Abstract
Children receive significant exposure to psychotropic drugs. Some psychiatric disorders are diagnosed and treated in children as young as 2 years old, resulting in exposure to prescription stimulants, antidepressants, and mood stabilizers during brain development. Difficulties in diagnoses at such young ages increase the likelihood that children who are not affected by these disorders receive drug exposure inadvertently. Additionally, the increased availability of caffeine-containing beverages in schools has facilitated exposure to this stimulant in children. However, the consequences of exposure to psychotropic drugs during brain development are not understood. When we exposed rats to the prescription stimulant methylphenidate during early adolescence, we discovered long-lasting behavioral and molecular alterations that were consistent with dramatic changes in the function of brain reward systems. In future work, it will be important to determine if other classes of psychotropic drugs cause these same effects, and whether these effects will also occur if drug exposure begins during other periods of development. Moreover, it will be critical to use more powerful behavioral methods that are sensitive to high-level aspects of motivation and cognitive function, and to establish causal links between developmental exposure-related alterations in these complex behaviors and specific alterations in the molecular biology of key brain regions. This approach may identify classes of psychotropic drugs that have high or low propensities to cause behavioral and molecular adaptations that endure into adulthood. It may also identify periods of development during which administration of these agents is particularly safe or risky.
Keywords: Addiction, Psychotropic, Developmental, Exposure, Rats
1. Introduction
Children receive significant exposure to psychotropic drugs. A substantial component of this exposure involves the illicit use of drugs such as nicotine, alcohol, marijuana, cocaine, heroin, and ‘Ecstasy’ (3–4 methylenedioxymethamphetamine). However, increasing numbers of children are exposed to other psychotropics because the drugs are prescribed by clinicians, or because they seem inherently benign. Disorders such as attention deficit hyperactivity disorder (ADHD), major depression (MD), and bipolar disorder (BD) are being diagnosed and treated in children as young as 2 years old. This trend results in significant exposure to drugs such as methlyphenidate (Ritalin™), desipramine (Norpramin™), and fluoxetine (Prozac™) during critical periods of brain development (e.g., Zito et al., 2000). It is becoming clear that exposure to these agents is not always medically justified. Difficulties in diagnoses at such young ages (Teicher et al., 1996) increase the likelihood that children who are not affected by these disorders are subjected to drug exposure inadvertently. In addition, progressive increases in the consumption of carbonated beverages among children (Harnack et al., 1999) and the appearance of soft drink vending machines in public schools (Pollack and Bright, 2003) has resulted in significant exposure to caffeine, a psychomotor stimulant (Wise and Bozarth, 1987). Six of the seven most popular soft drinks contain caffeine (www.beverage-digest.com), and children and adolescents often show signs of caffeine addiction including impaired attention during withdrawal (Bernstein et al., 1998, 2002). Surprisingly, there are few studies in which exposure to psychotropic drugs during brain development has been studied systematically in humans or laboratory animals.
It is conceivable that early exposure to these psychotropic drugs is creating public health issues that will only become apparent many years from now. For example, we (Andersen et al., 2002; Carlezon et al., 2003) and others (Bolaños et al., 2003) have discovered that exposure to methylphenidate (MPH) early in brain development causes behavioral and molecular adaptations that reflect dramatic changes in the function of brain reward systems that endure into adulthood (Sections 3.1 and 2.2). Some of the changes appear to be consistent with increases in depressive-like behaviors, including anhedonia (reduced ability to experience pleasurable things as being pleasurable) and dysphoria (feelings of unwellness). These behaviors are accompanied by long-lasting changes in the expression of key molecules (Andersen et al., 2002) including the transcription factor CREB (cAMP response element binding protein) within the nucleus accumbens (NAc) shell, a brain region known to mediate the rewarding effects of stimuli such as drugs of abuse, food, and sexual behavior (Wise and Bozarth, 1987). The significance of these behavioral and molecular adaptations is not understood, but one way to interpret these data is that exposure to psychotropic drugs in young animals (and, by extension, children) may produce relatively permanent changes that are not invariably beneficial. More research is needed on this important topic before firm conclusions can be reached (Volkow and Insel, 2003; Hyman, 2003). Ideally, future research will accomplish at least three important goals: one, to examine whether other classes of psychotropic drugs produce the same types of effects; two, to use more complex behavioral models to examine how these agents affect high level motivation and cognitive function; and three, to examine if there are periods of development in which it is particularly safe or risky to use psychotropic drugs. Each of these lines of study is critical, but each involves factors that ensure that this type of research will be complicated and controversial.
2. Traditional views of the consequences of exposure to psychotropic drugs
2.1. Behavioral consequences
Repeated administration of most psychotropic drugs (e.g., stimulants, opiates) causes ‘exposure-dependent’ alterations in responsiveness to their pharmacological actions. Such alterations are often described in terms of tolerance, which is characterized by progressive reductions in responsiveness to certain drug effects, or sensitization (also known as reverse-tolerance), which is characterized by progressive increases in responsiveness to certain drug effects. There are many comprehensive reviews that describe the behavioral and neurobiological correlates of tolerance and sensitization (e.g., Pierce and Kalivas, 1997; Robinson and Berridge, 2001; Carlezon and Nestler, 2002; Everitt and Wolf, 2002; Vezina, 2004). For the purposes of the present review, tolerance and sensitization provide examples of neural plasticity within which drug-induced changes in behavior can be linked to drug-induced changes in molecular processes. In the context of National Institute on Drug Abuse (NIDA)-sponsored research, if tolerance or sensitization to the motivational aspects of drugs of abuse occurs with repeated exposure, then these processes may contribute to the development and expression of addictive behaviors in laboratory animals and humans (Robinson and Berridge, 2001).
It has become generally accepted that repeated intermittent exposure to psychomotor stimulant drugs such as amphetamine, cocaine, morphine (see Carlezon and Nestler, 2002)—and even caffeine (Schenk et al., 1990)—causes sensitization to their stimulant properties in rats. Perhaps more importantly, repeated intermittent exposure to these agents appears to subsequently render animals more sensitive to rewarding drug effects (Lett, 1989; Piazza et al., 1989; Horger et al., 1991). Although there is little consistency among these types of studies in the ages of the animals at the first exposure to drug or at testing, the reliability and reproducibility of the general effect have fostered the notion that prior exposure to psychotropic drugs typically results in sensitization (or cross-sensitization) to key drug actions (Kalivas and Stewart, 1991). Such data raise general concerns that prior (and presumably illicit) exposure to psychomotor stimulant drugs will increase vulnerability to addictive-like behaviors in humans (Robinson and Berridge, 2001). Importantly, cross-sensitization can be caused by many classes of drugs: for example, repeated exposure to antipsychotic drugs can sensitize rats to the rewarding effects of cocaine (Kosten et al., 1996). Although there are few studies in which the consequences of exposure to antidepressant drugs on sensitivity to drugs of abuse have been explored, it is clear that exposure-dependent neuroplasticity is required before these agents have therapeutic effects in humans (Nestler et al., 2002). When considered together, data in the literature raise the possibility that previous exposure to drugs used in the treatment of psychiatric disorders such as ADHD (including MPH and amphetamine) and schizophrenia (including dopamine antagonists) makes normal lab animals (and, by extension, humans) more sensitive—and vulnerable—to the stimulant and rewarding aspects of drugs of abuse.
2.2. Molecular consequences
Repeated exposure to psychotropic drugs has profound effects on the molecular biology of the brain. Alterations in gene and protein expression within various brain regions have been observed after repeated administration of drugs of abuse (Nestler, 2001; Carlezon and Nestler, 2002), antidepressant drugs (Duman, 2002), mood stabilizing drugs (Manji and Duman, 2001), and antipsychotic drugs (Konradi and Heckers, 2001). The molecular consequences of exposure to stimulants and opiates have been studied most extensively within the mesocorticolimbic system and its neural inputs. These drugs regulate diverse classes of molecules including enzymes (e.g., tyrosine hydroxylase; Beitner-Johnson and Nestler, 1993), structural elements (e.g., neurofilaments; Beitner-Johnson and Nestler, 1993), immediate-early genes (IEGs; e.g., c-fos and fos-related proteins; for review, see Nestler et al., 1999), transcription factors (e.g., CREB; Turgeon et al., 1997), and neurotrophic factors (e.g., BDNF; Grimm et al., 2003). Exposure to antidepressant treatments regulates some of the same molecules (CREB, neurotrophic factors), although most of this research has focused on the hippocampus (Duman, 2004) and the NAc shell (Takahashi et al., 1999). Less is known about mood stabilizers, several of which appear to have common actions on signal transduction pathways (Manji et al., 2003), and antipsychotic drugs, which regulate IEGs in striatum and related tissues (Hiroi and Graybiel, 1996; Atkins et al., 1999; Konradi and Heckers, 2001; Grande et al., 2004). Regardless of the neural circuits within which psychotropic drugs have their most prominent actions, it has become abundantly clear that, without exception, these agents leave profound molecular signatures upon the brain.
2.3. Linking behavior with molecules
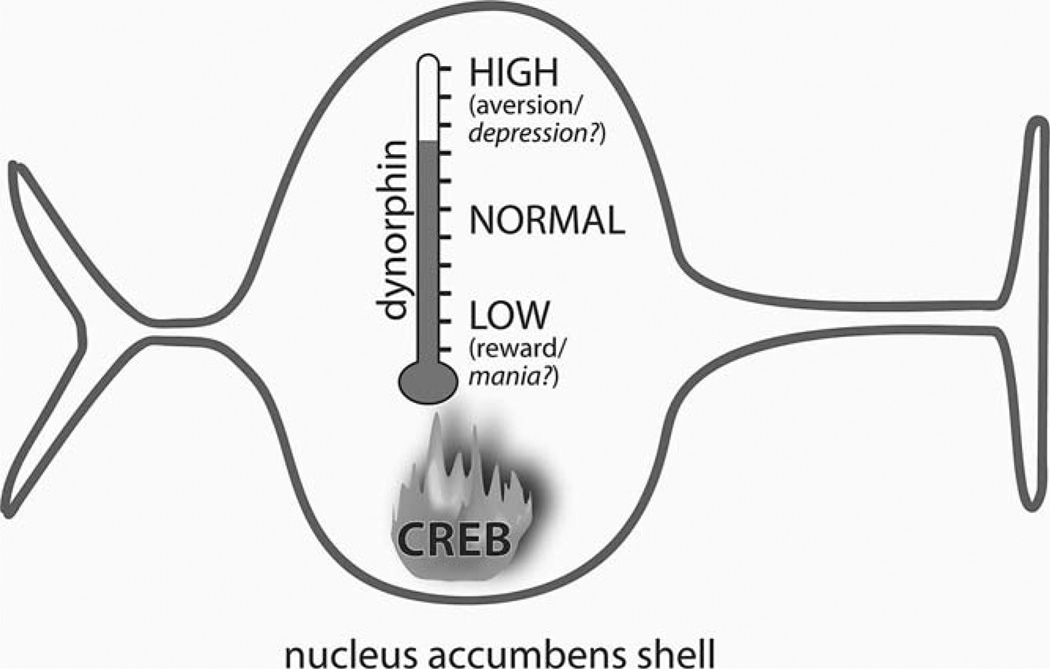
An emerging priority is to establish links between drug-induced changes in the molecular biology of the brain and drug-induced changes in complex behaviors. Molecular biology has identified multitudes of drug-induced alterations in gene and protein expression, but only in a small number of cases has the behavioral relevance of any particular change been established. Experiments that combine molecular and behavioral approaches can establish such links; improvements in genetic engineering techniques such as viral-mediated gene transfer (Carlezon et al., 2000), conditional mutations (Chen et al., 1998; Lewandoski, 2001), and short interfering RNA (RNAi) (Hommel et al., 2003) in rodents have shown that temporally specific alterations in the expression of single genes can have dramatic effects on complex behaviors. As one example, repeated intermittent exposure to morphine elevates expression of GluR1, a glutamate (AMPA) receptor subunit, in the ventral tegmental area (VTA) (Fitzgerald et al., 1996). The use of viral-mediated gene transfer to elevate GluR1 expression within the VTA demonstrated that this neuroadaptation can cause sensitized responses to the stimulant and rewarding effects of morphine (Carlezon et al., 1997). As another example, repeated exposure to drugs of abuse elevates ΔFosB in the NAc shell (Chen et al., 1997). Strategies that combined conditional transgenic mice and viral-mediated gene transfer demonstrated that increased expression of this transcriptionally active protein and one of its targets (the AMPA receptor subunit GluR2) causes sensitized responses to the rewarding effects of cocaine (Kelz et al., 1999; Nestler, 2001). Finally, recent evidence suggests that elevated activity of CREB in the NAc shell can lead directly to the expression of depressive-like signs in rats. Repeated exposure to stimulant drugs and stress each elevate the activity of CREB within the NAc (Turgeon et al., 1997; Pliakas et al., 2001; Barrot et al., 2002). Viral vector-induced elevations of CREB activity within the NAc shell cause depressive-like signs including anhedonia and dysphoria in rats (Carlezon et al., 1998). Elevated CREB activity within the NAc shell also increases immobility behaviors in the forced swim test (FST), a depressive-like effect (Pliakas et al., 2001) that may reflect ‘behavioral despair’ (Porsolt et al., 1977). In contrast, disruption of CREB function by overexpression of a dominant negative form of CREB (mCREB) in the NAc shell increases cocaine reward (Carlezon et al., 1998) and produces antidepressant-like effects (Pliakas et al., 2001). These behavioral adaptations appear related to the ability of CREB to regulate transcription of dynorphin (Cole et al., 1995; Carlezon et al., 1998), an endogenous κ-opioid receptor ligand (Chavkin et al., 1982). Indeed, the κ-antagonist nor-binaltorphimine (norBNI) attenuates the depressive-like signs caused by elevated CREB function within the NAc shell (Carlezon et al., 1998; Pliakas et al., 2001), most likely by blocking κ-opioid receptors that inhibit neurotransmitter release from mesolimbic DA neurons (Di Chiara and Imperato, 1988; Maisonneuve et al., 1994; Shippenberg and Rea, 1997; Svingos et al., 1999). Similarly, κ-agonists have depressive-like effects in the FST (Mague et al., 2003) and the intracranial self-stimulation paradigm (Todtenkopf et al., 2004), whereas κ-antagonists have antidepressant-like effects (Mague et al., 2003). Microinjections of κ-agonists directly into the NAc also cause conditioned place aversions (Bals-Kubik et al., 1993) that likely reflect, at least in part, dysphoric states. Considered together, these studies raise the possibility that CREB-regulated increases in dynorphin activity within the NAc shell can trigger key depressive-like signs that resemble anhedonia, dysphoria, and despair (Fig. 1). Future studies may reveal whether a more extensive blockade of CREB or dynorphin activity in the NAc shell can produce mania-like signs (mental and physical hyperactivity associated with elevated mood and irritability) in more powerful animal models.
Fig. 1.
Schematic depiction of how CREB activity in the nucleus accumbens (NAc) shell may act as a ‘hedonic thermostat’. Elevated expression of CREB increases transcription of dynorphin, which in turn causes aversive and/or depressive-like states (including dysphoria and anhedonia). Conversely, disruption of CREB activity decreases dynorphin transcription, enabling hedonic processes. (Based on Carlezon et al., 1998; Pliakas et al., 2001.)
3. Early developmental exposure to methylphenidate: atypical effects
3.1. Behavior
As described above, the preponderance of evidence from laboratory animals indicates that exposure to stimulants produces sensitization to their rewarding effects, a process that in humans would be expected to increase vulnerability to substance abuse. However, therapeutic administration of stimulants such as MPH in children with ADHD reportedly reduces the risk of substance abuse (Biederman et al., 1999; Wilens et al., 2003). Results from studies in rats that have focused on the long-term effects of exposure to MPH during development are inconsistent. We showed that exposure to MPH during early adolescence (P20–35) decreases the rewarding effects of cocaine and increases its aversive effects in place conditioning studies conducted during adulthood (Andersen et al., 2002). It is generally accepted that the place conditioning paradigm reflects rewarding and aversive drug actions (see Section 4.1.1, below); a reduced ability of normally effective doses of cocaine to establish conditioned place preferences may reflect anhedonia, whereas an increased ability of normally ineffective doses of cocaine to establish conditioned place aversions may reflect dysphoria. This treatment also reduces sensitivity to natural rewards such as sucrose, novelty, and sexual behavior, and increases anxiety- and depressive-like signs (Bolaños et al., 2003; Carlezon et al., 2003). Exposure to cocaine during the same developmental period produces some of the same behavioral effects (Carlezon et al., 2003). Similarly, exposure to oral MPH during late adolescence (P41–67) reduces sensitivity to methamphetamine, an effect that is not consistent with increased sensitivity to the stimulant and rewarding effects of drugs of abuse (Kuczenski and Segal, 2002). In contrast, exposure to MPH at mid adolescence (P35–42) facilitates the acquisition of intravenous cocaine self-administration during adulthood, suggesting enhanced sensitivity to the rewarding properties of the drug (Brandon et al., 2001). Methodological differences among these studies, including the age of the first exposure to psychotropic drugs, may explain the different conclusions among studies. Regardless, these studies challenge the view that early developmental exposure to stimulants invariably results in the subsequent development and expression of sensitized responses to the stimulant and rewarding effects of drugs of abuse. In fact, the data may indicate that early exposure causes tolerance to drug reward.
3.2. Physiology and molecular biology
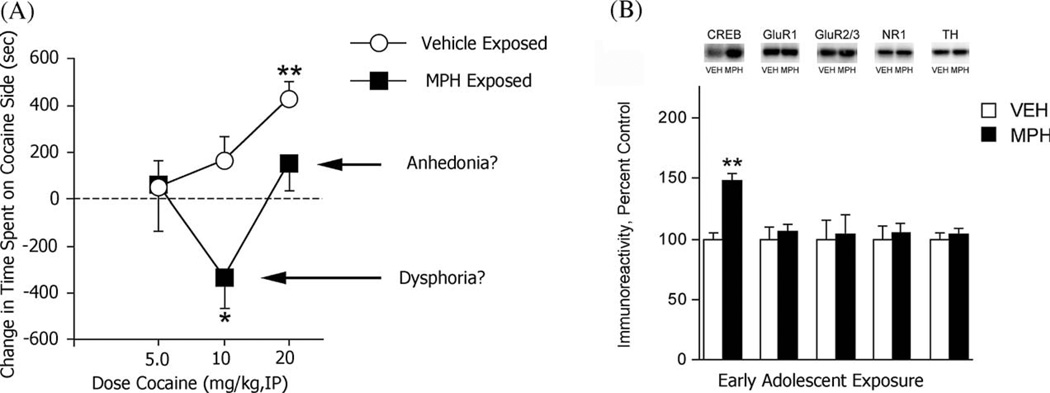
Little is known about the molecular consequences of developmental exposure to MPH. Exposure to MPH at P35–42 causes changes in the burst firing activity of mesolimbic dopamine cells that are consistent with increased addiction liability (Brandon et al., 2003). In contrast, we showed that rats exposed to MPH at P20–35 had large increases in CREB expression within the NAc shell during adulthood (Andersen et al., 2002). The fact that the depressive-like behavioral consequences of early developmental exposure to MPH (Fig. 2A) are accompanied by increases in CREB expression in the NAc shell (Fig. 2B)—a neuroadaptation previously associated with depressive-like signs in numerous behavioral assays (Carlezon et al., 1998; Pliakas et al., 2001; Barrot et al., 2002)—provides additional support for our simple working hypothesis that CREB activity in the NAc shell can trigger depressive-like behaviors by increasing local levels of dynorphin. Because it is unlikely that early exposure the psychotropic drugs such as MPH causes only a single neuroadaptation in a single brain region, further evaluation of other molecules and other regions is needed to develop detailed profiles of the molecular signatures that these agents leave upon the brain.
Fig. 2.
Effect of early exposure to MPH on behavior and the molecular biology of the NAc shell. (A) Exposure to MPH during early adolescence made intermediate doses of cocaine aversive (possibly reflecting dysphoria) and high doses less rewarding (possibly reflecting anhedonia) when the rats were tested during adulthood. (B) Exposure to MPH during pre-adolescence caused substantial increases in CREB levels within the NAc shell during adulthood, but it did not regulate any of the other drug-sensitive proteins studied. (Modified from Andersen et al., 2002.)
4. Types of studies that are needed for the future
4.1. Behavioral
It is clearly of interest to determine whether early developmental exposure to psychotropic drugs causes alterations in drug-taking behaviors in people. The most direct method with which to study drug-taking behaviors in laboratory animals is intravenous drug self-administration (IVSA). Rats intravenously self-administer many classes of drugs (see Collins et al., 1984), and modifications of the procedure to incorporate progressive ratio schedules have enabled strong conclusions about treatment-induced alterations in the reinforcing or incentive-motivational properties of drugs (Mendrek et al., 1998; Morgan and Roberts, 2004; Vezina, 2004). Progressive ratio studies may help to determine if early exposure to psychotropic drugs causes tolerance rather than sensitization to drugs of abuse. However, treatment-induced alterations in the shape of IVSA dose-effect functions can be difficult to interpret (Piazza et al., 2000), raising the possibility that this procedure reflects a variety of drug actions beyond those directly involving reinforcement.
To complement IVSA, it will also be important to develop detailed behavioral profiles of how early developmental exposure to psychotropic drugs affects more general aspects of motivation and cognition during adulthood. While IVSA is optimal for the study of drug-taking behaviors, there are no single ‘gold-standard’ tests of reward, aversion, and cognitive functions in rats. As such, it is necessary to use several assays to examine the behavioral consequences of developmental exposure to each of the psychotropic drugs under study. Each assay models different aspects of complex motivational, mood, and cognitive states that occur in humans, and each has strengths and limitations. Some require exposure to stress or long periods of training, while others involve short-term conditioning. As detailed below, in our research programs we use a variety of behavioral assays that are complementary but not redundant: each provides multiple forms of unique information that reflect alterations in sensitivity to rewarding and aversive stimuli, and more generally, effects on learning and attentional processes. Although some of our studies have begun only recently, this approach should facilitate the generation of detailed behavioral profiles that emerge in response to developmental exposure to psychotropic drugs, and the generation of stronger hypotheses that can be tested in the future.
4.1.1. Place conditioning (PC)
PC is a classical conditioning paradigm in which rats learn to associate the effects of a drug (or other treatment) with a previously neutral environment (see Carlezon, 2003). It is often referred to as the ‘conditioned place preference (CPP)’ paradigm, but this designation fails to capture the flexibility of the assay: it identifies both conditioned place preferences and conditioned place aversions, and thus it can be used to study both rewarding and aversive effects. Advantages of this assay are that it requires brief training periods (as few as 1–2 days), and that the rats are tested in a drug-free state (which minimizes drug treatment effects on performance). Rats develop preferences for environments in which they have experienced rewarding drug effects, and aversions to environments in which they have experienced aversive drug effects. Changes in time spent in the drug-paired environment are indicators of the rewarding or aversive properties that the environment has acquired because of its association with a drug.
Most drugs of abuse (cocaine, amphetamine, opiates) establish conditioned place preferences in rats (see Carr et al., 1989). Pre-exposure to these same drugs facilitates their ability to establish conditioned place preferences, suggesting sensitization to their rewarding effects (Lett, 1989) and confirming the sensitivity of this assay to treatments (e.g., exposure to psychotropic drugs) administered before PC commences. PC is also sensitive to the aversive aspects of the drugs themselves, and of drug withdrawal. High doses of cocaine (~60 mg/kg, IP) cause place aversions (Kosten et al., 1994), presumably because the drug becomes anxiogenic. Indeed, the ß-carboline FG 7142—which causes anxiety and panic-like states in rats (Pellow and File, 1986) and humans (Dorow, 1987)—causes place aversions (Di Scala and Sandner, 1989), suggesting that anxiety produces aversive effects in the PC assay. Similarly, rodents avoid environments in which they have experienced symptoms of opiate (Stinus et al., 1990) or stimulant (Cabib et al., 1996) withdrawal. The effects of drugs including PCP, nicotine, ethanol in the PC model are inconsistent; some investigators report that these drugs establish place preferences, whereas others report place aversions (Carr et al., 1989). Not surprisingly, it is often difficult in rats to demonstrate self-administration of these same drugs (Collins et al., 1984). These data indicate that conclusions about drug reward made with PC are consistent with those made in other rat models that require a more substantial allocation of resources (e.g., IVSA).
Because of these strengths, PC is a good method with which to evaluate the enduring effects of developmental exposure to psychotropic drugs. We have already demonstrated that this strategy effectively identifies exposure-associated alterations in the function of brain reward systems (Andersen et al., 2002; Carlezon et al., 2003). Rats exposed to MPH or cocaine during early adolescence developed place aversions to environments associated with intermediate doses of cocaine, a putative sign of dysphoria, and did not develop normal place preferences at higher doses of cocaine, a putative sign of anhedonia. Dysphoria and anhedonia are key symptoms of depressive states in humans, suggesting that exposure to these stimulants during key periods of brain development can have enduring behavioral consequences. In future studies, PC can be used to determine if developmental exposure to the other psychotropic drugs has similar effects.
Because the PC assay involves conditioning, developmental manipulations that have general effects on learning, memory, or attention may complicate interpretation of data. Considering that cocaine established strong place aversions in MPH- and cocaine-exposed rats (Andersen et al., 2002; Carlezon et al., 2003), it is unlikely that early developmental exposure to these particular drugs had general effects on conditioning. Regardless, it is important to use additional behavioral assays that are sensitive to rewarding and aversive states but are less dependent upon learning and memory to complement PC studies.
4.1.2. ICSS (intracranial self-stimulation)
ICSS is an operant paradigm that is highly sensitive to the function of brain reward systems. In this assay, rodents respond in order to self-administer rewarding electrical stimulation through electrodes implanted within the limbic system. Changes in the rewarding efficacy of the stimulation cause shifts in the functions that relate response rates to stimulation frequency: leftward shifts imply that the stimulation is more rewarding as a result of a treatment (reflecting hyperfunction of brain reward systems), whereas rightward shifts imply that it is less rewarding (reflecting hypofunction of brain reward systems). Shifts are quantified by calculating ICSS ‘thresholds’ (an estimate of the point at which the stimulation becomes rewarding) before and after treatment. The effects of many types of treatments on ICSS thresholds have been described (see Wise, 1996). Most drugs of abuse decrease the amount of stimulation required to sustain responding, as indicated by leftward shifts in rate-frequency functions and decreased ICSS thresholds. Conversely, agents that block drug reward (dopamine or opiate receptor antagonists) increase the amount of stimulation required to sustain responding, as indicated by right-ward shifts in rate-frequency functions, and increased ICSS thresholds. These agents also block the ability of rewarding drugs to cause leftward shifts. Drug withdrawal also causes rightward shifts and elevations in ICSS thresholds (Markou et al., 1992; Barr et al., 2003) that could reflect states of anhedonia or dysphoria. As such, ICSS is sensitive to manipulations that increase reward, decrease reward, or increase aversion.
ICSS can be used to determine if developmental exposure to psychotropic drugs affects the function of brain reward systems during adulthood in two separate types of experiments. First, it is possible to determine whether early developmental exposure to psychotropic drugs affects sensitivity to brain stimulation reward (BSR) itself. Second, it can be used to determine if the drug exposure affects sensitivity to the reward-potentiating effects of cocaine (Wise, 1996). Preliminary evidence suggests that early developmental exposure to MPH reduces the reward-potentiating actions of cocaine in the ICSS assay (S.D. Mague, S.L. Andersen, W.A. Carlezon Jr., unpublished results).
ICSS complements rather than replaces PC studies because exposure regimens that affect response capabilities—making the rats press at higher or lower rates during cocaine testing—can complicate data interpretation in this assay. Although some methods of analysis (‘curve-shift’ assay; see Miliaressis et al., 1986) minimize the consequences of altered rates, the use of PC and ICSS in tandem increases the ability to identify effects of developmental exposure to psychotropic drugs on brain reward systems, and enables stronger conclusions about whether such exposure increases reward, decreases reward, or increases aversion.
4.1.3. Fear-potentiated startle (FPS)
FPS is a classical conditioning paradigm that is primarily sensitive to aversive-like states. In the FPS assay, states of fear are inferred from a behavioral response (Walker and Davis, 2002). Fear is quantified by measuring the amplitude of the acoustic startle reflex in the presence of a cue previously paired with footshock. When rats are repeatedly presented with a cue (e.g., light flash) followed by footshock, they learn to associate the cue with impending shock. Eventually, presentation of the cue in the absence of the shock elicits behaviors that are typically used to define a state of fear, one of which is a potentiated startle response. To quantify startle, rats are placed in cages and presented with startle-eliciting white-noise bursts (~100 dB). The cages are positioned on sensors that quantify startle responses. On some trials, the startle-eliciting stimulus is preceded by the cue previously paired with shock; typically, under these conditions the startle response is substantially larger than on trials without presentation of the cue. The difference between the amplitude of startle on these two trial types is the operational measure of fear. One strength of the FPS is its ability to identify drugs with anxiolytic effects in humans, although it has also been invaluable in identifying brain regions (e.g., amygdala), neurotransmitters (e.g., glutamate, GABA), intracellular signaling molecules (e.g., CREB), and physiological mechanisms involved in the generation and reduction of fear and anxiety (Josselyn et al., 2001; Tsvetkov et al., 2002; Walker and Davis, 2002; Lamprecht and LeDoux, 2004).
The FPS assay may be sensitive to psychotropic drug-exposure related alterations in aversive-like states (fear), but the fact that it reflects conditioning (learning and memory) provides important information that enables more powerful interpretations of results from the other assays. Reduced drug reward in the PC assay might reflect dysregulation of learning and memory processes associated with early developmental exposure to the psychotropic drugs. This type of dysregulation would be also reflected in the FPS assay, by deficits in fear conditioning.
4.1.4. The 5-choice serial reaction time task (5CSRTT)
The 5CSRTT is an operant paradigm that quantifies the effects of manipulations on various aspects of attention in rats (Robbins, 2002). It has been particularly useful in the study of attention deficits like those associated with ADHD. In studies assessing attention in humans, performance is often characterized by a decline in function over time. This decline is sensitive to manipulations affecting task difficulty, and to reversal by psychostimulant drugs. The 5CSRTT evaluates comparable characteristics of performance and is sensitive to psychostimulants with efficacy in humans, and thus it is of great utility in studying the attention deficits that characterize ADHD.
5CSRTT experiments in rats are typically analogous to the continuous performance tests used in humans in clinical settings to quantify deficits that characterize ADHD. Attention comprises several distinct processes, and each can be quantified in rats using the 5CSRTT (Robbins, 2002). One process is sustained attention (vigilance), which is a continuous allocation of processing resources for the detection of rare events. Deficits in sustained attention are typically manifested towards the end of long test sessions. Another process is divided attention, which is the simultaneous allocation of processing resources to several different contingencies within the same test setting. For example, a rat might be required to simultaneously monitor several different sensory channels, thus requiring optimal allocation of limited processing abilities. A third form of attention is selective attention, whereby attention must be focused on a restricted number of sensory channels while ignoring the rest. The 5CSRTT paradigm is best utilized to quantify the effects of manipulations on sustained and divided attention, although conditions can be modified to enable measurement of selective attention. The 5CSRTT can also be used to assess both compulsive and impulsive aspects of inhibitory control.
The 5CSRTT is an ideal assay to determine if early developmental exposure to psychotropic drugs affects aspects of attention in rats during periods of adulthood. Additionally, this assay may provide important information that enables more powerful interpretations of results from the other assays. For example, reduced FPS might reflect deficits in attention that are required to establish associations between the conditioning stimulus (e.g., a light) and the shock. Thus, use of the 5CSRTT in tandem with other behavioral tests should enable the development of improved hypotheses.
4.2. Molecular approaches: biological contexts for the study of psychotropics
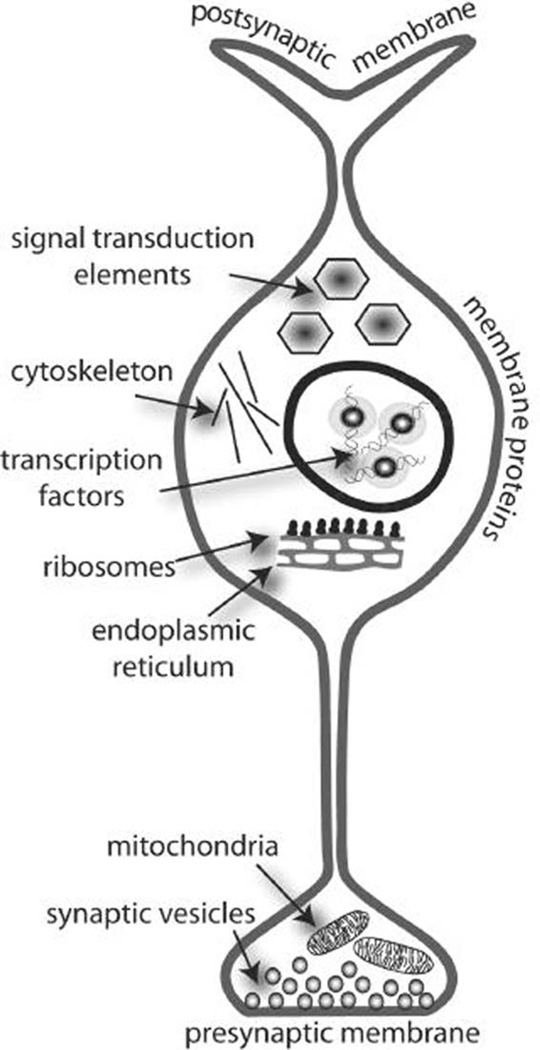
It is critical to identify molecular adaptations caused by early developmental exposure to psychotropic drugs so that the neurobiological consequences can be understood and, ultimately, predicted. New technologies such as gene array technologies and proteomics allow the detection of many mRNAs or proteins simultaneously (Henry et al., 2003). Proteomics can also aid in the analysis of phosporylation or glycosylation status of proteins and therefore give some indirect indication of protein activity (Steinberg et al., 2003). These technologies can contribute significantly to our understanding of the molecular consequences of treatment with psychotropic drugs. Assuming that all necessary precautions are taken to avoid false-positive or false-negative results, these studies yield a plethora of data; it is becoming abundantly clear that the most important question for this type of work is how to retrieve the most sensible information from such large datasets. Microarray or proteomics studies that focus on one or two genes or proteins often fail to exploit the power of these new technologies, and may even magnify some of their inherent limitations, particularly because in large datasets some data reach statistical significance purely by chance. If the data are analyzed in a biological context (i.e., the regulation patterns of genes or proteins of related function are examined together), the chance of false-positive results is dramatically reduced. Our working hypothesis is that functionally related genes or proteins contribute to the same pathways (i.e., second messenger pathways) or the same cellular structures (i.e., synapses, cell organelles, membranes; Fig. 3); thus if exposure to drugs affects a group of functionally related proteins or mRNAs, then the pathway or cellular structure of which they are a component is also likely affected by the drug. As one example, if early developmental exposure to a psychotropic drug alters the levels or phosphorylation state of a single protein involved in vesicle fusion, it would be difficult to attribute a functional significance to this adaptation. However, if many proteins involved in vesicle fusion are altered in a brain area, then there is stronger evidence that the psychotropic drug affects synapses and synaptic plasticity in this brain area. Examining a group of proteins that makes up a pathway or a cellular structure therefore provides a more comprehensive picture than simply examining a single component of that pathway. Furthermore, the up- or downregulation of gene or protein levels, or the extent of phosphorylation and glycosylation, may also provide indications of how the pathway or structure is affected by drug exposure. Thus, it is possible to deduce a substantial amount of information about the biologic effects of psychotropic drugs, even beyond the molecular realm, from gene array and proteomics technologies. This information can then be further verified with other experimental techniques.
Fig. 3.
To put gene array or proteomics data into biological contexts, genes or proteins can be grouped according to their cellular location (i.e., membrane, postsynaptic membrane, mitochondrion, etc.) or their function (i.e., transcription factor, signal transduction element, mitochondrial respiration, vesicular fusion, etc.). Furthermore, functions can overlap with cellular locations, such as ‘mitochondrion’ and ‘mitochondrial respiration’, ‘nucleus’ and ‘transcription factors’. Computer programs are available that calculate hypergeometric distributions to determine if particular functions or locations are more affected than would be expected by chance.
A biological context is critical for gene array and proteomics studies, and various computer programs with different approaches to this problem have become available and are improved on a continuous basis. Many non-commercial computer programs use the Gene Ontology (GO) database as a guideline for biological context. This database sorts genes into three separate ontologies defined as ‘molecular function’, ‘biological process’ and ‘cellular component’. For gene array analysis, computer programs such as Gene MicroArray Pathway Profiler (GenMAPP) (Dahlquist et al., 2002; Doniger et al., 2003) and DNA-Chip Analyzer (d-Chip) (Li and Wong, 2001) can classify results using the GO database. MAPPfinder, a component of the GenMAPP program, is furthermore able to utilize gene classifications defined by the investigator or by specialists in the field. Because the GO database is not brain-specific, questions such as the regulation of genes involved in the synthesis or reception of particular neurotransmitters, or questions pertaining to pre- or postsynaptic effects (Fig. 3) cannot be addressed with this database. However, investigators can use MAPPfinder to assemble their own database based on organizing principles that are specific to their work. MAPPfinder as well d-Chip use statistical tests involving hyper-geometric distributions. These tests calculate if more genes than expected are regulated in any group, and are an indication which structure or which biological function is most affected by psychotropic drug treatment in any brain area of interest.
5. Challenges for future work
Multidisciplinary approaches are needed to examine the full extent of the consequences of early developmental exposure to psychotropic drugs. As with any line of research that can benefit from multidisciplinary approaches, understanding how psychotropic drugs affect brain development and subsequent behavior will require expertise with a dazzling array of techniques, a familiarity with enormous literatures, and most importantly, a willingness to challenge dogma. Below are only a few of the complexities that face researchers interested in a field that promises to become progressively more important and relevant as the use of pharmacotherapies for psychiatric disorders becomes increasingly acceptable and prominent.
5.1. Periods of development in rodents
It is exceedingly difficult to model periods of human brain development in rats. Humans and rats are born at different times in brain development, and development proceeds at vastly different paces between species (see Spear, 2000). Even the definition of adolescence—a time of particular susceptibility to drug exposure in humans—is controversial. Adolescence is a period of development that involves a complex interplay of neurochemical (e.g., Andersen et al., 1997), anatomical (e.g., van Eden et al., 1990), molecular (e.g., Gelbard et al., 1989), cognitive (e.g., Spear and Brake, 1983), and behavioral (e.g., Laviola et al., 1995; Bolaños et al., 1998) adaptations. Often, the onset of puberty and sexual maturation (Graber et al., 1996), which occurs in rats at approximately P35, is considered to signal the onset of adolescence (Petersen, 1998). However, there is no consensus on which markers should define the onset, offset, and duration of adolescence. As an example, in only a small sample of recent studies in which consequences of developmental exposure to psychotropic drugs was examined, adolescence was defined as P35–42 (7 days; Brandon et al., 2001, 2003), P41–67 (26 days; Kuczenski and Segal, 2002), or P54–62 (8 days; Levin et al., 2003). Thus, any work designed to examine the effects of developmental exposure to psychotropic drugs will be complicated by differences in opinions about the biological boundaries of adolescence, as well as significant between- and even within-gender variability in the timing of changes associated with this period.
One approach is to consider adolescence to be a period of transition from childhood to adulthood that is marked by a variety of neurochemical, anatomical, molecular, and behavioral changes, but not defined by discrete events such as puberty (Spear, 2000). A conservative estimate of this period should be broad enough to account for individual variability in the exact timing of when these changes begin and end.
5.2. Drug doses, routes of administration, and gender
There are important differences between humans and rats in drug bioavailability, kinetics, and metabolism. Because these processes change with age and gender (Spear, 2000), differences can become magnified at various points of development and can vary dramatically between male and female rats. These factors influence—and complicate—all studies of drug-induced neuroplasticity. In the case of MPH, intraperitoneal (IP) injections in rats produce peak blood levels of drug that are much different from those seen after the drug is injected orally (by gavage, which involves insertion of a feeding needle through the mouth, throat, and esophagus into the stomach) in rats or oral administration of tablets in humans (Kuczenski and Segal, 2002). It is unclear whether differences in kinetics between species affect the reliability or relevance of preclinical studies in rats that do not precisely mimic the conditions seen in humans (Volkow) and Insel, 2003). More work is needed to determine if differences in route of administration and the time course of elevated blood levels produce profound effects on the outcomes of preclinical studies. However, one potentially relevant example worth consideration is the preclinical study of cocaine addiction in rats: many studies of the effects of cocaine on molecular and behavioral adaptations have involved IP or intravenous (IV) injections despite the fact that human addicts clearly do not favor these routes of administration. Addicts snort or smoke cocaine, and avoid using cocaine by IV routes because it causes dramatic and painful tissue necrosis at injection sites. Regardless, studies using IP or IV cocaine injections in laboratory animals have provided invaluable information that has improved our understanding of addiction, and significant findings have been confirmed in humans. As just one example, the ability of stimulants to increase dynorphin expression in the brains of rats (Daunais et al., 1993) has been observed in human addicts (Hurd and Herkenham, 1993) and in cell culture (Cole et al., 1995) despite profound differences in the ways that the drug was administered among the studies. Thus, to model the human condition, the optimal route of administration for many prescription psychotropics would be voluntary oral self-administration of drugs in tablet formulations; realistically, there should also be some allowance for the day-to-day variability in the timing at which people actually take their prescription medications. Considering that response-contingent and non-contingent administration of drugs can cause dramatically different neurobiological effects (Mantsch and Goeders, 2000), it appears likely that any route of drug administration other than voluntary oral self-administration at variable intervals will have the types of limitations inherent in any line of research conducted in laboratory animals.
5.3. The use of ‘normal’ rats
Difficulties in diagnoses of ADHD and MD at ages as young as 2 years old (Teicher et al., 1996) increase the likelihood that children who are not affected by these disorders receive exposure to powerful psychotropic drugs inadvertently. Moreover, the use of ‘benign’ psychotropic drugs such as caffeine is becoming widespread in children (Pollack and Bright, 2003). These factors provide strong rationale for conducting these studies in ‘normal’ rats that are not designed to model neuropsychiatric disorders. However, the results obtained address primarily the question of how psychotropics affect the normal brain and the consequences of early prescription of psychotropics to children who might not be destined to develop a psychiatric disorder. In the future, the development of improved animals models of these psychiatric conditions may ultimately reveal that the neurobiological consequences of early exposure to psychotropic drugs are dramatically different when there is underlying pathology.
5.4. Cataloguing of alterations in gene and protein expression
Gene microarray technology enables the simultaneous detection of thousands of mRNAs, and the specificity of the signal can be well-controlled through the use of multiple probes for each gene. One limitation of this technology is that gene microarrays examine levels of mRNAs rather than proteins, thus they cannot provide information on the regulation and modification of the proteins for which they code. In contrast, proteomics can potentially study the composition, expression level and chemical modification of proteins in a particular tissue. Proteomics can evaluate modifications such as phosphorylation or glycosylation, which affect the activity or location of proteins. The disadvantages of proteomics include complicated and expensive equipment and technology, and limits in the number of proteins that can be studied simultaneously (Archakov et al., 2003; Zhu et al., 2003). The simplest technology in proteomics is 2-dimensional gel electrophoresis, which does not require expensive equipment but is biased toward soluble and abundant proteins (Zhu et al., 2003). Furthermore, 2-dimensional gel electrophoresis requires either a priori knowledge of protein expression patterns of the tissue of interest in the gel, or the further identification of protein and peptide spots by mass spectrometry. Other approaches are currently limited by the availability of antibodies or specific protein-binding ligands and by access to mass spectrometry equipment. However, many of these obstacles will be overcome in the coming years, and it is reasonable to assume that these technologies will gradually become available to, and used routinely by, progressively more researchers.
Both gene microarrays and proteomics pose statistical challenges since they create large amounts of data with the potential for numerous false-positive findings. Moreover, researchers need to go beyond providing large catalogues of altered genes and proteins, and particular care must be taken to put findings into biological contexts. As described above, these techniques can be used to study the regulation of groups of genes or proteins involved in the same function, rather than individual genes and proteins that have no obvious biological connections. Furthermore, both techniques require verification by independent means (e.g., in situ hybridization, northern blot, real-time reverse transcriptase polymerase chain reaction [Q-PCR], protein immunoblotting, immunohistochemistry).
5.5. Summary and conclusions
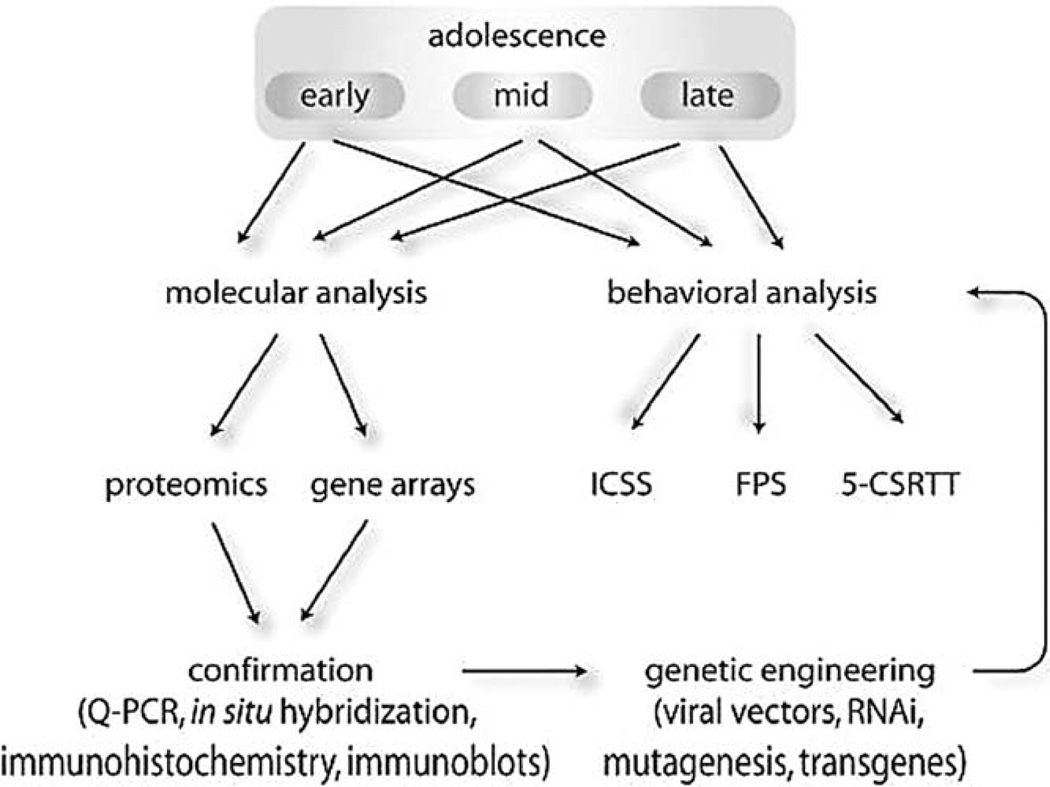
Because more children are being exposed to increasingly powerful psychotropic drugs at progressively younger ages, it has become critical to examine how exposure to these agents can alter brain development. In preclinical studies, an optimal approach is to study the behavioral and molecular consequences in parallel, and then to use genetic engineering to establish causal relations between brain biology and complex behaviors (Fig. 4). The identification of gross alterations in motivation and sensitivity to the rewarding effects of drugs of abuse and other important stimuli (food, sexual behavior) is a high priority. Additionally, the identification of equally important—but perhaps more subtle and difficult to detect—alterations in cognitive function and attention will also be critical, since these capabilities contribute to the quality of life and the potential to excel.
Fig. 4.
Schematic description of one strategy to establish causal relationships between psychotropic drug exposure-induced alterations in molecular biology and alterations in behavior. Ideally, molecular and behavioral analyses initially proceed in parallel, and involve drug exposure at different periods of brain development (e.g., early, mid, and late adolescence). When exposure-related molecular adaptations are identified, genetic engineering techniques can be used to mimic such changes to explore whether any particular adaptation is sufficient to cause alterations in motivation (e.g., using ICSS), learning (e.g., using FPS), or attention (e.g., using 5CSRTT).
Early intervention with psychotropic drugs against the background of underlying pathology may re-establish proper developmental trajectories, and thus has the potential to be a breakthrough innovation for psychiatry. However, until animal models that recapitulate the pathophysiology and behavioral symptomatology of disorders such as ADHD, MD, and BD become available—and until diagnosis of psychiatric disorders in young children is perfectly accurate—it will be important to understand how early developmental exposure to psychotropic drugs affect the normal brain. These types of studies will improve our understanding of biological vulnerability to addiction, as well as susceptibility to other mental conditions that may ultimately lead people to turn to drugs of abuse.
Acknowledgements
Research support is provided by DA12736 (to WC) and DA07134 (to CK). We thank Edward G. Meloni, Hilarie C. Tomasiewicz, and Tracie Paine for contributions to this manuscript.
References
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during early development. Nat. Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Archakov AI, Govorun VM, Dubanov AV, Ivanov YD, Veselovsky AV, Lewi P, Janssen P. Protein-protein interactions as a target for drugs in proteomics. Proteomics. 2003;3:380–391. doi: 10.1002/pmic.200390053. [DOI] [PubMed] [Google Scholar]
- Atkins JB, Chlan-Fourney J, Nye HE, Hiroi N, Carlezon, Nestler EJ. Region-specific induction of deltaFosB by repeated administration of typical versus atypical antipsychotic drugs. Synapse. 1999;33:118–128. doi: 10.1002/(SICI)1098-2396(199908)33:2<118::AID-SYN2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and κ-agonists are centrally mediated. Psychopharmacology. 1993;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol. Sci. 2003;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc. Natl. Acad. Sci. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner-Johnson D, Nestler EJ. Chronic morphine impairs axoplasmic transport in the rat mesolimbic dopamine system. Neuroreport. 1993;5:57–60. doi: 10.1097/00001756-199310000-00014. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Carroll ME, Dean NW, Crosby RD, Perwien AR, Benowitz NL. Caffeine withdrawal in normal school-age children. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37:858–865. doi: 10.1097/00004583-199808000-00016. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Carroll ME, Thuras PD, Cosgrove KP, Roth ME. Caffeine dependence in teenagers. Drug Alcohol Depend. 2002;66:1–6. doi: 10.1016/s0376-8716(01)00181-8. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20–e24. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res. Dev. Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol. Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol. Psychiatry. 2003;54:1338–1344. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S, Genua C, Simon H, LeMoal M, Piazza PV. Dose-dependent aversive and rewarding effects of amphetamine as revealed by a new place conditioning apparatus. Psychopharmacology. 1996;125:92–96. doi: 10.1007/BF02247398. [DOI] [PubMed] [Google Scholar]
- Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Mol. Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson V, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ, Neve RL. Viral-mediated gene transfer as a tool for neuropsychiatric research. Crit. Rev. Neurobiol. 2000;14:47–68. doi: 10.1080/08913810008443546. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol. Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: EdLiebman JM, Cooper SJ, editors. The Neuropharmacological Basis of Reward. Oxford: Clarendon Press; 1989. pp. 264–319. [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J. Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, Picciotto MR, Duman RS, Nestler EJ. Transgenic animals with inducible, targeted gene expression in brain. Mol. Pharmacol. 1998;54:495–503. doi: 10.1124/mol.54.3.495. [DOI] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology. 1984;82:6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat. Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scala G, Sandner G. Conditioned place aversion produced by FG 7142 is attenuated by haloperidol. Psychopharmacology. 1989;99:176–180. doi: 10.1007/BF00442804. [DOI] [PubMed] [Google Scholar]
- Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawlor SC, Conklin BR. MAPPfinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003;4:R7. doi: 10.1186/gb-2003-4-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorow R. FG 7142 and its anxiety-inducing effects in humans. Br. J. Clin. Pharmacol. 1987;23:781–782. [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Synaptic plasticity and mood disorders. Mol. Psychiatry. 2002;7:S29–S34. doi: 10.1038/sj.mp.4001016. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J. Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Brain Res. Dev. Brain Res. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Graber JA, Petersen AC, Brooks-Gunn J. Pubertal processes: methods, measures, and models. In: Graber JA, Petersen AC, Brooks-Gunn J, editors. Transitions during Adolescence: Interpersonal Domains and Context. Marwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 23–53. [Google Scholar]
- Grande C, Zhu H, Martin AB, Lee M, Ortiz O, Hiroi N, Moratalla R. Chronic treatment with atypical neuroleptics induces striosomal FosB/DeltaFosB expression in rats. Biol. Psychiatry. 2004;55:457–463. doi: 10.1016/j.biopsych.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnack L, Stang J, Story M. Soft drink consumption among US children and adolescents: nutritional consequences. J. Am. Diet. Assoc. 1999;99:436–441. doi: 10.1016/S0002-8223(99)00106-6. [DOI] [PubMed] [Google Scholar]
- Henry GL, Zito K, Dubnau J. Chipping away at brain function: mining for insights with microarrays. Curr. Opin. Neurobiol. 2003;13:570–576. doi: 10.1016/s0959-4388(03)00107-7. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Graybiel AM. Atypical and typical neuroleptic treatments induce distinct programs of transcription factor expression in the striatum. J. Comp. Neurol. 1996;374:70–83. doi: 10.1002/(SICI)1096-9861(19961007)374:1<70::AID-CNE5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat. Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- Horger BA, Wellman PJ, Morien A, Davies BT, Schenk S. Caffeine exposure sensitizes rats to the reinforcing effects of cocaine. Neuroreport. 1991;2:53–56. doi: 10.1097/00001756-199101000-00013. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Methylphenidate-induced plasticity: what should we be looking for? Biol Psychiatry. 2003;54:1310–1311. doi: 10.1016/j.biopsych.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Shi CJ, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response-element binding protein overexpression in the amygdala. J. Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen JS, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann A, Steffan C, Zheng YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Piciotto MR, Nestler EJ. Expression of the transcription factor AFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol. Psychiatry. 2001;50:729–742. doi: 10.1016/s0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotion or conditioned place aversion. J. Pharmacol. Exp. Ther. 1994;269:137–144. [PubMed] [Google Scholar]
- Kosten TA, DeCaprio JL, Nestler EJ. Long-term haloperidol administration enhances and short-term administration attenuates the behavioral effects of cocaine in a place conditioning procedure. Psychopharmacology. 1996;128:304–312. doi: 10.1007/s002130050138. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J. Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat. Rev. Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J. Pharmacol. Exp. Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Conditional control of gene expression in the mouse. Nat. Rev. Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Click SD. U50,488, a κ opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci. Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol. Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- Manji HK, Gottesman II, Gould TD. Signal transduction and genes-to-behaviors pathways in psychiatric diseases. Sci. STKE. 2003 doi: 10.1126/stke.2003.207.pe49. (pe49) [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Effects of cocaine self-administration on plasma corticosterone in rats: relationship to hippocampal type II glucocorticoid receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2000;24:633–646. doi: 10.1016/s0278-5846(00)00098-1. [DOI] [PubMed] [Google Scholar]
- Markou A, Hauger RL, Koob GF. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology. 1992;109:305–314. doi: 10.1007/BF02245878. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology. 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiol. Behav. 1986;37:85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DC. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci. Biobehav. Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc. Natl. Acad. Sci. USA. 1999;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol. Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Petersen AC. Adolescence. In: Blechman EA, Brownell KD, editors. Behavioral Medicine and Women: A Comprehensive Handbook. New York, NY: Guilford Press; 1998. pp. 45–50. [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J. Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J. Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack CP, Bright D. Caffeine consumption and weekly sleep patterns in US seventh-, eighth-, and ninth-graders. Pediatrics. 2003;111:42–46. doi: 10.1542/peds.111.1.42. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger B, Snow S. Caffeine preexposure sensitizes rats to the motor activating effects of cocaine. Behav. Pharmacol. 1990;1:447–451. [PubMed] [Google Scholar]
- Shippenberg TS, Rea W. Sensitization to the behavioral effects of cocaine: modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol. Biochem. Behav. 1997;57:449–455. doi: 10.1016/s0091-3057(96)00450-9. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev. Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Steinberg TH, Agnew BJ, Gee KR, Leung W-Y, Goodman T, Schulenberg B, Hendrickson J, Beechem JM, Haugland RP, Patton WF. Global quantitative phosphoprotein analysis using Multiplexed Proteomics technology. Proteomics. 2003;3:1128–1144. doi: 10.1002/pmic.200300434. [DOI] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J. Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Terwilliger R, Lane C, Mezes PS, Conti M, Duman RS. Chronic antidepressant administration increases the expression of cAMP-specific phosphodiesterase 4A and 4B isoforms. J. Neurosci. 1999;19:610–618. doi: 10.1523/JNEUROSCI.19-02-00610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Ito Y, Glod CA, Barber NI. Objective measurement of hyperactivity and attentional problems in ADHD. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35:334–342. doi: 10.1097/00004583-199603000-00015. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa opioid ligands on intracranial self-stimulation in rats. Psychopharmacology. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Jr, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Pollack AE, Fink JS. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 1997;749:120–126. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Kros JM, Uylings HB. The development of the rat prefrontal cortex. Its size and development of connections with thalamus, spinal cord and other cortical areas. Prog. Brain Res. 1990;85:169–183. doi: 10.1016/s0079-6123(08)62680-1. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biol. Psychiatry. 2003;54:1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol. Biochem. Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu. Rev. Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Zhu H, Bilgin M, Snyder M. Proteomics. Annu. Rev. Biochem. 2003;72:783–812. doi: 10.1146/annurev.biochem.72.121801.161511. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. J. Am. Med. Assoc. 2000;283:1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]