Abstract
Post-natal mammary gland development requires complex interactions between the epithelial cells and various cell types within the stroma. Recent studies have illustrated the importance of immune cells and their mediators during the various stages of mammary gland development. However, the mechanisms by which these immune cells functionally contribute to mammary gland development are only beginning to be understood. This review provides an overview of the localization of immune cells within the mammary gland during the various stages of post-natal mammary gland development. Furthermore, recent studies are summarized that illustrate the mechanisms by which these cells are recruited to the mammary gland and their functional roles in mammary gland development.
Keywords: mammary gland, immune cell, immune mediator, macrophage, mast cell, eosinophil
Introduction
Post-natal mammary gland development is characterized by well-defined phases of ductal development during puberty, alveolar development during pregnancy, production and secretion of milk during lactation, and regression during involution (1). These processes involve cycles of proliferation, differentiation and apoptosis, which require a complex orchestration of numerous events in both the epithelial cells and the surrounding stroma. Numerous cell types, including various immune cells, can be found within the stroma surrounding the epithelial structures at all stages of mammary gland development. There has been recent interest in characterizing the types of immune cells and understanding their functional roles during mammary gland development. Many of the immune cells observed in the mammary gland have been linked to the process of wound healing. As outlined in this review, the same immune cells and mechanistic events involved in wound healing may play a role in mammary gland development. Therefore, understanding the events of wound healing may allow us to gain insight into processes of normal mammary gland growth and development. The purpose of this review is to describe which immune cell types have been localized to the mammary gland and to outline recent studies that have been performed to address the functional significance of these cell types during mammary gland development.
Overview of immune cell function
Because a number of different immune cell types are mentioned in this review, this section will introduce each cell type and briefly describe the normal functioning of these cells within the context of the wound healing process. In order to understand how immune cells might contribute to mammary gland development, it is important to first understand how they typically function to promote immune responses.
Neutrophils are primarily involved in the wound healing response, and are typically the first cell type to enter the wound site. They appear immediately after injury and peak around 24–48 hours (2). Neutrophils are primarily responsible for phagocytosis of bacteria introduced during injury and thereby act to protect against bacterial infection. Neutrophils also release free radicals to destroy bacteria as well as proteases to aid in the breakdown of damaged tissue. Once the neutrophils have performed their functions, they undergo apoptosis and are phagocytosed by macrophages.
The second cell type to enter the wound site, and the major mediator of phagocytosis, is the macrophage (2, 3). Macrophage infiltration occurs 48–96 hours post-injury and peaks around day 3. Regan et al. have demonstrated that removing or inhibiting macrophage function results in impaired wound healing and a delay in the recruitment and expansion of fibroblast cell populations (2). These results demonstrate the essential role of macrophages in wound healing and maintenance of fibroblast activity. Macrophages are responsible for secretion of cytokines that recruit and regulate other cells involved during wound healing, including fibroblasts and other types of immune cells (3–5). These cytokines include transforming growth factor β (TGFβ), tumor necrosis factor α (TNFα), and interleukin-1 (IL-1). Macrophages also secrete factors that increase collagen synthesis and induce angiogenesis, which are crucial for restoration of dead or damaged tissue (6). Recently, macrophages have been categorized according to their functions during the wound healing process (7–9). For example, macrophages in the initial stages of inflammation have been named M1 macrophages and are primarily responsible for antigen presentation, killing of parasites and secreting factors that activate cytotoxic T cells. Macrophages associated with the later stage of inflammation, also known as resolution (10), are termed M2 macrophages and are responsible for promoting angiogenesis and tissue remodeling to facilitate tissue repair. These differences illustrate the wide range of functions macrophages are capable of performing depending on environmental signals.
While certain cell types such as mast cells and eosinophils are typically associated with allergy and asthma, they have also been implicated in the wound healing process (11). Mast cells contain cytoplasmic granules that are filled with inflammatory mediators, which are released in response to activating stimuli. These mediators include histamine, serine proteases, carboxypeptidase A and proteoglycans. Mast cells are also able to release lipid mediators and inflammatory cytokines, such as TNFα, and chemokines, which contribute to the wound healing process [reviewed in (12)]. Furthermore, mast cells serve as critical mediators in recruitment of polymorphonuclear leukocytes (PMNs) such as eosinophils and neutrophils (13).
Eosinophils are a family of leukocytes that are recruited out of the circulation to sites of inflammation where they mediate immune responses through a variety of mechanisms including secretion of inflammatory cytokines (14). Eosinophil-induced cytokines are important for cell trafficking, regulating vascular permeability, and upregulating adhesion molecule interactions (14). Eosinophils are highly responsive to IL-5, which binds to its specific receptor (IL-5R) located on mature eosinophils in both mice and humans (15). IL-5 is thought to prime eosinophils to respond to chemoattractants such as eotaxin (16–20). In addition to acting as a chemotactic agent for eosinophils, IL-5 also promotes eosinophil survival, maturation, and adhesion to surrounding cells including endothelial cells (15). Once localized to the inflammatory site, eosinophils secrete growth factors, including TGFβ, transforming growth factor α (TGFα) and epidermal growth factor (EGF), which support epithelial cell maintenance during processes of tissue development, repair, and remodeling (20–22).
T lymphocytes are not required for the initiation or early events of wound healing and instead they appear at the wound site following neutrophils and macrophages at approximately 5 days post-injury (2). The population of T cells peaks at day 7 and then slowly decreases until completion of wound repair. T cells are responsible for upregulating the activity of other wound healing cells, including macrophages, endothelial cells, and fibroblasts. The process of wound healing is characteristic of cell-mediated immunity, also known as a T helper cell 1 response (Th1). Th1 cytokines, including IL-2 and interferon-γ (IFNγ), are responsible for directing cell-mediated immunity, including activation of macrophages, and are important for the initial phase of wound healing involving immune cell infiltration and destruction of pathogens (23). Th2 cytokines such as IL-4, IL-5, IL-6, IL-10, and IL-13 are involved in the later phase of wound healing, including matrix deposition and tissue remodeling (23). Furthermore, these cytokines facilitate humoral immunity through activation of antibody-producing B cells (6).
As described in detail below, these various cell types have been associated with various stages of mammary gland development. The remainder of this review will highlight the recent studies that have provided insights into the functions of these cell types, and their mediators, during post-natal mammary gland development.
Immune cells associated with post-natal mammary gland development
Mammary gland development, unlike most other organs, occurs predominantly after birth in a series of specific stages. The stages of post-natal development, as outlined in this review, include pubertal development, pregnancy, lactation, and involution [(reviewed in (1)]. Interestingly, there are several characteristic traits common to both wound repair and mammary gland development including epithelial growth and proliferation, stromal reorganization and immune cell infiltration. Moreover, the same cells that compose the mammary gland and its surrounding microenvironment are those involved in wound healing. These cell types include epithelial cells, endothelial cells, fibroblasts, and immune cells. Immune cells such as eosinophils, mast cells, and macrophages occupy unique sites during the various stages of mammary gland development where they contribute to a diversity of effector functions. Initially, these cells are involved in ductal elongation and branching during the onset of puberty (20, 24, 25). During pregnancy, immune cells are thought to be involved in regulating epithelial cell rearrangement and differentiation characteristic of the developing alveolar structures (26). This is followed by lactation during which immunoglobulin-secreting cells migrate to the mammary gland, releasing factors into the milk that are critical for immunity in the newborn (27, 28). Finally, macrophages are prominent in the environment of the involuting mammary gland and recent studies have linked macrophages to stromal rearrangements during this stage (29, 30).
In addition to the similarities between mammary gland development and wound healing, it is important to note that there are also likely to be differences in immune cell recruitment and function during these two processes. During wound healing, platelet aggregation at the site of damage leads to chemokine secretion and subsequent immune cell infiltration into the damaged tissue (23). In contrast, it is likely that immune cell infiltration in the mammary gland occurs in response to signals from the epithelial or stromal cells at the various stages of development. Mammary gland development is highly dependent upon hormonal cues, such as estrogen during ductal elongation and progesterone during alveolar development (1). For example, estrogen regulates expression of cytokines in immune cell types, including macrophages (31). Furthermore, progesterone can also regulate macrophage function and has also been shown to regulate the Th1/Th2 phenotype (32). Interestingly, previous studies have suggested that cyclical levels of estrogen and progesterone are capable of regulating macrophage distribution in the uterus (33). Therefore, it is interesting to speculate that immune cell function in the mammary gland might be regulated by the circulating ovarian hormones at various stages during development, although the relationship between immune cells in the mammary gland and ovarian hormones remains to be determined.
While our understanding of the activities of all the various immune cell types during mammary gland development is still quite basic, this review will outline the most recent studies focusing on the ability of these various cell types, and their secreted inflammatory mediators, to regulate the various stages of mammary gland development.
Immune Cells Contribute to Mammary Ductal Morphogenesis
At birth, the mammary gland is composed of the mammary fat pad surrounding a rudimentary epithelial structure, which expands into the fat pad during puberty beginning at approximately 3 to 4 weeks of age (1). At the tips of developing ducts are bulb-like structures known as terminal end buds (TEBs), which invade through the mammary fat pad and give rise to new primary ducts. TEBs are sites of high levels of proliferation and their growth and development depends on recruitment and activation of immune cells such as mast cells, macrophages and eosinophils. These cells occupy unique sites during pubertal ductal elongation and contribute a diversity of effector functions to mammary gland development, as described in detail below.
Mast Cells
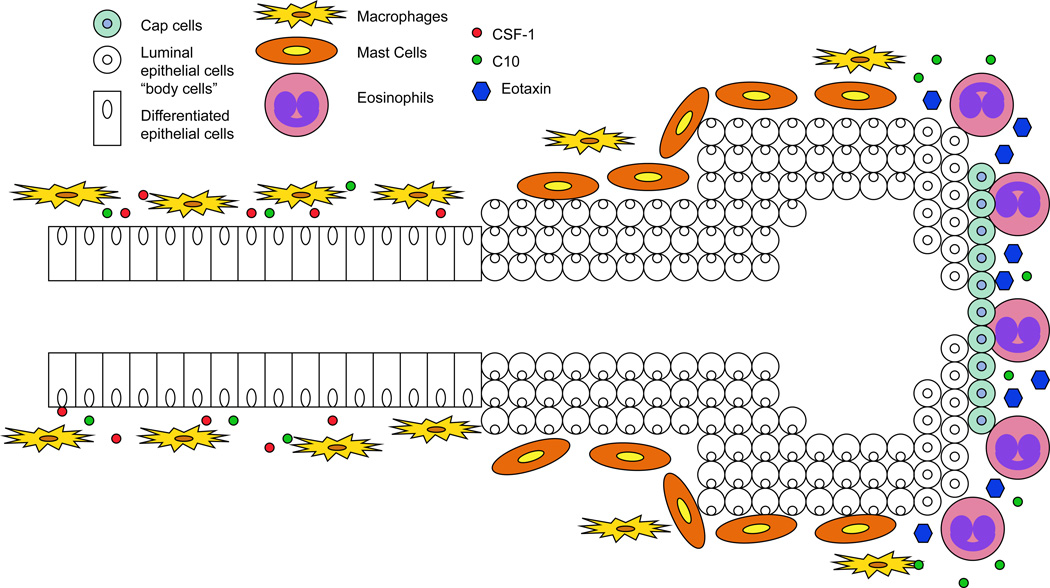
Mast cells are found in the microenvironment of mammary glands at all stages of development. Until recently, the role of mast cells in normal pubertal development remained unknown. However, Lilla and Werb have recently shown that mast cells contribute to proliferation and ductal branching during pubertal mammary gland development (25). Prior to the onset of pubertal development mast cells are low in number and do not localize to specific regions within the mammary gland. However, between 5 and 8 weeks of development, mast cell numbers are elevated and are associated with the stromal regions immediately surrounding proliferating TEBs (Figure 1). Using a mast cell deficient mouse model (KitW-sh), mast cells were found to be required for normal pubertal gland development (25). In the absence of mast cells, the number of ducts and TEBs was significantly reduced at 5 weeks of age compared to wild-type controls. Moreover, the average ductal length was also decreased at five weeks, which persisted at 12 weeks of age. Of important note, this decrease in ductal formation did not inhibit lactation thereby indicating the specificity of mast cell function in early gland development. In addition to mast cells, macrophages and eosinophils play important roles in ductal morphogenesis. The importance of these immune cells will be discussed in detail below, however, it is critical to point out that loss of mast cells did not influence the presence of macrophages and eosinophils at sites of TEBs, thus confirming that the role of mast cells in ductal elongation is independent of macrophage and eosinophil functioning.
Figure 1. Model of immune cell localization at the terminal end buds during post-natal mammary ductal morphogenesis.
Immune cells including mast cells, eosinophils, and macrophages contribute to numerous effector functions during mammary ductal morphogenesis. Mast cells localize to the stromal regions surrounding proliferating terminal end buds (TEBs) (25). Next, macrophages are recruited to TEBs. After being recruited, macrophages migrate and localize to the neck of TEBs in response to CSF-1. Finally, eosinophils are recruited to the head of TEBs in response to expression of epithelial cell-secreted eotaxin (24). At the head of TEBs, eosinophils secrete the chemokine C10 which acts to recruit additional macrophages (36).
Further studies were performed to define the mechanisms by which mast cells contribute to ductal morphogenesis. Inhibition of mast cell degranulation was found to reduce TEB formation, suggesting that mast cell-released factors are critical for ductal morphogenesis (25). Further analysis of mice deficient for dipeptidyl peptidase I (DPPI), an enzyme critical for activation of mast cell serine proteases, demonstrated that these proteases are important for proper TEB formation and subsequent ductal development. These studies clearly demonstrate a requirement for mast cells and their released factors during mammary ductal morphogenesis.
In contrast to understanding the effects of loss of mast cells on ductal formation, another study has evaluated the effects of increased mast cell accumulation in the mammary gland. In these studies, mice were fed with conjugated linoleic acid (CLA), which is a family of chemopreventive fatty acids found in food products made from ruminants (34). Feeding CLA to mice has been shown to induce changes in the mammary stroma, such as inhibition of angiogenesis and increased deposition of collagen rich extracellular matrix in the stroma surrounding the epithelium (34, 35). Interestingly, CLA feeding was also found to induce an increase in mast cells associated with the stroma surrounding mammary epithelial structures. These studies suggest that increased recruitment of mast cells to the mammary epithelium may elicit alterations in the stromal compartment, although the mechanisms by which these alterations are regulated remain to be determined.
Macrophages
Macrophages are known to play an important role in normal mammary gland development, specifically during puberty when they are recruited to the neck region of the TEBs (36) (Figure 1). Colony stimulating factor-1 (Csf1) has been shown to be a key player in macrophage proliferation and survival (37). In mice that are deficient for Csf1 (Csf1op/op), mammary glands had fewer TEBs and displayed inhibited ductal elongation and branching, which correlated with loss of macrophages in the mammary gland (36, 38). In addition to overall reduction in TEB number, the shape of the TEBs in Csf1op/op mice was altered indicating that macrophages participate in organization of TEB structure (38). TEBs from mice heterozygous for the Csf-1 mutation, +/Csf1op, were shown to be oblong in shape. Conversely, homozygous mice, Csf1op/op, harbored TEBs that were shorter and more circular. Furthermore, the change in TEB shape could be rescued by reconstituting the TEB-associated macrophage population in female Csf1op/op mice by expression of a tetracycline-regulated mouse mammary tumor virus (MMTV)-driven CSF-1 transgene (39). The TEBs from these mice were oblong in shape similar to +/Csf1op mice. These results suggest that macrophages are responsible for directing the shape of TEBs. Furthermore, using imaging techniques an inverse relationship between circularity and the presence of collagen fibers surrounding the TEB was determined. The absence of macrophages was directly linked to reduced collagen organization and therefore increased circularity in TEB shape. These results suggest that macrophages are responsible for assembling collagen into long organized fibers surrounding TEBs, which are responsible for enhancing ductal elongation.
Interestingly, studies by Gyorki et al. have revealed a critical contribution of macrophages to mammary stem cell function during post-natal development. Upon transplantation of an enriched population of mammary stem cells into the fat pads of Csf1op/op mice, in which macrophages are absent, the mammary stem cells exhibited reduced ability to reconstitute ductal outgrowths in fat pads cleared of endogenous epithelium (40). Only one single small outgrowth was observed in 18 mice compared to significant outgrowth in 18 of 24 wild-type mice. A second method of macrophage depletion was utilized to validate these studies. Prior to transplantation, clodronate liposomes were injected into the mammary fat pads at the same time as the mammary stem cell transplantation. Consistent with the data obtained from the Csf1op/op mice, there was a significant decrease in the ability of the mammary stem cells to form outgrowths. These data reflect the contribution of macrophages to normal mammary stem cell function during ductal morphogenesis. It is interesting to speculate that macrophage-derived factors might be acting on the epithelial cells and/or the stromal environment to impact stem cell function, and a better understanding of these mechanisms will provide critical insights into the ability of immune cells to regulate the stem cell niche during mammary gland development.
Eosinophils
Another immune cell important for mammary gland development is the eosinophil. Eosinophils have been observed in abundance within stromal tissue adjacent to the head of proliferating TEBs (20) (Figure 1). Recruitment of eosinophils to the head of TEBs occurs primarily in response to expression of the chemokine eotaxin. Expression of eotaxin remains relatively low until 5 weeks of age when it is enhanced in the mammary gland (14). This peak in eotaxin levels is quickly followed by eosinophil infiltration. It has been shown that in eotaxin knock-out mice (eotaxin−/−) (41), ductal branching and TEB formation were reduced due to loss of eosinophil infiltration (24). Moreover, mice deficient in IL-5 (IL-5−/−), which promotes eosinophil recruitment and activation, exhibited fewer TEBs, inhibited ductal branching, and decreased overall density suggesting that IL-5 plays a role in mammary gland development (42). Together, these studies demonstrate that interactions between stromal eosinophils and TEBs drive ductal elongation and branching.
Previously, the only IL-5 mouse models that existed were those examining deficiencies in eosinophil activity and not systemic eosinophilia. Eosinophilia is characterized by constitutive overexpression of IL-5, which causes a 10-fold increase in eosinophil number in the blood and tissues (43). Since IL-5 is known to play an important role in eosinophil recruitment and activation, Sferruzzi-Perri et al. have developed a transgenic mouse model with which to examine the effects of an overabundance of eosinophils on mammary gland development (20). IL-5 transgenic mice (IL-5Tg) were generated by linking the IL-5 genomic sequence to the CD2 regulatory sequence, which results in overexpression of IL-5 in T-lymphocytes (43). By 7 and 10 weeks of age, mice overexpressing IL-5 demonstrated a 4-fold increase in the number of TEB-associated eosinophils in comparison to wild-type mice (20). Analysis of ductal development in IL-5Tg mice revealed impaired TEB formation, ductal branching and ductal elongation. Mammary gland whole mounts from 5-week old IL-5Tg mice demonstrated a 38% reduction in the average number of branch points and a 43% decrease in the average number of TEBs. Moreover, 7-week old mice exhibited a 33% decrease in mean ductal length when compared to wild-type mice. By 10 weeks of age, however, these differences in branching number and ductal elongation were indistinguishable. This absence in measurable differences by 10 weeks may be explained by a reduction in overall eosinophil number marked by the completion of post-pubertal development. This suggests that the number of eosinophils present after pubertal development reflects the number of eosinophils required for optimal ductal branching and elongation as well as TEB formation. Eosinophil counts that either exceed or fall below this threshold contribute to abnormal TEB and ductal formation. It was suggested that a potential mechanism for the effects of increased eosinophils on ductal morphogenesis is that eosinophils are known to express high levels of TGFβ, which is a well-known inhibitor of mammary epithelial cell proliferation during pubertal development (20, 44). While this hypothesis has yet to be validated, the results from these studies, in conjunction with the results from the loss of eosinophil studies, clearly demonstrate that a proper balance of eosinophils is required for ductal morphogenesis to occur normally during mammary gland development.
Although many studies focus on understanding the importance of specific immune cells in isolation, is it important to keep in mind that immune cells often regulate each other. For example, current research indicates that eosinophils are capable of regulating mast cell function. Specifically, eosinophils produce eosinophil-derived stem cell factor, which promotes mast cell growth (14). Furthermore, eosinophils produce nerve growth factor (NGF), which is essential to mast cell survival and activation (14). In the pre-pubertal glands, mast cells are dispersed throughout the stroma and, unlike eosinophils, do not localize to any specific structure (14). However, during puberty at approximately 5 and 8 weeks of age, mast cells are found in the stroma directly in front of the leading edge of invading TEBs (14). Furthermore, Lilla and Werb showed by immunohistochemistry that even though some mast cells remained in the stroma, the majority were observed at mature ducts and blood vessels during later stages of development (14). These observations suggest that mast cells are not present during pre-pubertal development but that they arrive after eosinophil-mediated recruitment during later stages of development. Further analysis of the effects of eosinophil loss on mast cell recruitment or function during mammary gland development would strengthen the idea that immune cells are acting in concert to promote mammary gland development.
In addition to regulating mast cell activity, eosinophils also play a role in recruitment of macrophages to the TEB. In mice, eosinophils secrete the chemokine C10, which acts as a chemoattractant for macrophages [(reviewed in (36)]. This observation suggests an important role for eosinophils in recruitment of macrophages to the TEB. Unlike eosinophil populations that localize to the head of proliferating TEBs, macrophages, after being recruited to the TEB by eosinophils, migrate and localize to the neck of TEBs. Macrophages exclusively harbor the colony stimulating factor-1 receptor (CSF-1R), which binds to the ligand CSF-1. CSF-1 is synthesized by mammary epithelial cells during gland development and as a result stimulates mobilization of macrophages to the neck of the TEB. Therefore, it is likely that macrophages migrate to developing glands in response to a combination of growth factors secreted by mammary epithelial cells as well as chemoattractant cues from other leukocytes, such as eosinophils, within the stroma.
Immune cells and their regulators during pregnancy and lactation
In response to pregnancy-induced hormones, including progesterone and prolactin, the mammary gland undergoes extensive alterations in the alveolar compartment (1). This process includes proliferation of the alveolar epithelial cells and differentiation into milk-producing cells. In response to progesterone withdrawal at parturition, the mammary gland begins to produce and secrete milk, which is then provided to the newborn. In contrast to other stages of mammary gland development, little is known regarding the roles of immune cells in the development of the mammary gland during pregnancy and lactation. As described below, infiltration of various cell types into the mammary gland and expression of specific immune cell mediators have been observed during pregnancy and lactation. In addition, it has been well-established that immunoglobulins are produced by infiltrating immune cells and are critical for passive immunity provided to the newborn through the milk (1, 28, 45). Therefore, it is likely that immune cells play an important role during the functional stages of mammary gland development.
Macrophages
Macrophages have been found to be associated with developing alveoli during pregnancy and with actively lactating alveoli during lactation (36). Studies of the Csf1op/op mice, which are deficient in mammary gland-associated macrophages, demonstrated that a significant number of dams were unable to nurse their pups, suggesting that the presence of macrophages is critical for functional lactation (26). Further analysis of the mammary glands from these mice during pregnancy revealed decreased ductal growth and branching accompanied by a lack of secretory activity and decreased functional differentiation (26). Interestingly, precocious formation of the alveolar structures, accompanied by precocious expression of milk protein genes, was also observed in mammary glands from the Csf1op/op mice during late pregnancy. Therefore, disrupting Csf1 expression, presumably leading to decreased presence of macrophages associated with the alveolar structures, clearly has effects on mammary gland development during pregnancy, which then leads to a decrease in the ability to lactate. However, the mechanisms by which macrophages might contribute to alveolar development and function during these stages are not known
Eosinophils
While not as numerous as the macrophages, eosinophils also localize to the developing alveolar structures in the mammary gland during pregnancy (36). While the effects of eosinophil loss on mammary gland development have not been reported, studies of the IL-5−/− mice demonstrated decreased overall litter size and increased mortality rate relative to IL-5+/− and IL-5+/+ mice (42). Further studies revealed that IL-5−/− pups that were nursed by wild-type females developed normally whereas IL-5+/+ pups that were nursed by IL-5−/− females failed to survive indicating that the small size and increased mortality were due to the quality or amount of the mother’s milk and not underlying developmental issues of the pup. As mentioned above, IL-5 deficient mice and Csf1op/op mice both exhibited alterations in ductal development, leading to the possibility that insufficient ductal development is involved in the lactation defect observed in these mice. However, further studies of alveolar development and differentiation during pregnancy and lactation in these mice would provide more information regarding the role of eosinophils in mammary gland development during these stages.
Immune cell mediators during pregnancy and lactation
Recent studies have highlighted a role for immune cell mediators in the specification of epithelial cell differentiation during pregnancy (46). Similar to the immune system, various cytokines produced by the epithelial cells can be used to distinguish between the differentiation states of the cells. For example, Th1-associated cytokines including IL-1β, TNFα, IL-12a and IFNγ are secreted by undifferentiated epithelial cells (47, 48). However, treatment of cells with lactogenic hormones, including prolactin, dexamethasone and insulin, leads to a switch in the production of the Th1-related cytokines to Th2-related cytokines, including IL-4, IL-13 and IL-5 (47). Furthermore, treatment of differentiated epithelial cells with the Th1-associated cytokine IL-1β leads to decreased expression of the milk protein gene β-casein (48), suggesting that the correct balance of these inflammatory mediators is critical for proper epithelial cell differentiation during the switch to pregnancy.
A key downstream target of the Th2 cytokines is Stat6, which was previously found to be elevated during mammary gland development (49). In order to study the involvement of Stat6 signaling in mammary gland development, Khaled et al. analyzed mammary gland development in the Stat6 knock-out (Stat6−/−) mice (47). In the absence of Stat6, mice displayed loss in the number of overall ductal side branches and alveolar buds during pregnancy. Furthermore, by days 10 and 15 of gestation there was a decrease in the density of alveolar structure when compared to wild-type mice. The diminished population of epithelial cells suggests that there may be a defect in cell proliferation upon loss of Stat6. In agreement with this hypothesis, immunohistochemical analysis demonstrated that the number of Ki67-positive cells decreased by 50% in mammary glands lacking Stat6 (47). Moreover, a 50% reduction in Akt phosphorylation was observed indicating a potential signaling mechanism that would explain the inhibition of cell proliferation observed in Stat6−/− mice. However, by gestation day 15 the number of proliferating cells was replenished, suggesting that an alternative mechanism exists to compensate for the loss of Stat6 in the mammary gland, which allows Stat6−/− mice to be able to lactate and nurse their pups.
IL-4 and IL-13 are Th2-related cytokines that activate Stat6 (47, 50). Therefore, studies were pursued using the IL-4/IL-13 double knockout mice (IL-4−/−/IL-13−/−) to further understand the ability of these mediators to regulate mammary gland development during pregnancy (47). These studies demonstrated that IL-4−/−/IL13−/− mice exhibited delayed alveolar development, indicating that these cytokines are essential for mammary gland development during pregnancy. In conclusion, IL-4 and IL-13 cytokines are important for regulating Stat6 signaling which is essential to normal mammary gland development. While it is clear that these cytokines can act directly on epithelial cells to regulate differentiation [reviewed in (46)], the effects that the Th2-related cytokines might have on immune cells infiltration and function in the mammary gland during pregnancy remain to be determined.
Immune cell function during lactation: link to newborn health
Lymphocytes, including both B cells and T cells, have been found to infiltrate the mammary gland during lactation (27, 28). Furthermore, immune cells and their secreted products are critical for the transfer of passive immunity to the newborn. Passive immunity, the transfer of active components of humoral immunity such as antibodies, is important for the health of the immunologically naïve newborn. The most prominent immunoglobulin found in milk is immunoglobulin A (IgA), which is secreted by IgA+ B cells and is important for protecting against intestinal and respiratory pathogens (51). IgA can be transferred into the milk by either transfer from the bloodstream or secretion by IgA+ B cells that have been recruited to the mammary gland (27). Therefore, recent studies have been performed to understand both the recruitment of these cells to the gland and the functions of their secreted factors that are passed through the milk to the newborn.
The IgA+ B cells found in the mammary gland during lactation are recruited by specific chemokines that are upregulated during lactation. One such chemokine is CCL28, which was found to be upregulated in the mammary gland during lactation and binds to its receptor CCR10, which is found specifically on IgA+ B cells (45). Inhibition of CCL28 in vivo using blocking antibodies was found to inhibit the recruitment of the IgA+ cells and subsequent transfer of IgA to the newborn, suggesting that this chemokine is critical for B cell recruitment during lactation (45). In addition to chemokines, immune cell recruitment also requires expression of specific vascular-expressed adhesion molecules. For example, vascular cell adhesion molecule-1 (VCAM-1) was found to be expressed in the blood vessels within the mammary gland (27). Furthermore, inhibiting VCAM-1 using function-blocking antibodies led to decreased IgA secreted into the milk (52). Together, these results suggest that there are complex mechanisms involved in properly recruiting the immune cells important in transferring passive immunity to the newborn that are only beginning to be understood.
Involution
Overview
Following lactation, the mammary gland undergoes a dramatic remodeling program involving extensive apoptosis of the epithelial cells and remodeling of the stroma (53). This process results in a glandular structure similar to that observed in the mature virgin that is capable of repeating the pregnancy, lactation and involution cycle as needed. As described below, numerous immune cell types have been observed in the mammary gland during involution, particularly mast cells and macrophages. However, the mechanisms involved in recruiting these cell types and studies focused on these cell types have only recently begun to shed light on their ability to participate in the involution process.
Immune cell infiltration during involution
Gene expression profiling has suggested that both innate and adaptive immunity might be involved during mammary gland involution. Using microarray analysis, Stein et al. identified a set of genes specifically upregulated during the initial 4 days of involution (54). Further analysis of gene function revealed a set of genes associated with a wound-healing response, specifically neutrophil and macrophage activation. The chemokine CXCL1, which is a neutrophil chemoattractant, was induced on day 1 of involution. Induced at days 3 and 4 of involution were genes associated with macrophage chemoattraction and differentiation. Consistent with these observations, immunohistochemical analysis of mammary gland tissue during involution confirmed neutrophil infiltration at day 1 followed by a later infiltration of macrophages at days 3 and 4 of involution. Furthermore, a set of 49 immunoglobulins was upregulated and remained elevated in comparison with virgin animals, suggesting a B lymphocyte response. Although no specific increase in B-220+ B cells was detected, there was an increase in plasma cells observed on days 3 and 4 of involution. These studies clearly demonstrate that immune cell infiltration is a normal event during the involution process.
In a similar study, gene expression profiling of mammary glands during involution also revealed regulation of numerous immune-related genes during involution (49). The genes clustered into three separate groups, including inflammation, acute phase response and humoral immunity. Early cytokines that were induced included the neutrophil chemoattractant CXCL1, as found in the study described above. However, no evidence for increased neutrophil infiltration was found in this study and furthermore, several inhibitors of neutrophil recruitment and activation were found. Whether the discrepancies in the neutrophil results from these two studies were due to differences in mouse strain, or differences in timing in terms of when the tissues were examined for neutrophil recruitment is not known. Similar to the previous study, immunoglobulins and innate soluble defense factors were also found to be associated with involution. It has been hypothesized that in addition to participating in the process of involution, the increase in cytokines and acute phase response genes may be linked to inhibition of infection that could potentially accompany milk stasis following weaning (46, 49). Together, these studies provide a wealth of information that can be further evaluated to better understand the roles of immune cells and specific immune cell-associated genes in regulating mammary gland involution following weaning.
Mast cells
Recent studies have demonstrated that mast cells can be found in the involuting mammary gland (55). As described above, mast cells are known to produce high levels of numerous inflammatory mediators, including histamine, cytokines and proteases. Recent studies have shown that mast cells contribute to mammary gland involution by binding to plasma kallikrein, a plasminogen activator found at high levels during involution (55, 56). Previous studies have shown that lack of plasminogen leads to incomplete involution (56). Furthermore, plasma kallikrein in the mammary gland was found to be critical in the conversion of plasminogen to plasmin during involution (55). To determine the cell-type specificity of plasma kallikrein during involution, localization studies were performed. These studies found that active plasma kallikrein localized specifically to mast cells during involution, and was found to be situated in mast cell granules. Further studies demonstrated that plasma kallikrein was required for normal involution as inhibition of plasma kallikrein significantly delayed involution accompanied by decreased epithelial apoptosis, decreased collagen and altered adipose stroma. These studies demonstrate that a mast cell-derived factor is critical for mammary gland involution and provide evidence for a functional role for this immune cell during involution.
Macrophages
Another immune cell type that has been localized to the involuting mammary gland is the macrophage. Macrophage infiltration has been observed in the mammary gland at approximately 3–4 days of involution (54). Because macrophages are well-known for their ability to phagocytose apoptotic cells and debris, it had been assumed that macrophages were recruited to the mammary gland during involution to facilitate removal of dead or dying epithelial cells. However, recent studies have demonstrated that the epithelial cells themselves are primarily responsible for removal of apoptotic epithelial cells, by acting as amateur phagocytes [reviewed in (57)]. These observations suggest that macrophages may regulate other aspects of the involution process.
Recent studies have revealed insights into the potential mechanisms of macrophage recruitment and function during involution (29). Initially, studies were performed to determine whether macrophage infiltration was found during involution in species other than mice. Macrophages were indeed found to be present during involution in mammary gland tissue of both rats and humans, demonstrating that macrophage infiltration during involution is not limited to the mouse model (29). Further studies revealed that the macrophages associated with the involuting lobules expressed Arginase I, which is a marker consistent with M2-type macrophages. Interestingly, M2 macrophages are the macrophages typically associated with the remodeling phase of the inflammatory response, involving tissue remodeling. This observation is consistent with the possibility that macrophages are associated with remodeling of the mammary gland during the involution process. Macrophages were localized to specific involuting lobules, rather than distributed equally throughout the mammary gland, suggesting that the involuting lobules themselves are recruiting the macrophages. Previous work has demonstrated that collagen fragments are a potent macrophage chemoattractant (58). Therefore, it has been hypothesized that as the collagen surrounding the involuting lobules is proteolyzed, the fragments then recruit the macrophages to stromal region surrounding the lobules (29). The results from these studies raise a number of interesting questions that warrant further investigation, such as the ability of the microenvironment to recruit macrophages during involution and the functional role of macrophages during the involution process.
Conclusions
In summary, immune cells are clearly a normal component of the mammary gland stroma during all stages of post-natal mammary gland development and recent studies have begun to provide some insights regarding immune cell function during these stages. Furthermore, immune cell mediators that are regulated throughout mammary gland development are likely to have pleiotropic effects on epithelial cells, infiltrating immune cells and other stromal cell types. Further utilization of genetic mouse models that modulate the levels of immune cells and their mediators will continue to provide critical information regarding the mechanisms by which these cells function in the mammary gland. Overall, it is clear that mammary gland development relies on complex interactions between the epithelial cells and the cells within the stroma, including immune cells, which are only beginning to be understood.
Table 1.
Summary of the mouse models described in this review that have been used to understand immune cell function in the mammary gland.
| Mouse Model | Wild Type Gene Function | Phenotype | Cell Tye Affected |
Reference |
|---|---|---|---|---|
| KitW-sh | Kit is a tyrosine kinase receptor expressed on the surface of hematopoietic stem cells that normally bind cytokines |
|
Mast Cell Deficient | (25) |
| DPP1−/− (dipeptidyl peptidase 1) | DPP1, also known as cathepsin C, activates serine proteases in immune cells |
|
Mast Cell Deficient | (25) |
| Csf1op/op (colony stimulating factor-1) | CSF1 is a secreted cytokine that binds to hematopoietic cells and stimulates their differentiation into macrophages |
|
Macrophage Deficient | (36, 38) |
| Eotaxin−/− | Eotaxin is a secreted cytokine that actively recruits eosinophils and is therefore often involved in allergic response |
|
Decreased Eosinophil Recruitment | (36, 41) |
| IL-5−/− | Interleukin 5 is produced by Th2 cells and mast cells and it acts as a key mediator of eosinophil activation |
|
Decreased Eosinophils | (42) |
| IL-5Tg | Interleukin 5 is produced by Th2 cells and mast cells and it acts as a key mediator of eosinophil activation. |
|
Increased Eosinophils | (20) |
| Stat6−/− | STAT6 is a transcription factor that translocates to the nucleus to activate gene transcription in response to cytokine and growth factor signaling. |
|
Mammary Epithelial Cell Defects, Impaired Generation of Th2 Cells | (47) |
| IL-4−/−/IL-13−/− | IL-4 induces differentiation of naïve helper T cells to Th2 cells. IL-13 stimulates secretion of immunoglobulins from active B cells and is a key effector of inflammatory allergic response. |
|
Mammary Epithelial Cell Differentiation, Impaired Development of Th2 Cells and Th2 Associated Cytokines | (47) |
Acknowledgements
The authors would like to thank Dr. Jodi Goldberg for critical reading of this manuscript. JRR is supported by a pre-doctoral fellowship on the T32 CA009138 Cancer Biology Training Grant.
Abbreviations
- TGFβ
transforming growth factor β
- TNFα
tumor necrosis factor α
- IL
interleukin
- PMN
polymorphonuclear leukocytes
- TGFα
transforming growth factor α
- EGF
epidermal growth factor
- Th1
T helper cell 1
- Th2
T helper cell 2
- IFNγ
interferon-γ
- TEB
terminal end bud
- CLA
conjugated linoleic acid
- Csf1
colony-stimulating factor 1
- MMTV
mouse mammary tumor virus
- Tg
transgenic
- NGF
nerve growth factor
- CSF-1R
colony stimulating factor 1 receptor
- IgA
immunoglobulin A
- VCAM-1
vascular cell adhesion molecule-1
References
- 1.Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135(6):995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 2.Regan MC, Kirk SJ, Wasserkrug HL, Barbul A. The wound environment as a regulator of fibroblast phenotype. J Surg Res. 1991;50(5):442–448. doi: 10.1016/0022-4804(91)90022-e. [DOI] [PubMed] [Google Scholar]
- 3.Adamson R. Role of macrophages in normal wound healing: an overview. J Wound Care. 2009;18(8):349–351. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 5.Glaros T, Larsen M, Li L. Macrophages and fibroblasts during inflammation, tissue damage and organ injury. Front Biosci. 2009;14:3988–3993. doi: 10.2741/3506. [DOI] [PubMed] [Google Scholar]
- 6.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187(5A):11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40(11):1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 11.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2) Suppl 2:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, et al. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108(8):1151–1158. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 15.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 16.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182(4):1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mould AW, Matthaei KI, Young IG, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99(5):1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothenberg ME. Eotaxin. An essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol. 1999;21(3):291–295. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- 19.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79(12):3101–3109. [PubMed] [Google Scholar]
- 20.Sferruzzi-Perri AN, Robertson SA, Dent LA. Interleukin-5 transgene expression and eosinophilia are associated with retarded mammary gland development in mice. Biol Reprod. 2003;69(1):224–233. doi: 10.1095/biolreprod.102.010611. [DOI] [PubMed] [Google Scholar]
- 21.Ohno I, Lea RG, Flanders KC, Clark DA, Banwatt D, Dolovich J, et al. Eosinophils in chronically inflamed human upper airway tissues express transforming growth factor beta 1 gene (TGF beta 1) J Clin Invest. 1992;89(5):1662–1668. doi: 10.1172/JCI115764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todd R, Donoff BR, Chiang T, Chou MY, Elovic A, Gallagher GT, et al. The eosinophil as a cellular source of transforming growth factor alpha in healing cutaneous wounds. Am J Pathol. 1991;138(6):1307–1313. [PMC free article] [PubMed] [Google Scholar]
- 23.Teller P, White TK. The physiology of wound healing: injury through maturation. Surg Clin North Am. 2009;89(3):599–610. doi: 10.1016/j.suc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127(11):2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 25.Lilla JN, Werb Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev Biol. 2010;337(1):124–133. doi: 10.1016/j.ydbio.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollard JW, Hennighausen L. Colony stimulating factor 1 is required for mammary gland development during pregnancy. Proc Natl Acad Sci U S A. 1994;91(20):9312–9316. doi: 10.1073/pnas.91.20.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourges D, Meurens F, Berri M, Chevaleyre C, Zanello G, Levast B, et al. New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Mol Immunol. 2008;45(12):3354–3362. doi: 10.1016/j.molimm.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Weisz-Carrington P, Roux ME, Lamm ME. Plasma cells and epithelial immunoglobulins in the mouse mammary gland during pregnancy and lactation. J Immunol. 1977;119(4):1306–1307. [PubMed] [Google Scholar]
- 29.O'Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176(3):1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien J, Schedin P. Macrophages in breast cancer: do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia. 2009;14(2):145–157. doi: 10.1007/s10911-009-9118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harkonen PL, Vaananen HK. Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann N Y Acad Sci. 2006;1089:218–227. doi: 10.1196/annals.1386.045. [DOI] [PubMed] [Google Scholar]
- 32.Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009;17(1):42–50. doi: 10.1111/j.1524-475X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 33.De M, Wood GW. Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol. 1990;126(3):417–424. doi: 10.1677/joe.0.1260417. [DOI] [PubMed] [Google Scholar]
- 34.Russell JS, McGee SO, Ip MM, Kuhlmann D, Masso-Welch PA. Conjugated linoleic acid induces mast cell recruitment during mouse mammary gland stromal remodeling. J Nutr. 2007;137(5):1200–1207. doi: 10.1093/jn/137.5.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masso-Welch PA, Zangani D, Ip C, Vaughan MM, Shoemaker S, Ramirez RA, et al. Inhibition of angiogenesis by the cancer chemopreventive agent conjugated linoleic acid. Cancer Res. 2002;62(15):4383–4389. [PubMed] [Google Scholar]
- 36.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4(4):155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14(11):628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7(2):147–162. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 39.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235(12):3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 40.Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ, Visvader JE. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 2009;11(4):R62. doi: 10.1186/bcr2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185(4):785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colbert DC, McGarry MP, O'Neill K, Lee NA, Lee JJ. Decreased size and survival of weanling mice in litters of IL-5−/ −mice are a consequence of the IL-5 deficiency in nursing dams. Contemp Top Lab Anim Sci. 2005;44(3):53–55. [PubMed] [Google Scholar]
- 43.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172(5):1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniel CW, Robinson S, Silberstein GB. The transforming growth factors beta in development and functional differentiation of the mouse mammary gland. Adv Exp Med Biol. 2001;501:61–70. doi: 10.1007/978-1-4615-1371-1_7. [DOI] [PubMed] [Google Scholar]
- 45.Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200(6):805–809. doi: 10.1084/jem.20041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson CJ. Immune cell regulators in mouse mammary development and involution. J Anim Sci. 2009;87(13 Suppl):35–42. doi: 10.2527/jas.2008-1333. [DOI] [PubMed] [Google Scholar]
- 47.Khaled WT, Read EK, Nicholson SE, Baxter FO, Brennan AJ, Came PJ, et al. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development. 2007;134(15):2739–2750. doi: 10.1242/dev.003194. [DOI] [PubMed] [Google Scholar]
- 48.Baratta M, Motta M, Accornero P. Leptin reduces the inhibitory effect of IL-1 beta on beta-casein gene expression in differentiated mammary cells. Vet Res Commun. 2005;29(Suppl 2):153–155. doi: 10.1007/s11259-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 49.Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6(2):R92–R109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4(3):313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 51.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1(1):11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 52.Low EN, Zagieboylo L, Martino B, Wilson E. IgA ASC accumulation to the lactating mammary gland is dependent on VCAM-1 and alpha4 integrins. Mol Immunol. 2010;47(7–8):1608–1612. doi: 10.1016/j.molimm.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115(1):49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- 54.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6(2):R75–R91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lilla JN, Joshi RV, Craik CS, Werb Z. Active plasma kallikrein localizes to mast cells and regulates epithelial cell apoptosis, adipocyte differentiation, and stromal remodeling during mammary gland involution. J Biol Chem. 2009;284(20):13792–13803. doi: 10.1074/jbc.M900508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lund LR, Bjorn SF, Sternlicht MD, Nielsen BS, Solberg H, Usher PA, et al. Lactational competence and involution of the mouse mammary gland require plasminogen. Development. 2000;127(20):4481–4492. doi: 10.1242/dev.127.20.4481. [DOI] [PubMed] [Google Scholar]
- 57.Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod. 2008;78(4):586–594. doi: 10.1095/biolreprod.107.065045. [DOI] [PubMed] [Google Scholar]
- 58.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]