Abstract
Major depressive disorder (MDD) is associated with low levels of omega-3 polyunsaturated fatty acids (PUFAs), holding promise for new perspectives on disease etiology and treatment targets. As aggressive and impulsive behaviors are associated with low omega-3 PUFA levels in some clinical contexts, we investigated plasma PUFA relationships with trait aggression and impulsivity in patients with MDD. Medication-free MDD patients (n=48) and healthy volunteers (HV, n=35) were assessed with the Brown-Goodwin Aggression Inventory. A subset (MDD, n=39; HV, n=33) completed the Barratt Impulsiveness Scale. Plasma PUFAs eicosapentaenoic acid (EPA, 20:5n-3), docosahexaenoic acid (DHA, 22:6n-3), and arachidonic acid (AA, 20:4n-6) were quantified and ln-transformed to mitigate distributional skew. Ln-transformed PUFA (lnPUFA) levels were predictors in regression models, with aggression or impulsivity scores as outcomes, and cofactors of sex and diagnostic status (MDD with or without a history of substance use disorder [SUD], or HV). Interactions were tested between relevant PUFAs and diagnostic status. Additional analyses explored possible confounds of depression severity, self-reported childhood abuse history, and, in MDD patients, suicide attempt history. Among PUFA, lnEPA but not lnDHA predicted aggression (F1,76=12.493, p=0.001), and impulsivity (F1,65=5.598, p=0.021), with interactions between lnEPA and history of SUD for both aggression (F1,76=7.941, p=0.001) and impulsivity (F1,65=3.485, p=0.037). Results remained significant when adjusted for childhood abuse, depression severity, or history of suicide attempt. In conclusion, low EPA levels were associated with aggression and impulsivity only in patients with MDD and comorbid SUD, even though in most cases SUD was in full sustained remission.
Keywords: aggression, impulsivity, omega-3 fatty acids, PUFA, depression, substance abuse, substance dependence
1. Objectives of the study and background
Current approaches to understanding depression pathophysiology include the study of dimensions of observable behavior as they relate to neurobiology (NIMH, 2008). One important behavioral domain, which has been studied most intensely in pediatric populations, is aggression and its co-occurrence with depression, other clinical characteristics, and environmental factors (Chen, Huang, Wang, & Chang, 2012; Ferguson, San Miguel, & Hartley, 2009; Fite, Stoppelbein, Greening, & Preddy, 2011; Min et al., 2012). However, less is known about biological factors that impact the development and expression of aggression. Low tissue levels of omega-3 polyunsaturated fatty acids (PUFAs) are found in depression (Lin, Huang, & Su, 2010), and supplementation with omega-3 PUFAs, especially eicosapentaenoic acid (EPA, 20:5n-3), appears to have antidepressant effects (Martins, 2009; Sublette, Ellis, Geant, & Mann, 2011b). Additionally, relationships between omega-3 PUFAs and aggressive and impulsive behaviors have been observed in various psychiatric contexts. For example, in patients with deliberate self-harm, low plasma levels of total omega-3 PUFAs and individual omega-3 PUFAs, docosahexaenoic acid (DHA, 22:6n-3) and EPA, correlated with higher impulsivity scores (Garland et al., 2007). Aggressive cocaine abusers had lower plasma levels of the omega-6 PUFA docosapentaenoic acid (DPA, 22:5n-6), DHA, and total omega-3 PUFAs, and a trend toward a higher ratio of omega-6 to omega-3 PUFAs (Buydens-Branchey, Branchey, McMakin, & Hibbeln, 2003). Likewise, male violent and impulsive offenders on a forensic psychiatric unit had lower plasma phospholipid DHA than normal males (Virkkunen, Horrobin, Jenkins, & Manku, 1987). To date, however, no one has investigated the relationship between omega-3 PUFA status and aggressive and impulsive traits in patients with major depressive disorder (MDD).
We therefore tested the hypothesis that omega-3 PUFAs would correlate negatively with aggression and impulsivity scores, and that the correlation would be stronger in patients with MDD than in healthy volunteers. The effects of potentially relevant clinical and demographic characteristics were also examined in our model, including history of substance use disorders (SUD). Exploratory analyses were performed post-hoc to rule out confounds due to depression severity or to conditions known to be associated with aggression and/or impulsivity and with mood disorders, namely suicidal behavior (Cremniter et al., 1999; Mann, Waternaux, Haas, & Malone, 1999) and childhood adversity (Barnow, Lucht, & Freyberger, 2001).
2. Materials and methods
2.1. Subjects
This cross-sectional study was conducted at the New York State Psychiatric Institute (NYSPI). MDD patients (n=48) and healthy volunteers (HV, n=35), aged 18–65 yrs, were recruited through advertisements and gave written informed consent to participate in mood disorders studies approved by the NYSPI Institutional Review Board. Pregnant women and individuals with active medical illnesses were excluded from participation, ascertained on the basis of history, physical examination, and laboratory tests. Patients were free of psychiatric medications for at least 14 days prior to blood sampling (6 weeks in the case of fluoxetine).
2.2. Assessments
All assessments were performed by masters-level psychologists who participated in a weekly rating reliability conference. Patients met entry criteria that included a DSM-IV diagnosis of MDE in the context of MDD with a 17-item Hamilton Depression Rating Scale (HDRS-17) score of at least 16. The Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I) (First, Spitzer, Gibbon, & Williams, 2002) was used to determine major mental disorders, including the presence of and remission specifiers for SUD. All participants had a medical history, physical examination, and standard laboratory tests. The Structured Clinical Interview for DSM-IV Axis II disorders (SCID-II) (First, Gibbon, Spitzer, Williams, & Benjamin, 1997) was used to determine comorbid personality disorders. Other current comorbid Axis I disorders were not permitted, with the exception of dysthymia and anxiety disorders (in depressed patients) and a history of SUD. SCID-II diagnosis of personality disorder(s) was not an exclusion criterion. Diagnostic determinations were made by consensus of the research team, which was comprised of trained and experienced research psychologists and psychiatrists.
Depression severity was assessed by the HDRS-17 (Hamilton, 1960), conducted by clinicians, and the self-report Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). The Brown-Goodwin Aggression Inventory (B-G) (Brown, Goodwin, Ballenger, Goyer, & Major, 1979) and the Barratt Impulsiveness Scale (BIS) (Barratt, 1959) were administered to assess trait aggression and impulsivity, respectively. In patients with a history of suicide attempt, lethality was assessed according to the degree of medical damage caused by their most lethal attempt, using the Medical Lethality Scale (Beck, Beck, & Kovacs, 1975). In this scale, scores range from 0 (no injury) to 8 (fatal), with anchor points dependent on the method of attempt.
Clinical and demographic information included race, age, sex, height, weight, years of education, and smoking or nonsmoking status. Participants’ dietary intake of omega-3 PUFAs was assessed using a validated food frequency questionnaire (FFQ) (Sublette et al., 2011d). A subsample of 35 participants completed this FFQ, which was designed to assess dietary intake of omega-3 PUFA-containing foods during the previous six months.
2.3. Plasma analyses
The blood samples were collected into tubes containing ethylene-diamine-tetraacetic acid (EDTA) on ice and centrifuged at low speed for 10 min under refrigeration. The plasma was stored in cryotubes at −80°C until analyzed using a modifi cation of the Lepage procedure (Lepage et al., 1989). Briefly, methanol:acetyl-chloride (9:1) was added to aliquots of plasma and a combined standard, 17:0+23:0; after incubation at 100°C and cooling, 10% potassium carbonate was added, followed by addition of hexane, mixing, and centrifugation. The upper organic phase was transferred to a micro-vial and lyophilized. Hexane (30 ul) was then added and the vial capped prior to automatic injection onto a Model 6890 flame ionization gas chromatograph fitted with a DB-FFAP 30 m × 0.25 mm × 0.25 μm capillary column (Agilent Technologies, Foster City, CA). Hydrogen was used as carrier gas. Fatty acid methyl-esters (FAMEs) were separated over 52 min at a constant pressure of 13.2 psi with an optimized temperature program featuring a retention time lock for elution of the internal standard at a specific retention time. Analytical procedures were standardized against FAME retention times and response factors from a commercially available equal-weight mixture of 28 FAMEs (GLC462, Nu-Chek Prep, Elysian, MN). FAMEs of plasma DHA, EPA, and arachidonic acid (AA) were identified using the ChromPerfect Direct Data Acquisition system (Justice Innovations, Mountain View, CA) by retention times that were essentially constant between chromatographic runs. The intra- and inter-assay variances for all PUFAs (CV%) were less than 5% and 9%, respectively.
2.4. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics (version 19 for Mac [Apple, Inc., Cupertino, CA]). Plasma levels of EPA and DHA as well as the AA:EPA-ratio were skewed to the left due to a relatively high proportion of participants with very low levels of omega-3 PUFAs and significant outliers at the right-hand portion of the distribution. For this reason ln-transformations of all PUFAs were utilized. Similarly, as the dietary intake of omega-3 PUFAs had a skewed distribution, data were ln-transformed; zero intake values were avoided by addition of 0.000001 to all data points. MDD patients and HV were compared with respect to clinical and demographic factors as well as plasma concentrations and dietary intake of PUFAs, using t-tests or χ2 as appropriate.
Linear regression analyses were performed on the MDD and HV groups combined, with ln-transformed plasma PUFA levels (lnEPA, lnDHA, lnAA:EPA-ratio) as the predictor variables, diagnostic status (MDD vs. HV) as cofactor, and total aggression (B-G) or impulsivity (BIS) scores as the primary outcome measures, and testing for interactions between relevant lnPUFAs and significant covariates. Age, sex, race, BMI, smoking status, and history of SUD were separately tested in the model as covariates potentially related to omega-3 PUFA status, and non-significant covariates were removed. In the final model, history of SUD and diagnostic status were combined into one variable to prevent redundancy, with three categories: MDD with SUD, MDD without SUD, and HV.
For lnPUFA showing relationships with aggression or impulsivity, the following exploratory analyses were performed. Since childhood abuse history (Swogger, You, Cashman-Brown, & Conner, 2011) and depression have been associated with aggression (Chen et al., 2012; Ferguson et al., 2009; Fite et al., 2011; Min et al., 2012), regression analyses were repeated in the total sample with history of childhood abuse or HDRS-17 scores added to the model as possible confounders, and interactions between history of childhood abuse or HDRS-17 scores and lnPUFA were tested. The impact of omega-3 PUFA on aggression and impulsivity in patients at risk for suicide was explored by repeating the main regression model within the MDD group only, and including history of suicide attempt as a cofactor. As aggression or impulsivity may be more germane to high-lethality suicide attempters, the latter analyses were repeated excluding suicide attempters with a lethality of 0.
For the subsample that completed the FFQ, correlations were assessed between dietary PUFA and plasma levels of PUFA, using Pearson’s r. For all analyses, p≤0.05 was considered significant. Data are given as mean ± SD.
Portions of this dataset have been included in other analyses with different objectives (Liu et al., 2013; Sublette et al., 2011c; Sublette et al., 2011d).
3. Results
3.1. Clinical sample
Demographic and clinical characteristics and PUFA status of the 48 medication-free MDD patients and 35 HV are given in Table 1. No differences were seen between the three diagnostic groups (MDD with or without history of SUD, and HV) with regard to demographics. The MDD group exhibited moderate depression severity. A history of abuse - physical, sexual or both - during childhood was reported by a higher proportion of MDD patients (n=26, 52.1%) compared to the HV group (n=2, 5.7%) but did not differ between participants with and without SUD.
Table 1.
Demographic and clinical characteristics and fatty acid status of study participants by diagnostic group: depressed patients with and without comorbid substance use disorders, and healthy volunteers.
| Demographic Characteristics | HV (n=35) | MDD (n=48) | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Comorbid Substance Use Disorder | |||||||||
| No (n= 30) | Yes (n=18) | ||||||||
| N | n (%) | N | n (%) | N | n (%) | χ2 | df | p-value | |
| Male sex | 35 | 16 (45.7) | 30 | 11 (36.7) | 18 | 9 (50.0) | 0.949 | 2 | 0.622 |
| White race | 35 | 17 (48.6) | 30 | 19 (63.3) | 18 | 13 (72.2) | 3.108 | 2 | 0.211 |
|
| |||||||||
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | ||||
|
| |||||||||
| Personal income level ($1000/y) b | 35 | 19 (24) | 29 | 30 (48) | 18 | 18.5 (31.3) | 2.039 | 2 | 0.361 |
|
| |||||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | F-test | df | p-value | |
|
| |||||||||
| Age, years | 35 | 32.5 (12.7) | 30 | 34.8 (11.2) | 18 | 35.6 (13.2) | 0.460 | 2,80 | 0.633 |
| Body Mass Index, kg/m2 | 34 | 24.2 (4.3) | 30 | 24.6 (5.2) | 18 | 25.0 (5.1) | 0.183 | 2,79 | 0.833 |
| Education, years | 35 | 15.4 (2.2) | 29 | 15.2 (2.4) | 18 | 14.0 (2.5) | 2.210 | 2,79 | 0.116 |
|
| |||||||||
| Clinical Characteristics c | N | n (%) | N | n (%) | N | n (%) | χ2 | df | p-value |
|
| |||||||||
| Self-reported history of childhood abuse c | 35 | 2 (5.7) | 29 | 15 (51.7) | 18 | 10 (55.6) | 0.065 | 1 | 0.798 |
| Smokera ,c | 35 | 2 (5.7) | 29 | 6 (20.7) | 18 | 7 (38.9) | 1.838 | 2 | 0.199 |
| History of suicide attempt(s) c | 35 | 0 | 30 | 10 (33.3) | 18 | 8 (44.4) | 0.593 | 1 | 0.441 |
|
| |||||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | t-test | df | p-value | |
|
| |||||||||
| HDRS-17 c | 35 | 1.8 (2.7) | 30 | 19.5 (5.4) | 18 | 16.28 (5.4) | 2.010 | 46 | 0.050 |
| BDI c | 35 | 1.1 (1.9) | 30 | 27.1 (9.5) | 18 | 24.7 (9.0) | 0.878 | 46 | 0.384 |
| Aggression c | 35 | 12.7 (2.5) | 30 | 17.4 (5.1) | 18 | 21.2 (6.7) | −2.177 | 46 | 0.035* |
| Impulsivity c | 33 | 36.2 (11.4) | 25 | 55.5 (21.3) | 14 | 57.1 (19.3) | −0.236 | 37 | 0.815 |
|
| |||||||||
| PUFA levels | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | F-test | df | p-value |
|
| |||||||||
| Plasma levels, mg/Ld | |||||||||
| DHA | 35 | 64.7 (25.2) | 30 | 47.8 (18.0) | 18 | 63.7 (35.0) | 4.090 | 2.80 | 0.020* |
| EPA | 35 | 27.1 (15.5) | 30 | 16.1 (8.0) | 18 | 21.5 (10.0) | 6.620 | 2.80 | 0.002* |
| AA:EPA ratio | 35 | 12.5 ( 6.5) | 30 | 16.7 ( 6.5) | 18 | 14.7 ( 9.1) | 2.819 | 2.80 | 0.066 |
|
| |||||||||
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | F-test | df | P | |
|
| |||||||||
| Dietary levels, g/d b | |||||||||
| DHA | 15 | 0.020 (0.16) | 9 | 0.020 (0.07) | 11 | 0.010 (0.05) | 0.488 | 2 | 0.783 |
| EPA | 15 | 0.010 (0.08) | 9 | 0.010 (0.03) | 11 | 0.000 (0.13) | 1.355 | 2 | 0.508 |
Fisher’s exact test
Kruskal-Wallis test
For clinical characteristics, healthy volunteers were not included in the group comparisons.
PUFA levels are reported in non-ln-transformed values for easier comparison with other literature; however, statistical results reflect analyses performed with ln-transformed values.
p < 0.05.
Abbreviations: HV, healthy volunteers; MDD, major depressive disorder; IQR, interquartile range; SD, standard deviation; NA, not applicable; HDRS-17, 17-item Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; PUFA, polyunsaturated fatty acids; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; AA, arachidonic acid.
Clinical characteristics were tested between MDD with and without SUD, and aggression was the only clinical characteristic differentiating the groups, being higher in those with SUD. Out of 48 MDD patients, 18 were suicide attempters, and the ratio of attempters to nonattempters did not differ between the SUD and non-SUD groups. The majority (72.2%) of suicide attempters made their most recent attempt >2 years prior to the plasma PUFA sampling (median time since most recent attempt, 6.8 ± 9.8 yrs). Aggression scores did not differ between MDD attempters and MDD nonattempters. Lethality scores were quite low: median lethality of the most lethal attempt was 2, which corresponds to a level of injury requiring emergency room or outpatient treatment, and 50% of the most recent attempts had a lethality score of 0, i.e. having minimal to no medical consequences. Eleven participants did not complete the BIS self-report; no systematic bias was detected with regard to noncompletion. Five patients had a current SUD and thirteen had one or more SUDs in full or partial remission (see Table 2). Six patients had comorbid borderline personality disorder (BPD), of whom two had a history of SUD.
Table 2.
Distribution of substance use disorders comorbid with major depressive disorder in the study population.
| # of patients | Substance Disorder 1 | Remission Status | Substance Disorder 2 | Remission Status | Substance Disorder 3 | Remission Status |
|---|---|---|---|---|---|---|
| 2 | Alcohol Abuse | Full | ||||
| 2 | Alcohol Dep | Full | ||||
| 1 | Alcohol Dep | Partial | ||||
| 3 | Alcohol Dep | Current | ||||
| 1 | Alcohol Abuse | Full | Cannabis Dep | Full | ||
| 1 | Alcohol Abuse | Partial | Cannabis Dep | Current | ||
| 1 | Alcohol Abuse | Full | Cocaine Abuse | Full | Cannabis Abuse | Full |
| 2 | Alcohol Dep | Full | Cannabis Dep | Full | ||
| 1 | Alcohol Dep | Current | Cannabis Dep | Full | ||
| 1 | Alcohol Dep | Current | Opioid Dep | Full | ||
| 1 | Cocaine Dep | Full | ||||
| 1 | Cannabis Abuse | Full | ||||
| 1 | Cannabis Dep | Full |
Unexpectedly, omega-3 PUFA were lowest in MDD without SUD. Although plasma omega-3 PUFAs positively correlated with dietary intake (Pearson’s r=0.340, p=0.046 for EPA and r=0.510, p=0.002 for DHA), the subsample (n=20 MDD, n=15 HV) that completed the FFQ did not have adequate power to distinguish between-group differences in dietary DHA or EPA levels.
3.2. Effects of lnPUFA on aggression and impulsivity
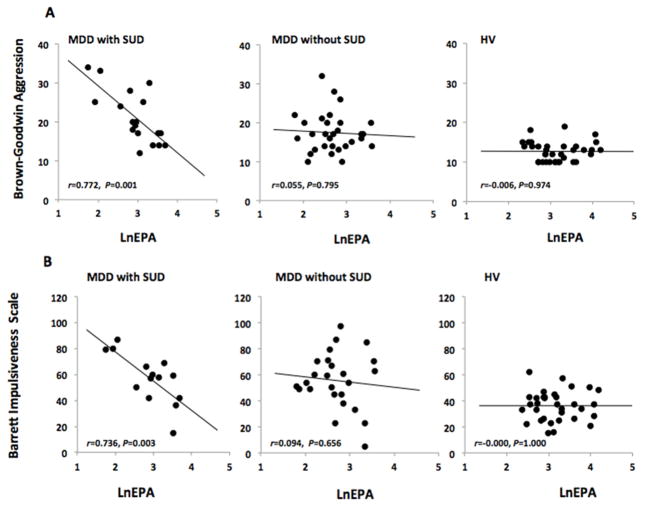
Plasma lnEPA correlated with total aggression and impulsivity scores after controlling for sex and diagnostic status (MDD with and without history of SUD, and HV); an interaction was seen between lnEPA and diagnostic status, such that only in MDD with comorbid SUD was there an effect of lnEPA on aggression or impulsivity (Table 3 and Figure 1). Significant effects with regard to aggression were also seen with the lnAA:EPA ratio. As expected for this measure of the ratio of omega-6 to omega-3 PUFA, the directionality was reversed: a positive correlation was seen (F1,76=11.142, p=0.001), and there was an interaction between diagnostic status and lnAA:EPAratio (F1,76=6.968, p=0.002). With regard to impulsivity, the lnAA:EPA-ratio showed a trend (p=0.094) toward a positive correlation. There were no associations between plasma lnDHA and aggression or impulsivity scores (data available upon request).
Table 3.
Linear regression model of relationships between ln-transformed eicosapentaenoic acid (EPA) and aggression or impulsivity in patients with major depressive disorder (MDD) and healthy volunteers (HV), taking sex and history of substance use disorders (SUD) into account.
| OUTCOME MEASURE: AGGRESSION (Brown-Goodwin
Aggression Inventory, Total Score). Diagnostic Groups: MDD with SUD,
n=18; MDD without SUD, n=30; HV,
n=35. | |||
|---|---|---|---|
| Variables | F | df | p-value |
| lnEPA | 12.493 | 1,76 | 0.001* |
| sex | 4.080 | 1,76 | 0.047* |
| diagnosis (MDD with SUD, MDD without SUD, HV) | 12.548 | 2,76 | <0.0001* |
| lnEPA*diagnosis interaction | 7.941 | 2,76 | 0.001* |
| OUTCOME MEASURE: IMPULSIVITY (BARRATT
IMPULSIVENESS SCALE, Total Score). Diagnostic Groups: MDD with SUD,
n=14; MDD without SUD, n=25; HV,
n=33. | |||
|---|---|---|---|
| Variables | F | df | p-value |
| lnEPA | 5.598 | 1,65 | 0.021* |
| sex | 0.973 | 1,65 | 0.328 |
| diagnosis (MDD with SUD, MDD without SUD, HV) | 5.158 | 2,65 | 0.008* |
| lnEPA* diagnosis interaction | 3.485 | 2,65 | 0.037* |
p < 0.05
Figure 1.
Relationships between ln-transformed eicosapentaenoic acid (EPA), and trait aggression and impulsivity scores, in patients with major depressive disorder (MDD) divided by presence or absence of comorbid substance use disorder (SUD) and healthy volunteers (HV).
3.3 Childhood abuse
Self-reported childhood abuse was a significant predictor of aggression (F=14.974; p<0.001), but when abuse was included as a cofactor in the full model, the association of lnEPA with aggression was still significant (F=11.190, p=0.001), and effects of abuse on aggression were reduced to a trend (F=3.163, p=0.079). On the other hand, childhood abuse did not predict impulsivity (F=0.066, p=0.798).
3.4. History of Suicide Attempt
Aggression and impulsivity scores were higher, and lnEPA was lower, in MDD patients compared with HV, but did not differ between MDD suicide attempters and non-attempters (data available on request). Addiiton of suicide attempt status to the analysis models (within the MDD group) did not affect aggression or impulsivity and did not change the effects of lnEPA, which retained its significance. Repeating this analysis without suicide attempters with a lethality of 0 did not affect the results.
3.5. Depression Severity
When severity of depression, as measured with the HDRS-17, was added to the regression models, it had no main effect on aggression (F1,75=0.159, p=0.691) or impulsivity (F1,64=0.020, p=0.887), and the effect on lnEPA as a predictor was negligible.
4. Discussion
This is the first study to report on relationships between plasma PUFAs and aggression or impulsivity in patients with MDD. We demonstrate that SUD comorbidity robustly drove associations between low plasma EPA and high trait aggression and impulsivity in MDD patients. Our findings support a body of literature in several contexts finding connections between aggression and low absolute levels of omega-3 PUFA or low omega-3 status relative to omega-6 PUFA, and we extend those findings by highlighting the importance of SUD, and ruling out a role for depression severity or childhood abuse in explaining the relationship of plasma levels of EPA to aggression in this MDD population. The finding that effects of EPA on aggression and impulsivity were only seen in context of SUD is remarkable in several respects. Firstly, the SUD group comprised mainly individuals in full sustained remission, so the effect is unlikely to be due to the general malnutrition sometimes seen with acute alcoholism; this, moreover, suggests that the connection between SUD and PUFA effects is an enduring one. Finally, plasma levels of EPA were higher in the SUD group than in MDD without SUD, so the effect was not simply a function of the severity of EPA deficiency.
Relationships between omega-3 PUFAs, depression, and aggression have been preliminarily explored in rodent studies. These studies have found increased aggressive behaviors with both omega-3 PUFA deficient (Demar et al., 2006) and high-omega-6 PUFA (Raygada, Cho, & Hilakivi-Clarke, 1998) intake, although depressive behaviors were increased on the omega-3 deficient diet and decreased on the elevated omega-6 diet. One study in dogs (Re, Zanoletti, & Emanuele, 2008) found that aggressive dogs were differentiable from normal animals by low DHA but not EPA levels, in contrast to our findings; however, they measured phospholipid rather than total plasma omega-3 PUFAs, and effects of substance use were not studied in the animal context.
In humans, a cross-national study found positive correlations between increases in omega-6 PUFA consumption and homicide rates (Hibbeln, Nieminen, & Lands, 2004). However, inconsistent results have been reported in the two clinical trials that assessed the effect of omega-3 PUFA supplementation on aggression: Zanarini et al. (Zanarini & Frankenburg, 2003) found that omega-3 PUFA supplements decreased both aggression and depression severity scores in BPD patients, whereas Hallahan et al. (2007) found that omega-3 PUFA supplementation improved depression and suicidality, but did not decrease aggression in a sample of patients with deliberate self-harm (Hallahan, Hibbeln, Davis, & Garland, 2007). A likely related behavioral domain, anger, was improved with omega-3 PUFA supplementation in a placebo-controlled study of a cohort of substance abusers with a lifelong history of aggression and problems with the law (n=24) (Buydens-Branchey & Branchey, 2008; Buydens-Branchey, Branchey, & Hibbeln, 2008). With regard to impulsivity, no correlation was seen with erythrocyte EPA levels in the closest comparator study, in a population of suicide attempters (Huan et al., 2004). Nor did Hallahan et al. (2007) find that omega-3 PUFA supplementation improved impulsivity scores in patients with deliberate self-harm (Hallahan et al., 2007). However, our findings raise the question as to whether past history of SUD could be a silent confound in other studies examining relationships between omega-3 PUFA levels or treatment, and aggression, impulsivity, anger, or hostility.
We have previously demonstrated associations between low levels of omega-3 PUFAs and altered biomarkers reflecting reduced dopaminergic but not serotonergic functioning, in a separate MDD population (Sublette et al., 2014). Baseline omega-3 PUFA levels correlated negatively with prolactin, an inverse indicator of dopaminergic tone, and with cerebrospinal fluid levels of homovanillic acid (HVA), the principal metabolite of dopamine. Low dopaminergic tone or responses likewise are found in substance use disorders. For example, in chronic alcohol use, D2 receptors are down-regulated and dopamine synthesis rates are impaired (Charlet, Beck, & Heinz, 2013); similarly, blunted dopaminergic responses are seen in cocaine dependency (Narendran & Martinez, 2008; Thomas, Kalivas, & Shaham, 2008). In rats, anticipation of alcohol ingestion induces dopamine but not serotonin activation in the nucleus accumbens (van Erp & Miczek, 2007). Alcohol also can induce aggressive behavior, an important public health problem (WHO, 2007). Thus we speculate that dopamine may be involved in the relationships between EPA intake, aggression, and SUD in MDD.
To explore additional sources of variance that may contribute to aggression, we examined possible effects of childhood abuse. As expected from prior studies (Bonomi, Cannon, Anderson, Rivara, & Thompson, 2008; Brodsky et al., 2001; Carey, Walker, Rossouw, Seedat, & Stein, 2008; Cong et al., 2012; Draper et al., 2008; Fergusson, Boden, & Horwood, 2008; Jumper, 1995; Kaplow & Widom, 2007; Kendler & Aggen, 2013; Kendler et al., 2000; Kendler, Kuhn, & Prescott, 2004; Levitan et al., 1998; Paolucci, Genuis, & Violato, 2001; Rohde et al., 2008; Young, Abelson, Curtis, & Nesse, 1997), a higher prevalence of self-reported childhood abuse was seen in MDD patients compared to HV, and childhood abuse was a significant predictor of aggression. However, we found that childhood abuse history did not mitigate the association between plasma EPA and SUD on aggression or impulsivity, indicating that the effects of EPA and childhood abuse on aggression are independent. Another factor known to be associated with aggression and impulsivity is comorbid BPD. We could not effectively assess the effects of this factor in our study, as the sample contained only six participants with BPD, two of whom also had comorbid history of SUD.
Correlations between EPA and aggression or impulsivity did not prove to be affected by suicide attempt status. This was contrary to our expectations, given that aggression distinguishes depressed attempters from nonattempters and predicts future suicidal behavior (Dumais et al., 2005; Keilp et al., 2006; Melhem et al., 2007; Oquendo et al., 2004) and that we (Sublette, Hibbeln, Galfalvy, Oquendo, & Mann, 2006) and others (Huan et al., 2004; Lewis et al., 2011) have found associations between low omega-3 PUFAs and suicide attempts and completion. Among the latter studies, Huan et al. (Huan et al., 2004) found both DHA and EPA to be lower in suicide attempters; Lewis et al. (Lewis et al., 2011) found significance for low DHA and a trend for low EPA in suicide deaths; while Sublette et al. (Sublette et al., 2006) found low DHA to predict future suicide attempts. However, in this study, the aggression scores did not differ between MDD attempters and nonattempters, the lethality scores were low, and a high proportion of the most recent attempts occurred more than two years ago. Thus this particular population may differ from previous studies that have found higher aggression scores in depressed suicide attempters.
We found that low plasma EPA, but not low DHA, was linked to higher aggression scores in MDD patients with a history of SUD, although both EPA and DHA were significantly lower in MDD than in HV. In the context of depression, several meta-analyses suggest a therapeutic benefit of EPA over DHA in clinical trials in depression (Lin et al., 2012; Martins, 2009; Martins, Bentsen, & Puri, 2012; Sublette, Ellis, Geant, & Mann, 2011a), despite the greater importance of DHA for brain functioning suggested by much higher brain concentrations of DHA than EPA, and the finding of selective deficits of DHA in postmortem depressed brains (McNamara et al., 2007). Similarly, a direct comparison study showed that EPA, but not DHA, as adjuvant to antidepressant medication lowered HDRS-17 scores after 12 weeks (Mozaffari-Khosravi, Yassini-Ardakani, Karamati, & Shariati-Bafghi, 2012). The biological mechanisms underlying differential effects of EPA with regard to depression are unknown; however, one possible explanation arises from certain anti-inflammatory effects specific to EPA. For example, EPA, to a much higher degree than DHA, competes with the omega-6 PUFA AA, its 20-carbon congener, for uptake into phospholipids in human lymphoma U937 cells (Obajimi et al., 2005) and for enzymatic metabolism via cyclooxygenase-2 in T-helper cells and monocytes (Jaudszus et al., 2013). Additionally, dietary studies in autoimmune-prone mice found that higher proportions of EPA to DHA in the diet results in a reduction of the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukins (IL) IL-6 and IL-1β (Bhattacharya, Sun, Rahman, & Fernandes, 2007). Similarly, EPA much more strongly than DHA suppresses mRNA expression and production of TNF-α and IL-1β, and production of leukotriene (LT) B4 and prostaglandin (PG) D2 in LPS-stimulated primary human asthmatic alveolar macrophages (Mickleborough, Tecklenburg, Montgomery, & Lindley, 2009). Oxidation of EPA may increase its anti-inflammatory activity (Sethi et al., 2002), and in animal studies EPA undergoes higher β-oxidation than DHA in the brain (Chen, Liu, Ouellet, Calon, & Bazinet, 2009) as well as in the liver (Willumsen, Vaagenes, Lie, Rustan, & Berge, 1996).
EPA’s anti-inflammatory effects may be important in this context because inflammation has been linked not only to depression (Raison & Miller, 2013), but also to anger and hostility, which can worsen after administration of the therapeutic inflammatory cytokine interferon-α [reviewed in (Lotrich, Sears, & McNamara, 2013)]. Higher ratios of omega-6 to omega-3 PUFAs, seen in depression (Lin et al., 2010), also have been associated with anger, irritability, and assaultiveness during exposure to elevated inflammatory cytokines, but not in their absence (Lotrich et al., 2013). This phenomenon was more pronounced in individuals carrying the polymorphism A-208G in the promoter region of TNF-α (Lotrich et al., 2013). Thus inflammation and inflammation-related genetic polymorphisms are two possible additional sources of variability that may interact with EPA to contribute to the etiology of aggressive states.
In this study population, both MDD and HV participants had extremely low median intakes of both DHA and EPA. In comparison, the acceptable micronutrient distribution range (AMDR) for EPA and DHA is set to 0.25 – 2 g/day by the Food and Agriculture Organization (FAO, 2010), and the U.S. Food and Drug Administration (FDA) has set a value of up to 3 g/day of omega-3 PUFAs as “Generally Regarded as Safe” (IOM, 2005). Hibbeln et al. (Hibbeln, Nieminen, Blasbalg, Riggs, & Lands, 2006) proposed that the intake for omega-3 PUFAs should be at least 3.5 g/day on a 2000 kcal diet in order to protect against cardiovascular and psychiatric diseases. The study participants in both diagnostic groups consumed only 2% of this recommendation, assuming their daily intake was on average 2000 kcal/day. Thus, if low EPA does predispose some individuals to aggression, improved nutrition in this regard could have important public health ramifications.
Limitations
The small sample size means the results need replication in a larger independent sample. Total plasma omega-3 PUFA levels have been found to be comparable to erythrocyte omega-3 PUFAs as indicators of dietary omega-3 PUFA intake (Hodson, Skeaff, & Fielding, 2008; Phillips & Dodge, 1967; Skeaff, Hodson, & McKenzie, 2006; Sullivan, Williams, & Meyer, 2006; Valles, Aznar, & Santos, 1984), but may give different results compared with plasma phospholipid PUFA, which are less affected by lipoprotein proportions (reviewed in Hodson et al., 2008). The lack of a significant relationship between suicide attempt history, omega-3 PUFAs, and aggression is inconclusive, as it may be that such an effect was diluted by the low lethality and long elapsed time from last attempt in most of the suicide attempters in this particular sample. Although our results are consistent with a hypothesis in which low EPA contributes to a higher inflammatory tone, which may lead to aggression and impulsivity, this cross-sectional study does not prove that low EPA has a causal role in aggression or impulsivity. However, the alternative that low EPA could be simply a result of poorer dietary choices made by individuals with high aggression/impulsivity is not likely, as EPA actually was lowest in the MDD group without a history of SUD, in whom EPA levels did not correlate with aggression or impulsivity. Additionally, as we did not collect information about the participants’ total dietary intake and level of activity, it is possible that low EPA itself is a confounder that does not contribute to the development of aggression and impulsivity, but is a marker of some related type(s) of dietary imbalance or other lifestyle differences. We also note that both dietary and plasma PUFA levels were measured acutely, while the aggression and impulsivity measures are lifetime measures.
5. Conclusions
This study is the first to report on associations of plasma EPA with aggression and impulsivity in medication-free adults with MDD. Our findings may indicate a therapeutic role for omega-3 PUFAs in aggressive MDD patients with SUD history. However, prospective studies of larger samples are needed to further understand the specific roles of EPA intake, other nutrients, inflammatory state, and genomics, with regard to depression, substance use, aggression, and impulsivity in MDD.
Highlights.
Omega-3 fatty acid levels correlate inversely with aggression, impulsivity in MDD
Omega-3 (n-3) fatty acid EPA but not DHA is involved
EPA effect only seen in MDD with comorbid substance use disorders
Acknowledgments
No acknowledgements to report.
Funding
This work was funded in part by MH079033 (PI:Sublette), NARSAD and MH076049 (PI:Grunebaum), MH040695 and MH062185 (PI:Mann), and MH48514 (PI:Oquendo).
Footnotes
Conflict of Interest
Dr Sublette received a grant of nutritional supplements from Unicity International for an unrelated study. Dr. Mann receives royalties from the Research Foundation of Mental Hygiene for commercial use of the C-SSRS and stock options in Qualitas Health, a manufacturer of an EPA supplement who did not fund this study, and was not involved in the design of the study, the data analyses or the preparation of the manuscript. Dr. Oquendo receives royalties for use of the Columbia Suicide Severity Rating Scale and received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to this study. She has received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Otsuko, Pfizer, Sanofi-Aventis, and Shire. Her family owns stock in Bristol Myers Squibb. All other authors report no conflicts.
Contributors
Dr. Sublette designed the study. Drs. Sublette, Oquendo, Grunebaum and Mann wrote the research protocols and acquired the data. Ms. Beier carried out the statistical analyses under the supervision of Dr. Galfalvy. Mr. Cooper performed the biochemical analyses and wrote the Methods section. Ms. Beier and Dr. Sublette wrote the manuscript. Dr. Lauritzen contributed to the design of the study and interpretation of the results. All authors participated in manuscript revision and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnow S, Lucht M, Freyberger HJ. Influence of punishment, emotional rejection, child abuse, and broken home on aggression in adolescence: an examination of aggressive adolescents in Germany. Psychopathology. 2001;34:167–173. doi: 10.1159/000049302. [DOI] [PubMed] [Google Scholar]
- Barratt ES. Anxiety and impulsiveness related to psychomotor efficiency. Perceptual and Motor Skills. 1959;9:7. [Google Scholar]
- Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. American Journal of Psychiatry. 1975;132:285–287. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Sun D, Rahman M, Fernandes G. Different ratios of eicosapentaenoic and docosahexaenoic omega-3 fatty acids in commercial fish oils differentially alter pro-inflammatory cytokines in peritoneal macrophages from C57BL/6 female mice. J Nutr Biochem. 2007;18:23–30. doi: 10.1016/j.jnutbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Bonomi AE, Cannon EA, Anderson ML, Rivara FP, Thompson RS. Association between self-reported health and physical and/or sexual abuse experienced before age 18. Child Abuse Negl. 2008;32:693–701. doi: 10.1016/j.chiabu.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Oquendo M, Ellis SP, Haas GL, Malone KM, Mann JJ. The relationship of childhood abuse to impulsivity and suicidal behavior in adults with major depression. American Journal of Psychiatry. 2001;158:1871–1877. doi: 10.1176/appi.ajp.158.11.1871. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M. Long-chain n-3 polyunsaturated fatty acids decrease feelings of anger in substance abusers. Psychiatry Research. 2008;157:95–104. doi: 10.1016/j.psychres.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:568–575. doi: 10.1016/j.pnpbp.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, McMakin DL, Hibbeln JR. Polyunsaturated fatty acid status and aggression in cocaine addicts. Drug and Alcohol Dependence. 2003;71:319–323. doi: 10.1016/s0376-8716(03)00168-6. [DOI] [PubMed] [Google Scholar]
- Carey PD, Walker JL, Rossouw W, Seedat S, Stein DJ. Risk indicators and psychopathology in traumatised children and adolescents with a history of sexual abuse. Eur Child Adolesc Psychiatry. 2008;17:93–98. doi: 10.1007/s00787-007-0641-0. [DOI] [PubMed] [Google Scholar]
- Charlet K, Beck A, Heinz A. The dopamine system in mediating alcohol effects in humans. Curr Top Behav Neurosci. 2013;13:461–488. doi: 10.1007/7854_2011_130. [DOI] [PubMed] [Google Scholar]
- Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;80:157–163. doi: 10.1016/j.plefa.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Chen X, Huang X, Wang L, Chang L. Aggression, peer relationships, and depression in Chinese children: a multiwave longitudinal study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53:1233–1241. doi: 10.1111/j.1469-7610.2012.02576.x. [DOI] [PubMed] [Google Scholar]
- Cong E, Li Y, Shao C, Chen J, Wu W, Shang X, Wang Z, Liu Y, Liu L, Gao C, Li Y, Wu J, Deng H, Liu J, Sang W, Liu G, Rong H, Gan Z, Li L, Li K, Pan J, Li Y, Cui Y, Sun L, Liu L, Liu H, Zhao X, Zhang Y, Zhang R, Chen Y, Wang X, Li H, Chen Y, Lin Y, Kendler KS, Flint J, Shi S. Childhood sexual abuse and the risk for recurrent major depression in Chinese women. Psychol Med. 2012;42:409–417. doi: 10.1017/S0033291711001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremniter D, Jamain S, Kollenbach K, Alvarez J-C, Lecrubier Y, Gilton A, Jullien P, Lesieur P, Bonnet F, Spreux-Varoquaux O. CSF 5-HIAA levels are lower in impulsive as compared to non-impulsive suicide attempters and control subjects. Biol Psychiatry. 1999;45:7. doi: 10.1016/s0006-3223(98)00382-5. [DOI] [PubMed] [Google Scholar]
- Demar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. Journal of Lipid Research. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- Draper B, Pfaff JJ, Pirkis J, Snowdon J, Lautenschlager NT, Wilson I, Almeida OP Depression, & Early Prevention of Suicide in General Practice Study G. Long-term effects of childhood abuse on the quality of life and health of older people: results from the Depression and Early Prevention of Suicide in General Practice Project. J Am Geriatr Soc. 2008;56:262–271. doi: 10.1111/j.1532-5415.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Dumais A, Lesage AD, Alda M, Rouleau G, Dumont M, Chawky N, Roy M, Mann JJ, Benkelfat C, Turecki G. Risk factors for suicide completion in major depression: a case-control study of impulsive and aggressive behaviors in men. American Journal of Psychiatry. 2005;162:2116–2124. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- FAO FaAO. Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr Pap. 2010;91:1–166. [PubMed] [Google Scholar]
- Ferguson CJ, San Miguel C, Hartley RD. A multivariate analysis of youth violence and aggression: the influence of family, peers, depression, and media violence. Journal of Pediatrics. 2009;155:904–908. e903. doi: 10.1016/j.jpeds.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Exposure to childhood sexual and physical abuse and adjustment in early adulthood. Child Abuse Negl. 2008;32:607–619. doi: 10.1016/j.chiabu.2006.12.018. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. American Psychiatric Press; 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. [Google Scholar]
- Fite PJ, Stoppelbein L, Greening L, Preddy TM. Associations between relational aggression, depression, and suicidal ideation in a child psychiatric inpatient sample. Child Psychiatry and Human Development. 2011;42:666–678. doi: 10.1007/s10578-011-0243-4. [DOI] [PubMed] [Google Scholar]
- Garland MR, Hallahan B, McNamara M, Carney PA, Grimes H, Hibbeln JR, Harkin A, Conroy RM. Lipids and essential fatty acids in patients presenting with self-harm. British Journal of Psychiatry. 2007;190:112–117. doi: 10.1192/bjp.bp.105.019562. [DOI] [PubMed] [Google Scholar]
- Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. British Journal of Psychiatry. 2007;190:118–122. doi: 10.1192/bjp.bp.106.022707. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:10. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcoholism, Clinical and Experimental Research. 2001;25:487–495. [PubMed] [Google Scholar]
- Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WEM. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:10. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Nieminen LR, Lands WE. Increasing homicide rates and linoleic acid consumption among five Western countries, 1961–2000. Lipids. 2004;39:1207–1213. doi: 10.1007/s11745-004-1349-5. [DOI] [PubMed] [Google Scholar]
- Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biological Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- IOM IoM. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) National Academies of Science; Washington DC: 2005. [DOI] [PubMed] [Google Scholar]
- Jaudszus A, Gruen M, Watzl B, Ness C, Roth A, Lochner A, Barz D, Gabriel H, Rothe M, Jahreis G. Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. Journal of Lipid Research. 2013;54:923–935. doi: 10.1194/jlr.P031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper SA. A meta-analysis of the relationship of child sexual abuse to adult psychological adjustment. Child Abuse Negl. 1995;19:715–728. doi: 10.1016/0145-2134(95)00029-8. [DOI] [PubMed] [Google Scholar]
- Kaplow JB, Widom CS. Age of onset of child maltreatment predicts long-term mental health outcomes. J Abnorm Psychol. 2007;116:176–187. doi: 10.1037/0021-843X.116.1.176. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Oquendo MA, Brodsky B, Ellis SP, Stanley B, John Mann J. Aggressiveness, not impulsiveness or hostility, distinguishes suicide attempters with major depression. Psychological Medicine. 2006;36:1779–1788. doi: 10.1017/S0033291706008725. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH. Clarifying the causal relationship in women between childhood sexual abuse and lifetime major depression. Psychol Med. 2013:1–9. doi: 10.1017/S0033291713001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers JE, Prescott CA. Childhood Sexual Abuse and Adult Psychiatric and Substance Use Disorders in Women: An Epidemiological and Cotwin Control Analysis. Arch Gen Psychiatry. 2000;57:7. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Lepage G, Levy E, Ronco N, Smith L, Galéano N, Roy CC. Direct transesterification of plasma fatty acids for the diagnosis of essential fatty acid deficiency in cystic fibrosis. Journal of Lipid Research. 1989;30:1483–1490. [PubMed] [Google Scholar]
- Levitan RD, Parikh SV, Lesage AD, Hegadoren KM, Adams M, Kennedy SH, Goering PN. Major depression in individuals with a history of childhood physical or sexual abuse: relationship to neurovegetative features, mania, and gender. Am J Psychiatry. 1998;155:1746–1752. doi: 10.1176/ajp.155.12.1746. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. Journal of Clinical Psychiatry. 2011 doi: 10.4088/JCP.11m06879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biological Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln JR, Belmaker RH, Su KP. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry. 2012;17:1161–1163. doi: 10.1038/mp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Galfalvy HC, Cooper TB, Oquendo MA, Grunebaum MF, Mann JJ, Sublette ME. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. Journal of Clinical Psychiatry. 2013;74:732–738. doi: 10.4088/JCP.12m07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Sears B, McNamara RK. Anger induced by interferon-alpha is moderated by ratio of arachidonic acid to omega-3 fatty acids. Journal of Psychosomatic Research. 2013;75:475–483. doi: 10.1016/j.jpsychores.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a Clinical Model of Suicidal Behavior in Psychiatric Patients. Am J Psychiatry. 1999;156:9. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. Journal of the American College of Nutrition. 2009;28:525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Molecular Psychiatry. 2012;17:1144–1149. doi: 10.1038/mp.2012.25. [DOI] [PubMed] [Google Scholar]
- McNamara R, Hahn C, Jandacek R, Rider T, Tso P, Stanford K, Richtand N. Selective Deficits in the Omega-3 Fatty Acid Docosahexaenoic Acid in the Postmortem Orbitofrontal Cortex of Patients with Major Depressive Disorder. Biological Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Melhem NM, Brent DA, Ziegler M, Iyengar S, Kolko D, Oquendo M, Birmaher B, Burke A, Zelazny J, Stanley B, Mann JJ. Familial pathways to early-onset suicidal behavior: familial and individual antecedents of suicidal behavior. American Journal of Psychiatry. 2007;164:1364–1370. doi: 10.1176/appi.ajp.2007.06091522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickleborough TD, Tecklenburg SL, Montgomery GS, Lindley MR. Eicosapentaenoic acid is more effective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clinical Nutrition. 2009;28:71–77. doi: 10.1016/j.clnu.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Min HJ, Jon DI, Jung MH, Hong N, Song MA, Kim YS, Harkavy-Friedman JM, Im HJ, Hong HJ. Depression, aggression, and suicidal ideation in first graders: a school-based cross-sectional study. Comprehensive Psychiatry. 2012;53:1145–1152. doi: 10.1016/j.comppsych.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Mozaffari-Khosravi H, Yassini-Ardakani M, Karamati M, Shariati-Bafghi SE. Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: A randomized, double-blind, placebo-controlled trial. European Neuropsychopharmacology. 2012 doi: 10.1016/j.euroneuro.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- NIMH. The National Institute of Mental Health Strategic Plan. 2008. [Google Scholar]
- Obajimi O, Black K, Macdonald D, Boyle R, Glen I, Ross B. Differential effects of eicosapentaenoic and docosahexaenoic acids upon oxidant-stimulated release and uptake of arachidonic acid in human lymphoma U937 cells. Pharmacological Research. 2005;52:183–191. doi: 10.1016/j.phrs.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. American Journal of Psychiatry. 2004;161:1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Paolucci EO, Genuis ML, Violato C. A meta-analysis of the published research on the effects of child sexual abuse. J Psychol. 2001;135:17–36. doi: 10.1080/00223980109603677. [DOI] [PubMed] [Google Scholar]
- Phillips GB, Dodge JT. Composition of phospholipids and of phospholipid fatty acids of human plasma. J Lipid Res. 1967;8:676–681. [PubMed] [Google Scholar]
- Raison CL, Miller AH. Do cytokines really sing the blues? Cerebrum. 2013;2013:10. [PMC free article] [PubMed] [Google Scholar]
- Raygada M, Cho E, Hilakivi-Clarke L. High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings’ aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase C activity. Journal of Nutrition. 1998;128:2505–2511. doi: 10.1093/jn/128.12.2505. [DOI] [PubMed] [Google Scholar]
- Re S, Zanoletti M, Emanuele E. Aggressive dogs are characterized by low omega-3 polyunsaturated fatty acid status. Veterinary Research Communications. 2008;32:225–230. doi: 10.1007/s11259-007-9021-y. [DOI] [PubMed] [Google Scholar]
- Rohde P, Ichikawa L, Simon GE, Ludman EJ, Linde JA, Jeffery RW, Operskalski BH. Associations of child sexual and physical abuse with obesity and depression in middle-aged women. Child Abuse Negl. 2008;32:878–887. doi: 10.1016/j.chiabu.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Ziouzenkova O, Ni H, Wagner DD, Plutzky J, Mayadas TN. Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood. 2002;100:1340–1346. doi: 10.1182/blood-2002-01-0316. [DOI] [PubMed] [Google Scholar]
- Skeaff CM, Hodson L, McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. Journal of Nutrition. 2006;136:565–569. doi: 10.1093/jn/136.3.565. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011a;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. Journal of Clinical Psychiatry. 2011b doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain, Behavior, and Immunity. 2011c;25:1272–1278. doi: 10.1016/j.bbi.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Hibbeln JR, Keilp JG, Malone KM, Oquendo MA, Mann JJ. Polyunsaturated fatty acid associations with dopaminergic indices in major depressive disorder. Int J Neuropsychopharmacol. 2014;17:383–391. doi: 10.1017/S1461145713001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential Fatty Acid status as a predictor of future suicide risk. American Journal of Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Segal-Isaacson CJ, Cooper TB, Fekri S, Vanegas N, Galfalvy HC, Oquendo MA, Mann JJ. Validation of a food frequency questionnaire to assess intake of n-3 polyunsaturated fatty acids in subjects with and without major depressive disorder. Journal of the American Dietetic Association. 2011d;111:117–123. e111–112. doi: 10.1016/j.jada.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BL, Williams PG, Meyer BJ. Biomarker validation of a long-chain omega-3 polyunsaturated fatty acid food frequency questionnaire. Lipids. 2006;41:845–850. doi: 10.1007/s11745-006-5039-0. [DOI] [PubMed] [Google Scholar]
- Swogger MT, You S, Cashman-Brown S, Conner KR. Childhood physical abuse, aggression, and suicide attempts among criminal offenders. Psychiatry Research. 2011;185:363–367. doi: 10.1016/j.psychres.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British Journal of Pharmacology. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles J, Aznar J, Santos MT. Influence of Some Plasma Fatty-Acids on the Phospholipid Fatty-Acid Pattern of Human-Platelets - an Exvivo Experience. Thromb Haemost. 1984;52:232–235. [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology. 2007;191:679–688. doi: 10.1007/s00213-006-0637-3. [DOI] [PubMed] [Google Scholar]
- Virkkunen ME, Horrobin DF, Jenkins DK, Manku MS. Plasma phospholipid essential fatty acids and prostaglandins in alcoholic, habitually violent, and impulsive offenders. Biological Psychiatry. 1987;22:1087–1096. doi: 10.1016/0006-3223(87)90051-5. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Expert Committee on Problems Related to Alcohol Consumption. Second report. World Health Organization Technical Report Series. 2007:1–53. 55–57. back cover. [PubMed] [Google Scholar]
- Willumsen N, Vaagenes H, Lie O, Rustan AC, Berge RK. Eicosapentaenoic acid, but not docosahexaenoic acid, increases mitochondrial fatty acid oxidation and upregulates 2,4-dienoyl-CoA reductase gene expression in rats. Lipids. 1996;31:579–592. doi: 10.1007/BF02523828. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Curtis GC, Nesse RM. Childhood adversity and vulnerability to mood and anxiety disorders. Depress Anxiety. 1997;5:66–72. [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR. omega-3 Fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry. 2003;160:167–169. doi: 10.1176/appi.ajp.160.1.167. [DOI] [PubMed] [Google Scholar]