Summary
Meningioma with extensive histiocytic changes is rare. We describe a case of histiocytic meningioma which occurred in a 55-year-old woman. The patient had a progressive headache and a decline in fine motor coordination and memory for the past four years. Magnetic resonance imaging demonstrated a well-demarcated, dura-based and contrast-enhancing mass lesion in the right superior frontoparietal region. Histopathologically, the tumor showed neoplastic meningothelial proliferation with extensive and multifocal histiocytic infiltration. The histiocytic component constituted approximately half of the entire tumor. Immunohistochemically, both meningothelial and histiocytic cells showed immunoreactivity for epithelial membrane antigen (EMA), while the histiocytic cells were also positive for CD4 and CD68. In addition, there were scattered S100-positive histiocytes throughout the tumor. Proliferative index highlighted by Ki67 immunostain was 1.6%. There were no high-grade changes such as frequent mitoses, necrosis, or brain parenchymal invasion in the specimen. With review of the literature, we propose that this type of meningioma should be considered as a separate subtype of meningioma. The biological basis and differential diagnosis are discussed.
Keywords: Meningioma, Histiocyte, Brain tumor
1. Introduction
Meningioma is the most common primary intracranial tumor and shows a significant histological diversity. The Word Health Organization (WHO) Classification of Tumours of the Central Nervous System recognizes multiple variants. Meningioma with extensive histiocytic changes is rare, and the biologic basis of this type of tumor is unclear. We present a case of a histiocytic meningioma and discuss the possible biologic origins and its differential diagnosis.
2. Case report
2.1. Clinical history
The patient was a 55-year old female who presented with a progressive headache, blurring vision and progressive decline in fine motor function and memory for four years. The patient previously had a CT scan after a head trauma more than four years ago, and a “very small” and clinically suspicious meningioma was noted at that time. The current magnetic resonance imaging demonstrated a well-demarcated, dura-based and contrast-enhancing mass measuring 4 cm in greatest dimension in the right superior frontoparietal region (Figure 1). The tumor was surgically removed subsequently
Figure 1.
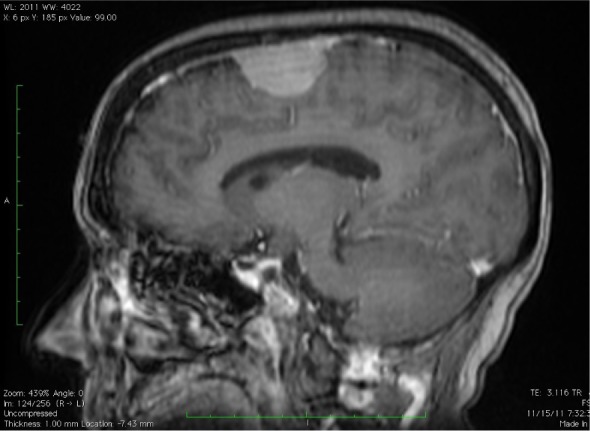
The T1-weighted MRI with contrast demonstrates a well-demarcated, dura-based and contrast-enhancing mass lesion of 4 cm in greatest dimension in the right superior frontoparietal region.
2.2. Macroscopy and microscopy
The excised brain specimen was grossly examined after fixation in 10% buffered formalin. The tissue was then embedded in paraffin. Sections with 4 µm thickness were generated. The sections were stained with hematoxylin and eosin as well as utilizing a immunohistochemical technique, an avidinbiotin-complex immunoperoxidase method. The immunohistochemical study used antibodies against epithelial membrane antigen (EMA), CD4, CD68, S-100 and Ki67. The tissue sections were blocked with 0.3% hydrogen peroxidase, washed in PBS for 30 minutes, and then mounted using 1% goat normal serum in PBS for 30 minutes. Subsequently, the primary specific antibody was added at a dilution of 1:100, and the sections were incubated at 4°C overnight. The stained sections were reviewed by two pathologists.
2.3. Results
Gross pathologic examination revealed multiple fragments of tan tissue. Under the microscope, the tumor showed atypical meningothelial proliferation with extensive and multifocal histiocytic infiltration (Figure 2A). The histiocytic component constituted approximately half of the entire tumor. There were no high-grade changes such as frequent mitoses, necrosis and brain parenchymal invasion in the specimen. Immunohistochemically, both meningothelial cells and histiocytic cells showed immunoreactivity to EMA (Figure 2B). The histiocytes also expressed both CD68 (Figure 2C) and CD4 (Figure 2D). In addition, there were few scattered S100-positive histiocytes throughout the tumor (Figure 2E). Proliferative index highlighted by Ki67 immunostain was 1.6%. Based on the morphologic and immunohistochemical features of this tumor, a diagnosis of histiocytic meningioma, WHO Grade I, was made.
Figure 2.
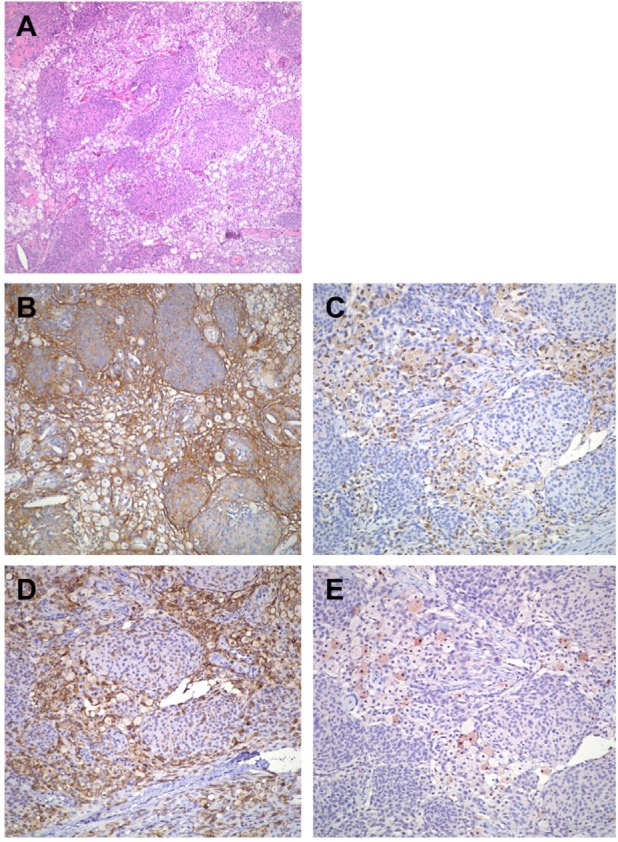
The tumor exhibits atypical meningothelial proliferation with extensive and multifocal histiocytic infiltration. The histiocytic component constitutes approximately half of the entire tumor (A). Both meningothelial cells and histiocytic cells show immunoreactivity to EMA (B). The histiocytic cells are positive for CD68 (C) and CD4 (D). Scattered S100-positive histiocytes are seen throughout the tumor (E). (A: hematoxylin and eosin stain, 100×; B: EMA stain, 200×; C: CD68 stain, 200×; D: CD4 stain, 200×; E: S100 stain, 200×).
3. Discussion
We present a case of a meningothelial meningioma that contained a significant amount of histiocytes. The biological origin of histiocytic cells in meningioma is not clear. There are two major hypotheses on the development of such tumors. The first is that the histiocytic cells represent a metaplastic change. Immunohistochemical and ultrastructural studies demonstrated that the histiocytes express epithelial membrane antigen and have ultrastructural features similar to the meningothelial cells (1,2). This theory considers that the histiocytic changes are secondary to the metabolic abnormality of neoplastic meningothelial cells, which results in cellular degeneration. In our case, all of the histiocytic cells express EMA, which signifies that they may share the same cell origin with meningothelium. An alternate hypothesis is that the histiocytic cells originate from monocytic lineage. A dura-based xanthogranuloma was reported in the literature by Husain et al (3). The histiocytes in that tumor expressed monocytic/histiocytic biomarkers CD68 and S100, but not meningothelial biomarker EMA, which indicates that the histiocytes derived from monocytic origin rather than meningothelium. Furthermore, Ikota et al (4) described a case they deemed a “xanthomatous meningioma” that exhibited a typical meningothelial meningioma with xanthomatous changes. In their case, some of the xanthomatous cells expressed EMA, while others were only positive for the histiocytic biomarkers, suggesting that both metaplasia and monocytic origination might play roles in the pathogenesis of that tumor. However, in this case, it is yet to be determined whether this histiocytic infiltration is neoplastic or inflammatory. It is worthy to note that our patient had a history of head trauma four years prior to this presentation. It has been known that xanthomas can be secondary to trauma (5). Perhaps, traumatic events or other tissue insults facilitate the histiocytic infiltration in otherwise typical meningiomas.
Histiocytic meningioma should be distinguished from other primary intracranial tumors such as microcystic meningioma and hemangioblastoma as well as from other intracranial histiocytic disorders such as xanthoma and Rosai-Dorfman disease. Microcystic meningiomas are generally hypocellular tumors characterized by a lacey and vacuolated appearance due to both clear cytoplasm and extracellular fluid filled spaces (6). The cytoplasm of these tumor cells is evenly clear and not foamy as in histiocytic meningioma. Furthermore, the neoplastic cells of microcystic meningiomas are reactive for EMA but not CD68. In contrast to histiocytic meningiomas that have two distinct cell populations, meningothelial cells and histiocytes, hemangioblastomas are composed of vascular and stromal components. The stromal component is characteristic of large and vacuolated stromal cells that are morphologically indistinguishable from foamy histiocytes. However, the stromal cells express inhibin and brachyury that are not expressed in the histiocytes of histiocytic meningiomas. In addition, EMA is not expressed in hemangioblastomas (7). Dura-based xanthomas or xanthogranulomas have been described as either primary disease or secondary to Histiocytosis X (8). They are characterized by nodular encapsulated infiltrates of histiocytes with lipid-filled clear cytoplasm mixed with small lymphocytes. However, a typical neoplastic meningothelial component is not present. Intra-cranial Rosai-Dorfman disease (RDD) is rare and may occur in the presence or absence of lymph node involvement and present as a dural mass (9). RDD is characterized by a polymorphic infiltration of histiocytes, lymphocytes and plasma cells. Emperipolesis, the hallmark of this disease, is often present. The histiocytes in RDD only express histiocytic biomarkers, but not epithelial markers, indicating their non-meningothelial origin. Although rare, it is important to be aware of histiocytic meningioma when encountering a dura-based lesion with infiltrates of histiocytic cells, because different histiocytic or histiocyte-containing diseases have different pathogenesis and prognosis and they require different treatment.
The WHO classifies the meningiomas containing a non-epithelial component such as fat, bone, cartilage or myxoid tissue as metaplastic meningioma (10). Whether the histiocytic meningioma should be grouped into the metaplastic meningioma category or considered as an independent entity needs more cases for further investigation. Since not all histiocytes in this type of tumor have the same biological features as meningothelial cells, we believe that it may be reasonable to separately classify this group of tumors as histiocytic meningioma. Our case does not exhibit atypical or anaplastic histopathological features and is graded as WHO grade I.
References
- 1. Kepes JJ. Lipidized meningothelial tumor cells in “xanthomatous” meningioma express macrophage antigen. J Neuropathol Exp Neurol. 1994; 53:384-388 [DOI] [PubMed] [Google Scholar]
- 2. Taraszewska A, Matyja E, Bogucki J. Xanthomatous changes in atypical and anaplastic meningiomas. Light and electron microscopic investigations. Folia Neuropathol. 2000; 38:125-134 [PubMed] [Google Scholar]
- 3. Husain S, Alkhalidi HM, Raddaoui E. A 38-year-old woman with a dural based lesion. Brain Pathol. 2012; 22:433-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikota H, Nakazato Y. A case of metaplastic meningioma with extensive xanthomatous change. Neuropathology. 2008; 28:422-426 [DOI] [PubMed] [Google Scholar]
- 5. Walker EE, Musselman MM. Xanthomas at sites of infection and trauma. Arch Surg. 1967; 94:39-40 [DOI] [PubMed] [Google Scholar]
- 6. Manwaring J, Ahmadian A, Stapleton S, Gonzalez-Gomez I, Rodriguez L, Carey C, Tuite GF. Pediatric microcystic meningioma: A clinical, histological, and radiographic case-based review. Childs Nerv Syst. 2013; 29:361-365 [DOI] [PubMed] [Google Scholar]
- 7. She DJ, Xing Z, Liu Y, Cao DR. Supratentorial hemangioblastomas: Three case reports and review of the literature. Clin Neuroradiol. 2013; 23:243-247 [DOI] [PubMed] [Google Scholar]
- 8. Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002; 33:1211-1226 [DOI] [PubMed] [Google Scholar]
- 9. Zhang JT, Tian HJ, Lang SY, Wang XQ. Primary intracerebral Rosai-Dorfman disease. J Clin Neurosci. 2010; 17:1286-1288 [DOI] [PubMed] [Google Scholar]
- 10. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97-109 [DOI] [PMC free article] [PubMed] [Google Scholar]


