Summary
Interferon-induced transmembrane protein 5 (IFITM5) is an osteoblast-specific membrane protein that plays an important role in the mineralization of the matrix in mature osteoblasts. However, understanding of the regulatory mechanism of IFITM5 expression is limited. Emerging evidence indicates that microRNAs (miRNAs) act as pivotal regulators in various biological processes including osteoblast proliferation and differentiation. This study aimed to investigate the impact of miRNAs on IFITM5 expression. Bioinformatic analyses predicted that miR-762 would be a potential regulator of IFITM5. A Dual-Luciferase Reporter Assay System indicated that miR-762 could bond with the 3′untranslated region (3′UTR) of IFITM5 via wild-type or mutant recombinant vectors and Western blotting verified that miR-762 negatively regulated IFITM5 expression. Collectively, these data indicate that miR-762 is a novel regulator of IFITM5 and that it suppresses the expression of IFITM5 in Saos-2 cells.
Keywords: IFITM5 gene, miR-762, mineralization, target gene
1. Introduction
MicroRNAs (miRNAs) are an abundant class of endogenous, small (22-nucleotide), single strand, and noncoding RNA molecules that can bind to the 3′untranslated region (3′UTR) of target mRNA and regulate the stability and translation of mRNA, resulting in either inhibition of translation or degradation of the target mRNA (1–3). MiRNAs play essential roles in diverse biological processes, including cell proliferation, differentiation, apoptosis, and tumor oncogenesis (4–6). Recently, numerous studies have revealed that miRNAs play critical roles in osteoblast differentiation. For example, miR-34s reportedly inhibits osteoblast differentiation by targeting special AT-rich sequence-binding protein 2 (SATB2) (7). MiR-210 is upregulated during BMP4-induced osteoblastic differentiation of bone marrow stromal cells, promoting osteoblastic differentiation by downregulating activin A receptor type IB (Acvr1b) expression (8). MiR-141 and miR-200a are involved in preosteoblast differentiation through the translational repression of distal-less homeobox 5 (Dlx5), a bone-generating transcription factor expressed in preosteoblast differentiation (9).
Interferon-induced transmembrane protein 5 (IFITM5) is a member of the interferon-induced transmembrane (IFITM) protein family, of which there are at least four closely related members in humans (IFITM1, -2, -3, and -10) clustered on chromosome 11 (10–12). IFITM5 encodes a 132-amino acid protein that has two transmembrane domains, such that it has extracellular N and C termini and an intracellular loop (13). IFITM5 has an aspartate-rich domain in the C-terminal region, that may be involved in calcium binding (10). Previous studies have confirmed the expression of IFITM5 in bone tissues in humans and that expression increases under culture conditions favoring osteogenic differentiation (14–16). Moreover, IFITM5 is a positive modulator of mineralization according to overexpression and knockdown studies in cultured osteoblasts (13). Little is presently known about regulation of the IFITM5 gene. The aim of the current study was to identify appropriate miRNAs and investigate their impact on IFITM5 expression. In human osteosarcoma Saos-2 cells, IFITM5 expression is closely correlated with differentiation and mineralization in vitro (13), so Saos-2 cells were chosen as a model of pro-mineralizing cells. Results revealed miR-762 can directly target the mineralization-related gene IFITM5 and inhibit its expression, providing a theoretical basis for further study of the mechanisms by which miR-762 regulates the progress of bone mineralization.
2. Materials and Methods
2.1. Cell culture and miRNA mimics transfection
Human osteosarcoma Saos-2 cells, acquired from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), were cultured in McCoy's 5A (Gibco, Carlsbad, CA, USA) supplemented with 15% (v/v) fetal bovine serum (Gibco, Carlsbad, CA, USA). The cells were incubated at 37°C in a humid chamber containing 5% CO2. MiRNA mimics and miRNA negative control (miR-NC) were purchased from a commercial manufacturer (GenePharma, Shanghai, China). Saos-2 cells were seeded in a 6-well plate (2 × 105/well) 24 h before transfection and cells were transfected with miRNA mimics (50 nM) or miR-NC (50 nM) using FuGENE® HD transfection reagent (Promega, Madison, WI, USA) in accordance with the manufacturer's protocol. The empty pmirGLO vector served as a blank control.
2.2. Bioinformatic prediction
MiRNAs can be predicted using a computational approach. First, the potential binding sites in the messenger RNA 3′UTR were identified according to specific base-pairing rules, and second, cross-species conservation requirements were implemented. A predictive search for miRNAs targeting IFITM5 was performed using the programs TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org) and DIANA-microT (http://diana.cslab.ece.ntua.gr).
2.3. Construction of plasmids and luciferase activity assay
IFITM5 3′UTR including the predicted binding site of miR-762 was amplified by a reverse transcriptasepolymerase chain reaction (RT-PCR) (Table 1) and inserted into multiple cloning sites of the T-Vector pMD19 (pMD19-UTR) (Takara Bio, Otsu, Japan) using the SacI and XbaI restriction sites. A site-directed gene mutagenesis kit (Takara Bio) was used to construct a mutant type of miR-762-binding site vector (pMD19-mUTR) with 4 base mutations within the seed region in accordance with the manufacturer's protocol. The pmirGLO dual-luciferase miRNA target expression vector (pmirGLO vector) containing both the firefly luciferase gene and Renilla luciferase gene was purchased from Promega (Madison, WI, USA). The particular restriction enzyme fragment of pMD19-UTR and pMD19-mUTR wsa inserted into 3′UTR down-stream of the firefly luciferase gene of the pmirGLO vector (pmirGLO-UTR and pmirGLOmUTR). Both constructs were confirmed by restriction enzyme digestion and sequencing (Huada, Beijing, China). Saos-2 cells were seeded in a 96-well plate (2 × 104/well) 24 h before transfection. The cells were transfected with 50 nM of miRNA mimics or miR-NC and 1.0 µg/mL of reporter vectors using FuGENE® HD transfection reagent. Luciferase activity was measured 24 h after transfection using a Dual-Glo luciferase assay system (Promega), and firefly luciferase activity was normalized to Renilla luciferase activity. The empty pmirGLO vector served as a blank control, and values for cells with empty pmirGLO vector were set equally to 1 for each comparison. Experiments were performed in triplicate and repeated three times. Data are presented as means ± S.D.
Table 1. Summary of sequences of primers used in this study.
| Usage | Gene | Direction | Sequence(5′→3′) |
| Cloninga | IFITM5 | Forward | CGAGCTCCAGGCTGGGTCCTGATCTGGGGC |
| Reverse | GCTCTAGACTGGAACCAGGCACTTTTAAT | ||
| Mutagenesis | IFITM5 | Forward | TGATCCTGGGGCCCTCCTAATCCAACATGGGCAC |
| Reverse | GGATGGGGCAGGGATGGAGGCCCCACAGAAGGAG | ||
| qRT-PCR | IFITM5 | Forward | TTGATCTGGTCGGTGTTCAG |
| Reverse | GTCAGTCATAGTCCGCGTCA | ||
| GAPDH | Forward | CACCATCTTCCAGGAGC | |
| Reverse | AGTGGACTCCACGACGTA | ||
| miR-762 | Forward | ATTATGGGGCTGGGGCCGGG | |
| U6 snRNA | Forward | CTCGCTTCGGCAGCACA | |
| Reverse | AACGCTTCACGAATTTGCGT |
IFITM5: interferon-induced transmembrane protein 5; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; qRT-PCR: quantitative RT-PCR;
SacI and XbaI sequences were introduced into the cloned PCR product for subsequent subcloning.
2.4. Total RNA extraction and real-time quantitative RT-PCR (qRT-PCR) analysis
RNA was isolated using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer's protocol, and the yield and quality of RNA samples were determined with a NanoDrop 2000c spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Levels of expression were measured using qRT-PCR. For miRNA quantification, the first-strand miRNA-cDNA PCR template was generated from 1.0 µg of total RNA using a miRNA first-strand cDNA synthesis kit (Tiangen, Beijing, China) in accordance with the manufacturer's instructions. Using cDNAs as templates, qRT-PCR was performed with a LightCycler 480 II (Roche, Basel, Switzerland) and miRcute miRNA qPCR detection kit (Tiangen) in accordance with the manufacturer's instructions. Cycling conditions were 1 cycle of 94°C for 2 min and 40 cycles of 94°C for 20 s and 60°C for 34 s. For measurement of the IFITM5 transcript from total RNA, total cDNA was synthesized using a reverse transcription kit (Takara Bio). QPCR was performed using SYBR Green II Master Mix (Roche) and the LightCycler 480 II. Amplification conditions were 95°C for 5 min, followed by 45 cycles of denaturation at 95°C for 10 s, annealing 60°C for 20 s and extensionat 72°C for 30 s. All PCR assays were performed in triplicate. U6 snRNA and GAPDH were used as endogenous controls for miRNA and mRNA, respectively. The sequences of all primers are shown in Table 1. The −ΔΔCt method was used to determine relative quantitation of miRNA and mRNA expression in Saos-2 cells, and the fold change was determined using the formula 2 −ΔΔCt.
2.5. Western blotting
Eight days after transfection with miR-762 mimics or miR-NC, total protein extracts from the cells were homogenized in radio immunoprecipitation assay (RIPA) Lysis buffer (Beyotime, Shanghai, China) supplemented with phenylmethanesulfonyl fluoride (Beyotime, Shanghai, China) on ice for 60 min and then centrifuged at 14,000 g for 15 min at 4°C. The protein concentration was determined using the Bradford method (BioRad Laboratories, Carlsbad, CA, USA). Samples (20 µg) were suspended in Laemmli loading buffer and incubated at 100°C for 6 min. Proteins were separated using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and was then transferred onto a polyvinylidene fluoride (PVDF) membrane and blocked by incubation with 5% low fat milk in TBST (10 mM Tris, 100 mM NaCl, and 0.05% Tween-20) for 1 h. The membranes were incubated overnight at 4°C with the following primary antibodies: rabbit polyclonal anti-human IFITM5 (1:1,000) from Sigma (St. Louis, MO, USA) and rabbit polyclonal anti-human GAPDH (1:2,000) from Proteintech Group, Inc. (Wuhan, Hubei, China). Unbound antibody was removed by washing with TBST buffer three times (10 min/wash). The membranes were then incubated with horseradish peroxide-conjugated secondary antibody for 1 h at room temperature, after which they were washed with TBST buffer three times (10 min/wash). The blots were developed with ECL reagent (Millipore Corporation, Billerica, MA, USA) in accordance with the manufacturer's instructions.
2.6. Statistical analysis
For quantitative data, results are expressed as the mean ± S.D. of n observations. Statistical significance between groups was determined using an unpaired Student's t-test. Statistical significance was defined as p < 0.05.
3. Results
3.1. MiR-762 was predicted to bind to IFITM5 3′UTR
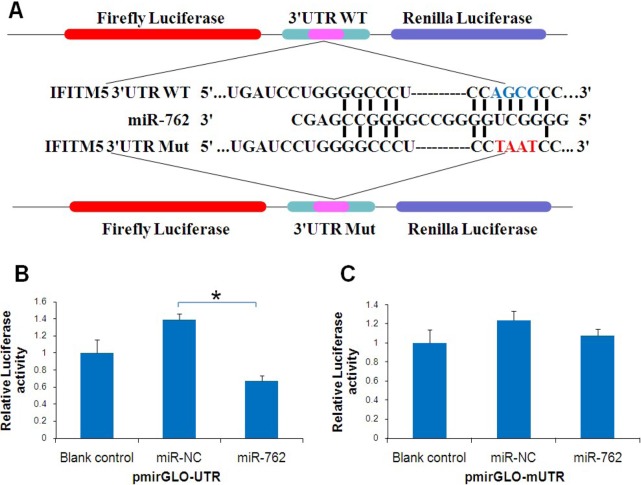
To explore the regulatory mechanism of IFITM5, a regulator of osteoblast differentiation, bioinformatic analyses were performed using TargetScan, miRanda, and DIANA-microT to predict the putative miRNAs binding to IFITM5 3′UTR. Although each program predicted dozens of different miRNAs, the common miRNA they all predicted was miR-762. The programs all predicted that there would be one binding site in IFITM5 3′UTR. Further analysis revealed that 12 bases of the IFITM5 3′UTR gene matched the miR-762 seed sequence, in which 7 bases were highly conserved, suggesting that IFITM5 may be a predicted target gene of miR-762 that is highly conserved (Figure 1A). A recent study found that miR-762 was involved in calcification of human vascular smooth muscle cells (VSMCs) calcification (17). Therefore, miR-762 was selected for further study.
Figure 1.
MiR-762 targets the 3′UTR of the IFITM5 gene in Saos-2 cells. (A) Schematic of the miR-762 putative target sites of IFITM5 3′UTR and alignment of miR-762 with the seed sites of wild-type IFITM5 3′UTR and mutant IFITM5 3′UTR. The 4 mutated nucleotides are red, and the corresponding wild-type nucleotides are blue. (B, C) Luciferase assay. Saos-2 cells were transiently transfected with a pmirGLO, pmirGLO-UTR, or pmirGLO-mUTR vector, each with or without cotransfection with 50 nM of miR-762. After 24 h, reduced luciferase activity was observed after cotransfection of pmirGLO-UTR vector with miR-762 compared with those transfected with miR-NC, but not pmirGLO-mUTR. Data are presented as means ± S.D. from three independent experiments. * p < 0.05 vs. miR-NC.
3.2. MiR-762 directly targeted IFITM5 3′UTR
To confirm that IFITM5 3′UTR was a direct target of miR-762, a pmirGLO-UTR vector containing the miR-762 binding sites was constructed to perform a reporter assay, and pmirGLO-mUTR containing mutant binding sites was used as a control. Both constructs were confirmed by restriction enzyme digestion and sequencing (Figure 2). The luciferase activity of the reporter vectors was assayed 24 h after cotransfection with miR-762 mimics or negative control in Saos-2 cells. When cotransfected with miR-762, the relative luciferase activity of pmirGLO-UTR was significantly suppressed by 52% (Figure 1B) in comparison to cotransfection with the negative control. In addition, the relative luciferase activity did not change when cotransfection was done with miR-762 and pmirGLO-mUTR containing a mutant binding site (Figure 1C), indicating that IFITM5 3′UTR is a direct target of miR-762.
Figure 2.
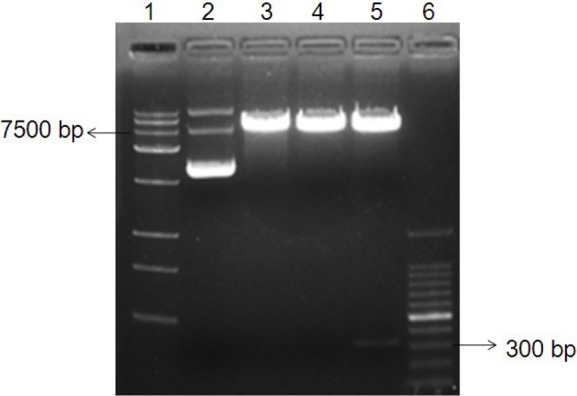
Restriction fragment analysis of the recombinant plasmid pmirGLO-UTR. The extracted recombinant plasmid was digested with two different restriction enzymes (SacI and XbaI) and separated with a 0.8% agarose gel. The linear plasmid pmirGLO (at 7500 bp) and IFITM5 3′UTR (at300 bp) are in lane 5. Lane 1: Trans15K DNA marker; Lane 2: undigested pmirGLO-UTR; Lane 3: SacI digested pmirGLO-UTR; Lane 4: XbaI digested pmirGLO-UTR; Lane 5: SacI and XbaI digested pmirGLO-UTR; Lane 6: 100 bp DNA ladder marker.
3.3. MiR-762 negatively regulated IFITM5 gene expression
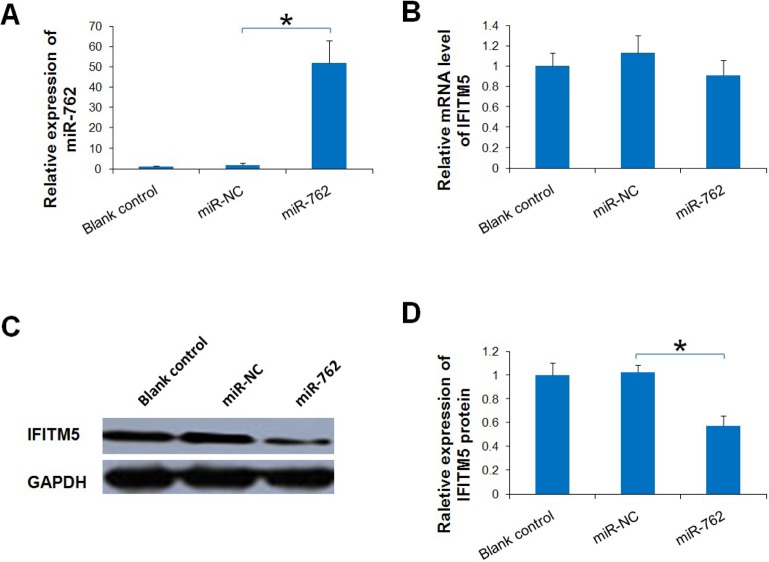
To further confirm that IFITM5 is a target gene for miR-762, qRT-PCR and Western blotting analysis were used to detect the expression of IFITM5 regulated by miR-762 in Saos-2 cells. Levels of miR-762 and IFITM5 mRNA in cells were determined using qRT-PCR. The expression of miR-762 was significantly up-regulated in comparison to miR-NC and the blank control in Saos-2 cells (Figure 3A). However, variations in the level of IFITM5 mRNA were not noted (p > 0.05) (Figure 3B). Interestingly, the protein level of IFITM5 was suppressed by miR-762 mimics (Figures 3C and 3D) in comparison to miR-NC in Saos-2 cells. Taken together, these results suggest that IFITM5 is a target gene of miR-762 and that IFITM5 is down-regulated by miR-762 only at the translational level in Saos-2 cells.
Figure 3.
Forced expression of miR-762 negatively regulated IFITM5 expression at the translational level. (A) Significantly upregulated expression of miR-762 in Saos-2 cells transfected with 50 nM miR-762 mimics was detected with qRT-PCR. U6 snRNA was the internal control gene. (B) MiR-762 did not inhibit the expression of IFITM5 at the mRNA level in Saos-2 cells. The mRNA expression of IFITM5 and internal control GAPDH was detected with qRT-PCR. (C, D) Significantly downregulated expression of IFITM5 at the translational level in Saos-2 cells was detected with Western blotting. The ratio of band intensity is relative to that of GAPDH. The band intensity was measured using ImageJ software. Data are presented as means ± S.D. from three independent experiments. * p < 0.05 vs. miR-NC.
4. Discussion
Bone homeostasis is balanced between bone formation and bone absorption by osteoblasts and osteoclasts, respectively (18). Therefore, study of osteoblast differentiation is essential to provide a better understanding of the skeletal disorders associated with bone mineralization, such as ankylosing spondylitis. MiRNAs display a novel mode of regulating gene expression and may regulate 30%–50% of human gene expression, though miRNAs account for only about 1% of all RNAs (19). Thus, important miRNAs should be identified as potential therapeutic targets for treatment of diseases related to bone mineralization.
Three different biological prediction programs (TargetScan, miRanda, and DIANA-microT) predicted that the 7 seed sequence of miR-762 combined with IFITM5 3′UTR at the same time. The 2–8 nucleotides of miRNA, known as the “seed region”, are thought to be the most important for recognition (20). Comparison of the relative luciferase activity of the wild-type and mutant recombinant vectors to miR-NC revealed that miR-762 combines with IFITM5 3′UTR and then suppresses the expression of the target gene. There was a low level of miR-762 expression in Saos-2 cells and miR-762 was significantly up-regulated after miR-762 mimics were transfected. Furthermore, expression of IFITM5 was significantly down-regulated by over-expressed miR-762 at the translational level and not at the mRNA level. These findings suggest that miR-762 downregulated expression of IFITM5 via imperfect complementarity to IFITM5 3′UTR in Saos-2 cells.
MiR-762 is reportedly involved in tumorigenesis and in the development and progression of diseases such as diabetes and immune system diseases; miR-762 acts by targeting a number of important genes (21–23). Significance analysis of microarrays initially revealed that miR-762 is significantly upregulated in oral squamous cell carcinoma, suggesting that miR-762 plays a role in oral carcinogenesis (21). Recently, the neural precursor cell-enriched miR-762 was found to translationally downregulate adenosyl methionine decarboxylase 1 (Amd1), a key enzyme required for the synthesis of the polyamines spermine and spermidine, as it regulates both embryonic stem cell self-renewal and differentiation into a neural lineage (24). MiR-762 is also reported to be upregulated in human corneal epithelial cells in response to tear fluid and pseudomonas aeruginosa antigens and it negatively regulates the expression of innate host defense genes encoding RNase7 and ST2 (23). More importantly, Gui et al. (17) found that miR-762 disrupts calcium transport, thereby increasing the concentration of intracellular Ca2+ and thus resulting in Pi- and Ca- induced human VSMC calcification both in vivo and in vitro. Interestingly, IFITM5 has an aspartate-rich domain in the C-terminal region that could be involved in calcium binding. IFITM5 overexpression in primary rat osteoblasts was found to result in a 60% increase in Ca2+ uptake (13). Hence, miR-762 is more likely to influence osteoblast mineralization through contact with IFITM5.
At present, two independent studies found that a mutation in the 5′UTR of the IFITM5 gene is the cause of osteogenesis imperfecta (OI) type V (14,25). OI type V is characterized by an autosomal-dominant inheritance pattern, propensity to hyperplastic callus formation, and calcification of the fore-arm interosseous membrane (26). Research has fully demonstrated that IFITM5 plays a major role in human skeletal physiology. Overexpression of IFITM5 in osteoblasts results in increased mineralization in vitro (13) and in the current study a low level of miR-762 in Saos-2 cells increased the expression of IFITM5, which may possibly contribute to hyperplastic callus formation. Furthermore, FK506-binding protein 11 (FKBP11) was identified as the only known binding partner of IFITM5 (27). Recent studies have revealed that the S-palmitoylation on IFITM5 promotes interaction with FKBP11 and cumulatively forms IFITM5-FKBP11-CD81, the prostaglandin F2 receptor negative regulator (FPRP) complex. Formation of this complex also leads to osteoblast-specific increased expression of 5 interferon-induced genes (28–29). Accordingly, speculation is that IFITM5 and FKBP11 might cooperatively regulate bone formation.
In conclusion, the current data indicate that miR-762 is a novel regulator of IFITM5. MiR-762 suppresses the expression of IFITM5 at the translational level in Saos2 cells and probably contributes to the progression of bone mineralization. These findings shed new light on the roles of miRNAs in osteoblast differentiation.
References
- 1. Ambros V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell. 2003; 113:673-676 [DOI] [PubMed] [Google Scholar]
- 2. Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003; 301:336-338 [DOI] [PubMed] [Google Scholar]
- 3. Ambros V. The functions of animal microRNAs. Nature. 2004; 431:350-355 [DOI] [PubMed] [Google Scholar]
- 4. Zhang N, Wei X, Xu L. MiR-150 promotes the proliferation of lung cancer cells by targeting P53. FEBS Lett. 2013; 587:2346-2351 [DOI] [PubMed] [Google Scholar]
- 5. Yin C, Wang PQ, Xu WP, Yang Y, Zhang Q, Ning BF, Zhang PP, Zhou WP, Xie WF, Chen WS, Zhang X. Hepatocyte nuclear factor-4αreverses malignancy of hepatocellular carcinoma through regulating miR-134 in the DLK1-DIO3 region. Hepatology. http://onlinelibrary.wiley.com/doi/10.1002/hep.26573/full (accessed June 14, 2013). [DOI] [PubMed] [Google Scholar]
- 6. Yang G, Wu D, Zhu J, Jiang O, Shi Q, Tian J, Weng Y. Upregulation of miR-195 increases the sensitivity of breast cancer cells to adriamycin treatment through inhibition of Raf-1. Oncol Rep. 2013; 30:877-889 [DOI] [PubMed] [Google Scholar]
- 7. Wei J, Shi Y, Zheng L, Zhou B, Inose H, Wang J, Guo XE, Grosschedl R, Karsenty G. MiR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol. 2012; 197:509-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizuno Y, Tokuzawa Y, Ninomiya Y, Yagi K, Yatsuka-Kanesaki Y, Suda T, Fukuda T, Katagiri T, Kondoh Y, Amemiya T, Tashiro H, Okazaki Y. MiR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Letters. 2009; 583:2263-2268 [DOI] [PubMed] [Google Scholar]
- 9. Itoh T, Nozawa Y, Akao Y. MicroRNA-141 and -200a are involved in bone morpho-genetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J Biol Chem. 2009; 284:19272-19279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siegrist F, Ebeling M, Certa U. The small interferon-induced transmembrane genes and proteins. J Interferon Cytokine Res. 2011; 31:183-197 [DOI] [PubMed] [Google Scholar]
- 11. Hickford D, Frankenberg S, Shaw G, Renfree MB. Evolution of vertebrate interferon inducible transmembrane proteins. BMC Genomics. 2012; 13:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sällman Almén M, Bringeland N, Fredriksson R, Schiöth HB. The dispanins: A novel gene family of ancient origin that contains 14 human members. PLoS One. 2012; 7: e31961-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moffatt P, Gaumond MH, Salois P, Sellin K, Bessette MC, Godin E, de Oliveira PT, Atkins GJ, Nanci A, Thomas G. Bril: A novel bone-specific modulator of mineralization. J Bone Miner Res. 2008; 23:1497-1508 [DOI] [PubMed] [Google Scholar]
- 14. Cho TJ, Lee KE, Lee SK, et al. A single recurrent mutation in the 5′-UTR of IFITM5 causes osteogenesis imperfecta type V. Am J Hum Genet. 2012; 91:343-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Liu H, Titus L, Boden SD. Natural antisense transcripts enhance bone formation by increasing sense IFITM5 transcription. Bone. 2012; 51:933-938 [DOI] [PubMed] [Google Scholar]
- 16. Hanagata N, Takemura T, Monkawa A, Ikoma T, Tanaka J. Phenotype and gene expression pattern of osteoblast-like cells cultured on polystyrene and hydroxyapatite with pre-adsorbed type-I collagen. J Biomed Mater Res A. 2007; 83:362-371 [DOI] [PubMed] [Google Scholar]
- 17. Gui T, Zhou G, Sun Y, Shimokado A, Itoh S, Oikawa K, Muragaki Y. MicroRNAs that target Ca(2+) transporters are involved in vascular smooth muscle cell calcification. Lab Invest. 2012; 92:1250-1259 [DOI] [PubMed] [Google Scholar]
- 18. Hu W, Ye Y, Zhang W, Wang J, Chen A, Guo F. MiR-142-3p promotes osteoblast differentiation by modulating Wnt signaling. Mol Med Rep. 2013; 7:689-693 [DOI] [PubMed] [Google Scholar]
- 19. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005; 120:15-20 [DOI] [PubMed] [Google Scholar]
- 20. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007; 27:91-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu T, Wang XY, Gong RG, Li A, Yang S, Cao YT, Wen YM, Wang CM, Yi XZ. The expression profile of microRNAs in a model of 7,12-dimethyl-benz[a] anthrance-induced oral carcinogenesis in Syrian hamster. J Exp Clin Cancer Res. 2009; 28:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen YQ, Wang XX, Yao XM, Zhang DL, Yang XF, Tian SF, Wang NS. Abated microRNA-195 expression protected mesangial cells from apoptosis in early diabetic renal injury in mice. J Nephrol. 2012; 25:566-576 [DOI] [PubMed] [Google Scholar]
- 23. Mun J, Tam C, Chan G, Kim JH, Evans D, Fleiszig S. MicroRNA-762 is up-regulated in human corneal epithelial cells in response to tear fluid and pseudomonas aeruginosa antigens and negatively regulates the expression of host defense genes encoding RNase7 and ST2. PLoS One. 2013; 8:e57850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang D, Zhao T, Ang HS, Chong P, Saiki R, Igarashi K, Yang H, Vardy LA. AMD1 is essential for ESC self-renewal and is translationally down-regulated on differentiation to neural precursor cells. Genes Dev. 2012; 26:461-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Semler O, Garbes L, Keupp K, Swan D, Zimmermann K, Becker J, Iden S, Wirth B, Eysel P, Koerber F, Schoenau E, Bohlander SK, Wollnik B, Netzer C. A mutation in the 5′-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am J Hum Genet. 2012; 91:349-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, Lalic L, Glorieux DF, Fassier F, Bishop NJ. Type V osteogenesis imperfecta: A new form of brittle bone disease. J Bone Miner Res. 2000; 15:1650-1658 [DOI] [PubMed] [Google Scholar]
- 27. Hanagata N, Li X, Morita H, Takemura T, Li J, Minowa T. Characterization of the osteoblast-specific transmembrane protein IFITM5 and analysis of IFITM5-deficient mice. J Bone Miner Metab. 2011; 29:279-290 [DOI] [PubMed] [Google Scholar]
- 28. Hanagata N, Li X. Osteoblast-enriched membrane protein IFITM5 regulates the association of CD9 with an FKBP11-CD81-FPRP complex and stimulates expression of interferon-induced genes. Biochem Biophys Res Commun. 2011; 409:378-384 [DOI] [PubMed] [Google Scholar]
- 29. Tsukamoto T, Li X, Morita H, Minowa T, Aizawa T, Hanagata N, Demura M. Role of S-palmitoylation on IFITM5 for the Interaction with FKBP11 in Osteoblast Cells. PLoS One. 2013; 8:e75831. [DOI] [PMC free article] [PubMed] [Google Scholar]