Abstract
Opioid therapy offers the promise of reducing the burden of chronic pain in not just individual patients, but among the broad population of patients with chronic pain. Randomized trials have demonstrated that opioid therapy for up to 12–16 weeks is superior to placebo, but have not addressed longer term use. In the US, opioid sales have quadrupled during 2000–2010 with parallel increases in opioid accidental overdose deaths substance abuse admissions. Clinical use of long-term opioid therapy is characterized by a pattern of adverse selection, where high-risk patients are prescribed high-risk opioid regimens. This adverse selection may link these trends in use, abuse and overdose. Long-term opioid therapy appears to be associated with iatrogenic harm to the patients who receive the prescriptions and to the general population. The US has conducted an experiment of population-wide treatment of chronic pain with long-term opioid therapy. The benefits have been hard to demonstrate, but the harms are now well demonstrated.
Introduction: the promise of opioid therapy
“Among the remedies which it has pleased Almighty God to give to man to relieve his sufferings, none is so universal and so efficacious as opium.
” --Thomas Sydenham (1692)
In the past few decades, we have witnessed the success of the ethical argument for pain treatment. This success was first accomplished by the hospice and palliative care community with the argument that dying in pain is ethically unacceptable. Arguments about turning dying patients into addicts were definitively and universally rejected. Even if pain treatment hastens death, this treatment is now considered ethically appropriate if the patient or authorized proxies choose to receive it [31]. In subsequent years, the ethical mandate for pain relief as basic care has been extended from end of life to all cancer pain. In 1985 and in 1996, the World Health Organization (WHO) issued guidelines on cancer pain relief [76]. Moderate to severe pain is common in cancer patients, and unfortunately many patients still do not get adequate relief [13].
This ethical mandate for pain treatment has recently been extended to chronic non-cancer pain [12]. We have previously made the argument that chronic non-cancer pain produces suffering and disability at least equal to that of cancer pain, but with higher prevalence and longer duration. (Sullivan and Farrell, 2005) [64]. In 2010, the IASP issued the Declaration of Montreal, which argued for “access to pain management without discrimination… on the basis of age, sex, gender, medical diagnosis, race or ethnicity, religion, culture, marital, civil or socioeconomic status, sexual orientation, and political or other opinion [39].” Prohibition of discrimination on the basis of “medical diagnosis” is meant to prohibit favoring cancer pain treatment over non-cancer pain treatment. In 2011, the Institute of Medicine in the United States issued a report on Relieving Pain in America [38]. In this report, prevalent pain is considered to be undertreated pain. This suggests that all pain justifies treatment, regardless of origin.
The above arguments for pain management as a fundamental human right have addressed pain treatment broadly. Indeed, some of them have specifically mentioned that this does not imply a universal right to receive opioid therapy. There has been a separate and more specific ethical argument made for opioid treatment. In 1986, Portenoy and Foley published a review of 38 cases of chronic non-cancer pain successfully treated with opioids [55]. In this article, they argue that “opioid maintenance therapy can be a safe, salutary and more humane alternative… in those patients with intractable non-malignant pain and no history of drug abuse.” In more recent years, these authors have acknowledged problems associated with widespread prescribing of opioids for non-cancer pain, but have reiterated their support for the practice [54]. For example, they have resisted opioid dosing guidelines that mandate specialist review if a patient exceeds a specific dose without clear improvement in pain and function. In 2011, Foley et al stated: “We disagree with the concept of setting a maximum dose. The pharmacology of opioid use in the treatment of pain is based on dose titration to effect.” Other authors have made a broader recommendation for opioids, recommending a trial of opioids for anyone with severe pain.[72] These statements are consistent with a strong sense of responsibility to relieve pain and suffering that has its roots in palliative care. What we might call “the opioid interpretation of the duty to relieve pain and suffering” was articulated most clearly by Sidney Wanzer in the New England Journal of Medicine in 1989: “The proper dose of pain medication is the dose that is sufficient to relieve pain and suffering… To allow a patient to experience unbearable pain or suffering is unethical medical practice [69].” This statement was meant to be applied in the palliative care context, but has since been applied much more broadly.
Rapid escalation in prescription opioid use, abuse, and overdose
Opioid use varies over a thousand-fold across different countries, from over 10mg morphine per capita in the most developed countries to less than 0.01mg per capita in the least developed countries. Beginning in the mid-1980’s prescription opioid sales began to escalate in the US. Global consumption also rose significantly, though this was driven almost entirely by increased use in the US. Figure 1 is reproduced from the University of Wisconsin Pain and Policy Studies Group. We have added selected events associated with increased opioid prescribing. In 1986, the article by Portenoy and Foley suggesting opioid treatment of chronic non-cancer pain was published. In 1996, the American Academy of Pain Medicine and the American Pain Society issued a consensus statement on The Use of Opioids for the Treatment of Chronic Pain which argued that opioids should have a role in the treatment of patients with chronic non-cancer pain [1]. It included statements such as, “Studies indicate that the de novo development of addiction when opioids are used for the relief of pain is low.” This was followed in many states by the passage of “Intractable Pain Acts” which removed sanctions for prescription of long-term and high-dose opioid therapy that had previously been in place [32]. Through the late 1990’s and early 2000’s, sustained release opioids were aggressively marketed as less prone to abuse than other opioids. In 2007, Purdue Pharma paid a $634.5 million fine for claiming the Oxycontin was less addictive and less subject to abuse than other pain medications [2].
Figure 1.
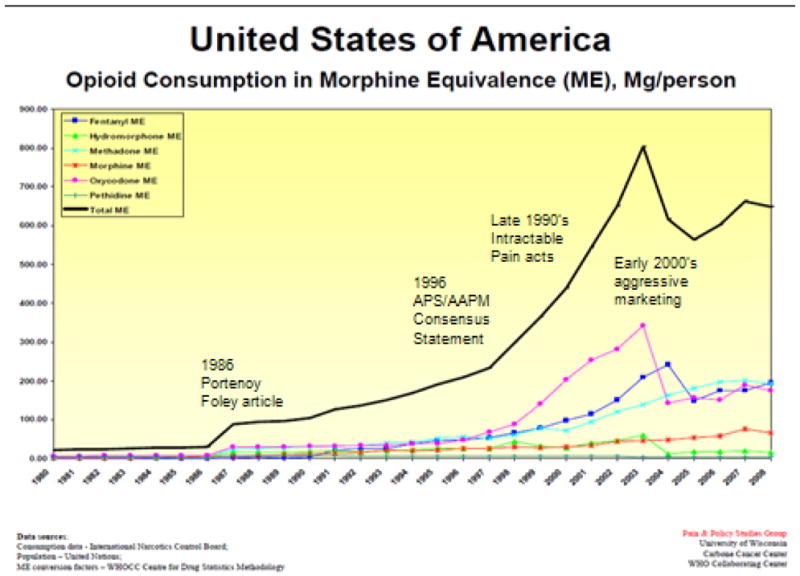
Opioid consumption in USA
Figure 2 is a graph created by the Centers for Disease Control and Prevention in the US which demonstrates parallel increases in opioid sales, deaths, and substance abuse admissions. Opioid sales have quadrupled during the 2000–2010 period. This is the most recent national data available. Deaths from accidental opioid overdose have also increased. These now exceed motor vehicle deaths in 30 states.
Figure 2.
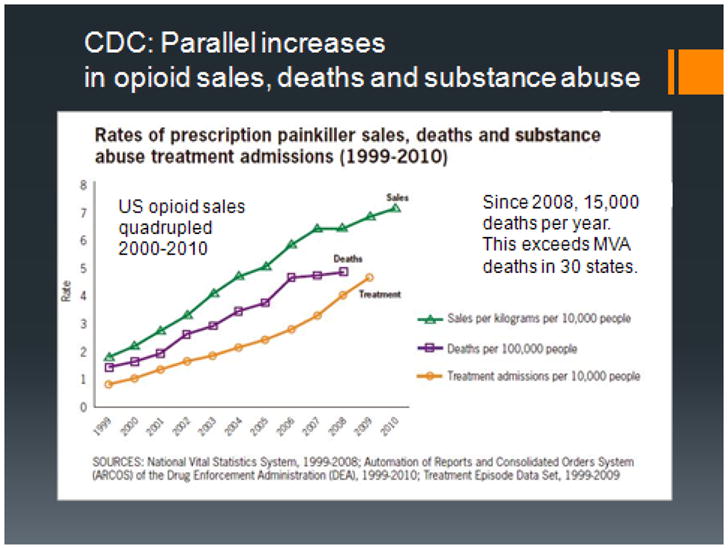
prescription opioid sales, deaths and substance abuse admissions in US
Opioid efficacy for chronic non-cancer pain: short-term and long-term studies
The ethical argument that patients with chronic pain deserve opioid treatment, is not complete without proof that opioid treatment can provide lasting pain relief. Several systematic reviews in recent years have summarized the results of RCTs on the efficacy of opioid therapy for chronic non-cancer pain [3; 30; 41; 43]. These RCTs were generally less than 16 weeks in duration, although 6 trials included in the review by Kalso et al had an open label follow-up of 6 to 24 months. Overall, opioids show efficacy over the usual 12-week duration of trials with pain intensity reduced approximately 30% more than placebo, with variable effects on function. Often, function of a body part (e.g., grip strength) was assessed rather than the ability of the whole person to perform daily tasks. Furthermore, pain relief did not always produce the expected effect on function. For example, the Furlan et al meta-analysis concluded that whereas strong opioids in general provided better pain relief than weak opioids or nonopioids, function was improved by weak opioids and nonopioids, but not strong opioids.[30] This is one demonstration of the complicated relationship between pain relief and functional improvement. These trials generally excluded all patients with mental health or substance use disorders. They also used enriched designs (e.g., excluding patients who responded to placebo) in order to increase the chance of showing opioid effectiveness. Some randomized trials of opioids allowed open label follow-up, but the small self-selected group of patients that went on to long-term opioid management do not permit any clear conclusion on the long-term effects of opioids. Most patients stopped opioids due to lack of efficacy or side effects, though the minority of patients who continued opioids did report significant pain relief. In a systematic review of opioid treatment of chronic low back pain, Martell et al concluded that “the evidence in favor of opioids (for CLBP) is not always consistent, and when supportive, only supports this treatment for short periods. [44]” In summary, randomized controlled trials have demonstrated analgesic efficacy of short-term, but not long-term, opioid treatment of chronic pain. We have assumed that the evidence of efficacy from these short-term trials can be extrapolated to long-term use, but this may not be the case.
It is not possible to randomize patients to years of opioid therapy, but it has been possible to prospectively study inception cohorts of opioid-treated injured workers. Both these injured workers, and their providers alike, have hoped that opioid treatment may facilitate rehabilitation and return to work. But now seven prospective studies suggest that this is not true. In each case, opioid treated workers were less likely to return to work than those who did not receive opioids. Opioid therapy retards return to work for injured workers in dose-dependent manner [11; 16; 29; 68; 71; 75]. It is important to be cautious in interpreting these non-randomized studies, because the patients who receive opioids are not the same patients as those who do not receive opioid. Those who receive opioids tend to have higher pain levels, greater activity interference, and higher levels of depression and anxiety. However, these trials have attempted to control for these baseline differences and a significant disability-promoting effect of opioids persists. There is some suggestion from other prospective cohort studies that opioid therapy may prolong pain and retard functional recovery. In the Women’s Health Initiative Observational Cohort study of post-menopausal women, opioid therapy at baseline was associated with lack of pain improvement and worse physical functioning over 3 years of follow-up after controlling for demographic and clinical differences women [10]. A Danish cohort study has shown decreased rates of recovery from chronic pain for patients treated with opioids.[63] Danish population-based cross-sectional studies have also shown an associate of opioid use with reporting of moderate/severe or very severe pain, poor self-rated health, unemployment, high health care utilization, and low quality of life.[23] These observational studies must be interpreted cautiously, but they suggest that extrapolating from proof of the efficacy of short-term opioid therapy to efficacy of long term opioid therapy may not be valid.
Risks of opioid addiction, abuse and misuse associated with opioid therapy
Early estimates on the addiction risk associated with opioid therapy were based on inpatient samples and outpatient samples from randomized trials which excluded patients with mental health and substance abuse disorders, now known to be the patients most likely to receive and continue opioids in clinical practice. Porter and Jick in 1980 gave initial estimates of opioid addiction at 0.05% and abuse 0.4% based upon patients treated with short-term opioid therapy while in-patients [56]. More recent estimates are highly variable due to differing definitions of opioid addiction and the populations studied. In 2008, Fishbain et al conducted a systematic review of 67 studies, and found that the rates of opioid abuse or addiction varied between 0.2% and 3.27%, and that the overall rates of aberrant drug-related behavior were approximately 15–20% [25]. When interpreting abuse rates, it is important to monitor the nature of the population sampled. In many of the above studies, patients with pre-existing substance use or mental health disorders were excluded. In studies without these exclusions, abuse rates tend to be much higher. For example, Chelminsky et al reported the rate of substance misuse to be at 32% among the patients enrolled in a primary care, multi-disciplinary pain management program [14]. Fleming et al estimated the rate of lifetime aberrant drug behavior to be at 80% among patients who receive opioids for chronic pain in primary care setting [26]. These higher abuse rates are likely closer to those seen in clinical practice. The pharmacology of opioids has been extensively studied and the search for non-addictive opioids has been long and fruitless. So we should not be surprised that addiction rates are higher than we had hoped.
Careful selection vs. adverse selection of patients for long-term opioid therapy
Opioid treatment guidelines emphasize careful risk assessment and selection of low-risk patients for long-term opioid therapy (LtOT). Generally, LtOT is recommended only for patients with intractable pain and no history of substance abuse. Caution is urged for those with a history of mental health disorder [15]. Contrary to the recommendations found in opioid treatment guidelines, studies of actual clinical practice reveal that patients with substance abuse or mental health disorders are in fact more likely to receive LtOT. We have named the process by which high risk patients are placed on high risk opioid regimens, “adverse selection.” The data documenting this process of adverse selection comes from two pharmaco-epidemiological studies completed in the last decade that have examined the trends in opioid therapy and how psychiatric disorders affect these trends. The TROUP study (Trends and Risks of Opioid Use for Pain) analyzed claims data from 2000 through 2005 on two disparate populations: a national, commercially insured population (HealthCore) and Arkansas Medicaid enrollees. The CONSORT study (CONsortium to Study Opioid Risks and Trends) looked at claims data from two HMOs, Group Health in Washington State and Kaiser Permanente of Northern California, between 1997 and 2005. Both studies used chart diagnosis to assess for the presence of chronic pain conditions, as well as psychiatric disorders, and defined LtOT as greater than 90 days of opioid use. The CONSORT study also included a one-time telephone survey of eligible participants.
The TROUP study found that in both the commercially insured and publicly insured populations, rates of non-cancer chronic pain diagnosis and opioid use increased linearly during the 6-year study period. The increase in cumulative yearly opioid dose received by patients who received chronic pain diagnoses was due to increases in the number of days of opioids supplied rather than the dose per day supplied. This indicates that opioid therapy for non-cancer pain increased in duration but not intensity on average over this study period [66]. In fact, the overall growth in opioid use appears to be due to an increase in long-term high-dose use, rather than a change in the dose received by the modal patient. In fact, opioid use by patients with chronic pain diagnoses is highly concentrated in a small proportion of this population. In our commercially insured sample, 5% of patients with chronic pain accounted for 70% total opioid consumption in 2005, while in our Medicaid sample the top 5% of patients with chronic pain accounted for 48% of total opioid use. Patients with substance use or mental health disorder diagnoses were over represented in this top 5% of opioid users [21]. This concentration of substance use and mental health disorders in high-dose LtOT users has been found in other studies as well [42; 49]. Between 2000 and 2005, the prevalence of LtOT in the samples studied in TROUP started higher and grew faster among patients with mental health or substance use problems compared to those without such a diagnosis [20].
The CONSORT study also identified a linkage between common mental health and substance use disorders and increased likelihood of receiving long-term opioid therapy. For example, a chart diagnosis of depression over the previous two years was associated with three times higher likelihood of receiving long-term opioids, a higher average daily dose, greater days supplied, and more use of DEA Schedule II opioids [9]. The CONSORT study also found that patients with a prior substance abuse diagnosis were more likely to receive long-term opioid therapy, to receive higher opioid dosages and to receive DEA Schedule II opioids [73]. Independent studies of veteran populations have also found a strong association between mental health disorders and opioid use [62]. Among Iraq and Afghanistan veterans with pain, 6% of veterans without mental health disorders, 12% with non-Post-Traumatic Stress Disorder (PTSD) mental health disorders, and 18% with PTSD were prescribed opioids within a year of their pain diagnosis. Among those prescribed opioids, patients with PTSD were more likely than those without mental health disorders to receive higher daily opioid dose, receive two or more opioids, and receive sedative hypnotics concurrently. This study confirms that adverse selection also occurs in the VA system where there is generally more access to mental health and addiction services.
In summary, “adverse selection” describes a process where the likelihood of a patient receiving an opioid regimen increases as the associated risks increase. For example, a history of depression or other common mental health disorders increases the likelihood of receiving LtOT by 3–4 fold, a history of alcohol or non-opioid drug abuse increases the likelihood by 4–5 times, and a history of opioid abuse or dependence makes it 5–10 times more likely to receive LtOT. Furthermore, these high risk patients with substance abuse and mental health disorders are more likely to receive high risk opioid regimens involving high opioid daily doses, high potency Drug Enforcement Agency Schedule II opioids (opioids with medical use but high risk for abuse) and concurrent sedative-hypnotic medications [61].
Adverse selection occurs not only at the initiation of opioid therapy, but also in patients’ decisions about continuing LtOT. Despite the rapidly increasing rates of LtOT for chronic non-cancer pain, the vast majority of opioid therapy is short-term. Most patients discontinue therapy before reaching 90 days of therapy, either due to side-effects or lack of efficacy [30; 50; 59]. The TROUP study asked the question of who stays on opioid therapy, and found that once 90 days of continuous therapy is received, two thirds of the patient will remain on opioids years later [45]. This suggests that LtOT may become a self-perpetuating or lifelong therapy. LtOT continuation is predicted by high daily opioid dose (>120mg morphine equivalent) and opioid misuse. Other prospective studies have shown similar findings [28; 67]. These data suggest that the population of patients on LtOT is progressively enriched with high-risk patients.
Medical harms associated with long-term opioid therapy
While the benefits of LtOT have been hard to demonstrate on a population basis, the harms are well documented. The most significant harm associated with opioid therapy is death from accidental overdose. There were approximately 15,000 prescription opioid overdose deaths in the Unites States in 2008, which was three times the rate in 1999. Demographic groups at the highest risk are men, middle-aged, rural, poor, whites and Native Americans. An investigation of 295 opioid deaths in West Virginia in 2006 suggested that bad patient behaviors, such diversion and doctor shopping, caused the deaths [35]. This suggested that the most important source of risk was the patients, not the drugs. But multiple studies have now shown that accidental overdose and death increase in proportion to the prescribed opioid dose, with a significant increases at doses greater than 100mg morphine equivalent dose (MED) per day [6; 17; 33; 52]. These studies tie opioid risk to prescribed dose and thus point to the inherent dangerousness of opioids.
The Center for Disease Control and Prevention reports half a million emergency department (ED) visits in 2009 due to misuse of opioids. Opioids are now involved in 40% of all drug-related ED visits. Among patients prescribed long-term opioids, 20–30% make an ED visit per year. These visits are especially likely in patients with a substance use or mental health disorder, and those receiving short-acting Schedule II opioids [8]. Visits for alcohol and drug problems among recipients of Lt OT are also increased in those with substance use and mental health disorders, but these are associated with long-acting Schedule II opioids. This is likely due to the increased overdose risk with the long-acting opioids.
Opioids pose special risks to the oldest and youngest patients. For patients age 60 and older, LtOT at 50mg or greater MED doubles the risk of fractures [60]. With the growth of opioid therapy among women of childbearing age, the rates of neonatal abstinence syndrome increased 2.8 times from 2000 to 2009 [51]. This was associated with increased rates of low birth weight, respiratory complications, and costs, especially in patients receiving Medicaid. For women who are on LtOT that become pregnant, clinicians face the dilemma of potentially precipitating premature labor by withdrawing opioid therapy or inducing neonatal abstinence syndrome by continuing opioid therapy. Adolescents have relatively low rates of LtOT, but high rates of opioid misuse and abuse. One contributing factor may be that adolescents with chronic pain who also have mental health disorders are nearly 3 times more likely to progress from short-term to long-term opioid use.[58]
Behavioral harms associated with long-term opioid therapy
Research aiming to quantify the risks for opioid misuse and abuse posed by prescription of opioid therapy is underway. We developed administrative criteria for opioid misuse consisting of excess days supplied, multiple opioid prescribers, and multiple opioid pharmacies. We were able to show that a misuse score based on these was linearly related to the risk of receiving an opioid abuse diagnosis. We estimated possible misuse at 20–24% and probable misuse at 3–6% of LtOT recipients. Younger age, back pain, multiple pain complaints and substance abuse disorders identify patients at high risk for misuse. Treatment with high daily dose opioids (especially >120 mg MED per day) and short-acting Schedule II opioids appears to increase the risk of misuse. This is contrary to the “pseudoaddiction” concept which postulates that aberrant opioid behaviors will disappear once a dose adequate to treat the pain problem has been reached.
We have shown in research reviewed above that patients with documented opioid abuse are more likely to be placed on LtOT. We have also shown that LtOT is associated with increased risk of receiving a new chart diagnosis of Opioid Abuse or Opioid Dependence. The likelihood of receiving these diagnoses is increased in high risk patients characterized by: lower age, and previous substance abuse or mental health disorders. It is also increased in patients who receive high risk treatments including : more days supplied of opioids, higher daily opioid doses, and DEA Schedule II opioids [19]. When we studied patients with new episodes of chronic pain, we were able to determine that chronic high dose use (>90 days) poses much more risk of being diagnosed with opioid abuse or dependence than acute high-dose use. Compared to those whose chronic pain episode was not treated with opioids, those treated with acute high-dose use had 3 times the risk of opioid abuse, while those with chronic high-dose use has 107 times the risk [18]. Unfortunately, as prescription opioids become harder to obtain and the street price increases, some individuals who were taking prescription opioids are switching to heroin. In Seattle, 40% of patients using heroin report first “getting hooked on” prescription opioids [53].
Increased prescription of opioids holds risks for the public beyond those experienced by the patients themselves. Rates of opioid misuse and abuse in the general public have also increased rapidly in the past decade. The National Survey on Drug Use and Health reports that 5% of the general population admitted to “non-medical” use of prescription opioids in 2010. This includes all use of the medication by someone other than the prescription recipient or for a purpose other than that for which it was prescribed [46]. The majority of these non-medical users obtained their opioids from friends or family, not from drug dealers or the internet. By 2006, there were more new initiators of prescription opioids than any other drug, including marijuana.
Understanding unintended consequences of opioid therapy: endogenous opioids, physical pain and social pain
The endogenous opioid system serves many other functions besides the stress-induced analgesia for which it is most well-known. These include: maternal-infant bonding, reward associated with alcohol use, sexual reward and sex drive, mood regulation, social status and stress, as well as eating, drinking, and other appetitive behaviors [5]. It is to be expected that long-term use of exogenous opioids would affect these other functions as well. For example, an intact endogenous opioid system is essential for normal maternal-infant bonding. Mice lacking the mu-opioid receptor gene (OPRM1) do not show normal attachment behaviors with little evidence of distress with separation from their mothers [48]. On the other hand, early postnatal maternal separation increases the rewarding value of morphine in genetically normal rat pups [47]. The opioid system is also involved in regulating social affiliative behaviors. Opioid agonists have been found to reduce efforts at social affiliation in mammals [36], while opioid antagonists increase efforts at social affiliation [24]. These findings suggest that opioids substitute for the rewards of social connection, and may explain the epidemiological association between emotional distress and the likelihood and receiving chronic opioid therapy.
Our research has focused on the role that substance use and mental health disorders have played in the use and abuse of prescription opioids. Much of the literature on opioid abuse distinguishes between legitimate opioid use for physical pain and illegitimate opioid abuse to produce euphoria or relieve emotional pain. But there is now a large body of evidence suggesting that physical and emotional pain states share many of the same neurobiological substrates. Neuroimaging studies have found that emotional distress associated with social disconnection in primates (i.e., rejection, humiliation, isolation) may arise from some of the same brain structures that underlie experiences of physical pain such as the dorsal anterior cingulate cortex and the anterior insula [22]. These areas have a high density of mu-opioid receptors [4]. Eisenberger has hypothesized that the primate social attachment system has “borrowed” the system evolved in simpler mammals for physical pain. This system makes social separation immediately aversive for the primate, thus promoting survival for species where social cooperation is essential.
The promises and perils of long-term opioid therapy for chronic pain: results from the US experiment
On the basis of the above, it is fair to say that the promises of general safety and effectiveness of long-term opioid therapy for chronic pain are unfulfilled. Clinical use of long-term opioid therapy is characterized by a pattern of adverse selection, where high-risk patients are prescribed high-risk opioid regimens. This pattern is unlikely to be reversed through renewed efforts at careful selection of opioid recipients, because opioids treat emotional as well as physical pain and the most distressed patients will always seek opioids most assiduously. Harms associated with long-term opioid therapy perhaps can be limited through focus on lower dose use, intermittent use, and avoiding use in younger patients [27]. There are patients who do well with improved function and quality of life on opioid therapy. These are often older adults with low dose intermittent use.[57] This evidence base is still evolving.
It is clear that the perils of long-term opioid therapy go beyond opioid addiction in patients prescribed these medications to a broader set of iatrogenic harms. If we borrow the framework for iatrogenesis used by Ivan Illich in his Medical Nemesis, we can describe three types of harms, proceeding from the most well-defined to the least well-defined [37]. First, there is clinical iatrogenesis, including: overdose, abuse, and adverse effects in patients. Second, there is social iatrogenesis, including: escalating opioid diversion, abuse, overdose in adolescents and poor rural middle-aged adults. Third, there is cultural iatrogenesis, including: an erosion of our ability to manage pain in non-medical ways, and unrealistic expectations of relief. The US has conducted an experiment of population-wide treatment of chronic pain with long-term opioid therapy. The benefits have been hard to demonstrate, but the harms are now well demonstrated.
So where do we go from here? We need to consider those patients not yet on long-term opioid therapy and those patients already on long-term opioid therapy separately. We should be much more vigilant about letting patients drift into long-term opioid therapy without an explicit decision, including patient informed consent. It is unclear whether brief trials of opioid therapy for patients with chronic pain are informative, because patients often do well for a month or two and then need a dose increase to maintain these gains. We should be more cautious about placing patients on daily long-acting opioids simply because nothing else worked. This often results in the highest risk patients on the highest risk opioid regimens. It appears that lower dose intermittent opioid therapy is safer and adequate for many patients. The “Principles for more selective and cautious opioid prescribing” prepared by the faculty of the National Summit for Opioid Safety held in Seattle, Washington in November 2012 provide an excellent set of guidelines.[34] The newly expanded Washington State Guideline for Prescribing Opioids to Treat Pain in Injured Workers issued in July 2013 provide detailed guidance on use of opioids after a workplace injury.[70]
For patients who are already on long-term opioid therapy and not showing clear improvements in pain, function, and quality of life, other strategies are needed. Since long-term opioid therapy has no natural endpoint, once long-term opioid therapy is established, it can go on for years.[45] For patients who have opioid addiction, long-term opioid maintenance with buprenorphine or methadone will be necessary.[74] For patients without a current substance use disorder, a randomized trial is exploring whether opioid taper support will assist patients who wish to discontinue opioids.[65] A multimodal intervention for patients with opioid misuse has shown efficacy in a pilot study.[40] Multiple other studies for prescription opioid misuse and abuse are underway.[7] Though these efforts to limit the adverse outcomes from opioid therapy are welcome, we believe the best clinical practice is to be quite sure that the patient is showing robust and broad benefit (i.e., pain, mood, physical and social function) from opioid use before proceeding to long-term opioid therapy.
Table 1.
Possible reasons for the US “opioid epidemic”
| Physician-related | Inadequate and inaccurate training on opioid pharmacology and risks |
| Lack of access to multidisciplinary chronic pain care | |
| Ease of prescribing opioids compared to other chronic pain therapies | |
| Patient-related | Strong appeal of immediate pain relief provided by opioids |
| Focus on pain rather than psychological distress as a treatment target | |
| More value placed on pain relief than functional improvement | |
| Society and health-system related | Acceptance of right to pain treatment and interpretation of this right in terms of access to opioid therapy |
| Better insurance coverage for medications than for other chronic pain therapies | |
| Aggressive marketing of sustained-release opioids by pharmaceutical companies |
Footnotes
Conflict of interest statement:
Mark Sullivan has received educational grants from Pfizer, Covidien, and Endo. Catherine Howe has no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark D. Sullivan, University of Washington.
Catherine Q. Howe, University of Washington.
References
- 1.The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13(1):6–8. [PubMed] [Google Scholar]
- 2.Associated Press. Book Purdue Pharma, Execs to Pay $634.5 Million Fine in OxyContin Case. City: 2007. Purdue Pharma, Execs to Pay $634.5 Million Fine in OxyContin Case. http://www.cnbc.com/id/18591525. [Google Scholar]
- 3.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24(6):469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, Hohnemann S, Piel M, Rosch F, Wester HJ, Henriksen G, Stoeter P, Bartenstein P, Treede RD, Schreckenberger M. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30(3):692–699. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Bodnar RJ. Endogenous opiates and behavior: 2011. Peptides. 2012;38(2):463–522. doi: 10.1016/j.peptides.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 7.Boston Medical Center. Transforming Opioid Prescribing in Primary Care (TOPCARE) National Institute on Drug Abuse (NIDA); May, 2013. ClinicalTrials.gov Identifier: NCT01909076. http://clinicaltrialsgov/show/NCT01909076. [Google Scholar]
- 8.Braden J, Russo J, Fan M, Edlund M, Martin B, DeVries A, Sullivan M. Emergency Department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170(16):1425–1432. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31(6):564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braden JB, Young A, Sullivan MD, Walitt B, Lacroix AZ, Martin L. Predictors of change in pain and physical functioning among post-menopausal women with recurrent pain conditions in the women’s health initiative observational cohort. J Pain. 2012;13(1):64–72. doi: 10.1016/j.jpain.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brede E, Mayer TG, Gatchel RJ. Prediction of failure to retain work 1 year after interdisciplinary functional restoration in occupational injuries. Arch Phys Med Rehabil. 2012;93(2):268–274. doi: 10.1016/j.apmr.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105(1):205–221. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- 13.Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 14.Chelminski PR, Ives TJ, Felix KM, Prakken SD, Miller TM, Perhac JS, Malone RM, Bryant ME, DeWalt DA, Pignone MP. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity. BMC Health Serv Res. 2005;5(1):3. doi: 10.1186/1472-6963-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillie KS, Fleming MF, Mundt MP, French MT. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008;21(2):108–117. doi: 10.3122/jabfm.2008.02.070144. [DOI] [PubMed] [Google Scholar]
- 17.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edlund M, Martin B, Fan M, Devries A, Braden J, MDS The Role of Opioid Prescription in Incident Opioid Abuse and Dependence Among Individuals with Chronic Non-Cancer Pain: The Role of Opioid Prescription. Clin J Pain. doi: 10.1097/AJP.0000000000000021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edlund M, Martin B, Fan M, DeVries A, Braden J, Sullivan M. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP Study. Drug Alcohol Depend. 2010;112:90–98. doi: 10.1016/j.drugalcdep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edlund MJ, Martin BC, Fan MY, Braden JB, Devries A, Sullivan MD. An analysis of heavy utilizers of opioids for chronic noncancer pain in the TROUP Study. J Pain Symptom Manage. 2010 doi: 10.1016/j.jpainsymman.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13(6):421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 23.Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1–2):172–179. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Fabre-Nys C, Meller RE, Keverne EB. Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacol Biochem Behav. 1982;16(4):653–659. doi: 10.1016/0091-3057(82)90432-4. [DOI] [PubMed] [Google Scholar]
- 25.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9(4):444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 26.Fleming MF, Davis J, Passik SD. Reported lifetime aberrant drug-taking behaviors are predictive of current substance use and mental health problems in primary care patients. Pain Med. 2008;9(8):1098–1106. doi: 10.1111/j.1526-4637.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55(4):325–331. doi: 10.1002/ajim.21998. [DOI] [PubMed] [Google Scholar]
- 28.Franklin GM, Rahman EA, Turner JA, Daniell WE, Fulton-Kehoe D. Opioid use for chronic low back pain: A prospective, population-based study among injured workers in Washington state, 2002–2005. Clin J Pain. 2009;25(9):743–751. doi: 10.1097/AJP.0b013e3181b01710. [DOI] [PubMed] [Google Scholar]
- 29.Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine (Phila Pa 1976) 2008;33(2):199–204. doi: 10.1097/BRS.0b013e318160455c. [DOI] [PubMed] [Google Scholar]
- 30.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174(11):1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavrin JR. Ethical considerations at the end of life in the intensive care unit. Crit Care Med. 2007;35(2 Suppl):S85–94. doi: 10.1097/01.CCM.0000252909.52316.27. [DOI] [PubMed] [Google Scholar]
- 32.Gilson AM, Joranson DE. Controlled substances and pain management: changes in knowledge and attitudes of state medical regulators. J Pain Symptom Manage. 2001;21(3):227–237. doi: 10.1016/s0885-3924(00)00263-3. [DOI] [PubMed] [Google Scholar]
- 33.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- 34.Group Health Cooperative. Principles For More Selective And Cautious Opioid Prescribing. 2012 http://www.ghinnovates.org/?p=3573.
- 35.Hall WJ. Centenarians: metaphor becomes reality. Arch Intern Med. 2008;168(3):262–263. doi: 10.1001/archinte.168.3.262. [DOI] [PubMed] [Google Scholar]
- 36.Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9(2):213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- 37.Illich I. Medical Nemesis: The Expropriation of Health. New York: Pantheon Books; 1976. [Google Scholar]
- 38.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Treatment, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 39.International Pain Summit Of The International Association For The Study Of P. Declaration of Montreal: declaration that access to pain management is a fundamental human right. J Pain Palliat Care Pharmacother. 2011;25(1):29–31. doi: 10.3109/15360288.2010.547560. [DOI] [PubMed] [Google Scholar]
- 40.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain. 2010;150(3):390–400. doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Kobus AM, Smith DH, Morasco BJ, Johnson ES, Yang X, Petrik AF, Deyo RA. Correlates of higher-dose opioid medication use for low back pain in primary care. J Pain. 2012;13(11):1131–1138. doi: 10.1016/j.jpain.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manchikanti L, Ailinani H, Koyyalagunta D, Datta S, Singh V, Eriator I, Sehgal N, Shah R, Benyamin R, Vallejo R, Fellows B, Christo PJ. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011;14(2):91–121. [PubMed] [Google Scholar]
- 44.Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 45.Martin BC, Fan MY, Edlund MJ, Devries A, Braden JB, Sullivan MD. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med. 2011;26(12):1450–1457. doi: 10.1007/s11606-011-1771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCabe SE, West BT, Teter CJ, Boyd CJ. Medical and nonmedical use of prescription opioids among high school seniors in the United States. Arch Pediatr Adolesc Med. 2012;166(9):797–802. doi: 10.1001/archpediatrics.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michaels CC, Holtzman SG. Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J Pharmacol Exp Ther. 2008;325(1):313–318. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- 48.Moles A, Kieffer B, D’Amatol F. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304(25):1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 49.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151(3):625–632. doi: 10.1016/j.pain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605. doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 52.Paulozzi LJ, Kilbourne EM, Shah NG, Nolte KB, Desai HA, Landen MG, Harvey W, Loring LD. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13(1):87–95. doi: 10.1111/j.1526-4637.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 53.Peavy KM, Banta-Green CJ, Kingston S, Hanrahan M, Merrill JO, Coffin PO. “Hooked on” prescription-type opiates prior to using heroin: results from a survey of syringe exchange clients. J Psychoactive Drugs. 2012;44(3):259–265. doi: 10.1080/02791072.2012.704591. [DOI] [PubMed] [Google Scholar]
- 54.Portenoy RK. Appropriate use of opioids for persistent non-cancer pain. Lancet. 2004;364(9436):739–740. doi: 10.1016/S0140-6736(04)16951-1. [DOI] [PubMed] [Google Scholar]
- 55.Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25(2):171–186. doi: 10.1016/0304-3959(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 56.Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123. doi: 10.1056/nejm198001103020221. [DOI] [PubMed] [Google Scholar]
- 57.Reid MC, Henderson CR, Jr, Papaleontiou M, Amanfo L, Olkhovskaya Y, Moore AA, Parikh SS, Turner BJ. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11(7):1063–1071. doi: 10.1111/j.1526-4637.2010.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richardson LP, Russo JE, Katon W, McCarty CA, DeVries A, Edlund MJ, Martin BC, Sullivan M. Mental health disorders and long-term opioid use among adolescents and young adults with chronic pain. J Adolesc Health. 2012;50(6):553–558. doi: 10.1016/j.jadohealth.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roth SH, Fleischmann RM, Burch FX, Dietz F, Bockow B, Rapoport RJ, Rutstein J, Lacouture PG. Around-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: placebo-controlled trial and long-term evaluation. Arch Intern Med. 2000;160(6):853–860. doi: 10.1001/archinte.160.6.853. [DOI] [PubMed] [Google Scholar]
- 60.Saunders KW, Dunn KM, Merrill JO, Sullivan M, Weisner C, Braden JB, Psaty BM, Von Korff M. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25(4):310–315. doi: 10.1007/s11606-009-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saunders KW, Von Korff M, Campbell CI, Banta-Green CJ, Sullivan MD, Merrill JO, Weisner C. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J Pain. 2012;13(3):266–275. doi: 10.1016/j.jpain.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 63.Sjogren P, Gronbaek M, Peuckmann V, Ekholm O. A population-based cohort study on chronic pain: the role of opioids. Clin J Pain. 2010;26(9):763–769. doi: 10.1097/AJP.0b013e3181f15daf. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan M, Ferrell B. Ethical challenges in the management of chronic nonmalignant pain: Negotiating through the cloud of doubt. J Pain. 2005;6(1):2–9. doi: 10.1016/j.jpain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan MD. Pilot Trial of Opioid Taper Support (POTS) University of Washington. National Institute on Drug Abuse (NIDA); Jun, 2013. ClinicalTrials.gov Identifier: NCT01883882.. http://clinicaltrialsgov/show/NCT01883882. [Google Scholar]
- 66.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thielke SM, Turner JA, Shortreed SM, Saunders K, Leresche L, Campbell CI, Weisner CC, Korff MV. Do Patient-perceived Pros and Cons of Opioids Predict Sustained Higher-Dose Use? Clin J Pain. 2013 doi: 10.1097/AJP.0b013e31828e361b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volinn E, Fargo JD, Fine PG. Opioid therapy for nonspecific low back pain and the outcome of chronic work loss. Pain. 2009;142(3):194–201. doi: 10.1016/j.pain.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 69.Wanzer SH, Federman DD, Adelstein SJ, Cassel CK, Cassem EH, Cranford RE, Hook EW, Lo B, Moertel CG, Safar P, et al. The physician’s responsibility toward hopelessly ill patients. A second look. N Engl J Med. 1989;320(13):844–849. doi: 10.1056/NEJM198903303201306. [DOI] [PubMed] [Google Scholar]
- 70.Washington State Department of Labor & Industries. Guideline for Prescribing Opioids to Treat Pain in Injured Workers to Treat Pain in Injured Workers. 2013 Jul 1; http://wwwlniwagov/ClaimsIns/Files/OMD/MedTreat/FINALOpioidGuideline010713.pdf.
- 71.Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976) 2007;32(19):2127–2132. doi: 10.1097/BRS.0b013e318145a731. [DOI] [PubMed] [Google Scholar]
- 72.Webster LR, Fine PG. Approaches to improve pain relief while minimizing opioid abuse liability. J Pain. 2010;11(7):602–611. doi: 10.1016/j.jpain.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Weisner CM, Campbell CI, Ray GT, Saunders K, Merrill JO, Banta-Green C, Sullivan MD, Silverberg MJ, Mertens JR, Boudreau D, Von Korff M. Trends in prescribed opioid therapy for non-cancer pain for individuals with prior substance use disorders. Pain. 2009;145(3):287–293. doi: 10.1016/j.pain.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White KT, Dillingham TR, Gonzalez-Fernandez M, Rothfield L. Opiates for chronic nonmalignant pain syndromes: can appropriate candidates be identified for outpatient clinic management? Am J Phys Med Rehabil. 2009;88(12):995–1001. doi: 10.1097/PHM.0b013e3181bc006e. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization. Cancer Pain Relief. Book Cancer Pain Relief. City: World Health Organization; 1996. [Google Scholar]


