Summary
Has the recent identification of iron deficiency as a risk factor for ischaemic stroke in patients with pulmonary arteriovenous malformations (AVMs) unmasked a new paradigm for stroke/infarct pathogenesis? This commentary reviews evidence that spans associations between iron deficiency and ischaemic strokes, iron deficiency enhancement of platelet aggregation in response to serotonin/5HT, settings in which plasma 5HT is elevated, and clinical trial confirmation that 5HT receptor antagonists prevent ischaemic stroke. The critical leap which directs attention away from atherothrombotic events at the neurovascular wall is that ischaemic strokes due to pulmonary AVMs are attributable to compromised pulmonary capillary bed filtration of venous blood. Right-to-left shunting is continuous through pulmonary AVMs, but also occurs intermittently in approximately 30% of the general population with intracardiac shunts such as patent foramen ovale (PFO). The testable hypothesis presented is that paradoxical embolism of venous platelet-based aggregates may constitute part of the causal chain between iron deficiency and ischaemic stroke, not only in the rare disease state of pulmonary AVMs, but also in major subgroups of the general population.
Keywords: Iron deficiency; 5HT (5 hydroxytryptamine, serotonin); right-to-left shunt; pulmonary capillary filter; paradoxical embolism; patent foramen ovale; platelet aggregation
1. Introduction
Strokes are the fourth ranked cause of death, and each year, leave millions of people severely disabled. The most common are ischaemic strokes which occur when a region of the brain is deprived of oxygenated blood supply, usually due to direct occlusion of arterial vessels. There is Class I, Level A evidence that ischaemic strokes can be prevented by anti-platelet agents (1).
The conventional list of ischaemic stroke risk factors is headed by smoking, hypertension, hypercholesterolaemia, obesity and diabetes, with a focus on atherosclerotic-based mechanisms. However, these risk factors do not explain the very high burden of ischaemic stroke in low income countries (2).
The rare disease state of pulmonary arteriovenous malformations (PAVMs) provides an alternative perspective. PAVMs are abnormal dilated vascular channels that provide a direct communication between pulmonary arteries and pulmonary veins, and hence a right-to-left shunt (Figure 1). In cross-sectional studies, approximately one in eight patients with PAVMs experience a clinical stroke (3,4) with imaging revealing a far higher ischaemic burden: in a study performed using 20th century scanners, 34/67 (51%) patients with median age 41 year had evidence of cortical or subcortical infarcts (5).
Figure 1.
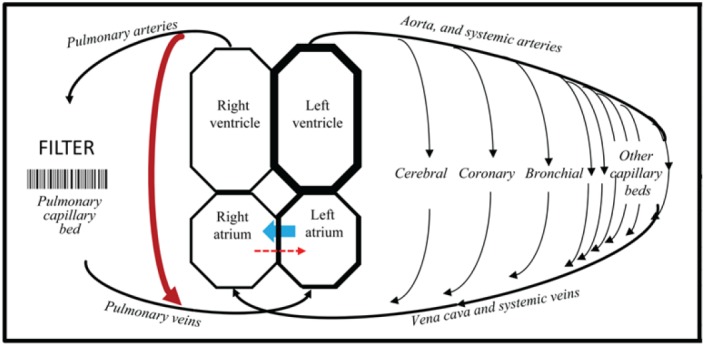
The pulmonary capillary filter and right-to-left shunts. Cartoon of the circulations, indicating the pulmonary capillary bed and the mechanical filter through which venous blood should pass before returning to the systemic arterial tree. Red arrows represent right-to-left shunts which allow venous blood to return to systemic arteries having bypassed filtration, gas exchange, and other pulmonary capillary functions. Right-to-left shunting is continuous through pulmonary AVMs, but only intermittent through intracardiac shunts such as patent foramen ovale, when flow is predominantly left-to-right (blue arrow, see text). Note that the lungs have a second arterial supply from the systemic circulation (bronchial vessels indicated) that can preserve arterial supply to lung tissue if the pulmonary circulation is compromised.
The general presumption was that the infarcts/strokes in PAVM patients developed as a result of paradoxical emboli of venous thromboemboli (VTE), and that stroke risk would increase with the severity of PAVMs. Surprisingly however, a prospective series of 219 patients published in 2008 demonstrated no clear link between ischaemic stroke and severity of PAVMs, venous thromboemboli, or conventional stroke risk factors (3). Further exploration of factors that contributed to the risks of these strokes was recommended (3).
2. Iron deficiency and ischaemic strokes
Understanding which PAVM patients are at higher risk of ischaemic stroke is important for patients, because while treatment by embolisation reduces stroke risk (3), individual PAVMs are often too small for embolisation, and most treated patients are left with residual right-toleft shunts. Additionally, it seemed plausible that the stroke risks for patients with right-to-left shunts due to PAVMs may also be relevant to individuals with other right-to-left shunts such as patent foramen ovale (3) which persist in approximately 30% of adults in the general population (6) (Figure 1).
2.1. Iron deficiency and ischaemic strokes due to PAVMs
Earlier this year, a study in 497 consecutive adults with PAVMs identified low serum iron levels, and high levels of fibrinogen (the predominant plasma protein for platelet adhesion), as new risk factors for ischaemic strokes in the population (4). The age/gender adjusted odds ratio of 0.96 (95% CI: 0.92, 1.00), per µmol/L increase in serum iron, implied that a modest reduction in serum iron would be associated with approximately double the risk of stroke. In the PAVM patients, iron deficiency was attributed to inadequate replacement of haemorrhagic iron losses due to underlying hereditary haemorrhagic telangiectasia (HHT), particularly nosebleeds (7).
As in the previous study, there were no associations between ischaemic stroke and known vascular risk factors: almost half of all strokes (29/61, 47.5%) occurred in lifelong non-smokers without any documented conventional stroke risk factors (hypertension, hypercholesterolemia, diabetes, or arrhythmias) (3,4). There was also no association between stroke risk and markers or outcomes of conventional venous thromboemboli (often considered responsible for paradoxical embolic events), or with platelet number or haemoglobin that are commonly cited as possible mediators of iron deficiency risks (4). There was an overlap with myocardial infarction, attributed to a common paradoxical process (4).
2.2. Iron deficiency and ischaemic stroke in the general population
This was not the first report to link iron deficiency to ischaemic stroke. Case reports and small series have generally focused on paediatric populations (8, and references), but major epidemiological studies in adults also support a link between iron deficiency and ischaemic stroke, operating independently to known stroke risk factors (9,10, and references). Iron deficiency would offer a plausible missing risk factor for stroke pathogenesis, because it affects in excess of 1 billion individuals worldwide, and is a particular problem in countries with restricted diets, and where iron losses are increased, for example due to hookworm and/or urinary schistosomiasis (7,11).
3. Iron deficiency and platelets
Iron deficiency has multiple effects that could be implicated in stroke pathogenesis, including anaemia/reduced blood oxygen content, high cardiac output with lower systemic vascular resistance, increased blood viscosity, and thrombocythaemia (12,13). Many were discussed in the respective stroke manuscripts (8–10 and references), but do not really stand up to careful scrutiny as likely primary mechanisms for focal ischaemic strokes (4). Given the proven efficacy of anti-platelet agents in stroke prevention (1), it seems surprising that a study linking iron deficiency to exuberant platelet aggregation(14) has been overlooked until now (4).
Four decades ago, the Oxford-based authors demonstrated increased platelet aggregation to serotonin (5-hydroxytryptamine, 5HT) (14). As confirmed and recently reviewed (4), the platelet response is relatively specific, with no difference observed in platelet aggregation responses to adenosine diphosphate (ADP). In many countries, 5HT is considered a minor platelet agonist, but evidence particularly from Japan suggests otherwise: Plasma 5HT concentrations increase after platelet activation, and in a number of pathologies and disease states including atherosclerosis (4,15,16). Critically, antagonists to the receptor 5HT2A result in dose-dependent inhibition of platelet aggregation in ischemic stroke patients (15), and achieved therapeutic equivalence with aspirin in secondary prevention of ischaemic stroke (16). Conversely, cardiovascular side effects are recognised for drugs that increase extracellular 5HT/serotonin, such as serotonin reuptake inhibitors (SSRIs) (17).
Taken together, these data suggest that for individuals with concurrent iron deficiency and a state associated with elevated plasma 5HT, exuberant platelet aggregation might be predicted.
4. PAVMs and compromised pulmonary capillary filtration
The 5HT/platelet data could be relevant to platelet aggregation during atherothrombotic events at arterial walls. However, multiple studies implicate iron deficiency in ischaemic stroke pathogenesis in children too young to have accumulated a significant atherosclerotic load (8 and references). Furthermore, as noted above, conventional atherosclerotic-based risk factors were not a feature of the PAVM patients with ischaemic strokes (3,4). A different paradigm seems to be needed.
4.1. The pulmonary capillary filter
After forming or entering the venous circulation, particulate matter and multicellular aggregates should lodge safely in pulmonary capillaries/arterioles. In man, morphometric, perfusion, and echocardiographic studies indicate that the cut off size for pulmonary capillary transit just exceeds the 7µm diameter of erythrocytes (4). The filter is exploited by conventional nuclear perfusion scans performed to diagnose pulmonary emboli: technetium-labelled albumin macroaggregates are injected intravenously, and impact in pulmonary capillaries receiving pulmonary arterial flow.
4.2. PAVMs allow blood-bourne particles to bypass pulmonary capillary filtration
If the pulmonary capillary filter were breached, for example if venous blood could pass through the right-to-left shunts of PAVMs, it would be expected that a proportion of venous particulate matter would impact not in the lungs, but in next (systemic) capillary bed. This is observed if perfusion scans are performed in patients with PAVMs, with striking cerebral images (4). The final clinical outcome following neurovascular impaction is more difficult to predict, and will depend on end organ thrombo-inflammatory and other vascular/tissue responses -clearly very few impactions result in a clinical stroke.
5. Patent foramen ovale (PFO) and intracardiac shunts
Could intracardiac shunts that affect at least 1 in 3 of the general population, provide a rationale for the iron deficiency- ischaemic stroke associations in children and adults? Recent AHA guidelines detail management strategies for ischaemic strokes associated with PAVMs in the same section as patent foramen ovale (PFO), recommending anti-platelet agents for secondary prevention in both conditions (Class IIa, Level B Evidence) (1).
In contrast to PAVMs, only a small proportion of individuals with PFO suffer ischemic strokes, but stroke rates are higher in the subgroup of PFO patients with permanent right-to-left shunts (18).
The discrepant stroke rates make intuitive sense in the light of physiological comparisons of right-to-left shunting through pulmonary AVMs, compared to intracardiac defects such as PFOs. Pulmonary AVMs provide almost continuous right-to-left shunts because the pressure in the pulmonary artery generally exceeds that of the pulmonary vein: shunt quantifications are highly reproducible within the same patient (4,12). PFOs and other intracardiac septal defects normally exhibit left-to-right flow, due to the higher pressure at equivalent points in the systemic compared to pulmonary circulation (Figure 1). At the end of valsalva manouvres however, pressure changes result in reversal of flow across such septal defects, and a transient right-to-left shunt (18).
This is important because valsalva manouvres occurs surprisingly frequently during daily life, for example during nasal/sinus clearance and strained bowel evacuations (18). Times when PFO right-to-left shunts would be in operation also include sleep apnoea, now recognised to be associated with ischaemic stroke and other adverse cardiovascular events (19,20). Associated pressure changes are well recognised, but valsalva provocation of right-to-left shunting, allowing the particulate constituents of venous blood to bypass the mechanical filter provided by the pulmonary capillary bed, has not been emphasised to date.
6. Future studies
Examining whether paradoxical embolism of venous platelet-based aggregates is likely to be contributing to ischaemic stroke risks in the general population could be relatively easy to address, particularly given the lead through iron deficiency.
First, future epidemiological studies of associations between iron deficiency and ischaemic stroke could test the null hypothesis that the presence of a PFO, or any form of right-to-left shunt, does not modify the odds ratio for stroke attributable to iron deficiency. It may be possible to address this retrospectively using subgroups of published series in which contrast echocardiographic studies have been undertaken (8–10).
Prospective studies could also test whether exuberant platelet aggregation to 5HT is associated with enhanced risk of ischaemic stroke, and whether contribution of iron deficiency to the stroke model is reduced once adjusted for the platelet aggregation phenotype.
Most importantly, it would seem wise that for future randomised controlled trials examining the potential efficacy of prevention/treatment of iron deficiency in stroke prevention, additional assessments should be incorporated in order to allow appropriate risk stratifications of physiological groupings. Suggestions include contrast echocardiographic studies to evaluate right-to-left shunts, capturing a history of valsalva-precipitating clinical events in study populations, and concurrent assessments of platelet 5HT aggregation responses. For secondary prevention when it would be unethical to withhold anti-platelet therapy, further comparisons of the relative efficacy of 5HT receptor antagonists versus compounds such as aspirin or clopidogrel could be made, capturing whether any differences were more or less evident in subgroups stratified by iron deficiency or echocardiographic evidence of shunting.
7. Conclusion
For society and individuals, the ultimate burden of ischaemic strokes both directly, and through contributions of small ischaemic strokes to vascular dementia, is profound. The identification of iron deficiency as a risk factor for ischaemic strokes in the rare disease of PAVMs appears to introduce new paradigms for stroke pathogenesis. Given the current evidence base, and prevalence of both compromised pulmonary capillary filtration and iron deficiency, it seems appropriate to now test the platelet-serotonin-shunt hypothesis, and establish the extent to which the pulmonary capillary filter prevents ischaemic strokes and other infarct-mediated pathologies such as myocardial infarction.
Acknowledgements
Dr. Shovlin has received funding support from the European Respiratory Society (2012 Rare Disease Achievement Award), National Institute of Health Research (London (NW) Comprehensive Local Research Network and Imperial Biomedical Research Centre), and patient donations. They played no role in the writing of the manuscript or the decision to submit it for publication.
References
- 1. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischaemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:227-276 [DOI] [PubMed] [Google Scholar]
- 2. Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: Estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009; 8:345-354 [DOI] [PubMed] [Google Scholar]
- 3. Shovlin CL, Jackson JE, Bamford KB, Jenkins IH, Benjamin AR, Ramadan H, Kulinskaya E. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax. 2008; 63:259-266 [DOI] [PubMed] [Google Scholar]
- 4. Shovlin CL, Chamali B, Santhirapala V, Livesey JA, Angus G, Manning R, Laffan MA, Meek J, Tighe HC, Jackson JE. Ischaemic strokes in patients with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia: Associations with iron deficiency and platelets. PLoS One. 2014; 9:e88812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moussouttas M, Fayad P, Rosenblatt M, Hashimoto M, Pollak J, Henderson K, Ma TY, White RI. Pulmonary arteriovenous malformations: Cerebral ischemia and neurologic manifestations. Neurology. 2000; 55:959-964 [DOI] [PubMed] [Google Scholar]
- 6. Hagan PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984; 59:17-20 [DOI] [PubMed] [Google Scholar]
- 7. Finnamore H, Le Couteur J, Hickson M, Busbridge M, Whelan K, Shovlin CL. Hemorrhage-adjusted iron requirements, hematinics and hepcidin define hereditary hemorrhagic telangiectasia as a model of hemorrhagic iron deficiency. PLoS One. 2013; 8:e76516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azab SF, Abdelsalam SM, Saleh SH, Elbehedy RM, Lotfy SM, Esh AM, Srea MA, Aziz KA. Iron deficiency anemia as a risk factor for cerebrovascular events in early childhood: A case-control study. Ann Hematol. 2014; 93:571-576 [DOI] [PubMed] [Google Scholar]
- 9. Gillum RF, Sempos CT, Makuc DM, Looker AC, Chien C-Y, Ingram DD. Serum transferrin saturation, stroke incidence, and mortality in women and men. The NHANES I epidemiologic followup study. National Health and Nutrition Examination Survey. Am J Epidemiol. 1996; 144:59-68 [DOI] [PubMed] [Google Scholar]
- 10. Chang YL, Hung SH, Ling W, Lin HC, Li HC, Chung SD. Association between ischemic stroke and iron-deficiency anemia: A population-based study. PLoS One. 2013; 8:e82952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva: 2002. Available http://www.who.int/whr/2002/en/whr02_en.pdf (accessed March 28, 2014). [Google Scholar]
- 12. Santhirapala V, Williams LC, Tighe HC, Jackson JE, Shovlin CL. Arterial oxygen content is precisely maintained by graded erythrocytotic responses in settings of high/normal serum iron levels, and predicts exercise capacity: An observational study of hypoxaemic patients with pulmonary arteriovenous malformations. PLoS One. 2014; 9:e90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hébert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin. 2004; 20:187-212 [DOI] [PubMed] [Google Scholar]
- 14. Woods HF, Youdim MBH, Boullin D, Callender S. Monoamine metabolism and platelet function in iron-deficiency anaemia. In: Iron metabolism. In CIBA Foundation Symposium 51 (new series). Amsterdam: Elsevier, 1977; pp. 227-248 [DOI] [PubMed] [Google Scholar]
- 15. Uchiyama S, Ozaki Y, Satoh K, Kondo K, Nishimaru K. Effect of sarpogrelate, a 5-HT(2A) antagonist, on platelet aggregation in patients with ischaemic stroke: Clinical-pharmacological dose-response study. Cerebrovasc Dis. 2007; 24:264-270 [DOI] [PubMed] [Google Scholar]
- 16. Shinohara Y, Nishimaru K, Sawada T, et al. Sarpogrelate-Aspirin Comparative Clinical Study for Efficacy and Safety in Secondary Prevention of Cerebral Infarction (S-ACCESS): A randomized, double-blind, aspirin-controlled trial. Stroke. 2008; 39:1827-1833 [DOI] [PubMed] [Google Scholar]
- 17. Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: Population based cohort study. BMJ. 2011; 343:d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rigatelli G1, Dell'Avvocata F, Cardaioli P, Giordan M, Braggion G, Aggio S, Chinaglia M, Mandapaka S, Kuruvilla J, Chen JP, Nanjundappa A. Permanent right-to-left shunt is the key factor in managing patent foramen ovale. J Am Coll Cardiol. 2011; 58:2257-2261 [DOI] [PubMed] [Google Scholar]
- 19. Beelke M, Angeli S, Del Sette M, De Carli F, Canovaro P, Nobili L, Ferrillo F. Obstructive sleep apnea can be provocative for right-to-left shunting through a patent foramen ovale. Sleep. 2002; 25:856-862 [PubMed] [Google Scholar]
- 20. Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012; 5:720-728 [DOI] [PubMed] [Google Scholar]


