Summary
Phenylketonuria (PKU) is a treat-able and prevent-able inborn error of metabolism which leads to severe mental retardation and neurobehavioral abnormalities. A screening program, especially for early detection, combined with a Phe-restricted therapeutic diet can help to control the process of PKU of most patients. The China government has put more emphasis on newborn screening and treatment against PKU, yet by comparing the situation of newborn screening and treatment against PKU in China and the relatively developed countries — United States, United Kingdom and Japan, the newborn screening and treatment against PKU in China is relatively weak and many deficiencies are found. More studies concerning multi-stage target blood Phe concentration criteria, a policy that requires newborn screening has to be taken, better financial support for newborn screening, publicity for newborn screening, and national guidelines for treatment of PKU may be prospects in China and may provide some support for better development of newborn screening and treatment against PKU in China.
Keywords: Phenylketonuria (PKU), newborn screening, treatment, guidelines, policy
1. Introduction
Phenylketonuria (PKU) is an inborn error of metabolism, usually caused by a deficiency of phenylalanine hydroxylase which can lead to mental retardation and neurobehavioral abnormalities (1). The overall incidence of PKU in the world varies widely in different human populations such as 1 in 15,000 births in the United States (2,3), 1 in 10,000 births in United Kingdom (4,5), 1 in 2,600 births in Turkey (6) and fewer than 1 in 100,000 births in Japan (7,8). However, PKU is a treat-able and prevent-able disease (9,10). A screening program, especially early detection, combined with a Phe-restricted therapeutic diet can help to control the development of PKU for most patients (11–14).
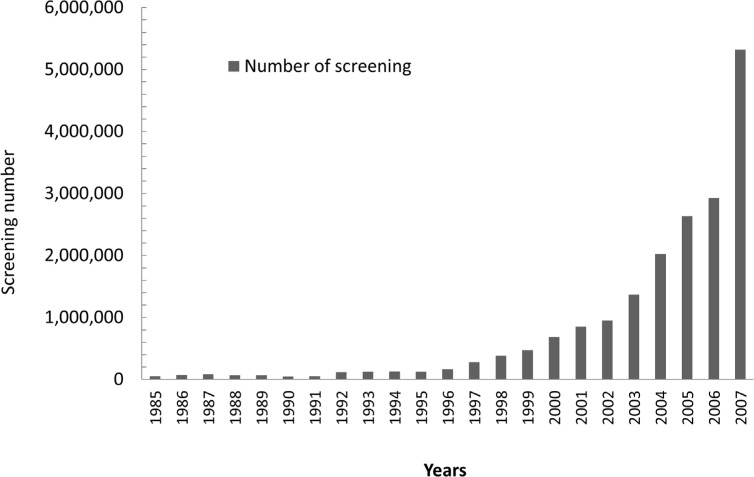
In China, the overall incidence of PKU is approximately 1/11,144 (15,16). Since 1981, a newborn screening program has been implemented by the China government, which mainly focused on congenital hypothyroidism (CH) and PKU. Between 1985 and 2006, according to the accessible data (17,18), a total number of 13,666,750 newborns had been tested for PKU and 1,170 cases had been confirmed as PKU patients, with a positive result rate of 1/11,680, and screening numbers for PKU increased remarkably after 1999 (Figure 1). In recent years, the China government also started to put more emphasis on the response to PKU, but since the diagnosis and treatment for PKU in China is still a relatively new area, many deficiencies may exist.
Figure 1.
Annual number of newborn screenings in China between 1985 and 2007.
This paper aims to compare the situation of newborn screening and treatment for PKU in China and the relatively developed countries — United States, United Kingdom and Japan, and provides some valuable experience for further development of a response policy for PKU in China.
2. Newborn screening for PKU
At present, for almost all patients with PKU, diagnosis from newborn screening and starting treatment immediately leads to a better effect than diagnosis with clinical symptoms (19). Newborn screening combined with a Phe-restricted therapeutic diet throughout childhood can help to control the progress of PKU for most patients (11). Research shows that with a higher newborn screening rate, earlier treatment can be received by patients, and the prognosis will be better (12–14). Therefore, an effective newborn screening for PKU is indeed needed.
A newborn screening program was launched by the China government in 1981, and screening for PKU is a part of the national newborn screening program. The very first screening started in Shanghai in October 1981 (20). After that, the screening program in China has made significant progress. The number of screening centers increased from only 3 in the 1980s to 46 in 2002 and further to 179 by the end of 2009 (18). The coverage of the newborn screening program also increased from 3.86% in 2003 to 59.01% in 2009 (18).
At the policy level, the China government issued a series of policies to enhance the coverage of newborn screenings (Table 1), including identification of the importance of newborn screening as a law. This stipulated that PKU is one of the screening diseases, included newborn screening as part of the basic maternal and infant health care services, and issued a standardized path and technical specification for China's newborn screening — in order to improve the quality of PKU screening and testing, and indicated the specific objectives of the newborn screening program (21–25).
Table 1. The characteristic policy related with newborn screening for PKU in China.
| Year | Publisher | Policy | Feature |
| 1994 | The Central People's Government of the People's Republic of China | Law of Maternal and infant health care | First time identified the importance of newborn screening as a law. |
| 2001 | The Central People's Government of the People's Republic of China | The measure for the implementation of the Law of Maternal and infant health care in China | Included the newborn screening into the maternal and infant health care services. |
| 2009 | NHFPC | The measure of newborn screening management | Indicated that PKU as one of the screening disease and provided a standardized path for China's newborn screening program. |
| 2009 | NHFPC | The plan of newborn screening program | Indicated the objective of newborn screening program, including PKU screening coverage. |
| 2010 | NHFPC | The technical specification of newborn screening | Improving the quality of PKU screening and tests. |
The newborn screening program for PKU in China has made significant progress since the number of screening centers increased, the coverage of the newborn screening program increased and the number of screened children increased as we mentioned above. However, compared with the newborn screening rate in developed countries, such as 99% in United States, the approximately 60% newborn screening rate in China is still low. To provide a better view of future prospects of newborn screening for PKU in China, we have summarized some potential valuable experience in the United States, UK and Japan.
2.1. Required by law
The government enforcement plays an important role in the process of newborn screening for PKU (Table 2). In 1963, in Massachusetts of the United States first passed a law that required the PKU screening test for all infants born in the state and represented the start of the newborn screening program in the United States (26). Today, all states of the United States have newborn screening programs for PKU and nearly every newborn is screened shortly after birth (27). According to the data, nearly all states required all newborns receive testing by law and only 4 states, Vermont, Maryland, Minnesota and Idaho, indicated that parents can decline screening (28). Thanks to these state laws, the launch of the newborn screening program for PKU has legislative authority, as well as improvement of the screening rate in the United States. Participation in the newborn screening program in the United States has reached 99.9% or higher (29). For example, Maryland reported that during the last several years, fewer than 5 families opted out of newborn screening each year and in Wyoming, there were 6,800 infants born in 2007, but only 2 families opted out of screening (29).
Table 2. The policy of newborn screening and treatment for PKU in United States, UK, Japan and China.
| Policy of newborn screening against PKU |
Policy of treatment against PKU |
||||
| Required by law | Financial support | Publicity supporting | National standardized guidelines | Charging policy | |
| United States | All states required except 4 states: Vermont, Maryland, Minnesota and Idaho. | The test is only free in New York, Pennsylvania, Kansas and District of Columbia. However in other states, health insurance programs and the State Children's Health Insurance Program or Medicaid pay for the fee. | Having some policies and regulations. | Published since 1993 | 33 states (64.71%) make private insurance requirements about the reimbursement for medical foods for PKU, and 42 states (82.35%) provide state services and assistance for PKU. Only 3 States (Mississippi, Oklahoma and West Virginia) do not have both private insurance requirements and state services and assistance for PKU. |
| United Kingdom | Do not require yet highly recommend. | The test is covered by National Health Service, and the screening service is free. | Having some policies and regulations. | Published since 1993 | The fee of amino acids and low protein foods are fully reimbursed by National Health Service, yet the patients over the age of 16 pay a prescription charge. |
| Japan | Do not require yet highly recommend. | The test is covered by the government. | Having some policies and regulations. | Published since 1995 | The charge for PKU's treatment is covered by national health insurance and special public subsidies. |
| China | Do not require in most area except Shanghai and Guangzhou. | Free-charge policy in HongKong and Shanghai. Other areas need to charge a fee for screening. | No such specific policy or regulation has been retrieved. | No such specific guideline has been retrieved. | The charge had been covered by URBMI in some places yet no such policy in most regions has been retrieved. Government of Guangzhou covers the fee of PKU children until 8 years of age. |
In some areas of China, local government also implemented some specific policies to demonstrate that newborn screening for PKU must be carried out. Shanghai and Guangzhou are two of the earliest areas that carried out newborn screening for PKU in China and also two of the areas with the highest screening rate including 97.0% in Shanghai and 99.0% in Guangzhou (30,31). In 1996, the Shanghai government issued regulation of maternal and infant health care in Shanghai which indicated the newborn screening program must be carried out in Shanghai (32). While in Guangzhou, regulation of maternal and infant health care was issued in 1998 which formulated that Guangdong province must carry out the newborn screening program and particularly proposed that the screening should include the PKU test (33).
Based on the experience in the United States and partly China, government enforcement may be able to help improve newborn screening coverage in China, yet current enforcement is still weak.
2.2. Multi-stage target blood Phe concentration criterion
Although the definition of PKU had been determined all round the world, the target blood Phe concentration criterion of PKU still differs significantly among different countries (11,34–38) (Table 3).
Table 3. Target blood phenylalanine concentrations (µmol/L) as recommended for treatment of PKU in different countries.
| Age group | United States | UK | Japan | Turkey | China |
| < 2 years | 120 – 360 | 120 – 360 | 120 – 240 | 60 – 240 | 120 – |
| 2–6 years | 120 – 360 | ||||
| 7–9 years | 120 – 480 | 180 – 360 | |||
| 10–12 years | 180 – 480 | ||||
| 13–15 years | 120 – 600 | 120 – 700 | 180 – 600 | ||
| > 16 years | 120 – 900 | 180 – 900 | |||
| Incidence | 1/15,000 | 1/10,000 | 1/70,000 | 1/4,000 | 1/11,144 |
Based on the information, the United States, UK and Japan have implemented a multi-stage target blood Phe concentration criterion. In Japan, the country with the lowest incidence, has implemented a 6 stage target blood Phe concentration criterion focused on different age groups. For children younger than 2 years old, between 2 and 6 years old, between 7 and 9 years old, between 10 and 12 years old, between 13 and 15 years old, and older than 16 years old, the target blood Phe concentration criterion respectively is 120–240, 120–360, 180–360, 180–480, 180–600, and 180–900. Research shows that after the new treatment guidelines with a more stringent restriction of phenylalanine levels issued in 1995, the mean blood phenylalanine levels of Japanese decreased (8). The United States and UK also implemented its own multi-stage target blood Phe concentration criterion based on the national circumstances and received a positive result (39,40). In some cases, the exquisite grouping of target blood Phe concentration criteria helps these countries to detect PKU patients more effectively and earlier in order to make a contribution in the direction of PKU control.
According to experience in the United States, UK and Japan, an appropriate multi-stage target blood Phe concentration criterion can help to screen patients more effectively to a certain extent. However, the multi-stage target blood Phe concentration criterion has still not been issued in China. According to the technical specification of newborn screening issued by National Health and Family Planning Commission of the People's Republic of China (NHFPC), the lower target Phe concentration of PKU in China is 120 µmol/L (> 2 mg/dL) and the upper target blood Phe concentration is not specific (25). There is no independent lower target Phe concentration for different age groups (25).
2.3. Financial support
Economic factors play an important role in the process of newborn screening for PKU. In 4 states of the United States, New York, Pennsylvania, Kansas and the District of Columbia, the NBS test is free, while the other states collect a fee. The charge is between $15 (in Florida) and $157.54 (in Rhode Island). Many health insurance programs pay the fees for newborn screening and the State Children's Health Insurance Program or Medicaid can pay the fees for families in need (41). Therefore, the vast majority of Americans do not need to pay for the newborn screening for PKU, actually. In Japan, the charge for newborn screening is also covered by the government (8,42,43). In UK, the Newborn Blood Spot Screening Programme is part of Public Health England (44) covered by the National Health Service, and the screening service is for free (45). The same situation has been seen in Japan, the newborn screening for PKU is also covered by the government (Table 2).
The fee for newborn screening for PKU in China is variable in different areas just as in the United States. However, except in Hong Kong, the children in the rest of the areas of China still need to pay a fee (46).
2.4. Publicity supporting
One of the most important problems during the development of the newborn screening program is publicity — awareness and understanding of newborn screening for the public. In developed countries, the supporting publicity has been implemented for a period and some valuable experience should be reviewed.
In the United States, Federal government support for newborn screening has continued through the years in various ways. Most visible has been the funding of publicity programs. In 2000, for example, the National Newborn Screening and Genetics Resource Center (NNSGRC), the Health Resources and Services Administration (HRSA) and the Centers for Disease Control (CDC) jointly sponsored a working group meeting to review the issues of implementing tandem mass spectrometry (MS/MS) testing as a means of screening newborns for rare metabolic diseases. The meeting represented the idea that “proposals for planning, operating and evaluating MS/MS for analyzing dried blood spots routinely collected from newborns” and “the public should receive accurate information regarding expanded and comprehensive newborn screening and the evolving knowledge regarding its strengths and weaknesses” (47). Combined with the other policies issued in the period (48,49), not only awareness towards newborn screening of health practitioners and politicians have been aroused, but also a media awareness campaign during 2000 which led to public attention towards newborn screening (50).
The situation in Japan and UK are the same (51–58). For example, in Japan, research shows that the awareness ratio of newborn screening in Japan was 26.6% at first, yet after a brief explanatory note on NBS was provided, 71.7% of respondents recognized the necessity of newborn screening (51) (Table 2).
In China, there was some research about how to improve the public's awareness of newborn screening (58–61) and also some nonprofit websites tried to provide the public with information for newborn screening. However, the situation is still weak.
3. Treatment for PKU
Untreated PKU can lead to mental retardation, seizures, and other serious medical problems (62). The acknowledged mainstream treatment for PKU patients is a strict Phe-restricted diet supplemented by a medical formula containing amino acids and other nutrients (63). However, the treatment for PKU is variable among different countries.
In China, the idea about early diagnosis and early treatment for PKU had been proposed for a long time (64,65), and some reimbursement system has been established (66–69). However, there are many issues in the aspect of treatment for PKU in China that still need to be carried out since treatment in China is relatively new. We will expound the situation of treatment for PKU in the aspect of national guidelines and charge policies.
3.1. National standardized guideline
In the United States, the Committee on Genetics of the American Academy of Pediatrics and the National Institutes of Health were trying to set up guidelines for PKU since 1993 (70), and treatment guidelines against PKU were finally published in 1994 (71). In the United States guidelines, the treatment should be initiated no later than 7-10 days after birth and the frequency of monitoring differed by patient's age as weekly until age 1 year, twice monthly from age 1 to 12 years, and monthly after an age of 12 years. As far as treatment duration, treatment throughout life is highly recommended (71–73). In UK, the Medical Research Council Working Party on PKU published a series of treatment guidelines for PKU in 1993 (74,75). In UK's guideline, the frequency of monitoring is divided into 3 groups as weekly between 0–4 years old, fortnightly between 5-9 years old, and monthly after 9 years old. In Japan, the guideline for the treatment of PKU was revised in 1995 with a more stringent restriction of phenylalanine levels (8,43) (Table 2). Although there are some differences between each country's guidelines, guidelines all included the same details as age at start of diet treatment, recommended blood Phe levels, frequency of monitoring, duration of diet treatment and etc.
Even though treatment for PKU has been carried out in China for a long time, no national standardized guidelines or regulations about the treatment of PKU issued have been retrieved by us.
3.2. Charge policy
In the aspect of charging policy, because of state laws, the situation in different states of America are different — 33 states (64.71%) have private insurance requirements about reimbursement for medical foods for PKU, and 42 states (82.35%) provide state services and assistance for PKU. Only 3 States (Mississippi, Oklahoma and West Virginia) do not have both private insurance requirements and state services and assistance for PKU. Reimbursements focus on patients under 18 to 22 years old, yet Colorado and North Dakota also dictate age limits specially for females 35 years old in Colorado and 45 years old in North Dakota (76,77). However, the idea of promoting social support for PKU's treatment has been proposed in the United States since 1993 (78,79). In UK, the fee for amino acids and low protein foods are fully reimbursed by the National Health Service, yet patients over the age of 16 pay a prescription charge (80–82). In Japan, the charge for PKU's treatment is covered by national health insurance and special public subsidies (83,84) (Table 2).
In China, treatment for PKU charged between 15,000 and 25,000 RMB (Chinese Yuan) annually (85). This charge had been covered by Urban Residents' Basic Medical Insurance (URBMI) in Fujian province and Hefei city and the reimbursement ratio is more than 70% (66–68) yet no such policy in other regions has been retrieved. For rural residents, the China government started a pilot program in January 2013 to cover 70% of the charge of PKU treatment as a reimbursement for serious illness for less than 10 years old patients with the New Rural Co-operative Medical System (NRCMS) (69). Besides these policies, some special bailouts have also been implemented in some regions. For example, in Guangzhou city, children confirmed to have PKU can receive the treatment from the Newborn Screening Center of the City of Guangzhou for free until 8 years of age (85).
4. Discussion
4.1. Prospects for newborn screening for PKU in China
Although the newborn screening program for PKU in China has made some progress, there are still some challenges among the existing newborn screenings and more effective strategies are expected to be implemented in the future.
The experience of the United States has already showed that appropriate legislation can help to improve screening coverage. Since the policy has been provided its importance during the process of newborn screening for PKU this kind of enforcement has already been implemented in some areas of China and generates an improvement of newborn screening for PKU. Further development about improving China's newborn screening program for PKU, and how to establish the law appropriately needs further discussion and the experience of Shanghai and Guangzhou indicates that the other areas in China can try to implement their own enforcement based on the practical situation.
The multi-stage target blood Phe concentration criteria can not only increase the standardization of the current concentration criteria by subdividing the patients into different age groups, but also can be a symbol of scientific management of PKU. Even though China's PKU incidence is lower than UK's according to the data, the experience of multi-stage target blood Phe concentration criteria that were implemented in these countries can be useful for China to improve the current criteria. However, because the incidence of PKU in the world varies widely in different human populations, research about suitable multi-stage target blood Phe concentration criteria for China still needs to be carried out.
Research also indicates that the important role that economic factors play in the process of newborn screening (86), as parents are more willing to let their children be screened when there is less cost for screening. These results remind us that a new charging policy of newborn screening for PKU should focus more on reducing the charge by increasing national financial support or reimbursement in China. The financial support for newborn screening for PKU in the United States, UK and Japan provide a hint of increasing the screening rate. The financial support of newborn screening in China is relatively weak as the majority of families still need to pay for the screening (87). The financial support in the United States, UK and Japan is a good example for further study of China's newborn screening policies.
The publicity of newborn screening showed a good impact in the United States, UK and Japan, yet this kind of education in China is still missing. In some areas, researchers try to use health education to introduce newborn screening for PKU to the public, and this kind of research needs to be carried out further in China.
4.2. Prospects for treatment for PKU in China
As me mentioned above, the national policy of treatment for PKU in China is still missing, and therefore the standardization of treatment for PKU in China still needs to be enhanced. According to the experience of the United States, UK and Japan, treatment guidelines including details can help to improve the degree of standardization. In the aspect of treatment time, treatment throughout a lifetime can be considered a further aim as lifetime treatment needs the support of quite a lot of health resources. A suitable treatment time for Chinese patients needs more study.
As for charging policy, the current reimbursement ratio of PKU's treatment in China is almost 70% with a 10 years old limitation. Comparing with the situation in United States, UK and Japan, a higher reimbursement ratio and age limitation is a direction for further discussion. However, the local government's attention to reimbursement may be an effective method, such as Guangzhou, HongKong and the situation in different states of the United States.
5. Conclusion
Newborn screening and treatment for PKU in China is relatively weak. Compared with the newborn screening and treatment for PKU in the United States, UK and Japan, the multi-stage target blood Phe concentration criteria, policy that requires newborn screening has to be taken, better financial support for newborn screening, publicity of newborn screening, national guidelines of treatment for PKU including details, and a higher reimbursement ratio and age limitation may be prospects that should be further studied in China and may provide some support for better development of newborn screening and treatment for PKU in China.
Acknowledgements
This study was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan. We also thank Satveer Kour who provided critical information about UK's newborn screening program on behalf of UK National Screening Committee/NHS Screening Programmes.
References
- 1. Vantyghem MC, Dobbelaere D, Mention K, Wemeau JL, Saudubray JM, Douillard C. Endocrine manifestations related to inherited metabolic diseases in adults. Orphanet J Rare Dis. 2012; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Institutes of Health Consensus Development Conference Statement: Phenylketonuria: Screening and management, October 16–18, 2000. Pediatrics. 2001; 108:972-982 [DOI] [PubMed] [Google Scholar]
- 3. Targum SD, Lang W. Neurobehavioral problems associated with phenylketonuria. Psychiatry (Edgmont). 2010; 7:29-32 [PMC free article] [PubMed] [Google Scholar]
- 4. Loeber JG. Neonatal screening in Europe; the situation in 2004. J Inherit Metab Dis. 2007; 30:430-438 [DOI] [PubMed] [Google Scholar]
- 5. The National Society for Phenylketonuria (United Kingdom) Limited. Management of PKU. http://www.nspku.org/sites/default/files/publications/Management%20of%20PKU.pdf (accessed November 1, 2013)
- 6. Ozalp I, Coskun T, Tokatli A, Kalkanoglu HS, Dursun A, Tokol S, Koksal G, Ozguc M, Kose R. Newborn PKU screening in Turkey: At present and organization for future. Turk J Pediatr. 2001; 43:97-101 [PubMed] [Google Scholar]
- 7. Guldberg P, Henriksen KF, Sipila I, Guttler F, de la Chapelle A. Phenylketonuria in a low incidence population: Molecular characterisation of mutations in Finland. J Med Genet. 1995; 32:976-978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aoki K, Ohwada M, Kitagawa T. Long-term follow-up study of patients with phenylketonuria detected by the newborn screening programme in Japan. J Inherit Metab Dis. 2007; 30:608. [DOI] [PubMed] [Google Scholar]
- 9. Bickel H, Gerrard J, Hickmans EM. Influence of phenylalanine intake on phenylketonuria. Lancet. 1953; 265:812-813 [DOI] [PubMed] [Google Scholar]
- 10. Haberle J, Boddaert N, Burlina A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012; 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010; 376:1417-1427 [DOI] [PubMed] [Google Scholar]
- 12. Olusanya BO. Highlights of the new WHO Report on Newborn and Infant Hearing Screening and implications for developing countries. Int J Pediatr Otorhinolaryngol. 2011; 75:745-748 [DOI] [PubMed] [Google Scholar]
- 13. Huang LH, Zhang L, Tobe RY, et al. Cost-effectiveness analysis of neonatal hearing screening program in China: Should universal screening be prioritized? BMC Health Serv Res. 2012; 12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO. Prevention of hearing impairment. http://www.who.int/entity/pbd/deafness/en/english.pdf (accessed November 1, 2013)
- 15. Zhou YA, Ma YX, Zhang QB, Gao WH, Liu JP, Yang JP, Zhang GX, Zhang XG, Yu L. Mutations of the phenylalanine hydroxylase gene in patients with phenylketonuria in Shanxi, China. Genet Mol Biol. 2012; 35:709-713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mei L, Song PP, Xu LZ. Newborn screening and related policy against Phenylketonuria in China. Intractable Rare Dis Res. 2013; 2:72-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu YH, Qin YF, Zhao ZY. Retrospective study on neonatal screening for congenital hypothyroidism and phenylketonuria in China in the past 22 years. Zhonghua Er Ke Za Zhi. 2009; 47:18-22 (in Chinese) [PubMed] [Google Scholar]
- 18. Shi XT, Cai J, Wang YY, Tu WJ, Wang WP, Gong LM, Wang DW, Ye YT, Fang SG, Jing PW. Newborn screening for inborn errors of metabolism in mainland china: 30 years of experience. JIMD Rep. 2012; 6:79-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Spronsen FJ. Phenylketonuria: A 21st century perspective. Nat Rev Endocrinol. 2010; 6:509-514 [DOI] [PubMed] [Google Scholar]
- 20. Chen RG, Chen HY, Shi SZ, et al. Preliminary report on neonatal screening for hypothyroidism, phenylketonuria and galactosemia. Shanghai Medical Journal. 1983; 344-349 [Google Scholar]
- 21. The Central People's Government of the People's Republic of China. Law of Maternal and infant health care in China. http://www.gov.cn/banshi/2005-08/01/content_18943.htm (accessed November 2, 2013) (in Chinese)
- 22. The Central People's Government of the People's Republic of China. The measure for the implementation of the Law of Maternal and infant health care in China. http://www.gov.cn/banshi/2005-08/01/content_19126.htm (accessed November 2, 2013) (in Chinese)
- 23. NHFPC. The measure of newborn screening management. http://www.moh.gov.cn/fzs/s3576/200903/c53a0c97e68740c286ceb96e9ac56280.shtml (accessed November 2, 2013) (in Chinese)
- 24. NHFPC. The planning of newborn screening program. http://www.moh.gov.cn/cmsresources/mohfybjysqwss/cmsrsdocument/doc6649.doc (accessed November 2, 2013) (in Chinese)
- 25. NHFPC. The technical specification of newborn screening. http://www.moh.gov.cn/cmsresources/mohfybjysqwss/cmsrsdocument/doc10798.doc (accessed November 2, 2013) (in Chinese)
- 26. National Institutes of Health. September Is Newborn Screening Awareness Month. http://www.nichd.nih.gov/news/resources/spotlight/Pages/092413-sept-newbornscreening.aspx#screening (accessed November 2, 2013)
- 27. National Institutes of Health. How many infants are screened in the United States? http://www.nichd.nih.gov/health/topics/newborn/conditioninfo/pages/infantsscreened.aspx (accessed November 2, 2013)
- 28. National Newborn Screening & Global Resource Center. Newborn Screening. http://genes-r-us.uthscsa.edu/resources/consumer/statemap.htm (accessed November 2, 2013)
- 29. The President's Council on Bioethics. The changing moral focus of newborn screening: An ethical analysis by the president's council on bioethics. http://bioethics.georgetown.edu/pcbe/reports/newborn_screening/chapter4.html (accessed November 2, 2013)
- 30. The health department of Shanghai. Shanghai's 2008 public health work joint conference. http://www.smhb.gov.cn/website/b/37912.shtml (accessed November 3, 2013) (in Chinese)
- 31. The newborn screening center in Guangzhou. The general information about newborn screening in Guangdong province. http://www.gdnsn.com/qiansengaikuan/qsgk.htm (accessed November 3, 2013) (in Chinese)
- 32. The health department of Shanghai. The regulation of maternal and infant health care in Shanghai. http://www.shanghai.gov.cn/shanghai/node2314/node3124/node3125/node3127/userobject6ai250.html (accessed November 3, 2013) (in Chinese)
- 33. The standing committee of the people's congress in Guangdong province. The management regulation of maternal and infant health care in Guangdong province. http://www.gdnsn.com/falifagui/gdsmybjtl.htm (accessed November 3, 2013) (in Chinese)
- 34. National Institutes of Health (NIH) to host a consensus development conference on screening and management for phenylketonuria (PKU). Pediatr Nurs. 2000; 26:539. [PubMed] [Google Scholar]
- 35. van Spronsen FJ, Ahring KK, Gizewska M. PKU-what is daily practice in various centres in Europe? Data from a questionnaire by the scientific advisory committee of the European Society of Phenylketonuria and Allied Disorders. J Inherit Metab Dis. 2009; 32:58-64 [DOI] [PubMed] [Google Scholar]
- 36. Blau N, Belanger-Quintana A, Demirkol M, Feillet F, Giovannini M, MacDonald A, Trefz FK, van Spronsen F, European PKU centers . Management of phenylketonuria in Europe: Survey results from 19 countries. Mol Genet Metab. 2010; 99:109-115 [DOI] [PubMed] [Google Scholar]
- 37. Ahring K, Belanger-Quintana A, Dokoupil K, Gokmen-Ozel H, Lammardo AM, MacDonald A, Motzfeldt K, Nowacka M, Robert M, van Rijn M. Blood phenylalanine control in phenylketonuria: A survey of 10 European centres. Eur J Clin Nutr. 2011; 65:275-278 [DOI] [PubMed] [Google Scholar]
- 38. Koch R, Azen C, Friedman EG, Fishler K, Baumann-Frischling C, Lin T. Care of the adult with phenylketonuria. Eur J Pediatr. 1996; 155(Suppl 1):S90-S92 [DOI] [PubMed] [Google Scholar]
- 39. Demirkol M, Gizewska M, Giovannini M, Walter J. Follow up of phenylketonuria patients. Mol Genet Metab. 2011; 104(Suppl):S31-S39 [DOI] [PubMed] [Google Scholar]
- 40. McPheeters ML, Lindegren ML, Sathe N, Reimschisel T. Adjuvant Treatment for Phenylketonuria: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 56. http://www.ncbi.nlm.nih.gov/books/NBK137776/pdf/TOC.pdf (accessed November 4, 2013) [PubMed]
- 41. National Institutes of Health. Who pays for newborn screening? http://www.nichd.nih.gov/health/topics/newborn/conditioninfo/how-used/pages/pays.aspx (accessed November 4, 2013)
- 42. Aoki K. Newborn screening in Japan. Southeast Asian J Trop Med Public Health. 2003; 34(Suppl 3):80. [PubMed] [Google Scholar]
- 43. Kitagawa T. Newborn screening for inborn errors of metabolism in Japan. A history of the development of newborn screening. Pediatr Endocrinol Rev. 2012; 10(Suppl 1):8-25 [PubMed] [Google Scholar]
- 44. UK National Screening Committee. About US. http://www.screening.nhs.uk/about (accessed November 4, 2013)
- 45. Department of Health. Payment by Results: Mental health payments for 2013 to 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/232162/Mental_Health_PbR_Guidance_for_201314.pdf (accessed November 4, 2013)
- 46. Padilla CD, Therrell BL. Newborn screening in the Asia Pacific region. J Inherit Metab Dis. 2007; 30:490-506 [DOI] [PubMed] [Google Scholar]
- 47. CDC. Using tandem mass spectrometry for metabolic disease screening among newborns. A report of a work group. MMWR Recomm Rep. 2001; 50:1-34 [PubMed] [Google Scholar]
- 48. Serving the family from birth to the medical home. Newborn screening: A blueprint for the future — a call for a national agenda on state newborn screening programs. Pediatrics. 2000; 106:389-422 [PubMed] [Google Scholar]
- 49. Howse JL, Katz M. The importance of newborn screening. Pediatrics. 2000; 106:595. [DOI] [PubMed] [Google Scholar]
- 50. Therrell BL, Jr. U.S. newborn screening policy dilemmas for the twenty-first century. Mol Genet Metab. 2001; 74:64-74 [DOI] [PubMed] [Google Scholar]
- 51. Fujii C, Sato Y, Harada S, Kakee N, Gu YH, Kato T, Shintaku H, Owada M, Hirahara F, Umehashi H, Yoshino M. Attitude to extended use and long-term storage of newborn screening blood spots in Japan. Pediatr Int. 2010; 52:393-397 [DOI] [PubMed] [Google Scholar]
- 52. Moody L, Choudhry K. Parental views on informed consent for expanded newborn screening. Health Expect. 2013; 16:239-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cleary M, Walter JH. Assessment of adult phenylketonuria. Ann Clin Biochem. 2001; 38:450-458 [DOI] [PubMed] [Google Scholar]
- 54. Angela D, Davina F, Hugh D, Peter O. New mothers' awareness of newborn screening, and their attitudes to the retention and use of screening samples for research purposes. Genomics, Society and Policy. 2005; 1:41-50 [Google Scholar]
- 55. Song P, Gao J, Inagaki Y, Kokudo N, Tang W. Intractable and rare diseases research in Asia. Biosci Trends. 2012; 6:48-51 [PubMed] [Google Scholar]
- 56. Inagaki Y, Song PP. Necessity of cooperation with government on publication of scientific research results for intractable diseases. Intractable Rare Dis Res. 2013; 2:69-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang W, Makuuchi M. Intractable and rare diseases research. Intractable Rare Dis Res. 2012; 1:1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Song PP, Gao JJ, Inagaki Y, Kokudo N, Tang W. Rare diseases, orphan drugs, and their regulation in Asia: Current status and future perspectives. Intractable Rare Dis Res. 2012; 1:3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng SX. The influence of implementing newborn health education on the rate of newborn screening. Chinese Journal of Modern Drug Application. 2011; 5:136 (in Chinese) [Google Scholar]
- 60. Zhao H, Cui YZ, Zhou XY, Pang JX, Zhang XM, Xu SQ, Han JX. Study and analysis of the state of rare disease research in Shandong Province, China. Intractable Rare Dis Res. 2012; 1:161-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Han JX, Cui YZ, Zhou XY. Rare diseases research in China: Opportunities, challenges, and solutions. Intractable Rare Dis Res. 2012; 1:10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Filiano JJ. Neurometabolic diseases in the newborn. Clin Perinatol. 2006; 33:411-479 [DOI] [PubMed] [Google Scholar]
- 63. Macleod EL, Ney DM. Nutritional Management of Phenylketonuria. Ann Nestle Eng. 2010; 68:58-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yu WM, Xu L, Li XW, He C, Shen M, Zhang ZX, Jin YY, Zhou ZS, Qiao F. An Eighteen-year Study on Phenylketonuria. Acta Academiae Medicinae Sinicae. 2003; 2:218-222 (in Chinese) [PubMed] [Google Scholar]
- 65. Zhang L, Xu XH, Zhang SJ. Research advance in the treatment of phenylketonuria. Chinese Journal of Contemporary Pediatrics. 2009; 11:786-789 (in Chinese) [PubMed] [Google Scholar]
- 66. Fujian province human resources and social security bureau. The notification about further work towards 2011 urban residents' basic medical insurance of Fujian province. http://www.fjlss.gov.cn/action/article/article_show.action?vo.aid=41976 (accessed November 5, 2013) (in Chinese)
- 67. Human resources and social security bureau of Hefei City. Further adjustment of urban residents' basic medical insurance range. http://www.ahhfld.gov.cn/n1105/n32819/n169172/n173056/16683609.html (accessed November 5, 2013) (in Chinese)
- 68. Xiamen Municipal Government, P.R. China. The interpreting of the notification about further work towards 2011 urban residents' basic medical insurance. http://www.xm.gov.cn/zwgk/flfg/wjjd/201108/t20110803_417653.htm (accessed November 5, 2013) (in Chinese)
- 69. NHFPC. The determining of key points of health work in 2013. http://www.moh.gov.cn/wsb/pxwfb/201301/53164cc1472446a48c47734a005ff5da.shtml (accessed November 5, 2013) (in Chinese)
- 70. Schweitzer-Krantz S, Burgard P. Survey of national guidelines for the treatment of phenylketonuria. Eur J Pediatr. 2000; 159(Suppl 2):S70-73 [DOI] [PubMed] [Google Scholar]
- 71. National PKU News. U.S. PKU Screening and Treatment Guidelines. http://www.pkunews.org/diet/guide2.htm (accessed November 5, 2013)
- 72. National PKU News. Diet Intervention Guidelines for Adults with Untreated PKU. http://www.pkunews.org/adults/guide.htm (accessed November 5, 2013)
- 73. Agency for Healthcare Research and Quality. Adjuvant treatment for phenylketonuria: Future research needs. http://www.ncbi.nlm.nih.gov/books/NBK137776/pdf/TOC.pdf (accessed November 5, 2013) [PubMed]
- 74. Recommendations on the dietary management of phenylketonuria. Report of Medical Research Council Working Party on Phenylketonuria. Arch Dis Child. 1993; 68:426-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Phenylketonuria due to phenylalanine hydroxylase deficiency: An unfolding story. Medical Research Council Working Party on Phenylketonuria. BMJ. 1993; 306:115-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Health Resources and Services Administration. State statutes and regulations on dietary treatment of disorders identified through newborn screening. http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reportsrecommendations/reports/statelaws.pdf (accessed November 5, 2013)
- 77. National PKU News. State Laws and Policies. http://pkunews.org/rights/lobby6.htm#9 (accessed November 5, 2013)
- 78. Reimbursement for medical foods for inborn errors of metabolism. American Academy of Pediatrics Committee on Nutrition. Pediatrics. 1994; 93:860. [PubMed] [Google Scholar]
- 79. Millner BN. Insurance coverage of special foods needed in the treatment of phenylketonuria. Public Health Rep. 1993; 108:60-65 [PMC free article] [PubMed] [Google Scholar]
- 80. Belanger-Quintana A, Dokoupil K, Gokmen-Ozel H, Lammardo AM, MacDonald A, Motzfeldt K, Nowacka M, Robert M, van Rijn M, Ahring K. Diet in phenylketonuria: A snapshot of special dietary costs and reimbursement systems in 10 international centers. Mol Genet Metab. 2012; 105:390-394 [DOI] [PubMed] [Google Scholar]
- 81. European society for phenylketonuria and allied disorders treated as phenylketonuria. Reimbursement PKU products in Europe. http://www.espku.org/images/stories/reimbursement_ESPKU_2011_vs3.pdf (accessed November 5, 2013)
- 82. Gao JJ, Song PP, Tang W. Rare disease patients in China anticipate the sunlight of legislation. Drug Discov Ther. 2013; 7:126-128 [PubMed] [Google Scholar]
- 83. Japan Intractable Diseases Information Center. Specific treatment program. http://www.nanbyou.or.jp/entry/512 (accessed November 5, 2013) (in Japanese)
- 84. Boshi-Aiiku-Kai IGF. Program of free infant formula against congenital metabolic abnormalities disease treatment. http://www.boshiaiikukai.jp/milk.html (accessed November 5, 2013)
- 85. Guangdong Newborn Screening Network. Summary of Experiences with Newborn Screening for and Free Treatment of PKU in Guangzhou. http://www.gdnsn.com/qiansengaikuan/pkuzl.htm (accessed November 5, 2013) (in Chinese)
- 86. Mak CM, Lam CW, Law CY, Siu WK, Kwong LL, Chan KL, Chan WT, Chow KM, Lee KW, Chan WP, Chan AY. Parental attitudes on expanded newborn screening in Hong Kong. Public Health. 2012; 126:954-959 [DOI] [PubMed] [Google Scholar]
- 87. Gong SW, Jin S. Current progress in the management of rare diseases and orphan drugs in China. Intractable Rare Dis Res. 2012; 1:45-52 [DOI] [PMC free article] [PubMed] [Google Scholar]