Summary
Fibrodysplasia ossificans progressiva (FOP) is a disabling heritable disorder of connective tissue characterized by progressive heterotopic ossification in various extraskeletal sites. Early correct diagnosis of FOP is important to prevent additional iatrogenic harm or trauma. Congenital malformation of the great toes is a well-known diagnostic clue, but some patients show normal-appearing great toes. The thumb shortening and cervical spine abnormalities are other skeletal features often observed in FOP. This study aimed to address the quantitative assessment of these features in a cohort of patients with FOP, which potentially helps early diagnosis of FOP. Radiographs of the hand and cervical spine were retrospectively analyzed from a total of 18 FOP patients (9 males and 9 females) with an average age of 13.9 years (range 0.7–39.3 years). The elevated ratio of the second metacarpal bone to the distal phalanx of the thumb (> +1SD) was a consistent finding irrespective of the patient's age and gender. Infant FOP patients, in addition, exhibited an extremely high ratio of the second metacarpal bone to the first metacarpal bone (> +3SD). The height/depth ratio of the C5 vertebra increased in patients over 4 years of age (> +2SD). Additionally, the ratio of (height+depth) of the C5 spinous process to the C5 vertebral depth was markedly elevated in young patients (> +2SD). We quantitatively demonstrated the hand and cervical spine characteristics of FOP. These findings, which can be seen from early infancy, could be useful for early diagnosis of FOP even in patients without great toe abnormalities.
Keywords: Fibrodysplasia ossificans progressiva, early diagnosis, radiographic characteristics
1. Introduction
Fibrodysplasia ossificans progressiva (FOP) is a severely disabling genetic disorder of connective tissues characterized by congenital malformations of the great toes and progressive heterotopic ossification (HO) in various extraskeletal sites including muscles, tendons, ligaments, fascias, and aponeuroses. FOP is caused by a recurrent activating mutation (c.617G > A, p.R206H) in the gene encoding activin receptor IA/activin-like kinase 2 (ACVR1/ALK2), a bone morphogenetic protein (BMP) type I receptor (1). HO typically begins to form during the first decade of life preceded by painful soft tissue swelling and inflammation (flareups), which are sometimes mistaken for aggressive fibromatosis or musculoskeletal tumors. Surgical resection of HO leads to explosive new bone formation (2). Since there is no definitive treatment to prevent progressive HO in FOP to date (3), early correct diagnosis is necessary to maintain their mobility by preventing additional iatrogenic harm (4).
Malformations of the great toes, such as hallux valgus, deformed proximal phalanges and shortened first metatarsal bones, are well-known pre-osseous features of FOP (5). A reported incidence of these deformities is 95%, suggesting that there exists rare FOP cases without the great toe abnormalities (6). We demonstrated additional early radiographic signs of FOP including shortening of the first metacarpal bones and hypertrophy of the posterior element of the cervical spine (7). Clinical awareness of these deformities can aid clinicians in making early diagnosis of FOP, but quantitative assessment of these deformities has not yet been determined.
In this study, we retrospectively examined radiographs of the hand and cervical spine in FOP patients and demonstrated various abnormal radiographic parameters helpful for early diagnosis of this specific disorder.
2. Materials and Methods
2.1. Demographics
This study represents a retrospective case-control study consisting of Japanese FOP patients followed up at health care facilities where members of the Research Committee on Japanese Fibrodysplasia Ossificans Progressiva practiced. After approval from the Institutional Review Board of the Nagoya University Hospital, we collected the hand and/or cervical spine radiographs from 18 FOP patients (9 males and 9 females) with an average age of 13.9 years (range 0.7–39.3 years) at the time of this study. The patients were diagnosed clinically and radiographically based on various characteristic findings of FOP including deformities of the great toes, extraskeletal HO, joint contractures, cervical fusions, broad femoral necks, and osteochondroma-like lesions. Molecular testing was performed on fourteen patients. Thirteen showed the common ACVR1/ALK2 mutation within the glycine/serine-rich regulatory (GS) domain (c.617G > A, p.R206H), and one patient had an atypical mutation within the protein kinase domain (c.774G > T, p.R258S). Molecular studies were not conducted for the remaining 4 patients who showed characteristic skeletal features of FOP. We examined anteroposterior (AP) radiographs of the hands and lateral radiographs of the cervical spine in each individual. The earliest hands and cervical spine films were analyzed using image processing and analysis software ImageJ®.
2.2. Radiographic assessment of the hand
According to the measurement method by Poznanski et al. (8), the length of each phalanx and metacarpal bone was measured. In brief, the tangent lines were drawn at both ends of each bone, which were perpendicular to the bone axis, and a bone length was defined as the distance between these two lines (Figure 1). We measured a length of the distal (D1) and proximal (P1) phalanges of the thumb as well as that of the first and second metacarpal bones (MET1 and MET2), and calculated the following bone length ratios, MET2/MET1, MET2/P1, MET2/D1, MET1/P1, MET1/D1, and P1/D1. Radiographs of both hands from one patient were separately analyzed to obtain the average value of the measurements. Reference ranges of these measurements in different ages and genders were used based on the literature from Poznanski et al. (8). The control data of these measurements in infant (n = 21) were determined by the radiographic database in Nagoya University Hospital.
Figure 1.
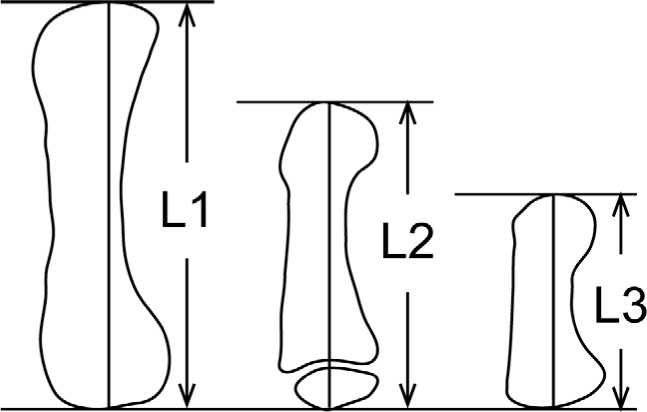
A schematic diagram illustrating the measurement method of bone length in the hand. Bone length was defined as the distance between the tangents drawn to each end of the bone, which were perpendicular to the bone axis. The entire bone length was measured for adults (L1), children (L2), and infants (L3).
2.3. Radiographic assessment of the cervical spine
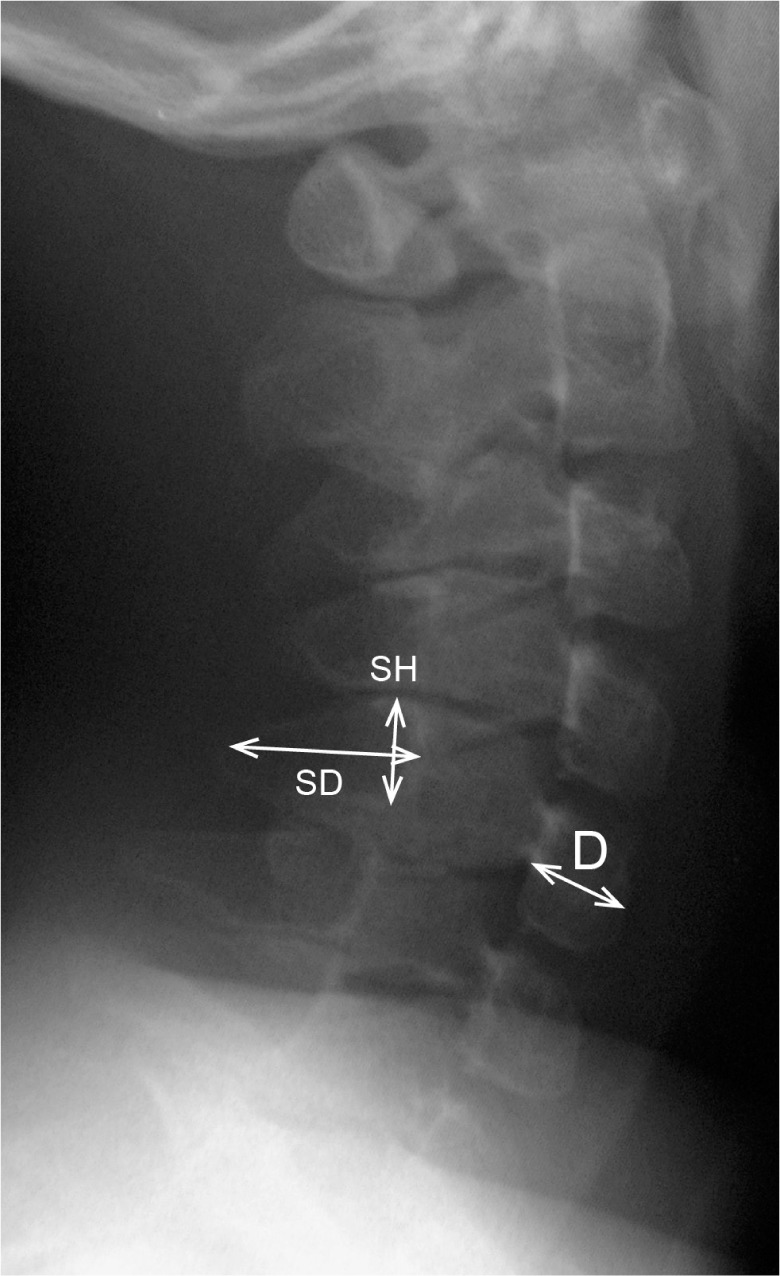
According to the measurement method proposed by Remes et al. (9), the height and depth of the C5 vertebral body were measured. Briefly, vertebral body height (H) was measured at the midpoint of the vertebra, perpendicular to the lower end plate. The vertebral body depth (D) was measured at the midpoint of the body from the anterior wall to the posterior wall (Figure 2). The H/D ratios of the C5 vertebra were then calculated and compared to normal reference values established by Remes et al. in different age and gender groups (9). In addition, we measured the height and depth of the C5 spinous process. The height of the spinous process (SH) was defined as the distance from the cranial to caudal margin at the junction of the spinous process and lamina. The depth of spinous process (SD) was measured from the midportion of the anterior wall to that of the posterior rim demarcating a thick cortex shadow (Figure 2). The sum of SH and SD measurements was used for the evaluation of spinous process size, then the (SH + SD)/D ratio of the C5 vertebra was calculated. Reference values of the (SH + SD)/D ratio were established from the radiographic database of normal controls in Nagoya University Hospital.
Figure 2.
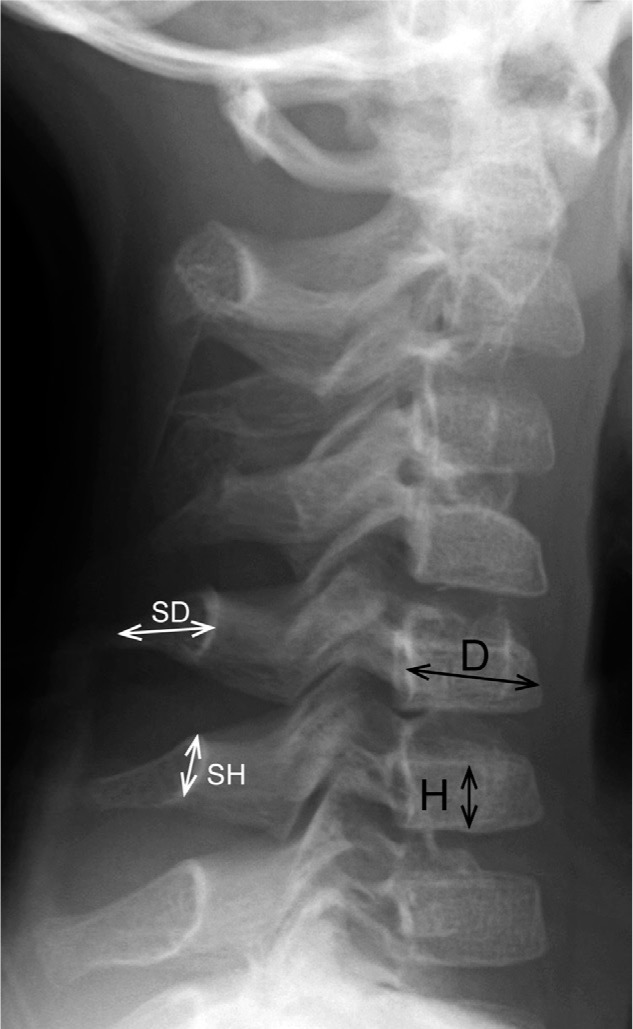
A radiograph depicting the measurements of the bone length in the cervical spine. The height (H) and depth (D) of the C5 vertebral body was measured at the midportion of the body. The height of the C5 spinous process (SH) was defined as the distance from the cranial to the caudal rim at the juxta-laminar zone. The depth of the spinous process (SD) was measured from the midpoint of the anterior wall to that of the posterior rim.
3. Results
3.1. Characteristics of the study cohort
Patients' characteristics and quantitative indices of the measurements are shown in Table 1. Deviation of the bone length ratios in the hand and cervical spine was calculated based on age-matched reference values.
Table 1. Characteristics and quantitative indices for the study population.
| Patient | Sex | ALK2 mutation | Age at X-ray (yrs) |
Deviation of the bone length ratios (SD) |
|||
|---|---|---|---|---|---|---|---|
| Hand/Cervical spine | MET2/D1 | MET2/D1 | H/D | (SH+SD)/D | |||
| 1 | M | R206H | 0/0 | 1.0 | 1.0 | 0.6 | 0.1 |
| 2 | M | R206H | 0/0 | 2.4 | 2.4 | 0.6 | 7.1 |
| 3 | M | R206H | 1/3 | 3.1 | 3.1 | 0.9 | 2.8 |
| 4 | F | R206H | 5/6 | 2.8 | 2.8 | 3.3 | 8.3 |
| 5 | M | R206H | 8/7 | 6.2 | 6.2 | 2.8 | 3.7 |
| 6 | M | R206H | 12/18 | 4.1 | 4.1 | 1.9 | 1.5 |
| 7 | F | R206H | 17/17 | 4.0 | 4.0 | 4.1 | NA |
| 8 | F | R206H | 20/NA | 2.2 | 2.2 | NA | NA |
| 9 | M | R206H | 29/NA | 2.7 | 2.7 | NA | NA |
| 10 | M | R206H | 34/NA | 3.5 | 3.5 | NA | NA |
| 11 | F | R206H | 36/NA | 1.0 | 1.0 | NA | NA |
| 12 | M | R206H | 39/16 | 1.9 | 1.9 | 3.0 | 1.8 |
| 13 | F | R206H | NA/18 | NA | NA | 0.6 | 2.4 |
| 14 | F | R258S | 14/14 | 1.7 | 1.7 | 4.9 | NA |
| 15 | M | ND | NA/4 | NA | NA | 3.2 | 8.8 |
| 16 | F | ND | NA/8 | NA | NA | 9.2 | 7.9 |
| 17 | F | ND | NA/16 | NA | NA | 4.4 | NA |
| 18 | F | ND | 5/5 | 5.3 | 5.3 | 5.3 | 5.4 |
M denotes male; F, female; ND, not determined; NA, not applicable; SD, standard deviation.
3.2. Radiographic characteristics of the hand
Mean and standard deviation of the MET2/D1 and MET2/MET1 ratio in control infants (n = 21) are 2.9 ± 0.29 and 1.64 ± 0.08, respectively. Twenty-six hand radiographs from 14 patients (8 males and 6 females) were available. Regardless of age and gender, all FOP patients showed a MET2/D1 ratio larger than +1SD of normal controls (Figure 3A and 3B). In infant patients without an epiphyseal ossification center of the first metacarpal bone, the MET2/MET1 ratio was extremely large (> +3SD of normal controls) (Figure 4A and 4B). The MET2/P1 ratio was higher in infant patients, but it scattered around the mean value with increasing age (data not shown). There were no characteristic features in the values of the MET1/P1, MET1/D1, and P1/D1 ratios in FOP patients, although the MET1/P1 and MET1/D1 ratios were relatively small (< -1SD of normal controls) in infant FOP patients (data not shown).
Figure 3.
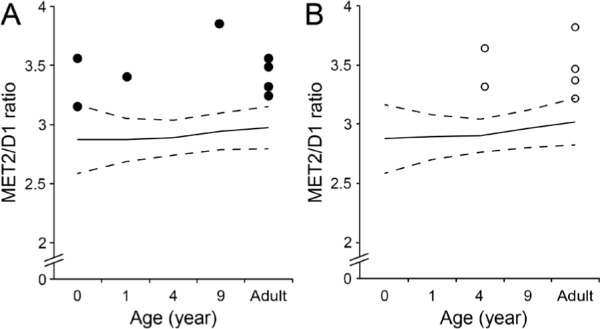
Scatter plots showing the bone length ratio of the second metacarpal bone (MET2) to the distal phalanx of the thumb (D1) in male (A) and female (B) patients with FOP. Solid and dash lines denote the normal value and the standard deviation (SD) of the MET2/D1 ratio, respectively.
Figure 4.
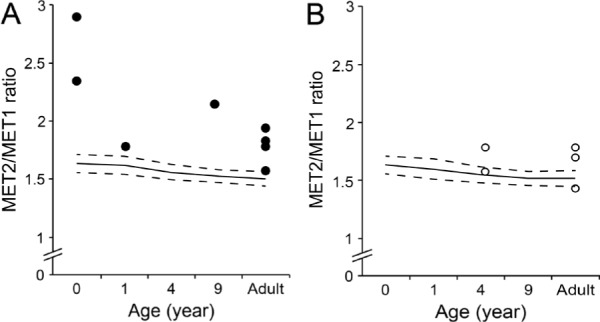
Scatter plots showing the bone length ratio of the second metacarpal bone (MET2) to the first metacarpal bone (MET1) in male (A) and female (B) patients with FOP. Solid and dash lines denote the normal value and the standard deviation (SD) of the MET2/MET1 ratio, respectively.
3.3. Radiographic characteristics of the cervical spine
Reference values of the (SH + SD)/D ratio of the C5 vertebra are shown in Table 2. There were 14 (7 males and 7 females) cervical spine radiographs available for analysis. Among them, three radiographs were excluded from analysis of the (SH + SD)/D ratio for insufficient resolution. The H/D ratio of the C5 vertebra exceeded +2SD of normal controls in patients over 4 years of age except one female adult patient (Figure 5A and 5B). Similarly, the (SH + SD)/D ratio of the C5 vertebra was larger than +2SD of normal controls in young patients except one male infant (Figure 6).
Table 2. Mean and standard deviation of normal controls for the (SH+SD)/D ratio of the C5 vertebra.
| Age group | <1 | 1–2 | 2–3 | 3–4 | 4–5 | 5–6 | 6–7 | 7–8 | 8–9 | 9–10 | 10–11 | 11–12 | 12–13 | 13–14 | 14–15 | 15–16 | 16–17 | 17–18 | 18–19 | 19–20 | 20–21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 1.05 | 1.10 | 1.09 | 1.20 | 1.21 | 1.43 | 1.37 | 1.47 | 1.33 | 1.47 | 1.50 | 1.53 | 1.51 | 1.57 | 1.69 | 1.86 | 1.76 | 1.73 | 1.71 | 1.78 | 1.86 |
| SD | 0.13 | 0.15 | 0.15 | 0.13 | 0.11 | 0.18 | 0.18 | 0.17 | 0.19 | 0.18 | 0.16 | 0.18 | 0.20 | 0.19 | 0.12 | 0.16 | 0.22 | 0.22 | 0.23 | 0.24 | 0.25 |
| N | 11 | 21 | 17 | 13 | 6 | 13 | 25 | 19 | 20 | 17 | 16 | 17 | 14 | 20 | 16 | 21 | 23 | 31 | 28 | 38 | 20 |
SD denotes standard deviation; N, number of control subjects; SH, height of the spinous process; SD, depth of the spinous process; D, depth of the vertebral body.
Figure 5.
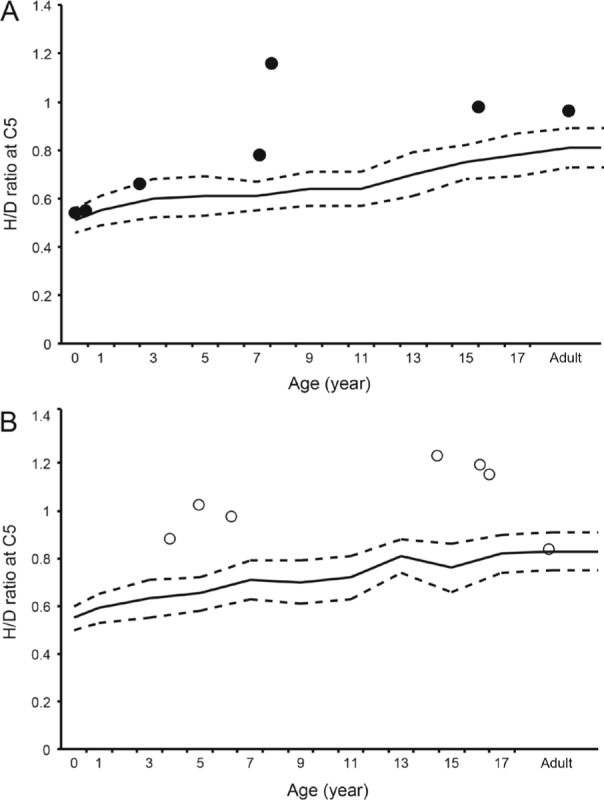
Scatter plots showing the bone length ratio of the C5 vertebral height (H) to depth (D) in male (A) and female (B) patients with FOP. Solid and dashed lines denote the normal value and the standard deviation (SD) of the H/D ratio, respectively.
Figure 6.
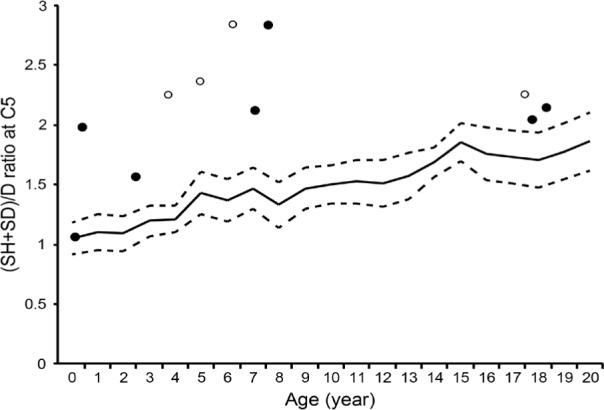
Scatter plots showing the bone length ratio of the C5 spinous process height (SH) + depth (SD) to the C5 vertebral depth (D). Solid and open circles indicate male and female, respectively. Solid and dashed lines denote the normal value and the standard deviation (SD) of the (SH+SD)/D ratio, respectively.
4. Discussion
In the present study, we quantitatively proved the hand and cervical spine abnormalities in FOP including shortened thumbs as well as tall and narrow vertebral bodies and hypertrophic posterior elements of the cervical spine (7,10). Especially in young patients, shortening of the first metacarpal bone and enlargement of the cervical spinous processes were pathognomonic findings useful for early diagnosis of FOP before the appearance of HO.
Previous studies have reported that thumb shortening was seen in 50% of FOP patients (6). In the present study, all patients had a MET2/D1 ratio larger than +1SD of normal controls, and 85% (11/13) of the patients showed an increased MET2/MET1 ratio. The thumb shortening, therefore, seems to be more common than previous reports in FOP. Furthermore, an extremely high MET2/MET1 ratio in infant patients suggested that disproportionate shortening of the first metacarpal bone was an important early radiographic finding in FOP (Figure 7).
Figure 7.
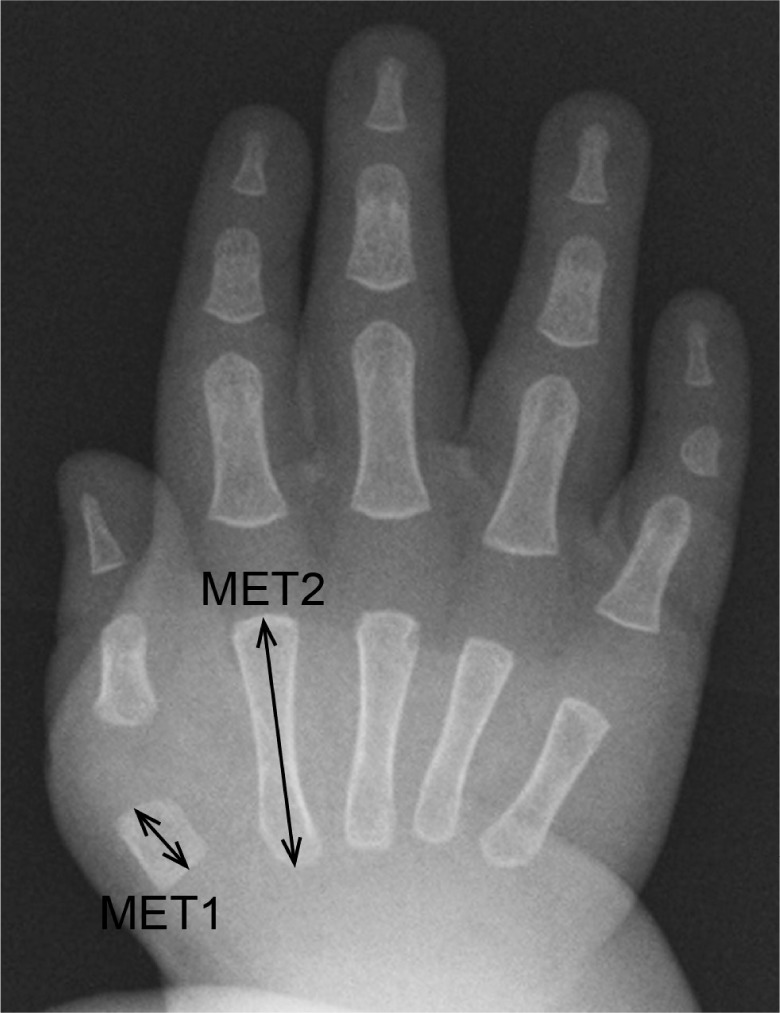
An anteroposterior radiograph of the right hand of Patient 1 at the age of eight months showing marked shortening of the first metacarpal bone. The MET2/MET1 ratio and the corresponding SD value is 2.9 and 16.3, respectively.
It is an intriguing feature of FOP that thumb morphogenesis is exclusively disrupted in the development of digit formation (11). The thumb is the last digit in the autopod to form, and it is different from other digits in terms of its relative position, shape, size, and number of phalanges. These unique thumb identities may be attributed to the expression profile of HoxD genes, which are pivotal transcriptional factors regulating limb patterning and growth (12). All four HoxD10 to D13 genes are expressed in the future digit II-V area in the autopod during the hand plate formation, whereas sole expression of the HoxD13 gene in the presumptive digit I area is of great significance (13). Mutations in the homeodomain of the HoxD13 gene cause brachydactyly type D that is characterized by variable shortening of the distal phalanx of the thumb. This mutated HoxD13 proteins responsible for its decreased affinity for the double-stranded DNA target containing a cognitive sequence of the homeodomain (14). Interestingly, previous research has revealed that BMP signaling-dependent Smad1/4 proteins prevented HoxD10 and HoxD13 from binding to DNA targets (15). Constitutively-activated BMP signaling in FOP thus is likely to impair HoxD13-mediated transcriptional regulation by direct interactions between BMP-induced Smads and HoxD13. Mesenchymal condensation and chondrocyte proliferation of the presumptive digit I area could be suppressed by down-regulated HoxD13 function, whereas in presumptive digits II to V areas, it could be preserved by compensating expressions of other HoxD genes (HoxD11 and HoxD12). Dysregulated BMP signal transduction during embryogenesis seems to cause relative shortening of the first metacarpals and distal phalanges of the thumb in FOP.
More than 90% of adult FOP patients showed fusion of the facet joints, which is a type of orthotopic ossification (6). To our knowledge, however, there are no reports delineating the precise prevalence of tall and narrow vertebral bodies and enlarged posterior elements of the cervical vertebrae. Here we demonstrated that the H/D and (SH + SD)/D ratios in the C5 vertebrae were larger than +2SD of normal values in 64% and 73% of patients, respectively (Figure 8). In addition to neck stiffness, which seemed to be an important early clinical sign before the appearance of HO (6), tall and narrow vertebrae and hypertrophic spinous processes of the cervical spine are radiographic characteristics in young FOP patients.
Figure 8.
A lateral radiograph of the cervical spine of Patient 16 at the age of eight years showing enlarged spinous process of the C5 vertebra. The (SH+SD)/D ratio and the corresponding SD value is 2.8 and 7.9, respectively.
In a previous in vivo study, genetically-engineered overexpression of BMP-2/4 both dorsally and laterally to the neural tube manifested combined phenotypes of hypertrophic spinous processes and large deletion of the lateral and ventral parts of vertebral bodies (16). Thus, mesenchymal condensations at the paraxial mesoderm in FOP, where BMP-2 signaling is aberrantly activating, could be responsible for both enlarged spinous processes and relatively tall vertebral bodies.
The common ACVR1/ALK2 mutation (c.617G > A, p.R206H) shows a homogeneous phenotype including congenital malformation of the great toes and the skeletal features in the thumb and cervical spine (17). In contrast, several atypical mutations in the ALK2/ACVR1 gene, such as L196P, R258S, R375P, G328R, and P197_F198 del insL, have been identified in patients who showed normal-appearing great toes (18). In this study, one patient (Patient 14) with an atypical mutation (c.774G > C, p.R258S) showed normal-appearing great toes. She also lacked the shortened thumb but exhibited exceptionally tall and narrow vertebral bodies. Another patient (Patient 4) who showed neither malformed great toes nor shortening of the first metacarpal bone also manifested distinctive features of the cervical spine in spite of the common ACVR1/ALK2 mutation. We believe that radiographic characteristics of the cervical spine are potent diagnostic clues for FOP especially in cases without typical deformities of the great toes.
Acknowledgements
This work was supported partly by Research Committee on Fibrodysplasia Ossificans Progressiva from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006; 38:525-527 [DOI] [PubMed] [Google Scholar]
- 2. Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005; 116:e654-e661 [DOI] [PubMed] [Google Scholar]
- 3. Kitoh H, Achiwa M, Kaneko H, Mishima K, Matsushita M, Kadono I, Horowitz JD, Sallustio BC, Ohno K, Ishiguro N. Perhexiline maleate in the treatment of fibrodysplasia ossificans progressiva: An open-labeled clinical trial. Orphanet J Rare Dis. 2013; 8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: Diagnosis, management, and therapeutic horizons. Pediatr Endocrinol Rev. 2013; 10 (Suppl 2):437-448 [PMC free article] [PubMed] [Google Scholar]
- 5. Nakashima Y, Haga N, Kitoh H, Kamizono J, Tozawa K, Katagiri T, Susami T, Fukushi J, Iwamoto Y. Deformity of the great toe in fibrodysplasia ossificans progressiva. J Orthop Sci. 2010; 15:804-809 [DOI] [PubMed] [Google Scholar]
- 6. Kaplan FS, Glaser DL, Shore EM, Deirmengian GK, Gupta R, Delai P, Morhart P, Smith R, Le Merrer M, Rogers JG, Connor JM, Kitterman JA. The phenotype of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005; 3:183-188 [Google Scholar]
- 7. Mishima K, Kitoh H, Katagiri T, Kaneko H, Ishiguro N. Early clinical and radiographic characteristics in fibrodysplasia ossificans progressiva: A report of two cases. J Bone Joint Surg Am. 2011; 93:e52. [DOI] [PubMed] [Google Scholar]
- 8. Poznanski AK, Garn SM, Holt JF. The thumb in the congenital malformation syndromes. Radiology. 1971; 100:115-129 [DOI] [PubMed] [Google Scholar]
- 9. Remes VM, Heinanen MT, Kinnunen JS, Marttinen EJ. Reference values for radiological evaluation of cervical vertebral body shape and spinal canal. Pediatr Radiol. 2000; 30:190-195 [DOI] [PubMed] [Google Scholar]
- 10. Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008; 22:191-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: Clinical and genetic aspects. Orphanet J Rare Dis. 2011; 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oberg KC. Review of the molecular development of the thumb: Digit primera. Clin Orthop Relat Res. 2014; 472:1101-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deschamps J. Tailored Hox gene transcription and the making of the thumb. Genes Dev. 2008; 22:293-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson D, Kan SH, Oldridge M, Trembath RC, Roche P, Esnouf RM, Giele H, Wilkie AO. Missense mutations in the homeodomain of HOXD13 are associated with brachydactyly types D and E. Am J Hum Genet. 2003; 72:984-997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Nie S, Chang C, Qiu T, Cao X. Smads oppose Hox transcriptional activities. Exp Cell Res. 2006; 312:854-864 [DOI] [PubMed] [Google Scholar]
- 16. Monsoro-Burq AH, Duprez D, Watanabe Y, Bontoux M, Vincent C, Brickell P, Le Douarin N. The role of bone morphogenetic proteins in vertebral development. Development. 1996; 122:3607-3616 [DOI] [PubMed] [Google Scholar]
- 17. Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J, Sillence D, Zackai E, Ravitsky V, Zasloff M, Ganguly A, Shore EM. Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics. 2008; 121:e1295-e1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaplan FS, Xu M, Seemann P, et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009; 30:379-390 [DOI] [PMC free article] [PubMed] [Google Scholar]


