Abstract
Ingestion of marine debris can have lethal and sublethal effects on sea turtles and other wildlife. Although researchers have reported on ingestion of anthropogenic debris by marine turtles and implied incidences of debris ingestion have increased over time, there has not been a global synthesis of the phenomenon since 1985. Thus, we analyzed 37 studies published from 1985 to 2012 that report on data collected from before 1900 through 2011. Specifically, we investigated whether ingestion prevalence has changed over time, what types of debris are most commonly ingested, the geographic distribution of debris ingestion by marine turtles relative to global debris distribution, and which species and life-history stages are most likely to ingest debris. The probability of green (Chelonia mydas) and leatherback turtles (Dermochelys coriacea) ingesting debris increased significantly over time, and plastic was the most commonly ingested debris. Turtles in nearly all regions studied ingest debris, but the probability of ingestion was not related to modeled debris densities. Furthermore, smaller, oceanic-stage turtles were more likely to ingest debris than coastal foragers, whereas carnivorous species were less likely to ingest debris than herbivores or gelatinovores. Our results indicate oceanic leatherback turtles and green turtles are at the greatest risk of both lethal and sublethal effects from ingested marine debris. To reduce this risk, anthropogenic debris must be managed at a global level.
Análisis Global de la Ingesta de Residuos Antropogénicos por Tortugas Marinas
La ingesta de residuos marinos puede tener efectos letales y subletales sobre las tortugas marinas y otros animales. Aunque hay investigadores que han reportado la ingesta de residuos antropogénicos por tortugas marinas y la incidencia de la ingesta de residuos ha incrementado con el tiempo, no ha habido una síntesis global del fenómeno desde 1985. Por esto analizamos 37 estudios publicados, desde 1985 hasta 2012, que reportan datos colectados desde antes de 1900 y a lo largo del 2011. Investigamos específicamente si el predominio de la ingesta ha cambiado con el tiempo, qué tipos de residuos se ingieren comúnmente, la distribución geográfica de la ingesta de residuos por tortugas marinas en relación a la distribución global de residuos y cuáles especies y etapas de vida tienen más probabilidad de ingerir residuos. La probabilidad de que las tortugas verdes (Chelonia mydas) y laúd (Dermochelys coriacea) ingieran escombros incrementa significativamente con el tiempo; plástico fue el residuo que más se ingirió. Las tortugas en casi todas las regiones estudiadas ingieren residuos, pero la probabilidad de ingesta no estuvo relacionada con las densidades modeladas de residuos. Además de esto, tortugas más pequeñas, en etapa oceánica de vida, tuvieron una mayor probabilidad de ingerir residuos que las tortugas forrajeras terrestres, mientras que las especies carnívoras tuvieron menos probabilidad de ingerir residuos que las herbívoras o las gelatinívoras. Nuestros resultados indican que las tortugas verdes y laúd tienen el mayor riesgo de efectos letales y subletales de la ingesta de residuos marinos. Para reducir el riesgo, los residuos antropogénicos deben manejarse en un nivel global.
Keywords: Caretta caretta, Dermochelys coriacea, Eretmochelys imbricata, garbage, Lepidochelys kempii, litter, rubbish, trash, basura, Caretta caretta, Dermochelys coriacea, escombros, Eretmochelys imbricata, Lepidochelys kempii, residuos
Introduction
Plastics in the Environment
Although there are little to no empirical data on the quantity of anthropogenic debris (hereafter debris) entering the marine environment, estimates place it at approximately 6.4 million tons annually (UNEP 2005), about 80% of which is thought to originate from land-based sources (Faris & Hart 1994). However, these estimates do not take into account aperiodic events that can cause dramatic point-source increases, such as the 2011 Japanese tsunami which created an estimated 1.5 million tons of floating debris (NOAA 2012). Because there is presently no way to map the movement of debris in real time, best estimates of where debris accumulates come from oceanographic models. Recent work by Lebreton et al. (2012) predicts that floating debris accumulates in 5 main oceanic gyres and occurs predominantly in subtropical regions. Debris gathers in drift lines and convergence zones, which are also important feeding areas for many oceanic species, including sea birds, pelagic fish, and sea turtles (Ashmole & Ashmole 1967; Carr 1986).
Plastic is the primary type of debris found in marine and coastal environments (Derraik 2002), and plastics are the most common form of debris ingested by wildlife (Mrosovsky et al. 2009; van Franeker et al. 2011; Schuyler et al. 2012). With the exponential increase in global plastic production over the past 60 years (PlasticsEurope 2009), it is likely that effects on marine wildlife from ingestion of plastic have also increased. Ingestion of marine debris affects over 170 species (Laist 1997). Debris ingestion can result in death by perforation or impaction of the gastrointestinal system and toxic compounds in plastics may have sublethal effects on development and population dynamics (Oehlmann et al. 2009).
Six of the world’s 7 species of sea turtles have been found to ingest debris, with the exception of the flatback sea turtle (Natator depressus) (Balazs 1985; Ceccarelli 2009). All 6 are listed as globally vulnerable or endangered (IUCN 2012). In 1985, Balazs summarized all known cases of sea turtle interactions with marine debris. Since then, researchers from around the world have investigated debris ingestion by turtles on local or regional scales (e.g., Tomas et al. 2002; Lazar & Gracan 2011; Schuyler et al. 2012). Results of a historical analysis of debris ingestion by leatherback turtles showed a long-term increase in ingestion frequency (Mrosovsky et al. 2009), but there has been no global review of debris ingestion for all turtle species since 1985. Understanding the factors that affect debris ingestion by turtles, including types of debris ingested, global distribution of debris, and life history and feeding ecology, may help focus management priorities on reducing plastics in the marine environment and decreasing the potential for debris ingestion.
Turtle Life History and Feeding Ecology
Sea turtle species have different lifestyles. At various stages of their lives, they may live and feed primarily in open ocean, predominantly in neritic areas, or they may switch back and forth. (Walker & Parmenter 1990; Bolten 2003; Godley et al. 2008; Rees et al. 2012). Turtles living in oceanic or coastal environments and feeding pelagically or benthically may encounter very different densities and types of marine debris and may therefore have different probabilities of debris ingestion.
Feeding preference may also affect the probability of debris ingestion by turtles. Most neonate turtles have generalist diets that become more specialized as they recruit to the coastal environment (Plotkin et al. 1993; Boyle & Limpus 2008). Adult green turtles are primarily herbivorous (Bjorndal 1997), whereas loggerhead (Caretta caretta) and Kemp’s ridley (Lepidochelys kempii) turtles are primarily carnivorous and eat crustaceans, molluscs, and other hard-bodied organisms (Bjorndal 1997). Although flatback turtles are also carnivorous, they eat primarily soft-bodied invertebrates (Sperling et al. 2007). Olive ridley (Lepidochelys olivacea) and hawksbill turtles (Eretmochelys imbricata) are omnivorous, although hawksbills feed mostly on sponges and algae (Bell 2012). Leatherback turtles feed exclusively on jellyfish and other gelatinous organisms (Shaver 1991; Bjorndal 1997). These different feeding preferences may affect the types and amount of debris turtles encounter and are likely to ingest.
Estimating Frequency of Plastic Ingestion
There is currently no reliable method for assessing plastic ingestion in live turtle populations. Results of some dietary studies in which lavage (Seminoff et al. 2002; Witherington 2002) or fecal analyses were used showed turtles ingested plastics (e.g., Seminoff et al. 2002; Casale et al. 2008), but these techniques almost certainly underestimate debris ingestion because only a small subset of the gastrointestinal tract is sampled. Seminoff et al. (2002) found 1.9% of 101 lavaged turtles had ingested debris: 41 of these turtles were kept in a tank and their feces collected. Of these, 19% excreted debris, 10 times the amount found through lavage. Seven turtles from the same population died and their stomach contents were analyzed; 2 had ingested debris. Necropsy, therefore, is the most effective method of identifying debris ingestion by turtles; however, necropsy limits the study population to deceased animals.
We analyzed literature published since 1985 to compile a global assessment of the prevalence of marine debris ingestion by sea turtles. We focused on factors that might be useful in prioritizing management actions by investigating whether ingestion prevalence changed over time, the types of debris most commonly ingested, the geographic distribution of debris ingestion by marine turtles relative to global debris distribution, and the species of turtle and life-history stages at which turtles are most likely to ingest debris.
Methods
We reviewed the literature on the gastrointestinal contents of sea turtles published after Balazs’ (1985) review. We searched ISI Web of Knowledge and the Aquatic Sciences and Fisheries Abstracts for the terms feeding ecology, foraging ecology, or diet and plastic, debris, marine debris, litter, flotsam, detritus, or tar balls. In each string of terms we included sea turtle plus the genus and species names of all 7 species of sea turtles. Because analysis of gastrointestinal contents is the most accurate way to determine the presence or absence of marine debris, we used only studies in which a systematic necropsy of at least 7 individuals was conducted. Most of the articles we included in our study were peer-reviewed publications, but we also included 3 conference proceedings (Sadove & Morreale 1989; Plotkin & Amos 1990; Duguy et al. 2000) and 3 government reports (Duronslet et al. 1991; Cannon 1998; Shaver 1998). For papers that did not explicitly report debris ingestion, we asked authors whether debris had not been found or whether it was not reported. When we were unable to contact an author, we assumed debris was not found. When studies reported on the same set of turtles (Plotkin & Amos 1990; Plotkin et al. 1993; Duguy 1997; Duguy et al. 2000; Mrosovsky et al. 2009), we counted each turtle only once in our analyses.
Because each study varied in length and no study specified how many turtles were analyzed each year or the proportion that ingested plastic in each year, we used a Monte Carlo simulation to determine whether the likelihood of marine debris ingestion by turtles changed over time (sensu Efron & Tibshirani 1994). We randomly assigned turtles with and without debris from each study to years, drawn with replacement, for the duration of each study. We then fit a logistic regression to the full simulated data set across all studies. We repeated this process to generate 100 logistic regressions fit to independently simulated data and calculated the median slope, intercept, and p value from all regressions. To determine whether there were differences among species, we ran the same analyses individually for each species. Although we analyzed only papers published after Balazs’ 1985 review, one paper reported on a compilation of studies of leatherback turtles since 1895 (Mrosovsky et al. 2009). Because we did not conduct an exhaustive literature search for other studies in this time frame, we conducted a second analysis for leatherbacks excluding the Mrosovsky data.
We calculated the total number of studies reporting ingestion of multiple types of debris. We mapped the percentage of turtles found to have ingested debris at each study site overlaid on a global map of marine debris accumulation, as modeled by Lebreton et al. (2012). Due to a lack of standardized reporting in studies, we were unable to investigate quantitatively the effects of debris ingestion on different life-history stages; however, we considered these effects in qualitative terms. To determine which species were most likely to ingest debris, we aggregated reports from all studies for each species and used logistic regression to determine the species’ effect on the probability of ingesting debris.
Results
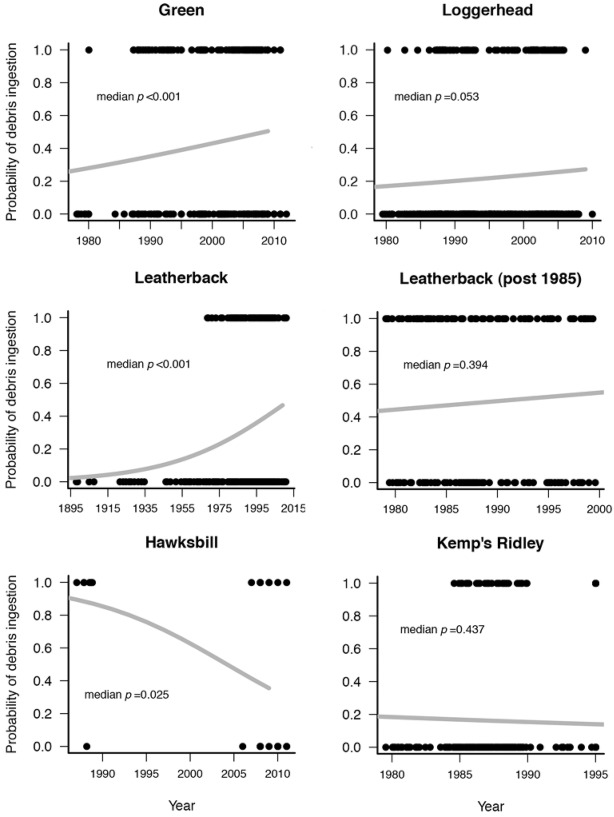
Thirty-seven studies met our criteria (Table 1). Over 116 years (1895–2012), the probability of debris ingestion increased significantly for green and leatherback turtles (median p < 0.001) and increased nonsignficantly for loggerhead turtles (median p = 0.053) (Fig. 1). The probability of leatherback turtles ingesting debris did not change significantly from 1985 to 2012. The probability of Kemp’s ridley turtles ingesting debris also did not change over time. The probability of debris ingestion for hawksbill turtles decreased from 1985 to 2012.
Table 1.
Articles published since 1985 that report on studies in which a systematic survey of turtles (n ≥ 7 animals) was conducted and necropsies were performed to determine contents of the gastrointestinal system
| Reference | Study dates | Country or region | Number of turtles in study | Species | Turtles with ingested debris (%) |
|---|---|---|---|---|---|
| Bjorndal et al. (1994 | 1988–1993 | USA | 51 | multiple | 49 |
| Boyle and Limpus (2008) | 2002–2006 | Australia | 54 | green, loggerhead | 65 |
| Bugoni et al. (2001) | 1997–1998 | Brazil | 50 | multiple | 50 |
| Burke et al. (1994) | 1985–1989 | USA | 18 | Kemp’s ridley | 0 |
| Cannon (1998) | 1994 | USA | 158 | multiple | 11 |
| Casale et al. (2008) | 2001–2005 | central Mediterranean | 33 | loggerhead | 52 |
| Duguy (1997) | 1978–1995 | France | 141 | multiple | 17 |
| Duguy et al. (2000) | 1979–1999 | France | 87 | leatherback | 55 |
| Duronslet et al. (1991) | 1987–1989 | USA | 32 | multiple | 59 |
| Foley et al. (2007) | 2000–2001 | USA | 44 | green | 2 |
| Frick et al. (2009) | 1986–2001 | Azores | 12 | loggerhead | 25 |
| Garnett et al. (1985) | 1979 | Australia | 44 | green | 0 |
| Guebert-Bartholo et al. (2011) | 2004–2007 | Brazil | 76 | green | 70 |
| Hasbun et al. (2000) | 1997 | UAE | 13 | green | 0 |
| Kaska et al. (2004) | 2001 | Turkey | 65 | loggerhead | 5 |
| Lazar and Gracan (2011) | 2001–2004 | Eastern Adriatic | 54 | loggerhead | 35 |
| Limpus et al. (2001) | 1989–1998 | Australia | 47 | loggerhead | 0 |
| Lopez-Mendilaharsu (2005) | 2000–2002 | USA | 24 | green | 0 |
| Mrosovsky et al. (2009) | 1885–2007 | Global | 408 | leatherback | 34 |
| Parker et al. (2005) | 1990–1992 | northern Pacific | 52 | loggerhead | 35 |
| Parker et al. (2011) | 1990–2004 | USA | 10 | green | 70 |
| Peckham et al. (2011) | 2003–2007 | USA | 82 | loggerhead | 0 |
| Plotkin & Amos (1990) | 1986–1988 | Texas | 23 | green, hawksbill | 61 |
| Plotkin et al. (1993) | 1986–1988 | Texas | 82 | loggerhead | 51 |
| Quinones et al. (2010) | 1987 | Peru | 192 | green | 42 |
| Revelles et al. (2007) | 2002–2004 | Mediterranean | 19 | loggerhead | 37 |
| Ross (1985) | 1977–1979 | Oman | 9 | green | 0 |
| Russo et al. (2003) | 1994–1998 | Mediterranean | 45 | green, loggerhead | 18 |
| Sadove and Morreale (1989) | 1979–1988 | USA | 116 | multiple | 12 |
| Santos et al. (2011) | 2007–2008 | Brazil | 15 | green | 20 |
| Schuyler et al. (2012) | 2006–2011 | Australia | 115 | multiple | 33 |
| Seminoff et al. (2002) | 1995–1999 | Mexico | 7 | green | 29 |
| Seney and Musick (2007) | 1983–2002 | USA | 166 | loggerhead | 0 |
| Shaver (1991) | 1983–1989 | USA | 101 | Kemp’s ridley | 29 |
| Shaver (1998) | 1984 | USA | 37 | Kemp’s ridley | 19 |
| Tomas et al. (2002) | N/A | Spain | 54 | loggerhead | 80 |
| Tourinho et al. (2010) | 2006–2007 | Brazil | 34 | green | 100 |
Figure 1.
Change in probability of ingestion of debris over time for different species of sea turtles (black dots, presence [1.0] or absence [0.0] of debris in turtles from one iteration of a Monte Carlo function; gray lines, inverse logit calculation of the probability of a turtle ingesting debris on the basis of the median slope and intercept for 100 iterations of the Monte Carlo function; p values, median values for 100 iterations of the Monte Carlo function). For the leatherback turtle graph, data are for all leatherback turtles, and for the leatherback post 1985 graph, data from Mrosovsky et al. (2009) are excluded.
Of 31 studies providing details of ingested debris, 96.8% (n = 30), reported that sea turtles ingested some form of plastic. Some studies differentiated between soft (n = 19) and hard plastic (n = 12). Rope, fishing line, Styrofoam, tar, and fishhooks were other commonly ingested items (Fig. 2). About half the studies that reported debris ingestion (n = 16) did not report whether ingestion was the primary cause of death. In 15 studies, researchers determined whether debris ingestion resulted in mortality. Of these studies, 11 reported debris was responsible for 2–17% of total turtle mortality; 5–35% of the turtles that ingested plastic were reported as being killed by it. Four studies, of 12–37 animals each, reported that debris ingestion killed no turtles.
Figure 2.
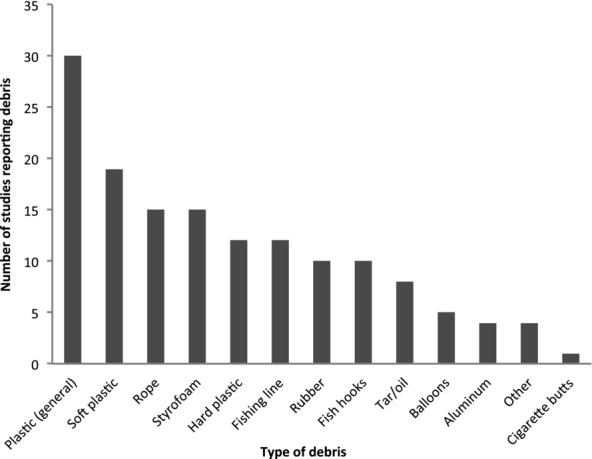
Total number of studies reporting on ingestion of particular types of marine debris by sea turtles. In many cases, multiple types of debris were found, so a study could be counted in more than one category.
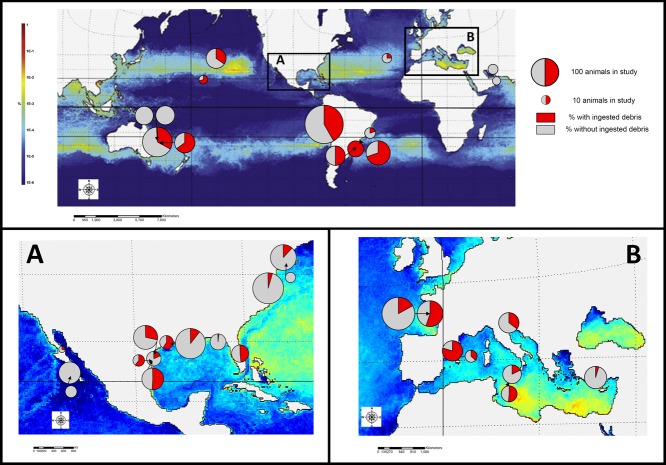
There was no discernible geographic pattern of debris ingestion relative to global models of debris distribution (Fig. 3). In all regions studied, aside from the Persian Gulf, turtles ingested debris.
Figure 3.
Locations of studies of ingested debris by sea turtles worldwide (enlargements: [a] the Gulf of Mexico and [b] the Mediterranean) overlaid on a 30-year model of global debris distribution (red and yellow areas on maps, high debris concentration) (Lebreton et al. 2012). Circles are sized relative to the total number of turtles necropsied (large, 100 turtles; small, 10 turtles). Red areas in circles indicate the percentage of turtles in each study found with ingested debris. All species have been amalgamated. (Background map reprinted from Marine Pollution Bulletin [Vol. 64], L. C.-M. Lebreton, S. D. Greer, and J. C. Borrero. Numerical Modelling of Floating Debris in the World’s Oceans. Pages 653–661. Copyright 2012, with permission from Elsevier.)
Hawksbill turtles were most likely to ingest debris, followed by green and leatherback turtles (Fig. 4). The carnivorous species (loggerhead and Kemp’s ridley) were least likely to ingest debris. Aside from the hawksbill, which did not differ significantly from either green or loggerhead turtles, all species differed significantly from one another in probability of ingesting debris (logistic regression, p < 0.0148 for all factor levels). Ingestion of debris by a flatback turtle was reported only once, so we excluded it from our analyses.
Figure 4.
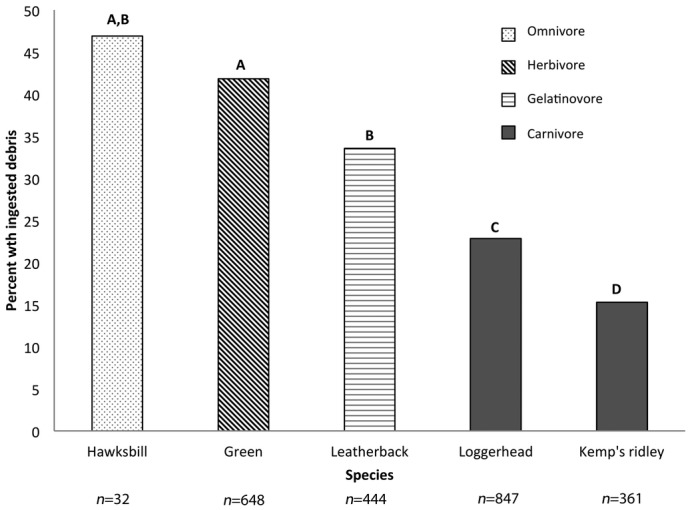
Percentage of the total number of each species of turtle across all studies that were reported to have ingested debris. Different letters above bars indicate significant differences between species (p < 0.05) (n, total number of turtles necropsied for each species).
Discussion
Debris Ingestion over Time and Debris Types
The majority of debris consumed by all turtles was composed of plastic (Fig. 2). Even in 1985, when plastic production levels were still relatively low, plastic was the most widely reported debris item ingested (Balazs 1985).
The likelihood of a green turtle ingesting debris nearly doubled from an approximate 30% likelihood in 1985 to nearly 50% in 2012 (Fig. 1). Leatherbacks showed a significant increase in debris ingestion when historical data were included in the analyses, but the increase leveled off after 1985. Data from 1985 to 2012 did not show a significant increase in the probability of debris ingestion. This result is consistent with that of Mrosovsky et al. (2009), who also found that debris ingestion by leatherback turtles leveled off in the 1980s. The results with leatherback turtles suggest the environment has reached a saturation point, at least with respect to debris distribution. When running oceanic debris models similar to C. W. Lebreton’s (unpublished data) noted that after releasing hypothetical debris particles for about 10 years, debris distribution stabilized (i.e., debris continued to enter the system, but it ended up in the same areas). This possible saturation might also explain our results with Kemps’ ridley turtles.
The decrease in hawksbill turtle ingestion of debris we found may be due to small sample size. Only 2 studies reported on hawksbill gut contents, and these studies were conducted at the very beginning and very end of the literature-review period (Plotkin & Amos 1990; Schuyler et al. 2012).
It is possible that increasing awareness of debris ingestion may have affected necropsy methods. As more studies were published on debris ingestion, researchers may have become more meticulous in their necropsy techniques. However, because our analyses included feeding studies, in which gut contents are investigated carefully, and studies reporting null ingestion, it is reasonable to expect that observed increases were not due to differences in necropsy methods among studies. Additionally, our finding of increasing plastic ingestion is consistent with findings of other researchers for both turtles and seabirds (e.g., Mrosovsky et al. 2009; van Franeker et al. 2011).
Many of the turtles examined in the studies we reviewed did not ingest large quantities of debris. However, even small amounts of ingested debris can result in gut obstruction and mortality (Bjorndal et al. 1994). Although many studies did not report mortality of turtles, for those that did, about 4% of the total number of turtles necropsied (n = 1106) were reportedly killed by plastic ingestion. Of those turtles that ingested debris (n = 454) 42 (9%) were killed by it (range 0–35%). Although this number is relatively small, mortality is not the only risk associated with debris ingestion. Plasticizers, such as bisphenol-A (BPA) and phthalates, incorporated into plastics at production can leach into the environment or into tissue (Oehlmann et al. 2009). One group of researchers hypothesizes that plasticizers function as endocrine disruptors (Krishnan et al. 1993) and thus may have population-level effects on seabirds (van Franeker & SNS Fulmar Study Group 2011). Floating plastics also readily absorb heavy metals and other toxins from the ocean and can release these into the tissues of animals upon ingestion (Teuten et al. 2009), although little is known about the effects of metal or toxin release on marine species.
Location of Turtles and Debris
Debris ingestion by sea turtles occurs worldwide. Although not every study reported turtles with ingested debris, in every region of the world where gastrointestinal contents were examined, debris was detected. Similarly, Balasz (1985) reported debris ingestion by turtles at 19 locations worldwide, including all continents except Antarctica, where turtles do not occur.
No relation was observed between high proportions of debris ingestion at locations where stranded turtles were found and areas of high debris concentrations as determined from ocean-current modeling. We considered analyzing the correlation between coastal human population density and debris ingestion by turtles at study sites, but decided this analysis would have little relevance due to the large-scale migratory paths and motility of turtles and the wide distribution of marine debris from its source. For instance, results of a study conducted in the New York Bight, adjacent to the New York City metropolitan area (1990 population 16.4 million inhabitants) (Bureau of the Census 1990), showed only 12% of turtles ingested debris (Sadove & Morreale 1989). The results of a second study 5 years later in the same region showed no evidence of debris ingestion (Burke et al. 1994). Conversely, Tourinho et al. (2010) studied turtles in a “relatively undeveloped” area of southern Brazil. Here, over 200 km from Porto Alegre (2010 metropolitan area population 4.4 million) (IBGE 2010), 100% of turtles surveyed had ingested debris. Because most turtles migrate long distances during their posthatchling pelagic phase and during breeding migrations (Musick & Limpus 1997; Luschi et al. 2003), they are highly likely to encounter ocean-borne debris at some life stage, particularly when they passively drift in oceanic gyres, where debris accumulates. Because debris does not decompose as rapidly as food items and given that the physiology of turtles does not permit regurgitation or expulsion (Sheavly & Register 2007), turtles may encounter and ingest the debris far from where they strand.
Life-History Stage of Turtles
Anthropogenic debris accumulates in oceanic gyres far from shore (sensu Lebreton et al. 2012) (Fig. 3). Accordingly, one might expect oceanic-phase turtles to be more likely to ingest debris than coastal foragers. The 4 studies that reported on turtles sampled from oceanic waters found an average of 49.2% of turtles (n = 128) ingested debris (Parker et al. 2005; Boyle & Limpus 2008; Frick et al. 2010; Parker et al. 2011). Casale et al. (2008) investigated loggerhead turtles accidentally caught in oceanic waters on longlines and those accidentally caught in nearby benthic waters by trawl fishers. Of the oceanic turtles (n = 13), 64% had ingested debris, whereas 22% (n = 9) of benthic turtles had ingested debris. Similarly, results of a comparison of 2 populations of similarly sized juvenile loggerhead turtles with different foraging strategies showed that 35% of animals that foraged in the open ocean had ingested debris (Parker et al. 2005), whereas none of the coastal benthic-feeding turtles ingested debris (Peckham et al. 2011). Other studies in which stranded turtles were analyzed report that smaller oceanic turtles are more likely to ingest debris than larger turtles (Plotkin & Amos 1990; Schuyler et al. 2012). Balazs (1985) presented similar results: 69% of immature turtles ingested debris, whereas 31% of adult turtles ingested debris. This means young oceanic turtles may be more at risk from debris ingestion than older benthic-feeding turtles. Not only are they more likely to ingest debris, but their relatively small, thinner digestive systems will be more vulnerable to impaction by and perforation from the debris (Schuyler et al. 2012).
Species
All species studied ingested debris, but green and leatherback turtles were significantly more likely to ingest debris than were Kemp’s ridley or loggerhead turtles. Hawksbills were the most likely to ingest debris, but the sample size was small (n = 32) and came from only 2 studies, so other factors such as geography or life stage may have skewed results (Plotkin & Amos 1990; Schuyler et al. 2012). Our results differ from Balazs’ (1985), who reported that green turtles were most likely to ingest debris (32%), followed by loggerhead (26%), leatherback (24%), and hawksbill (19%) turtles. However, his data were reported only as the total number of cases for each species, not on the basis of the percentage of the total number of animals of that species that had ingested debris, given all animals sampled.
Carnivorous species (e.g., loggerhead and Kemp’s ridley turtles) appear less susceptible to debris ingestion than herbivores (green), gelatinovores (leatherback), and omnivores (hawksbill), or perhaps they are less likely to retain the ingested debris. One possible explanation of the lower incidence of debris ingestion in carnivorous species is that noncarnivores may be more likely to ingest debris or be more likely to die from ingestion of debris than carnivorous turtles. This could be because they have a greater affinity for gelatinous organisms and eat soft plastic because of its similarity to their prey, because they are less selective and feed on a variety of items including plastics, or because they feed in areas that accumulate debris.
The differences in debris ingestion by species may also be attributed to differences in the biology of the animals and how their digestive systems cope with debris once ingested. Adult and subadult loggerhead sea turtles have a larger-diameter digestive tract than green turtles of a similar age class; thus, they may more readily pass ingested materials (Bugoni et al. 2001) or perhaps they have different enzymes or microflora that act differently on ingested debris (Bjorndal 1997).
Debris Management
The differences in how debris ingestion is investigated and reported make it challenging to develop relevant global analyses on which to base management recommendations. Standardized reporting methods on debris effects on wildlife, including debris type and size, species, and life-history stage of animals affected, would go a long way toward creating a globally consistent and comparable data set. Furthermore, increased efforts to understand debris effects in underresearched areas where turtles occur in great numbers (especially Southeast Asia, western and northern Australia, South America, and Africa), and in mid-ocean pelagic turtles would be beneficial.
Our results show clearly that debris ingestion by sea turtles is a global phenomenon of increasing magnitude. Our finding that oceanic-stage green and leatherback turtles are at higher risk than benthic-feeding carnivorous turtles means management actions to target these species and life stages should be considered. This is particularly important for leatherback turtles that spend the bulk of their lives in oceanic waters, and are listed as critically endangered (IUCN 2012).
Ingestion prevalence at stranding locations was not related to predicted debris density, likely due to the long migrations of turtles. Thus, conducting coastal cleanups will not solve the problem of debris accumulation in the pelagic environment, where animals are most commonly affected, although it is an important step in preventing marine debris input into the ocean. Anthropogenic debris is not only a problem for endangered turtles and other marine wildlife, but also affects human health and safety (e.g., discarded sharps and medical waste and ship encounters with large items). Debris also has aesthetic and economic consequences and may result in decreased tourism (Ballance et al. 2000), reduced economic benefits from fisheries (Havens et al. 2008), and damage to vessels (Jones 1995). Furthermore, debris destroys habitats and aids in the transport of invasive species (Sheavly & Register 2007). It is therefore a high priority to address this global problem. An estimated 80% of debris comes from land-based sources; hence, it is critical to implement effective waste management strategies and to create and maintain a global survey and comprehensive database of marine debris ingestion and entanglement. Additionally, it is worth engaging with industry to create and implement appropriate innovations and controls to assist in decreasing marine debris.
Acknowledgments
This work was funded by CSIRO Wealth from Oceans Flagship and Ecosystem Sciences, Goldring Earthwatch Emerging Marine Scientist Fellowship, ARC Linkage grant LP110200216, Goodman Family Foundation, the Australian Postgraduate Award, the Margaret Middleton Foundation, and the Shell Social Investment Program. We thank the staff of the Moreton Bay Research Station for help throughout this project, J. Roberts for assistance in the early stages of manuscript writing, and constructive comments from D. Ceccarelli, M. Hamann, N. FitzSimmons, and 4 anonymous reviewers.
Literature Cited
- Ashmole NP, Ashmole MJ. Comparative feeding ecology of sea birds of a tropical oceanic island. New Haven, Connecticut: Peabody Museum of Natural History, Yale University; 1967. [Google Scholar]
- Balazs G. Impact of ocean debris on marine turtles: entanglement and ingestion. In: Shomura RS, Yoshido HO, editors. Proceedings of the workshop on the fate and impact of marine debris. U.S. National Oceanic and Atmospheric Administration (NOAA) Technical memorandum 54. Honolulu: National Marine Fisheries Service; 1985. pp. 387–429. [Google Scholar]
- Ballance A, Ryan PG, Turpie JK. How much is a clean beach worth? The impact of litter on beach users in the Cape Peninsula, South Africa. South African Journal of Science. 2000;96:210–213. [Google Scholar]
- Bell I. Algivory in hawksbill turtles: Eretmochelys imbricata food selection within a foraging area on the Northern Great Barrier Reef. Marine Ecology. 2012;34:43–55. [Google Scholar]
- Bjorndal K. Foraging ecology and nutrition of sea turtles. In: Lutz PL, Musick JA, editors. The biology of sea turtles. Boca Raton, Florida: CRC Press; 1997. pp. 199–232. [Google Scholar]
- Bjorndal KA, Bolten AB, Lagueux CJ. Ingestion of marine debris by juvenile sea turtles in coastal Florida habitats. Marine Pollution Bulletin. 1994;28:154–158. [Google Scholar]
- Bolten AB. Variation in sea turtle life history patterns: neritic vs. oceanic developmental stages. The Biology of Sea Turtles. 2003;2:243–257. [Google Scholar]
- Boyle MC, Limpus CJ. The stomach contents of post-hatchling green and loggerhead sea turtles in the southwest Pacific: an insight into habitat association. Marine Biology. 2008;155:233–241. [Google Scholar]
- Bugoni L, Krause L, Petry MV. Marine debris and human impacts on sea turtles in southern Brazil. Marine Pollution Bulletin. 2001;42:1330–1334. doi: 10.1016/s0025-326x(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Bureau of the Census. 1990 Census of population general population characteristics: New York. U.S. Washington, D.C: Department of Commerce; 1990. [Google Scholar]
- Burke VJ, Morreale SJ, Standora EA. Diet of the Kemp’s ridley sea turtle, Lepidochelys kempii, in New York waters. U.S. National Marine Fisheries Service Fishery Bulletin. 1994;92:26–32. [Google Scholar]
- Cannon AC. Gross necropsy results of sea turtles stranded on the upper Texas and western Louisiana coasts, 1 January–31 December 1994. Pages 81–85 in Characteristics and causes of Texas marine strandings. Seattle: National Marine Fisheries Service; 1998. U.S. National Oceanic and Atmospheric Administration (NOAA) technical report 143. [Google Scholar]
- Carr A. Rips, FADS, and little loggerheads. BioScience. 1986;36:92–100. [Google Scholar]
- Casale P, Abbate G, Freggi D, Conte N, Oliverio M, Argano R. Foraging ecology of loggerhead sea turtles Caretta caretta in the central Mediterranean Sea: evidence for a relaxed life history model. Marine Ecology-Progress Series. 2008;372:265–276. [Google Scholar]
- Ceccarelli DM. Impacts of plastic debris on Australian marine wildlife. Report. Canberra: Department of the Environment, Water, Heritage and the Arts; 2009. Australia. [Google Scholar]
- Derraik J. The pollution of the marine environment by plastic debris: a review. Marine Pollution Bulletin. 2002;44:842–852. doi: 10.1016/s0025-326x(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Duguy R. Marine turtles of the Gulf of Gascony. Annales de la Societe des Sciences Naturelles de la Charente-Maritime. 1997;8:633–645. [Google Scholar]
- Duguy R, Moriniere P, Meunier A. The ingestion of floating debris by Luth’s turtle Dermochelys coriacea (Vandelli, 1761) in the Gulf of Gascony. Annales de la Societe des Sciences Naturelles de la Charente-Maritime. 2000;8:1035–1038. [Google Scholar]
- Duronslet MJ, Revera DB, Stanley KM. Man-made marine debris and sea turtle strandings on beaches of the upper Texas and southwestern Louisiana coasts, June 1987 through September 1989. National Oceanic and Atmospheric Administration (NOAA) technical memorandum 279. Texas: National Marine Fisheries Service Galveston; 1991. [Google Scholar]
- Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall/CRC; 1994. [Google Scholar]
- Faris J, Hart KM. Seas of debris: a summary of the Third international conference on marine debris. Raleigh, North Carolina: North Carolina Sea Grant College Program; 1994. [Google Scholar]
- Foley AM, Singel KE, Dutton PH, Summers TM, Redlow AE, Lessman J. Characteristics of a green turtle (Chelonia mydas) assemblage in northwestern Florida determined during a hypothermic stunning event. Gulf of Mexico Science. 2007;25:131–143. [Google Scholar]
- Frick MG, Williams KL, Bolten AB, Bjorndal KA, Martins HR. Foraging ecology of oceanic-stage loggerhead turtles Caretta caretta. Endangered Species Research. 2010;9:91–97. [Google Scholar]
- Garnett ST, Price IR, Scott FJ. The diet of the green turtle, Chelonia mydas (L), in Torres Strait. Australian Wildlife Research. 1985;12:103–112. [Google Scholar]
- Godley B, Blumenthal J, Broderick A, Coyne M, Godfrey M, Hawkes L, Witt M. Satellite tracking of sea turtles: Where have we been and where do we go next? Endangered Species Research. 2008;4:3–22. [Google Scholar]
- Guebert-Bartholo FM, Barletta M, Costa MF, Monteiro-Filho ELA. Using gut contents to assess foraging patterns of juvenile green turtles Chelonia mydas in the Paranagua Estuary, Brazil. Endangered Species Research. 2011;13:131–143. [Google Scholar]
- Hasbun CR, Lawrence AJ, Samour JH, Al-Ghais SM. Preliminary observations on the biology of green turtles, Chelonia mydas, from the United Arab Emirates. Aquatic Conservation-Marine and Freshwater Ecosystems. 2000;10:311–322. [Google Scholar]
- Havens KJ, Bilkovic DM, Stanhope D, Angstadt K, Hershner C. The effects of derelict blue crab traps on marine organisms in the lower York River, Virginia. North American Journal of Fisheries Management. 2008;28:1194–1200. [Google Scholar]
- IBGE (Brazilian Institute of Geography and Statistics) Censo 2010. IBGE, location. 2010. Available from http://censo2010.ibge.gov.br/en/sobre-censo (accessed April 2013)
- IUCN (International Union for Conservation of Nature) IUCN red list of threatened species. Version 2012.2. IUCN, Gland, Switzerland. 2012. Available from http://www.iucnredlist.org (accessed December 2012)
- Jones MM. Fishing debris in the Australian marine environment. Marine Pollution Bulletin. 1995;30:25–33. [Google Scholar]
- Kaska Y, Celik A, Bag H, Aureggi M, Ozel K, Elci A, Kaska A, Elca L. Heavy metal monitoring in stranded sea turtles along the Mediterranean coast of Turkey. Fresenius Environmental Bulletin. 2004;13:769–776. [Google Scholar]
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2279. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Laist D. Impacts of marine debris: entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In: Coe JM, Rogers DB, editors. Marine debris: sources, impacts, and solutions. New York: Springer; 1997. pp. 99–139. [Google Scholar]
- Lazar B, Gracan R. Ingestion of marine debris by loggerhead sea turtles, Caretta caretta, in the Adriatic Sea. Marine Pollution Bulletin. 2011;62:43–47. doi: 10.1016/j.marpolbul.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Lebreton LCM, Greer S, Borrero J. Numerical modelling of floating debris in the world’s oceans. Marine Pollution Bulletin. 2012;64:653–661. doi: 10.1016/j.marpolbul.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Limpus CJ, de Villiers DL, de Villiers MA, Limpus DJ, Read MA. The loggerhead turtle, Caretta caretta in Queensland: feeding ecology in warm temperate waters. Memoirs of the Queensland Museum. 2001;46:631–645. [Google Scholar]
- Lopez-Mendilaharsu M, Gardner SC, Seminoff JA, Riosmena-Rodriguez R. Identifying critical foraging habitats of the green turtle (Chelonia mydas) along the Pacific coast of the Baja California peninsula, Mexico. Aquatic Conservation-Marine and Freshwater Ecosystems. 2005;15:259–269. [Google Scholar]
- Luschi P, Hays GC, Papi F. A review of long-distance movements by marine turtles, and the possible role of ocean currents. Oikos. 2003;103:293–302. [Google Scholar]
- Mrosovsky N, Ryan G, James M. Leatherback turtles: the menace of plastic. Marine Pollution Bulletin. 2009;58:287–289. doi: 10.1016/j.marpolbul.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Musick JA, Limpus CJ. Habitat utilization and migration in juvenile sea turtles. The Biology of Sea Turtles. 1997;1:137–163. [Google Scholar]
- NOAA (National Oceanic and Atmospheric Organization) Japan Tsunami debris: information and FAQs. NOAA, Silver Spring, Maryland. 2012. Available from http://marinedebris.noaa.gov/info/japanfaqs.html (accessed June 2012)
- Oehlmann J, et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:2047–2062. doi: 10.1098/rstb.2008.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DM, Cooke WJ, Balazs GH. Diet of oceanic loggerhead sea turtles (Caretta caretta) in the central North Pacific. Fishery Bulletin. 2005;103:142–152. [Google Scholar]
- Parker DM, Dutton PH, Balazs GH. Oceanic diet and distribution of haplotypes for the green turtle, Chelonia mydas, in the Central North Pacific. Pacific Science. 2011;65:419–431. [Google Scholar]
- Peckham SH, Maldonado-Diaz D, Tremblay Y, Ochoa R, Polovina J, Balazs G, Dutton PH, Nichols WJ. Demographic implications of alternative foraging strategies in juvenile loggerhead turtles Caretta caretta of the North Pacific Ocean. Marine Ecology Progress Series. 2011;425:269–280. [Google Scholar]
- PlasticsEurope. The compelling facts about plastics 2009: an analysis of European plastics production, demand and recovery for 2008. Brussels: PlasticsEurope; 2009. [Google Scholar]
- Plotkin P, Amos A. Effects of anthropogenic debris on sea turtles in the northwestern Gulf of Mexico. In: Shomura R, Yoshida H, editors. Proceedings of the 2nd international conference on marine debris. Honolulu: National Oceanic and Atmospheric Administration; 1990. pp. 736–743. [Google Scholar]
- Plotkin P, Wicksten M, Amos A. Feeding ecology of the loggerhead sea turtle Caretta caretta in the Northwestern Gulf of Mexico. Marine Biology. 1993;115:1–5. [Google Scholar]
- Quinones J, Gonzalez Carman V, Zeballos J, Purca S, Mianzan H. Effects of El Nino-driven environmental variability on black turtle migration to Peruvian foraging grounds. Hydrobiologia. 2010;645:69–79. [Google Scholar]
- Rees A, Al-Kiyumi A, Broderick A, Papathanasopoulou N, Godley B. Conservation related insights into the behaviour of the olive ridley sea turtle Lepidochelys olivacea nesting in Oman. Marine Ecology Progress Series. 2012;450:195–205. [Google Scholar]
- Revelles M, Cardona L, Aguilar A, Fernandez G. The diet of pelagic loggerhead sea turtles (Caretta caretta) off the Balearic archipelago (western Mediterranean): relevance of long-line baits. Journal of the Marine Biological Association of the United Kingdom. 2007;87:805–813. [Google Scholar]
- Ross JP. Biology of the green turtle, Chelonia mydas, on an Arabian feeding ground. Journal of Herpetology. 1985;19:459–468. [Google Scholar]
- Russo G, Di Bella C, Loria GR, Insacco G, Palazzo P, Violani C, Zava B. Notes on the influence of human activities on sea chelonians in Sicilian waters. Ibex Journal of Mountain Studies. 2003;7:37–41. [Google Scholar]
- Sadove S. Marine mammal and sea turtle encounters with marine debris in the New York Bight and the northeast Atlantic. Pages 2–7. In: Shomura R, Morreale S, editors. Proceedings of the 2nd international conference on marine debris. Honolulu: National Oceanic and Atmospheric Administration; 1989. [Google Scholar]
- Santos RG, et al. Coastal habitat degradation and green sea turtle diets in Southeastern Brazil. Marine Pollution Bulletin. 2011;62:1297–1302. doi: 10.1016/j.marpolbul.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Schuyler Q, Hardesty BD, Wilcox C, Townsend K. To eat or not to eat? Debris selectivity by marine turtles. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0040884. DOI: 10.1371/journal.pone.0040884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney EE, Musick JA. Historical diet analysis of loggerhead sea turtles (Caretta caretta) in Virginia. Copeia. 2007;2007:478–489. [Google Scholar]
- Seminoff JA, Resendiz A, Nichols WJ. Diet of east Pacific green turtles (Chelonia mydas) in the central Gulf of California, Mexico. Journal of Herpetology. 2002;36:447–453. [Google Scholar]
- Shaver DJ. Feeding ecology of wild and head-started Kemp’s Ridley sea turtles in South Texas waters. Journal of Herpetology. 1991;25:327–334. [Google Scholar]
- Shaver DJ. Sea turtle strandings along the Texas coast, 1980–94. Pages 57–72 in Characteristics and causes of Texas marine strandings. U.S. National Oceanic and Atmospheric Administration (NOAA) technical report 143. Seattle: National Marine Fisheries Service; 1998. [Google Scholar]
- Sheavly S, Register K. Marine debris & plastics: environmental concerns, sources, impacts and solutions. Journal of Polymers and the Environment. 2007;15:301–305. [Google Scholar]
- Sperling JB, Grigg GC, Beard LA, Limpus CJ. Respiratory properties of blood in flatback turtles (Natator depressus. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2007;177:779–786. doi: 10.1007/s00360-007-0174-3. [DOI] [PubMed] [Google Scholar]
- Teuten EL, et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:2027–2045. doi: 10.1098/rstb.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas J, Guitart R, Mateo R, Raga JA. Marine debris ingestion in loggerhead sea turtles, Caretta caretta from the Western Mediterranean. Marine Pollution Bulletin. 2002;44:211–216. doi: 10.1016/s0025-326x(01)00236-3. [DOI] [PubMed] [Google Scholar]
- Tourinho PS, do Sul JAI, Fillrnann G. Is marine debris ingestion still a problem for the coastal marine biota of southern Brazil? Marine Pollution Bulletin. 2010;60:396–401. doi: 10.1016/j.marpolbul.2009.10.013. [DOI] [PubMed] [Google Scholar]
- UNEP (United Nations Environment Programme) Marine litter, an analytical overview. Nairobi: UNEP; 2005. [Google Scholar]
- van Franeker JA, et al. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environmental Pollution. 2011;159:2609–2615. doi: 10.1016/j.envpol.2011.06.008. [DOI] [PubMed] [Google Scholar]
- van Franeker JA. SNS Fulmar Study Group. Chemicals in marine plastics and potential risks for a seabird like the Northern Fulmar (Fulmarus glacialis. In: Carswell B, McElwee K, Morison S, editors. Fifth international marine debris conference. Honolulu: National Oceanic and Atmospheric Adminstration; 2011. pp. 415–418. [Google Scholar]
- Walker T, Parmenter C. Absence of a pelagic phase in the life cycle of the flatback turtle, Natator depressa (Garman) Journal of Biogeography. 1990;17:275–278. [Google Scholar]
- Witherington BE. Ecology of neonate loggerhead turtles inhabiting lines of downwelling near a Gulf Stream front. Marine Biology. 2002;140:843–853. [Google Scholar]