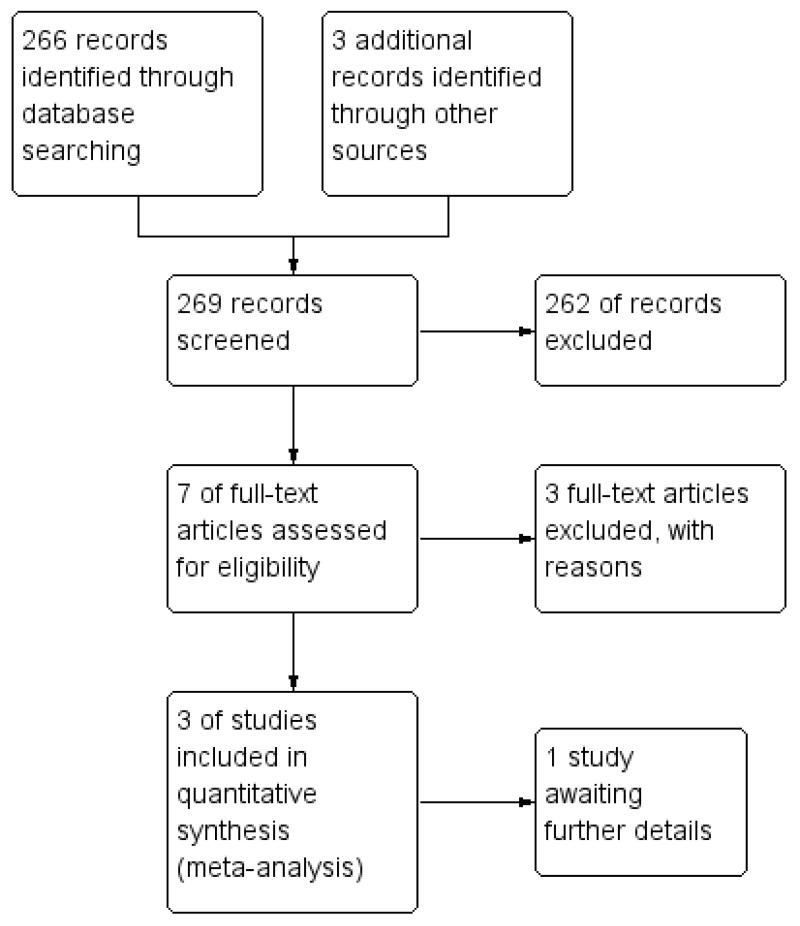
After the 2010 update the total number of included studies has increased to eight. These eight studies randomised a total of 1144 people (Fenton 1979; Fenton 1998; Hoult 1983; Howard 2010; Johnson 2005; Muijen 1992; Pasamanick 1964a; Stein 1975). Please also see Figure 1.
Figure 1 .
Study flow diagram (2010 UPDATE).
2. Previous searches
The initial search yielded 2446 references (Joy 2000). An initial electronic and subsequent paper scan of all abstracts produced a final database of 61 possible reports. A full copy of each of these was obtained and sorted into 18 separate studies. From the original 2446 references only five studies met the specified inclusion criteria. When the search was run again in 2003 the resulting numbers of possible references and actual studies was very similar. Because the search term is so broad, the 2003 search term yielded over 2000 references (Joy 2004). Again, these were scanned and narrowed down to a possible nine trials, none of which met the inclusion criteria and we added all of these to the excluded studies table. A rethinking of the search term for the 2006 update resulted in a much more manageable list of hits but again, none of the new trials met our inclusion criteria. For the 2010 update, the initial search produced 266 studies, which produced a possible database of seven reports. When full reports were obtained for all of these, the authors agreed that three new studies (Fenton 1998, Howard 2010 and Johnson 2005) met the specified inclusion criteria.
We presented the eleven main outcomes as follows: death/harm, hospital use, leaving the study early, global state, mental state, burden, satisfaction, economic costs, quality of life, social functioning and clinical and social problems.
Included studies
For detailed descriptions please also see Characteristics of included studies.
1. Length of trials
The shortest trial was Howard 2010 having a duration of three months, and Pasamanick 1964a, was the longest trial, lasting two years.
2. Participants
The eight included studies all focused on severely mentally ill adults who were in crisis and required or were in need of immediate hospitalisation. The majority of participants were psychotic (most suffering from schizophrenia), but there was a substantial representation of other diagnoses such as depression and severe neuroses. Three studies stated how they had used diagnostic criteria for diagnosis (Fenton 1979; Hoult 1983; Muijen 1992). All included people aged 18 years and above of both sexes (except Howard 2010 who included women only).
3. Setting
Due to the inclusion criteria, all included studies needed to take place in hospital and the community. The trials were based in Australia (Hoult 1983), Canada (Fenton 1979; Fenton 1998), the USA (Pasamanick 1964a, Stein 1975) and the UK (Howard 2010, Johnson 2005 and Muijen 1992).
4. Size
Trial size ranged from from 41 participants (Howard 2010) to 260 participants (Johnson 2005).
5. Interventions
5.1. Crisis Intervention
Johnson 2005 investigated care provided by crisis resolution home teams versus standard care. Five other trials (Fenton 1979; Hoult 1983; Muijen 1992; Pasamanick 1964a; Stein 1975) had home-based treatments similar to each other where crisis intervention was included as part of a package. Two studies investigated crisis houses providing residential home-like care. For all studies, a multidisciplinary team, usually comprising psychiatrists, psychologists, nurses, occupational therapists and social workers, delivered care. To be included in this review, the teams had to treat crises occurring in and out of office hours. All included studies provided emergency care although the type of cover varied. Six had members of staff on call ready to visit 24 hours a day if needed (Fenton 1979; Fenton 1998; Hoult 1983; Howard 2010; Johnson 2005; Stein 1975). Muijen 1992 provided a telephone answering service only, but if people wanted further help they could use the walk-in emergency clinic at the local hospital. Pasamanick 1964a initially provided a telephone service with home visits but then switched to an answer-machine instructing callers to call back during office hours. If it was truly an emergency they could contact the local police station.
5.2. Standard care
Standard care for all the included studies involved hospitalisation if required. The majority of standard care patients were hospitalised immediately after allocation. Once hospitalised, people received the standard level of care for that hospital. This tended to be short and intense care with the overall aim being early discharge. As well as medication, various forms of treatment programmes such as counselling, physiotherapy and occupational therapy were available on site. Social workers were also available. After discharge all trials used their normal outpatient services.
6. Outcomes
6.1 Missing
None of the studies evaluated staff satisfaction, medication concordance, or number of carers (professional or lay) needed to maintain the well-being of an individual. For some studies, readmission to hospital was evaluated, but it was not clear if all relapses necessitated readmission so it is impossible to see if crisis intervention helped postpone relapse. Only two studies provided data on readmission (Fenton 1998; Johnson 2005). Stein 1975 did attempt to evaluate the living situation of participants but did not report usable data.
6.2 Scales
Eighteen different instruments were used to collect continuous data. Only nine of these rating scales, however, collected data useful to this review. The primary reason for exclusion of these data were that the scales had never been validated. To prevent bias in data collection, the quality and validity of scales need to be assessed through unbiased peer review. Recent research shows trials using non-validated scales are more likely to find significant differences in outcomes than trials using peer-reviewed scales (Marshall 2000). Other methodological problems in data collection are recorded in the Characteristics of included studies.
Below are details of the scales that provided useful data.
6.2.1 Brief Psychiatric Rating Scale (BPRS, Overall 1962)
A brief clinician-rated scale used to assess the global severity of a range of psychiatric symptoms. Scores range from 24 (not present) to 168 (extremely severe impairment). Used in Hoult 1983, Johnson 2005 and Howard 2010.
6.2.2 Client Satisfaction Questionnaire (Larsen 1979)
Eight-item patient-rated scale measuring patients’ satisfaction with different aspects of their care (quality of service, amount of support received, needs and preferences). Measured on a scale of one to four for each item. Higher scores indicate greater satisfaction. Used in Johnson 2005 and Muijen 1992.
6.2.3 Global Assessment Scale (GAS, Endicott 1976)
A clinician-rated assessment of overall functioning on a scale of one to 100. Lower scores indicate poorer functioning. Used in Muijen 1992 and Howard 2010.
6.2.4 Health of the Nation Outcome Scores (HoNOS, Wing 1998)
Twelve-item scale covering clinical problems and social functioning in mentally ill people on a scale of zero to four each item, range zero to 48. Higher scores indicate poorer functioning. Used in Johnson 2005.
6.2.5 Manchester Short Assessment of quality of life (MANSA, Priebe 1999)
Sixteen-item scale covering quality if life, some items rated on a scale of one to seven, some items yes/no. Higher scores indicate higher quality of life. Used in Johnson 2005, short form used in Howard 2010.
6.2.6 Life Skills Profile (LSP, Parker 1991)
Instrument assessing functioning in persons with severe mental illnesses, 39-items, higher scores reflect better functioning. Used in Johnson 2005.
6.2.7 Present State Examination - 9th Edition (PSE, Wing 1974)
Clinician-rated scale measuring mental status. One hundred and forty symptom items are rated and combined to give various syndrome and sub-syndrome scores. Higher scores indicate greater clinical impairment. Used in Hoult 1983.
6.2.8 Psychiatric Evaluation Form (PEF, Endicott 1972)
A clinician-rated scale used to assess psychological functioning during the week prior to interview. Consists of 24 individual and eight summary scales. Scoring on each scale ranges from one to five with higher scores indicating greater impairment. Used in Fenton 1979.
6.2.9 Social Adjustment Scale (SAS, Weissman 1971)
Measures social functioning in a number of life domains (work, social, extended family, marital, parental, family unit, and economic adequacy) on a scale of one to seven. Lower scores indicate poorer functioning. Used in Muijen 1992.
6.2.10 EuroQuol - 5 dimension (EQ-5D, Brooks 1996)
Measures quality of life across five dimensions, scores from zero to one with one being perfect health and zero being death, used in Howard 2010.
Excluded studies
For detailed descriptions please also see Characteristics of excluded studies.
1. Excluded studies
We have now excluded twenty-eight studies from this review, three of which we excluded after the 2010 search (Grawe 2006, Power 2007 and Warner 2006). and four of which we excluded after the 2006 search (Harrison 2003; Jones 2003; Kuipers 2004; Metcalfe 2005). Only four of the excluded studies were not randomised (Bond 1989; Harrison 2003; Mosher 1975; Pai 1982). One, Kuipers 2004, did randomise treatments but did not randomise a homecare package with standard care. Four studies focused on people who did not meet the eligibility criteria (Bush 1990; Muijen 1994; Pasamanick 1964b; Van Minnen 1997). Although severely mentally ill, it was unclear if they were in crisis and in need of immediate hospitalisation. Two studies, Henlegger 1999 and Mattejat 2001 focused on children and young people who were severely ill and in crisis rather than our population of interest which was adults.
Most of the trials (17), were judged to have unsuitable home care intervention. Some specifically did not provide 24-hour emergency cover (Gater 1997; Merson 1992) or diverted people from hospital to attendance at a daily clinic (Levenson 1997). We had to exclude eight (Ghandi 2001; Herz 2000; Linszen 1998; Rosenheck 1995; Sledge 1996; Taylor 1998; Tyrer 1995; Warner 2006) in this category as they were investigating ‘home care packages’ versus hospital care rather than crisis intervention. Power 2007 was investigating general practitioner access to crisis teams. Three recent studies (Jones 2003, Metcalfe 2005 and Grawe 2006) used forms of intensive case management.
Finally, Burns 1993 met most eligibility criteria but, because of the design of the study, many people were lost after allocation (48%). We felt that data with such a degree of loss incorporated too great a level of assumption (see ‘Methods’) so we excluded these. We also excluded Polak 1976 as too much of the data were unusable.
2. Awaiting assessment
One study (Bindman 2008) requires further assessment. This is an unpublished study referred to in Johnson 2008. Many outcomes are not reported. Mean number of days in hospital after initial admission and patient satisfaction are reported, but no SDs are provided. We have written to the author and await his reply.
3. Ongoing
We are not aware of any ongoing trials relevant to this review.
Risk of bias in included studies
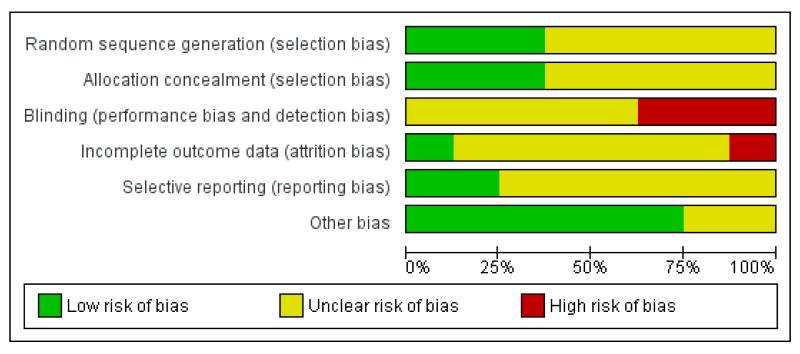
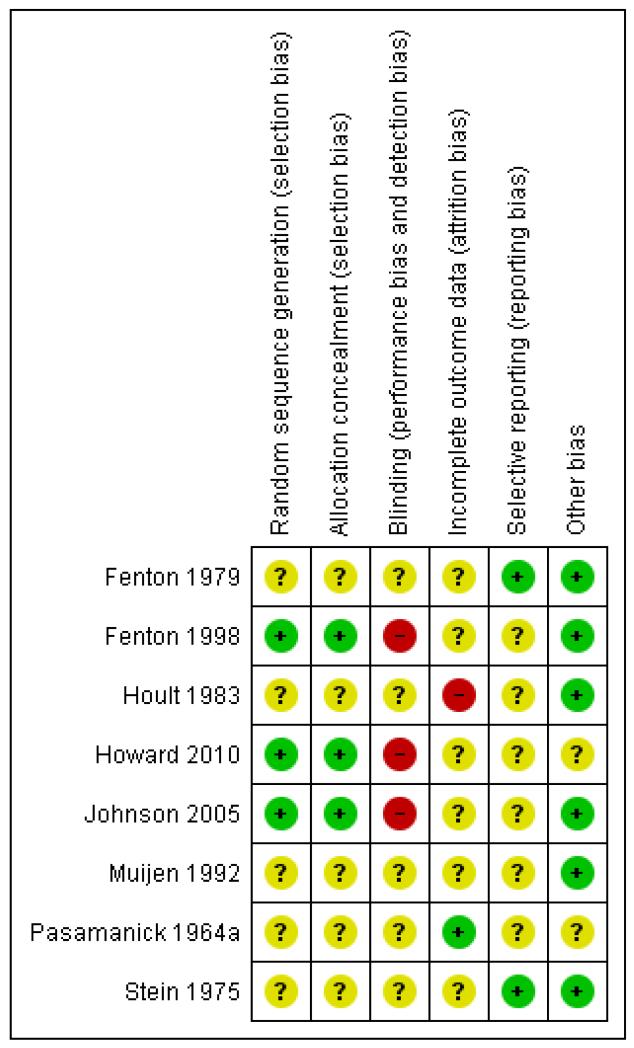
For summary of risk of bias across included studies please see Figure 2 and Figure 3.
Figure 2 .
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 3 .
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
All trials were randomised but two studies did not describe how this took place (Fenton 1979; Stein 1975) and, therefore, we categorised these as moderate risk of bias with some doubt about the results (see ‘Methods 3. Assessment of a trial’s methodological quality’). Other trials used sealed envelopes to blind the sequence of allocation (Hoult 1983; Muijen 1992) or allocated by a deck of randomly sequenced cards (Pasamanick 1964a). Both systems are open to the possibility of selection bias operating. Fenton 1998, Howard 2010 and Johnson 2005 used independent, computer-generated 24-hour randomisation services.
Blinding
Due to the nature of the intervention it is impossible to blind participants to the type of treatment received. All studies, therefore were single-blind with raters either blind to treatment allocation or not part of treatment teams. Five studies used independent raters who were not part of the treatment teams (Fenton 1979; Fenton 1998; Hoult 1983; Muijen 1992; Stein 1975). Four of these studies did not state if these raters were blind to treatment group but Muijen 1992 did report that raters were not blinded for reasons of safety. Pasamanick 1964a was the only trial where the raters were clearly not independent. In this study, nurses and clinicians responsible for care completed follow-up ratings. ForJohnson 2005, ratings of patient satisfaction, mental state and quality of life were taken by independent researchers at eight weeks but clinical problems and social functioning were taken at eight weeks and six months by clinicians involved in care. Furthermore, Johnson 2005 and Howard 2010 stated that it was not possible to blind researchers collecting the data.
Incomplete outcome data
Proportions of follow-up varied with outcome. For example, for the outcome of ‘hospital admission and readmission’, four studies had no loss to follow-up. The two exceptions (Hoult 1983; Johnson 2005) did not report data for 19/119 and 34/260 people respectively. The follow-up assessments of clinical state and ‘satisfaction with treatment’ were not so good but only Fenton 1979 had greater than 30% loss (57/157). Most of the attrition was clearly explained as the result of refusal or inability to complete the assessments. Pasamanick 1964a did lose 21 people, seven of whom were impossible to trace. Loss of data from relatives was more substantial. Again, it was primarily due to inability or refusal to complete assessments but the logistics of this made the attrition understandable. Consent had to be obtained from the patient as well as the relative, and, in some cases the person in crisis had to be present at the interview. Reasons for loss of data were, however, well explained in five of the studies but reasons for the loss in Fenton 1998, Howard 2010 and Johnson 2005 were not explained.
Selective reporting
The majority of data in this review originates from published reports. We have had no opportunity to see protocols of these trials to compare the outcomes reported in the full publications with what was measured during the conduct of the trial. Most other problems arose with continuous data. Several studies failed to present the standard deviation/error of the means, making data unusable. Several outcomes were presented as P values alone. These were also reported as ‘P < 0.05 or P > 0.05’ rather than their exact value thus making it impossible to extract data. Other problems were (a) data given for one group only (Muijen 1992; Pasamanick 1964a; Stein 1975), (b) data combined and not presented by randomised group (Fenton 1979), (c) data obtained using non-validated scales (Muijen 1992; Stein 1975) and (d) in some cases, no data presented on specific outcomes (Hoult 1983; Muijen 1992). We tried to contact authors some time ago for additional data but it now seems unlikely that further information will become available.
Other potential sources of bias
All studies had small sample sizes, Howard 2010 deals with a women-only sample. We could detect no other sources of bias such as biased sources of funding to authors.
Effects of interventions
1. Introduction
The review now includes eight studies, six of these have investigated mobile programmes delivered in the patients’ own homes and two report on home-like residential crisis houses. As these programmes differ somewhat, the outcomes below were analysed both including and excluding the two studies examining residential alternatives. Generally, analyses are reported including available data from all studies, however, where the exclusion of the two residential studies produced substantially different findings, results are reported both with and without these.
2. COMPARISON 1: CRISIS INTERVENTION versus STANDARD CARE
2.1 Death/harm
Overall, the number of deaths was similar for both groups, two studies, Howard 2010 and Pasamanick 1964a did not report any deaths. For the outcome of death by natural causes, pooled data showed no statistical difference between treatment groups (n = 980, 6 RCTs, risk ratio (RR) 0.63, 95% confidence interval (CI) 0.18 to 2.24). Death by suicide also showed no statistical difference for pooled data (n = 980, 6 RCTs, RR 1.06, 95% CI 0.36 to 3.11). Combining these groups for the outcome death by any cause produced similar results (n = 980, 6 RCTs, RR 0.88, 95% CI 0.37 to 2.07).
We analysed two outcomes of harm. Again, pooled data for ‘attempted suicide’ showed no difference between crisis interventions and standard care (n = 369, 3 RCTs, RR 2.62, 95% CI 0.21 to 32.02). There were two studies (Johnson 2005 and Muijen 1992) with at least one homicide. These occurred in the crisis arm (n = 568, 3 RCTs, RR 2.96, 95% CI 0.31 to 28.28).
2.2 Hospital use
We assessed hospital admission in several ways to reflect the differing measures used in the included studies. For some of the studies, it is misleading to compare treatment groups on the ‘number of hospital admissions’ as those in standard care had an index admission as part of their care package. This ‘result’, in effect, records only the treatment given rather than its outcome. In order to present the difficulty the crisis resolution care teams experienced in keeping people out of hospital, the relative success at ‘keeping to initial trial protocol’ was assessed instead. This presentation of the data was used for Fenton 1979; Hoult 1983; Muijen 1992; Pasamanick 1964a; Stein 1975. in comparisons 2.2.1 and 2.2.2. below.
However, Fenton 1998 and Johnson 2005 did report data for admission to acute services after initial index admission to hospital, a residential crisis house or care by a crisis resolution team. These data are therefore, presented separately below (from 2.2.3 to 2.2.7) to distinguish it from data from Fenton 1979; Hoult 1983; Muijen 1992; Pasamanick 1964a; Stein 1975 where index and other admission were not differentiated. Howard 2010 did not report hospital admission data.
2.2.1 Unable to keep to initial trial protocol as regards admission
The difference between the groups was highly significant with more crisis intervention care ‘failures’. These data also show the difficulty encountered by the crisis intervention care teams in keeping people from admission. By 12 months pooled data from all the trials showed 44.8% of those allocated to crisis intervention care on presentation were admitted.
2.2.2 Repeat admissions including index admission
A second analysis looked at repeat admissions. At 12 months there was a non-significant difference between groups (n = 465, 3 RCTs, RR 0.71, 95% CI 0.31 to 1.61), furthermore, there was significant heterogeneity for this latter outcome (I2 86%). At 20 months, data from Muijen 1992 similarly did not show a statistically significant effect (n = 188, 1 RCT, RR 1.10, 95% CI 0.75 to 1.60).
2.2.3 Number of repeat admissions per participant
Fenton 1998 reported data for mean number of admissions per participant. However, the data are highly skewed and descriptive statistics only are presented; the mean for admissions is slightly higher for standard care.
2.2.4. Repeat admissions excluding index admission
Fenton 1998 and Johnson 2005 reported data for admission to acute services after initial index admission to hospital, a residential crisis house or care by a crisis resolution team by numbers of participants. At three months Johnson 2005 reported that there were significantly fewer number of participants readmitted after the initial crisis for the crisis arm than for the standard arm (n = 260,1 RCT, RR, 0.53, 95% CI 0.41 to 0.68). At six months, Fenton 1998 and Johnson 2005 data combined indicated again that there were fewer participants readmitted for the crisis arm than for the standard arm although this difference was not significant (n = 369, 2 RCTs, RR 0.75, 95% CI 0.50 to 1.13).
However, there was significant heterogeneity for the outcome at six months (I2 80%) and it should be remembered that Fenton 1998 was investigating a residential programme, whilst Johnson 2005 looked at a mobile crisis resolution team. Taking Johnson 2005 data only, the number of participants readmitted after the initial crisis for the crisis arm rather than the standard arm was significantly lower (n = 258,1 RCT, RR 0.6.20, 95% CI 0.51 to 0.76.
2.2.5. Repeat admissions: Compulsory detentions under Mental Health Act
One study, Johnson 2005, reported numbers of participants admitted by compulsory detention under the Mental Health Act at three months and six months. There were fewer compulsory detentions in the crisis arm, although not to a significant extent (n = 260, 1 RCT, RR 0.62, 95% CI 0.34 to 1.11) and (n = 258, 1 RCT, RR 0.69, 95% CI 0.43 to 1.11), respectively.
2.2.6 Treatment failure
Fenton 1998 defined ‘treatment failure’ as transfer to another in-patient facility without a prior return to the community, 13% of participants in the crisis arm required transfer and 4% of those in the standard arm. This was not a significant difference (n = 119, 1 RCT, RR 3.26, 95% CI 0.74 to 14.44).
2.2.7 Number of days in acute care
Data were unusable for some studies. Three studies (Fenton 1979; Muijen 1992; Stein 1975) included ‘index admission’ in their data and the remaining two did not report standard deviations (Hoult 1983; Pasamanick 1964a). ‘Acute care’ here is defined as admission to hospital or to crisis house after the initial index crisis.
Data at three months were reported by Johnson 2005, and show that the crisis group had significantly fewer days in acute care post-crisis (n = 260, 1 RCT, mean difference (MD) −10.30, 95% CI −14.77 to −5.83). Data at six months are reported by Fenton 1998 and Johnson 2005. The crisis group again had fewer days in acute care post-crisis, but not to a significant extent (n = 365, 2 RCTs, MD −10.54, 95% CI −26.49 to 5.42).
As with repeat admissions above however, it appears that the exclusion of the data from Fenton 1998 which relates to a residential programme, produces a different result, With the inclusion of Johnson 2005 data only, the crisis group had significantly fewer days in acute care post-crisis (n = 257,1 RCT, MD −17.30, 95% CI −27.80 to −6.80).
2.2.8 Number of visits
Skewed data were presented. One study reported that the crisis intervention care group had significantly fewer staff contacts during the six- to 12-month period (P = 0.005) but by 12 months there was no statistical difference between the two groups use of staff time (P > 0.05) (Fenton 1979).
2.3 Leaving the study early
If data for this outcome were not clearly presented in the tables, we took relevant data from the text of each report.
At three months, there was no difference between treatment groups (n = 463, 3, RCTs, RR 0.80 95% CI 0.55 to 1.15). Small but significant differences favouring the standard care group were found for pooled data at six (n = 718, 5 RCTs, RR 0.73, 95% CI 0.55 to 0.97,) and 12 months (n = 594, 4 RCTs, RR 0.74, 95% CI 0.56 to 0.98). By 20 months a very slight but not statistically significant effect favouring the crisis group was found (n = 475, 3 RCTs, RR 0.78, 95% CI 0.57 to 1.06).
Only Hoult 1983 presented data for all relatives of those randomised. We found no difference in attrition between the groups (n = 120, RR 1.09, 95% CI 0.52 to 2.28). Muijen 1992 reported only on those relatives who were living with the randomised person and again found no clear differences between groups at 20 months (n = 76, RR 0.71, 95% CI 0.43 to 1.17).
2.4 Global state
Global state did not vary greatly between the two groups. Two scales were used, the GAS and SAS. Data for GAS in Howard 2010 at three months showed no difference in scores between the crisis and standard groups. Data for GAS in Muijen 1992 were equivocal at six, 12 and 20 months (six months: n = 129, MD 5.10, 95% CI −0.86 to 11.06; 12 months: n = 131, MD 3.50, 95% CI −3.15 to 10.15; 20 months: n = 142, MD 5.70, 95% CI −0.26 to 11.66). SAS data from Muijen 1992 were also not significantly different over the same time periods (six months: n = 130, MD −0.20, 95% CI −0.75 to 0.35; 12 months: n = 120, MD −0.30, 95% CI −0.85 to 0.25; 20 months: n = 139, MD −0.60 95% CI −1.15 to −0.05).
Muijen 1992 also reported change in global state during the first three months. They found no difference for GAS change scores (n = 129, MD 5.20, 95% CI −1.19 to 11.59) or for SAS change scores (n = 127, MD 0.09, 95% CI −0.31 to 0.13). The data, however, were likely to be skewed. Hoult 1983 found the crisis intervention care patients had significantly higher scores on the HSRS (P < 0.05) but reported no variance of these data.
2.5 Mental state
2.5.1 Numbers unwell
The crisis intervention packages investigated within this review seem to have little discernible effect on mental state. Hoult 1983 gave numbers unwell at 12 months and reported a slight, statistically non-significant effect favouring the crisis intervention care group (n = 120, RR 0.65, 95% CI 0.40 to 1.07).
2.5.2 Scale data
2.5.2.1 Endpoint data
Johnson 2005 and Howard 2010 assessed mental state using the BPRS at three months and found that the crisis group scored better although not significantly (n = 248, 2 RCTs, MD −4.03, 95% CI −8.18 to 0.12 ). However, we wish to determine whether there are differences between residential programmes such as Howard 2010 and mobile teams investigated in Johnson 2005. When data from Howard 2010 are removed, the difference between the crisis group and the standard group becomes significant (n = 226, 1 RCT, MD −2.90, 95% CI 5.50 to 0.30).
Muijen 1992 also assessed mental state using the BPRS and found no significant difference between the groups by six or 12 months (n = 129, 1 RCT, MD −2.10, 95% CI −6.40 to 2.20; n = 131, 1 RCT, MD −2.2, 95% CI −6.03 to 2.03, respectively) but a statistically significant difference favouring the crisis intervention care group by 20 months (n = 142, 1 RCT, MD −4.5, 95% CI −8.68 to −0.32). Hoult 1983 also assessed mental state using the BPRS and claimed no difference between the groups but reported no data to support this.
Fenton 1979 used the PEF and found no effect at three months (n = 118, 1 RCT, MD 0.20, 95% CI −0.22 to 0.62) or at six months (n = 111, 1 RCT, MD 0.10, 95% CI −0.42 to 0.62). By 12 months, there was a small difference suggesting mental state of crisis intervention care group to be more improved (n = 97, 1 RCT, MD −0.40, 95% CI −0.84 to 0.04). This slight advantage was lost by 20 months when the difference between the groups was not significant (n = 100, 1 RCT, MD 0.10, 95% CI −0.47 to 0.67).
Muijen 1992 used the PSE but data were skewed. No significant difference was found at six or 12 months (P not reported) but they found a slight difference favouring the crisis intervention care group at 20 months (P = 0.09, trend only). Hoult 1983 also reported data for the PSE endpoint scores. They found a significant difference favouring the crisis intervention care group but did not report standard deviations.
Fenton 1998 used PANSS at six months follow-up. There were no significant differences between the groups (n = 111, 1 RCT, MD 4.00, 95% CI −3.45 to 11.45).
2.5.2.2 Change data
Muijen 1992 presented skewed data for change in mental state. At three months, they found no significant differences for scores on the BPRS (n = 129, 1 RCT, MD −3.50, 95% CI −8.92 to 1.92) or the PSE (n = 129, 1 RCT, MD −2.70, 95% CI −7.69 to 2.29).
2.5.3 Specific symptoms or behaviour
Hoult 1983 found no significant difference between the crisis intervention care and standard care groups for patient sociability at three months (n = 129, 1 RCT, RR 0.86, 95% CI 0.66 to 1.12) but the crisis care group was significantly more ‘sociable’ by six months (n = 120, 1 RCT, RR 0.43, 95% CI 0.30 to 0.64). This study also reported that there was no significant difference in aggressive behaviour at three or six months (n = 120, 1 RCT, RR 0.97, 95% CI 0.72 to 1.31; n = 120, 1 RCT, RR 0.70, 95% CI 0.39 to 1.25, respectively).
Hoult 1983 also recorded various behaviours such as agitation, depression, disorientation, psychotic thoughts, substance abuse and withdrawal (at four months).
The crisis intervention care group was less agitated (n = 120, 1 RCT, RR 0.59, 95% CI 0.36 to 0.95) and disorientated (n = 120, 1 RCT, RR 0.47, 95% CI 0.28 to 0.79) than the standard care group. There was a very small effect favouring the crisis intervention care group on the outcomes of ‘psychotic behaviour’ (n = 120, 1 RCT, RR 0.58, 95% CI 0.30 to 1.11) and ‘withdrawal’ (n = 120, 1 RCT, RR 0.72, 95% CI 0.48 to 1.07) but these results are not statistically significant. No differences were found for ‘depression’ (n = 120, 1 RCT, RR 0.80, 95% CI 0.57 to 1.13) or ‘substance abuse’ (n = 120, 1 RCT, RR 0.67, 95% CI 0.33 to 1.36).
2.6 Burden
Studies measured two types of burden; the burden placed on the families of the patients and burden placed on the community.
In general, the families of patients in the crisis intervention care group reported less burden than those of standard care patients. Significantly fewer crisis intervention care families reported disruption to their daily routine at three months than their standard care counterparts (n = 220, 2 RCTs, RR 0.76, 95% CI 0.59 to 0.97). This was a non-significant difference at six months (n = 220, 2 RCTs, RR 0.67, 95% CI 0.37 to 1.21,).
Significantly fewer crisis intervention care families reported significant disruption to their social life at three months (n = 220, 2 RCTs, RR 0.69, 95% CI 0.53 to 0.91). By six months, this was a non-significant difference (n = 220, 2 RCTs, RR 0.72, 95% CI 0.43 to 1.22). The crisis intervention care families also reported significantly less physical illness over the same time periods (n = 100, 1 RCT, RR physically ill by three months 0.78, 95% CI 0.65 to 0.95; n = 100, 1 RCT, RR physically ill by six months 0.71, 95% CI 0.55 to 0.92,) than those allocated to standard care. Data from Hoult 1983 show no significant difference in financial burden at three months (n = 120, RR 0.76, 95% CI 0.52 to 1.10) and at six months (n = 120 RR 0.84, 95% CI 0.53 to 1.33). This study also reported the number of families who felt that the overall burden was great; the outcome favoured the crisis intervention care group at three months (n = 120 RR 0.57, 95% CI 0.41 to 0.80) and also at six months (n = 120, RR 0.34, 95% CI 0.20 to 0.59).
Fenton 1979 assessed family burden using the FEF but presented combined data. The two significant items (assuming patient’s responsibilities and finding extra chores difficult) were the only items presented as individual data. A total of 61 items were analysed. No differences were found between those allocated to the crisis intervention care and standard care for ‘community burden’. The number of people with paid work at six months (Fenton 1998) and without full time employment at 20 months (Muijen 1992) was not significantly different (n = 112, 1 RCT, RR 1.41, 95% CI 0.65 to 3.04 and n = 189, 1 RCT, RR 0.97, 95% CI 0.85 to 1.12, respectively) as were the risk of using the emergency services at least once (n = 120, 1 RCT, RR 0.81, 95% CI 0.43 to 1.54). Stein 1975 reported slightly fewer crisis intervention care patients having had at least one arrest by 12 months although this result was not statistically significant (n = 120, 1 RCT, RR 0.71, 95% CI 0.46 to 1.12), Fenton 1998 reported only three arrests in total, all of these occurred in the crisis arm but this was not a significant difference (n = 111, 1 RCT, RR 5.36, 95% CI 0.28 to 101.35). Fenton 1998 also reported that at six months follow-up, there was no significant difference in the numbers of participants who were homeless (n = 113, 1 RCT, RR 1.23, 95% CI 0.59 to 2.57). Hoult 1983 commented on community burden. They did not claim significant difference between the groups but no data were reported.
2.7 Satisfaction
One trial gave count data for patient and relative satisfaction (Hoult 1983). Overall people allocated to crisis intervention care and their relatives were more satisfied with their treatment and level of support than those given standard care. By 12 months significantly fewer people in the crisis intervention care groups felt ‘unimproved’ (n = 119, 1 RCT, RR 0.48 95% CI 0.31 to 0.74) when compared to the standard care group. Also data significantly favoured the crisis intervention care group when it came to dissatisfaction with the level of treatment received (n = 119, 1 RCT, RR 0.66 95% CI 0.50 to 0.88) and or feeling less able to cope than before their treatment (n = 119, 1 RCT, RR 0.36 95% CI 0.21 to 0.62). More people allocated to standard care felt they would have preferred community treatment when compared to the numbers of crisis intervention care patients preferring to have received hospital care (n = 119, 1 RCT, RR 0.46 95% CI 0.27 to 0.77). There was a small effect suggesting more crisis intervention care patients felt they would need extra help in the future but the difference was not statistically significant (n = 119, 1 RCT, RR 1.48 95% CI 0.88 to 2.48).
Three other studies used satisfaction scales to obtain continuous data (Johnson 2005, Muijen 1992 and Howard 2010). Johnson 2005 measured patient satisfaction at 3 months using the CSQ and found borderline significant differences favouring the crisis intervention care group (n = 226, 1 RCT, MD 1.60, 95% CI −0.22 to 3.42), Muijen 1992 also measured patient satisfaction using the CSQ and found significant differences favouring the crisis intervention care group at six months (n = 115, 1 RCT, MD 5.10 95% CI 3.16 to 7.04), at 12 months (n = 121, 1 RCT, MD 4.80 95% CI 3.12 to 6.49) and also at 20 months (n = 137, 1 RCT, MD 5.4O 95% CI 3.91 to 6.89). Howard 2010 measured patient satisfaction by three months using the VSSS scale but found no significant differences (n = 24, 1 RCT, MD 0.20, 95% CI −0.20 to 0.60)
Only Hoult 1983 assessed relative satisfaction using count data. At three months, slightly fewer relatives in the crisis intervention care group were dissatisfied with the patients’ improvement (n = 120, 1 RCT, RR 0.79, 95% CI 0.60 to 1.04). By six months the difference was statistically significant (n = 120, 1 RCT, RR 0.71, 95% CI 0.53 to 0.97). Significantly fewer crisis intervention care relatives were dissatisfied with the treatment the patient was receiving at three months (n = 120, 1 RCT, RR 0.63, 95% CI 0.44 to 0.89), six months (n = 120, 1 RCT, RR 0.57, 95% CI 0.42 to 0.78) and one year (n = 120, 1 RCT, RR 0.46, 95% CI 0.29 to 0.72). There was no difference in the number of relatives preferring the patient to have been allocated to the other treatment at any of the time points. There was a slight tendency towards more crisis intervention care relatives being satisfied with their allocated treatment as time progressed although this was not significant at any time point (n = 120, 1 RCT, RR at three months 1.27, 95% CI 0.63 to 2.57; n = 120, 1 RCT, RR at six months 1.11, 95% CI 0.49 to 2.54; n = 120, 1 RCT, RR at one year 0.81, 95% CI 0.43 to 1.54).
Significantly fewer relatives in the crisis intervention care group felt unable to cope at 12 months than they had felt before treatment began (n = 120, 1 RCT, RR 0.57, 95% CI 0.42 to 0.78) when compared with the standard care group. A small effect was found at 12 months showing more crisis intervention care relatives felt they would need future help. This difference was not statistically significant (n = 120, 1 RCT, RR 1.21, 95% CI 0.91 to 1.60).
2.8 Economic
The two trials that reported relevant data found crisis intervention care for those in crisis was significantly cheaper than standard care (P < 0.001) but all data presented were highly skewed (Fenton 1979; Muijen 1992). The other two trials (Hoult 1983; Stein 1975) also found crisis intervention care to be significantly cheaper but gave no variance of the average cost.
2.9 Quality of Life
Johnson 2005 measured quality of life by three months using the MANSA scale but found no significant differences (n = 226, 1 RCT, MD −1.50, 95% CI −5.15 to 2.15). Howard 2010 measured quality of life by three months using the short form MANSA scale and found a significantly better quality of life for the crisis intervention arm over the standard care group (n = 28, 1 RCT, MD 0.70, 95% CI 0.14 to 1.26).
Howard 2010 measured also quality of life by three months using the EQ-5D scale but found no significant differences between the crisis and standard care group (n = 26, 1 RCT, MD 0.01, 95% CI −0.32 to 0.34).
2.10 Social functioning
Johnson 2005 measured social functioning by three months and six months using the LSP scale but found no significant differences at either time point (n = 260, 1 RCT, MD 3.00, 95% CI −0.72 to 6.72 and MD 1.00, 95% CI −2.76 to 4.76, respectively). Fenton 1998 reported the mean number of social contacts per week for participants at six months, rates for the two arms were very similar and showed no significant difference (n = 107, 1 RCT, MD 0.43, 95% CI −0.30 to 1.16).
2.11 Clinical and social problems
Johnson 2005 measured clinical and social problems by three months and six months using the HoNOS scale but found no significant differences at either time point (n = 257, 1 RCT, MD −1.90, 95% CI −3.2 to −0.60 and MD −0.60, 95% CI −2.07 to 0.87, respectively).
2.12 Unmet needs
Howard 2010 measured participants’ unmet needs using the CAN scale. However, the data are highly skewed and descriptive statistics only are presented, the mean for the standard care group is higher (more unmet needs) than for the crisis arm.
2.13 Outcomes with no data - staff satisfaction
No data were presented for this outcome although three trials (Hoult 1983; Muijen 1992; Pasamanick 1964a) mentioned considerable problems with staff recruitment, despondency and ‘burnout’ within the crisis intervention care team.
2. COMPARISON 2: CRISIS INTERVENTION (Mobile teams) versus CRISIS INTERVENTION (residential)
A stated objective of the review was to compare crisis intervention carried out by mobile teams operating in the homes of patients versus crisis intervention taking place in home-like residential units. It has not been possible to make this comparison as the studies we have identified make comparisons between either mobile teams or residential units and standard care, usually hospital admission.
DISCUSSION
Summary of main results
1. General
Overall, the description of the methodology within the included studies was poor. Trials were small and data reporting problematic. We had great difficulty in acquiring a definitive description of ‘crisis intervention’ for studies included in 2006 and earlier and used the criteria that it should involve an intense, time-limited, input of care during a crisis period and that this care should be available 24 hours. None of the included studies included before 2006 investigated ‘crisis intervention’ in a pure form. All employed packages of home care that included an element of crisis intervention according to the above criteria. The crisis intervention elements ranged from an automated 24-hour telephone help line to on-call staff who could provide an immediate response. The 2006 results of this review therefore related to this type of home care (i.e. home care designed to treat those in psychiatric crisis) compared with standard hospital care. To complicate matters further, as the home care intervention was not only implemented during a crisis but also lasted well beyond, results also relate to the effects of this ongoing treatment.
However, in this 2010 update we have now included three new studies. Johnson 2005 has gathered data in the UK after government policy established crisis resolution teams (CRTs) (Department of Health 2000). This study investigated ‘crisis intervention’ given in a form specified for CRTs which, unlike the earlier studies, provided short-term input but did not provide ongoing home care intervention. The two other new studies included (Fenton 1998; Howard 2010) investigated alternatives to hospitalisation, home-like ‘crisis houses’ aiming to provide care in a residential setting and support to continue daily routines as far as possible.
It should be noted that one of the included studies (Pasamanick 1964a), took place almost fifty years ago. In general, the care of people with schizophrenia has changed enormously since then and the relevance of this trial is questionable. It does, however, meet all the criteria necessary for inclusion and the two results obtained from this trial (hospital admission and leaving the study early) are in line with findings from other studies.
2. COMPARISON 1. CRISIS INTERVENTION versus STANDARD CARE
2.1 Death or harm
There were few episodes of self-harm and even less of death. There were two homicides altogether in the total sample, both occurring in the crisis intervention treatment arms of studies. However, with a total sample size of 984 for all the studies combined, it is difficult to draw conclusions from this. There is no indication of any effect crisis intervention may have on these important outcomes. The only firm conclusion possible is that much larger studies are needed if this is to be investigated within the context of trials.
2.2. Hospital use
Care needs to be taken when interpreting hospital admission rates. For studies included in the 2006 update and earlier, comparing the treatment groups on overall number of admissions is misleading as admission was an integral part of the standard care and this ‘index’ admission was not differentiated from subsequent admissions taking place within the timescale of the study.
However, with two of the new studies, (Fenton 1998; Johnson 2005), admission data were differentiated and therefore, data from these two studies have been presented separately. There is a further distinction to be made between these studies, as Fenton 1998 reports data from a crisis house whereas Johnson 2005 reports on the work of a mobile crisis team treating patients within their own homes.
Data from Johnson 2005 at three months and six months show a clear, significant advantage for the crisis arm, this is also the case for admissions due to compulsory detentions, which were analysed separately. Once data from Fenton 1998 at six months are included, the crisis arm retains a more favourable outcome than standard care, but not to a significant extent. It appears that the crisis intervention package provided by the Crisis Resolution Teams in the UK succeeded in reducing hospital admissions, which was one of their main stated aims.
Data for hospital admission rates for the home care package group i.e. all included studies dating before 1998, were presented as the number of repeat admissions.
Pooled data from three studies (Fenton 1979; Hoult 1983; Muijen 1992) suggested crisis intervention as part of a home-care package was superior, with significantly less repeat admissions by 12 months. However, this result contains a considerable amount of heterogeneity (I2 86%), with one very positive study (Hoult 1983) affecting data from the other two studies which found no differences in repeat admissions. There is no clear reason why Hoult 1983 sits apart from the other two trials. Data from Fenton 1979 suggested the home care group had fewer staff contacts, but this information was not supported by usable data and more research is needed. In conclusion, the picture for hospital readmission is mixed with two studies finding a distinct advantage for the crisis arm and three studies finding no difference. These varied findings may be caused by the different ways in which ‘crisis intervention’ has been applied and also by the different methods by which hospital readmission has been measured.
2.3 Leaving the study early
Homogeneous data suggest that people who were allocated to have their crisis managed within the home care group were more likely to stay in care for at least a year. This is an important finding and even though findings for several other important effects of this package may be unremarkable these data alone may be enough to promote the use of a crisis ethos within home care teams (see Implications for practice).
2.4 Global state and mental state
Howard 2010 and Muijen 1992 were the only studies reporting usable data for global measures of outcome. Although there was some suggestion that within the GAS score there was an effect favouring the crisis group, no major differences between the two treatments were found and the clinical meaning is unclear.
Pooled data on mental state as measured by the BPRS for Howard 2010 and Johnson 2005 found a clear, significant advantage for the crisis arm after three months. Muijen 1992 found no differences in the crisis and standard care arms at six and 12 months, but at 20 months again, the advantage was significant to the crisis arm. Hoult 1983 was the only trial to give binary data based on relatives’ observations. Some differences in behaviours such as sociability, agitation and disorientation were found favouring the crisis intervention care group but it would be prudent to replicate these findings as they are all from one very positive small study (n = 120, Hoult 1983). Overall, one may cautiously suggest that results favour crisis intervention over hospital admission, however, all studies are small and not of high quality (see Summary of findings for the main comparison)
2.5 Burden
Overall, specific burden on families such as ‘disruption to daily routine’ (CI 3 to 30), ‘physical illnesses experienced’ (CI 2 to 14), and ‘disruption to social life’ (CI 3 to 30), favoured the crisis intervention group. None of these findings are based on large numbers and, again, all should be replicated. The direction of effect, however, is consistent within and across trials. These data, at the very least are hypotheses-generating for further studies and may suggest that families find routine admission more disruptive and burdensome than well-motivated crisis intervention.
Little can be said about the effect of crisis intervention regarding ‘community burden’ in terms of employment, numbers of people being arrested, homelessness or using emergency teams, except perhaps that the results are resolutely equivocal. Unfortunately few of the included studies recorded and reported these important outcomes.
2.6 Satisfaction
Patient and relatives’ satisfaction was higher in the crisis intervention care group than in those allocated to standard care. This finding was generally consistent over several measures although all continuous measures are difficult to interpret, one study only found that relatives may have preferred more help outside of working hours (Hoult 1983). These data would fit with the findings relating to ‘burden’ and further supports the suggestion that the experimental intervention is acceptable to both those with serious mental illness and their ‘lay’ carers.
2.7 Economic
The limited data available found crisis intervention care to be significantly cheaper than standard care. Again, data were difficult to interpret, as they were either very skewed or unusable. We recognise the difficulty in recording such data but nevertheless such outcomes are of crucial importance if research is to be relevant to managers and policy makers.
2.8 Quality of Life
Limited data from two studies found no significant differences between treatment arms, however, one very small study Howard 2010 did find a significant advantage for the crisis arm.
2.9 Social functioning
Limited data from one study found no significant differences between treatment arms.
2.10 Clinical and social problems
Limited data from one study found no significant differences between treatment arms.
2.11 Missing outcome: staff satisfaction
It is unfortunate that no data are available for staff satisfaction. Issues such as staff recruitment, despondency and burnout are essential to the successful implementation of crisis intervention care packages. Several of the studies mentioned these as notable problems affecting the running of the project. If such problems were prominent in these usually well-resourced and well-motivated research teams, they may amount to insurmountable obstacles to the implementation of similar projects in routine psychiatric settings.
2. COMPARISON 1. CRISIS INTERVENTION (mobile) versus CRISIS INTERVENTION (residential)
It was not possible to compare these outcomes as none of the studies directly compared residential units providing crisis care versus the mobile teams. However, inclusion of the data from the two studies assessing the residential crisis houses with the data from the six studies examining mobile care generally produced consistent results. Where results were at variance this has been indicated above. For hospital admission, mobile teams did appear to be slightly more successful at preventing readmission compared with standard care.
Overall completeness and applicability of evidence
1. Completeness
No outcomes in this review involve large numbers of people. Some important measures and more subtle findings are not recorded. Most importantly, it was possible to assess whether crisis interventions were achieving their chief aim, that is, to reduce hospital admissions for psychiatric treatment on one study only. Furthermore, we identified few data on quality of life, social functioning and patient satisfaction. Staff satisfaction was identified in the literature as an important factor but was not measured by any of the studies although some did mention problems with staff recruitment and stress.
2. Applicability
Only one of the most recent trials, Johnson 2005, evaluated crisis intervention as practiced by Crisis Resolution Home Care teams currently in the UK, that is, as a brief, time-limited intervention. The other, older trials, all comprised crisis interventions which were part of an on-going healthcare package. The extent to which these are relevant to current treatment is debated in Johnson 2008. An important limitation is that the older studies, unlike the present system, continued care for a longer term after the resolution of the initial crisis. Two other studies were included because they evaluated crisis houses, home-like units based in residential areas, as an alternative to hospital admission.
Quality of the evidence
The included trials were of similar size, ranging from 41 to 260 participants and follow-up ranged from three months to 24 months. Trials of this relatively small size are unlikely to detect subtle but important differences with any confidence, and it was not possible to look at subgroups such as different kinds of programmes and patients at different clinical stages. Overall, the quality of reporting of these trials was poor. Some studies were not clearly blinded or did not have independent assessors collecting outcome data. Allocation concealment was not always described and loss of some participants to follow-up unaccounted for. We are unsure if data are incomplete or selectively reported, or if other biases were operating.
Potential biases in the review process
1. Missing studies
Every effort was made to identify relevant trials. However, trials in this area are very few in number. Johnson 2005 point out that recruitment to a trial during a psychiatric crisis is a challenge as crises tend to occur suddenly and unexpectedly. To fulfil the consent requirements for present ethical regulations, waiting for a researcher to arrive and conduct an interview before formulating a treatment plan is often unsafe and unfeasible, and patients often lack the capacity to make informed decisions. These problems probably account for the lack of recent randomised evaluations of crisis services. It seems unlikely therefore, that we have failed to identify large relevant studies in accessible literature. We were unable to include one unpublished study where we are awaiting a response from the author.
2. Introducing bias
This review group has now updated this review several times and incorporated the new methodology of the Cochrane Schizophrenia Group. We have tried to be balanced in our appraisal of the evidence, RD is a statistician who is unconnected with this field, but we could have inadvertently introduced bias. We welcome comments or criticisms.
Agreements and disagreements with other studies or reviews
Johnson 2008 have reviewed the evidence for crisis intervention for adults requiring psychiatric admission for any condition, and included non-randomised studies as well as RCTs. They concluded that trends have emerged suggesting that crisis intervention has an impact on hospital admission and that service users are more satisfied with them than with standard hospital care. However, they cautioned that the evidence base remains very limited and that high-quality randomised trials are needed, particularly on the functioning of well-established crisis intervention teams which have not, as yet, been assessed. Shepperd 2009 have reviewed alternatives to inpatient mental health care for children and young people, including crisis intervention. They identified one study only for this age group, for children with emotional and behavioural disorders experiencing psychiatric crises requiring hospitalisation. By comparison to standard care, children in the crisis intervention group showed small, significant favourable differences in family cohesion, social behaviour and competency and self-esteem in the short-term, again, providing some limited support for the crisis approach.
AUTHORS’ CONCLUSIONS
Implications for practice
The effects of crisis intervention in a ‘pure’ form apply to only one of the studies included in the review. Older studies have evaluated crisis intervention on top of an ongoing package of community-based care and two other new studies have evaluated crisis houses. Some conclusions, therefore, apply to home-care packages as a package as a whole and some to crisis houses, although where findings differ between these different modes of delivery, this has been taken into account.
1. For people with serious mental illnesses and their families
After this 2010 update including three new studies, the data relating to readmission, length of stay, general functioning and mental state remain inconclusive. However, if a person with serious mental illness is experiencing a crisis, a well-organised team using a crisis intervention ethos within their care may provide support and treatment that is more acceptable to both sufferers and their families and less burdensome for the families than if the person was admitted to standard hospital care. Perhaps, as a result, the ill person would be more likely to stay in care.
2. For clinicians
Crisis intervention also seems to be a more acceptable type of care than standard hospital treatment whether stand-alone or as part of an ongoing homecare package. Where clinicians intend to establish a service, it may be advisable to consider better defined care packages or, if this is not feasible, introduce a crisis intervention ethos within the context of a well-designed trial.
3. For policy makers and managers
The results of this review have to be considered carefully in the context of other community packages already evaluated and reviewed. Lessons from crisis intervention theorists have been learnt by those formulating better defined care packages such as Intensive Case Management. More robust data from another Cochrane review illustrate how this package may have many of the desired effects originally envisaged for crisis intervention (Dieterich 2010). Results from searches of 2003 and 2006 found only trials that were investigating packages of ‘community care’ rather than ‘crisis intervention’, and the 2010 search found only one study investigating the work of crisis intervention teams. These studies should be incorporated into reviews and then policy makers and funders would be in a better position for decision making.
Implications for research
1. General
Should we acquire more data from existing studies, we would probably know much more about the effects of this widely implemented ethos of care. Much important data within the included studies were not reported clearly and therefore clinicians, funders and recipients of care may feel that they have been let down by the research community. If the CONSORT recommendations (Begg 1996; Moher 2001) were to be followed in reporting of future studies, this would greatly assist synthesis of data in reviews.
2. Specific
There are very few data on the role crisis intervention plays in treatment of people with severe mental illnesses. Currently it is implemented without good evidence. The earlier Cochrane reviews on this subject were only able to include studies in which crisis intervention was part of a package of longer-term community care rather than stand-alone crisis intervention. These earlier studies suggested that a trial of home care treatment such as the assertive community treatment (ACT) approach, with crisis intervention versus a similar home care treatment without crisis intervention would be informative. However, now that UK government policy mandated that crisis resolution teams (CRTs) are established throughout England (Department of Health 2000), and that there has been one study of the effectiveness of one of these teams (Johnson 2005), then it would be productive for future research to adopt a similar approach to Johnson 2005 to evaluating current services and crisis resolution. The use of strict randomisation to crisis intervention versus standard care means that there are considerable ethical hurdles to be overcome, however, Johnson 2005 do provide a model for dealing with this. Future trials should be large and simple. The interesting dichotomous outcomes that have been used in individual studies in this review could be incorporated with the addition of clear measures of the burden on the community and staff involved. Certainly researchers should use well-validated instruments for outcome measurement. Table 2 provides a suggestion for future design of trials in this area.
Table 2 .
Suggestions for trial design
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. Blindness: single. Duration: 12 months at least. Raters: independent. |
| Participants | Diagnosis: schizophrenia or related psychoses. N = 450* History: in need of psychiatric admission. Sex: both. Age: any. |
| Interventions | 1. Mobile crisis team, providing treatment in patients’ homes, multidisciplinary, 24-hour service, drug treatment, psychotherapy, instruction in living skills. N = 150 2. Crisis houses, providing treatment in residential areas, multidisciplinary, 24-hour service, drug treatment, psychotherapy, instruction in living skills. N = 150 3. Admission to psychiatric wards N = 150. In all three treatment arms, continuing treatment likely to be given by community mental health teams after initial crisis |
| Outcomes | Death. Serious harm to self and others. Service outcomes: hospital admission, readmissions. Leaving the study early. Global and mental state (CGI, binary outcome).** Satisfaction: family burden, patient satisfaction, relative satisfaction, staff burden (binary data) Economic data. Quality of life. |
| Notes | * Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. ** Primary outcome |
PLAIN LANGUAGE SUMMARY.
Crisis intervention for people with severe mental illnesses
The move from hospital to community-based care can be a frightening and difficult experience for people with severe mental illness (SMI). People with mental health problems may not have networks of support such as family, friends and carers. They often have no one they know personally to help them when they go home. To complicate matters, people with SMI can have critical downturns in their mental health creating a revolving-door of care, where service users are discharged from hospital when considered stable and well, only to go back into hospital again when their mental health becomes worse during an acute episode or crisis. Crisis intervention and home-care packages have been developed as a possible solution to these problems.
Crisis care, where support is provided during a crisis for service users, either in their home or a community setting, was found by this review to provide a package of support that was worthwhile, acceptable and less expensive than standard care. Furthermore, crisis care avoided repeat admission to hospital (at three and six months after crisis, in some cases by 50%); improved the mental state of services users more than standard care (at three months after crisis); was more acceptable and satisfactory to service users, their families and carers; placed less burden on families and carers; and reduced the stigma of hospitalisation. Burden (such as disruption to daily routine, social life and susceptibility to physical illness) was also reduced for service users, their families and carers. There were no differences in death rates between crisis and standard care.
The review, however, looks at only six studies with a total of 984 people. The methods of these six studies were considered poor and there was no definitive description of crisis intervention or crisis care, meaning there was a lack of focus on crisis care in its pure form. Most studies excluded service users with alcohol or drug misuse, and those who were a danger of being harmful to themselves or others. The authors of the review suggest more studies are needed to create a stronger evidence base. Crisis care may be currently delivered without sound and good evidence. For example, no data or information were available on carer input, concordance or the willingness of service users to take medication and the number of relapses experienced by service users. Finally despite reports of staff ‘burn-out’, staff satisfaction with crisis care was not assessed.
This plain language summary has been prepared by Ben Gray of Rethink Mental Illness: Benjamin Gray, Service User and Service User Expert, Rethink Mental Illness. ben.gray@rethink.org.
ACKNOWLEDGEMENTS
Thanks to Professor Tom Burns for help with defining crisis intervention, Professor Max Marshall who also helped with difficult definitions and Leanne Roberts for administrative support. Thanks also to Gill Rizzello and Tessa Grant for their help with the 2003 and 2006 updates and many thanks to Judith Wright for narrowing the 2006 search down to a manageable number of trials. Thanks to Samantha Roberts for help with the 2010 searches. Also thanks to Mark Fenton of the James Lind Alliance for his suggestions for Table 2. We would also like to acknowledge the significant contribution of Karl Rice for his help with trial selection, data extraction, completion of report for the original review publication and for his help with trial selection in the 2003 and 2006 updates.
We have updated the methods section substantially to reflect the new methods used by the Cochrane Schizophrenia Group since this review was last published. We have used their standard template for the methods section and adapted it as required.
The trial search co-ordinator of the Cochrane Schizophrenia Group, Samantha Roberts, developed and ran the searches.
SOURCES OF SUPPORT
Internal sources
Gwent Community Health NHS Trust, Adult Services, UK.
NHS Executive Anglia and Oxford R&D Directorate, UK.
University of Leeds, UK.
External sources
Welsh Office of Research and Development for Health and Social Care, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised. Blindness: single. Duration: 12 months. Raters: independent. |
|
| Participants | Diagnosis: schizophrenia 41.9%, psychosis 30.3%, neurosis 27.8% (ICD-8). N = 162.* History: in need of psychiatric admission, 40% first admissions. Sex: 40% M, 60% F. Age: over 18 yrs, modal range 24-35 yrs. Exclusions: organic brain syndrome, alcoholism, drug dependency, violent or suicidal behaviour, non-English speaking, non resident of Montreal |
|
| Interventions | 1. Home care: assessment & treatment in home environment, multidisciplinary team, 24-hour service, drug treatment, psychotherapy, instruction in living skills. N = 78. 2. Standard care: short-term, intensive care in hospital, normal staffing levels, social work, follow-up visits after discharge. N = 84 |
|
| Outcomes | Death.** Hospital admission: unable to keep to initial protocol. Readmission. Leaving the study early (patients). Staff contact.*** Mental state: PEF. Economic cost. Unable to use - Days in hospital: includes index admission. Leaving the study early (relatives): no individual data available for each group. Family burden: FEF (reported only 2 ‘significant’ items out of 61 analysed) |
|
| Notes | * Demographic data on 155 patients only. ** Assumed deaths occurred at 6 months. ***Home care: number of visits made by team to families or patients in community. Standard care: number of visits made by patient to OPD |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described, raters ‘independent of treatment teams’. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Loss of participants presented and described, no LOCF. |
| Selective reporting (reporting bias) | Low risk | All outcomes had data presentation. |
| Other bias | Low risk | Grant funded research. |
| Methods | Allocation: randomised. Blindness: not blinded. Duration: 6 months. Raters: independent. |
|
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, other psychoses 56%, bipolar 21%, other major mood disorder 20%, other 4%, co-morbid drug or alcohol dependence 27%, co-morbid axis II disorder among patients without schizophrenia, schizoaffective disorder or bipolar 77% N = 119. Gender: female 48%, male 52%. Age: mean = 38 years, standard deviation = 10.5. History: insured patients (USA) with documented severe and persistent mental illness characterised by repeated hospitalisation, voluntary admitted patients. Exclusions: uninsured or private third party insurance, patients requiring detoxification or acute general medical intervention |
|
| Interventions | 1. Home-like acute residential facility - 8-bed crisis alternative house in residential neighbourhood 2. Standard care: psychiatric unit in general hospital. |
|
| Outcomes | Death. Hospital admission: number of days for index admission for both interventions. Hospital admissions: admission for acute care not including the index admission. Treatment failure rate: defined as transfer to another in-patient facility Mental State: PANSS. Social functioning: number of participants with paid work, homeless, arrested Number of social contacts per week per participant. Unable to use - Patient satisfaction data - questionnaire adapted by authors |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent and computer-generated sequence. |
| Allocation concealment (selection bias) | Low risk | As above, clinicians blinded to randomisation. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Not described, but research interviewers collected some data from medical records, therefore unlikely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Missing data for hospital arm between 0 and 20 (maximum 40%) participants depending on outcome Missing data for residential arm between 0 and 29 (42%) participants depending on outcome 95% of patients located for follow-up interview, missing data not explained |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit clear judgment. The original protocol is not available, therefore it is not clear if all measured outcomes have been reported |
| Other bias | Low risk | No conflicting interests declared. Exclusion of uninsured and third-party insured participants reduces generalisability of findings |
| Methods | Allocation: randomised. Blindness: single, independent raters. Duration: 12 months. |
|
| Participants | Diagnosis: severe psychosis (PSE), 50.4% schizophrenia (DSM III). N = 120. History: presenting for admission to psychiatric hospital. Sex: 45.8% M, 54.2% F. Age: 15-65 yrs. Exclusions: dual diagnosis, organic brain disorder, mental retardation, non resident of local area |
|
| Interventions | 1. Home care: multidisciplinary team, 24-hour crisis service, drug treatment, counselling, training in basic living skills, family intervention, support & education, intensive treatment during acute phase. N = 60. 2. Standard care: admission (mean of 3 weeks), normal staffing levels, day programmes, discussion groups, arts & crafts, sporting activities, after care by standard community mental health centres. N = 60 |
|
| Outcomes | Death. Harm. Hospital admission: unable to keep to initial protocol. Readmissions. Leaving the study early (patients, relatives-total). Mental state.* Family burden. Patient satisfaction. Relative satisfaction (total and subgroup). Unable to use - Number of days in hospital: no SD. Loss (relatives-sub): not clear how many relatives lived with patients. Global State: HSRS (no SD). Mental State: BPRS (no data). Mental state: PSE (no SD). Community burden: no data. Economic cost: no SD. |
|
| Notes | * 19 symptoms, rated by relatives. For purposes of this review these were grouped into affective symptoms, psychotic symptoms, behaviour, physical problems, social functioning, substance abuse & most relevant symptom taken from each category for analysis | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised - nurse selected randomly mixed sealed envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Loss of some participants not described. |
| Selective reporting (reporting bias) | Unclear risk | Most outcomes had data presented, apart from community burden |
| Other bias | Low risk | Grant funded research |
| Methods | Allocation: randomised, random 24-hour assignment by private company (author correspondence). Blindness: independent raters, not blinded (author correspondence). Duration: 12 weeks. Raters: independent (author correspondence). |
|
| Participants | Diagnosis: schizophrenia 20%, mood/anxiety disorder 29%, bipolar 29%, personality disorder 23% N = 41.* History: All women requiring voluntary admission to either psychiatric in-patient ward or women’s crisis house Sex: 100% F. Age: crisis arm M = 40 years (SD 13.1), ward arm M = 34 years (SD 11.0) Exclusions: participants not clinically appropriate for crisis house e.g. violent behaviour, needing detoxification, or needing intensive observation and constant supervision |
|
| Interventions | 1. Admission to crisis house: Home-like environment in residential area 2. Stantard care: admission to psychiatric in-patient ward. |
|
| Outcomes | Global functioning: GAS. Mental state: BPRS. Quality of life: MANSA and EQ-5D. Patient satisfaction - VSSS. Unable to use - Quality of life: EQ-5D data highly skewed, descriptive statistics only used Assessment of need: CAN data highly skewed, descriptive statistics only used |
|
| Notes | *41 participants randomised - the study also followed up non-randomised patients but these were not used in the review | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Private company providing 24-hour randomisation. |
| Allocation concealment (selection bias) | Low risk | As above, central allocation. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Researchers collecting measures not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Those not consenting to randomisation were also followed-up, consenting to randomisation = 42. 1 lost as subsequently did not enter ward or house. 5 (26%) lost to follow-up in crisis arm, 8 (36%) lost to ward arm, losses not explained |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgment of ‘yes’ or ‘no’. The original protocol is not available, therefore it is not clear if all measured outcomes have been reported |
| Other bias | Unclear risk | Funded by MRC, no conflicting interests declared. |
| Methods | Allocation: randomised. Blindness: not blinded. Duration: 6 months. Raters: independent for some outcome measures only. |
|
| Participants | Diagnosis: 25% schizophrenia/schizoaffective disorder, 10% bipolar affective disorder, 7% other psychosis, 30% unipolar depression, 13% personality disorder, 4% other non-psychotic disorder, 5% substance misuse only (substance misuse or dependence sole diagnosis or co-morbid 43%). N = 260. History: in need of admission to psychiatric hospital. Sex: 51% M, 49% F. Age: 18-65 Mean = 37.9 years. Exclusions: non-residents of London Borough of Islington. |
|
| Interventions | 1. Crisis care: standard care augmented by multi-disciplinary Crisis Resolution Teams available 24 hours with the aim of managing patients at home if feasible 2. Standard care from in-patient unit, crisis houses and Community Mental Health Teams |
|
| Outcomes | Death. Harm. Hospital admission: total and also due to compulsory detention only. Leaving the study early (patients). Mental state: BPRS. Patient satisfaction: CSQ. Quality of life: MANSA. Social functioning: LSP*, HONOS*. |
|
| Notes | *Measures taken by staff involved in care, not independent. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation by Aberdeen University 24-hour randomisation service |
| Allocation concealment (selection bias) | Low risk | As above, randomisation at central location. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Article states ‘blinding of researchers, clinicians or patients was not possible’ |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Missing outcome data balanced across both intervention arms (n = 17 both) which is only a 12% loss, however, reasons for this loss not accounted for in the article |
| Selective reporting (reporting bias) | Unclear risk | Original protocol unavailable, unclear if all measured outcomes reported. Insufficient information to permit a clear judgement of yes/no |
| Other bias | Low risk | Grant funded research, no competing interests declared. Some bias for the analyses in this review as the standard care group includes participants admitted to crisis houses |
| Methods | Allocation: randomised. Blindness: single. Duration: 20 months. Raters: independent. |
|
| Participants | Diagnosis: serious mental illness (PSE), 53% met criteria for schizophrenia. N = 189. History: in need of immediate hospitalisation. Sex: 49.7% M, 50.3% F. Age: 17-64 yrs, mean ~35yrs. Exclusions: dual diagnosis. |
|
| Interventions | 1. Home care: DLP’s home based care, multidisciplinary team, crisis clinics, 24-hour answering service, problem solving, home visits & relative support, life skills training, assistance with financial & housing problems. N = 92. 2. Standard care: hospital care, normal staffing levels, standard outpatient services, CPN. N = 97 |
|
| Outcomes | Death. Hospital admission: unable to keep to initial protocol.* Readmission.* Leaving the study early (patients and relatives-sub**). Mental state: PSE, BPRS. Global state: GAS, SAS. Patient satisfaction: CSQ. Economic cost. Unable to use- Harm to self: incomplete information, data given refers only to patients who were admitted. Days in hospital: includes index admission.* Daily living: DLS (was adapted for use in the SAS by authors) Relative’s satisfaction: RSQ (devised by authors, as yet not peer reviewed). Service Use: no data for standard care group. |
|
| Notes | * After 31 months change in policy meant DLP team lost control of admission & discharges ** Only relatives living with patient were followed up. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised: not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Loss to follow up presented and described. LOCF not used. Attrition low |
| Selective reporting (reporting bias) | Unclear risk | Protocol outcomes reported, some poor data reporting. |
| Other bias | Low risk | Grant funded research. |
| Methods | Allocation: randomised. Blindness: single. Duration: 24 months. Raters: not blind. |
|
| Participants | Diagnosis: schizophrenia. N= 163.* History: recently hospitalised or in need of hospitalisation. Sex: 68% F, 32% M. Age: mean ~37 yrs. Exclusions: homicidal or suicidal tendencies. |
|
| Interventions | 1. Home-drug care: home based nurse visits, drug treatment, practical assistance & support for patient & family, multidisciplinary team, 24hour answering service. N = 64. 2. Home-placebo care: as above except placebos given instead of prescribed medication. N = 45.*** 3. Standard care: hospitalisation & medication, normal staffing levels & treatment programmes. N = 54 |
|
| Outcomes | Hospital admission: unable to keep to initial protocol. Leaving the study early (patients). Unable to use- Readmission: individual data not presented. Days in hospital: no SD. Mental state: IMPS, MSPP, SORR & PHNR (no SD). Family burden: no data for standard care group. Role fulfilment: no data for standard care group. Social activity: no data for standard care group. |
|
| Notes | * A second cohort recruited from community centres - only randomised to home-drug or home-placebo care - not used in this review ** Once a patient from the home-care group was admitted they were no longer treated by the community team, follow-up interviews still conducted *** Not used in this analysis. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised: using random deck of cards. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Loss to follow up presented and explained. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, results paper unclear what outcomes initially intended, poor data reporting, many outcomes no data presented for standard care group |
| Other bias | Unclear risk | funded by a US Public Health Service Research Grant from the National Institute of Mental Health Sandoz, Inc., and Smith and Kline and French Laboratories supplied drugs used in the trial |
| Methods | Allocation: randomised. Blindness: single. Duration: 14 months. Raters: independent. |
|
| Participants | Diagnosis: any severe psychiatric disorder. N = 130. History: in need of psychiatric hospital admission. Sex: 55% M, 45% F. Age: 18-62 yrs, mean ~31 yrs. Exclusions: dual diagnosis. |
|
| Interventions | 1. Home care: CLP’s home based care, multidisciplinary team, 24hr service, drug treatment, coping skills taught, family support given, use of community agencies - for 14 months & then withdrawn. N = 65 2. Standard care: hospitalisation, aim of returning to community as soon as possible, normal staffing levels, standard outpatient follow-up. N = 65 |
|
| Outcomes | Death. Harm. Hospital admission: unable to keep to initial protocol.* Leaving the study early (patients). Community burden. Unable to use - Readmission: no data for home care group. Days in hospital: includes index admission. Leaving the study early (relatives): not clear if all relatives followed up or just relatives living with the patient. Mental state: SCRS (no mean or SD). Global State: CAF (devised by authors, as yet not peer reviewed). Family burden: FBS (devised by authors, as yet not peer reviewed). Life satisfaction: LSS (no mean or SD). Self Esteem: SES (no mean or SD). Economic cost: no SD. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised: not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Loss to follow up described for patients, not for relatives. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Grant funded research. |
Abbreviations 1. Diagnostic systems
DSM III: Diagnostic Statistical Manual, version 3
ICD - 8: International Classification of Diseases - 8th Review
PSE: Present State Examination
2. Scales/Forms used to collect data
BPRS: Brief Psychiatric Rating Scale
CSQ-8: Client Satisfaction Questionnaire
CAF: Community Adjustment Form
CAN: Camberwell Assessment of needs
DLS: Daily Living Score
EQ-5D: EuroQuol 5 dimension quality of life measure
FBS: Family Burden Scale
FEF: Family Evaluation Form
HSRS: Health and Sickness Rating Scale
HONOS: Health of the Nation Outcomes Scales
IMPS: Inpatient Multidimensional Psychiatric Scale
LSP: Life Skills Profile
LSS: Life Satisfaction Scale
MANSA: Manchester Short Assessment of quality of life
MSPP: Multidmensional Scale for Rating Psychiatric Patients
PANSS: Positive and Negative Syndrome Scale
PEF: Psychiatric Evaluation Form
PHNR: Public Health Nursing Report
SAS: Social Adjustment Scale
SCRS: Short Clinical Rating Scale
SES: Self Esteem Scale
SORR: Significant Other Rating Report
RSQ: Relative’s Satisfaction Questionnaire
Other
ACT: Assertive Community Treatment
CI: confidence interval
M: Male
F: Female
N: Number
CLP: Community Living Programme
CPN: Community Psychiatric Nurse
DLP: Daily Living Programme
OPD: Outpatient department relatives-sub - sub group of relatives actually living with the patient.
RR: relative risk
SD: Standard deviation
MD: mean difference
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bond 1989 | Allocation: not randomised, parallel case series. |
| Burns 1993 | Allocation: randomised - but 332 allocated yet only 162 entered study. Participants: anyone presenting for treatment to the mental health services in relevant catchment area, majority not severely ill, only 35% met PSE category ‘psychotic’ |
| Bush 1990 | Allocation: randomised. Participants: people with severe psychosis + high rate of rehospitalisation - not necessarily in ‘crisis’ or need of readmission at time of allocation. Interventions: community intensive outreach versus hospital care |
| Gater 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: multi-disciplinary community team versus hospital care but the community care did not involve an ‘out of hours’ emergency service, this was only provided on the day of referral |
| Ghandi 2001 | Allocation: randomised. Participants: 55% people with schizophrenia, others with bipolar affect disorder, depressive disorders or other psychiatric conditions. Interventions: community teams versus standard care but not care for those in crisis |
| Grawe 2006 | Allocation: randomised. Participants: 100% schizophrenic disorders DSM-IV. I Interventions: Integrated biomedical and psychosocial treatments in routine care versus standard treatment, not crisis care |
| Harrison 2003 | Allocation: not randomised. |
| Henlegger 1999 | Allocation: randomised. Participants: adolescents (mean age ~13 years) requiring psychiatric hospitalisation, majority not suffering from schizophrenia |
| Herz 2000 | Allocation: randomised. Participants: people with schizophrenia or schizoaffective disorder. Interventions: intensive community aftercare versus standard community aftercare |
| Jones 2003 | Allocation: randomised. Participants: homeless people with severe mental illness. Interventions: critical time intervention (an adapted form of intensive case management) versus standard care, not specific to care during a crisis |
| Kuipers 2004 | Allocation: randomised. Participants: people with functional psychosis. Interventions: COAST versus treatment as usual, both interventions were multidisciplinary team-based community care but COAST included specialised psychological interventions and information geared towards early intervention issues, not specifically crisis intervention |
| Levenson 1997 | Allocation: randomised. Participants: people with acute schizophrenia. Intervention: admission versus ‘community care’; non hospitalised group sent home but not treated there - required to attend outpatient clinic daily, treatment not delivered by multidisciplinary team, not available 24 hours |
| Linszen 1998 | Allocation: randomised. Participants: young people with recent onset schizophrenia. Interventions: family intervention, not crisis intervention. |
| Mattejat 2001 | Allocation: randomised. Participants: children and adolescents with severe psychiatric disorders (mean age ~ 11 years) Interventions: home treatment and hospital admission, nature of intervention unclear |
| Merson 1992 | Allocation: randomised. Participants: anyone with a psychiatric disorder referred as a psychiatric emergency from the accident and emergency department or GP. Intervention: early intervention service (EIS) designed to treat people as quickly as possible versus standard care; EIS assessment at home and then case managers assigned - not a crisis intervention, not available 24 hours a day |
| Metcalfe 2005 | Allocation: randomised. Participants: people with severe psychosis complicated by additional needs. Interventions: intensive case management (10-15 cases) versus standard case management (30-35 cases), not crisis intervention |
| Mosher 1975 | Allocation: quasi randomisation. Participants: people with schizophrenia, first admission. Interventions: treated in a residential home versus hospital care |
| Muijen 1994 | Allocation: randomised. Partcipants: people with serious mental illness in home care for 18 months (Phase I of study) - not in acute phase |
| Pai 1982 | Allocation: quasi randomised. |
| Pasamanick 1964b | Allocation: randomised. Participants: people with serious mental illness referred to the study from community centres; not necessarily in a crisis, not allocated to standard care as not in need in of hospitalisation - instead were allocated to home-drug or home-placebo group. See included studies table (Pasmanick-Ohio) for more detail |
| Polak 1976 | Allocation: randomised. Participants: people with psychiatric illness requiring hospitalisation in a setting where a crisis ethos being practiced. Intervention: home based care via multidisciplinary team with 24 hours on-call service available based care. Outcomes: denominators unclear, no usable data. |
| Power 2007 | Allocation: randomised. Paticipants: 150 patients with first-episode psychosis. Interventions: GP training for early detection versus standard care |
| Rosenheck 1995 | Allocation: randomised. Paticipants: people with schizophrenia or other serious psychiatric illness. Intervention: Intensive Psychiatric Community Care (IPCC) versus hospitalisation; IPCC form of ACT (Assertive Community Treatment) rather than crisis intervention |
| Sledge 1996 | Allocation: randomised. Participants: people in acute phase of psychiatric disorder. Intervention: partial hospitalisation versus standard hospitalisation - both hospital-based packages |
| Taylor 1998 | Allocation: randomised. Participants: people with psychosis. Interventions: intensive community care versus standard community care |
| Tyrer 1995 | Allocation: randomised. Participants: people who were psychiatrically vulnerable. Interventions: close supervision by key-worker versus standard psychiatric follow-up |
| Van Minnen 1997 | Allocation: randomised. Participants: people with both “mental retardation and severe mental illness” Intervention: Outreach treatment, not crisis intervention. |
| Warner 2006 | Allocation: randomised. Participants: older individuals with severe mental illness. Interventions: home treatment versus hospital care, not specifically for those in crisis |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Allocation: randomised. Blindness: single. Duration: 6 months. Raters: not known. |
| Participants | Diagnosis: not given. N = 240. History: in need of psychiatric admission. Sex: not known. Age: not known. Exclusions: not known. |
| Interventions | 1. Crisis intervention by Crisis resolution teams. 2. Standard care: hospital admission plus follow-up by Community Mental Health Teams |
| Outcomes | No useable outcomes reported. Details of mean number of days hospital admissions given, but no SDs Patient satisfaction also reported, but no Ns or SDs given. |
| Notes | Unpublished study, many details not known. |
SD: standard deviation
DATA AND ANALYSES
Comparison 1.
CRISIS INTERVENTION vs STANDARD CARE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death: 1. Any cause | 6 | 980 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.37, 2.07] |
| 2 Death: 2. By cause | 6 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 natural causes | 6 | 980 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.18, 2.24] |
| 2.2 suicide or death in suspicious circumstances | 6 | 980 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.36, 3.11] |
| 3 Harm to self or others | 5 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 attempted suicide | 3 | 369 | Risk Ratio (M-H, Random, 95% CI) | 2.62 [0.21, 32.02] |
| 3.2 homicide | 3 | 568 | Risk Ratio (M-H, Random, 95% CI) | 2.96 [0.31, 28.28] |
| 4 Hospital use: 1. Unable to keep to initial trial protocol as regards admission | 5 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 by 6 months | 3 | 427 | Risk Ratio (M-H, Random, 95% CI) | 35.76 [6.76, 189.25] |
| 4.2 by 12 months | 5 | 713 | Risk Ratio (M-H, Random, 95% CI) | 51.79 [14.92, 179.86] |
| 4.3 by 20 months | 2 | 306 | Risk Ratio (M-H, Random, 95% CI) | 67.69 [9.48, 483.15] |
| 4.4 by 24 months | 1 | 118 | Risk Ratio (M-H, Random, 95% CI) | 39.77 [2.47, 639.78] |
| 5 Hospital use: 2. Repeat admissions including index admission | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5.1 by 12 months | 3 | 465 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.31, 1.61] |
| Participants5.2 by 20 months | 1 | 188 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.75, 1.60] |
| 6 Hospital Use: 3. Number of repeat admissions per participant | Other data | No numeric data | ||
| 6.1 By 6 months | Other data | No numeric data | ||
| 7 Hospital use: 4. Repeat admissions excluding index admission | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 7.1 by 3 months | 1 | 260 | Risk Ratio (M-H, Random, 95% CI) | 0.53 [0.41, 0.68] |
| 7.2 by 6 months | 2 | 369 | Risk Ratio (M-H, Random, 95% CI) | 0.75 [0.50, 1.13] |
| 8 Hospital use: 5. Repeat admissions excluding index admission - compulsory detentions only | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 8.1 by 3 months | 1 | 260 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.34, 1.11] |
| 8.2 by 6 months | 1 | 258 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.43, 1.11] |
| 9 Hospital use: 6. Treatment failure | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 9.1 By 6 months | 1 | 119 | Risk Ratio (M-H, Random, 95% CI) | 3.26 [0.74, 14.44] |
| 10 Hospital use: 7. Days in acute care | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 By 3 months | 1 | 260 | Mean Difference (IV, Random, 95% CI) | −10.3 [−14.77, −5.83] |
| 10.2 By 6 months | 2 | 365 | Mean Difference (IV, Random, 95% CI) | −10.54 [−26.49, 5.42] |
| 11 Hospital use: 8. Home or outpatient visits (data likely to be skewed) | Other data | No numeric data | ||
| 11.1 6 - 12 months | Other data | No numeric data | ||
| 11.2 by 12 months | Other data | No numeric data | ||
| 12 Leaving the study early (unwilling or unable to provide infomation): 1. Patients | 8 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 12.1 by 3 months | 3 | 463 | Risk Ratio (M-H, Random, 95% CI) | 0.80 [0.55, 1.15] |
| 12.2 by 6 months | 5 | 718 | Risk Ratio (M-H, Random, 95% CI) | 0.73 [0.55, 0.97] |
| 12.3 by 12 months | 4 | 594 | Risk Ratio (M-H, Random, 95% CI) | 0.74 [0.56, 0.98] |
| 12.4 by 20 months | 3 | 475 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.57, 1.06] |
| 13 Leaving the study early (unwilling or unable to provide information) 2. Relatives | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 13.1 total in study | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 1.09 [0.52, 2.28] |
| 13.2 subgroup of those living with patient | 1 | 76 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.43, 1.17] |
| 14 Global state: 1. GAS (endpoint score, range 1-100, low = poor) (loss in some cases is greater than 30%) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 By 3 months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.0 [−12.82, 12.82] |
| 14.2 by 6 months | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 5.10 [−0.86, 11.06] |
| 14.3 by 12 months | 1 | 131 | Mean Difference (IV, Random, 95% CI) | 3.5 [−3.15, 10.15] |
| 14.4 by 20 months | 1 | 142 | Mean Difference (IV, Random, 95% CI) | 5.70 [−0.26, 11.66] |
| 15 Global state: 2. SAS (endpoint score, high = poor) (loss in some cases in greater than 30%) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 by 6 months | 1 | 130 | Mean Difference (IV, Random, 95% CI) | −0.20 [−0.75, 0.35] |
| 15.2 by 12 months | 1 | 120 | Mean Difference (IV, Random, 95% CI) | −0.30 [−0.85, 0.25] |
| 15.3 by 20 months | 1 | 139 | Mean Difference (IV, Random, 95% CI) | −0.60 [−1.15, −0.05] |
| 16 Global state: 3. GAS scale change data by 3 months (+ve change = good, data likely to be skewed) | 2 | 156 | Mean Difference (IV, Random, 95% CI) | 4.17 [−1.56, 9.89] |
| 17 Global state: 4. SAS change data by 3 months (−ve change = good, data likely to be skewed) | 1 | 127 | Mean Difference (IV, Random, 95% CI) | −0.09 [−0.31, 0.13] |
| 18 Mental state - general: 1. Unwell by 12 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.65 [0.40, 1.07] |
| 19 Mental state - general: 2. BPRS (endpoint score, range 24-168, high = poor) (loss in standard group >30% for 6 months or more) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 by 3 months | 2 | 248 | Mean Difference (IV, Random, 95% CI) | −4.03 [−8.18, 0.12] |
| 19.2 by 6 months | 1 | 129 | Mean Difference (IV, Random, 95% CI) | −2.10 [−6.40, 2.20] |
| 19.3 by 12 months | 1 | 131 | Mean Difference (IV, Random, 95% CI) | −2.0 [−6.03, 2.03] |
| 19.4 by 20 months | 1 | 142 | Mean Difference (IV, Random, 95% CI) | −4.5 [−8.68, −0.32] |
| 20 Mental state - general: 3. PEF (endpoint score, range 0-5, high = poor) (loss is greater than 30%) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 by 3 months | 1 | 118 | Mean Difference (IV, Random, 95% CI) | 0.20 [−0.22, 0.62] |
| 20.2 by 6 months | 1 | 111 | Mean Difference (IV, Random, 95% CI) | 0.10 [−0.42, 0.62] |
| 20.3 by 12 months | 1 | 97 | Mean Difference (IV, Random, 95% CI) | −0.40 [−0.84, 0.04] |
| 20.4 by 20 months | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.10 [−0.47, 0.67] |
| 21 Mental state - general: 4. PSE (endpoint score, high score = poor, data likely to be skewed) | Other data | No numeric data | ||
| 21.1 by 6 months | Other data | No numeric data | ||
| 21.2 by 12 months | Other data | No numeric data | ||
| 21.3 by 20 months | Other data | No numeric data | ||
| 22 Mental state - general: 5. BPRS scale change data by 3 months (−ve change = good, data likely to be skewed) | 1 | 129 | Mean Difference (IV, Random, 95% CI) | −3.5 [−8.92, 1.92] |
| 23 Mental state - general: 6. PSE scale change data by 3 months (−ve change = good, data likely to be skewed). | 1 | 129 | Mean Difference (IV, Random, 95% CI) | −2.70 [−7.69, 2.29] |
| 24 Mental state: general PANSS (endpoint score, range 30-210, high = poor) | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [−3.45, 11.45] |
| 24.1 By 6 months | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [−3.45, 11.45] |
| 25 Mental state - specific: 1. Unsociable (reported by relatives) | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 25.1 by 3 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.86 [0.66, 1.12] |
| 25.2 by 6 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.43 [0.30, 0.64] |
| 26 Mental state - specific: 2. Aggression (reported by relatives) | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 26.1 by 3 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.72, 1.31] |
| 26.2 by 6 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.7 [0.39, 1.25] |
| 27 Mental state - specific: 3. Various problems at 4 months (reported by relatives) | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 27.1 agitation | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.59 [0.36, 0.95] |
| 27.2 depression | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.8 [0.57, 1.13] |
| 27.3 disorientation | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.47 [0.28, 0.79] |
| 27.4 psychotic behaviour | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.58 [0.30, 1.11] |
| 27.5 substance abuse | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.67 [0.33, 1.36] |
| 27.6 withdrawal | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.72 [0.48, 1.07] |
| 28 Burden - family: 1. Disruption to daily routine | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 28.1 by 3 months | 2 | 220 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.59, 0.97] |
| 28.2 by 6 months | 2 | 220 | Risk Ratio (M-H, Random, 95% CI) | 0.67 [0.37, 1.21] |
| 29 Burden - family: 2. Disruption to social life | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 29.1 by 3 months | 2 | 220 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.53, 0.91] |
| 29.2 by 6 months | 2 | 220 | Risk Ratio (M-H, Random, 95% CI) | 0.72 [0.43, 1.22] |
| 30 Burden - family: 3. Financial strain | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 30.1 by 3 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.52, 1.10] |
| 30.2 by 6 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.53, 1.33] |
| 31 Burden - family: 4. Physical illness due to patient’s illness | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 31.1 by 3 months | 1 | 100 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.65, 0.95] |
| 31.2 by 6 months | 1 | 100 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.55, 0.92] |
| 32 Burden - family: 5. Overall burden is great | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 32.1 by 3 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.57 [0.41, 0.80] |
| 32.2 by 6 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.20, 0.59] |
| 33 Burden - community: 1. Not employed by 20 months | 1 | 189 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.85, 1.12] |
| 34 Burden - community: 1a. In paid work by 6 months | 1 | 112 | Risk Ratio (M-H, Random, 95% CI) | 1.41 [0.65, 3.04] |
| 34.1 By 6 months | 1 | 112 | Risk Ratio (M-H, Random, 95% CI) | 1.41 [0.65, 3.04] |
| 35 Burden - community: 2. Various outcomes by 12 months | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 35.1 at least one arrest | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.46, 1.12] |
| 35.2 at least one use of emergency services | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.81 [0.43, 1.54] |
| 36 Burden - community: 3. Arrested | 1 | 111 | Risk Ratio (M-H, Random, 95% CI) | 5.36 [0.28, 101.35 |
| 36.1 By 6 months | 1 | 111 | Risk Ratio (M-H, Random, 95% CI) | 5.36 [0.28, 101.35] |
| 37 Burden - community: 4. Homelessness | 1 | 113 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.59, 2.57] |
| 37.1 By 6 months | 1 | 113 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.59, 2.57] |
| 38 Satisfaction - patient: 1. Various outcomes by 12 months | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 38.1 feels unimproved | 1 | 119 | Risk Ratio (M-H, Random, 95% CI) | 0.48 [0.31, 0.74] |
| 38.2 dissatisfied with treatment received | 1 | 119 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.50, 0.88] |
| 38.3 prefered to get other treatment | 1 | 119 | Risk Ratio (M-H, Random, 95% CI) | 0.46 [0.27, 0.77] |
| 38.4 feels less able to cope | 1 | 119 | Risk Ratio (M-H, Random, 95% CI) | 0.36 [0.21, 0.62] |
| 38.5 feels will need more help outside working hours in the future | 1 | 119 | Risk Ratio (M-H, Random, 95% CI) | 1.48 [0.88, 2.48] |
| 39 Satisfaction - patient: 2. Patient satisitfied with care: Satisfaction Scale (endpoint score, range 0 −32, low = poor) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 39.1 by 3 months | 1 | 226 | Mean Difference (IV, Random, 95% CI) | 1.60 [−0.22, 3.42] |
| 39.2 by 6 months | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 5.10 [3.16, 7.04] |
| 39.3 by 12 months | 1 | 121 | Mean Difference (IV, Random, 95% CI) | 4.80 [3.11, 6.49] |
| 39.4 by 20 months | 1 | 137 | Mean Difference (IV, Random, 95% CI) | 5.40 [3.91, 6.89] |
| 40 Satisfaction: Patient: 3. VSSS Scale (low = poor) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.20 [−0.20, 0.60] |
| 41 Satisfaction - relatives: 1. Feels patient is not improved | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 41.1 by 3 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.60, 1.04] |
| 41.2 by 6 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.53, 0.97] |
| 42 Satisfaction - relatives: 4. Various outcomes by 12 months | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 42.1 feel themselves less able to cope | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 42.2 feel themsleves to need more help outside working hours in the future | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 1.21 [0.91, 1.60] |
| 43 Satisfaction - relatives: 3. Would have preferred patient to have received other treatment | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 43.1 by 3 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [0.63, 2.57] |
| 43.2 by 6 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.49, 2.54] |
| 43.3 by 12 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.81 [0.43, 1.54] |
| 44 Satisfaction - relatives: 2. Dissatisfied with treatment received | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 44.1 by 3 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.44, 0.89] |
| 44.2 by 6 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 44.3 by 12 months | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.46 [0.29, 0.72] |
| 45 Economic cost per patient (data likely to be skewed) | Other data | No numeric data | ||
| 45.1 total cost for trial period - as assessed by researchers | Other data | No numeric data | ||
| 45.2 total cost for trial period - as assessed by finance department | Other data | No numeric data | ||
| 45.3 per week | Other data | No numeric data | ||
| 46 Quality of Life MANSA patient endpoint score, range 0 - 88, low = poor | 1 | 226 | Mean Difference (IV, Random, 95% CI) | −1.5 [−5.15, 2.15] |
| 47 Quality of Life MANSA-short form patient endpoint score, low = poor | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.14, 1.26] |
| 48 Quality of life: EQ-5D range 0 to 1, low = poor | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [−0.32, 0.34] |
| 48.1 By 3 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [−0.32, 0.34] |
| 49 Social contacts | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.43 [−0.30, 1.16] |
| 49.1 By 6 months | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.43 [−0.30, 1.16] |
| 50 Social functioning: LSP-staff endpoint score, range 0-156, low = poor | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 50.1 by 3 months | 1 | 260 | Mean Difference (IV, Random, 95% CI) | 3.0 [−0.72, 6.72] |
| 50.2 by 6 months | 1 | 260 | Mean Difference (IV, Random, 95% CI) | 1.0 [−2.76, 4.76] |
| 51 Clinical and social problems HoNOS - staff endpoint score, range 0-48, high = poor | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 51.1 by 3 months | 1 | 257 | Mean Difference (IV, Random, 95% CI) | −1.90 [−3.20, −0.60] |
| 51.2 by 6 months | 1 | 255 | Mean Difference (IV, Random, 95% CI) | −0.60 [−2.07, 0.87] |
| 52 Unmet needs: CAN scale range 0-22, high = poor | Other data | No numeric data |
FEEDBACK
General comments
Summary
Background
The historical background to the development of crisis intervention is useful and important. However it can be argued that this form of intervention dates back at least to the 1950s where it was well established in Amsterdam (Querido 1968).
The ethos of crisis intervention is given and accurately reflects the desire to avoid hospitalisation. This review incorporated major misunderstandings concerning the nature of crisis intervention which was wrongly assumed to be designed to replace hospital care, a claim not made in any of the five studies included in the review. Even in early studies it was not usually claimed that hospitalisation could be entirely avoided. For example in discussing crisis intervention Stein and Test make reference to minimal hospital use as necessary for some of those given “training in community living” (see Trial ID, Stein - Madison, citation Stein 1980). Later authors were more explicit in their expectation that hospitalisation was inevitable for some patients. For example Muijen et al state that brief hospitalisation where this is unavoidable is one of the “principles of the daily living programme” (see Trial ID, Muijen - London, citation Muijen 1992 p. 380). The assumption in other parts of the review that admission to hospital reflects a “failure” of crisis intervention is hence questionable and is not supported by the authors of the main studies in this field.
In the final sentence of the introduction a number of statements are made concerning possible problems of crisis intervention. None of these is referenced and each is questionable, with some published evidence to the contrary particularly for the issues of family burden (Dean 1993). Indeed the issue of burden is discussed later in the review and evidence given appears to contradict this part of the introduction.
Data synthesis
The treatment of dichotomous data seems appropriate.
The decision to treat rating scales as continuous data is questionable. Despite checks for normal distribution it cannot be concluded that data from such instruments is parametric. To do so implies that for example a BPRS score of 40 indicates a person is twice as unwell as a patient with a score of 20. Although this error is often made in published trials, including those presented in this review, this does not justify replication of this fault. The subsequent difficulties in quantitative analysis of data from the studies may partly reflect methodological inadequacies in the review. If the RevMan software is not designed to cope appropriately with data from psychiatric rating scales then either a different package should be used, at least some of these issues should be discussed.
Description of studies
Excluded studies
It is not clear why certain important crisis intervention studies that do not meet the selection criteria are not listed here (for example Dean 1993).
Results
Hospital use
No mention is made of the dramatic reduction in mean number of days in hospital, encountered in every study. There is no explanation why this outcome was excluded. It is reasonable to note that a direct comparison of number of hospital admissions gives unfair advantage to the crisis group. However this does not justify excluding a comparison of mean number of days in hospital. Number of days in hospital was stated as an outcome measure in the methods section of the review and yet there is no mention of this in the results. The information is available in the references cited. Despite the fact that the nature of the control treatment necessitated admission to hospital it is still valid and important to compare mean number of days in hospital. If there are concerns about the interpretation of the findings because the hospital-treated patients inevitably spent at least one night in hospital this can be discussed, but does not justify omission of meta-analysis of these data. Another possible cause for omission of this data may have been its likelihood of skew. Any study which examines length of stay inevitably will include a small number of individuals whose admission was much longer than average for good clinical reasons. If the data is analysed using non-parametric means this should not prevent meaningful comparison between the groups, which are both likely to display this effect.
Discussion
The review uncovered an interesting possible confounding influence, which may have favoured the crisis teams in the main studies. This was the fact that crisis intervention continued for the duration of the studies, and hence presumably for much longer than the episode of acute disturbance that would have required hospitalisation. Hence it can be argued that the results from longer-term follow-up of patients reflect a service similar to assertive community treatment. This issue has not been widely recognised in the past. However the results of assessments made within the mean period of hospital treatment of the control group could be said to reasonably reflect the effectiveness of home treatment as an adjunct to hospitalisation. Perhaps separate analysis of such data may be possible in future amendments.
The assumptions that hospital admission reflects home care failure have been discussed earlier and are again repeated in this section.
Conclusions
The implications for policy makers do not include the conclusions made for patients, families and clinicians, that home care may have significant advantages in terms of patient acceptability and burden to family, with no evidence of significant differences in social or clinical outcomes.
It is important to emphasise the need for high quality hospital care, and the report rightly implies that crisis intervention should not replace inpatient care. Given that there is little difference in outcomes between crisis intervention and standard care, and that crisis intervention is more acceptable to patients and their carers, it is surprising that no recommendation is made to encourage development of home treatment services. If the issue is considered from another perspective it could be argued that there is even less evidence for the efficacy and desirability of hospitalisation. The proposal for future research that attempts to control for the effect of the crisis team continuing its input well beyond the initial episode is reasonable. However if the effect of the period of acute illness were to be studied in more detail this may be more relevant to current home treatment interventions, which are often short in duration and directly comparable to a typical inpatient admission.
Miscellaneous
A number of the charts (e.g. GAS) place crisis on the right although in the methods section it is stated that it would be to the left.
Conflicts of interest
Given the considerable debate that the issue of home treatment has generated, often with highly polarised views, the opinions of the reviewers prior to the report should perhaps have been given as potential conflicts of interest. This may explain the conclusions which are unduly negative towards home treatment, and which may lack objectivity.
Recommendations
The review does not reflect an accurate objective appraisal of the current evidence concerning crisis intervention. It is recommended that:
An analysis of mean number days in hospital is included. If required any potential problems of such a comparison could be included.
The data from rating scales should be re-examined and if possible re-analysed as non-continuous using appropriate tests for significance.
The nature of crisis intervention as an adjunct to, not a replacement for hospitalisation should be explicitly stated, and those sections, which wrongly interpret hospital admission as a failure of home treatment, should be corrected.
The potential problems of home treatment mentioned at the end of the introduction should either be referenced, including evidence to the contrary, or removed.
Conclusions should take more account of the almost total lack of evidence from randomised controlled trials which support hospitalisation as a treatment. Thus a more objective conclusion and recommendations could be made.
Intellectual or clinical conflicts of interest should be declared.
Conflict of Interest
I believe both from experience working in home treatment teams and hospital based services that crisis intervention is an important and more acceptable adjunct to hospitalisation for those with acute psychiatric disorders. I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
Background
The reviewers have incorporated some of the recommendations but cannot accept others.
The additional helpful reference (Querido 1968) has been sought and the Background amended.
The ethos of crisis intervention does reflect the desire to avoid hospitalisation but the reviewers continue to contend that this review incorporated major misunderstandings concerning the nature of crisis intervention. The commentator stated that we assumed that crisis intervention was “assumed to be designed to replace hospital care”. This was not stated and we are sorry if it was implied. We have scrutinised the ‘Background’ of the review and tried to modify text that could have been misinterpreted.
Although early studies usually did not claim that hospitalisation could be entirely avoided this was the desired outcome. Hospitalisation was indeed seen as a failure of community care (see Trial ID, Muijen - London, citation Muijen 1992 page 753, paragraph 4, line 1 “Early in the programme, hospital admission of home care patients was seen as a failure but gradually positive indications for admission were identified.”) It was only well after the trials started that the tone as regards hospitalisation became more realistic and balanced. One of the studies even refers to ‘home care failures’ (see Trial ID, Pasamanick-Ohio, citation Pasamanick 1964, page 179, paragraph 3, line 2 “Some patients of course, do not succeed on home treatment and are admitted to the hospital.”) Other studies describe how “every effort is made to avoid hospitalisation (see Trial ID, Stein - Madison, citation Stein 1975 page 518, paragraph 4, line 5). Finally Hoult 1984 (Trail ID Hoult - Sydney) page 360, paragraph 4 describes the aims of the study being “to demonstrate that is feasible to treat psychiatric patients in the community as an alternative to hospital admission.” The final sentence of the introduction did present a number of statements concerning possible problems with crisis intervention. None of these were referenced and because each is questionable, this text has been modified.
Data synthesis
The Cochrane Schizophrenia Group has widely consulted on the management of these problematic and unsatisfying data. The problems, for the purposes of this comment, fall into two large categories - analysis and interpretation. After discussion with the ALLSTAT discussion list and personal communication with key members in the Cochrane Statistical Methods Working Group the Editors of the Cochrane Schizophrenia Group decided to advise a conservative line to reviewers. Statisticians, acknowledged the world over for their expertise in the field of meta-analysis, are unable to give clear answers at the present time. There is no right way of analysis of these data - although there are many ways that are wrong. In this case we accepted the advice of the Cochrane Schizophrenia Group’s editors but, essentially, the commentator suggests that a yet more conservative line should have been followed.
The commentator states that the scale derived data are not in fact continuous, although have been described as such. This is true and we have amended the text accordingly. These scales provide ordinal, and not interval data. However, the exceedingly fine gradation of such scales, does result, in a few instances that have been studied, in them behaving as if they were continuous in analysis. For statisticians that have had access to individual patient data the fine categorical scale did not benefit from a more sophisticated analysis in which ranking was incorporated. There is, however, as far as the Cochrane Schizophrenia Group’s statistical advisors are aware, no published literature to replicate this impression. It is felt, and there is no greater evidence than this at present, that RevMan’s relatively simple analysis is entirely adequate. The decision to treat these data as continuous is, as the commentator states, worthy of question, but practical solutions have not been presented.
Scales are largely research tools used for the subtle purposes of research by researchers. Assuming the scales are used reliably and are validated for the outcome they are measuring in the population that they are rating, even if the data are then valid their clinical interpretation is problematic. Scales are unusual in clinical practice and interpretation of any correctly analysed data is problematic for front-line clinicians.
Description of studies
Excluded studies
We have re-read Dean 1993. For those undertaking reviews there are difficult decisions to take regarding exclusion of studies. The usual rule is that the studies in the excluded section of a Cochrane review should be presented as a service to the reader. Should a paper, from its title or abstract, be so obviously not appropriate, presentation in the Excluded studies section serves little purpose. Usually studies in the excluded section are those that have caused the reviewers to be sufficiently in doubt as to need to acquire full copies. This is not a hard and fast rule and sometimes it is worth including an oft-cited study even if its exclusion is not in doubt. Dean 1993 is not a randomised trial. The title and abstract provided enough information for the reviewers to come to this conclusion and the study is therefore not presented in the ‘Excluded studies’ table.
Results
Hospital use
The commentator states that “no mention is made of the dramatic reduction in mean number of days in hospital, encountered in every study. There is no explanation why this outcome was excluded.” We made mention of this important outcome in the ‘Included studies’ table. In the column containing information on outcomes recorded in each trial average stay is frequently recorded as being part of the trial design. The reason for exclusion of these data is always reported. Several of the studies include index admission in the data and others provide no measure of variance, making data impossible to interpret. This should have been highlighted in the text of the review and it has been amended.
As was noted by the commentator, it is reasonable to note that a direct comparison of number of hospital admissions gives “unfair advantage” to the crisis group. The reviewers were concerned at how to present data, especially when “unfair advantage” is bound to be evident. This is also why little credence is given to outcome “04 Hospitalisation: Unable to keep to initial protocol as regards admission” in the text. The reviewers thank the commentator for stressing the point that days in hospital should have been presented - but remain doubtful. The reviewers will not amend this version of the review but in the following months will seek advice and respond fully to this criticism. As the commentator states, all such data are likely to be skewed and difficult to present.
Discussion
The commentator rightly draws the reader’s attention to the likely confounding of the longer-term effects of crisis intervention by ongoing community care packages. This was clearly stated in the text of the review. It would indeed be desirable to tease out any effects of ‘pure’ crisis intervention should data be made available.
Conclusions
The ‘Implications’ section is divided into separate sections for specific named groups. The reviewers do not wish to imply that each set of implications do not have meaning for the other groups.
The commentator finds it surprising that, because this review find little differences between crisis intervention and standard care (excepting some measures of burden and satisfaction), that no recommendation is made to encourage development of home treatment services. The largest combined data set (two trials) in the series of measures of burden and satisfaction was 220 people. All studies were undertaken by teams of such quality that it is difficult to generalise any results to more usual clinical care. The reviewers suggest that it would have been surprising if objective appraisal of this interesting and important data had not reached the conclusions as presented in the original review.
Miscellaneous
It was not possible to present the GAS data with the data favouring the experimental outcome to the left of the line. All graphs, however, were appropriately labelled. The ‘Methods’ 4.4 General has been amended with the words ‘where possible’.
Conflicts of interest
The commentator may be correct in suggesting that the reviewers should have pre-stated their views on the effects of crisis intervention, in order to protect themselves from accusations of bias and lack of objectivity. The reviewers restate their original claim that they have no conflicts of interest that would affect their objectivity with regard to this review.
References
Dean C, Phillips J, Gadd EM, Joseph M, England S. Comparison of community based service with hospital based service for people with acute, severe psychiatric illness BMJ 1993; 307: 473-6.
Querido A. The shaping of community mental health care. British Journal of Psychiatry 1968; 114: 293-302.
Both these citations are now added to the main text of the review.
Contributors
Comment received from Andrew Owens, Warwick, UK, September 1999. Reply by Claire Joy, York, and Clive Adams, Oxford, UK, January 2000.
Results and conclusions
Summary
NNTs are used somewhat incautiously. If the summary OR is the constant across all studies say for loss to follow-up at 6 or 12 months, the NNT cannot be constant too. Thus the range of NNTs with which the included trials are compatible is not the ‘summary NNT’ quoted, but the summary OR applied to the range of baseline risks actually occurring in the included studies.
The impact on family burden appears to be rather overstated given that only two out of the five included trials contributed data on this outcome, and the size of effect differed depending on which of the five specific measures of family burden was examined.
Reply
We would like to thank the commentator for highlighting these points and we are sorry not to have addressed then for such a long time. We have taken them into account in the 2003-4 update.
We have changed the way we calculate the NNT and now take into account the risk in the control group and hope this addresses the concern above.
In 2003-4 we substantially rewrote the review, taking into account all comments, and hope the emphasis is now not overstated.
Contributors
Comment received from Chris Hyde, Birmingham, UK, July 2000.
Comment replied to by Clive Adams, Leeds, UK, July 2004.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
CRISIS INTERVENTION compared to STANDARD CARE for people with severe mental illnesses
Patient or population: patients with people with severe mental illnesses
Settings:
Intervention: CRISIS INTERVENTION
Comparison: STANDARD CARE
| Outcomes |
Illustrative comparative risks*
(95% CI) |
Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | CRISIS INTERVENTION | |||||
|
Global state Global Assessment Scale (GAS) Follow-up: 12 months |
The mean global state in the control groups was 64.4 | The mean global state in the intervention groups was 5.7 higher (0.26 lower to 11.66 higher) | 142 (1 study) | ⊕ ⊕ ⊕ ○ moderate1,2 | Outcome measure favours crisis intervention over standard care, although not to a significant extent | |
| Mental state - general Brief Psychiatric Rating Scale (BPRS) Follow-up: 3 months | The mean mental state - general in the control groups was 43.5 | The mean mental state - general in the intervention groups was 4.03 lower (8.18 lower to 0.12 higher) | 248 (2 studies) | ⊕ ⊕ ○ ○ low3,4 | Outcome measure favours crisis intervention, although not to a significant extent | |
| Patient Satisfaction Client Satisfaction Questionnaire (CSQ-8) Follow-up: 20 months | The mean patient satisfaction in the control groups was 22.0 | The mean patient satisfaction in the intervention groups was 5.4 higher (3.91 to 6.89 higher) | 137 (1 study) | ⊕ ⊕ ⊕ ○ moderate1,2 | Outcome favours crisis intervention to a significant extent | |
| Quality of Life Manchester Short Assessment (MANSA) Follow-up: 6 months | The mean quality of life in the control groups was 41.7 | The mean quality of life in the intervention groups was 1.5 lower (5.15 lower to 2.15 higher) | 226 (1 study) | ⊕ ⊕ ○ ○ low3,4 | Outcome favours standard care although not to a significant extent | |
| Burden on family Numbers of families stating that overall burden is great Follow-up: 6 months | 583 per 1000 | 198 per 1000 (117 to 344) | RR 0.34 (0.2 to 0.59) | 120 (1 study) | ⊕ ⊕ ○ ○ low2,4 | Outcome significantly favours crisis intervention |
| Hospital use Repeat admissions excluding index admission Follow-up: 6 months | 758 per 1000 | 470 per 1000 (387 to 576) | RR 0.62 (0.51 to 0.76) | 258 (1 study) | ⊕ ⊕ ⊕ ⊕ high | Outcome significantly favours crisis intervention |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Details of randomisation not described.
Blinding not described
Raters not blinded
Loss of some participants not described
HISTORY
Protocol first published: Issue 2, 1998
Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 14 April 2010 | Amended | Contact details updated. |
| 5 August 2009 | Amended | Contact details updated. |
| 23 April 2008 | Amended | Converted to new review format. |
| 23 August 2006 | New citation required and conclusions have changed | Substantive amendment |
| 18 January 2006 | New search has been performed | Four new studies added to excluded studies table and references. Text changed to reflect new findings of the update. |
| 4 July 2003 | New search has been performed | Nine studies added to excluded studies table and references. Statistics changed from OR to RR. Results updated. Conclusions updated. Methodology changed to current format. Included studies table changed to current format. Text changes to reflect findings of the update. |
| 18 February 2000 | Feedback has been incorporated | Response to feedback. |
| 2 February 2000 | Feedback has been incorporated | Feedback added. |
| 25 August 1999 | Amended | Reformatted. |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
Protocol states that random 20% samples of selected full reports will be independently re-inspected by RD to ensure reliability. In the event, only four full reports met the selection criteria so 100% of these were inspected by RD. Similarly, the protocol states that RD will extract data from a random sample of 10% of data from included studies, as only three additional study was included, RD extracted data from 100% of all included studies.
This update has also had the methodology section substantially updated to reflect the methods employed by the Cochrane Schizophrenia Group.
WHAT’S NEW
Last assessed as up-to-date: 12 February 2012.
| Date | Event | Description |
|---|---|---|
| 12 January 2012 | New citation required but conclusions have not changed | Substantial update: conclusions not significantly changed. |
| 31 March 2011 | New search has been performed | Results of 2010 search added: three new included studies with usable data added to analysis, four new excluded studies and one new study for which we await further details. Results and conclusions not significantly altered |
Footnotes
DECLARATIONS OF INTEREST There was no potential conflict of interest.
References to studies included in this review
- Fenton 1979 {published data only} .Fenton FR, Tessier L, Contandriopoulos AP, Nguyen H, Struening EL. A comparative trial of home and hospital psychiatric treatment: financial costs. Canadian Journal of Psychiatry. 1982;27:177–85. doi: 10.1177/070674378202700301. [DOI] [PubMed] [Google Scholar]; Fenton FR, Tessier L, Struening EL. A comparative trial of home and hospital psychiatric care: one-year follow-up. Archives of General Psychiatry. 1979;36:1073–9. doi: 10.1001/archpsyc.1979.01780100043003. [DOI] [PubMed] [Google Scholar]; Fenton FR, Tessier L, Struening EL, Smith FA, Benoit C, Contandriopoulos AP. A two-year follow-up of a comparative trial of the cost-effectiveness of home and hospital psychiatric treatment. Canadian Journal of Psychiatry. 1984;29:205–11. doi: 10.1177/070674378402900304. [DOI] [PubMed] [Google Scholar]; Smith FA, Fenton FR, Benoit C, Barzell E, Tessier L. Home care treatment of acutely ill psychiatric patients. Candian Psychiatric Association Journal. 1978;23(2):73–6. doi: 10.1177/070674377802300201. [DOI] [PubMed] [Google Scholar]; *
- Fenton 1998 {published data only} .Fenton WS, Hoch JS, Herrell JM, Mosher L, Dixon L. Cost and cost-effectiveness of hospital vs residential crisis care for patients who have serious mental illness. Archives of General Psychiatry. 2002;59:357–64. doi: 10.1001/archpsyc.59.4.357. [DOI] [PubMed] [Google Scholar]; Fenton WS, Mosher LR. Crisis residential care for patients with serious mental illness. In: Martindale B, Bateman A, editors. Psychosis: Psychological Approaches and their Effectiveness. Gaskell, Royal College of Psychiatrists; London UK: 2000. pp. 157–76. [Google Scholar]; Fenton WS, Mosher LR, Herrell JM, Blyler CR. Randomized trial of general hospital and residential alternative care for patients with severe and persistent mental illness. American Journal of Psychiatry. 1998;155(4):516–22. doi: 10.1176/ajp.155.4.516. [DOI] [PubMed] [Google Scholar]; *
- Hoult 1983 {published data only} .Hoult J. Community care of the acutely mentally ill. British Journal of Psychiatry. 1986;149:137–44. doi: 10.1192/bjp.149.2.137. [DOI] [PubMed] [Google Scholar]; Hoult J, Reynolds I. Schizophrenia. A comparative trial of community orientated and hospital orientated psychiatric care. Acta Psychiatrica Scandinavica. 1984;69:359–72. doi: 10.1111/j.1600-0447.1984.tb02506.x. [DOI] [PubMed] [Google Scholar]; Hoult J, Reynolds I, Charbonneau Powis M, Coles P, Briggs J. A controlled study of psychiatric hospital versus community treatment: The effect on relatives. Australian and New Zealand Journal of Psychiatry. 1981;15:323–8. doi: 10.3109/00048678109159455. [DOI] [PubMed] [Google Scholar]; Hoult J, Reynolds I, Charbonneau Powis M, Weekes P, Briggs J. Psychiatric hospital versus community treatment: the results of a randomised trial. Australian and New Zealand Journal of Psychiatry. 1983;17:160–7. doi: 10.3109/00048678309160000. [DOI] [PubMed] [Google Scholar]; Hoult J, Rosen A, Reynolds I. Community orientated treatment compared to psychiatric hospital orientated treatment. Social Science and Medicine. 1984;18:1005–10. doi: 10.1016/0277-9536(84)90272-7. [DOI] [PubMed] [Google Scholar]; Reynolds I, Hoult JE. The relatives of the mentally ill. A comparative trial of community-oriented and hospital-oriented psychiatric care. Journal of Nervous and Mental Diseases. 1984;172:480–9. [PubMed] [Google Scholar]; *
- Howard 2010 {published data only} .Howard L, Flach C, Leese M, Byford S, Killaspy H, Cole L, et al. Effectiveness and cost-effectiveness of admissions to women’s crisis houses compared with traditional psychiatric wards: pilot patient-preference randomised controlled trial. British Journal of Psychiatry. 2010;197:32–40. doi: 10.1192/bjp.bp.110.081083. [DOI] [PubMed] [Google Scholar]; Howard L, Leese M, Byford S, Killaspy H, Cole L, Lawlor C, et al. Methodological challenges in evaluating the effectiveness of women’s crisis houses compared with psychiatric wards. Journal of Nervous and Mental Disease. 2009;197(10):722–7. doi: 10.1097/NMD.0b013e3181b97621. [DOI] [PubMed] [Google Scholar]; *
- Johnson 2005 {published data only} .Johnson S, Nolan F, Pilling S, Sandor A, Hoult J, McKenzie N, et al. Randomised controlled trial of acute mental health care by a crisis resolution team: The North Islington crisis study. BMJ. 2005;331(7517):599–602. doi: 10.1136/bmj.38519.678148.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muijen 1992 {published data only} .Knapp M, Beecham J, Koutsogeorgopoulou V, Hallam A, Fenyo A, Marks IM, et al. Service use and costs of home-based versus hospital-based care for people with serious mental illness. British Journal of Psychiatry. 1994;164:195–203. doi: 10.1192/bjp.165.2.195. [DOI] [PubMed] [Google Scholar]; Marks I, Connolly J, Muijen M, Audini B, McNamee G, Lawrence R. Home-based versus hospital-based care for people with serious mental illness. British Journal of Psychiatry. 1994;165:179–94. doi: 10.1192/bjp.165.2.179. [DOI] [PubMed] [Google Scholar]; Muijen M, Marks I, Connolly J, Audini B. Home based care and standard hospital care for patients with severe mental illness: A randomised controlled trial. BMJ. 1992;304:749–54. doi: 10.1136/bmj.304.6829.749. [DOI] [PMC free article] [PubMed] [Google Scholar]; Muijen M, Marks IM, Connolly J, Audini B, McNamee G. The daily living programme. Preliminary comparison of community versus hospital-based treatment for the seriously mentally ill facing emergency admission. British Journal of Psychiatry. 1992;160:379–84. doi: 10.1192/bjp.160.3.379. [DOI] [PubMed] [Google Scholar]; Simpson CJ, Seager CP, Robertson JA. Home-based care and standard hospital care for patients with severe mental illness: A randomised controlled trial. British Journal of Psychiatry. 1993;162:239–43. doi: 10.1192/bjp.162.2.239. [DOI] [PubMed] [Google Scholar]; *
- Pasamanick 1964a {published data only} .Davis AE, Dinitz S, Pasamanick B. The prevention of hospitalization in schizophrenia: Five years after an experimental program. American Journal of Orthopsychiatry. 1972;42:375–88. doi: 10.1111/j.1939-0025.1972.tb02504.x. [DOI] [PubMed] [Google Scholar]; Pasamanick B, Scarpitti FR, Dinitz S. Schizophrenics in the Community: An Experimental Study in the Prevention of Hospitalization. Appleton-Century-Crofts; New York: 1967. [Google Scholar]; Pasamanick B, Scarpitti FR, Lefton M, Dinitz S, Wernert JJ, McPheeters H. Home versus hospital care for schizophrenics. JAMA. 1964;187:177–81. doi: 10.1001/jama.1964.03060160005001. [DOI] [PubMed] [Google Scholar]; *
- Stein 1975 {published data only} .Stein L, Test M, Marx AJ. Alternative to the hospital: A controlled study. American Journal of Psychiatry. 1975;132:517–22. doi: 10.1176/ajp.132.5.517. [DOI] [PubMed] [Google Scholar]; Stein LI, Test MA. Alternative to mental hospital treatment. I. Conceptual model, treatment program, and clinical evaluation. Archives of General Psychiatry. 1980;37:392–7. doi: 10.1001/archpsyc.1980.01780170034003. [DOI] [PubMed] [Google Scholar]; Test MA, Knoedler W, Allness D, Burke S, Brown R, Wallisch L. Community care of schizophrenia: two-year findings. Schizophrenia Research. 1989;3:1–16. [Google Scholar]; Test MA, Stein LI. Alternative to mental hospital treatment. III. Social cost. Archives of General Psychiatry. 1980;37:409–12. doi: 10.1001/archpsyc.1980.01780170051005. [DOI] [PubMed] [Google Scholar]; Test MA, Stein LI. Training in community living: A follow-up look at a gold-award program. Hospital and Community Psychiatry. 1976;27:193–4. doi: 10.1176/ps.27.3.193. [DOI] [PubMed] [Google Scholar]; Test MA, Stein LI. Training in community living: Research design and results. In: Stein LI, Test MA, editors. Alternatives to Mental Hospital Treatment. Plenum Publishing Corporation; New York, USA: 1978. pp. 57–74. [Google Scholar]; Weisbrod BA, Test MA, Stein LI. Alternative to mental hospital treatment. II. Economic benefit cost analysis. Archives of General Psychiatry. 1980;37:400–5. doi: 10.1001/archpsyc.1980.01780170042004. [DOI] [PubMed] [Google Scholar]; *
References to studies excluded from this review
- Bond 1989 {published data only} .Bond GR, Miller LD, Krumwied RD, Ward RS. Assertive case management in three CMHCs: A controlled study. Hospital and Community Psychiatry. 1988;39:411–8. doi: 10.1176/ps.39.4.411. [DOI] [PubMed] [Google Scholar]; Bond GR, Witheridge TF, Wasmer D, Dincin J, McRae SA, Mayes J, et al. A comparison of two crisis housing alternatives to psychiatric hospitalization. Hospital and Community Psychiatry. 1989;40:177–83. doi: 10.1176/ps.40.2.177. [DOI] [PubMed] [Google Scholar]; *
- Burns 1993 {published data only} .Burns T, Beadsmoore A, Bhat AV, Oliver A, Mathers C. A controlled trial of home-based acute psychiatric services. I. Clinical and social outcome. British Journal of Psychiatry. 1993;163:49–54. doi: 10.1192/bjp.163.1.49. [DOI] [PubMed] [Google Scholar]; Burns T, Raftery J. Cost of schizophrenia in a randomized trial of home-based treatment. Schizophrenia Bulletin. 1991;17:407–10. doi: 10.1093/schbul/17.3.407. [DOI] [PubMed] [Google Scholar]; Burns T, Raftery J, Beadsmoore A, McGuigan S, Dickson M. A controlled trial of home-based acute psychiatric services. II. Treatment patterns and costs. British Journal of Psychiatry. 1993;163:55–61. doi: 10.1192/bjp.163.1.55. [DOI] [PubMed] [Google Scholar]; *
- Bush 1990 {published data only} .Bush CT, Langford MW, Rosen P, Gott W. Operation outreach: intensive case management for severely psychiatrically disabled adults. Hospital and Community Psychiatry. 1990;41:647–9. doi: 10.1176/ps.41.6.647. [DOI] [PubMed] [Google Scholar]
- Gater 1997 {published data only} .Gater R, Goldberg D, Jackson G, Jennett N, Lowson K, Ratcliffe J, et al. The care of patients with chronic schizophrenia: a comparison between two services. Psychological Medicine. 1997;27:1325–36. doi: 10.1017/s0033291797005631. [DOI] [PubMed] [Google Scholar]
- Ghandi 2001 {published data only} .Ghandi N, Tyrer P, Evans K, McGee A, Lamont A, Harrison-Read P. A randomized controlled trial of community-oriented and hosptial-oriented care for discharged psychiatric patients: Influence of personality disorder on police contacts. Journal of Personality Disorders. 2001;15(1):94–102. doi: 10.1521/pedi.15.1.94.18644. [DOI] [PubMed] [Google Scholar]
- Grawe 2006 {published data only} .Grawe RW, Falloon IRH, Widen JH, Skogvoll E. Two years of continued early treatment for recent-onset schizophrenia: A randomised controlled study. Acta Psychiatrica Scandinavica. 2006;114:328–36. doi: 10.1111/j.1600-0447.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- Harrison 2003 {published data only} .Harrison J, Marshall S, Marshall P, Marshall J, Creed F. Day hospital versus home treatment. Social Psychiatry and Psychiatric Epidemiology. 2003;38:541–6. doi: 10.1007/s00127-003-0672-x. [DOI] [PubMed] [Google Scholar]
- Henlegger 1999 {published data only} .Henlegger SW, Rowland MD, Randall J, Ward DM, Pickrel SG, Cunningham PB, et al. Home-based multisystemic therapy as an alternative to the hospitalization of youths in psychiatric crisis: Clinical outcomes. Journal of American Academy of Child and Adolescent Psychiatry. 1999;38(11):1331–9. doi: 10.1097/00004583-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Herz 2000 {published data only} .Herz MI, Lamberti JS, Mintz J, Scott R, O’Dell SP, McCartan L, et al. A program for relapse prevention in schizophrenia: A controlled study. Archives of General Psychiatry. 2000;57:277–83. doi: 10.1001/archpsyc.57.3.277. [DOI] [PubMed] [Google Scholar]
- Jones 2003 {published data only} .Jones K, Colson PW, Holter MC, Lin S, Valencia E, Susser E, et al. Cost- effectiveness of critical time intervention to reduce homelessness among persons with mental illness. Psychiatric Services. 2003;54:884–90. doi: 10.1176/appi.ps.54.6.884. [DOI] [PubMed] [Google Scholar]; *
- Kuipers 2004 {published data only} .Kuipers E, Holloway F, Rabe-Hesketh S, Tennakoon L. An RCT of early intervention in psychosis: Croydon Outreach and Assertive Support Team (COAST) Social Psychiatry and Epidemiology. 2004;39:358–63. doi: 10.1007/s00127-004-0754-4. [DOI] [PubMed] [Google Scholar]; *
- Levenson 1997 {published data only} .Levenson AJ. Acute schizophrenia: An efficacious outpatient treatment approach as an alternative to full-time hospitalization. Diseases of the Nervous System. 1977;38:242–5. [PubMed] [Google Scholar]
- Linszen 1998 {published data only} .Linszen D, Lenoir M, De Haan L, Dingemans P, Gersons B. Early intervention, untreated psychosis and the course of early schizophrenia. British Journal of Psychiatry. 1998;172:84–9. [PubMed] [Google Scholar]
- Mattejat 2001 {published data only} .Mattejat F, Hirt BR, Wilken J, Schmidt MH, Remschmidt H. Efficacy of inpatient and home treatment in psychiatrically disturbed children and adolescents. European Child & Adolescent Psychiatry. 2001;10:I71–9. doi: 10.1007/s007870170008. [DOI] [PubMed] [Google Scholar]
- Merson 1992 {published data only} .Merson S, Tyrer P, Carlen D, Johnson T. The cost of treatment of psychiatric emergencies: A comparison of hospital and community services. Psychological Medicine. 1996;26:727–34. doi: 10.1017/s0033291700037740. [DOI] [PubMed] [Google Scholar]; Merson S, Tyrer P, Onyett S, Lack S, Birkett P, Lynch S, Johnson T. Early intervention in psychiatric emergencies: A controlled clinical trial. Lancet. 1992;339:1311–4. doi: 10.1016/0140-6736(92)91959-c. [DOI] [PubMed] [Google Scholar]; *
- Metcalfe 2005 {published data only} .Metcalfe C, White IR, Weaver T, Ukoumunne OC, Harvey K, Tattan T, et al. Intensive case management for severe psychotic illness: is there a general benefit for patients with complex needs? A secondary analysis of the UK700 trial data. Social Pyschiatry and Epidemiology. 2005;40:718–24. doi: 10.1007/s00127-005-0954-6. [DOI] [PubMed] [Google Scholar]; *
- Mosher 1975 {published data only} .Bola JR, Mosher LR. Treatment of acute psychosis without nueroleptics: Two-year outcomes from the Soteria Project. Journal of Nervous and Mental Disease. 2003;191:219–29. doi: 10.1097/01.NMD.0000061148.84257.F9. [DOI] [PubMed] [Google Scholar]; Mosher LR, Menn A, Matthew SM. Soteria: Evaluation of a home-based treatment for schizophrenia. American Journal of Orthopsychiatry. 1975;45:455–67. doi: 10.1111/j.1939-0025.1975.tb02556.x. [DOI] [PubMed] [Google Scholar]; Mosher LR, Menn AZ. Community residential treatment for schizophrenia: Two-year follow-up. Hospital and Community Psychiatry. 1978;29:715–23. doi: 10.1176/ps.29.11.715. [DOI] [PubMed] [Google Scholar]; *
- Muijen 1994 {published data only} .Audini B, Marks IM, Lawrence R, Connolly J, Watts V. Home-based versus out-patient/in-patient care for people with serious mental illness. Phase II of a controlled study. British Journal of Psychiatry. 1994;164:204–10. doi: 10.1192/bjp.165.2.204. [DOI] [PubMed] [Google Scholar]; Knapp M, Marks I, Wolstenholme J, Beecham J, Astin J, Audini B, et al. Home-based versus hospital-based care for serious mental illness. British Journal of Psychiatry. 1998;172:506–12. doi: 10.1192/bjp.172.6.506. [DOI] [PubMed] [Google Scholar]; *
- Pai 1982 {published data only} .Pai S, Kapur RL. Evaluation of home care treatment for schizophrenic patients. Acta Psychiatrica Scandinavica. 1983;67:80–8. doi: 10.1111/j.1600-0447.1983.tb06726.x. [DOI] [PubMed] [Google Scholar]; Pai S, Kapur RL. Impact of treatment intervention on the relationship between dimensions of clinical psychopathology, social dysfunction and burden on the family of psychiatric patients. Psychological Medicine. 1982;12:651–8. doi: 10.1017/s0033291700055756. [DOI] [PubMed] [Google Scholar]; Pai S, Nagarajaiah Treatment of schizophrenic patients in their homes through a visiting nurse - some issues in the nurse’s training. International Journal of Nursing Studies. 1982;19:167–72. doi: 10.1016/0020-7489(82)90034-7. [DOI] [PubMed] [Google Scholar]; Pai S, Roberts E. Follow-up study of schizophrenic patients initially treated with home care. British Journal of Psychiatry. 1983;143:447–50. doi: 10.1192/bjp.143.5.447. [DOI] [PubMed] [Google Scholar]
- Pasamanick 1964b {published data only} .Davis AE, Dinitz S, Pasamanick B. The prevention of hospitalization in schizophrenia: Five years after an experimental program. American Journal of Orthopsychiatry. 1972;42:375–88. doi: 10.1111/j.1939-0025.1972.tb02504.x. [DOI] [PubMed] [Google Scholar]; Pasamanick B, Scarpitti FR, Dinitz S. Schizophrenics in the community:An experimental study in the prevention of hospitalization. Appleton-Century-Crofts; New York: 1967. [Google Scholar]; Pasamanick B, Scarpitti FR, Lefton M, Dinitz S, Wernert JJ, McPheeters H. Home versus hospital care for schizophrenics. JAMA. 1964;187:177–81. doi: 10.1001/jama.1964.03060160005001. [DOI] [PubMed] [Google Scholar]; *
- Polak 1976 {published data only} .Polak PR, Kirby MW. A model to replace psychiatric hospitals. Journal of Nervous and Mental Diseases. 1976;162:13–22. doi: 10.1097/00005053-197601000-00003. [DOI] [PubMed] [Google Scholar]
- Power 2007 {published data only} .Power P, Iacoponi E, Reynolds N, Fisher H, Morris R, Garety P, et al. The Lambeth early onset crisis assessment team study: General practitioner education and access to an early detection team in first-episode psychosis. British Journal of Psychiatry. 2007;191(Suppl 51):s133–39. doi: 10.1192/bjp.191.51.s133. [DOI: 10.1192/bjp.191.51.s133] [DOI] [PubMed] [Google Scholar]
- Rosenheck 1995 {published data only} .Rosenheck R, Neale M, Leaf P, Milstein R, Frisman L. Multisite experimental cost study of intensive psychiatric community care. Schizophrenia Bulletin. 1995;21(1):129–40. doi: 10.1093/schbul/21.1.129. [DOI] [PubMed] [Google Scholar]; Rosenheck RA, Neale MS. Cost-effectiveness of intensive psychiatric community care for high users of inpatient services. Archives of General Psychiatry. 1998;55:459–65. doi: 10.1001/archpsyc.55.5.459. [DOI] [PubMed] [Google Scholar]; *
- Sledge 1996 {published data only} .Sledge WH, Tebes J, Rakfeldt J, Davidson L, Lyons L, Druss B. Day hospital/crisis respite care versus inpatient care. Part I. Clinical outcomes. American Journal of Psychiatry. 1996;153:1065–73. doi: 10.1176/ajp.153.8.1065. [DOI] [PubMed] [Google Scholar]; Sledge WH, Tebes J, Wolff N, Helminiak TW. Day hospital/crisis respite care versus inpatient care. Part II. Service utilization and costs. American Journal of Psychiatry. 1996;153:1074–83. doi: 10.1176/ajp.153.8.1074. [DOI] [PubMed] [Google Scholar]; *
- Taylor 1998 {published data only} .Becker T, Holloway F, McCrone P, Thornicroft G. Evolving service interventions in Nunhead and Norwood: PRiSM Psychosis Study 2. British Journal of Psychiatry. 1998;173:371–5. doi: 10.1192/bjp.173.5.371. [DOI] [PubMed] [Google Scholar]; Taylor RE, Leese M, Clarkson P, Holloway F, Thornicroft G. Quality of life outcomes for intensive versus standard community mental health services: PRiSM Psychosis Study 9. British Journal of Psychiatry. 1998;173:416–22. doi: 10.1192/bjp.173.5.416. [DOI] [PubMed] [Google Scholar]; *
- Tyrer 1995 {published data only} .Tyrer P, Morgan J, Van Horn E, Jayakody M, Evans K, Brummell R, et al. A randomised controlled study of close monitoring of vulnerable psychiatric patients. Lancet. 1995;345:756–9. doi: 10.1016/s0140-6736(95)90640-1. [DOI] [PubMed] [Google Scholar]
- Van Minnen 1997 {published data only} .Van Minnen A, Hoogduin CA, Broekman TG. Hospital versus outreach treatment of patients with mental retardation and psychiatric disorders: A controlled study. Acta Psychiatrica Scandinavica. 1997;95:515–22. doi: 10.1111/j.1600-0447.1997.tb10140.x. [DOI] [PubMed] [Google Scholar]
- Warner 2006 {published data only} .Warner J. Randomised controlled evaluation of home treatment for older people with mental illness. National Research Register.
References to studies awaiting assessment
- Bindman 2008 {unpublished data only} .Johnson S, Bindman JP. Recent research on crisis resolution teams: Findings and limitations. In: Johnson S, Needle J, Bindman JP, Thornicroft G, editors. Crisis Resolution and Home Treatment in Mental Health. Cambridge University Press; Cambridge: 2008. p. 54. [Google Scholar]
Additional references
- Altman 1996 .Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audit Comm 1986 .Audit Commission . Making a Reality of Community Care. HMSO; London: 1986. [Google Scholar]
- Begg 1996 .Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–9. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- Bland 1997 .Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ. 1997;315:600. doi: 10.1136/bmj.315.7108.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler 1974 .Bleuler MJN. The long-term course of the schizophrenic psychoses. Psychological Medicine. 1974;4:244–54. doi: 10.1017/s0033291700042926. [DOI] [PubMed] [Google Scholar]
- Boissel 1999 .Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d’efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d’etude des indices d’efficacite] Therapie. 1999;54(4):405–11. [PUBMED: 10667106] [PubMed] [Google Scholar]
- Borschmann 2011 .Borschmann R, Henderson C, Hogg J, Phillips R, Moran P. Crisis interventions for people with borderline personality disorder. Cochrane Database of Systematic Reviews. 2011;(Issue 10) doi: 10.1002/14651858.CD009353.pub2. [DOI: 10.1002/14651858.CD009353; : CD009353] [DOI] [PubMed] [Google Scholar]
- Brooks 1996 .Brooks R, EuroQol Group EQ-5D: The current state of play. Health Policy. 1996;37:3–20. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Buchan 2001 .Buchan IE. StatsDirect Statistical Software.1.9.8 Edition. CamCode; Cambridge: 2001. [Google Scholar]
- Caplan 1964 .Caplan GK. Principles of Preventive Psychiatry. Basic Books; New York: 1964. [Google Scholar]
- Deeks 2000 .Deeks J. Issues in the selection for meta-analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25-28; Cape Town; Cape Town. The Cochrane Collaboration; 2000. [Google Scholar]
- Department of Health 2000 .Department of Health . The NHS Plan. The Stationary Office; London: 2000. [Google Scholar]
- Dieterich 2010 .Dieterich M, Irving CB, Park B, Marshall M. Intensive case management for severe mental illness. Cochrane Database of Systematic Reviews. 2010;(Issue 10) doi: 10.1002/14651858.CD007906.pub2. [DOI: 10.1002/14651858.CD007906.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divine 1992 .Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians’ patient care behavior. Journal of General Internal Medicine. 1992;7:623–9. doi: 10.1007/BF02599201. [DOI] [PubMed] [Google Scholar]
- Donner 2002 .Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine. 2002;21:2971–80. doi: 10.1002/sim.1301. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne 2002 .Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta-analyses involving crossover trials: Methodological issues. International Journal of Epidemiology. 2002;31(1):140–9. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- Ellison 1974 .Ellison D, Rieker P, Marx J. Organisational adaptation to community mental health. In: Roma P, editor. Sociological perspectives on community mental health. Davis Company; Philadelphia, PA: 1974. [Google Scholar]
- Endicott 1972 .Endicott J, Spitzer RL. What! Another rating scale? The psychiatric evaluation form. Journal of Nervous and Mental Disease. 1972;154(2):88–104. doi: 10.1097/00005053-197202000-00002. [DOI] [PubMed] [Google Scholar]
- Endicott 1976 .Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale. Archives of General Psychiatry. 1976;33:766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Finch 1991 .Finch SJ, Burgess PM, Herrman HE. The implementation of community-based crisis services for people with acute psychiatric illness. Australian Journal of Public Health. 1991;15:122–9. doi: 10.1111/j.1753-6405.1991.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Furukawa 2006 .Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology. 2006;59(7):7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Geller 1995 .Geller JL, Fisher WH, McDermeit M. A national survey of mobile crisis services and their evaluation. Psychiatric Services. 1995;46:893–7. doi: 10.1176/ps.46.9.893. [DOI] [PubMed] [Google Scholar]
- Gulliford 1999 .Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: Data from the Health Survey for England 1994. Journal of American Epidemiology. 1999;149:876–83. doi: 10.1093/oxfordjournals.aje.a009904. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2011 .Higgins JPT, Green S, editors. [updated March 2011];Cochrane Handbook for Systematic Reviews of Interventions. (Version 5.1.0). www.cochrane-handbook.org Available from.
- Hoult 1984a .Hoult J, Reynolds I. Schizophrenia: A comparative trial of community-oriented and hospital-oriented psychiatric care. Acta Psychiatrica Scandinavica. 1984;69:359–72. doi: 10.1111/j.1600-0447.1984.tb02506.x. [DOI] [PubMed] [Google Scholar]
- Hoult 1984b .Hoult J, Rosen A, Reynolds I. Community oriented treatment compared to psychiatric hospital oriented treatment. Social Sciences Medicine. 1984;11:1005–10. doi: 10.1016/0277-9536(84)90272-7. [DOI] [PubMed] [Google Scholar]
- Hoult 1986 .Hoult J. The community care of the acutely mentally ill. British Journal of Psychiatry. 1986;149:137–44. doi: 10.1192/bjp.149.2.137. [DOI] [PubMed] [Google Scholar]
- Johnson 2008 .Johnson S, Needle J, Bindman JP, Thornicroft G. Crisis Resolution and Home Treatment in Mental Health. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Kay 1986 .Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. Multi-Health Systems; North Tonawanda, NY: 1986. [Google Scholar]
- Lamb 1979 .Lamb RH. Alternatives to Acute Hospitalization. Jossey-Bass; San Fransico, CA: 1979. [Google Scholar]
- Langsley 1968 .Langsley DG, Pittman FS, Machotka P, Flomenhaft K. Family crisis therapy - results and implications. Family Process. 1968;7:145–58. [Google Scholar]
- Larsen 1979 .Larsen DH, Attkison CC, Hargreaves WA. Assessment of client/patient satisfaction: Development of a general scale. Evaluation and Program Planning. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- Leucht 2005 .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry. 2005;187:366–71. doi: 10.1192/bjp.187.4.366. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
- Leucht 2005a .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophrenia Research. 2005;79(2-3):231–8. doi: 10.1016/j.schres.2005.04.008. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
- Leucht 2007 .Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF? Schizophrenia Bulletin. 2007;33(1):183–91. doi: 10.1093/schbul/sbl025. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann 1944 .Lindemann E. Symptomatology and management of acute grief. American Journal of Psychiatry. 1944;101:141–8. doi: 10.1176/ajp.151.6.155. [DOI] [PubMed] [Google Scholar]
- Marshall 2000 .Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry. 2000;176:249–52. doi: 10.1192/bjp.176.3.249. [DOI] [PubMed] [Google Scholar]
- McGorry 1996 .McGorry PD, Edwards J, Mihalopoulos C, Harrigan SM, Jackson HJ. EPPIC: An evolving system of early detection and optimal management. Schizophrenia Bulletin. 1996;22:305–26. doi: 10.1093/schbul/22.2.305. [DOI] [PubMed] [Google Scholar]
- Moher 2001 .Moher D, Schulz KF, Altman D. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–91. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- Overall 1962 .Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Parker 1991 .Parker G, Rosen A, Emdur N, Hadzi-Pavlov D. The life skills profile: Psychometricproperties of a measure assessing function and disability in schizophrenia. Acta Psychiatrica Scandinavia. 1991;83:145–52. doi: 10.1111/j.1600-0447.1991.tb07381.x. [DOI] [PubMed] [Google Scholar]
- Pasamanick 1967 .Pasamanick B, Scarpitti F, Dinitz S. Schizophrenics in the community: An experimental study in the prevention of hospitalization. Appleton Century Crofts; New York: 1967. [Google Scholar]
- Priebe 1999 .Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (Mansa) International Journal of Social Psychiatry. 1999;45(1):7–12. doi: 10.1177/002076409904500102. [DOI] [PubMed] [Google Scholar]
- Querido 1968 .Querido A. The shaping of community mental health care. British Journal of Psychiatry. 1968;114:293–302. doi: 10.1192/bjp.114.508.293. [DOI] [PubMed] [Google Scholar]
- Schoenfeld 1986 .Schoenfeld P, Halvey J, Hemley-Van Der Velden E, Ruhf L. Long term outcome of network therapy. Hospital and Community Psychiatry. 1986;37:373–6. doi: 10.1176/ps.37.4.373. [DOI] [PubMed] [Google Scholar]
- Schünemann 2008 .Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. pp. 359–83. [Google Scholar]
- Shepperd 2009 .Shepperd S, Doll H, Gowers S, James A, Fazel M, Fitzpatrick R, et al. Alternatives to inpatient mental health care for children and young people. Cochrane Database of Systematic Reviews. 2009;(Issue 2) doi: 10.1002/14651858.CD006410.pub2. [DOI: 10.1002/14651858.CD006410.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein 1978 .Stein LI, Test MA. Alternatives to mental hospital treatment. Plenum Press; New York: 1978. [Google Scholar]
- Test 1978 .Test MA, Stein LI. Community treatment of the chronic patient: Research overview. Schizophrenia Bulletin. 1978;4:350–64. [Google Scholar]
- Thomas 1970 .Thomas CS, Weisman GK. Emergency planning: The practical and theoretical backdrop to an emergency treatment unit. International Journal of Social Psychiatry. 1970;16:283–7. [Google Scholar]
- Ukoumunne 1999 .Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area-wide and organisation-based interventions in health and health care: A systematic review. Health Technology Assessment. 1999;3(5):iii–92. [MEDLINE: 10982317] [PubMed] [Google Scholar]
- Weisman 1989 .Weisman GK. Crisis Intervention. In: Bellack AS, editor. A clinical guide for the treatment of schizophrenia. Plenum Press; New York: 1989. pp. 101–34. [Google Scholar]
- Weissman 1971 .Weissman MM, Paykel ES, Siegel R, Klerman GL. The social role performance of depressed women: Comparisons with a normal group. American Journal of Orthopsychiatry. 1971;41:390–405. doi: 10.1111/j.1939-0025.1971.tb01126.x. [DOI] [PubMed] [Google Scholar]
- WHO 1987 .World Health Organization . Report on European Study. World Health Organization; Copenhagen: 1987. Mental Health Services in Pilot Study Areas. [Google Scholar]
- Wing 1974 .Wing JK, Cooper JE, Sartorius N. Measurement and classification of psychiatric symptoms: An instruction manual for the PSE and Catego program. Cambridge University Press; Cambridge: 1974. [Google Scholar]
- Wing 1998 .Wing JK, Beevor AS, Curtis RH, Park SB, Hadden S, Burns A. Health of the Nation Outcome Scales (HoNOS). Research and Development. British Journal of Psychiatry. 1998;172:11–8. doi: 10.1192/bjp.172.1.11. [DOI] [PubMed] [Google Scholar]
- Xia 2009 .Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Loss to outcomes stakeholder survey: The LOSS study. Psychiatric Bulletin. 2009;33(7):254–7. [Google Scholar]
References to other published versions of this review
- Joy 2000 .Joy CB, Adams CE, Rice K. Crisis intervention for people with severe mental illnessees: a Cochrane review. Schizophrenia Research. 2000;41(1):230–1. doi: 10.1002/14651858.CD001087. [DOI: 10.1002/14651858.CD001087] [DOI] [PubMed] [Google Scholar]
- Joy 2004 .Joy CB, Adams CE, Rice K. Crisis intervention for people with severe mental illnesses. Cochrane Database of Systematic Reviews. 2004;(Issue 4) doi: 10.1002/14651858.CD001087.pub2. [DOI: 10.1002/14651858.CD001087.pub2] [DOI] [PubMed] [Google Scholar]
- Joy 2006 .Joy CB, Adams CE, Rice K. Crisis intervention for people with severe mental illnesses. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD001087.pub3. [DOI: 10.1002/14651858.CD001087.pub3; PUBMED: 17054133] [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study