Abstract
Researchers and clinicians have recently begun using Virtual Reality (VR) to create immersive and interactive cue exposure paradigms. The current study aimed to assess the effectiveness of individual cue exposure therapy (CET), using smoking-related VR cues (smoking-VR) as a smoking cessation treatment compared to a placebo-VR (neutral cue) treatment. The sample consisted of healthy treatment-seeking cigarette smokers, who underwent bi-weekly cognitive behavioral group therapy (CBT) plus either smoking-VR CET or placebo-VR CET (random assignment). Smoking-VR CET participants had a higher quit rate than placebo-VR CET participants (P = 0.015). Smoking-VR CET treated participants also reported smoking significantly fewer cigarettes per day at the end of treatment than placebo-VR CET treated participants (P = 0.034). These data indicate that smoking-related VR CET may prove useful in enhancing the efficacy of CBT treatment for tobacco dependence.
Keywords: Tobacco Dependence, Virtual Reality, Cognitive-behavioral Therapy, Smoking Cessation, Nicotine
Introduction
Tobacco-dependent cigarette smokers develop associations between cigarette smoking and people, places, and objects. Subsequently, these smoking-related cues generally elicit craving and induce withdrawal when presented to smokers (Abrams, Monti, Carey, Pinto, & Jacobus, 1988; R. Niaura, Abrams, Pedraza, Monti, & Rohsenow, 1992; R. S. Niaura, et al., 1988; Tiffany & Hakenewerth, 1991). This cue-induced craving propagates smoking behavior and may provoke relapse in abstinent smokers (Abrams, et al., 1988; Abrams, et al., 1987; R. Niaura, Abrams, Demuth, Pinto, & Monti, 1989; R. Niaura, Abrams, Monti, & Pedraza, 1989). Smoking cessation therapies aimed at reducing overall craving have proven successful in increasing the likelihood of quitting; however, few therapies have demonstrated success in attenuating cue-induced craving (Drummond, 2000; Ferguson & Shiffman, 2008).
Extinction learning involves the repeated presentation of a cue, previously paired with reinforcement, in the absence of that reinforcement. This process eventually ceases to elicit the originally learned behavior (Shaham, Shalev, Lu, De Wit, & Stewart, 2003). The original association between cue and reinforcement remains intact, however, and may reemerge under a number of different scenarios. Animal models have shown that reinstatement of drug-seeking to previously extinguished cues occurs upon returning to the context where drug-cue conditioning took place (renewal effect), the presentation of a drug-cue some time after extinction training (spontaneous recovery), re-exposure to the reinforcing stimulus (reinstatement), or by the cue predicting the availability of reinforcement (instrumental learning) (Self & Nestler, 1998). Each of these antecedents parallels situations reported to elicit relapse in drug-dependent individuals (Katz & Higgins, 2003), and therefore must be taken into consideration when developing behavioral therapy intended to reduce drug craving and relapse (Conklin & Tiffany, 2002).
Based on preclinical models of extinction learning, treatments have been developed to examine the efficacy of cue exposure therapy (CET) in humans (Hammersley, 1992; Heather & Bradley, 1990; Heather & Greeley, 1990). During CET, drug-dependent individuals are repeatedly exposed to drug cues (e.g., guided imagery or paraphernalia) in the absence of reinforcement (drug administration) in an attempt to extinguish previously learned drug-cue associations. Coping strategies commonly used in cognitive behavioral therapy (CBT) may also be included in CET to provide guidance on managing craving during high-risk situations (Rohsenow et al., 2001). CET has been administered in a variety of forms to treat tobacco (R. Niaura et al., 1999), alcohol- (Monti et al., 2001; Rohsenow et al., 2001), opiate- (Franken, de Haan, van der Meer, Haffmans, & Hendriks, 1999), and cocaine- (O’Brien, Childress, McLellan, & Ehrman, 1990) dependence with varying levels of success (Conklin & Tiffany, 2002).
Recently, clinicians have used Virtual Reality (VR) to create immersive and interactive cue exposure paradigms. These systems have been successfully applied to CET for the treatment of anxiety, specific phobias, posttraumatic stress (Parsons & Rizzo, 2007; Rizzo et al., 2009) and substance-dependent disorders (J. Lee et al., 2004; J. H. Lee, Kwon, Choi, & Yang, 2007). Clinical trials have shown that VR elicits significantly greater cue-induced craving than either neutral cues (Baumann & Sayette, 2006; Bordnick et al., 2004; Bordnick, Graap, Copp, Brooks, & Ferrer, 2005; Bordnick et al., 2008; Kuntze et al., 2001; J. H. Lee et al., 2003; Saladin, Brady, Graap, & Rothbaum, 2006) or traditional models of cue exposure (Kuntze et al., 2001; J. H. Lee et al., 2003). Applying immersive, multimodal VR cue exposure to appropriately spaced CET sessions may alleviate deficiencies in traditional cue exposure methods and significantly enhance the efficacy of smoking cessation treatment (Conklin & Tiffany, 2002).
The current study aimed to assess the effectiveness of individual CET, conducted with VR smoking-related cues (smoking-VR) as a smoking cessation treatment, compared to a placebo-VR treatment. To ensure that all participants received treatment for tobacco dependence, group CBT was administrated in combination with individual CET. We hypothesized that participants treated with smoking-VR would have higher abstinence rates and report greater reductions in craving for cigarettes than participants treated with placebo-VR.
Materials and Methods
Participants
Healthy treatment-seeking cigarette smokers (≥10 cigarettes/day), who met DSM-IV criteria for tobacco dependence, were recruited through Internet advertisements. Potential participants underwent telephone and in-person screenings. During the telephone screening, participants provided medical, psychiatric, and substance-abuse histories without personal identifiers. The in-person screenings included administration of the Smoker’s Profile, Fagerström Test for Nicotine Dependence (FTND) (Fagerstrom, 1978; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), Urge to Smoke (UTS) Scale (Brody et al., 2002; Jarvik et al., 2000b), and Hamilton Depression (HAM-D) (Hamilton, 1967) and Anxiety (HAM-A) (Hamilton, 1969) rating scales. Potential participants provided breath samples for measuring carbon monoxide (CO) levels using a MicroSmokerlyzer (Bedfont Scientific Ltd, Kent, United Kingdom), at the time of initial screening to verify recent smoking. All participants received detailed verbal and written descriptions of the study procedures before giving informed consent, as approved by the VA Greater Los Angeles Healthcare System Institutional Review Board.
Exclusion criteria included: 1) history of any Axis I psychiatric diagnosis other than tobacco dependence, 2) medical conditions that might interfere with treatment, 3) and current illicit drug use, except occasional marijuana use. Potential participants were required to test negative for drug use in a urine toxicology test during the in-person screening. Participants reporting recreational alcohol (≤ 1 drink/day) or marijuana (≤ 1 use/week) use, not meeting criteria for abuse/dependence, were allowed to participate, but were instructed to abstain for at least 24 h prior to each treatment session.
Cue-Induced Craving Assessment
Prior to the initiation of treatment, participants completed a one-hour cue-induced craving assessment. During this assessment, participants engaged in two VR sessions (smoking-VR and placebo-VR) for 10-min each with a 10-min break between sessions (presentation order was randomized between participants). Participants provided self-reports of cigarette craving prior to (time = 0), during (time = 5), after (time = 10), and following (time = 15) each VR session. Self-reports of craving were determined using the Urge to Smoke (UTS) Scale (Jarvik et al., 2000a).
Cognitive Behavioral Therapy (CBT)
All participants attended bi-weekly (Tuesday & Thursday) CBT group therapy sessions (60 min) over eight weeks. Participants initiated CBT on a rolling schedule following the completion of the cue-induced craving assessment. A licensed psychotherapist (S.S.) performed twelve continuous CBT sessions from a standardized manual for small groups of participants (group size varied from 2–6 participants). Participants provided self-reports of the number of cigarettes smoked the previous day and exhaled CO during each group CBT session. CBT specifically included: 1) education about smoking addiction, withdrawal, and relapse; 2) making preparations for a quit date; 3) recognizing dangerous situations (triggers) that could lead to relapse; 4) developing coping skills, such as avoiding temptation, coping with negative affective states, reducing overall stress, and distracting attention from smoking urges with other activities; and 5) social support (Abrams et al., 2003).
Virtual Reality Cue Exposure Therapy (CET)
Participants were randomly assigned to either smoking-VR or placebo-VR CET prior to the initiation of treatment. For CET, participants attended bi-weekly 30-min individual VR sessions prior to or following group CBT (depending on availability). The VR sessions included two 10-min exposures to VR cues (smoking-related or placebo), separated by a 10-min break. During the 10-min break, participants reviewed the coping skills taught in the most recent group CBT session, and were encouraged to apply the skills during the second VR exposure. Successful completion of VR treatment required attendance of at least eight CET sessions.
The VR environments used here were constructed and presented in a similar manner to those in a previous report by our group (Culbertson et al., 2010). The smoking-VR sessions were individualized for each participant’s self-reported triggers (e.g., objects, people, places, and music associated with smoking). Varieties of environments were created and accessed using Second Life, a freely available online gaming program. These environments included a modern apartment with outdoor seating area, a driving simulation, a replica of Venice beach, a bus stop in Los Angeles, an outside area in front of a bar/restaurant, and a coffee shop. During each session, the participant navigated the VR environment from the first-person point of view while their virtual self, or avatar, smoked a virtual cigarette. Each participant’s avatar was modified to replicate his or her physical appearance. Additional avatars were added to each environment and modified to perform cigarette smoking animations. Other individualized smoking paraphernalia (e.g., box of preferred brand of cigarette, lighter, coffee, etc.) were also placed in each environment where possible (Figure 1).
Figure 1.
Screenshots from the smoking-related virtual reality cue environment (smoking-VR) on top and placebo-related.
The placebo-VR cues sessions were selected for each participant’s personal interest from a variety of settings available in Second Life. These environments included an art show, a carnival, an outdoor sports center, a space museum, and a university. Participants listened to their preferred genre of music during the placebo cue exposure. Each environment was inspected prior to participant exposure to ensure the absence of any smoking-related cues.
Treatment Response Measures: Smoking and Craving
Participants provided self-reports of smoking (cigs/day) and exhaled breath CO samples during each CET session to monitor recent smoking behavior. Abstinence was determined by a self-report of no cigarettes per day and an exhaled CO ≤ 5ppm. Participants also provided craving ratings using the UTS scale prior to (time = 0), during (time = 15), and following (time = 30) each treatment session.
Statistical Analysis
Means (± standard deviations) of demographic and treatment variables were determined independently for each treatment group. Student’s t-tests and a Fisher’s exact test (for gender) were compared between treatment groups for the demographic variables. To evaluate treatment outcomes, groups were compared together, and independently, using unpaired and paired Student’s t-tests, respectively, for the primary smoking outcome measures. A Fisher’s exact test was applied to assess differences in quit rates between groups.
UTS-raw and UTS-change scores were analyzed to measure overall and cue-induced craving (craving change in response to each cue condition). The UTS-change score was calculated by subtracting the baseline rating (time = 0) for each cue condition from the following ratings (time = 5, 10, 15). This method eliminates baseline variability between participants, while also accounting for carry-over effects between cue conditions. A within-subjects general linear model (GLM) for repeated measures was used to assess the effect of cue condition and time on UTS-raw and UTS-change scores independently. An unpaired Student’s t-test was used to compare UTS-raw and UTS-change scores between cue conditions at each time point. Additionally, a Pearson correlation was determined to assess relationships between demographic and smoking characteristics, and UTS-raw and UTS-change scores.
To examine cigarette craving during treatment, a within-subject GLM for repeated measures, including a between-group variable, was used to test for interactions and/or effects of treatment type (smoking-VR and placebo-VR CET), repeated individual CET treatments (from session 1 to 8), and treatment time (time = 0, 15, 30) on self-reported UTS-raw scores. An unpaired Student’s t-test was used to assess group differences in UTS-raw scores at the start and completion of CET. A paired Student’s t-test was also used to assess the UTS-change score from the first to last CET session in all participants, and each treatment group separately. Statistical analyses were performed using SPSS 17 for Mac OS X.
Results
Demographic and Smoking Characteristics
The study sample included craving assessment of 15 (13 men, two women) tobacco-dependent, treatment-seeking adults (mean ± SD age 42.2 ± 12.5 yr) with, on average, two years of post high school education (mean ± SD 13.8 ± 2.6 yr). Eleven participants completed treatment (smoking-VR: n = 5; placebo-VR: n = 6), and these participants reported smoking 19.8 ± 3.9 cigs/day for 20.8 ± 9.8 years, and had CO levels of 17.9 ± 12.5 prior to treatment. No significant differences were observed prior to treatment between groups on demographic or smoking characteristics (Table 1), other than the placebo-VR participants having smoked for significantly more years than smoking-VR participants (Student’s t-tests, two-tailed, P < 0.05).
Table 1.
Demographic and Smoking Characteristics for Smoking-VR and Placebo-VR Treated Participants
| Treatment Group | ||
|---|---|---|
| Smoking-VR (n = 5) | Placebo-VR (n = 6) | |
| Gender | ||
| Male (%) | 80 | 100 |
| Female (%) | 20 | 0 |
| Ethnicity | ||
| White (Not Hispanic) (%) | 60 | 100 |
| Hispanic or Latino (%) | 0 | 0 |
| African American (%) | 0 | 0 |
| Other (%) | 40 | 0 |
| Age | 36.4±4.7 | 46.8±3.8 |
| Education | 15.2±1.4 | 13.2±0.8 |
| Cigarette Use | ||
| Cigarettes per Day | 17.6±1.1 | 21.7±1.7 |
| Years Smoking | 13.9±2.8* | 27.2±3.6* |
| Exhales Carbon Monoxide (ppm) | 22.0±6.0 | 16.5±6.0 |
Values represent mean ± S.E.M.;
= p < 0.05
Cue-Induced Craving Assessment
A within-subject GLM for repeated measures demonstrated a significant effect of cue type (smoking-VR versus placebo-VR) on self-reported UTS-raw scores (F1, 14 = 11.19, P = 0.005) and UTS-change scores (F1, 14= 15.54, P = 0.001) (Figure 2). No effect of time was observed for self-reported UTS-raw or UTS-change scores. A paired Student’s t-test demonstrated that participants reported significantly greater UTS-change scores during (time = 5) (t14 = 4.18, P = 0.001), after (time = 10) (t14= 3.08, P = 0.008), and following (time = 15) (t14 = 2.76, P = 0.015) the smoking-VR cues, compared to the placebo-VR cues. No significant difference in UTS-raw scores was observed between cue conditions at any time point. A negative association was observed between age and UTS-raw scores during (time = 5) and following (time = 15) the smoking-VR cues, and during (time = 5) and after (time = 10) the placebo-VR cues (P < 0.05 for all). A positive association was observed between FTND scores and UTS-raw scores after (time = 10) and following (time = 15) the smoking-VR cues (P < 0.05 for both), with a trend towards an association between FTND scores and UTS-change scores for the after (time = 10) and following (time = 15) smoking-VR cues (P < 0.06 for both).
Figure 2.
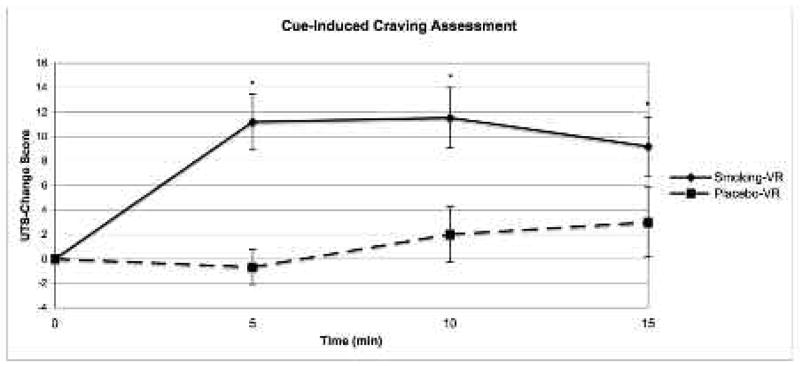
Self-reported cigarette craving during (time = 5), after (time = 10), and following (time = 15) each cue condition (smoking-VR and placebo-VR) presented in the cue-craving assessment (values represent mean change in UTS score from baseline ± standard error mean for each treatment group).
Between Group Treatment Outcomes
Smoking-VR CET participants had a significantly higher quit rate than placebo-VR CET participants (Fisher’s exact test, P = 0.015). Smoking-VR CET participants also reported smoking significantly fewer cigarettes per day at the end of treatment than placebo-VR CET participants (t9 = 2.54, P = 0.034). Smoking-VR CET participants had (non-significantly) lower exhaled CO levels at the completion of treatment than placebo-VR CET participants (2.8 ppm vs. 8.5 ppm, respectively) (Figure 3).
Figure 3.
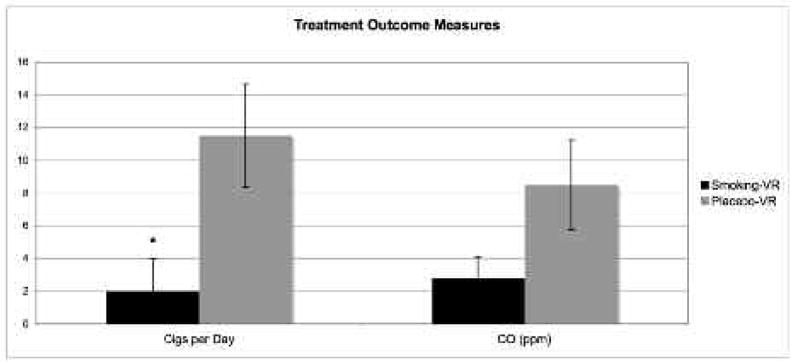
Treatment outcome measures: self-reported cigarettes per day and exhaled CO (ppm) measured at the completion of treatment (values represent treatment group mean value ± standard error mean).
Cigarette Craving During Treatment
A within-subject GLM for repeated measures, including a between-group variable, revealed a significant effect of repeated CET sessions on self-reported UTS-raw scores (F1, 10 = 3.64, P = 0.032); however, no effect of treatment type was observed. An exploratory analysis (using a within subject GLM for repeated measures) revealed a significant effect of time when considering UTS scores provided following each VR exposure (time = 15 and time = 30) in smoking-VR CET treated participants (F1, 4 = 3.64, P = 0.046), but not placebo-VR CET treated participants. The study group as a whole demonstrated a significant reduction in UTS scores from the first to last treatment session (t10 = 3.96, P = 0.003). This effect was also observed in each group independently (P < 0.05).
Discussion
Smoking-VR cues elicited significantly higher levels of overall craving and greater increases in cue-induced craving than placebo-VR cues. Tobacco-dependent smokers treated with smoking-VR CET demonstrated a significantly higher quit rate and reported smoking significantly fewer cigarettes per day at the completion of treatment. Although not significant, smoking-VR treated participants also provided substantially lower exhaled CO levels than placebo-VR treated participants at the end of treatment. All participants reported significant decreases in cigarette craving across treatment, as well as significant reductions in craving from the start to the completion of treatment. Taken together, these preliminary findings establish the potential for smoking-VR CET, in combination with CBT, to be a useful treatment for tobacco dependence.
Previous studies of CET in substance-dependent individuals have used a range of treatment paradigms and outcome variables, leading to varied success (Conklin & Tiffany, 2002). The largest and most well-controlled study to apply CET in tobacco dependence did not demonstrate enhanced smoking cessation rates when combined with CBT;(R. Niaura, et al., 1999) however, the current report is distinct since the previous study used CET consisting of participants imagining themselves in smoking situations, which may have accounted for the disparate results.
Relapse to smoking following treatment commonly occurs when a recently abstinent smoker encounters an environment previously associated with smoking (Wikler, 1973). Studies of CET in smokers have, for the most part, been conducted in non-smoking environments (e.g. clinic, laboratory) (Conklin & Tiffany, 2002), which may present a problem since extinction learning is context dependent, and may not generalize across environments (Bouton & Moody, 2004). Furthermore, environments paired with smoking elicit greater cue-induced urges to smoke than environments not paired with smoking (Dols, Willems, van den Hout, & Bittoun, 2000), even in the absence of smoking-related cues (Conklin, 2006; Thewissen, van den Hout, Havermans, & Jansen, 2005). For these reasons, the present study supports the theory that CET must incorporate multiple, context-relevant environments for extinction learning to translate beyond the treatment setting. Some researchers have addressed this issue by treating people with drug dependence in the environment where they use drugs (Dawe, et al., 1993; Kasvikis, Bradley, Powell, Marks, & Gray, 1991). The method used here has potential advantages for practicality and safety. More recently, VR drug cue environments have been applied to CET and demonstrated efficacy in reducing craving and cue-induced brain activation in smokers (J. Lee, et al., 2004; J. H. Lee, Lim, Wiederhold, & Graham, 2005).
Preclinical models of reinstatement have discovered that manipulating intra- and inter- session intervals of extinction learning may reduce cue-associated relapse. Short, repeated presentation of cues, with sufficient time between cue exposures to allow for recovery of responding, increases the rate and duration of extinction learning (Berman & Katzev, 1972; Mackintosh, 1974). Spreading out the intervals between extinction learning sessions to allow responding to reemerge also attenuates spontaneous recovery of extinguished behaviors (Bouton, 1993). Previous studies of CET in smokers have applied long extinction sessions, focusing on singular cues, with short inter-session intervals (Conklin & Tiffany, 2002). In the current study, smoking-VR CET treated participants received twice-weekly CET sessions across eight weeks, with each session including two cue exposures. Appropriate spacing of CET sessions would lead to higher craving during the initial cue exposure in a CET session (i.e. return to responding), followed by an incremental decrease in craving after each subsequent cue exposure (i.e. re-extinguish responding). Smoking-VR CET treated participants demonstrated significantly greater levels of craving following the first smoking-VR cue exposure than the second exposure across treatment. This difference was greatest during the first half of the CET session, presumably when extinction learning was first developing. Furthermore, this effect was absent in placebo-VR CET treated participants suggesting that this reduction in craving was not attributable to general CET procedures, which were matched between groups.
To our knowledge, previous applications of CET for tobacco dependence did not specifically address lapse episodes during treatment. Following a self-reported lapse in the current study, smoking-VR CET treated participants were exposed to a VR context intended to replicate the environment where the lapse occurred. Although no direct measure was applied to assess the efficacy of this intervention, 80% of smoking-VR treated participants proved able to remain abstinent following lapses, while all placebo-VR treated participants returned to smoking.
This study had several limitations. Although the observed dropout rate (~ 27%) is within the expected range, the exclusion of four participants led to a low sample size. Consequently, the study population reported here lacked gender and ethnic diversity, and was not entirely balanced (e.g. placebo-VR CET treated participants reported more years of smoking). Demographic variables such as age (negatively associated with overall craving to smoking-VR and placebo-VR cues) and tobacco dependence (positively associated with overall craving to smoking-VR cues) may have also influenced individual treatment responses. Applying CET as an outpatient treatment constrains the number of controls and measures (e.g. physiological reactivity) that can be taken to assess treatment efficacy, which could enhance extinction learning and abstinence. This lack of control (e.g. time since last cigarette) also led to between subject variability in self-reported cravings during CET sessions, though this method may be more applicable to actual clinical treatment situations.
In conclusion, smoking-related VR cues proved useful in eliciting cue-induced craving and, when applied with the parameters used here, may significantly enhance the efficacy of CET as a treatment for tobacco dependence.
Acknowledgments
Supported by the National Institute on Drug Abuse (A.L.B. [R01 DA20872]; C.S.C. [DA245482]), the Tobacco-Related Disease Research Program (19XT-0135), a Department of Veterans Affairs Type I Merit Review Award, the Richard Metzner Chair in Clinical Neuropharmacology (A.L.B.), and the ARCS Foundation. The authors would also like to acknowledge the UCLA Experiential Technology Center for their assistance in building the virtual reality cigarette smoking-related environment.
References
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Re activity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther. 1988;26(3):225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Abrams DB, Monti PM, Pinto RP, Elder JP, Brown RA, Jacobus SI. Psychosocial stress and coping in smokers who relapse or quit. Health Psychol. 1987;6(4):289–303. doi: 10.1037//0278-6133.6.4.289. [DOI] [PubMed] [Google Scholar]
- Abrams DB, Niaura RS, Brown RA, Emmons KM, Goldstein MG, Monti PM. The Tobacco Dependence Treatment Handbook: A Guide to Best Practices. The Guilford Press; 2003. [Google Scholar]
- Baumann SB, Sayette MA. Smoking cues in a virtual world provoke craving in cigarette smokers. Psychol Addict Behav. 2006;20(4):484–489. doi: 10.1037/0893-164X.20.4.484. [DOI] [PubMed] [Google Scholar]
- Berman JS, Katzev RD. Factors involved in the rapid elimination of avoidance behavior. Behav Res Ther. 1972;10(3):247–256. doi: 10.1016/0005-7967(72)90041-1. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Graap KM, Copp H, Brooks J, Ferrer M, Logue B. Utilizing virtual reality to standardize nicotine craving research: a pilot study. Addict Behav. 2004;29(9):1889–1894. doi: 10.1016/j.addbeh.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Graap KM, Copp HL, Brooks J, Ferrer M. Virtual reality cue reactivity assessment in cigarette smokers. Cyberpsychol Behav. 2005;8(5):487–492. doi: 10.1089/cpb.2005.8.487. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Traylor A, Copp HL, Graap KM, Carter B, Ferrer M, et al. Assessing reactivity to virtual reality alcohol based cues. Addict Behav. 2008;33(6):743–756. doi: 10.1016/j.addbeh.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Moody EW. Memory processes in classical conditioning. Neurosci Biobehav Rev. 2004;28(7):663–674. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59(12):1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14(1):12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Culbertson C, Nicolas S, Zaharovits I, London ED, De La Garza R, 2nd, Brody AL, et al. Methamphetamine craving induced in an online virtual reality environment. Pharmacol Biochem Behav. 2010;96(4):454–460. doi: 10.1016/j.pbb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Powell J, Richards D, Gossop M, Marks I, Strang J, et al. Does post-withdrawal cue exposure improve outcome in opiate addiction? A controlled trial. Addiction. 1993;88(9):1233–1245. doi: 10.1111/j.1360-0443.1993.tb02146.x. [DOI] [PubMed] [Google Scholar]
- Dols M, Willems B, van den Hout M, Bittoun R. Smokers can learn to influence their urge to smoke. Addict Behav. 2000;25(1):103–108. doi: 10.1016/s0306-4603(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Drummond DC. W hat does cu e-reactivity have to offer clinical research? Addiction. 2000;95(Suppl 2):S129–144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3–4):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2008 doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Franken IH, de Haan HA, van der Meer CW, Haffmans PM, Hendriks VM. Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. J Subst Abuse Treat. 1999;16(1):81–85. doi: 10.1016/s0740-5472(98)00004-x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. Br J Soc Clin Psychol. 1969;3:76–79. [Google Scholar]
- Hammersley R. Cue exposure and learning theory. Addict Behav. 1992;17(3):297–300. doi: 10.1016/0306-4603(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Heather N, Bradley BP. Cue exposure as a practical treatment for addictive disorders: why are we waiting? Addict Behav. 1990;15(4):335–337. doi: 10.1016/0306-4603(90)90043-w. [DOI] [PubMed] [Google Scholar]
- Heather N, Greeley J. Cue exposure in the treatment of drug dependence: the potential of a new method for preventing relapse. Drug Alcohol Rev. 1990;9(2):155–168. doi: 10.1080/09595239000185211. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacology Biochemistry and Behavior. 2000a;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. 2000b;66(3):553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Kasvikis Y, Bradley B, Powell J, Marks I, Gray JA. Postwithdrawal exposure treatment to prevent relapse in opiate addicts: a pilot study. Int J Addict. 1991;26(11):1187–1195. doi: 10.3109/10826089109062154. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168(1–2):21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kuntze MF, Stoermer R, Mager R, Roessler A, Mueller-Spahn F, Bullinger AH. Immersive virtual environments in cue exposure. Cyberpsychol Behav. 2001;4(4):497–501. doi: 10.1089/109493101750527051. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim Y, Graham SJ, Kim G, Wiederhold BK, Wiederhold MD, et al. Nicotine craving and cue exposure therapy by using virtual environments. Cyberpsychol Behav. 2004;7(6):705–713. doi: 10.1089/cpb.2004.7.705. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ku J, Kim K, Kim B, Kim IY, Yang BH, et al. Experimental application of virtual reality for nicotine craving through cue exposure. Cyberpsychol Behav. 2003;6(3):275–280. doi: 10.1089/109493103322011560. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kwon H, Choi J, Yang BH. Cue-exposure therapy to decrease alcohol craving in virtual environment. Cyberpsychol Behav. 2007;10(5):617–623. doi: 10.1089/cpb.2007.9978. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback. 2005;30(3):195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The Psychology of Animal Learning. Oxford: Oxford University Press; 1974. [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, et al. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001;25(11):1634–1647. [PubMed] [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking -related stimuli and early relapse to smoking. Addict Behav. 1989;14(4):419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Monti PM, Pedraza M. Reactivity to high risk situations and smoking cessation outcome. J Subst Abuse. 1989;1(4):393–405. [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Pedraza M, Monti PM, Rohsenow DJ. Smokers’ reactions to interpersonal interaction and presentation of smoking cues. Addict Behav. 1992;17(6):557–566. doi: 10.1016/0306-4603(92)90065-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction. 1999;94(5):685–695. doi: 10.1046/j.1360-0443.1999.9456856.x. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97(2):133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15(4):355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Rizzo AA. Affective outcomes of virtual reality exposure therapy for anxiety and specific phobias: A meta-analysis. J Behav Ther Exp Psychiatry. 2007 doi: 10.1016/j.jbtep.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Rizzo AA, Difede J, Rothbaum BO, Johnston S, McLay RN, Reger G, et al. VR PTSD exposure therapy results with active duty OIF/OEF combatants. Stud Health Technol Inform. 2009;142:277–282. [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, et al. Cue exposure with coping skills training and communication skills training for alcohol dependence : 6- and 12- month outcomes. Addiction. 2001;96(8):1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Brady KT, Graap K, Rothbaum BO. A preliminary report on the use of virtual reality technology to elicit craving and cue reactivity in cocaine dependent individuals. Addict Behav. 2006;31(10):1881–1894. doi: 10.1016/j.addbeh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51(1–2):49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Thewissen R, van den Hout M, Havermans RC, Jansen A. Context-dependency of cue-elicited urge to smoke. Addiction. 2005;100(3):387–396. doi: 10.1111/j.1360-0443.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Hakenewerth DM. The production of smoking urges through an imagery manipulation: psychophysiological and verbal manifestations. Addict Behav. 1991;16(6):389–400. doi: 10.1016/0306-4603(91)90047-l. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28(5):611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]



