Abstract
Background
People with schizophrenia from families that express high levels of criticism, hostility, or over involvement, have more frequent relapses than people with similar problems from families that tend to be less expressive of emotions. Forms of psychosocial intervention, designed to reduce these levels of expressed emotions within families, are now widely used.
Objectives
To estimate the effects of family psychosocial interventions in community settings for people with schizophrenia or schizophrenia-like conditions compared with standard care.
Search strategy
We updated previous searches by searching the Cochrane Schizophrenia Group Trials Register (September 2008).
Selection criteria
We selected randomised or quasi-randomised studies focusing primarily on families of people with schizophrenia or schizoaffective disorder that compared community-orientated family-based psychosocial intervention with standard care.
Data collection and analysis
We independently extracted data and calculated fixed-effect relative risk (RR), the 95% confidence intervals (CI) for binary data, and, where appropriate, the number needed to treat (NNT) on an intention-to-treat basis. For continuous data, we calculated mean differences (MD).
Main results
This 2009-10 update adds 21 additional studies, with a total of 53 randomised controlled trials included. Family intervention may decrease the frequency of relapse (n = 2981, 32 RCTs, RR 0.55 CI 0.5 to 0.6, NNT 7 CI 6 to 8), although some small but negative studies might not have been identified by the search. Family intervention may also reduce hospital admission (n = 481, 8 RCTs, RR 0.78 CI 0.6 to 1.0, NNT 8 CI 6 to 13) and encourage compliance with medication (n = 695, 10 RCTs, RR 0.60 CI 0.5 to 0.7, NNT 6 CI 5 to 9) but it does not obviously affect the tendency of individuals/families to leave care (n = 733, 10 RCTs, RR 0.74 CI 0.5 to 1.0). Family intervention also seems to improve general social impairment and the levels of expressed emotion within the family. We did not find data to suggest that family intervention either prevents or promotes suicide.
Authors’ conclusions
Family intervention may reduce the number of relapse events and hospitalisations and would therefore be of interest to people with schizophrenia, clinicians and policy makers. However, the treatment effects of these trials may be overestimated due to the poor methodological quality. Further data from trials that describe the methods of randomisation, test the blindness of the study evaluators, and implement the CONSORT guidelines would enable greater confidence in these findings.
Medical Subject Headings (MeSH): *Expressed Emotion, *Family Therapy, *Social Support, Family Relations, Randomized Controlled Trials as Topic, Recurrence [prevention & control], Schizophrenia [*therapy]
MeSH check words: Humans
BACKGROUND
In 1972 an influential study showed that people with schizophrenia from families that express high levels of criticism, hostility, or over involvement have more frequent relapses than people with similar problems from families that tend to be less expressive of their emotions (Brown 1972). A variety of psychosocial interventions designed to reduce these levels of expressed emotions within families now exist. The aim of using these psychosocial approaches is to decrease stress within the family as well as the rate of relapse. These interventions are proposed as adjuncts rather than alternatives to drug treatments.
Description of the condition
Schizophrenia is a chronic, relapsing mental illness and has a worldwide lifetime prevalence of about 1% irrespective of culture, social class and race. Schizophrenia is characterised by positive symptoms such as hallucinations and delusions and negative symptoms such as emotional numbness and withdrawal. One-quarter of those who have experienced an episode of schizophrenia recover and the illness does not recur. Another 25% experience an unremitting illness. Half do have a recurrent illness but with long episodes of considerable recovery from the positive symptoms. Current medication is effective in reducing positive symptoms, but negative symptoms are fairly resistant to treatment. In addition, drug treatments are associated with adverse effects and the overall cost of the illness to the individual, their carers and the community is considerable.
Description of the intervention
Psychosocial family interventions may have a number of different strategies. These include: (a) construction of an alliance with relatives who care for the person with schizophrenia; (b) reduction of adverse family atmosphere (that is, lowering the emotional climate in the family by reducing stress and burden on relatives); (c) enhancement of the capacity of relatives to anticipate and solve problems; (d) reduction of expressions of anger and guilt by the family; (e) maintenance of reasonable expectations for patient performance; (f) encouragement of relatives to set and keep to appropriate limits whilst maintaining some degree of separation when needed; and (g) attainment of desirable change in relatives’ behaviour and belief systems.
How the intervention might work
By reducing levels of expressed emotion, stress, family burden, and enhancing the capacity of relatives to solve problems, whilst maintaining patient compliance with medication, family intervention aims to reduce relapse and subsequent hospitalisation.
Why it is important to do this review
Many important qualitative reviews highlight the possible advantages of using family interventions for those with serious mental illnesses (Leff 1995). Quantitative reviews are less common (Mari 1994).
OBJECTIVES
To estimate the effects of family psychosocial interventions in community settings for the care of people with schizophrenia or schizophrenia-like conditions.
METHODS
Criteria for considering studies for this review
Types of studies
We included all relevant randomised or quasi-randomised controlled trials.
Types of participants
We included families of people who have a diagnosis of schizophrenia and/or schizoaffective disorder. As a result of Szmukler 2003, we reconsidered the inclusion criteria. This study evaluated family interventions for a group that included people without schizophrenia-like illnesses (less than 17%). It would seem harsh to exclude this study because everyone did not have schizophrenia, and therefore devalue the results of this review for clinicians dealing with a mixed group for whom they feel family intervention may be indicated. Because this decision is post hoc we have included and excluded the data from Szmukler 2003 in order to see if inclusion made a substantive difference. We have discussed the results of these sensitivity analyses below. The objectives of the review remain to estimate the effects of family psychosocial interventions in community settings for the care of people with schizophrenia or schizophrenia-like conditions. Entry criteria for this update have changed and now studies are eligible where most (more than 75%) families include one member with a diagnosis of schizophrenia and/or schizoaffective disorder.
Types of interventions
Any psychosocial intervention with relatives of those with schizophrenia that required more than five sessions.
Standard care, but this was not restricted to an in-patient context/environment.
Types of outcome measures
Primary outcomes
-
1
Suicide and all causes of mortality
-
2
Service utilisation
-
2.1
Hospital admission
-
3
Clinical global response
-
3.1
Relapse
Secondary outcomes
-
1
Service utilisation
-
1.2
Days in hospital
-
2
Clinical global response
-
2.2
Global state - not improved
-
2.3
Average change or endpoint score in global state
-
2.4
Leaving the study early
-
2.5
Compliance with medication
-
3
Mental state and behaviour
-
3.1
Positive symptoms (delusions, hallucinations, disordered thinking)
-
3.2
Negative symptoms (avolition, poor self-care, blunted affect)
-
3.3
Average change or endpoint score
-
4
Social functioning
-
4.1
Average change or endpoint scores
-
4.2
Social impairment
-
4.3
Employment status (employed/unemployed)
-
4.4
Work related activities
-
4.5
Unable to live independently
-
4.6
Imprisonment
-
5
Family outcome
-
5.1
Average score/change in family burden
-
5.2
Patient and family coping abilities
-
5.3
Understanding of the family member with schizophrenia
-
5.4
Family care and maltreatment of the person with schizophrenia
-
5.5
Expressed emotion
-
5.6
Quality of life/satisfaction with care for either recipients of care or their carers
-
6
Economic outcomes
-
6.1
Cost of care
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group Trials Register (update September 2008)
We searched the register using the phrase:
[(*family* or family*) in title, abstract, index terms of REFERENCE] or [(*family* or family*) in interventions of STUDY
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
2. Previous searches from earlier versions of this review
Please see (Appendix 1).
Searching other resources
1. Handsearching
We searched the reference lists of the review articles and the primary studies to identify possible articles missed by the computerised search.
2. Personal contact
We contacted authors for information regarding unpublished trials.
Data collection and analysis
Selection of studies
We independently inspected all reports. We resolved any disagreement by discussion, and where doubt remained, we acquired the full article for further inspection. Once we obtained the full articles, we independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we sought further information and added these trials to the list of those awaiting assessment.
Data extraction and management
We independently extracted data from selected trials. When disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting classification.
Assessment of risk of bias in included studies
We assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases.
If disputes arose as to which category a trial has to be allocated, again, we achieved resolution by discussion, after working with a third reviewer.
Earlier versions of this review used a different, less well-developed, means of categorising risk of bias (see Appendix 2).
Measures of treatment effect
1. Binary data
For binary outcomes we calculated the relative risk (RR) and its 95% confidence interval (CI) based on the fixed-effect model. Relative risk is more intuitive (Boissel 1999) than odds ratios, and odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. When the overall results were significant we calculated the number needed to treat (NNT) and the number needed to harm (NNH). Where people were lost to follow up at the end of the study, we assumed that they had had a poor outcome and once they were randomised they were included in the analysis (intention-to-treat/ITT analysis).
Where possible, we made efforts to convert outcome measures to binary data. This can be done by identifying cut-off points on rating scales and dividing participants accordingly into “clinically improved” or “not clinically improved”. It is generally assumed that if there is a 50% reduction in a scale-derived score such as the Brief Psychiatric Rating Scale (BPRS Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986, this could be considered a clinically significant response (Leucht 2005a, Leucht 2005). It is recognised that for many people, especially those with chronic or severe illness, a less rigorous definition of important improvement (e.g. 25% on the BPRS) would be equally valid. If individual patient data are available, we used the 50% cut-off point for non-chronically ill people and a 25% cut-off point for those with chronic illness. If data based on these thresholds were not available, we used the primary cut-off presented by the original authors.
2. Continuous data
2.1 Skewed data
Continuous data on outcomes in mental health trials are often not normally distributed. To avoid the pitfall of applying parametric tests to non-parametric data, we applied the following standards to all endpoint data derived from continuous measures. The criteria were used before inclusion: (a) standard deviations and means had to be obtainable; and, for finite scores, such as endpoint measures on rating scales, (b) the standard deviation (SD), when multiplied by two, had to be less than the mean (as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution) (Altman 1996). If a scale starts from a positive value (such as PANSS, which can have values from 30 to 210) the calculation described above in (b) should be modified to take the scale starting point into account. In these cases skew is present if 2SD>(S-Smin), where S is the mean score and Smin is the minimum score.
We did not show skewed endpoint data from studies with fewer than 200 participants graphically, but added these to the ‘Other data’ tables and briefly commented on in the text. However, skewed endpoint data from larger studies (200 or more participants) pose less of a problem and we entered the data for analysis.
For continuous mean change data (endpoint minus baseline) the situation is even more problematic. In the absence of individual patient data it is impossible to know if change data are skewed. The RevMan meta-analyses of continuous data are based on the assumption that the data are, at least to a reasonable degree, normally distributed. Therefore we included such data, unless end-point data were also reported from the same scale.
2.2 Final endpoint value versus change data
Where both final endpoint data and change data were available for the same outcome category, we presented only final endpoint data. We acknowledge that by doing this much of the published change data may be excluded, but argue that endpoint data is more clinically relevant and that if change data were to be presented along with endpoint data, it would be given undeserved equal prominence. We have contacted authors of studies reporting only change data for endpoint figures.
2.3 Crossover design
Where we have included crossover design studies, we have negated the potential additive effect in the second or later stages on these trials by only analysing data from the first stage.
2.4 Scale-derived data
A wide range of instruments are available to measure mental health outcomes. These instruments vary in quality and many are not valid, and are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore we included continuous data from rating scales only if the measuring instrument had been described in a peer-reviewed journal.
Whenever possible we took the opportunity to make direct comparisons between trials that used the same measurement instrument to quantify specific outcomes. Where continuous data were presented from different scales rating the same effect, we presented both sets of data and inspected the general direction of effect.
2.5 Tables and figures
Where possible we entered data into RevMan in such a way that the area to the left of the line of no effect indicated a favourable outcome for family intervention.
Unit of analysis issues
Studies increasingly employ cluster randomisation (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a unit-of-analysis error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes Type I errors (Bland 1997, Gulliford 1999).
Where clustering had not been accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra-class correlation co-efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non-cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a design effect. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co-efficient (ICC) (Design effect = 1+(m−1)*ICC) (Donner 2002). If the ICC was not reported we assumed it to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
Dealing with missing data
We excluded data from studies where more than 50% of participants in any group were lost to follow up (this did not include the outcome of ‘leaving the study early’). In studies with less than 50% dropout rate, people leaving early were considered to have had the negative outcome, For example, we treated those lost to follow up for the outcome of relapse as having relapsed in the analysis. We also treated suicide as relapse.
Assessment of heterogeneity
Firstly, we considered all the included studies within any comparison to judge for clinical heterogeneity. Then we visually inspected graphs to investigate the possibility of statistical heterogeneity. We supplemented this by using primarily the I2 statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I2 estimate was greater than or equal to 50%, we interpreted this as indicating the presence of considerable levels of heterogeneity (Higgins 2003).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We are aware that funnel plots may be useful in investigating reporting biases, but are of limited power to detect small-study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
Where possible, we used a fixed-effect model for analyses. We understand that there is no closed argument for preference for use of fixed-effect or random-effects models. The random-effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us, however, random-effects does put added weight onto the smaller studies - those trials that are most vulnerable to bias.
Subgroup analysis and investigation of heterogeneity
When we found heterogeneous results, we investigated the reasons for this. Where heterogeneous data substantially altered the results and we identified the reasons for the heterogeneity, we did not summate these studies in the meta-analysis, but presented them separately and discussed them in the text.
Sensitivity analysis
Earlier versions of this review did not undertake any sensitivity analyses. This 2010 update also had not pre-planned any. However, because we have added so many new studies from China, and because of concern regarding the quality of trials from China (Wu 2006), we decided to undertake a sensitivity analysis testing, for the primary outcomes, to determine whether addition of the Chinese trials did have any substantial effect on the overall results.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
For substantive descriptions of studies, please see Characteristics of included studies and Characteristics of excluded studies
Results of the search
1. The search
We found 855 references from 327 studies during the September 2008 search. In earlier searches, the 2002 update search yielded 1078 citations, and the June 2005 update identified 104 citations.
Included studies
1. Included trials
We were able to include 53 studies in total; this includes 21 added in the 2010 update.
2. Methods
All trials were described as ‘randomised’. Hogarty 1997, used a quasi-random method by allocating (‘on alternate weeks or months’) participants before they were admitted. The demographic data suggests that this process resulted in evenly balanced groups so we have included data, although they must be viewed with caution. Also, Gong 2007 and Liu 2003 used a quasi-randomised method of allocation. Ran 2003 block randomised participants into clusters using six different townships as units. Chen 2005 also used a cluster randomised design but did not report the number of clusters used. Szmukler 2003 (unpublished data) reports using an exploratory randomised controlled trial to evaluate the effectiveness of a carers intervention, using permuted blocks with varying block sizes and sample stratification. The majority of studies did not describe the method used to randomly allocate participants to treatment. However, some studies reported using computer generated randomisation, or block randomisation to achieve balanced groups. Carra 2007 described concealment of allocation by the use of an external statistician who was not involved in enrolling participants and was responsible for the method of sequence generation; all other included studies did not describe how sequence generation was concealed from the investigators and participants, and doubt remains as to how impervious all methods of allocation are to the introduction of bias.
Most trials did not achieve full blindness although many studies attempted to single blind at least some measurements (Barrowclough 2001; Falloon 1981; Goldstein 1978; Leavey 2004; Leff 1989;Linszen 1996; Merinder 1999; Tarrier 1988; Vaughan 1992; Xiong 1994; Zhang 1994).
3. Length of treatment
Length of treatment varied from six weeks (Bloch 1995; Goldstein 1978) to three years (Hogarty 1997). Hogarty 1997 also followed participants up for an additional three years.
4. Setting
Studies were conducted in Australia (two trials), Canada (one trial), Europe (12 trials), the People’s Republic of China (28 trials) and the USA (10 trials).
5. Participants
Participants in all the included trials (except Szmukler 2003 and Leavey 2004) were diagnosed as having schizophrenia or schizoaffective disorder. Most studies used structured clinical assessments to determine the diagnosis (DSM 20 studies, CCMD 15 studies, ICD-10 seven studies, RDC two studies, New Haven Index one study, and PSE six studies). Szmukler 2003 included more than 80% with a diagnosis of schizophrenia-like illnesses, whilst the remainder suffered from bipolar affective disorder, or psychotic depression. Leavey 2004 included people described as having a psychotic illness. Overall, the age of participants ranged from 16 to 80 years. Of those studies which reported the sex of the participants, most included both men and women, although Glynn 1992, Liu 2007, Zhang 1994, and Zhang 2006a included only male patients. Patients had varied histories. Most studies involved families whose relatives had had multiple admissions, although three trials did involve substantial proportions of people with first episodes of illness (Goldstein 1978; Linszen 1996; Zhang 1994).
6. Interventions
6.1 Intervention group
All participants received family interventions and some had an educational component. Thirteen trials included family therapy in the presence of patients (Barrowclough 2001; De Giacomo 1997; Dyck 2002; Falloon 1981; Glynn 1992; Goldstein 1978;Herz 2000; Leff 1982; Leff 2001; Linszen 1996; Mak 1997;Xiong 1994; Zhang 1994) whilst eight restricted the groups to relatives (Bloch 1995; Buchkremer 1995; Chien 2004; Hogarty 1997; Leavey 2004; Posner 1992; Tarrier 1988; Vaughan 1992). Szmukler 2003 conducted family sessions mostly without the patient being present. Overall, the main aim of the family-based interventions, when reported, was to improve family atmosphere and reduce relapse of schizophrenia.
In addition to ‘standard’ family intervention (i.e. schizophrenia education and behavioural modification), the family intervention groups used other non-pharmacological approaches as part of their strategy. Barrowclough 2001 used motivational interviewing and cognitive behavioural intervention. Falloon 1981 provided 24-hour support for the family therapy group. Goldstein 1978 utilised a ‘crisis-orientated’ family intervention as part of the family intervention and Hogarty 1997 incorporated relaxation training for the intervention group and educated the ‘family’ on stressors for schizophrenia and prodromal symptoms. Role-play was used by Tarrier 1988 as a means of educating family members on how to manage schizophrenia, whereas Vaughan 1992 incorporated homework exercises for the family members.
6.2 Comparison group
The control groups were all given standard care or usual level of care that involved pharmacological interventions. Bloch 1995 provided the control group with a single session discussion about the study, and also gave participants educational material describing schizophrenia. Leff 2001 gave two sessions of education about schizophrenia to the control group. Szmukler 2003 provided a single one-hour session for the control group in which the study was described and the carers discussed their problems; carers were also provided with the same written and video information as the intervention group. Further measures were employed by Falloon 1981 who used supportive psychotherapy for the control arm of the study. Linszen 1996 and Merinder 1999 provided psychosocial support in an individualised context without family involvement.
7. Outcomes
Data we were able to extract included the outcomes of death, mental state, compliance (including compliance with medication and leaving the study early), quality of life, social functioning and measures of family functioning. Ran 2003 reported data as if from a non-cluster randomised study; the analyses were based on the numbers of individual families, with no account taken of the clustering effect. We sought statistical advice from the MRC Biostatistics Unit, Cambridge, UK. Dr Julian Higgins advised that the binary data as presented in the report should be divided by a ‘design effect’ and that this should be calculated using the mean number of families in the groups (m) and the intraclass correlation coefficient (ICC) (Design effect = 1+(m−1)*ICC). We contacted Dr Ran to obtain the ICC. Dr Ran kindly replied but ICC values were not available, so we assumed this to be 0.1 (Ukoumunne 1999). We have listed scales below that provided data for the review.
7.1 Global state
7.1.1 Global Assessment of Functioning - GAF
The GAF (APA 1987) allows the clinician to express the patient’s psychological, social and occupational functioning on a continuum extending from superior mental health, with optimal social and occupational performance to profound mental impairment when social and occupational functioning are precluded. Ratings are made on a scale of 0 to 90. Higher scores indicate a better outcome. Barrowclough 2001, Merinder 1999 and Xiong 1994 reported data from this scale.
7.2 Mental state
7.2.1 Brief Psychiatric Rating Scale - BPRS
The BPRS is an 18-item scale measuring positive symptoms, general psychopathology and affective symptoms (Overall 1962). The original scale has 16 items, but a revised 18-item scale is commonly used. Scores can range from 0-126. Each item is rated on a seven-point scale varying from ‘not present’ to ‘extremely severe’, with higher scores indicating more severe symptoms. The BPRS was used in Linszen 1996, Fernandez 1998, Merinder 1999, Xiong 1994 and Zhang 1994 as part of the definition of relapse, and Magliano 2006, Merinder 1999 and Xiong 1994 reported BPRS mental state scores.
7.2.2 Positive and Negative Syndrome Scale - PANSS
This scale was developed to evaluate the positive, negative and general symptoms in schizophrenia (Kay 1987). It has 30 items, and each of these can be defined on a seven-point scoring system varying from one (absent) to seven (extreme). The scale can be divided into three sub-scales for measuring the severity of general psychopathology, positive symptoms (PANSS-P) and negative symptoms (PANSS-N). Higher scores indicate more symptoms. This scale was used by Barrowclough 2001, Dai 2007 and Liu 2003 to monitor treatment changes in schizophrenia.
7.2.3 Frankfurt Complaint Inventory - FBF-3
This is a 98-item self-application questionnaire in which the patient assesses the presence of subjective complaints on 10 clinical scales (loss of control, simple perception, complex perception, speech, cognition and thought, memory, motor behaviour, loss of automatisms, anhedonia, and anxiety and irritability due to stimuli overload) (Süllwold 1986). Higher scores indicate greater symptomology. A Spanish version of this scale (Jimeno 1996) was used in Fernandez 1998.
7.2.4 Insight Scale - IS
This is an eight-item questionnaire (Birchwood 1994). Three factors are scored: awareness of illness; need for treatment; and attribution of symptoms on a three-point scale. Higher scores indicate improvement in insight. This was used in Merinder 1999 to assess insight into psychosis and need for treatment.
7.2.5 Symptom Checklist 90 - SCL-90
The SCL-90 is a self-report clinical rating scale of psychiatric symptomatology (Derogatis 1976). It consists of 90 items, with 83 items representing nine sub-scales: somatization (n = 12 items), obsessive-compulsive (n = 10 items), interpersonal sensitivity (n = 9 items), depression (n = 13 items), anxiety (n = 10 items), angerhostility (n = 6 items), phobic anxiety (n = 7 items), paranoid ideation (n = 6 items) and psychoticism (n = 10 items). Seven additional items include disturbances in appetite and sleep. The SCL-90 also utilises three global distress indices: Global Severity Index (GSI), Positive Symptom Distress Index (PSDI), Positive Symptom Total (PST). Items are rated on a five-point Likert scale, ranging from “not at all distressing” (0) to “extremely distressing” (4), with higher scores indicating greater symptomatology. Li 2005a reported data from this scale.
7.2.6 Present State Examination - 9th Edition - PSE
This is a clinician-rated scale measuring mental status (Wing 1974). It rates 140 symptom items, which are combined to give various syndrome and sub-syndrome scores. Higher scores indicate greater clinical impairment. Tarrier 1988 and Vaughan 1992 used the PSE to help define relapse.
7.2.7 Scale for the Assessment of Negative Symptoms - SANS
This scale was used in Bradley 2006 and Xiong 1994 to assess negative symptoms (Andreasen 1982). This is a six-point scale, providing a global rating of the following negative symptoms: alogia; affective blunting; avolition-apathy; anhedonia-asociality and attention impairment. Higher scores indicate more symptoms.
7.2.8 Scale for the Assessment of Positive Symptoms - SAPS
This scale was used in Xiong 1994 to assess positive symptoms (Andreasen 1982). This is a six-point scale providing a global rating of positive symptoms such as delusions, hallucinations and disordered thinking. Higher scores indicate more symptoms.
7.3 Social functioning
7.3.1 Health of the Nation Outcome Scale - HoNOS
The HoNOS scale is used to rate various aspects of mental and social health, on a scale of 0-4 (Amin 1999). It is designed to be used by clinicians before and after interventions, so that changes attributable to the interventions can be measured. Higher scores indicate a worse outcome. Bradley 2006 reported data from this scale.
7.3.2 Social Disability Screening Schedule - SDSS
THE SDSS is a Chinese simplified version of the World Health Organization’s Disability Assessment Schedule and assesses 10 different aspects of social functioning (WHO 1988). Higher scores indicate a worse outcome. Dai 2007 and Tan 2007 reported data from this scale.
7.3.3 Social Functioning Scale - SFS
This scale (Birchwood 1990) was used in Barrowclough 2001, Fernandez 1998 and Leff 2001 to measure the ability of people with schizophrenia to function in the community.
7.4 Family outcome
7.4.1 Coping with Life Events and Difficulties Interview - COPI
A semi-structured interview with 29 items reflecting the carer’s subjective response or style of coping with a severe event or marked difficulty in terms of problem tackling, and cognitive and emotional responses (Bifulco 1996). A high score is poor. Szmukler 2003 reported data from this scale.
7.4.2 Family Support Service Index - FSSI
The Family Support Service Index measures the formal support services needed and their usage by psychiatric patients and their families (Heller 1991). Higher scores indicate a greater need for family support. This index was used by Chien 2004.
7.4.3 Family Assessment Device - FAD
The Family Assessment Device assesses multiple dimensions of family functioning for patients with mental disorders and other conditions (Epstein 1983). It consists of 60 items, each of which is rated on a four-point Likert scale (from 1= strongly disagree, to 4= strongly agree) along seven dimensions: problem solving; communication; roles; affective responsiveness; affective involvement; behavioural control and general functioning. The total scores range from four to 28, with higher scores reflecting poorer family functioning. This scale was used by Chien 2004.
7.4.4 Family Burden Interview Schedule - FBIS
The Family Burden Interview Schedule is a 25-item semi-structured interview schedule to assess the burden of care placed on families of a psychiatric patient living in the community (Pai 1981). It comprises six categories of perceived burden (with 2-6 items in each category): family finance, routine, leisure, interaction, physical and mental health. The items are rated on a three-point Likert scale (0 = no burden, 1 = moderate burden and 2 = severe burden). The total scores ranged from 0 to 50 with higher scores indicating higher burden or care. This scale was used by Chien 2004.
7.4.5 Camberwell Family Interview - CFI
The CFI is a measure of expressed emotions, of criticisms and of unfavourable attention (Vaughn 1976). The CFI is a long, complex and difficult structured interview for which extensive training is needed. Reliability is usually acceptable, but for ‘warmth’ it is low even after extensive training. Yet it seems to be superior to most alternative measures of expressed emotions and family atmosphere. The ratings are undertaken from videos of family interaction and focuses on the number of critical comments expressed. A high score is poor. Leff 2001 and Tarrier 1988 reported data from this scale.
7.4.6 Clinical Interview Schedule Revised - CIS-R
This scale provides a global score of psychological morbidity (Lewis 1992). The main purpose of the CIS-R is to identify the presence of neurosis and to establish the nature and severity of neurotic symptoms. There are 15 sections to the scale covering: somatic symptoms; fatigue; concentration and forgetfulness; sleep problems; irritability; worry about physical health; depression; depressive ideas; anxiety; phobias; panic; compulsions; obsessions and overall effects. A high score is poor. Szmukler 2003 reported data from this scale.
7.4.7 Experience of Care giving Inventory - ECI
A self-report measure of the experience of caring for a relative with a serious mental illness, with care giving conceptualised in a stress-appraisal-coping framework (Szmukler 1996). A 66-item version taps dimensions of care giving distinct from, but linked with, coping and psychological morbidity. This scale was dichotomised by the authors of Bloch 1995. A high score is poor. Continuous data from this scale were reported in Szmukler 2003.
7.4.8 Family Questionnaire - FQ
The Family Questionnaire is a brief self-rating scale for assessing the expressed emotional status of relatives of people with schizophrenia (Wiedemann 2002). The scale comprises 20 questions on a four-point scale with a low score indicating a better outcome, and was used by Merinder 1999.
7.4.9 Verona Service Satisfaction Scale - VSSS
This is a self-administered questionnaire that assesses satisfaction with services on a five-point scale across seven dimensions: overall satisfaction; professional skills and behaviour; information; access; efficacy; types of intervention and relatives’ involvement (Ruggeri 1993). The five points are 0-1 = “terrible”, 1-2 = “mostly dissatisfied”, 2-3 = “mixed”, 3-4 = “mostly satisfied”, 4-5 = “excellent”. This scale was used by Merinder 1999.
7.4.10 Ways of Coping - WOC
This scale describes cognitive and behavioural strategies for coping with stressful events over the preceding month (MacCarthy 1989b). Higher scores indicate poorer coping. This scale was dichotomised by the authors of Bloch 1995.
7.4.11 Self Evaluation and Social Support Schedule - SESS
This is a structured interview schedule to assess availability of confidants (Andrews 1991). It contains detailed questions about the relationship with the primary confidant, including closeness, confiding, intimacy, dependency, and negative interactions. This involves three to four hours administration time and extensive interviewer and rater training. It is not appropriate for large-scale epidemiologic studies. Szmukler 2003 reported data from this scale.
7.4.12 Adaptability, Partnership, Growth, Affection, and Resolve - APGAR
This scale assesses a family member’s perception of family functioning by examining his/her satisfaction with family relationships (Smilkstein 1978).The measure consists of five parameters of family functioning: Adaptability, Partnership, Growth, Affection, and Resolve. The response options were designed to describe frequency of feeling satisfied with each parameter on a 3-point scale ranging from 0 (hardly ever) to 2 (almost always).The items were developed on the premise that a family member’s perception of family functioning could be assessed by reported satisfaction with the five dimensions of family functioning listed above. Higher scores indicate better family functioning. Du 2005 reported data from this scale.
7.5 Behaviour
7.5.1 The Nurses’ Observation Scale for Inpatient Evaluation - NOSIE
The Nurses’ Observation Scale for Inpatient Evaluation (NOSIE) is a highly sensitive ward behaviour rating scale (Honigfeld 1965a). Final item selection includes the best 30 of an original pool of 100 items. Higher scores indicate a poor outcome. Dai 2007 reported data from this scale.
Two summary scales were used:
-
Confidents or Very Close Others (Brown 1986)
The Confidents or Very Close Others summary scale assesses the relationship between the carer and two core contacts, i.e. the first two people a carer would confide in about a problem. Seven questions cover degree of confiding, emotional support, absolution from guilt, practical support, negative verbal and behavioural response and perception of helpfulness. Szmukler 2003 reported data from this scale.
-
General Community Support (Brown 1986)
The General Community Support subscale comprises five questions on the broader network or more diffuse social contacts of the carer, e.g. non-core relatives and acquaintances including possible neighbours, local shop keepers, pub-owners and church personnel. An estimate is made of the number of people having a positive or negative attitude towards the patient’s illness and the degree of practical or emotional support given or negative response made. Szmukler 2003 reported data from this scale.
7.6 Quality of Life
7.6.1 Quality of Life - QoL
This is a 21 item scale which measures both quality of life and negative/deficit symptoms of schizophrenia in adults and utilises a semi-structured interview (Heinrichs 1984). The categories covered are physical functioning, occupational role, interpersonal relationships and psychological functioning. Each item is rated on a seven-point scale, which requires the clinical judgement of the interviewer. Data from this scale were reported by Bradley 2006 and Shi 2000.
7.7 Redundant data
A large number of scales were used in the studies. Many measures, even those within included studies, were reported in such a way as to render the results unusable. Data were either not reported at all or did not distinguish treatment groups. Where data were presented it was common not to have means or variances reported or inaccurate P values presented.
Excluded studies
1. Excluded studies
We excluded 79 studies. Of these, 21 (28%) were not randomised and 23 (30%) involved people in hospital or interventions that could not be described as family intervention compared with standard care. Thirty-one studies (42%) did not report outcome data or presented it in a form that we could not use.
2. Awaiting assessment
No studies are awaiting assessment.
3. Ongoing studies
We are not aware of any ongoing studies.
Risk of bias in included studies
Only eight studies, from the total of 53, described the method of randomisation, and only one was explicit about the method of allocation concealment. Blinding was not always possible for family intervention, although few studies attempted, or at least failed to report whether the investigators assessing the participants were blind to treatment allocation. Similarly, study attrition was often omitted from the results, yet it is unlikely that group sizes remained constant throughout the investigation period. We did not have access to the included studies protocols, and were therefore unable to judge whether various tests of effectiveness had been omitted from the published findings. The effect of these potential biases is that the outcomes in this review may overestimate effects.
Allocation
Random allocation to treatment group was only described in eight of the 53 included studies, although all included studies were stated to be randomised. Three trials used a quasi-randomisation technique (Gong 2007; Hogarty 1997; Liu 2003) and it is possible that these studies are unacceptably open to the introduction of bias at the point of allocation. Only Carra 2007 described measures to conceal the sequence of allocation from participants and investigators. Ran 2003 was a cluster randomised trial. This methodology is likely to become more common but should be accompanied by reporting of the intra-class correlation coefficient (ICC). We have been forced to estimate this coefficient and, as a result, may be underemphasising the importance of this study.
Blinding
Trialists were aware of the possibility of the introduction of observer bias by not blinding the raters to the group to which people or families were allocated. Ten studies reported that no form of blinding was used (Bloch 1995; Buchkremer 1995; De Giacomo 1997; Fernandez 1998; Glynn 1992; Herz 2000; Leavey 2004;Leff 1982; Shi 2000; Szmukler 2003). A further 16 studies did not mention whether blinding had been used. Hogarty 1997 was also not blinded but considerable efforts were made to ensure that decisions, for example regarding relapse, were both reliable and valid. In other studies an attempt was made to ensure that raters were blind for part or the entire recording of outcome. No study tested the integrity of this blinding.
Incomplete outcome data
Study attrition was often not reported and these trials may have carried forward the last observation of the participants, which may have introduced some uncertainty into the results, as it is unlikely that the participants clinical state remained stable.
Selective reporting
We identified no under reporting of outcomes that had been collected by the trialists, although we did not have access to the protocols of the studies to determine whether all the outcome measures were reported.
Other potential sources of bias
A large number of Chinese trials were added to this review. Evidence has emerged that many trials from the People’s Republic of China which were stated to be randomised are not (Wu 2006). We did not contact the authors to verify the process by which they randomised but took the descriptions and statements as being correct. Nor did we identify any overt bias in the results. However, inclusion of these studies may increase the risk of biased data favouring family intervention.
Effects of interventions
I. Comparison I. Any family based interventions (more than five sessions) versus standard care
More information on this comparison is available in Summary of findings for the main comparison.
I.I Service utilisation
1.1.1 Hospital admission
Hospital admissions at six months were equivocal (n = 132, three RCTs, RR 0.85 CI 0.4 to 1.7). In the 2002 update of this review, the evidence that family intervention reduces hospital admission at one year was equivocal, and this was a change from earlier versions of the review which found family intervention to significantly reduce hospital admission (Mari 1996). In this 2010 update, however, there is again some suggestion that family intervention does significantly reduce hospital admission at one year (n = 481, eight RCTs, RR 0.78 CI 0.6 to 1.0, NNT 8 CI 6 to 13). Longer follow up (to 18 months) also finds that family intervention does significantly reduce admission (n = 228, three RCTs, RR 0.46 CI 0.3 to 0.7, NNT 4 CI 3 to 8), although data beyond that time (two years, n = 145, five RCTs, RR 0.83 CI 0.7 to 1.1; three years, n = 122, 2 RCT, RR 0.91 CI 0.7 to1.2) are equivocal. Removing trials from China from the analyses for this outcome made no substantive difference.
1.1.2 Days in hospital
The total number of days spent in hospital at three months was significantly lower in the family intervention group (Chien 2004, n = 48, MD −6.67 CI −11.6 to −1.8). Xiong 1994 also reported data for the time spent in hospital. These data are not normally distributed (skewed) and are not presented on a graph. In this study the 33 people in the intervention group spent an average of 7.9 days in hospital by the end of the 12-month follow-up period (SD 22.4). The 28 people in the control group spent an average of 24 days in hospital (SD 43.6). These findings were reported by the authors to be statistically significantly different (P = 0.03), favouring the group given family intervention.
1.2 Global state
1.2.1 Relapse
Please see Analysis 1.4.
For the purposes of this review, suicide is considered as a relapse. There is, however, no universally accepted definition of relapse (please see Characteristics of included studies). Some definitions required the recurrence of symptoms for patients with full remission at discharge, and others required a deterioration of symptoms for people who presented residual symptoms at baseline assessment. Finally, some studies stipulated that relapse was indicated by a managerial event such as hospitalisation or substantial change of medication.
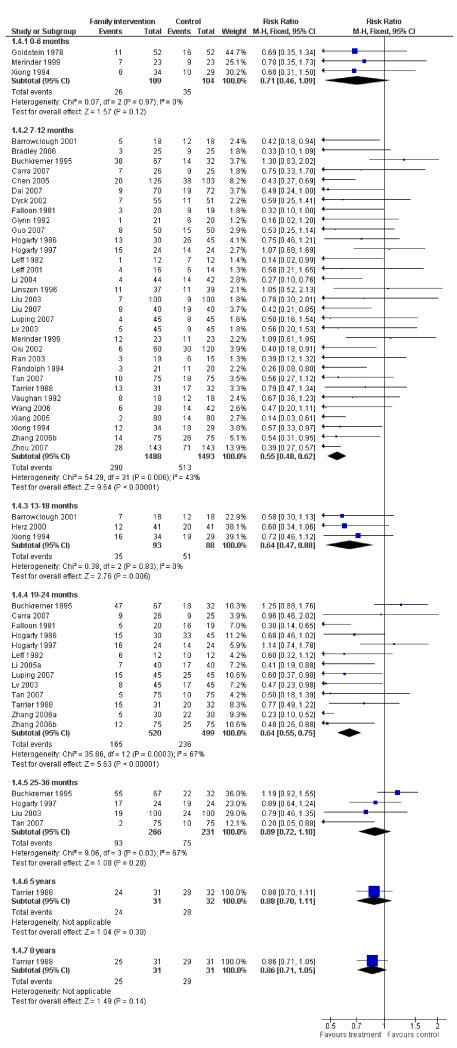
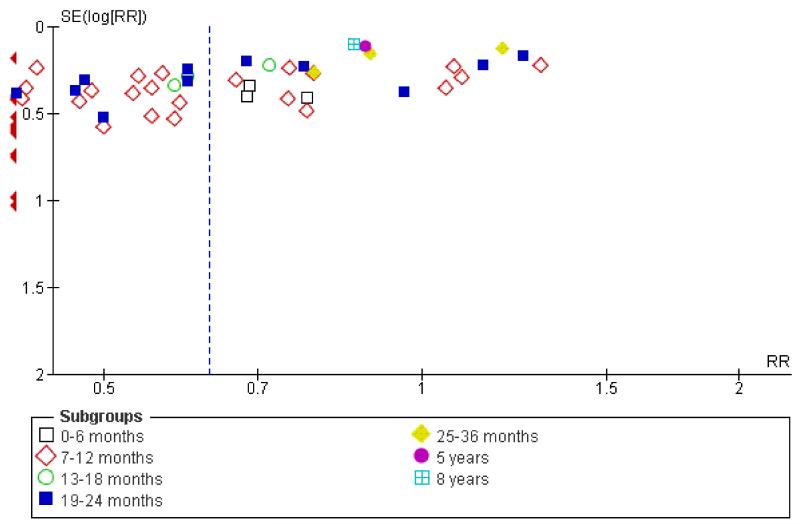
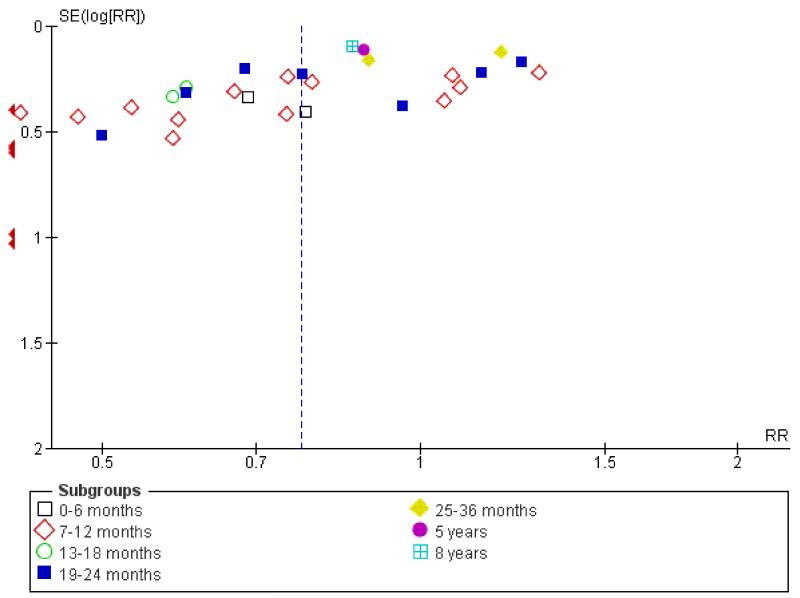
Family intervention did not reduce the rate of these ‘relapse events’ at six months (n = 213, 3 RCTs, RR 0.71 CI 0.5 to 1.1). By 12 months, family intervention did reduce relapse events (n = 2981, 32 RCTs, RR 0.55 CI 0.5 to 0.6, NNT 7 CI 6 to 8), as well as at 18 months (n = 181, 3 RCTs, RR 0.64 CI 0.5 to 0.9, NNT 5 CI 4 to 15) and at 24 months (n = 1019, 13 RCTs, RR 0.64 CI 0.6 to 0.8), although data are heterogeneous (I2 = 67%). When trials from China are removed findings tend to be a little less positive (Figure 1) but not dramatically so. Funnel plots of this outcome - before (Figure 2) and after (Figure 3) removal of the Chinese studies does not really suggest that there is a ‘small study bias’ operating.
Figure 1. Forest plot of comparison: 1 ANY FAMILY-BASED INTERVENTIONS (> 5 sessions) vs STANDARD CARE, outcome: 1.4 Global state: 1. Relapse (without use of data from China).
Figure 2. Funnel plot of comparison: 1 ANY FAMILY-BASED INTERVENTIONS (> 5 sessions) vs STANDARD CARE, outcome: 1.4 Global state: 1. Relapse.
Figure 3. Funnel plot of comparison: 1 ANY FAMILY-BASED INTERVENTIONS (> 5 sessions) vs STANDARD CARE, outcome: 1.4 Global state: 1. Relapse (minus Chinese trials).
Data regarding relapse at three years are not significantly different (n = 497, 4 RCTs, RR 0.89 CI 0.7 to 1.1). Data from longer follow up (five and eight years) are, in each case, reported by a single small study, and are non-significant.
1.2.2 ‘Not improved’
Xiang 1994 and Ran 2003 continue to suggest significantly fewer people in the control group improved than in the family intervention group (n = 112, 2 RCTs, RR 0.40 CI 0.2 to 0.7, NNT 2 CI 2 to 4).
1.2.3 Global Assessment of Functioning (GAF)
Average endpoint scores on the GAF scale at one year (Barrowclough 2001, n = 32, MD −10.28 CI −20.3 to −0.2) were borderline significant (0.05) for family intervention. Average endpoint scores by two years also favoured family intervention (n = 90, 2 RCTs, MD −8.66 CI −14.4 to −2.9). Merinder 1999 reported mean change data after eight family intervention sessions and at 12 months. Both sets of data contain considerable skew and are not significantly different.
1.2.4 Self-reported psychiatric symptom scores (SCL-90)
Self-reported psychiatric symptom scores favoured family intervention (Li 2005a, n = 80, MD −22.01 CI −30.9 to −13.0) compared with the control group.
1.3 Mental state
1.3.1 Average scores - Brief Psychiatric Rating Scale
BPRS data favoured family intervention at one year (n = 170, 3 RCTs, MD −8.32 CI −10.9 to −5.7), although data were heterogeneous (I2 = 79%). BPRS negative scores (n = 62, 1 RCT, MD −0.30 CI −0.9 to 0.3) are equivocal. We found, skewed, BPRS change data were not significantly different (n = 156, 3 RCTs, MD −0.30 CI −0.8 to 0.2). In Merinder 1999 we found no significant difference in the short term skewed data.
1.3.2 Average scores - Positive and Negative Symptom Score
We found PANSS endpoint total scores (n = 174, 2 RCTs, MD −7.90 CI −11.9 to −3.8) favoured family intervention compared with the control group at one year. However, PANSS positive and negative scores were not significant. PANSS general psychopathology data favoured family intervention (Dai 2007, n = 142, MD -3.60 CI -5.8 to -1.4). Barrowclough 2001 reported 18-month outcome data, and we found PANSS total and positive scores were not significant. But PANSS negative scores did favour the family intervention (n = 29, MD −5.23 CI −8.4 to −2.0) group. One Chinese study (Liu 2003, n = 149) reported data at three years, and we found PANSS total scores (MD −10.20 CI −13.6 to −6.9), PANSS positive scores (MD −2.60 CI −4.1 to −1.1) and PANSS negative scores (MD −3.70 CI −4.9 to −2.5) favoured family intervention. We found PANSS positive change scores (Dai 2007, n = 142, MD −2.00 CI −3.5 −0.5) and PANSS negative change scores (Dai 2007, n = 142, MD −4.00 CI −5.8 to −2.2) favoured the family intervention group compared with the control group.
1.3.3 Average scores - SAPS/SANS
Xiong 1994 used Chinese versions of the SAPS and SANS scales. Data at 18 months however, were skewed for both, with the SANS-CV outcome not statistically significant, although the SAPS-CV outcome was significant (P = 0.03), favouring family intervention. Bradley 2006 also reported SANS endpoint scores, but the data were too skewed to report here.
1.3.4 Insight
Average change in general mental state scores for insight were only reported by Merinder 1999. Data for an unspecified period after eight sessions of family intervention and at one year were both equivocal.
1.3.5 Average scores - Frankfurt scale
Finally, Fernandez 1998 reported on the average endpoint score of the mental state rating scale, the Frankfurt Scale. Data were too skewed to present graphically. They were not statistically significant.
1.4 Behaviour
1.4.1 Average scores - Nursing observation (NOSIE)
We found NOSIE endpoint scores favoured the control group (Dai 2007, n = 142, MD 59.10 CI 54.6 to 63.6) compared with participants given family intervention. NOSIE positive factor scores also failed to show any benefit for the family intervention group (Dai 2007, n = 142, MD 33.40 CI 30.5 to 36.3) over the 12-month assessment period.
1.5 Compliance
1.5.1 Leaving the study early
No studies reported data for the short term (0 to 12 weeks). By three months, study attrition was occurring but was no greater for the family intervention group than for the control (n = 552, 7 RCTs, RR 0.92 CI 0.6 to 1.4). Results from seven months to one year were not significant (n = 733, 10 RCTs, RR 0.74 CI 0.5 to 1.0) but revealed a trend in favour of family intervention (P = 0.07). Loss to follow up from 13 months to two years (n = 887, 10 RCTs, RR 0.74 CI 0.6 to 1.0) favoured family intervention NNT 22 to prevent one participant leaving the study (CI not estimable). Long-term data from 25 months to three years favoured family intervention (n = 290, 3 RCTs, RR 0.42 CI 0.3 to 0.7, NNT 6 CI 5 to 10), but results for more than three years (Tarrier 1988, n = 63, RR 1.72 CI 0.7 to 4.2) were equivocal. Tarrier 1988 reported loss to follow-up data at eight years and we found no significant difference between groups (n = 63, RR 1.72 CI 0.7 to 4.2).
1.5.2 Compliance with medication
Compliance with medication improved for people whose relatives received family intervention (n = 695, 10 RCTs, RR 0.60 CI 0.5 to 0.7, NNT 6 CI 5 to 9).
1.5.3 Compliance with community care
No significant differences were found in compliance with community care at one year (Carra 2007, n = 29, RR 0.68 CI 0.4 to 1.1), or by two years (Carra 2007, n = 29, RR 0.85 CI 0.6 to 1.3).
1.5.4 Months on medication
The data on months taking medication are from a single study (Xiong 1994). This small study (n = 63) suggests that those receiving family therapy do stay on medication for longer, although no findings are statistically significant.
1.6 Adverse events - death
The majority of deaths were due to suicide. Of the 377 people in the studies that reported death as an outcome, 17 (5%) committed suicide. There were five deaths due to other causes. Family intervention had no clear effect on the numbers of people who killed themselves during the studies (n = 377, 7 RCTs, RR 0.79 CI 0.4 to 1.8). Personal communication with Professor Tarrier suggested that there might be a few more deaths in the long-term follow up of Tarrier 1988, but numbers and group of allocation have not been clarified.
1.7 Social functioning
1.7.1 Generally socially impaired
Falloon 1981 and Xiang 1994 report on a rating of overall social impairment up to nine months. Results suggest that family intervention does significantly reduce general social impairment (n = 116, 2 RCTs, RR 0.51 CI 0.4 to 0.7). Data are heterogeneous (I2 = 75%). We also found general social functioning scores (n = 90, 3 RCTs, MD −8.05 CI −13.3 to −2.8) favoured family intervention, however, again data are heterogeneous (I2 = 63%).
1.7.2 Work
The majority of the studies did not provide data for specific aspects of social functioning. Four, however, reported on employment. The results at one year are equivocal (n = 285, 5 RCTs, RR unemployed 1.06 CI 0.9 to 1.3) as are those at two years (Carra 2007, n = 51, RR 1.33 CI 0.8 to 2.1), and three years (Buchkremer 1995, n = 99, RR unemployed 1.19 CI 0.9 to 1.6). Xiang 1994 (n = 77) evaluated whether family intervention helped with a person’s abilities to perform work tasks. It did not (RR 0.31 CI 0.1 to 1.0). Similarly, Ran 2003 also reported no differences in a person’s ability to perform work tasks (n = 35, RR 1.68 CI 0.2 to 16.9). Xiong 1994 reported skewed data for months spent in employment. At the end of a year in this study, the 33 people in the intervention group spent an average of 5.6 months in employment (SD 5.0) compared with the 28 in the control group who spent 3.1 months employed (SD 5.1). This was statistically significant.
1.7.3 Living independently
Three studies reported whether or not patients whose families received family intervention were able to move towards more independent living. The results for this show a trend towards increased ability to live independently at one year (n = 164, 3 RCTs, RR 0.83 CI 0.7 to 1.0) but total numbers are small and the results are not statistically significant. Three-year data (Buchkremer 1995) also did not indicate increased ability to live independently for either group.
1.7.4 Imprisonment
A single small study reported on imprisonment (Falloon 1981, n = 39) and we found no clear effect of family intervention for this outcome (RR 0.95 CI 0.2 to 4.1).
1.7.5 Disability Assessment Scale
Only skewed data were available, and data suggest that participants given family intervention had worse levels of disability.
1.7.6 Social Disability Screening Schedule (SDSS)
Participants given family intervention for two years did not reveal any significant differences between groups (Tan 2007, n = 150, MD −0.51 CI −1.4 to 0.4) based on the Social Disability Screening Schedule. However, at three years, results favoured family intervention (n = 150, MD −1.94 CI −2.90 to −1.0). Further endpoint data favouring family intervention at one year were reported by Wang 2006, and contained wide confidence intervals. Bradley 2006 also reported skewed data from the HoNOS scales and data are added to other data tables.
1.8 Family outcomes
1.8.1 Ability to cope
Bloch 1995 reports on the families’ ability to cope. This is not clearly increased by the experimental intervention (n = 63, RR 0.79 CI 0.6 to 1.0). Falloon 1981 suggests that there is no difference in the ability of the patient to cope with the key relative within the family as a result of the intervention (n = 39, RR 1.11 CI 0.5 to 2.7). Regarding families’ ability to understand the patients’ needs, only Bloch 1995 reported this outcome and suggested that family intervention decreases poor understanding of patients’ needs (n = 63, RR 0.58 CI 0.4 to 0.9). Insufficient care or maltreatment by the family was reported by two studies, Xiang 1994 at six months and Ran 2003 at nine months. The results suggests a trend favouring family intervention, although this is not statistically significant (P = 0.06) and a larger study may have rendered the outcome significant (n = 111, 2 RCT, RR 0.49 CI 0.2 to 1.0).
Szmukler 2003 reported on continuous measures of coping by the carers (Coping with Life-events & Difficulties Interview). Coping skills were all equivocal (n = 49, MD effective coping −0.5 CI −1.9 to 0.9; MD ineffective coping 0.30 CI -0.7 to 1.3), with no benefit being shown for the carers in the intervention group compared with those in the control group.
In Chien 2004, using the Family Support Service Index scale, we found the family intervention group required significantly more support than the control group (n =48, MD 0.86 CI 0.2 to 1.5).Chien 2004 also reported data from the Family Assessment Device scale and we found those receiving family intervention had significantly better outcomes in family functioning (n = 48, MD −6.56 CI −10.50 CI −10.5 to −2.6).
1.8.2 Burden
In Chien 2004 we found participants given family intervention were perceived as less of a burden according to the Family Burden Interview Schedule (n = 48, MD −7.01 CI −10.8 to −3.3). Data from Xiong 1994 also suggests a significant reduction in the burden felt by family carers (n = 60, MD −0.4 CI −0.7 to −0.1). Carra 2007 reported dichotomous data (n = 51) on burden and all data were equivocal. Leff 2001 and Bradley 2006 reported continuous data for burden but these were skewed and are not reported in the text.
1.8.3 Expressed emotion within the family
In Hogarty 1986 we found the overall level of expressed emotion was equivocal. However, we found families given the intervention reported a statistically significant decreases in levels of over-involvement (Tarrier 1988, n = 63, RR 0.40 CI 0.2 to 0.7, NNT 3 CI 2 to 6) and criticism (n = 63, RR 0.44 CI 0.2 to 0.8, NNT 3 CI 3 to 9). Hostility was also significantly lower in the family intervention group (n = 87, 2 RCTs, RR 0.35 CI 0.2 to 0.7, NNT 3 CI 3 to 6). When we combined the results of three studies, significant findings in favour of family intervention for high expressed emotion became evident (n = 164, 3 RCTs, RR 0.68 CI 0.5 to 0.9) but data were heterogeneous (I2 = 68%). Leff 2001 reported equivocal results for expressed emotion on continuous scores, and skewed data on critical comments, and over-involvement. Merinder 1999 reported (skewed data) expressed emotion from the Family Questionnaire which were also equivocal. Knowledge Scores reported by Leff 2001 were skewed and could not be reported due to the wide variations around the mean.
1.8.4 Psychological morbidity of carers
Szmukler 2003 reported continuous data for this outcome. Data were skewed with no statistically significant difference between carers in the family intervention or standard care group.
1.8.5 Care giving
Szmukler 2003 provided data on the family’s experience of care giving. These data were also skewed and did not show clear differences between the experiences of the different groups of families.
1.8.6 Social support
Szmukler 2003 reported on the role of support given to carers by close confidants and the attitudes of people in the wider community. The data were too skewed to present graphically, but the study report stated that no significant differences were found between the two groups.
1.8.7 Stress of care giving
Szmukler 2003 reported on the amount of stress experienced, but data were skewed and are not presented here.
1.8.8 Change in expressed emotion by the caregiver
Merinder 1999 reported on change in expressed emotion by caregivers but only skewed data were available, and are not presented.
1.8.9 Satisfaction
Merinder 1999 (n = 46) rated the satisfaction of carers and patients using the Verona Service Satisfaction Scale. All data are skewed and difficult to interpret. None are statistically significant but there is a consistent impression that carers in the family intervention group are more satisfied with care than those allocated to standard care.
1.8.10 Family APGAR
We found family APGAR (Du 2005, n = 146, MD −2.90 CI −3.4 to −2.4) scores favoured participants given family intervention during 12 months’ assessment.
1.8.11 Quality of life
In Shi 2000 we found families in the family intervention group had significantly higher level of quality of life than family members of the control group (n = 213, MD 19.18 CI 9.8 to 28.6) at the two-year endpoint. However, no significant differences in quality of life were found one small study (n = 50) at one year (MD −5.05 CI −15.4 to 5.3).
1.9 Economic analyses
Falloon 1981, Tarrier 1988 and Xiong 1994 include an economic analysis. In Falloon 1981 and Xiong 1994 direct and indirect costs of community management to patients, families, health, welfare, and community agencies were recorded, while Tarrier 1988 restricted the economic analysis to direct costs. Falloon 1981 suggests that, after one year, the overall costs of the family approach were approximately 20% less than those of the control condition (Cardin 1985). In Tarrier 1988 there was a decrease of 27% in the mean cost per patient in family intervention group. In Xiong 1994 the intervention resulted in a net saving of 58% of the per capita yearly income (in China), but the proportion of this saving that directly benefited the family would vary depending on whether or not the patient had medical insurance and received work disability payment.
2. COMPARISON 2. BEHAVIOURAL FAMILY-BASED versus SUPPORTIVE FAMILY BASED INTERVENTIONS (>5 sessions)
2. Comparison 2. Behavioural family-based versus supportive family-based interventions (more than five sessions)
2.1 Service utilisation
2.1.1 Hospital admission
The single large study in this comparison, Schooler 1997, reported equivocal results at two years (n = 528, RR 0.98, CI 0.9 to 1.1).
2.2 Global state
Schooler 1997 rated the stability of a person’s global state. By six months there was no difference in the numbers of people being rated as unstable (n = 528, RR 1.08 CI 0.9 to 1.3).
2.3 Compliance: leaving the study early or poor compliance with treatment protocol
Seventy-nine percent of people left Schooler 1997 early or did not/could not adhere to the treatment protocol. Family intervention did not change this attrition (n = 528, RR 0.96 CI 0.9 to 1.1).
3. Comparison 3. Group family-based interventions versus individual family-based interventions (more than five sessions)
3.1 Global state
Leff 1989 and McFarlane 1995 provide data for relapse at one year (n = 195, 2 RCTs, RR 0.70 CI 0.4 to 1.2). At two years results were also not statistically significant (n = 197, 3 RCTs, RR 0.71 CI 0.5 to 1.1). McFarlane 1995 reported data for the outcome of ‘more than one relapse’ between 19 and 24 months. Wide confidence intervals render the result equivocal (n = 172, RR 0.71 CI 0.3 to 1.5).
3.2 Compliance
Leff 1989 and McFarlane 1995 report data for people leaving the study early with no clear difference between group-based and individual-based family intervention techniques (n = 195, 2 RCTs, RR 1.35, CI 0.8 to 2.2). Only McFarlane 1995 provided data for poor compliance with medication and these too were equivocal (n = 172, RR 1.0 CI 0.5 to 2.0).
3.3 Social functioning
Leff 1989 found a statistically favourable outcome for the individual family based intervention. More people allocated to individual family intervention were able to live independently compared with those who had been randomised to the group-based family intervention (n = 23, RR 2.18 CI 1.1 to 4.4).
3.4 Family outcomes
Leff 1989 reported on the amount of expressed emotion by relatives. Data comparing the interventions are equivocal (n = 23, RR 0.94 CI 0.5 to 1.9), although the authors reported a significant (P < 0.05) reduction in expressed emotion between baseline and at two years.
DISCUSSION
Summary of main results
1. Comparison 1. Any family-based interventions (more than five sessions) versus standard care
1.1 Service utilisation
Previous versions of this review produced equivocal data suggesting that family intervention does not reduce hospital admission by one year compared with standard care (seven RCTs, n = 374 families) (Pharoah 2000; Pharoah 2003). Even earlier versions suggested that family intervention did reduce hospital admission significantly more than standard care alone (Mari 1994a; Mari 1996). Again, in this 2010 update, the evidence suggests that family intervention significantly reduces hospital admission at one year, with one patient out of every eight treated with family intervention prevented from hospitalisation compared with standard care.
We do recognise the enormous difficulty of conducting randomised trials in this area, but, nevertheless as family intervention is widely used, it could be expected that its implementation should be based on more stable and convincing data than these. The data reported by Falloon 1981 (n = 39) Xiong 1994 (n = 63) and Zhang 1994 (n = 83) strongly favours family intervention, and when these studies are excluded from the meta-analysis hospital admission becomes non-significant at all time points. Days spent in hospital is reduced but this outcome is based on one small study (Chien 2004).
1.2 Global state
Despite the definition of relapse varying across studies, we felt that the summation of data to be reasonable as definitions in routine clinical practice may also vary. Inclusion of the cluster randomised trial Ran 2003, with data managed as described in the ‘Unit of analysis issues’ section above also made little difference to the overall finding. People allocated to family interventions may relapse less compared with those in the standard care group. It is a concern that these findings may contain an element of small study bias (Figure 1). Small, less positive studies may remain unpublished or inaccessible. This must weaken the findings but currently the best available evidence suggests that the approximate number of families needed to be given family intervention in order to avoid one relapse at the end of a year is about seven. These figures could be seen as supportive of the general use of family intervention or prohibitive of its introduction into everyday use. Data from the People’s Republic of China (Xiang 1994), support the impression of better overall global improvement in the family intervention group compared with the control. Continuous scores from the Global Assessment of Functioning (GAF) and the (SCL-90) also support this in favour of family intervention.
1.3 Mental state
Participants and trialists invested time and effort rating mental state using several scales. It is difficult to know if the result justifies the effort on the part of everyone concerned. The overall impression is mixed with both favourable and equivocal findings for family intervention. Some agreement across trials on design of study could have rendered these data more useful.
1.4 Behaviour
Family intervention was not beneficial in terms of behavioural measures from the NOSIE scale compared with those patients given standard care, although this result is based on a single study (Dai 2007).
1.5 Compliance
About 14% of participants left the study before completion by one year. The addition of several Chinese trials has resulted in retention rates further improving from the finding reported previously (17%). Compared with other trials for the care of people with schizophrenia, this level of follow up is excellent (Thornley 1998). Family intervention did not seem to promote or hinder this attrition. The experimental intervention did, however, promote compliance with medication. It can be speculated that it is by this means that family intervention has its main effect. Hogarty 1997 did suggest that although compliance with medication was indeed improved by family intervention, this did not fully account for the findings favouring family intervention. In the short term, however, compliance with medication is predictive of a better outcome (Dencker 1986) and this could well be the means by which family intervention contributes to decreased relapse.
1.6 Adverse events - death
That 5% of the 377 people in the studies which reported death as an outcome committed suicide during the follow-up period suggests that these studies were dealing with a disturbed group of people. It is expected that the life-time rate of suicide for people with schizophrenia is about 14-20% (Jablensky 2000). From the limited data we have, there is no suggestion that family intervention makes any difference to this outcome.
1.7 Social functioning
Measuring social impairment is difficult, but from the different ratings there is an impression that family intervention does improve general functioning in this domain. Interpreting the various scale-derived outcomes is problematic. Continuous data from the Social Functioning Scale is in favour of the family intervention group, but doubts remain for its robustness given the small numbers of participants. The outcome of employment is more readily understandable and family intervention did not seem to have much of an effect, if any. Other clear outcomes relating to social functioning, living independently and imprisonment were also equivocal. This may be an example of rating scales being sensitive to slight changes that may not have repercussions for routine life.
1.8 Family outcomes
The numerous measures used in this area by several trialists suggest that this is seen as an important area for research but that there is no consensus on what to measure. A small study (n = 63) suggests that family intervention may help increase the families’ understanding of the patients’ needs. Measures of insufficient care or maltreatment by the family did not suggest family intervention lowers levels of maltreatment, although perhaps larger powered studies would have shown a treatment effect. Similarly, coping with life events was equivocal and underpowered. Need of service usage was found to be significantly lower in the standard care group. However, data from the same study found family functioning to be significantly improved in the family intervention group. Two small studies both found family intervention lessened burden, but again this family outcome is weakened by small numbers. The levels of emotion expressed within the family may indeed be reduced by family intervention.
1.9 Quality of life
Finally, the overall quality of life of family members may be increased by the family intervention package but we are unsure of the practical meaning and applicability of the result (MD 19.18 CI 9.8 to 28.6). The measure was a scale referenced in Mandarin, for which we have no adequate description and additional change data were equivocal (Bradley 2006).
1.10 Economic analyses
Reports that include an economic analysis all favour family intervention in terms of net saving in direct or indirect costs (Falloon 1981; Tarrier 1988; Xiong 1994). This is a consistent and important finding.
2. Comparison 2. Behavioural family-based versus supportive family-based interventions (more than five sessions)
Schooler 1997 involved more than 500 families. For the simple and clear outcomes reported (hospital admission and global state) there is no clear difference between the two forms of family intervention. The enormous attrition (79% by 30 months) is a major concern as regards the design and applicability of the results of this trial.
3. Comparison 3. Group family-based interventions versus individual family-based interventions (more than five sessions)
Group-based family interventions should be more economical than an individual approach. However, no economic data were reported. For global outcomes, no clear differences are apparent, with wide confidence intervals precluding firm conclusions. The same applies for outcomes related to compliance. The small trial Leff 1989 found that more people allocated to individual family intervention were able to live independently compared with those who had been randomised to the group-based family intervention (n = 23, RR 2.18 CI 1.1 to 4.4). This important outcome should be replicated.
4. Sensitivity analyses
Studies from the People’s Republic of China now make up the majority of the included studies, and evidence has emerged that many trials from China are not randomised even when stated to be (Wu 2006). We did not find any clear evidence that these studies were not truly randomised, although the absence of demographic data made judgements difficult. Nevertheless, the potential remains that these trials could contain biases. Where relevant for key outcomes, we added and subtracted these studies. Inclusion certainly tightened confidence intervals but never materially affected the direction of result or the conclusion drawn.
Overall completeness and applicability of evidence
Participants in studies that contributed to the results were not only from several types of care-cultures, but also involved both men and women, with a wide range of ages, people with long histories of illness and those in their first episode. There were no clear dissimilarities between the trials from Australia, Europe, the People’s Republic of China and the USA. Even Buchkremer 1995, which did add heterogeneity to certain results (see below), had similar methods, inclusion criteria, interventions and outcomes to the other studies. The provision of health care for mentally ill people in the countries in which the trials were undertaken is diverse, but the relative consistency of results suggests that their outcomes may be generalised to other health service traditions.
Quality of the evidence
The quality of reporting in most studies was poor (Figure 4). Only eight studies from 53 described the method of randomisation, and only one study described the method of allocation concealment. Blinding was not always possible for family intervention, although few studies attempted, or at least failed to report, whether the investigators assessing the patients were blind to treatment allocation. Similarly, study attrition was often omitted from the results, yet it is unlikely that group sizes remained constant throughout the investigation period. We did not have access to the included studies protocols, and were therefore unable to judge whether various tests of effectiveness had been left out of the published finding. The effect of these poorly reported studies is that the outcomes in this review may be biased with an overestimate of effect (Juni 2001).
Figure 4. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
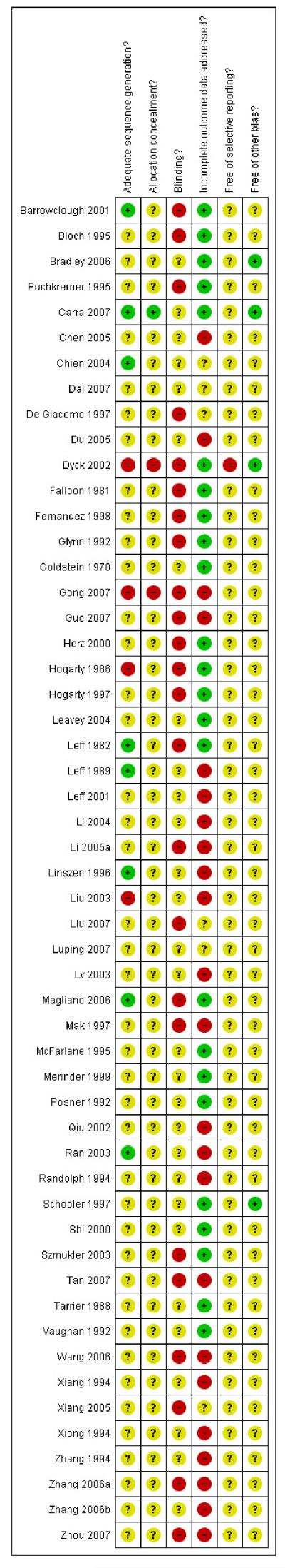
Potential biases in the review process
We have now updated this review several times and it is entirely possible that foreknowledge of the data could bias how we present them. We are now aware of this but try to ensure that this is offset by gaining wide peer review. We are aware of the issues with biases from trials from China. We have tried to investigate this in outcomes where most of the data are from China. It is possible that some of the outcomes have confidence intervals that are too narrow because of addition of data from China that we have not been able to show is overtly inappropriate to use. At no point do the trials from China materially affect direction of result or, we think, interpretation we have made.
Agreements and disagreements with other studies or reviews
This 2010 update substantially expands and improves upon earlier versions. It, however, generally agrees with findings from previous versions.
AUTHORS’ CONCLUSIONS
Implications for practice
1. People with schizophrenia and their families
The main benefit of family intervention for people with schizophrenia is that it may decrease the risk of relapse. It may also help people with schizophrenia to consistently take their medication. Family intervention can also make family life less burdensome and tense and may reduce re-hospitalisation. For this gain, which could be perceived as of moderate certainty, people with schizophrenia and their families should be willing to spend a significant amount of time in contact with services.
2. Clinicians
Clinicians may feel that family intervention is worth the time and effort, assuming that a high-quality family service is available. Prevention of relapse is a cornerstone of psychiatric care. Should highquality services not be available, clinicians will have difficult decisions to take in view of the fact that any benefit from family therapy is moderate and other equally or more effective interventions may be more accessible (Marshall 1999).
3. Managers or policy makers
As always, service managers and funders have to weigh up the costs and benefits of this treatment and whether it significantly improves outcomes for individuals and for families. They may feel, with the relatively high number needed to treat, that the resources required to adequately implement family interventions might be better used in other ways. Alternatively, if families could be clearly shown to benefit from this approach as well as patients, it may be considered worth the cost.
Implications for research
1. General
If the CONSORT recommendations (Begg 1996; Moher 2001) were followed in the reporting of future studies, the effects of family intervention would be clearer. Much important data within the included studies were so poorly reported that clinicians, funders and recipients of care might have reason to feel let down by the research community.
2. Specific
2.1 Future trials
Large simple, well-designed and reported trials continue to be justified. All data in this review are unstable and a large, pragmatic study should be undertaken to settle arguments about the value of this widely used therapy. A design is suggested in Table 1. Entry criteria should be broad, interventions accessible and outcomes clear and well reported. If studies employ a cluster randomised design, such as Ran 2003, they should not be reported as a standard randomised study and intra-class correlation coefficients should be provided.
Table 1. Suggestions for design of future study.
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. Blindness: single, tested. Duration: 12 months beyond end of intervention at least. Raters: independent. |
| Participants | Families of patients who have a diagnosis of schizophrenia and/or schizoaffective disorder. N = 450.* |
| Interventio ns |
|
| Outcomes | Healthy life: days of 'healthy' life.** General state: relapse, frequency and intensity of minor and major exacerbations. Social role and performance: not using social adjustment because of the normative bias of this measure. Quality of life: binary measure. Distress among relatives: binary measure. Burden on family: binary measure. Mental state: depressive spells and/or suicide attempts. Service outcomes: admitted, number of admissions, length of hospitalisation, contacts with psychiatric services. Compliance with drugs. Economic evaluations: cost-effectiveness, cost-benefit. |
| Notes | * Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. ** Primary outcome. |
Most of the included studies focused primarily on the decrease of relapse when assessing the effects of family interventions. The relapse criterion, when used as the main outcome measure, has the disadvantage that the patient leaves the trial when the event occurs and data regarding the period following relapse may not be collected. More complete follow-up data would produce a more valid picture of the lasting effect of family intervention on the course of the illness.
A variety of outcomes could be considered as important assessments in future family interventions. These could be:
a method to monitor days of ‘healthy’ life - not having relapse as the main criterion but the frequency and intensity of minor and major exacerbations (Marder 1987; Carpenter 1990);
an assessment of social role and social performance, not using social adjustment because of the normative bias of this measure (Corin 1990);
a quality of life assessment (Heinrichs 1984);
measures of distress among relatives;
inclusion of subjective reports of patients (Strauss 1989) and relatives;
assessment of burden on the family; vii. measurement of depressive spells and/or suicide attempts;
the number of patients admitted, number of admissions per patient and length of hospitalisation;
the counting of contacts with psychiatric services (Tarrier 1988); and
the assessment of compliance with drugs and random check of blood tests for those taking oral medications (Tarrier 1988).
All such measures, however, should be readily understandable by all users of this research, and binary as well as continuous data should be reported.
Data collection should allow for economic evaluations (cost-effectiveness and cost-benefit) of the two intervention strategies being compared.
PLAIN LANGUAGE SUMMARY.
Family intervention for schizophrenia
People with schizophrenia are more likely to experience a relapse within family groups when there are high levels of expressed emotion (hostility, criticism or over involvement) within the family, compared to families who tend to be less expressive of their emotions. There are several psychosocial interventions available involving education, support and management to reduce expressed emotion within families. In this review we compare the effects of family psychosocial interventions in community settings for the care of people with schizophrenia or schizophrenia-like illnesses.
Studies were conducted in Europe, Asia and North America with packages of family intervention varying among studies, although there were no clear differences in study design. Results indicated that family intervention may reduce the risk of relapse and improve compliance with medication. However data were often inadequately reported and therefore unusable. As this package of care is widely employed, there should be further research to properly clarify several of the short-term and long-term outcomes.
ACKNOWLEDGEMENTS
We acknowledge the helpful comments of those who refereed this review. Thank you to Mingming Zhang, Huilin Liu and Jun Xia for their invaluable assistance translating articles written in Chinese. Thank you to Angelica Izquierdo de Santiago for kindly translating Spanish articles. Thank you also to Dr Julian Higgins of the MRC Biostatistics Unit, Cambridge, for advice. We are grateful to Dr Kurt Hahlweg for providing additional research papers.
We would like to thank David Streiner for his contributions to earlier versions of this review.
SOURCES OF SUPPORT
Internal sources
McMaster University, Ontario, Canada.
Universidade Federal de Sao Paulo, Brazil.
Hinchingbrook Health Care, Cambridgeshire, UK.
External sources
International Clinical Epidemiology Network (INCLEN), USA.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
ANY FAMILY BASED INTERVENTIONS (>5 sessions) compared to STANDARD CARE for schizophrenia
Patient or population: patients with schizophrenia
Settings: mostly hospital-based
Intervention: ANY FAMILY BASED INTERVENTIONS (>5 sessions)
Comparison: STANDARD CARE
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
|
| ||||||
| Assumed risk | Corresponding risk | |||||
|
| ||||||
| STANDARD CARE | ANY FAMILY BASED INTERVENTIONS (>5 sessions) | |||||
| Service utilisation: Hospital admission - at about 12 months |
Low risk population |
RR 0.78 (0.63 to 0.98) | 532 (9 studies) | ⊕⊕○○ low 1,2 | ||
| 100 per 1000 | 78 per 1000 (63 to 98) | |||||
|
Medium risk population
|
||||||
| 500 per 1000 | 390 per 1000 (315 to 490) | |||||
|
High risk population
|
||||||
| 800 per 1000 | 624 per 1000 (504 to 784) | |||||
|
| ||||||
| Global state: Relapse - at about 12 months |
Low risk population
|
RR 0.55 (0.48 to 0.62) | 2981 (32 studies) | ⊕⊕○○ low 1,2 | ||
| 100 per 1000 | 55 per 1000 (48 to 62) | |||||
|
Medium risk population
|
||||||
| 500 per 1000 | 275 per 1000 (240 to 310) | |||||
|
High risk population
|
||||||
| 800 per 1000 | 440 per 1000 (384 to 496) | |||||
|
| ||||||
| Compliance: Poor compliance with medication |
Low risk population
|
RR 0.6 (0.49 to 0.73) | 695 (10 studies) | ⊕⊕○○ low 1,2 | ||
| 100 per 1000 | 60 per 1000 (49 to 73) | |||||
|
Medium risk population
|
||||||
| 500 per 1000 | 300 per 1000 (245 to 365) | |||||
|
High risk population
|
||||||
| 800 per 1000 | 480 per 1000 (392 to 584) | |||||
|
| ||||||
| Social functioning: Specific - unemployed - at about 1 year |
Low risk population
|
RR 1.06 (0.89 to 1.25) | 285 (5 studies) | ⊕⊕○○ low 1,2 | ||
| 200 per 1000 | 212 per 1000 (178 to 250) | |||||
|
Medium risk population
|
||||||
| 500 per 1000 | 530 per 1000 (445 to 625) | |||||
|
High risk population
|
||||||
| 900 per 1000 | 954 per 1000 (801 to 1000) | |||||
|
| ||||||
| Social functioning: Specific - unable to live independently - at about 1 year |
Low risk population
|
RR 0.83 (0.66 to 1.03) | 164 (3 studies) | ⊕⊕○○ low 1,2 | ||
| 200 per 1000 | 166 per 1000 (132 to 206) | |||||
|
Medium risk population
|
||||||
| 500 per 1000 | 415 per 1000 (330 to 515) | |||||
|
High risk population
|
||||||
| 900 per 1000 | 747 per 1000 (594 to 927) | |||||
|
| ||||||
| Family outcome: Burden - not improved/worse (objective burden related to self-sufficiency) |
Low risk population
|
RR 0.53 (0.21 to 1.37) | 51 (1 study) | ⊕○○○ very low 1,2,3 | ||
| 200 per 1000 | 106 per 1000 (42 to 274) | |||||
|
Medium risk population
|
||||||
| 500 per 1000 | 265 per 1000 (105 to 685) | |||||
|
High risk population
|
||||||
| 900 per 1000 | 477 per 1000 (189 to 1000) | |||||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Randomisation not well described.
Best quality funnel plot of review suggests small negative studies not identified.
Single small study
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised - computer generated random list. Blindness: assessor blind. Duration: 9 months, with follow up at 12 and 18 months. Setting: Tameside and Glossop, Stockport and Oldham, England |
|
| Participants | Diagnosis: comorbid schizophrenia and substance use disorders (ICD 10 and DSM IV). N = 36. Age: range 17-62 years, mean 30.5. Sex: 33 M, 3 F. History: median duration 4 years, range 1-19 years, informed consent obtained |
|
| Interventions |
Family intervention consisted of 10-16 sessions and the individual interventions (CBT and motivational intervention) occurred on ~ 29 sessions |
|
| Outcomes | Death. Global state: GAF. Mental state: PANSS. Social functioning: SFS. Relapse. Unable to use - Addiction Severity Index: no usable data. The Drugs Attitude Inventory: no usable data. The Leeds Dependence Questionnaire: no usable data. The Alcohol Use Scale: no usable data. Drug Use Scale of the Clinician Rating Scale: no usable data |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomised, computer generated |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Single, untested |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised - no further details. Blindness: not blind. Duration: 6 weeks treatment, follow up 6 months. Setting: Melbourne, Australia. |
|
| Participants | Diagnosis: schizophrenia or schizo-affective disorder (DSM-III-R). N = 63. Age: mean ~ 30 years. Sex: not reported. History: acutely ill, past admissions ~ 4, duration ill ~ 8 years |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Hospital admission. Family experience: ECI, WOC. Unable to use - Global state: GHQ (no usable data). Mental state: PANAS (no data). Social functioning: LSP (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blinded |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised (by a staff member who drew names from a canister and, without looking at the names). Blindness: single (Independent researchers who were blind to study condition, conducted the assessments). Duration: 12 months with 18-month follow up. Setting: Australia. |
|
| Participants | Diagnosis: schizophrenia (DSM IV). N = 59*. Age: mean 34. Sex: 15 M, F 35. History: 21 had received hospital treatment before study entry; ten participants had a substance disorder. Inclusion criteria: who had a diagnosis of schizophrenia, schizoaffective disorder, or schizophreniform disorder; who were aged between 18 and 55 years; and who had a minimum of 10 hours of contact with family members each week |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Mental state: BPRS, SANS. QoL. Social functioning: HoNOS. Family outcome: Family Burden Scale. |
|
| Notes | *Nine participants completed the data collection procedure after treatment Family intervention - 26 sessions over 12 months | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised - no further details |
| Allocation concealment? | Unclear | Randomised by a staff member who drew names from a canister and, without looking at the names |
| Blinding? All outcomes |
Unclear | Single blind, independent researchers who were blind to study condition, untested |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Yes | Principally funded by grant 1997-0219 from the Victorian Health Promotion Foundation |
| Methods | Allocation: ’randomly assigned’. Blindness: not blind. Duration: 10 weeks family therapy, follow up 1 year. Setting: Italy. |
|
| Participants | Diagnosis: schizophrenia (DSM-III). N = 99. Age: range 18-48 years, mean 27. Sex: 72 M, 27 F. History: > 2 episodes or clinically deteriorating, mean previous episodes 2.6, mean duration ill 5.5 years. Exclusions: psychiatric secondary diagnoses. |
|
| Interventions |
|
|
| Outcomes | Death. Relapse. Hospital admission. Unemployed. Independent living. Unable to use - Mental state: AMDP (no usable data). Global state: CGI, GAS (no usable data). Hospitalisation: no usable data. Length of admission: no data reported. Additional medication: no usable data. Family experience: CFI, FKI, MFB (no usable data). |
|
| Notes | The therapeutic relative groups and self help groups are added in this review | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blinded |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised using random number table. Blindness: ’both relatives and clinicians in the IG groups programme were blind as to successive participation to the SG’. Duration 2 years. Setting: Italy. |
|
| Participants | Diagnosis: schizophrenia. N = 101. Age: mean 29 years. Sex: 73 M, 28 F. History: clinically stable. Inclusion criteria: relatives living with someone suffering from schizophrenia and had not attended family groups or other support services before the study intervention; the patient was clinically stable (having had no psychiatric hospitalisation or any relapse for six months prior to study entry) and was not receiving any psychosocial or rehabilitative treatment other than standard care; absence of alcohol or drug dependence or organic disease |
|
| Interventions |
All groups received standard antipsychotic care. |
|
| Outcomes | Relapse. Hospitalisation. Compliance with standard community care. Objective burden: self-sufficiency, social functioning, worsened. Relatives’ EE was evaluated by the CFI. |
|
| Notes | The family support programme is consists of two components that roughly correspond to the phases of the group. The first phase involves training on communication and coping skills, stress identification and management, and multiple family group-based problem solving, basically derived from the second stage of the psychoeducational multiple family group approach used by McFarlane Weekly sessions composed of 16-18 relatives for 24 sessions (1.75 h per session) and leaflets. The second element comprises weekly meetings for 48 sessions (1.5 h per session) over 2 years with a support group made up of 8-9 relatives who have previously attended the information group |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomised using random numbers table |
| Allocation concealment? | Yes | ’Allocation concealment was ensured by the external involvement of a statistician (C.M.), who was not involved in enrolling participants, and was responsible for the method of sequence generation’ |
| Blinding? All outcomes |
Unclear | Follow-up assessments were carried out by research assistants blind about the treatment assigned, untested |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Yes | Grant |
| Methods | Allocation: randomised (by cluster - no further details). Blindness: not reported. Duration: 9 months. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-3-R, ICD-10). N = 357*. Age: mean 43 years. Sex: M 128, F 198. History: age of onset about 31 years, median of length of illness about 10 years.. Excluded: patients with other medical illnesses. |
|
| Interventions |
|
|
| Outcomes | Relapse. Unable to use. Leaving the study early (no usable data). Not complying with medication (no usable data). Improvement scale (no usable data). |
|
| Notes | *31 participants did not complete the study due to, families unwilling to look after patients, family or patient thought the treatment was ineffective, and concern about discrimination from neighbours Family intervention - once a month for 9 months, 1.5-3.1hours each time. Including familyvisits, introducing basic information on schizophrenia, its available treatment and rehabilitation, crisis intervention, by trained psychiatrists and local physicians |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No details |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised, computer generated numbers. Blindness: not reported. Duration: 3 months. Setting: Hong Kong, China. |
|
| Participants | Diagnosis: schizophrenia (DSM IV). N = 48. Age: range 20-50+ years, mean 40. Sex: 27 M, 21 F. History: illness less than 3 years, with no comorbidity or other mental illness |
|
| Interventions |
Standard care, mostly chlorpromazine, haloperidol (88% in the experimental group and 85% in the control group), with > 70% taking the medium dose |
|
| Outcomes | Leaving the study early. Global state: hospital admission. Family outcome: family Burden Interview Schedule. Family outcome: family Assessment Device. Family outcome: family Support Service Index. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomsied, by computer generation |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No reported |
| Incomplete outcome data addressed? All outcomes |
Unclear | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: no details. Duration: one year. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-3). N = 152*. Age: mean 25 years. Sex: men and women. History: no details. Excluded: patient with severe cardiac, liver and renal disease; mental disabled patient |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Mental state: PANSS, NOSIE. Unable to use: Leaving the study early. |
|
| Notes | *10 dropped out of study, unclear from which group. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No details |
| Incomplete outcome data addressed? All outcomes |
Unclear | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly assigned’. Blindness: not blind. Duration: 10 weeks family therapy, follow up 1 year. Setting: Italy. |
|
| Participants | Diagnosis: schizophrenia (DSM-III). N = 38. Age: not reported. Sex: not reported. History: duration of illness < 3 years. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Unable to use: Mental state: BPRS (no usable data). Social functioning: SCOS, FMSS (no usable data). Family experience: ACL (no usable data). Global state: CGI (no usable data). Family experience: SISCI-1 (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blind |
| Incomplete outcome data addressed? All outcomes |
Unclear | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: no details. Duration: 1 years. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (no further details). N = 146. Age: mean 37 years. Sex: male and female. History: no details. |
|
| Interventions |
|
|
| Outcomes | Family score: Family APGAR. Unable to use: Leaving the study early. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No details |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly assigned by pulling a piece of paper, labelled either MFGT or SC, out of a hat’. Blindness: open study. Duration: two years, with one year follow up. Setting: Washington, USA. |
|
| Participants | Diagnosis: schizophrenia, paranoid type, schizoaffective disorder, schizophrenia, other types (DSM IV). N = 106. Age: range 18-45 years, mean 32 years. Sex: 82 M, 24 F. History: mostly chronically ill, mean duration ~ 10 years, with relatively low levels of psychiatric symptoms at study entry. Inclusion criteria: required to have contact with a family member for 5 hours a week |
|
| Interventions |
Family intervention treatment intended to improve illness management, social support and coping skills for the patient and family members; this approach is based on the research by McFarlane and colleagues Standard care including medication management, case management and for some patients, therapeutic and rehabilitation services |
|
| Outcomes | Leaving the study early. Rehospitalisation. Relapse. |
|
| Notes | ITT analysis used with last observation carried forward. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | No | Randomised by pulling papers out of a hat labelled with study group |
| Allocation concealment? | No | No |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | No | Not all outcome data reported |
| Free of other bias? | Yes | Grant from the National Institute of Mental Health |
| Methods | Allocation: ’randomized procedure’ - no further details. Blindness: ’not blind’. Duration: 9 months treatment, 2 years follow up. Setting: LA, USA. |
|
| Participants | Diagnosis: schizophrenia (DSM-III, PSE). N = 39. Age: range 18-41 years, mean 25.8. Sex: not reported. History: stabilised after relapse, English speakers, mean previous admissions ~ 3, mean duration ill ~ 4 years, high EE (CFI) |
|
| Interventions |
|
|
| Outcomes | Relapse. Hospital admission. Leaving the study early. Drug compliance. Employment. Residential care. Imprisonment. Social impairment. Ability to cope. Unable to use - Mental state: “7 point scale” (no further details). Duration of exacerbation: no SD. Duration unstable: no SD. Social functioning: SBAS, SAS-SR (no usable data). Family knowledge: no data. Patient functioning: no usable data. Time in employment: no SD. Costs: no SD. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blind |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: not blind. Duration: 1 year treatment. Setting: Cantabria, Spain. |
|
| Participants | Diagnosis: schizophrenia (ICD 10). N = 46. Age: range 18-45 years. Sex: 26 M, 20 F. History: average length of illness 8.3 years. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Mental state: BPRS. Frankfurt Scale. Social functioning: SFS. Unable to use: Family experience: CFQ (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blind |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly assigned’ - methods not described. Blindness: not attempted - participant reports corroborated by family. Duration: 1 year treatment, 1 year follow up. Setting: LA, USA. |
|
| Participants | Diagnosis: schizophrenia or schizo-affective disorder (DSM-III, expanded PSE). N = 41. Age: 18-42 years, mean ~ 31. Sex: all male. History: illness ~ 10 years (SD ~ 7), consecutive admissions, stable for 4 weeks. Exclusions: substance abuse. |
|
| Interventions |
|
|
| Outcomes | Relapse: psychotic exacerbations documented > 2 weeks. Hospital admission. Employment. Leaving the study early. Unable to use: Medication use: chlorpromazine equivalents (no SD). Social functioning: SAS-SR (no mean). Mental state: BPRS, SANS (no mean). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blind |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised, stratified by premorbid psychosocial competence, sex - no further details. Blindness: single - definition of relapse + BPRS, single and non-blind - decision to rehospitalise. Duration: 6 weeks treatment, 6 months follow up. Setting: Ventura, USA. Design: factorial. |
|
| Participants | Diagnosis: schizophrenia (New Haven Index > 4). N = 104*. Age: mean 23.4 years. Sex: 57 M, 47 F. History: ’acute’, consecutive admissions, 1-2 previous admissions |
|
| Interventions |
Factored with:
|
|
| Outcomes | Relapse (full-time admission, partial hospitalisation or substantial change in medication). Leaving the study early. Unable to use: Mental state: BPRS (subgroup analysis, no SD). Suicide: N = 2, original allocation unclear. Service use: no usable data. |
|
| Notes | * total N is 103 in second paper - reasons unclear. Data relating to high and low dose fluphenazine not used in this review. Leaving the study early data is contradictory in different parts of report - first set of data chosen at random |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single blind, untested |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised according to admission sequence. Blindness: open study. Duration: three years. Setting: community, China. |
|
| Participants | Diagnosis: schizophrenia. N= 166. Age: mean 31 years intervention group; 37 control group. Sex: M and F. History: no details. |
|
| Interventions |
|
|
| Outcomes | Global state: compliance with medication. Leaving the study early. Unable to use:Recovery: scale not reported. Deteriation: scale not reported. Global state: relapse (n’s unclear). Mental state: BPRS (n’s unclear). Social functioning: SDSS (n’s unclear). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | No | Quasi-randomised |
| Allocation concealment? | No | According to admission sequence |
| Blinding? All outcomes |
No | Open |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: open study. Duration: one years. Setting: community, China. |
|
| Participants | Diagnosis: schizophrenia. N = 100. Age: mean 32 years. Sex: male and female. History: no details. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Unable to use: Global state: compliance with medication (data not based upon each patient). Mental state: not improved-BPRS (data not based upon each patient) |
|
| Notes | Family interventionwas given 3 sessions/week, 30-45 minutes/session. In addition, once a week there is a lecture on mental health education; once a month there is a seminar for family members of people with schizophrenia | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly assigned’. Blindness: not blind. Duration: 18 months. Setting: New York, USA. |
|
| Participants | Diagnosis: schizophrenia (DSM III). N = 82. Age: mean 29.7 years. Sex: 53 M, 29 F. History: patient’s having at least 1 hospitalisation in the last 3 years or 2 or more lifetime hospital admissions |
|
| Interventions |
Intervention group received 5 components: 1. Education for patients and family members about the process of relapse in schizophrenia and how to recognise prodromal symptoms and behaviours; 2. active monitoring for prodromal symptoms by treatment team members, patients, family members and others in frequent contact with the patient; 3. clinical interventions, within 24 to 48 hours, when prodromal episodes were detected; 4, one-hour weekly supportive group therapy emphasizing improving coping skills or 30 to 45 minute individual supportive therapy sessions if patients refused group treatment; 5, 90-minute multifamily psychoeducational groups that family members were encouraged to attend |
|
| Outcomes | Leaving the study early. Relapse. Rehospitalisation. Unable to use: Mental state: PANSS (no usable data). Global state: GAS (no usable data). Prodromal symptoms: ESQ (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blind |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’on alternate weeks or months’ (determined before patients admitted) - quasirandom method. Blindness: not blind but efforts made to make decisions reliable and valid. Duration: treatment 2 years, follow up 2 years. Setting: Pittsburgh, USA. |
|
| Participants | Diagnosis: schizophrenia + schizo-affective disorders (RDC). N = 75. Age: range 17-55 years, mean 27. Sex: not reported. History: consecutive admissions < 6 months, mean previous admissions ~ 2.7, high EE family (CFI). Exclusions: substance abuse, organic illness, bipolar disorder |
|
| Interventions |
More than 5 sessions. |
|
| Outcomes | Relapse. Drug compliance. Expressed emotion. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | No | Quasi-randomised |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blind |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: not blind. Duration: 3 years treatment, 3 years follow up. Setting: Pittsburgh, USA. Design: factorial. |
|
| Participants | Diagnosis: schizophrenia + schizo-affective disorders (RDC). N = 97. Age: range 16-55 years, mean 28.6. Sex: 56 M, 41 F. History: acute admissions, mean previous admissions 2.7, mean length of illness 6.2 years. Exclusions: organic brain syndrome, drug or alcohol dependence in past 6 months, medical conditions preventing use of antipsychotic medication |
|
| Interventions |
All groups received more than 5 sessions. |
|
| Outcomes | Relapse (psychotic). Leaving the study early. Unable to use: Drug compliance: no usable data. Therapeutic alliance: no usable data. |
|
| Notes | The paper reports two trials (N = 151), one studying patients who lived with families (N = 97) and one studying patients who lived alone. This review only looked at the data from the former trial. For this review supportive therapy is the control arm and family therapy is the intervention |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | No blind |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised by block. Blindness: single. Duration: 9 months. Setting: London, UK. |
|
| Participants | Diagnosis: psychotic illness (ICD-10). N= 106. Age: not reported. Sex: 68 M, 30 F. History: informed consent obtained, first episode psychotic illness within six months of study. Excluded: organic disorders or learning difficulties. |
|
| Interventions |
Seven interactive sessions lasting ~ one hour, usually in the carers home |
|
| Outcomes | Leaving the study early. Rehospitalisation. Unable to use: Days in hospital: no usable data. Family experience: CGSQ (no usable data). Perceived Severity of Illness: no usable data. Service satisfaction: VSSS (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised by block, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single blind, untested |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised using table of random numbers. Blindness: not blind. Duration: 9 months treatment, 2 years follow up. Setting: London, UK. |
|
| Participants | Diagnosis: schizophrenia or paranoid psychosis (PSE). N = 24. Age: range 16-65 years, mean ~ 34. Sex: 12 M, 12F. History: consecutive stabilised admissions, mean previous admissions 1.2- 2.3, high EE > 35 hours contact/week (CFI) |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Death. Relapse. Deliberate self harm. Drug compliance. Poor tolerance to medication. Independent living (face to face contact). Expressed emotion. Unable to use: Individual expressed emotion measures: no usable data. Hospital admission: no usable data. Poor compliance with treatment-relatives: no usable data. Knowledge of diagnosis: no usable data. Knowledge of symptoms: no usable data. Relatives’ knowledge about treatment: denominator unclear. Change in relatives’ attitude: no usable data. Length of time to relapse: no SD. Quality of life/employment: no usable data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomised, by random numbers table |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blind |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: random allocation using table of random numbers. Blindness: single. Duration: 9 months treatment, 2 years follow up. Setting: London, UK. |
|
| Participants | Diagnosis: schizophrenia (PSE). N = 23. Age: range 18-65 years, mean 26.5. Sex: 13 M, 10 F. History: high EE families, > 35 hours contact/week (CFI), mean previous admissions ~ 2.5. Exclusions: not reported. |
|
| Interventions |
|
|
| Outcomes | Relapse. Family experience: expressed emotion. High contact with families. Unable to use: Drug compliance: no numbers for each group. Social activity: no numbers for each group. Occupational activities: no numbers for each group. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomised, by random numbers table |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single, untested |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: single assessors blind. Duration: 1 year. Setting: London, UK. |
|
| Participants | Diagnosis: schizophrenia. N = 30. Age: range 16-65 years, mean 33.5. Sex: not reported. History: mean number of previous admissions to hospital 2.4. Exclusions: not reported. |
|
| Interventions |
Family intervention based on the work by Kuipers, consists of 3 months of didactic instruction followed by supervision of the trainees’ clinical work with families. Family intervention included techniques for improving communication within the family, reducing relatives’ criticism and over involvement, lowering contact between patient and high expressed emotion relatives, increasing the social networks of family members and setting realistic objectives. The approach includes cognitive and behavioural elements as well as techniques from strategic and systemic family therapy |
|
| Outcomes | Death. Relapse. Family experience: expressed emotion, assessment of burden. Knowledge of interview. Social functioning: SFS. Unable to use: Family experience: CFI (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single blind, untested |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised - no further details. Blindness: not stated. Duration: 9 months. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-2-R). N = 86. Age: mean age ~ 23 years. Sex: men and women. History: first episode. |
|
| Interventions |
|
|
| Outcomes | Mental state: BPRS. Global state: relapse. |
|
| Notes | *This intervention has several components, each component has its only frequency: 1) psychoeducation on the cause, development of SZ, 30 minutes/twice/week; 2) psychological intervention and crisis intervention including demonstration of communications skills and emotional expression skills, 1 hour/omonth; 3) seminars for patients and family to exchange experiences and encourage each other, 2 hours/2months | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No details |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: open study. Duration: 2 years. Setting: community, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-2-R). N = 80. Age: mean 20 years. Sex: male and female. History: no details. Excluded: severe illness; drug addict or alcoholic; pregnancy |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Global state: hospitalisation. Global state: SCL-90. Unable to use: Mental state: SANS (sub-scale data only). |
|
| Notes | Routine nursing, discharge advice and family intervention plus cognitive behavioural intervention. Once every 3 months for 2 years, there is a psychoeducation seminar; each familywas given a booklet on the prevention and family nursing techniques of the illness; also, they are invited back to clinic for check-ups once every 2-3 months | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: random allocation, stratified by EE level, by table of numbers. Blindness: single. Duration: 1 year treatment, 1 year follow up. Setting: Amsterdam, The Netherlands. |
|
| Participants | Diagnosis: schizophrenia + related disorders (DSM-III-R). N = 76. Age: range 15-26 years, mean 20.6. Sex: 53 M, 23 F. History: 43% > 1 psychotic episode, needing continuous anti-psychotic medication. Exclusions: primary substance dependence, drug related psychoses |
|
| Interventions |
|
|
| Outcomes | Relapse: BPRS + scrutiny of notes. Unable to use: Drug compliance: categorical scale, no data reported. |
|
| Notes | Before randomisation all families attended psychoeducational meetings | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomised, by random numbers table |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single, untested |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: quasi-randomised (hospital admission). Blindness: no details. Duration: three years. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (CCM-2-R). N = 200. Age: mean 26 years. Sex: men and women. History: no details. Excluded: no details. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | No | Randomised, according to hospital admission |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No details |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: open study. Duration: one year. Setting: China. |
|
| Participants | Diagnosis: chronic schizophrenia (CCMD-3). N = 80. Age: mean 29 years. Sex: men. History: no details. |
|
| Interventions |
|
|
| Outcomes | Global state: compliance with medication. Global state: relapse. Unable to use: Mental state: BPRS (no n values). |
|
| Notes | Family intervention is in the form of telephone consultation and consultation at the clinic, once a month for 1 year | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
Unclear | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: no details. Duration: two years. Setting: community, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-3). N = 90. Age: 18-26 years. Sex: no details. History: average length of illness ~ 6 years. |
|
| Interventions |
*Individual family intervention: family visit was made at the end of 2nd week after the start of the intervention, then at the end of every 4th week until the completion of the intervention |
|
| Outcomes | Global state: relapse. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No details |
| Incomplete outcome data addressed? All outcomes |
Unclear | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly sampled’. Blindness: no details. Duration: 2 years. Setting: community, Nanyang City, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-2-R). N = 90 Age: 18-60 years. Sex: male and female. History: no details. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Unable to use: Leaving the study early. |
|
| Notes | Family intervention involved providing information to family members; providing communication skill training; teaching coping strategies. Aim of the therapy is to improve family’s coping ability and improve family functioning and atmosphere, as well as their knowledge on schizophrenia. Therapy was provided by qualified psychiatrists for 50 minutes/month | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No details |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomisation by computer. Blindness: open study. Duration: 6 months. Setting: Italy. |
|
| Participants | Diagnosis: chronic schizophrenia (DSM IV). N = 71. Age: mean 56 years. Sex: 49 M, 22 F. History: previous suicide attempts ~ 10, chronic illness with average age of onset 21; average hospitalisation two |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Global state: compliance. Mental state: BPRS. Social functioning: Assessment of Disability, Social Network Questionnaire. Family outcome: Family Problems Questionnaire. |
|
| Notes | The intervention consists of four components (developed by Falloon): assessment of individual and family needs; information sessions with consumers and their relatives about clinical aspects of schizophrenia, its treatments and early signs of relapse; communication skills training; and problem-solving skills training. The training program included three monthly modules of two and a half days each. In the year after the training course, participants attended four supervision meetings and each month they received by-phone tutorial support on family work | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer randomisation |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | Grants from the M. Lugli Foundation and from Italy's National Institute of Health |
| Methods | Allocation: ’randomly allocated’ - no further details. Blindness: not blinded. Duration: 1 year. Setting: Hong Kong. |
|
| Participants | Diagnosis: chronic schizophrenia (DSM-III). N = 55. Age: range 18-63 years. Sex: M and F. History: duration of illness 2 years or more. |
|
| Interventions |
|
|
| Outcomes | Employment status at 6 months. Unable to use: Mental state: BPRS (no usable data). Knowledge about schizophrenia: no usable data. The WHO Psychiatric Disability Assessment Schedule: no usable data. Service satisfaction: CSQ: no usable data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blind |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly assigned’ - no further details. Blindness: psychiatrists and relapse field raters blind. Duration: treatment 2 years, follow up 2 years. Setting: New York, USA. |
|
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, schizophreniform disorder (DSM-III R). N = 172.* Age: range 18-45 years, mean 27.3. Sex: 126 M, 46 F. History: >/=10 hours family contact for 2 months preceding admission, mean age of onset of illness 19.5 years. Exclusions: physically dependent substance abuse. |
|
| Interventions |
|
|
| Outcomes | Relapse. Drug compliance. Leaving the study early. Unable to use: Hospital admission: data not given for each group. Mental state: BPRS (no usable data). Medication dose: no SD. Employment: no usable data. Relapse episodes: no usable data. Cost effectiveness: no usable data. |
|
| Notes | * 19 cases excluded, probably after randomisation (11 cases not discharged after 2 years, 8 dropped out during engagement period (1st week). No further data analysis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Partial blinding, untested |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: block randomisation. Blindness: single. Duration: follow up 1 year. Setting: Aarhus, Denmark. |
|
| Participants | Diagnosis: schizophrenia (ICD-10). N = 46. Age: range 30.3 - 39.6 years, mean 35.9. Sex: 24 M, 22 F. History: receiving treatment at time of inclusion in community psychiatric centres |
|
| Interventions |
|
|
| Outcomes | Relapse. Leaving the study early. Global state: GAF. Mental state: BPRS, IS. Service satisfaction: VSSS. Knowledge of schizophrenia. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised by block, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single, untested |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomised’ - no further details. Blindness: not stated. Duration: treatment 8 weeks, follow up 10 months. Setting: Winnipeg, Canada. |
|
| Participants | Diagnosis: schizophrenia (DSM-III R). N = 55. Age:< 40 years, mean 29.1. Sex: 39 M, 16 F. History: > 1 admissions (mean 4.4), last within past 2 years, regular contact with family |
|
| Interventions |
|
|
| Outcomes | Death. Hospital admission. Leaving the study early. Unable to use: Global state: GHQ (no usable data). Negative feelings for patient: no usable data. Family experience: WOC, FSS (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Not stated |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: no details. Duration: one year. Setting: community, Nanyang City, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-2-R). N = 120. Age: mean 33 years. Sex: men and women. History: average length of illness ~ 6 years. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Unable to use Mental state: BPRS, SDSS (ns not reported). Social functioning: SDSS (no usable data). Leaving the study early. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Not reported |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: block randomised by cluster (townships) by random numbers table. Blindness: ’assessors were blind to the study design’. Duration: 9 months. Setting: Chengdu, China. |
|
| Participants | Diagnosis: schizophrenia (ICD-10, CCMD-2-R). N = 6 townships (326 people). Age: mean 44 years. Sex: 128 M, 198 F. History: chronic and acute schizophrenia. |
|
| Interventions |
|
|
| Outcomes | Relapse. Compliance with medication. Social functioning. Clinical status. Family maltreatment. Unable to use: Mental disability: outcomes unclear. |
|
| Notes | ICC not reported, estimated to be 0.1. Data divided by Design Effect = (1+(m-1)*ICC). |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Randomised, computer generated |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Assessors blind, no further details |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised - no further details. Blindness: not stated. Duration: treatment 3 years. Setting: Los Angeles, USA. |
|
| Participants | Diagnosis: schizophrenia (DSM-III). N = 42. Age: range 21-55 years. Sex: not reported. History: chronic illness, average age at first hospital admission 22.2 years |
|
| Interventions |
25 behavioural family management sessions held with the families over a 12-month period on a declining contact basis (mean 21 sessions, SD 5.7) |
|
| Outcomes | Relapse. Leaving the study early. Hospital stay. Unable to use: Mental state: BPRS, PSE (no usable data). Family experience: CFI (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Not stated |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly assigned’ - method not described, stratified randomisation to medication. Blindness: not stated for family treatment. Duration: 30 months treatment and follow up. Setting: Atlanta, San Francisco, New Hyde Park, Philadelphia, New York |
|
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, schizophreniform disorder (DSM-III R). N = 528. Age: range 18-55 years, mean 29.6* Sex: 349 M, 179 F* History: acutely ill, hospital admissions, living with or > 4 hours face to face contact with family of origin. Exclusions: liver damage, organic brain disease, psychoactive substance dependence pregnancy |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Stabilisation. Hospital admission. Unable to use: Relapse: BPRS worsening of symptoms for 2 months or use of rescue medication (no usable data). First use of rescue medication: no usable data. 20 weeks use of rescue medication: no results given. Time to rehospitalisation: no usable data. |
|
| Notes | *Mean age and sex extrapolated from demographic data for 313 subjects followed to end of treatment | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Not stated |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Yes | Grant from NIMH |
| Methods | Allocation: randomised - no further details. Blindness: not reported. Duration: 2 years. Setting: Shanghai, China. |
|
| Participants | Diagnosis: schizophrenia. N = 214*. Age: mean 42.1 years. Sex: male and female. History: not reported. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Quality of life. Unable to use: Mental state: PANSS, Self Rating Anxiety Scale (no SD). Disability Assessment Scale: no SD. |
|
| Notes | *One participant not accounted for. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Not stated |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’exploratory randomised controlled trial’. Blindness: not blinded. Duration: 6 months. Setting: London, UK. |
|
| Participants | Diagnosis: schizophrenia (44), other diagnoses (17) schizoaffective disorder, bipolar disorder, psychotic depression. N = 61. Age: not stated. Sex: not stated. History: not stated. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Global state: Clinical Interview Schedule revised. Coping skills: Coping with Life-events & Difficulties Interview. Family experience: Experience of Care giving Inventory, Stressor-severity of care giving difficulty. Social functioning: Self-Evaluation & Social Support Schedule Unable to use: Hospital readmission: no usable data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Not blinded |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: open study. Duration: three years. Setting: China. |
|
| Participants | Diagnosis: chronic schizophrenia (CCMD-3, ICD-10). N= 150. Age: 18-55 years. Sex: men and women. History: no details. |
|
| Interventions |
|
|
| Outcomes | Relapse. Social functioning: Social Disability Screening Schedule |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly allocated’ - method not described, stratified by first/multiple episode, presence/absence of residual symptoms and EE. Blindness: single - CFI, PSE, relapse. Duration: 9 months treatment, 8 years follow up. Setting: Salford, UK. |
|
| Participants | Diagnosis: schizophrenia (PSE). N = 83*. Age: range 16-64 years, mean 35.3. Sex: 29 M, 54 F. History: acutely ill, hospital admissions, to be discharged to family having lived with them > 3 months, mean past admissions ~ 3, mean duration ill ~ 6 yrs. Excluded: organic illness. |
|
| Interventions |
Education only: 2 sessions with family. N = 16* high EE, 9 low EE. Control: routine multidisciplinary care in OPD. N = 16* high EE, 10 low EE More than 5 sessions. |
|
| Outcomes | Death. Relapse (recurrence/worsening of psychotic symptoms over 1 week, PSE). Hospital admission. Leaving the study early. Family experience: CFI. Unable to use: Contact with services: no data. Use of medication: no data. |
|
| Notes | Intervention group 1+2 both involved psychoeducational involvement of families undertaken by multidisciplinary team in clinics, 2 sessions of educational programme, 3 of stress management, and 8 of goal setting. These groups added for this analysis. Groups 3+4 not split in data reporting and used as comparison for this analysis *Only the 64 people from high EE families were randomised to group 1 +2 vs group 3+4, and are used in this analysis. 19 from low EE families were allocated to groups 3+4 only and are not included in this analysis |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single, untested |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly allocated’ - no further details. Blindness: rater blind. Duration: 10 weeks treatment, 9 months follow up. Setting: Sydney, Australia. |
|
| Participants | Diagnosis: schizophrenia (PSE). N = 36. Age: mean 26.3 years. Sex: 30 M, 6 F. History: mean previous admissions ~ 4, high EE families, newly admitted. Exclusions: heroin abuse, brain damage. |
|
| Interventions |
|
|
| Outcomes | Death. Relapse: PSE. Hospital admission. Drug compliance. Leaving the study early. Unable to use: Relatives satisfaction with care: no control group data. Family experience: CFI (ratings not reported because unreliable) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single blind |
| Incomplete outcome data addressed? All outcomes |
Yes | Study attrition reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: no details. Duration: one year. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-3). N = 89*. Age: no details. Sex: no details. History: average length of illness ~ 6 years. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Global state: compliance. Social functioning: Social Disability Screening Schedule (skewed) |
|
| Notes | *9 participants not accounted for. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | None |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly selected’ - no further details. Blindness: double. Duration: 4 months. Setting: Sichuan, China. |
|
| Participants | Diagnosis: schizophrenia and affective disorders (DSM-III-R). N = 77. Age: range 18-80 years, mean 40.5. Sex: not stated. History: not reported. |
|
| Interventions |
|
|
| Outcomes | Treatment compliance. Family care status. Clinical status. Clinical state. Social functioning. Unable to use: Medical records: no usable data. Mental state: PSE (no usable data). Social functioning: Social Disability Screening Schedule (no usable data) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Double blind, untested |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: open study. Duration: one year. Setting: community, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-2-R). N= 160. Age: mean 41 years. Sex: male and female. History: no details. Excluded: severely disabled. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Unable to use: Mental state: BPRS (no n’s). Social functioning: SDSS (no n’s). Treatment rate. (unclear outcome). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
Unclear | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly assigned’ - no further details. Blindness: assessments blinded. Duration: 18 months treatment, 18 months follow up. Setting: Shashi & Jingzhou, China. |
|
| Participants | Diagnosis: schizophrenia (DSM-III-R). N = 63*. Age: range 17-54 years, mean 31. Sex: 43 M, 20 F. History: mean previous admissions ~ 4, mean duration ill ~ 7.5 years, participants living with family |
|
| Interventions |
|
|
| Outcomes | Death. Relapse. Global state: GAF. Mental state: BPRS-R, SAPS-CV, SANS-CV. Hospital admission. Drug compliance. Family burden. |
|
| Notes | *One participant not accounted for. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single blind, untested |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: ’randomly assigned’ - no further details. Blindness: raters blinded. Duration: 18 months treatment, 18 months follow up. Setting: Suzhou, China. |
|
| Participants | Diagnosis: schizophrenia (Chinese Medical Association’s criteria). N = 83. Age: mean 23.8 years. Sex: 78 M, 5 F. History: no previous admissions, mean duration ill 2.8 years. Exclusions: concurrent medical illness. |
|
| Interventions |
|
|
| Outcomes | Hospital admission. Drug compliance. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | Single, untested |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: none. Duration: two years. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-3). N = 60. Age: range 19-45 years. Sex: male only. History: no details. Excluded: no details. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Global state: compliance. |
|
| Notes | *one session per week/4 sessions = 1 course and two courses in total | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: no details. Duration: two years. Setting: China. |
|
| Participants | Diagnosis: schizophrenia. N= 150. Age: 16-60 years. Sex: men and women. History: no details. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Unable to use: Mental state: PANSS (n’s not reported). Global state: GHQ (n’s not reported). Social functioning: DAS (n’s not reported). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
Unclear | No details |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
| Methods | Allocation: randomised. Blindness: none. Duration: one year. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (CCMD-2-R). N = 286. Age: range 17-68 years. Sex: men and women. History: no details. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised, no further details |
| Allocation concealment? | Unclear | No details |
| Blinding? All outcomes |
No | Open study |
| Incomplete outcome data addressed? All outcomes |
No | Study attrition not reported |
| Free of selective reporting? | Unclear | No details |
| Free of other bias? | Unclear | No details |
Diagnostic tools and scales:
CCMD-2-R - Chinese Classification of Mental Disorders 2nd edition revised
CCMD-3-R - Chinese Classification of Mental Disorders 3rd edition revised
DSM - Diagnostic and Statistical Manual
ESQ - Early Signs Questionnaire
ICD 10 - International Classification of Diseases
MPS - Munster Prognosis Score
RDC - Research Diagnostic Criteria
Mental state:
AMDP - Arbeitsgemeinschaft fur Methodik und Dokumentation in Psychiatrie
BPRS - Brief Psychiatric Rating Scale
CES-D - Centre for Epidemiologic Studies Depression Scale
IS - Insight Scale
PANSS - Positive and Negative Symptom Scale
PANAS - The Positive and Negative Affects Scale
PSE - Present State Examination
SANS - Scale for the Assessment of Negative Symptoms
SAPS - Scale for the Assessment ofPositive Symptoms
SCL-90 - Symptom Checklist 90
Global state:
CGI - Clinical Global Impression
ESQ - Early Signs Questionnaire
GAF - Global Assessment of Functioning
GAS - Global Assessment Scale
GHQ - General Health Questionnaire
Social functioning -
DAS - Disability Assessment Schedule
LSP - The Life Skills Profile
FMSS - Five Minutes Speech Sample
HoNOS - Health nf the Nation Outcome Scales
MRSS - Morningside Rehabilitation State Scale
SAS-SR - Social Adjustment Scale - self report
SBAS - Social Behaviour Assessment Schedule
SDSS - Social Disability Screening Schedule
SCOS - Strauss-Carpenter Outcome Scale
SFS - Social Functioning Scale
Family experience -
ACL - Adjective Check List
CFI - Camberwell Family Interview
CFQ - Questionnaire of Family Confrontation
CGSQ - Care Givers Strain Questionnaire
ECI - Experience of Caregiving Inventory
FKI - Family Conflict Inventory
FSS - Family satisfaction scale
MFB - Munster Family Questionnaire
SISCI-1 - Synthesis and Scission-1 Test
WOC - Ways ofCoping
Family outcome -
CSQ - The Client Satisfaction Questionnaire
VSSS - Verona Service Satisfaction Scale
Adverse events -
SAS - Simpson and Angus Scale
TESS - Treatment Emergent Symptom Scale
General -
EE = expressed emotion
ICC = Intraclass Correlation Coefficient
m = mean number within each group
Sz = schizophrenia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Abramowitz 1989 | Allocation: not randomised. |
| Barber 1988 | Allocation: randomised. Participants: families of people with schizophrenia. Intervention: family intervention versus standard care - 6 hour, 1 day workshop for families - fewer than 5 sessions. Outcomes: no usable data. |
| Barrowclough 1999 | Allocation: randomised. Participants: families of people with schizophrenia. Intervention: family support versus any appropriate psychosocial intervention and not specifically randomised to family intervention |
| Barrowclough 2002 | Allocation: randomised. Participants: people with schizophrenia who are substance misusers. Intervention: routine care versus routine care and integrated psychological and psychosocial treatment programme |
| Birchwood 1992 | Allocation: part sequential, part randomised. Participants: families of people with schizophrenia. Intervention: group education versus postal education versus video education - each with/without homework - fewer than 5 sessions. Outcomes: no usable data. |
| Brooker 1992 | Allocation: not randomised, case series. |
| Byalin 1985 | Allocation: randomised. Participants: people with schizophrenia. Interventions: observational evaluation of treatment refractory patients and a stable group of chronically ill people with schizophrenia. Outcomes: no usable data. |
| Cozolino 1988 | Allocation: randomised, stratified for EE. Participants: families of people with schizophrenia or schizoaffective disorder. Intervention: 3-hour family education session (fewer than 5 sessions) versus standard care. Outcomes: part of cohort did not receive FI because of participation in another ongoing study - no separate data available |
| Durell 1968 | Allocation: randomised. Participants: people with schizophrenia. Intervention: 3-hour family education session versus standard care - fewer than 5 sessions. Outcomes: no usable data. |
| Durr 1996 | Allocation: randomised. Participants: people with schizophrenia. Intervention: psychoeducational treatment with standard care versus psychoeducational treatment with prophylactic premedication |
| Dyck 2000 | Allocation: randomised by cohort. Participants: families of people with schizophrenia or schizoaffective disorder. Intervention: group family treatment versus standard care. Outcomes: no usable data because no intra class correlation coefficients given |
| Esterson 1965 | Allocation: not randomised, case series. |
| Fowler 2002 | Allocation: randomised. Participants: people with psychosis. Interventions: family intervention and cognitive behavioural therapy and standard care, number of groups and treatment assignments unclear. Outcomes: no data reported. |
| Freeman 2002 | Allocation: not randomised. |
| Glick 1985 | Allocation: randomised. Participants: families of people with schizophrenia, schizophreniform disorder, major affective disorder (DSM- III). Intervention: family psychotherapy versus multimodal hospital care - restricted to inpatients. Outcomes: not reported separately for schizophrenia. |
| Hahlweg 1999 | Allocation: randomised. Participants: people with schizophrenia and their closest relative. Interventions: behavioural family management using targeted medication versus behavioural family management with either targeted medication or standard dose |
| He 2005 | Allocation: quasi-randomised. Participants: people with schizophrenia. Interventions: family intervention versus standard care. Outcomes: no usable data. |
| Hogarty 1974 | Allocation: randomised. Participants: people with schizophrenia. Interventions: chlorpromazine or placebo and major role therapy versus intensive social case work plus vocational rehabilitation counselling |
| Hornung 1995 | Allocation: randomised. Participants: people with schizophrenia and their relatives. Interventions: psychoeducational medication training (PMT) and cognitive psychotherapy plus work with relatives’ groups versus psychoeducational medication training (PMT) and cognitive psychotherapy versus control group |
| Hu 2002 | Allocation: not randomised. |
| Jenner 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: care as usual versus hallucination focused integrative treatment |
| Jeppesen 1999 | Allocation: randomised. Participants: people with schizophrenia and care givers. Intervention: assertive community treatment, psychoeducational family treatment and social skill training versus standard care. Outcomes: no usable data. |
| Kelly 1990 | Allocation: randomised Participants: families of people with schizophrenia. Intervention: group education versus postal information versus video information |
| Kim 1997 | Allocation: not randomised. |
| Koettgen 1988 | Allocation: randomised. Participants: people with schizophrenia and their families. Intervention: therapy group (high EE) versus 1st control group (high EE) versus 2nd. control group (low EE) Outcomes: no usable data. |
| Kopelowicz 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: skills training versus standard care, not family intervention |
| Kottgen 1984 | Allocation: not randomised. |
| Langsley 1968 | Allocation: randomised. Participants: families of all admissions to hospital. Intervention: family crisis support and education versus standard care. Outcomes: not reported separately for schizophrenia. |
| Lenior 2001 | Allocation: randomised. Participants: parents of patients with recent onset schizophrenia. Intervention: standard intervention versus behavioural family intervention with standard intervention. Outcomes: no usable data. |
| Lenior 2003 | Allocation: randomised. Participants: people with schizophrenia. Intervention: family intervention versus standard care. Outcomes: no usable data. |
| Levene 1989 | Allocation: not randomised, case series. |
| Lewandowski 1988 | Allocation: not randomised. |
| Li 1996 | Allocation: not reported as being randomised. |
| Li 1998 | Allocation: randomised. Participants: people with schizophrenia. Interventions: family intervention versus standard care. Outcomes: no usable data. |
| Li 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: family intervention versus standard care. Outcomes: no usable data. |
| Lu 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: family intervention versus standard care. Outcomes: no usable data. |
| Ma 2003b | Allocation: not randomised. |
| MacCarthy 1989 | Allocation: randomised. Participants: families of people with schizophrenia, Asperger’s syndrome, manic-depressive illness, psychotic depression, and ’unsure’ diagnoses. Intervention: psycho-social intervention for families versus standard care. Outcomes: no outcomes reported separately for those with schizophrenia |
| Madew 1967 | Allocation: randomised. Participants: people with schizophrenia restricted to an inpatient environment - not family therapy |
| Mak 1996 | Allocation: randomised. Participants: people with schizophrenia and a family member. Intervention: family therapy and psychosocial education versus conventional treatment. Outcomes: no data presented. |
| Malm 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: integrated community care, with both arms receiving psychosocial family intervention |
| Mavreas 1992 | Allocation: randomised. Participants: families of people with schizophrenia. Intervention: family intervention versus standard treatment. Outcomes: no outcomes reported. |
| McCreadie 1991 | Allocation: not randomised. |
| McFarlane 1995b | Allocation: randomised in cohorts. Participants: families of people with schizophrenia or schizoaffective disorder. Intervention: psychoeducational single family treatment versus psychoeducational group family treatment versus dynamic group family treatment. Outcomes: not usable because no intra-class correlation coefficients provided |
| Merinder 1998 | Allocation: not randomised. |
| Motlova 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: family psychoeducation versus family psychoeducation, dosage study |
| Mottaghipour 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: early onset psychosis given family psychoeducation versus chronic psychosis given family psychoeducation |
| Nordentoft 1999 | Allocation: randomised. Participants: people with schizophrenia. Interventions: assertive community treatment, psychoeducational treatment and social skill training versus standard care. Outcomes: no data presented. |
| Olfson 1998 | Allocation: randomised. Participants: people with schizophrenia and their family members.. Intervention: ’medication algorithms’ versus patient and family education versus clinical support. Outcomes: no usable data. |
| Pereira 1994 | Allocation: not randomised. |
| Petersen 2005 | Allocation: randomised. Participants: people with 1st episode schizophrenia. Intervention: integrated treatment versus standard care (family intervention offered as an option to intervention group) |
| Pitschel 1993 | Allocation: randomised. Participants: not described. Intervention: psychoeducational groups and their relatives versus standard care. Outcomes: no usable data. |
| Pu 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: family nursing versus standard care - not family intervention |
| Ro-Trock 1977 | Allocation: randomised. Participants: adolescents with schizophrenia, adjustment reaction, drug problems. Intervention: family therapy versus individual therapy. Outcomes: not reported separately for schizophrenia. |
| Roncone 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: single family intervention versus multiple family intervention |
| Shimodera 2000 | Allocation: randomised. Participants: people with schizophrenia and their family members. Intervention: standard care versus standard care with single family treatment. Outcomes: no usable data. |
| Smith 1978 | Allocation: randomised. Participants: acutely ill psychiatric patients. Intervention: home care versus inpatient control group, not family intervention |
| Smith 1987 | Allocation: randomised. Participants: families of people with schizophrenia. Intervention: group family intervention versus postal information - no standard care comparison |
| Solomon 1996 | Allocation: randomised. Participants: families of those with schizophrenia, major affective disorder (DSM III-R). Intervention: individual family consultation versus group family psychoeducation. Outcome: no usable data. |
| Spencer 1988 | Allocation: randomised. Participants: people with schizophrenia within a inpatient setting, not family therapy |
| Spiegel 1987 | Allocation: randomised. Participants: people with schizophrenia and their families. Interventions: family home consultations versus standard care, with both groups having FI available |
| Stein 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: customary care versus standard care, unclear if family intervention. Outcomes: no usable data. |
| Tarrier 1989 | Allocation: not randomised, review. |
| Tarrier 2000 | Allocation: not randomised. |
| Telles 1995 | Allocation: randomised. Participants: families of people with schizophrenia (DSM-III-R) with florid symptoms (BPRS). Interventions: behavioural family management versus standard case management. Outcomes: no usable data, numbers in each group not reported |
| Valencia 1999 | Allocation: quasi-experimental - not randomised. Participants: people with schizophrenia. Interventions: psychosocial intervention - not family therapy |
| Ventegodt 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: assertive community treatment, psychoeducational treatment and social skills training versus ’standard treatment’. Outcomes: no usable data. |
| Victolo 1999 | Allocation: randomised. Participants: people with schizophrenia and their families. Interventions: standard care versus psychoeducational intervention. Outcomes: no usable data. |
| Wang 1999 | Allocation: not reported to be randomised. |
| Wellisch 1977 | Allocation: 3 year follow up study from a randomised study. Participants: 71% schizophrenic reaction and 29% non-schizophrenic. Interventions: individual therapy versus family therapy. Outcomes: no data presented for the schizophrenia group. |
| Wiedemann 1992 | Allocation: randomised. Participants: people with schizophrenia. Interventions: targeted medication and family therapy versus maintenance therapy with family therapy. Outcomes: no usable data. |
| Wiedemann 1994 | Allocation: randomised. Participants: families of people with schizophrenia, schizoaffective disorder (RDC). Intervention: behavioral family management with standard dose medication versus behavioral family management with targeted dose medication |
| Wu 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: psychoeducation, unclear if family intervention given |
| Yao 1997 | Allocation: not reported to be randomised. |
| Zastowny 1992 | Allocation: randomised. Participants: people with schizophrenia and their families within an inpatient setting, not family therapy |
| Zhang 1998 | Allocation: randomisation unclear, blinding not reported. |
| Zhang 1999 | Allocation: not randomised. |
| Zhang 2000b | Allocation: not randomised. |
| Zhang 2003 | Allocation: not reported. Participants: people with schizophrenia. Interventions: family intervention, open ward versus closed ward |
Diagnostic tool
DSM-III - Diagnostic Statistical Manual, version 3.
DSM-III-R - Diagnostic Statistical Manual, version 3, revised.
RDC - Research Diagnostic Criteria.
Mental state scale
BPRS - Brief Psychiatric Rating Scale.
DATA AND ANALYSES
Comparison 1. ANY FAMILY-BASED INTERVENTIONS (> 5 sessions) vs STANDARD CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Service utilisation: 1. Hospital admission | 14 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 0-6 months | 3 | 232 | Risk Ratio (M-H, Fixed, 95% CI) | 0.85 [0.44, 1.66] |
| 1.2 7-12 months | 9 | 532 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.63, 0.98] |
| 1.3 13-18 months | 3 | 228 | Risk Ratio (M-H, Fixed, 95% CI) | 0.46 [0.30, 0.69] |
| 1.4 19-24 months | 5 | 375 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.65, 1.07] |
| 1.5 25-36 months | 2 | 205 | Risk Ratio (M-H, Fixed, 95% CI) | 0.91 [0.72, 1.16] |
| 2 Service utilisation: 2. Days in hospital at 3 months | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | −6.67 [−11.59, −1.75] |
| 3 Service utilisation: 3. Days in hospital at 1 year (skewed data) | Other data | No numeric data | ||
| 4 Global state: 1. Relapse | 36 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 0-6 months | 3 | 213 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.46, 1.09] |
| 4.2 7-12 months | 32 | 2981 | Risk Ratio (M-H, Fixed, 95% CI) | 0.55 [0.48, 0.62] |
| 4.3 13-18 months | 3 | 181 | Risk Ratio (M-H, Fixed, 95% CI) | 0.64 [0.47, 0.88] |
| 4.4 19-24 months | 13 | 1019 | Risk Ratio (M-H, Fixed, 95% CI) | 0.64 [0.55, 0.75] |
| 4.5 25-36 months | 4 | 497 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.72, 1.10] |
| 4.6 5 years | 1 | 63 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 4.7 8 years | 1 | 62 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 5 Global state: 2. Not improved/deteriorated | 2 | 112 | Risk Ratio (M-H, Fixed, 95% CI) | 0.40 [0.23, 0.68] |
| .51 by 6 months | 1 | 77 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.17, 0.62] |
| 5.2 by 9 months | 1 | 35 | Risk Ratio (M-H, Fixed, 95% CI) | 0.70 [0.26, 1.88] |
| 6 Global state: 3. Average endpoint score (GAF, high score = better) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 0-12 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | −10.28 [−20.34, −0. 22] |
| 6.2 2 years | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | −8.66 [−14.37, −2.94] |
| 7 Global state: 4. Average change score (GAF, high score = better - skewed data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 post-intervention | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 4.88 [−3.87, 13.63] |
| 7.2 at one year | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [−3.18, 13.68] |
| 8 Global state: 5. Average endpoint score at 2 years (SCL-90, high score = poor) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −22.01 [−30.99, −13. 03] |
| 9 Mental state: 1a. Average endpoint score (BPRS, high score = poor) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 total score at 1 year | 3 | 170 | Mean Difference (IV, Fixed, 95% CI) | −8.32 [−10.92, −5.73] |
| 9.2 negative score at 6 months | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [−0.90, 0.30] |
| 10 Mental state: 1b. Average change score (BPRS total, high score = poor) | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [−0.76, 0.17] |
| 11 Mental state: 1c. Average change score (BPRS positive, high score = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 post intervention 8 sessions | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | −2.72 [−7.10, 1.66] |
| 12 Mental state: 2a. Average endpoint score (PANSS, 1 year, high score = poor) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 total | 2 | 174 | Mean Difference (IV, Fixed, 95% CI) | −7.90 [−11.96, −3.83] |
| 12.2 positive subscore | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | −2.72 [−6.27, 0.83] |
| 12.3 negative subscore | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | −2.02 [−5.88, 1.84] |
| 12.4 general psychopathology | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | −3.60 [−5.82, −1.38] |
| 13 Mental state: 2b. Average endpoint score (PANSS, 18 months, high score = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 total | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | −6.30 [−15.98, 3.38] |
| 13.2 positive subscore | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [−2.16, 4.04] |
| 13.3 negative subscore | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | −5.23 [−8.43, −2.03] |
| 14 Mental state: 2c. Average endpoint score (PANSS, 36 months, high score = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 total | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | −10.20 [−13.55, −6. 85] |
| 14.2 positive subscore | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | −2.60 [−4.12, −1.08] |
| 14.3 negative subscore | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | −3.70 [−4.94, −2.46] |
| 15 Mental state: 2d. Average change score (PANSS, high score = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 positive | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | −2.0 [−3.49, −0.51] |
| 15.2 negative | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | −4.0 [−5.81, −2.19] |
| 16 Mental state: 3. Average endpoint score (Positive and Negative Symptoms, skewed data) | Other data | No numeric data | ||
| 16.1 Scale for Assessment of Positive Symptoms (Chinese version) at 18 months | Other data | No numeric data | ||
| 16.2 Scale for Assessment of Negative Symptoms (Chinese version) at 18 months | Other data | No numeric data | ||
| 17 Mental state: 4. Average endpoint score (SANS high score = worse, skewed) | Other data | No numeric data | ||
| 18 Mental state: 5. Average change in insight (Insight Scale, high score = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 post intervention - 8 sessions | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [−1.03, 1.07] |
| 18.2 at 1 year | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [−0.50, 2.38] |
| 19 Mental state: 6. Average endpoint score (Frankfurt (FBF-3 scale) 1 year, high score = poor, skewed data) | Other data | No numeric data | ||
| 20 Behaviour: 1. Average endpoint score (NOSIE, 1 year, high score = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 total score | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 59.10 [54.57, 63.63] |
| 20.2 positive factor score | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 33.4 [30.52, 36.28] |
| 21 Behaviour: 2. Average endpoint score (NOSIE negative factor, 1 year, high score = poor) | Other data | No numeric data | ||
| 22 Compliance: 1. Leaving the study early | 28 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 22.1 by between 3 and 6 months | 7 | 552 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.59, 1.42] |
| 22.2 by between 7 months and 1 year | 10 | 733 | Risk Ratio (M-H, Fixed, 95% CI) | 0.74 [0.53, 1.03] |
| 22.3 by between 13 months and 2 years | 10 | 887 | Risk Ratio (M-H, Fixed, 95% CI) | 0.74 [0.55, 1.00] |
| 22.4 by between 25 months and 3 years | 3 | 290 | Risk Ratio (M-H, Fixed, 95% CI) | 0.42 [0.26, 0.67] |
| 22.5 by more than 3 years | 1 | 63 | Risk Ratio (M-H, Fixed, 95% CI) | 1.72 [0.71, 4.16] |
| 23 Compliance: 2. Poor compliance with medication | 10 | 695 | Risk Ratio (M-H, Fixed, 95% CI) | 0.60 [0.49, 0.73] |
| 24 Compliance: 3. Poor compliance with standard community care | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 24.1 at 1 year | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.41, 1.11] |
| 24.2 at 2 years | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 0.85 [0.55, 1.30] |
| 25 Compliance: 4. Months on medication | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 25.1 by 6 months follow up | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [−0.34, 1.14] |
| 25.2 by 12 months follow up | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [−0.54, 2.74] |
| 25.3 by 18 months follow up | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [−1.10, 4.30] |
| 26 Adverse events: Death | 8 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 26.1 suicide | 7 | 377 | Risk Ratio (M-H, Fixed, 95% CI) | 0.79 [0.35, 1.78] |
| 26.2 other cause | 4 | 176 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.19, 3.11] |
| 27 Social functioning: 1a. General - socially impaired (0-9 months) | 2 | 116 | Risk Ratio (M-H, Fixed, 95% CI) | 0.51 [0.35, 0.72] |
| 28 Social functioning: 1b. General - average endpoint score (Social Function Scale, 1 year, high score = good) | 3 | 90 | Mean Difference (IV, Fixed, 95% CI) | −8.05 [−13.27, −2.83] |
| 29 Social functioning: 2a. Specific - unemployed | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 29.1 at6-12 months follow up | 5 | 285 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.89, 1.25] |
| 29.2 at 2 years | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 1.33 [0.84, 2.10] |
| 29.3 at 3 years follow up | 1 | 99 | Risk Ratio (M-H, Fixed, 95% CI) | 1.19 [0.92, 1.55] |
| 30 Social functioning: 2b. Specific - unable to perform work activities | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 30.1 by 4 months | 1 | 77 | Risk Ratio (M-H, Fixed, 95% CI) | 0.31 [0.09, 1.03] |
| 30.2 by 9 months | 1 | 35 | Risk Ratio (M-H, Fixed, 95% CI) | 1.68 [0.17, 16.91] |
| 31 Social functioning: 2c. Specific - time in employment at one year (skewed) | Other data | No numeric data | ||
| 32 Social functioning: 2d. Specific - unable to live independently | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 32.1 by 1 year | 3 | 164 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.66, 1.03] |
| 32.2 by 3 years | 1 | 99 | Risk Ratio (M-H, Fixed, 95% CI) | 0.82 [0.59, 1.14] |
| 33 Social functioning: 2e. Specific - imprisonment | 1 | 39 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.22, 4.14] |
| 34 Social functioning: 3. Disability Assessment Schedule (3 year, high score = poor) | Other data | No numeric data | ||
| 35 Social functioning: 4. Average endpoint score (SDSS, high score = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 35.1 at two years | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | −0.51 [−1.38, 0.36] |
| 35.2 at three years | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | −1.94 [−2.90, −0.98] |
| 36 Social functioning: 5. Average SDSS endpoint score at one year (high score = poor, skewed) | Other data | No numeric data | ||
| 37 Social functioning: 6. Average endpoint score (HoNOS 1 year, high score = poor) | Other data | No numeric data | ||
| 38 Family outcome: 1a. Coping and understanding: general issues (dichotomised from WOC scale) | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 38.1 family not able to cope a lot better at 6 months | 1 | 63 | Risk Ratio (M-H, Fixed, 95% CI) | 0.79 [0.60, 1.03] |
| 38.2 patient coping poorly with key relatives at 9 months | 1 | 39 | Risk Ratio (M-H, Fixed, 95% CI) | 1.11 [0.45, 2.70] |
| 38.3 not understanding the patient a lot better at 6 months | 1 | 63 | Risk Ratio (M-H, Fixed, 95% CI) | 0.58 [0.39, 0.87] |
| 39 Family outcome: 1b. Coping and understanding: insufficient care or maltreatment by family | 2 | 111 | Risk Ratio (M-H, Fixed, 95% CI) | 0.49 [0.23, 1.04] |
| 39.1 by up to 6 months | 1 | 77 | Risk Ratio (M-H, Fixed, 95% CI) | 0.53 [0.22, 1.24] |
| 39.2 by up to 9 months | 1 | 34 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.08, 1.87] |
| 40 Family outcome: 1c. Coping: Average score (Coping with Life-events & Difficulties Interview, high = poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 40.1 effective coping endpoint score (6 months) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | −0.5 [−1.85, 0.85] |
| 40.2 ineffective coping endpoint score (6 months) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [−0.72, 1.32] |
| 41 Family outcome: 2. Service usage: Family Support Service Index, 3 months (high scores = worse) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.86 [0.21, 1.51] |
| 42 Family outcome: 3. Functioning (Family Assessment Device, 3 months, high scores = worse) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | −6.56 [−10.50, −2.62] |
| 43 Family outcome: 4a. Burden (Family Burden Interview Schedule, 3 months, high score = worse) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | −7.01 [−10.77, −3.25] |
| 44 Family outcome: 4b. Burden endpoint score at 0-18 months (high score = poor) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | −0.40 [−0.71, −0.09] |
| 45 Family outcome: 4c. Burden - not improved/worse (objective burden related to self-sufficiency) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 45.1 12 months | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 0.53 [0.21, 1.37] |
| 45.2 2 years | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 1.92 [0.19, 19.90] |
| 46 Family outcome: 4d. Burden -not improved/worse (objective burden related to social functioning) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 46.1 12 month | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 2.40 [0.51, 11.27] |
| 46.2 2 year | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 2.88 [0.64, 12.97] |
| 47 Family outcome: 4e. Burden -not improved/worse (subjective burden) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 47.1 12 months | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 1.44 [0.60, 3.46] |
| 47.2 2 years | 1 | 51 | Risk Ratio (M-H, Fixed, 95% CI) | 0.58 [0.15, 2.16] |
| 48 Family outcome: 4f. Burden -endpoint score (1 year, high score = worse, skewed) | Other data | No numeric data | ||
| 49 Family outcome: 5a. Expressed emotion | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 49.1 overall levels | 1 | 75 | Risk Ratio (M-H, Fixed, 95% CI) | 0.9 [0.68, 1.19] |
| 49.2 over involvement | 1 | 63 | Risk Ratio (M-H, Fixed, 95% CI) | 0.40 [0.22, 0.73] |
| 49.3 criticism | 1 | 63 | Risk Ratio (M-H, Fixed, 95% CI) | 0.44 [0.24, 0.81] |
| 49.4 hostility | 2 | 87 | Risk Ratio (M-H, Fixed, 95% CI) | 0.35 [0.18, 0.66] |
| 49.5 high EE family | 3 | 164 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.54, 0.86] |
| 50 Family outcome: 5b. Expressed emotion, warmth 1 year (CFI, high score = poor) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [−0.29, 1.23] |
| 51 Family outcome: 5c. Expressed emotion (1 year, skewed) | Other data | No numeric data | ||
| 51.1 critical comments | Other data | No numeric data | ||
| 51.2 over-involvement | Other data | No numeric data | ||
| 52 Family outcome: 6. Knowledge Score (1 year, skewed data) | Other data | No numeric data | ||
| 53 Family outcome: 7. Average endpoint score (Clinical Interview Schedule Revised, 6 months, skewed) | Other data | No numeric data | ||
| 54 Family outcome: 8. Average endpoint score (Experience of Caregiving Inventory, 6 months, skewed) | Other data | No numeric data | ||
| 55 Family outcome: 9. Average endpoint score (SESS, 6 months, skewed) | Other data | No numeric data | ||
| 55.1 general support | Other data | No numeric data | ||
| 55.2 confidant support | Other data | No numeric data | ||
| 56 Family outcome: 10. Average endpoint score (Stressor-severity of caregiving difficulty, 6 months, skewed) | Other data | No numeric data | ||
| 57 Family outcome: 11. Average change in emotion expressed by relatives (Family Q’aire - after 8 sessions) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | −3.25 [−8.24, 1.74] |
| 58 Family outcome: 12a. Satisfaction - average change in relatives’ satisfaction (VSSS , 1 year, data skewed) | Other data | No numeric data | ||
| 59 Family outcome: 12b. Satisfaction - relatives (VSSS -post intervention at 8 sessions, skewed) | Other data | No numeric data | ||
| 60 Family outcome: 12c. Satisfaction - patients (VSSS -post intervention at 8 sessions, skewed) | Other data | No numeric data | ||
| 61 Family outcome: 13.Average change score (APGAR, by 1 year, high score = better) | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | −2.90 [−3.40, −2.40] |
| 62 Quality of Life: 1. Average endpoint score (QoL, 2 years, high score = good) | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | 19.18 [9.78, 28.58] |
| 63 Quality of life: 2. Average endpoint change (QoL, 1 year, high score = good) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −5.05 [−15.44, 5.34] |
Comparison 2. BEHAVIOURAL FAMILY-BASED vs SUPPORTIVE FAMILY-BASED INTERVENTIONS (> 5 sessions).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Service utilisation: Hospital Admission by 19-24 months | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2 Global state: Unstable (0-6 months) | 1 | 528 | Risk Ratio (M-H, Fixed, 95% CI) | 1.08 [0.88, 1.33] |
| 3 Compliance: Leaving the study early +/− poor compliance with treatment protocol (up to 30 months) | 1 | 528 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
Comparison 3. GROUP FAMILY-BASED INTERVENTIONS vs INDIVIDUAL FAMILY-BASED INTERVENTIONS (> 5 sessions).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. Relapse | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 7-12 months | 2 | 195 | Risk Ratio (M-H, Fixed, 95% CI) | 0.70 [0.41, 1.22] |
| 1.2 19-24 months | 3 | 197 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.48, 1.05] |
| 2 Global state: 2. More than 1 relapse (19-24 months) | 1 | 172 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.34, 1.50] |
| 3 Compliance: 1. Poor compliance with treatment protocol | 2 | 195 | Risk Ratio (M-H, Fixed, 95% CI) | 1.35 [0.84, 2.17] |
| 4 Compliance: 2. Poor compliance with medication | 1 | 172 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.50, 1.99] |
| 5 Social functioning: Unable to live independently (by 1 year) | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 2.18 [1.09, 4.37] |
| 6 Family outcome: Emotion expressed at 2 years (high EE families) | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.45, 1.92] |
Analysis 1.4.
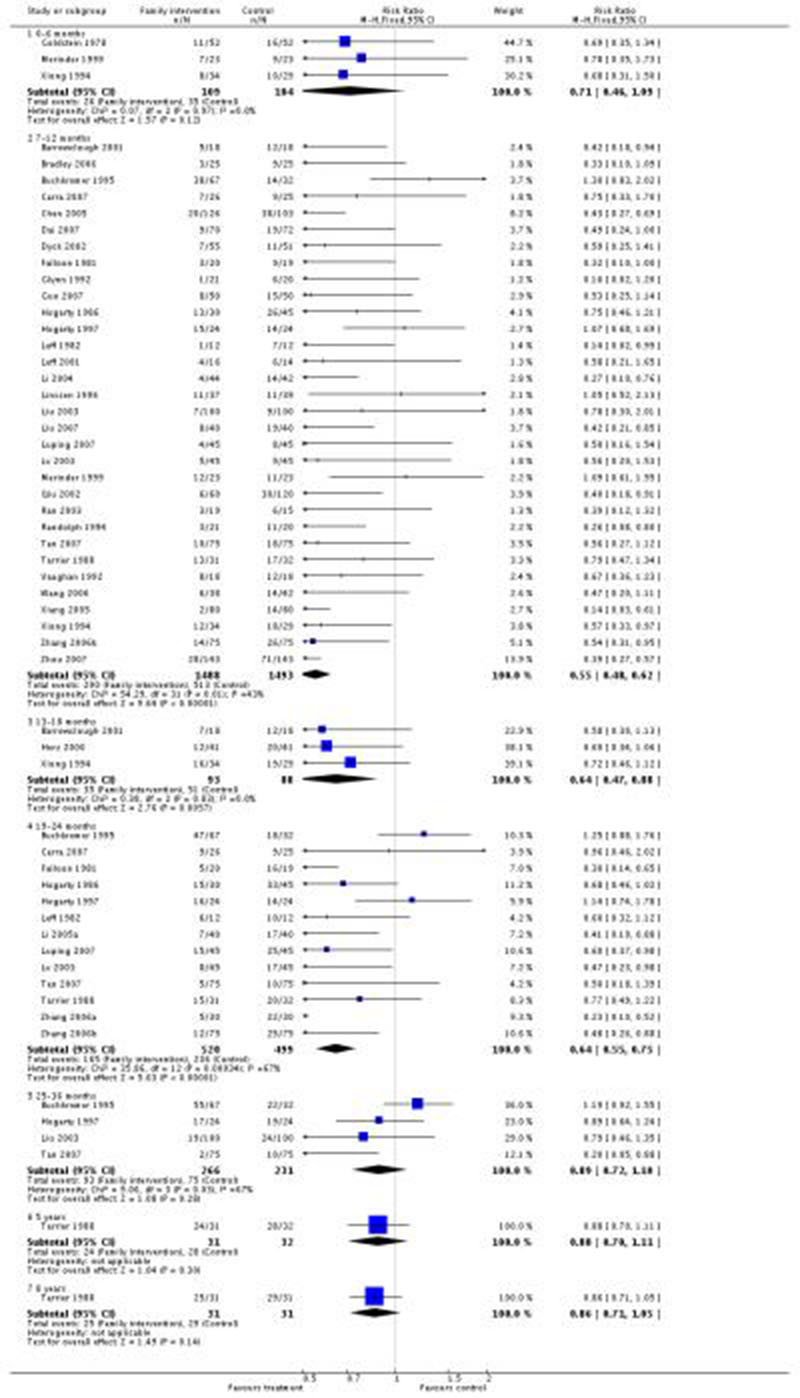
Review: Family intervention for schizophrenia
Comparison: 1 ANY FAMILY-BASED INTERVENTIONS (> 5 sessions) vs STANDARD CARE
Outcome: 4 Global state: 1. Relapse
FEEDBACK
Results
Summary
It is unclear from the review how many of the 12 included studies reported subsidiary outcome data and why all the subsidiary data were not used in analysis.
Reply
The review has been substantially rewritten and updated. The ‘Included trials’ table has been much expanded.
Contributors
Comment received from Christine Barrowclough, Salford, UK, June 1997
Reply from Fiona Pharoah, Cambridge, UK, February 1999
WHAT’S NEW
Last assessed as up-to-date: 14 January 2010.
| Date | Event | Description |
|---|---|---|
| 20 January 2010 | New search has been performed | Reformatted. 21 new studies added in 2010 update. |
HISTORY
Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 23 August 2006 | New citation required and conclusions have changed | Substantive amendment |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
In early versions of this review we used an I2 estimate of 75% or more to indicate the presence of heterogeneity. For the 2010 update we used a more conservative estimate of heterogeneity I2 more than 50%. In addition, 11 studies that were included in earlier versions, under a subsection of fewer than five family intervention sessions, were removed and are to be included in a separate review of family intervention using entry criteria of fewer than five family intervention sessions.
Appendix 1. Previous search strategies
-
1
Electronic searches for update June 2005
-
1.1
We searched the Cochrane Schizophrenia Group’s Trials Register (June 2005) using the phrase:
[(*family* or family*) in title, abstract, index terms of REFERENCE] or [(*family* or family*) in interventions of STUDY]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
-
2
Details of previous electronic searches
-
2.1
We searched the Cochrane Schizophrenia Group’s register of trials (November 2002) using the phrase:
[(*family* or family*) in title, abstract, index terms of REFERENCE] or [(*family* or family*) in interventions of STUDY]
-
2.2
We searched the Cochrane Library (Issue 2, 1998) using the Cochrane Schizophrenia Group’s terms for schizophrenia (see Group Module) combined with the phrase:
[famil*]
-
2.3
We searched the Cochrane Schizophrenia Group’s Register (June 1998) using the phrase:
[famil* or #42=105 or #42=107]
#42 is the field within this register that held codes for each intervention and 105 and 107 are the codes for family interventions.
-
2.4
We searched EMBASE (January 1981 to June 1995) using the Cochrane Schizophrenia Group’s terms for both randomised controlled trials and schizophrenia (see Group Module) combined with the phrase:
and (famil* and therap*)
-
2.5
We searched MEDLINE (January 1966 to June 1995) using the Cochrane Schizophrenia Group’s terms for both randomised controlled trials and schizophrenia (see Group Module) combined with the phrase:
and (explode/family in MeSH and famil*)
Appendix 2. Risk of bias
Assessment of methodological quality
We assessed the methodological quality of included trials in this review using the criteria described in the Cochrane Handbook (Higgins 2005) and the Jadad Scale (Jadad 1996). The former is based on the evidence of a strong relationship between allocation concealment and direction of effect (Schulz 1995). The categories are defined below:
Low risk of bias (adequate allocation concealment)
Moderate risk of bias (some doubt about the results)
High risk of bias (inadequate allocation concealment). For the purpose of the analysis in this review, we included trials if they met the Cochrane Handbook criteria A or B.
The Jadad Scale measures a wider range of factors that impact on the quality of a trial. The scale includes three items:
Was the study described as randomised?
Was the study described as double-blind?
Was there a description of withdrawals and drop outs?
Each item receives one point if the answer is positive. In addition, a point can be deducted if either the randomisation or the blinding/masking procedures described are inadequate. For this review we used a cut-off of two points on the Jadad scale to check the assessment made by the Handbook criteria. However, the Jadad Scale was not used to exclude trials. We only included quasi-randomised studies if it was clear that the demographic profile of each group was similar.
Footnotes
DECLARATIONS OF INTEREST
None known.
References to studies included in this review
- Barrowclough 2001 {published data only} .Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O’Brien R. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. American Journal of Psychiatry. 2001;158(10):1706–13. doi: 10.1176/appi.ajp.158.10.1706. [DOI] [PubMed] [Google Scholar]
- Haddock G, Barrowclough C, Tarrier N, Moring J, O’Brien R, Schofield N, Quinn J, Palmer S, Davies L, Lowens I, McGovern J, Lewis S. Cognitive-behavioural therapy and motivational intervention for schizophrenia and substance misuse. 18-month outcomes of a randomised controlled trial. British Journal of Psychiatry. 2003;183:418–26. doi: 10.1192/bjp.183.5.418. [DOI] [PubMed] [Google Scholar]
- Bloch 1995 {published data only} .Bloch S, Szmukler GI, Herrman H, Benson A, Colussa S. Counselling caregivers of relatives with schizophrenia: themes, interventions, and caveats. Family Process. 1995;34:413–25. doi: 10.1111/j.1545-5300.1995.00413.x. [DOI] [PubMed] [Google Scholar]
- Szmukler GI, Herrman H, Colusa S, Benson A, Bloch S. A controlled trial of a counselling intervention for caregivers of relatives with schizophrenia. Social Psychiatry and Psychiatric Epidemiology. 1996;31:149–55. doi: 10.1007/BF00785761. [DOI] [PubMed] [Google Scholar]
- Bradley 2006 {published data only} .Bradley GM, Couchman GM, Perlesz A, Nguyen AT, Singh B, Riess C. Multiple-family group treatment for English- and Vietnamese-speaking families living with schizophrenia. Psychiatric Services. 2006;57(4):521–30. doi: 10.1176/ps.2006.57.4.521. [DOI] [PubMed] [Google Scholar]
- Buchkremer 1995 {published data only} .Buchkremer G, Schulze Monking H, Holle R, Hornung WP. The impact of therapeutic relatives’ groups on the course of illness of schizophrenic patients. European Psychiatry. 1995;10:17–27. doi: 10.1016/0767-399X(96)80071-X. [DOI] [PubMed] [Google Scholar]
- Buchkremer G, Stricker K, Holle R, Kuhs H. The predictability of relapses in schizophrenic patients. European Archives of Psychiatry and Clinical Neuroscience. 1991;240:292–300. doi: 10.1007/BF02189543. [DOI] [PubMed] [Google Scholar]
- Schulze Monking H. Self-help groups for families of schizophrenic patients: formation, development and therapeutic impact. Social Psychiatry and Psychiatric Epidemiology. 1994;29:149–54. doi: 10.1007/BF00796496. [DOI] [PubMed] [Google Scholar]
- Carra 2007 {published data only} .Carrà G, Montomoli C, Clerici M, Lorenzo Cazzullo CL. Family interventions for schizophrenia in Italy: randomized controlled trial. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:23–30. doi: 10.1007/s00406-006-0677-z. [DOI] [PubMed] [Google Scholar]
- Chen 2005 {published data only} .Chen H. Schizophrenia in rural communities control study of family intervention. Chinese Journal of Clinical Medicinal Professional Research. 2005;11(1):21–23. [Google Scholar]
- Chien 2004 {published data only} .Chien WT, Norman I, Thompson DR. A randomized controlled trial of a mutual support group for family caregivers of patients with schizophrenia. International Journal of Nursing Studies. 2004;41(6):637–49. doi: 10.1016/j.ijnurstu.2004.01.010. MEDLINE: 15240088. [DOI] [PubMed] [Google Scholar]
- Dai 2007 {published data only} .Dai M, Liu F, Fan J. A study on influence of early comprehensive intervention on prognosis of incipient schizophrenia patients. Chinese Nursing Research. 2007;21(9):3293–4. [Google Scholar]
- De Giacomo 1997 {published data only} .De Giacomo P, Pierri G, Rugiu AS, Buonsante M, Vadruccio F, Zavoianni L. Schizophrenia: a study comparing a family therapy group following a paradoxical model plus psychodrugs and a group treated by the conventional clinical approach. Acta Psychiatrica Scandinavica. 1997;95(3):183–8. doi: 10.1111/j.1600-0447.1997.tb09618.x. [DOI] [PubMed] [Google Scholar]
- Du 2005 {published data only} .Du Z, Zhang L, Chen J, Li G, Cao Z. The study of family support and the quality of life changed of schizophrenes before and after family intervention. Chinese Nursing Mangagement. 2005;5(3):32–4. [Google Scholar]
- Dyck 2002 {published data only} .Dyck DG, Hendryx MS, Short RA, Voss WD, McFarlane WR. Service use among patients with schizophrenia in psychoeducational multiple-family group treatment. Psychiatric Services. 2002;53(6):749–54. doi: 10.1176/appi.ps.53.6.749. [DOI] [PubMed] [Google Scholar]
- Falloon 1981 {published data only} .Doane JA, Falloon IR, Goldstein MJ, Mintz J. Parental affective style and the treatment of schizophrenia. Predicting course of illness and social functioning. Archives of General Psychiatry. 1985;42:34–42. doi: 10.1001/archpsyc.1985.01790240036004. [DOI] [PubMed] [Google Scholar]
- Falloon IR, McGill CW, Boyd JL, Pederson J. Family management in the prevention of morbidity of schizophrenia: social outcome of a two-year longitudinal study. Psychological Medicine. 1987;17:59–66. doi: 10.1017/s0033291700012988. [DOI] [PubMed] [Google Scholar]
- Falloon IRH, Boyd JL, McGill CW, Razani J, Moss HB, Gilderman AM. Family management in the prevention of exacerbations of schizophrenia: a controlled study. New England Journal of Medicine. 1982;306:1437–40. doi: 10.1056/NEJM198206173062401. [DOI] [PubMed] [Google Scholar]
- Falloon IRH, Jeffery LB, McGill CW, Williamson M, Razani J, Moss HB, Gilderman AM, Simpson GM. Family management in the prevention of morbidity of schizophrenia: clinical outcome of a two-year longitudinal study. Archives of General Psychiatry. 1985;42:887–96. doi: 10.1001/archpsyc.1985.01790320059008. [DOI] [PubMed] [Google Scholar]
- Falloon IRH, Pederson J. Family management in the prevention of schizophrenia: the adjustment of the family unit. British Journal of Psychiatry. 1985;147:156–63. doi: 10.1192/bjp.147.2.156. [DOI] [PubMed] [Google Scholar]
- Falloon IRH, Razani J, Moss HB, Boyd JL, McGill CW, Pederson J. Community-based treatment of schizophrenia: a one-year check-up with families and individual therapy [Gemeindenahe versorgung von schizophrenen eine einjaehrige kontrolluntersuchung bei familienund einzeltherapie] Partnerberatung. 1983;20:73–9. [Google Scholar]
- Liberman RP, Cardin V, McGill CW, Falloon IR. Behavioral family management of schizophrenia: clinical outcome and costs. University of Maryland School of Medicine Symposium: Economic issues in schizophrenia (1986) Psychiatric Annals. 1987;17:610–19. [Google Scholar]
- McGill CW, Falloon IR, Boyd JL, Wood SC. Family educational intervention in the treatment of schizophrenia. Hospital and Community Psychiatry. 1983;34:934–8. doi: 10.1176/ps.34.10.934. [DOI] [PubMed] [Google Scholar]
- Rea M, Strachan A, Goldstein M, Falloon I, Hwang S. Changes in patient coping style following individual and family treatment for schizophrenia. British Journal of Psychiatry. 1991;158:642–7. doi: 10.1192/bjp.158.5.642. [DOI] [PubMed] [Google Scholar]
- Strang JS, Falloon IRH, Moss HB, Razini J, Boyd JL. Drug treatment and family intervention during the aftercare treatment of schizophrenics. Psychopharmacology Bulletin. 1981;17:87–8. [PubMed] [Google Scholar]
- Fernandez 1998 {published data only} .Fernandez OV, Lemos GS, Garcia SA, Otero GA, Alonso SM, Gutierrez Perez AM. Integrated psychological treatment for schizophrenic patients [Tratamiento psicologico integrado de pacientes esquizofrenicos] Psicothema. 1998;10(2):459–74. [Google Scholar]
- Glynn 1992 {published data only} .Glynn SM, Randolph ET, Eth S, Paz GG, Leong GB, Shaner AL, Van Vort W. Schizophrenic symptoms, work adjustment, and behavioral family therapy. Rehabilitation Psychology. 1992;37:323–38. [Google Scholar]
- Randolph ET, Eth S, Glynn SM, Paz GG, Leong GB, Shaner L, Strachan A, Van-Vort W, Escobar JI, Liberman RP. Behavioural family management in schizophrenia. Outcome of a clinic-based intervention. British Journal of Psychiatry. 1994;164:501–6. doi: 10.1192/bjp.164.4.501. [DOI] [PubMed] [Google Scholar]
- Goldstein 1978 {published data only} .Goldstein MJ, Kopeiken HS. Short- and long-term effects of combining drug and family therapy. In: Goldstein MJ, editor. New Developments in Interventions With Families of Schizophrenics. Jossey-Bass; San Francisco: 1981. pp. 5–26. [Google Scholar]
- Goldstein MJ, Rodnick EH, Evans JR, May PRA, Steinberg MR. Drug and family therapy in the aftercare of acute schizophrenics. Archives of General Psychiatry. 1978;35:1169–77. doi: 10.1001/archpsyc.1978.01770340019001. [DOI] [PubMed] [Google Scholar]
- Gong 2007 {published data only} .Gong C, Zhang J, Zheng G. Three-year follow-up study of family synthetically intervention for community family bed patients with schizophrenia. Chinese Journal of Behavioral Medical Science. 2007;16(10):903–5. [Google Scholar]
- Guo 2007 {published data only} .Guo Y, Gao Z, Wu Y, Yan Z. Influence of family intervention on the cl inic curative effect of schizophrenia patients. Chinese Journal of Health Education. 2007;23(7):550–1. [Google Scholar]
- Herz 2000 {published data only} .Herz MI, Lamberti JS, Mintz J, Scott R, O’Dell SP, McCartan L, Nix G. A program for relapse prevention in schizophrenia: a controlled study. Archives of General Psychiatry. 2000;57(3):277–83. doi: 10.1001/archpsyc.57.3.277. [DOI] [PubMed] [Google Scholar]
- Hogarty 1986 {published data only} .Anderson CM, Reiss DJ. Approaches to psychoeducational family therapy. International Journal of Family Psychiatry. 1982;3:501–17. [Google Scholar]
- Hogarty G, McEvoy J, Munetz M, DiBarry A, Bartone P, Cather R, Cooley SR, Carter M, Madonia M. Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia. Results of a two-year controlled study. Archives of General Psychiatry. 1988;45:797–805. doi: 10.1001/archpsyc.1988.01800330021002. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Anderson C. Bewaeltigung der Schizophrenie. Multidimensionale Konzepte Psychosoziale und Kognitive Therapien Angehoerigenarbeit und Autoprotektive Anstrengungen. Verlag Hans Huber; Bern: 1986. A controlled trial of family therapy, social skills training and drug therapy in the aftercare of schizophrenics. Effects on relapse and expressed emotion [Eine kontrollierte studie ueberfamilientherapie, training sozialer fertigkeiten und unterstuetzende chemotherapie in der nachbehandlung schizophrener vorlaeufige effekte auf rezidive und expressed emotion nach einem jahr bewaeltigung der schizophrenie] pp. 72–86. [Google Scholar]
- Hogarty GE, Anderson CM. Medication, family psychoeducation, and social skills training: first-year relapse results of a controlled study. Psychopharmacology Bulletin. 1986;22:860–2. [PubMed] [Google Scholar]
- Hogarty GE, Anderson CM, Reiss DJ, Kornblith SJ, Greenwald DP, Javna CD, Madonia MJ, Environmental/personal indicators in the course of schizophrenia research group Family psychoeducation, social skills training and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. One-year effects of a controlled study on relapse and expressed emotion. Archives of General Psychiatry. 1986;43:633–42. doi: 10.1001/archpsyc.1986.01800070019003. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Anderson CM, Reiss DJ, Kornblith SJ, Greenwald DP, Ulrich RF, Carter M, The Environmental-personal Indicators in the Course of Schizophrenia Research Group Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. II. Two-year effects of a controlled study on relapse and adjustment. Archives of General Psychiatry. 1991;48:340–7. doi: 10.1001/archpsyc.1991.01810280056008. [DOI] [PubMed] [Google Scholar]
- Hogarty 1997 {published data only} .Hogarty GE, Kornblith SJ, Greenwald D, DiBarry AL, Cooley S, Ulrich RF, Carter M, Flesher S. Three-year trial of personal therapy among schizophrenic patients living with or independent of family. I: Description of study and effects of relapse rates. American Jornal of Psychiatry. 1997;154(11):1504–15. doi: 10.1176/ajp.154.11.1504. [DOI] [PubMed] [Google Scholar]
- Leavey 2004 {published data only} .Leavey G, Gulamhussein S, Papadopoulos C, Johnson-Sabine E, Blizard BKM. A randomized controlled trial of a brief intervention for families of patients with a first episode of psychosis. Psychological Medicine. 2004;34(3):423–31. doi: 10.1017/s0033291703001594. MEDLINE: 15259827. [DOI] [PubMed] [Google Scholar]
- Leff 1982 {published data only} .Berkowitz R. Therapeutic intervention with schizophrenic patients and their families: a description of a clinical research project. Journal of Family Therapy. 1984;6:211–33. [Google Scholar]
- Berkowitz R, Eberlein-Fries R, Kuipers L, Leff J. Educating relatives about schizophrenia. Schizophrenia Bulletin. 1984;10:418–29. doi: 10.1093/schbul/10.3.418. [DOI] [PubMed] [Google Scholar]
- Leff J, Berkowitz R, Shavit N, Strachan A, Glass I, Vaughn C. A trial of family therapy versus a relatives’ group for schizophrenia. British Journal of Psychiatry. 1989;154:58–66. doi: 10.1192/bjp.154.1.58. [DOI] [PubMed] [Google Scholar]
- Leff J, Kuipers L, Berkowitz R, Eberlein-Fries R, Sturgeon D. A controlled trial of social interventions in the families of schizophrenic patients. British Journal of Psychiatry. 1982;141:121–34. doi: 10.1192/bjp.141.2.121. [DOI] [PubMed] [Google Scholar]
- Leff J, Kuipers L, Berkowitz R, Eberlein-Fries R, Sturgeon D. Psychosocial relevance and benefit of neuroleptic maintenance: experience in the United Kingdom. Journal of Clinical Psychiatry. 1984;45:43–9. [PubMed] [Google Scholar]
- Leff J, Kuipers L, Berkowitz R, Sturgeon D. A controlled trial of social intervention in the families of schizophrenic patients: two-year follow-up. British Journal of Psychiatry. 1985;146:594–600. doi: 10.1192/bjp.146.6.594. [DOI] [PubMed] [Google Scholar]
- Leff 1989 {published data only} .Leff J, Berkowitz R, Shavit N, Strachan A, Glass I, Vaughn C. A trial of family therapy versus a relatives’ group for schizophrenia. British Journal of Psychiatry. 1989;154:58–66. doi: 10.1192/bjp.154.1.58. [DOI] [PubMed] [Google Scholar]
- Leff J, Berkowitz R, Shavit N, Strachan A, Glass I, Vaughn C. A trial of family therapy versus a relatives’ group for schizophrenia: two-year follow-up. British Journal of Psychiatry. 1990;157:571–7. doi: 10.1192/bjp.157.4.571. [DOI] [PubMed] [Google Scholar]
- Leff 2001 {published data only} .Leff J, Sharpley M, Chisholm D, Bell R, Gamble C. Training community psychiatric nurses in schizophrenia family work: A study of clinical and economic outcomes for patients and relatives. Jounal of Mental Health. 2001;10(2):189–97. [Google Scholar]
- Li 2004 {published data only} .Li Y, Jia JD, Zhang MS. Influence of family mental intervention on social function, family environment and relapse rate in first-episode schizophrenics. Zhongguo Linchuang Kangfu. 2004;(21):4184–5. EMBASE: 2004428972. [Google Scholar]
- Li 2005a {published data only} .Li M, Li Z. Effect of family interference on rehabilitation of the patients with schizophrenia. Journal of Nursing Science. 2005;20(23):48–9. [Google Scholar]
- Linszen 1996 {published and unpublished data} .Linszen D, Dingemans P, Lenior M, Scholte W. Early individual and family intervention in schizophrenia; Proceedings of the10th World Congress of Psychiatry; 1996; Madrid, Spain. 1996.Aug 23-28, [Google Scholar]
- Linszen D, Dingemans P, Van Der Does JW, Nugter A, Scholte P, Lenoir R, Goldstein MJ. Treatment, expressed emotion and relapse in recent onset schizophrenic disorders. Psychological Medicine. 1996;26(2):333–42. doi: 10.1017/s0033291700034723. [DOI] [PubMed] [Google Scholar]
- Linszen DH, Dingemans P, Lenior ME, Nugter MA, Scholte WF, Van Der Does AJ. Relapse criteria in schizophrenic disorders: different perspectives. Psychiatry Research. 1994;54:273–81. doi: 10.1016/0165-1781(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Nugter A, Dingemans P, Van Der Does JW, Linszen D, Gersons B. Family treatment, expressed emotion and relapse in recent onset schizophrenia. Psychiatry Research. 1997;72:23–31. doi: 10.1016/s0165-1781(97)00086-3. [DOI] [PubMed] [Google Scholar]
- Nugter MA, Dingemans P, Linszen DH, Van Der Does AJW, Gersons BPR. Parental communication deviance: its stability and the effect of family treatment in recent-onset schizophrenia. Acta Psychiatrica Scandinavica. 1997;95(3):199–204. doi: 10.1111/j.1600-0447.1997.tb09620.x. [DOI] [PubMed] [Google Scholar]
- Liu 2003 {published data only} .Liu QH, Zhan LY. Effect of one to one system family intervention on the reduction of schizophrenia relapse: arandomized controlled trial. Chinese Journal of Clinical Rehabilitation. 2003;7(30):1114–5. [Google Scholar]
- Liu 2007 {published data only} .Liu TF, Cai XH. Family intervention on schizophrenia. Medical Journal of Chinese Peoples Health. 2007;19(3):223–25. [Google Scholar]
- Luping 2007 {published data only} .Jiang L, Xu XJ, Xu M. Effect of backup psychotherapy in family on schizophrenic recrudescence. China Journal of Health Psychology. 2007;15(4):368–9. [Google Scholar]
- Lv 2003 {published data only} .Lv XL, Tang QF. Individual family treatment on relapse of schizophrenia. Medical Journal of Chinese People Health. 2003;15(8):465–6. [Google Scholar]
- Magliano 2006 {published data only} .Magliano L, Fiorillo A, Malangone C, De Rosa C, Maj M. Patient functioning and family burden in a controlled, real-world trial of family psychoeducation for schizophrenia. Psychiatric Services. 2006;57(12):1784–91. doi: 10.1176/ps.2006.57.12.1784. [DOI] [PubMed] [Google Scholar]
- Mak 1997 {published data only} .Mak KY, Wong MC, Ma LK, Fung SC. A cost-effectiveness study of a community-based family management rehabilitation programme for schizophrenic outpatients in Hong Kong. Hong Kong Journal of Psychiatry. 1997;7(2):26–35. [Google Scholar]
- McFarlane 1995 {published data only} .McFarlane WR, Lukens E, Link B, Dushay R, Deakins SA, Newmark M, Dunne EJ, Horen B, Toran J. Multiple-family groups and psychoeducation in the treatment of schizophrenia. Archives of General Psychiatry. 1995;52:679–87. doi: 10.1001/archpsyc.1995.03950200069016. [DOI] [PubMed] [Google Scholar]
- Merinder 1999 {published data only} .Merinder LB, Viuff AG, Laugesen HD, Clemmensen K, Misfelt S, Espensen B. Patient and relative education in community psychiatry: a randomized controlled trial regarding its effectiveness. Social Psychiatry and Psychiatric Epidemiology. 1999;34(6):287–94. doi: 10.1007/s001270050146. [DOI] [PubMed] [Google Scholar]
- Posner 1992 {published data only} .Posner CM, Wilson KG, Kral MJ, Lander S, Mcllwraith RD. Family psychoeducational support groups in schizophrenia. American Journal of Orthopsychiatry. 1992;62(2):206–18. doi: 10.1037/h0079337. [DOI] [PubMed] [Google Scholar]
- Qiu 2002 {published data only} .Qiu YF, Ma LX, Tong Q. The role of family therapy in the rehabilitation of patients with schizophrenia. Chinese Journal of Clinical Rehabilitation. 2002;6(23):3551. [Google Scholar]
- Ran 2003 {published data only} .Ran M, Xiang M, Huang M. A control study of psychoeducational family intervention for relatives of schizophrenics in a Chinese rural community. Chinese Journal of Psychiatry. 2001;34(2):98–101. [Google Scholar]
- Ran MS, Xiang MZ, Chan LW, Leff J, Simpson P, Huang M-S, Shan YH, Li SG. Effectiveness of Psychoeducational intervention for rural Chinese families experiencing schizophrenia. Social Psychiatry and Psychiatric Epidemiology. 2003;38:69–75. doi: 10.1007/s00127-003-0601-z. [DOI] [PubMed] [Google Scholar]
- Randolph 1994 {published data only} .Randolph ET, Spencer ETH, Glynn SH, Paz GG, Leong GB. Behavioural family management in schizophrenia outcome of a clinic-based intervention. British Journal of Psychiatry. 1994;164:501–6. doi: 10.1192/bjp.164.4.501. [DOI] [PubMed] [Google Scholar]
- Schooler 1997 {published data only} .Falloon IRH, McGill CW, Matthews SM, Keith SJ, Schooler NR. Family treatment for schizophrenia - the design and research application of therapist training models. Journal of Psychotherapy Practice and Research. 1996;5:45–56. [PMC free article] [PubMed] [Google Scholar]
- Keith SJ, Bellack A, Frances A, Mance R, Matthews SM. The influence of diagnosis and family treatment on acute treatment response and short term outcome in schizophrenia. Psychopharmacology Bulletin. 1989;25:336–9. [PubMed] [Google Scholar]
- Schooler NJ, Keith SJ, Severe JB, Matthews SM, Bellack A, Glick ID, Hargreaves WA, Kane JM, Ninan PT, Frances A, Jacobs M, Lieberman JA, Mance R, Simpson GM, Woerner MG. Relapse and rehospitalisation during maintenance treatment of schizophrenia. The effects of dose reduction and family treatment. Archives of General Psychiatry. 1997;54:453–63. doi: 10.1001/archpsyc.1997.01830170079011. [DOI] [PubMed] [Google Scholar]
- Wuerker AK, Long JD, Haas GL, Bellack AS. Interpersonal control, expressed emotion, and change in symptoms in families of persons with schizophrenia. Schizophrenia Research. 2002;58:281–92. doi: 10.1016/s0920-9964(01)00320-6. [DOI] [PubMed] [Google Scholar]
- Shi 2000 {published data only} .Shi Y, Zhao B, Xu D, Sen J. A comparative study of life quality in schizophrenic patients after family intervention. Chinese Mental Health Journal. 2000;14(2):135–7. [Google Scholar]
- Szmukler 2003 {unpublished data only} .*; Szmukler G, Kuipers E, Joyce J, Harris T, Leese M, Maphosa W, Staples E. An exploratory randomised controlled trial of a support programme for carers of patients with a psychosis. Social Psychiatry and Psychiatric Epidemiology. 2003;38(8):411–8. doi: 10.1007/s00127-003-0652-1. [DOI] [PubMed] [Google Scholar]
- Tan 2007 {published data only} .Tan SQ, Liu GY, Zhang XB, Song JW, Xiang YQ. Family intervention on chronic schizophrenia in a rural 3 years follow-up study. Medical Forum. 2007;11(6):503–5. [Google Scholar]
- Tarrier 1988 {published data only} .Barrowclough C, Tarrier N. Social functioning in schizophrenic patients. Social Psychiatry and Psychiatric Epidemiology. 1990;25:125–9. doi: 10.1007/BF00782739. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Barrowclough C, Proceddu K, Fitzpatrick E. The Salford family intervention project: relapse rates of schizophrenia at five and eight years. British Journal of Psychiatry. 1994;165:829–32. doi: 10.1192/bjp.165.6.829. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Barrowclough C, Vaughn C, Bamrah JS, Porceddu K, Watts, Freeman H. Community management of schizophrenia: a two-year follow-up of a behavioural intervention with families. British Journal of Psychiatry. 1989;154:625–8. doi: 10.1192/bjp.154.5.625. [DOI] [PubMed] [Google Scholar]
- *; Tarrier N, Barrowclough C, Vaughn C, Bamrah JS, Porceddu K, Watts S, Freeman H. The community management of schizophrenia: a controlled trial of abehavioural intervention with families to reduce relapse. British Journal of Psychiatry. 1988;153:532–42. doi: 10.1192/bjp.153.4.532. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Lownson K, Barrowclough C. Some aspects of family interventions in schizophrenia. II. Financial considerations. British Journal of Psychiatry. 1991;159:481–4. doi: 10.1192/bjp.159.4.481. [DOI] [PubMed] [Google Scholar]
- Vaughan 1992 {published data only} .Vaughan K, Doyle M, McConaghy N, Blaszczynski A, Fox A, Tarrier N. The Sydney intervention trial: a controlled trial of relatives’ counselling to reduce schizophrenic relapse. Social Psychiatry and Psychiatric Epidemiology. 1992;27:16–21. doi: 10.1007/BF00788951. [DOI] [PubMed] [Google Scholar]
- Wang 2006 {published data only} .Wang Y, Zhang HW, Yue DC. The effect of family intervention in first onset schizophrenia patients during remission. Medical Journal of Chinese People’s Health. 2006;18(12):1065–6. [Google Scholar]
- Xiang 1994 {published data only} .Xiang M, Ran M, Li S. A controlled evaluation of psychoeducational family intervention in a rural Chinese community. British Journal of Psychiatry. 1994;8(5):201–5. doi: 10.1192/bjp.165.4.544. [DOI] [PubMed] [Google Scholar]
- Xiang 2005 {published data only} .Xiang TB, He E, Yu F. Effect of community prevention and treatment in patients with schizophrenia and their family economic burden. Chinese Journal of Clinical Rehabilitation. 2005;9(28):24–7. [Google Scholar]
- Xiong 1994 {published data only} .Xiong H, Xiong W, Lipeng F, Wang R. Comprehensive family treatment for schizophrenic patients: a prospective, randomized, single-blind control trial of 63 patients. Chinese Mental Health Journal. 1994;8(5):201–5. [Google Scholar]
- *; Xiong W, Phillips MR, Hu X, Wang R, Dai Q, Kleinman J, Kleinman A. Family-based intervention for schizophrenic patients in China: a randomised controlled trial. British Journal of Psychiatry. 1994;165:239–47. doi: 10.1192/bjp.165.2.239. [DOI] [PubMed] [Google Scholar]
- Zhang 1994 {published data only} .Zhang M, Wang M, Li J, Phillips MR. Randomised-control trial of family intervention for 78 first-episode male schizophrenic patients: an 18-month study in Suzhou, Jiangsu. British Journal of Psychiatry. 1994;165(Suppl 24):96–102. [PubMed] [Google Scholar]
- Zhang 2006a {published data only} .Zhang SL, Liu LL, Pan SX, Feng YF, Zhang Y, Zhu QH. Comparison of the effects of family therapy relapse rate in schizophrenia. Shandong Archives of Psychiatry. 2006;19(2):138–9. [Google Scholar]
- Zhang 2006b {published data only} .Zhang ML, Yuan GZ, Yao JJ, Ni SQ, Zhang X, An BF, Zhou DX, Bao ZH. Systematic family intervention for people with schizophrenia. Chinese Journal of Psychiatry. 2006;39(2):84. [Google Scholar]
- Zhou 2007 {published data only} .Zhou JH. Family drug intervention on relapse of schizophrenia. Health Medical Research. 2007;15(20):59–60. [Google Scholar]
References to studies excluded from this review
- Abramowitz 1989 {published data only} .Abramowitz IA, Coursey RD. Impact of an educational support group on family participants who take care of their schizophrenic relatives. Journal of Consulting and Clinical Psychology. 1989;57(2):232–6. doi: 10.1037//0022-006x.57.2.232. [DOI] [PubMed] [Google Scholar]
- Barber 1988 {published data only} .Barber M. The Effect of a Schizophrenic Family Workshop on Levels of Acceptance and Stress in Primary Family Caregivers of Schizophrenic Relatives; Univ. Texan Women’s; Houston, USA. 1988. [Google Scholar]
- Barrowclough 1999 {published data only} .Barrowclough C, Tarrier N, Lewis S, Sellwood W, Mainwaring J, Quinn J, Hamlin C. Randomised controlled effectiveness trial of a needs-based psychosocial intervention service for carers of people with schizophrenia. British Journal of Psychiatry. 1999;174:505–11. doi: 10.1192/bjp.174.6.505. [DOI] [PubMed] [Google Scholar]
- Sellwood W, Tarrier N, Quinn J, Barrowclough C. The family and compliance in schizophrenia: The influence of clinical variables, relatives’ knowledge and expressed emotion. Psychological Medicine. 2003;33(1):91–6. doi: 10.1017/s0033291702006888. [DOI] [PubMed] [Google Scholar]
- Barrowclough 2002 {published data only} .Barrowclough C, Haddock G, Tarrier N, Lewis SW. A randomised, controlled trial of a psychosocial intervention in dual diagnosis. Schizophrenia Research. 2002;53(3 Suppl 1):227. [Google Scholar]
- Birchwood 1992 {published data only} .Birchwood M, Smith J, Cochrane R. Specific and non-specific effects of educational intervention for families living with schizophrenia. A comparison of three methods. British Journal of Psychiatry. 1992;160:806–14. doi: 10.1192/bjp.160.6.806. [DOI] [PubMed] [Google Scholar]
- Brooker 1992 {published data only} .Brooker C, Falloon I, Butterworth A, Goldberg D, Graham-Hole V, Hillier V. The outcome of training community psychiatry nurses to deliver psychosocial intervention. British Journal of Psychiatry. 1994;165:222–30. doi: 10.1192/bjp.165.2.222. [DOI] [PubMed] [Google Scholar]
- Brooker C, Tarrier N, Barrowclough C, Butterworth A, Goldberg D. Training community psychiatric nurses for psychosocial intervention: report of a pilot study. British Journal of Psychiatry. 1992;160:836–44. doi: 10.1192/bjp.160.6.836. [DOI] [PubMed] [Google Scholar]
- Byalin 1985 {published data only} .Byalin K, Jed J, Lehmann S. International Review of Applied Psychology. Sage; London: 1985. Designing and evaluating intervention strategies for deinstitutionalized mental patients. Special Issue: schizophrenia; pp. 381–90. [Google Scholar]
- Cozolino 1988 {published data only} .Cozolino LJ, Goldstein MJ, Nuechterlein KH, West KL, Snyder KS. The impact of education about schizophrenia on relatives varying in expressed emotion. Schizophrenia Bulletin. 1988;14(4):675–87. doi: 10.1093/schbul/14.4.675. [DOI] [PubMed] [Google Scholar]
- Durell 1968 {published data only} .Durell J, Hanson EJ, Steinberg HR. The treatment of schizophrenia in a therapeutic community - a controlled study; Proceedings of the 124th Annual Meeting of the American Psychiatric Association; Boston, USA. 1968.May 13-17, 1968. [Google Scholar]
- Durr 1996 {published data only} .Durr H, Hahlweg K. Process analysis of behavioral family management with schizophrenia patients [Familienbetreuung bei schizophrenen patienten - analyse des therapieverlaufes] Zeitschrift fur Klinische Psychologie. 1996;25(1):33–46. [Google Scholar]
- Dyck 2000 {published data only} .Dyck DG, Short RA, Hendryx MS. Multi-family psychoeducation and schizophrenia - impact on consumer outcomes and family burden and health; Proceedings of the 4th International Conference on Schizophrenia - 1996: Breaking down the Barriers; Vancouver, Canada. Vancouver, BC. 1996.Oct 6-9, 1996. [Google Scholar]
- Dyck DG, Short RA, Hendryx MS, Norrell D, Myers M, Patterson T, McDonnell MG, Voss WD, McFarlane WR. Management of negative symptoms among patients with schizophrenia attending multiple family groups. Psychiatric Services. 2000;51(4):513–19. doi: 10.1176/appi.ps.51.4.513. [DOI] [PubMed] [Google Scholar]
- Dyck DG, Short RA, Hendryx MS, Voss WD, McFarlane WD. Multi-family groups and the management of schizophrenia: patient and family caregiver outcomes. Schizophrenia Research. 2001;1-2(Suppl):259. [Google Scholar]
- Hazel NA, McDonell MG, Short RA, Berry CM, Voss WD, Rodgers ML, Dyck DG. Impact of multiple-family groups for outpatients with schizophrenia on caregivers’ distress and resources. Psychiatric Services. 2004;55(1):35–41. doi: 10.1176/appi.ps.55.1.35. PUBMED: 14699198. [DOI] [PubMed] [Google Scholar]
- McDonell MG, Short RA, Berra CM, Dyck DG. Burden in schizophrenia caregivers: impact of family psychoeducation and awareness of patient suicidality. Family Process. 2003;42:91–103. doi: 10.1111/j.1545-5300.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- Esterson 1965 {published data only} .Esterson A, Cooper DG, Laing RD. Results of family oriented therapy with hospitalised schizophrenics. British Medical Journal. 1965;2:1462–65. doi: 10.1136/bmj.2.5476.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler 2002 {published data only} .Fowler D. Psychosocial prevention of relapse in psychosis: a multi-centre randomised controlled trial of cognitive behavioral therapy and family intervention. National Research Register. 2002 N0546102205. [Google Scholar]
- Freeman 2002 {published data only} .Freeman D. Psychological prevention of relapse in psychosis: a multi-centre randomised controlled trial of cognitive behavioral therapy and family intervention. National Research Register. 2002 N0577110745. [Google Scholar]
- Glick 1985 {published data only} .Clarkin JF, Glick ID, Haas GL, Spencer JH. The effects of inpatient family intervention on treatment outcome. In: Mirin SM, Gossett JT, Grob MC, editors. Psychiatric Treatment: Advances in Outcome Research. American Psychiatry Press; Washington DC: 1991. pp. 47–59. [Google Scholar]
- Glick ID, Clarkin JF, Haas GL, Spencer JH. A randomized clinical trial of inpatient family intervention. VI. Mediating variables and outcome. Family Process. 1991;30:85–99. doi: 10.1111/j.1545-5300.1991.00085.x. [DOI] [PubMed] [Google Scholar]
- Glick ID, Clarkin JF, Haas GL, Spencer JH. Clinical significance of inpatient family intervention: conclusions from a clinical trial. Hospital and Community Psychiatry. 1993;44:869–73. doi: 10.1176/ps.44.9.869. [DOI] [PubMed] [Google Scholar]
- Glick ID, Clarkin JF, Spencer JH, Haas GL, Lewis AB, Peyser J, DeMane N, Good-Ellis M, Harris E, Lestelle V. A controlled evaluation of inpatient family intervention. I. Preliminary results of the six-month follow-up. Archives of General Psychiatry. 1985;42:882–6. doi: 10.1001/archpsyc.1985.01790320054007. [DOI] [PubMed] [Google Scholar]
- Glick ID, Spencer JH, Clarkin JF, Haas GL, Lewis AB, Peyser J, DeMane N, Good-Ellis M, Harris E, Lestelle V. A randomized clinical trial of inpatient family intervention. III: Effects at 6 month and 18 month follow-ups. American Journal of Psychiatry. 1988;145:1115–21. doi: 10.1176/ajp.145.9.1115. [DOI] [PubMed] [Google Scholar]
- Glick ID, Spencer JH, Clarkin JF, Haas GL, Lewis AB, Peyser J, DeMane N, Good-Ellis M, Harris E, Lestelle V. A randomized clinical trial of inpatient family intervention. IV. Follow up results for subjects with schizophrenia. Schizophrenia Research. 1990;3:187–200. doi: 10.1016/0920-9964(90)90036-7. [DOI] [PubMed] [Google Scholar]
- Haas GL, Glick ID, Clarkin JF, Spencer JH, Lewis AB. Gender and schizophrenia outcome:a clinical trial of an inpatient family intervention. Schizophrenia Bulletin. 1990;16:277–92. doi: 10.1093/schbul/16.2.277. [DOI] [PubMed] [Google Scholar]
- Haas GL, Glick ID, Clarkin JF, Spencer JH, Lewis AB, Peyser J, DeMane N, Good-Ellis M, Harris E, Lestelle V. Inpatient family intervention: a randomized clinical trial. II. Results at hospital discharge. Archives of General Psychiatry. 1988;45:217–24. doi: 10.1001/archpsyc.1988.01800270025003. [DOI] [PubMed] [Google Scholar]
- Hahlweg 1999 {published data only} .Hahlweg K. Psychoeducative family care in schizophrenia [Psychoedukative familienbetreuung bei schizophrenen patienten] In: Lasar M, Ulrich T, editors. Psychotherapeutische Strategien der Schizophreniebehandlung. Lengerich; 1997. [Google Scholar]
- *; Hahlweg K, Wiedemann G. Principles and results of family therapy in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 1999;249(4):108–15. doi: 10.1007/pl00014179. [DOI] [PubMed] [Google Scholar]
- He 2005 {published data only} .He RF. Family education and cognitive insight therapy maintenance therapy in patients with schizophrenia: 3 years follow-up study. Chinese Journal of Nervous and Mental Disorders. 2005;31(3):227–9. [Google Scholar]
- Hogarty 1974 {published data only} .Hogarty GE, Goldberg SC, Schooler NR. Drug and sociotherapy in the aftercare of schizophrenic patients. III. Adjustment of nonrelapsed patients. Archives of General Psychiatry. 1974;31(5):609–18. doi: 10.1001/archpsyc.1974.01760170011002. [DOI] [PubMed] [Google Scholar]
- Hornung 1995 {published data only} .Hornung WP, Holle R, Schulze Monking H, Klingberg S, Buchkremer G. Psychoeducational-psychotherapeutic treatment of schizophrenic patients and their caregivers. Results of a 1 year catamnestic study [Psychoedukativ-psychotherapeutische Behandlung von schizophrenen Patienten und ihren Bezugspersonen. Ergebnisse einer 1-Jahres-Katamnese] Nervenarzt. 1995;66(11):828–34. [PubMed] [Google Scholar]
- Hu 2002 {published data only} .Hu F, Hu A, Zhao C. Rehabilitation effects of family talks on education about schizophrenia in village He ze city’s 3rdpeople hospital, Shan dong. Medical Journal of Chinese Civil Administration. 2002;14(4):255–6. 219. [Google Scholar]
- Jenner 2002 {published data only} .Jenner JA, Nienhuis F, Wiersma D, Van De Willige G. Integrative treatment significantly improves the burden of illness and controls symptoms in drug-resistant schizophrenia. Schizophrenia Research. 2002;53(3 Suppl 1):211. [Google Scholar]
- Jeppesen 1999 {published data only} .Jeppesen P, Abel MB, Krarup G, Jorgensen P, Nordentoft M. Family burden and expressed emotion in first episode psychosis; Proceedings of the 3rd International Conference of Early Psychosis; Copenhagen, Denmark. 2002.Sep 26-28, 2002. [Google Scholar]
- Jeppesen P, Nordentoft M, Abel M, Hemmingsen RP, Joergensen, Kassow P. Opus-project:a RCT of integrated psychiatric treatment for recent onset psychotic patients. Schizophrenia Research. 2001;49:262. [Google Scholar]
- *; Jeppesen P, Hemmingsen R, Reisby N, Jørgensen P, Nordentoft M, Abel M-B. The impact of mental disorder on caregivers; Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999.Aug 6-11, 1999. [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Abel M, Ohlenschlaeger JK, Jorgensen P, Christensen T. OPUS project: a randomised controlled trial of integrated psychiatric treatment in first episode psychosis. Schizophrenia Research. 2003;60:297. [Google Scholar]
- Kelly 1990 {published data only} .Kelly GR, Scott JE. Medication compliance and health education among outpatients with chronic mental disorders. Medical Care. 1990;28(12):1181–97. doi: 10.1097/00005650-199012000-00006. [DOI] [PubMed] [Google Scholar]
- Kim 1997 {published data only} .Kim C, Kang DH, Jang JH, Cho JS, Youn S, Shim KS. Two-year effects of psychosocial treatment on relapse of chronic schizophrenia; Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, USA. 1997; May 17-22, 1997. MEDLINE: 80221608. [Google Scholar]
- Koettgen 1988 {published data only} .Koettgen C. Schizophrenia - against the certainity of delusion in psychiatry (A 4-year follow-up study of outpatient work with relatives and patients) [Schizophrenie - wider die wahngewissheit der psychiatrie (Eine 4-jahres-katamnese nach ambulanter gruppenarbeit mit angehoerigen und patienten] Der Mensch in der Psychiatrie. 1988:298. [Google Scholar]
- Kopelowicz 2003 {published data only} .Kopelowicz A, Zarate R, Gonzalez Smith V, Mintz J, Liberman RP. Disease management in Latinos with schizophrenia: a family-assisted, skills training approach. Schizophrenia Bulletin. 2003;29(2):211–27. doi: 10.1093/oxfordjournals.schbul.a006999. [DOI] [PubMed] [Google Scholar]
- Kottgen 1984 {published data only} .Kottgen C, Sonnichsen I, Mollenhauer K, Jurth R. Group therapy with the families of schizophrenic patients: results of the Hamburg Camberwell Family Interview Study III. International Journal of Family Psychiatry. 1984;5:83–94. [Google Scholar]
- Langsley 1968 {published data only} .Langsley DG, Machotka P, Flomenhaft K. Avoiding mental hospital admission: a follow-up study. American Journal of Psychiatry. 1971;127:1391–4. doi: 10.1176/ajp.127.10.1391. [DOI] [PubMed] [Google Scholar]
- Langsley DG, Machotka P, Flomenhaft K. Follow up evaluation of family crisis therapy. American Journal of Orthopsychiatry. 1969;39:753–9. doi: 10.1111/j.1939-0025.1969.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Langsley DG, Pittman FS, Machotka P, Flomenhaft K. Family crisis therapy - results and implications. Family Process. 1968;7:145–58. [Google Scholar]
- Lenior 2001 {published data only} .*; Lenior ME, Dingemans PM, Linszen DH, De Haan L, Schene AH. Social functioning and the course of early-onset schizophrenia: five-year follow-up of a psychosocial intervention. British Journal of Psychiatry. 2001;179:53–8. doi: 10.1192/bjp.179.1.53. [DOI] [PubMed] [Google Scholar]
- Lenior ME, Linszen DH, Dingemans PM, Schene H. Family intervention and the course of parental expressed emotion: a longitudinal study. Schizophrenia Research. 2001;(1-2 Suppl):263. doi: 10.1016/s0920-9964(01)00305-x. [DOI] [PubMed] [Google Scholar]
- Lenior 2003 {published data only} .Lenior ME, Dingemans PM, Schene AH, Linszen DH. Predictors and five year outcome in early-onset schizophrenia. A path analysis. Schizophrenia Research. 2003;60:292. [Google Scholar]
- Levene 1989 {published data only} .Levene JE, Newman F, Jefferies JJ. Focal family therapy outcome study. I. Patient and family functioning. Canadian Journal of Psychiatry. 1989;34:641–7. doi: 10.1177/070674378903400704. [DOI] [PubMed] [Google Scholar]
- Lewandowski 1988 {published data only} .Lewandowski L, Buchkremer G. Theraputische group work with a national of schizophrenic patients. Results of two year course of studies [Theraputische gruppenarbeit mit angehorigen schizophrener patienten. Ergebnisse zweijahriger verlaufsuntersuchungen] Zeitschrift fur Klinische Psychologie. 1988;XVII(3):210–24. [Google Scholar]
- Li 1996 {published data only} .Li W. Family intervention for schizophrenic patients. Journal of Clinical Psychological Medicine. 1996;6(4):209–11. [Google Scholar]
- Li 1998 {published data only} .Li Y. Effect of family education on the rehabilitation of social function in schizophrenics. Medical Journal of Chinese Civil Administration. 1998;10(03):160–63. [Google Scholar]
- Li 2005 {published data only} .Li XY. The effect of cognitive therapy and family intervention in male patients with schizophrenia. Chinese Journal of Practical Nursing. 2005;21(9A):58–9. [Google Scholar]
- Lu 2000 {published data only} .Lu ZZ, Shi YB. The dynamic effects of psychosocial intervention on schizophrenic patients and caregivers. Modern Rehabilitation. 2000;4(5):716–7. [Google Scholar]
- Ma 2003b {published data only} .Ma A, Li S. Effect of family healthy education on recurrence of discharged schizophreniacs. Journal of Nursing Science. 2003;18(7):538–9. [Google Scholar]
- MacCarthy 1989 {published data only} .*; MacCarthy B, Kuipers L, Hurry J, Harper R, LeSage A. Counselling the relatives of the long-term adult mentally ill. I. Evaluation of the impact on relatives and patients. British Journal of Psychiatry. 1989;154:768–75. doi: 10.1192/bjp.154.6.768. [DOI] [PubMed] [Google Scholar]
- Madew 1967 {published data only} .Madew L, Singer G, Macindoe I. Family support and rehabilitation in the therapeutic community: second progress report. Medical Journal of Australia. 1967;1(9):435–7. doi: 10.5694/j.1326-5377.1967.tb21366.x. [DOI] [PubMed] [Google Scholar]
- Mak 1996 {published data only} .Mak KY. Group psychoeducation and family therapy; Proceedings of the 10th World Congress of Psychiatry; Madrid, Spain. 1996.Aug 23-28, 1996. [Google Scholar]
- Malm 2003 {published data only} .Malm U, Ivarsson B, Allebeck P, Falloon IR. Integrated care in schizophrenia: a 2-year randomized controlled study of two community-based treatment programs. Acta Psychiatrica Scandinavica. 2003;107(6):415–23. doi: 10.1034/j.1600-0447.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- Mavreas 1992 {published data only} .Mavreas VG, Tomaras V, Karydi V, Economou M, Stefanis CN. Expressed emotion in families of chronic schizophrenics and its association with clinical measures. Social Psychiatry and Psychiatric Epidemiology. 1992;27:4–9. doi: 10.1007/BF00788949. [DOI] [PubMed] [Google Scholar]
- McCreadie 1991 {published data only} .McCreadie RG, Phillips K, Harvey JA, Waldron G, Stewart M, Baird D. The Nithsdale schizophrenia surveys. VIII. Do relatives want family intervention - and does it help? British Journal of Psychiatry. 1991;158:110–13. doi: 10.1192/bjp.158.1.110. [DOI] [PubMed] [Google Scholar]
- McFarlane 1995b {published data only} .McFarlane WR, Link B, Dushay R, Marchal J, Crilly J. Psychoeducational multiple family groups: four year relapse outcome in schizophrenia. Family Process. 1995;34:127–44. doi: 10.1111/j.1545-5300.1995.00127.x. [DOI] [PubMed] [Google Scholar]
- Merinder 1998 {published data only} .Merinder LB, Viuff AG, Laugesen H, Clemendsen K, Misfelt S, Espensen B. Effects of psychoeducative methods: a randomized controlled study. Nordisk Psykiatrisk Tidsskrift. 1998;52(S41):144. [Google Scholar]
- Motlova 2003 {published data only} .Motlova L, Dragomirecka E, Spaniel F, Selepova P, Zalesky R, Goppoldova E. The influence of group psychoeducation on quality of life in schizophrenia patients and their relatives; Proccedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, USA. 2003; May 17-22, 2003. SCIENTIFIC AND CLINICAL REPORT SESSION 23-THE PROMISE OF SCIENCE: THE POWER OF HEALING]No. 69. [Google Scholar]
- Mottaghipour 2000 {published data only} .Mottaghipour Y, Woodland L, Sara G. Efficacy and effectiveness in early psychosis family education group program; Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000.2000. [Google Scholar]
- Nordentoft 1999 {published data only} .Jeppesen P, Abel MB, Krarup G, Jorgensen P, Nordentoft M. Family burden and expressed emotion in first episode psychosis. The OPUS-trial; Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002; Sep 26- 28, 2002. p. 59. S26.4. [Google Scholar]
- *; Nordentoft M, Jeppesen P, Kassow P, Abel M, Petersen L, Thorup A, Cristensen T, Øhlenschlæger J, Jørgensen P. Opus-project: a randomised controlled trial of integrated psychiatric treatment in first-episode psychosis-clinical outcome improved. Schizophrenia Research. 2002;51(3 Suppl):51. [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Abel M. OPUS project: a randomised controlled trial of integrated psychiatric treatment in first episode psychosis; Proceedings of the 9th International Congress on Schizophrenia Research; Colorado Springs, USA. 2003.2003. [Google Scholar]
- Nordentoft M, Reisby N, Jeppesen P, Abel M-B, Kassow P, Jírgensen P. Opus-project: differences in treatment outcome of a randomised controlled trial of integrated psychiatric treatment of first-episode psychotic patients; Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999.Aug 6-11, 1999. [Google Scholar]
- Olfson 1998 {published data only} .Olfson M. Implementing practice guidelines for schizophrenia; Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Canada. 1998.1998. [Google Scholar]
- Pereira 1994 {published data only} .Pereira MJ. Application of family therapy and psychoeducational model in a brief detention unit [Aplicación de la terapia familiar y del modelo psicoeducativo en una unidad de internamiento breve] Psiquis. 1994;15(1):43–47. [Google Scholar]
- Petersen 2005 {published data only} .Jørgensen P, Jeppesen P, Abel MB, Kassow P, Krarup G, Hemmingsen R, Nordentoft M. Early intervention in schizophrenia. Nordic Journal of Psychiatry. 2002;2:8. [Google Scholar]
- Petersen L, Nordentoft M, Jeppesen P, Ohlenschaeger J, Thorup A, Christensen TO, Krarup G, Dahlstrom J, Haastrup B, Jorgensen P. Improving 1-year outcome in first-episode psychosis: OPUS trial. British Journal of Psychiatry. 2005;48:98–103. doi: 10.1192/bjp.187.48.s98. [DOI] [PubMed] [Google Scholar]
- Pitschel 1993 {published data only} .Pitschel Walz G, Boerner R, Mayer C, Engel R, Peuker I, Welschehold M, Bauml J, Buttner P, Kissling W. Psychoeducational groups for schizophrenic patients and their relatives: influence on knowledge, attitudes and familial expressed emotions. Pharmacopsychiatry. 1993;26:186. [Google Scholar]
- Pu 2003 {published data only} .Pu J, Zhnag C, Liu N. Family nursing intervention and first onset schizophrenics rehabilitation. Journal of Nursing Science. 2003;18(3):163–5. [Google Scholar]
- Ro-Trock 1977 {published data only} .Ro-Trock GK, Wellisch DK, Schoolar JC. A family outcome study in an inpatient setting. American Journal of Orthopsychiatry. 1977;47(3):514–22. doi: 10.1111/j.1939-0025.1977.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Wellisch DK, Ro-Trock GK. A three year follow up offamily therapy. International Journal of Family Therapy. 1980;2(3):169–75. [Google Scholar]
- Roncone 2000 {published data only} .Roncone R, Morosini P, Falloon IRH, Casacchia M. Family interventions in schizophrenia in Italian mental health services; Proceedings of the 8th Keio University International Symposia for Life Sciences and Medicine on Comprehensive Treatment of Schizophrenia: Linking Neurobehavioral Findings to Psychosocial Approaches; Tokyo, Japan. 2000.Jun 5-7, 2000. pp. 284–9. [Google Scholar]
- Shimodera 2000 {published data only} .Shimodera S, Inoue S, Mino Y, Tanaka S, Kii M, Motoki Y. Expressed emotion and psychoeducational intervention for relatives of patients with schizophrenia: A randomized controlled study in Japan. Psychiatry Research. 2000;96(2):141–8. doi: 10.1016/s0165-1781(00)00193-1. [DOI] [PubMed] [Google Scholar]
- Smith 1978 {published data only} .Smith FA, Fenton FR, Benoit C, Barzell E, Tessier L. Home care treatment of acutely ill psychiatric patients. A one year follow up. Canadian Psychiatric Association Journal. 1978;23(2):73–6. doi: 10.1177/070674377802300201. [DOI] [PubMed] [Google Scholar]
- Smith 1987 {published data only} .Smith JV, Birchwood MJ. Specific and non-specific effects of educational intervention with families living with a schizophrenic relative. British Journal of Psychiatry. 1987;150:645–52. doi: 10.1192/bjp.150.5.645. [DOI] [PubMed] [Google Scholar]
- Solomon 1996 {published data only} .Solomon P, Draine J, Mannion E. The impact of individualized consultation and group workshop family education interventions in ill relative outcomes. Journal of Nervous and Mental Disease. 1986;184:252–5. doi: 10.1097/00005053-199604000-00009. [DOI] [PubMed] [Google Scholar]
- Solomon P, Draine J, Mannion E, Meisel M. Effectiveness of two models of brief family education: retention of gains by family members of adults with serious mental illness. American Journal of Orthopsychiatry. 1997;67(2):177–86. doi: 10.1037/h0080221. [DOI] [PubMed] [Google Scholar]
- *; Solomon P, Draine J, Mannion E, Meisel M. Impact of brief family psychoeducation on self-efficacy. Schizophrenia Bulletin. 1996;22:41–50. doi: 10.1093/schbul/22.1.41. [DOI] [PubMed] [Google Scholar]
- Spencer 1988 {published data only} .Spencer JH, Glick ID, Haas GL, Clarkin JF, Lewis AB, Peyser J, Demane N, Good-Ellis M, Harris E, Lestrelle V. A randomised clinical trial of inpatient family intervention, II: Effects at 6-month and 18-month follow-ups. American Journal of Psychiatry. 1988;145(9):1115–21. doi: 10.1176/ajp.145.9.1115. [DOI] [PubMed] [Google Scholar]
- Spiegel 1987 {published data only} .Spiegel D, Wissler T. Using Family Consultation as Psychiatric Aftercare for Schizophrenic Patients. Hospital and Community Psychiatry. 1987;38(10):1096–99. doi: 10.1176/ps.38.10.1096. [DOI] [PubMed] [Google Scholar]
- Stein 2003 {published data only} .Stein MK, Glynn SM, Shepherd JG, Rook KS, Vo M, Potkin SG. A test of a culturally appropriate family sponsorship program for caucasian and vietnamese caregivers of persons with schizophrenia. Schizophrenia Research. 2003;60:328–9. [Google Scholar]
- Tarrier 1989 {published data only} .Tarrier N. Effect of treating the family to reduce relapse in schizophrenia: A review. Journal of the Royal Society of Medicine. 1989;82(7):423–24. doi: 10.1177/014107688908200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrier 2000 {published data only} .Tarrier N. A family support and psychoeducation service for schizophrenia: effectiveness and training. National Research Register. 2000 [Google Scholar]
- Telles 1995 {published data only} .Telles C, Karno M, Mintz J, Paz G, Arias M, Tucker D, Lopez S. Immigrant families coping with schizophrenia. Behavioural family intervention versus case management with a low-income Spanish-speaking population. British Journal of Psychiatry. 1995;167:473–9. doi: 10.1192/bjp.167.4.473. [DOI] [PubMed] [Google Scholar]
- Valencia 1999 {published data only} .Valencia CM. Psychosocial intervention program for patients with chronic schizophrenia [Programa de intervencion psicosocial para pacientes esquizofrenicos cronicos] Salud Mental. 1999;22:128–37. [Google Scholar]
- Ventegodt 2001 {published data only} .Ventegodt AT, Jeppesen P, Petersen L, Abel M, Nordentoft M, Kassow P, Hemmingsen RP. Opus-project: a randomised controlled trial of first episode psychotic patients: genderdifferences, social network and negative symptoms. Schizophrenia Research. 2001;49(1-2 Suppl):267. [Google Scholar]
- Victolo 1999 {published data only} .Victolo Y, Warchavchik I, Romano F, Bassit D, Wang YP, Marcolin MA, Louza MR, Elkis H. Psycho-educational intervention for schizophrenics and their families: a randomized, controlled study in Sao Paulo, Brazil. Schizophrenia Research. 1999;36(1-3):334. [Google Scholar]
- Wang 1999 {published data only} .Wang X, Ma A, He X. The effect of family intervention on mental health of the family members of newly onset schizophrenics. Chinese Mental Health Journal. 1999;11(02):121–2. [Google Scholar]
- Wellisch 1977 {published data only} .Wellisch DK, Ro-Trock GK. A three year follow of family therapy. International Journal of Family Therapy. 1977;2(3):169–75. [Google Scholar]
- Wiedemann 1992 {published data only} .Wiedemann G, Hank G, Feinstein E, Muller U, Hahlweg K. Targeted vs maintenance neuroleptic medication and family therapy in the aftercare of acute schizophrenics. Clinical Neuropharmacology. 1992;15(Suppl 1 (Pt B)) [Google Scholar]
- Wiedemann G, Hank G, Feinstein E, Muller U, Hahlweg K. Targeted vs maintenance neuroleptic medication and family therapy in the aftercare of acute schizophrenics. Pharmacopsychiatry. 1992;25:93. [Google Scholar]
- Wiedemann 1994 {published data only} .Wiedemann G, Hahlweg K, Hank K, Feinstein E, Muller U, Dose M. Deliverability of psychoeducational family management. Schizophrenia Bulletin. 1994;20:547–56. doi: 10.1093/schbul/20.3.547. [DOI] [PubMed] [Google Scholar]
- Wu 2003 {published data only} .Wu SL. Effect of healthy education to patients family members on the recovery of social function in schizophreniapatients. Zhonghua Linchuang Kangfu Zazhi. 2003;7(27):3710–1. [Google Scholar]
- Yao 1997 {published data only} .Yao ZR. Study on the compliance in family education and maintenance treatment of schizophrenics. Medical Journal of Chinese Civil Administration. 1997;9(2):88–90. 127. [Google Scholar]
- Zastowny 1992 {published data only} .Zastowny TR, Lehman AF, Cole RE, Kane C. Family management of schizophrenia: a comparison of behavioural and supportive family treatment. Psychiatric Quarterly. 1992;63(2):159–87. doi: 10.1007/BF01065988. [DOI] [PubMed] [Google Scholar]
- Zhang 1998 {published data only} .Zhang M, Weng Z, Yan H. Two year experience of psychosocial education for relatives of schizophrenics. Chinese Journal of Psychiatry. 1998;31(2):90–3. [Google Scholar]
- Zhang 1999 {published data only} .Zhang X, Zhang J. Family psychotherapy and remission of symptoms of schizophrenia. Chinese Mental Health Journal. 1999;13(6):238–9. [Google Scholar]
- Zhang 2000b {published data only} .Zhang Z, Yu Y, Fan W. A prospective control study of the rehabilitation effects of family intervention in schizophrenic patients. Chinese Journal of Rehabilitation. 2000;15(1):26–7. [Google Scholar]
- Zhang 2003 {published data only} .Zhang Y, Chen M, Li R. Effect of family intervention on patients with schizophrenia. Health Psychology Journal. 2003;7475(3):226–7. [Google Scholar]
Additional references
- Altman 1996 .Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin 1999 .Amin S, Singh SP, Croudace T, Medley I, Harrison G. Evaluating the health of the nation outcome scales. Reliability and validity in a three-year follow-up of first-onset psychosis. British Journal of Psychiatry. 1999 May;:399–403. doi: 10.1192/bjp.174.5.399. [DOI] [PubMed] [Google Scholar]
- Andreasen 1982 .Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Archives of General Psychiatry. 1982;39(7):784–8. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andrews 1991 .Andrews B, Brown GW. Self Evaluation and Social Support (SESS) Manual. Royal Holloway College; London: Mimeo: 1991. [Google Scholar]
- APA 1987 .American Psychiatric Association . American Psychiatric Association. American Psychiatric Association; Washington D.C.: 1987. Diagnostic and Statistical Manual of Mental Disorders (DSM III-R) [Google Scholar]
- Begg 1996 .Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–9. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- Bifulco 1996 .Bifulco A, Brown G. Cognitive coping response to crises and onset of depression. Social Psychiatry and Psychiatric Epidemiology. 1996;48:207–10. doi: 10.1007/BF00785763. [DOI] [PubMed] [Google Scholar]
- Birchwood 1990 .Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale: the development and validation of a scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry. 1990;157:853–9. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Birchwood 1994 .Birchwood M, Smith J, Drury V, Healy J, MacMillian F, Slade M. A self-report insight scale for psychosis:reliability, validity and sensitivity to change. Acta Psychiatrica Scandinavica. 1994;89:62–7. doi: 10.1111/j.1600-0447.1994.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Bland 1997 .Bland JM. Statistics notes. Trials randomised in clusters. BMJ. 1997;315:600. doi: 10.1136/bmj.315.7108.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel 1999 .Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie. 1999;54(4):405–11. [PubMed] [Google Scholar]
- Brown 1972 .Brown GW, Birley JLT, Wing JK. Influence of family life on the course of schizophrenic disorders: a replication. British Journal of Psychiatry. 1972;121:241–58. doi: 10.1192/bjp.121.3.241. [DOI] [PubMed] [Google Scholar]
- Brown 1986 .Brown G, Andrew B, Harris T, Adler Z, Bridge L. Social support self esteem and depression. Psychologocal Medicine. 1986;16:813–31. doi: 10.1017/s0033291700011831. [DOI] [PubMed] [Google Scholar]
- Cardin 1985 .Cardin V, McGill C, Falloon I. Economic Analysis: costs, benefit and effectiveness in family management of schizophrenia: a study of clinical, social and economic benefits. Johns Hopkins Press; Baltimore: 1985. [Google Scholar]
- Carpenter 1990 .Carpenter WT, Hanlon TE, Heinrichs DW, Summerfelt AT, Kirkpatrick B, Levene J, Buchanan RW. Continuous versus targeted medication in schizophrenic out-patients: outcome results. American Journal of Psychiatry. 1990;147:1138–48. doi: 10.1176/ajp.147.9.1138. [DOI] [PubMed] [Google Scholar]
- Corin 1990 .Corin EE. Facts and meaning in psychiatry: an anthropological approach to the lifeworld of schizophrenics. Culture, Medicine and Psychiatry. 1990;14:153–88. doi: 10.1007/BF00046659. [DOI] [PubMed] [Google Scholar]
- Deeks 2000 .Deeks J. Issues in the selection for meta-analyses of binary data; Proceedings of the 8th International Cochrane Colloquium; Cape Town, South Africa. 2000.Oct 25-28, 2000. [Google Scholar]
- Dencker 1986 .Dencker SJ, Malm U, Lepp M. Schizophrenic relapse after drug withdrawal is predictable. Acta Psychiatrica Scandinavica. 1986;73:181–5. doi: 10.1111/j.1600-0447.1986.tb10584.x. [DOI] [PubMed] [Google Scholar]
- Derogatis 1976 .Derogatis LR, Rickels K, Rock AF. Symptom Checklist 90. The SCL-90 and the MMPI: a step in the validation of a new self-report scale. 1976;128:280–9. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- Divine 1992 .Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians’ patient care behavior. Journal of General Internal Medicine. 1992;7(6):623–9. doi: 10.1007/BF02599201. [DOI] [PubMed] [Google Scholar]
- Donner 2002 .Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine. 2002;21:2971–80. doi: 10.1002/sim.1301. [DOI] [PubMed] [Google Scholar]
- Epstein 1983 .Ellis C. The McMaster family assessment device. Journal of Maritial and Family Therapy. 1983;9:171–80. [Google Scholar]
- Gulliford 1999 .Gulliford MC. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology. 1999;149:876–83. doi: 10.1093/oxfordjournals.aje.a009904. [DOI] [PubMed] [Google Scholar]
- Heinrichs 1984 .Heinrichs DW, Hanlon ET, Carpenter WT., Jr The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10:388–96. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Heller 1991 .Heller T, Factor A. Premanency planning for adults with mental retardation living with family caregivers. American Journal on Mental Retardation. 1991;96:163–76. [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2005 .Higgins JPT, Green S. Cochrane Handbook for Systematic Review of Interventions 4.2.5 [updated may 2005]. The Cochrane Library. 3. John Wiley & Sons, Ltd; Chichester, UK: 2005. [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 1. John Wiley & Sons; Chichester: 2008. [Google Scholar]
- Honigfeld 1965a .Honigfeld G, Gillis RD, Klett CJ. Nurses’ observation scale for inpatient evaluation: a new scale for measuring improvement in chronic schizophrenia. Journal of Clinical Psychology. 1965;21:65–71. doi: 10.1002/1097-4679(196501)21:1<65::aid-jclp2270210122>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Jablensky 2000 .Jablensky A. Course and outcome of schizophrenia and their prediction. In: Gelder MG, Lopez-Ibor JJ, Andreason NC, editors. New Oxford Textbook of Psychiatry. Oxford University Press; Oxford: 2000. p. 612. [Google Scholar]
- Jadad 1996 .Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jimeno 1996 .Jimeno N, Jimeno A, Vargas ML. Conceptos y resultados. Springer Verlag; Iberica: 1996. The psychotic syndrome and the inventory of Frankfurt [El sindrome psicotico y el inventario de Franfurt] [Google Scholar]
- Juni 2001 .Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–6. doi: 10.1136/bmj.323.7303.42. PUBMED: 11440947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay 1986 .Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. Multi-Health Systems; North Tonawanda (NY): 1986. [Google Scholar]
- Kay 1987 .Kay SR, Fiszbein A, Opler LA. The Positive And Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Leff 1995 .Leff J, Shriqui CL. Family management of schizophrenia. In: Nasrallah HA, editor. Contemporary Issues in the Treatment of Schizophrenia. American Psychiatric Press; Washington DC: 1995. pp. 683–702. [Google Scholar]
- Leucht 2005 .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores. British Journal of Psychiatry. 2005;187:366–71. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- Leucht 2005a .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean? Schizophrenia Research. 2005;79:231–8. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Lewis 1992 .Lewis G. Assessing psychiatric disorders with a human interviewer or a computer. Journal of Epidemiology and Community Heath. 1992;48:207–10. doi: 10.1136/jech.48.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCarthy 1989b .MacCarthy B, Kuipers L, Hurry J, Harper R. Counselling the relatives of the long-term adult mentally ill. I. Evaluation of the impact on relatives and patients. British Journal of Psychiatry. 1989;154:768–75. doi: 10.1192/bjp.154.6.768. [DOI] [PubMed] [Google Scholar]
- Marder 1987 .Marder SR, Van Putten T, Mintz J, Lebell M, McKenzie J, Phillip RAM. Low and conventional dose maintenance therapy with fluphenazine decanoate: two-year outcome. Archives of General Psychiatry. 1987;44:518–21. doi: 10.1001/archpsyc.1987.01800180028005. [DOI] [PubMed] [Google Scholar]
- Mari 1994 .Mari J, Streiner D. An overview of family interventions and relapse on schizophrenia: meta-analysis of research findings. Psychological Medicine. 1994;23:565–78. doi: 10.1017/s0033291700027720. [DOI] [PubMed] [Google Scholar]
- Mari 1996 .Mari JJ, Streiner D. Family intervention for people with schizophrenia. Cochrane Database of Systematic Reviews. 1996;(1) doi: 10.1002/14651858.CD000088. DOI: 10.1002/14651858.CD000088. [DOI] [PubMed] [Google Scholar]
- Marshall 1999 .Marshall M, Lockwood A. Assertive community treatment for people with severe mental disorders. Cochrane Database of Systematic Reviews. 1999;(1) doi: 10.1002/14651858.CD001089. DOI: %3Chtml%3E%3Cbody%3E%3C!-StartFragment-%3E%3Cspan class=%22source-copyright%22%3E10.1002/14651858.CD001089%3C/span%3E%3C!-EndFragment-%3E%3C/body%3E%3C/html%3E. [DOI] [PubMed] [Google Scholar]
- Marshall 2000 .Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry. 2000;176:249–52. doi: 10.1192/bjp.176.3.249. [DOI] [PubMed] [Google Scholar]
- Moher 2001 .Moher D, Altman D, Schulz KF. The CONSORT statement: Revised recommendations for improving the quality of reports parrallel-group randomized trials. JAMA. 2001;285:1987–91. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- Overall 1962 .Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Pai 1981 .Pai S, Kapur RL. The burden on the family of a psychiatric patient: development of an interview schedule. British Journal of Psychiatry. 1981;138:332–35. doi: 10.1192/bjp.138.4.332. [DOI] [PubMed] [Google Scholar]
- Ruggeri 1993 .Ruggeri M, Dall Agnola R. The development and use of the Verona Expectations for Care Scale (VECS) and the Verona Service Satisfaction Scale (VSSS) for measuring expectations and satisfaction with community-based psychiatric services in patients, relatives and professionals. Psychological Medicine. 1993;23:511–23. doi: 10.1017/s0033291700028609. [DOI] [PubMed] [Google Scholar]
- Schulz 1995 .Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Smilkstein 1978 .Smilkstein G, Ashworth C, Montano D. Validity and reliability of the family APGAR as a test of family function. Journal of Family Practice. 1978;15:303–11. [PubMed] [Google Scholar]
- Strauss 1989 .Strauss JS. Subjective experiences of schizophrenia: toward a new dynamic psychiatry II. Schizophrenia Bulletin. 1989;15:179–87. doi: 10.1093/schbul/15.2.179. [DOI] [PubMed] [Google Scholar]
- Szmukler 1996 .Szmukler GI, Burgess P, Herrman H, Benson A. Caring for relatives with serious mental illness: the development of experience of caregiving inventory. Social Psychiatry and Psychiatric Epidemiology. 1996;31:137–48. doi: 10.1007/BF00785760. [DOI] [PubMed] [Google Scholar]
- Süllwold 1986 .Süllwold L, Huber G. Schizophrene Basisstörungen. Springer Verlag; Berlin: 1986. [PubMed] [Google Scholar]
- Thornley 1998 .Thornley B, Adams CE. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317:1181–4. doi: 10.1136/bmj.317.7167.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukoumunne 1999 .Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area-wide and organistation-based intervention in health and health care: a systematic review. Health Technology Assessment. 1999;3(5):1–75. [PubMed] [Google Scholar]
- Vaughn 1976 .Vaughn CE, Leff JP. The measurement of expressed emotion in families of psychiatric patients. British Journal of Social and Clinical Psychology. 1976;15:157–65. doi: 10.1111/j.2044-8260.1976.tb00021.x. [DOI] [PubMed] [Google Scholar]
- WHO 1988 .WHO Psychiatric Disability Assessment Schedule. World Health Organization Geneva; [Google Scholar]
- Wiedemann 2002 .Wiedemann G, Rayki O, Feinstein E, Hahlweg K. The family questionnaire: development and validation of a new self-report scale for assessing expressed emotion. Psychiatry Research. 2002;109(3):265–79. doi: 10.1016/s0165-1781(02)00023-9. [DOI] [PubMed] [Google Scholar]
- Wing 1974 .Wing JK, Cooper JE, Sartorius N. Measurement and Classification of Psychiatric Symptoms. Cambridge University Press; London: 1974. [Google Scholar]
- Wu 2006 .Wu T, Li Y, Liu G, Bian Z, Li J, Zhang J, Xie L, Ni J. Investigation of authenticity of ‘claimed’ randomized controlled trials (RCTs) and quality assessment of RCT reports published in China. Proceedings of the 14th Cochrane Colloquium; Dublin. 2006.Oct 23-36, 2006. [Google Scholar]
References to other published versions of this review
- Mari 1994a .Mari J, Streiner D. An overview of family interventions and relapse on schizophrenia: meta-analysis of research findings. Psychological Medicine. 1994;23:565–78. doi: 10.1017/s0033291700027720. [DOI] [PubMed] [Google Scholar]
- Mari 1996a .Mari JJ, Streiner D. Family intervention for people with schizophrenia. Cochrane Database of Systematic Reviews. 1996;(1) doi: 10.1002/14651858.CD000088. DOI: 10.1002/14651858.CD000088. [DOI] [PubMed] [Google Scholar]
- Pharoah 2000 .Pharoah FM, Mari JJ, Streiner D. Family intervention for schizophrenia. Cochrane Database of Systematic Reviews. 2000;(2) doi: 10.1002/14651858.CD000088. DOI: 10.1002/14651858.CD000088. [DOI] [PubMed] [Google Scholar]
- Pharoah 2003 .Pharoah FM, Rathbone J, Mari JJ, Streiner D. Family intervention for schizophrenia. Cochrane Databaseof Systematic Reviews. 2003;(4) doi: 10.1002/14651858.CD000088. DOI: 10.1002/14651858.CD000088. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study