Summary
As the world's most populous country, China has the world's largest number of rare disease groups in terms of prevalence. However, the country has no system of registering cases of most rare diseases, so there is very little documented information on the epidemiology of those diseases. The purpose of this study was to study the state of rare disease research and survey doctors in Shandong Province regarding their level of awareness of rare diseases. Types of rare diseases and numbers of cases were tallied and their geographical distribution over the decades was analyzed. Eight hundred and twenty-four doctors in tertiary hospitals and maternity and child care hospitals were surveyed by questionnaire. Data were descriptively analyzed and a map of disease distribution was created. Articles about rare diseases were retrieved from the Chinese Biomedical Literature Database to provide pertinent data. This study yielded 5,749 cases of 323 different types of rare diseases. The survey found that doctors lack awareness of research on rare diseases. An authoritative and information-rich platform for rare disease research is urgently needed. Key steps are to study epidemiological and statistical techniques and then obtain available data to provide a basis for the definition and regulation of rare diseases in China.
Keywords: Rare diseases, awareness survey, descriptive analysis
1. Introduction
Rare diseases are also known as “orphan diseases", but there is no satisfactory definition of rare diseases around the world. In the United States of America (USA), a rare disease is defined as a disease that affects fewer than 200,000 individuals, but in Japan the number is 50,000 and in Australia it is 2,000. The European Union (EU) definition is less than 5 in 10,000. The World Health Organization (WHO) defines a rare disease as all pathological conditions affecting 0.65–1 out of every 1,000 inhabitants (1). These numbers clearly relate to the population sizes of these countries, but even adjusting for that, the definitions vary from about 1 to 8 in 10,000 (2). Data on rare diseases are constantly collected and updated through the combined efforts of government, patient organizations, and medical and scientific institutions. Many organizations, such as orphanet (http://www.orpha.net) in the EU, have showed that these data play an important role in areas such as the prevention and treatment of rare diseases, policy-making, medical research, and social welfare.
Study and regulation of rare diseases has progressed worldwide, and this is especially true in the USA and EU. The USA adopted important legislation on rare disease and orphan drugs in 1983 that has successfully promoted investment in research and development (R&D) of new pharmaceutical products to treat rare diseases. Similar legislation was also enacted in Australia in 1997 and in the EU in 1999. This legislation explicitly recognized the unmet need for targeted treatments for rare diseases and it created regulatory pathways and incentives for manufacturers to develop orphan drugs (3–5). In Asia, Japan, South Korea, and Taiwan have established systematic economic and regulatory incentives to encourage R&D of drugs for rare diseases.
China is also actively promoting regulation of rare diseases, but these diseases have not been covered by the national health system and special legislation on orphan drugs was only recently enacted (6). Given its large population, China probably has a large number of patients with rare diseases according to the definition of the WHO. However, there are still no official data on “the prevalence of rare diseases, their variety, and the number of cases of rare diseases”. A crucial step is to collect data on rare diseases in China.
Shandong Province is one of China's most populous provinces, the sixth census recorded its population as 95,793,065, which represents 7.2% of China's total population. The province started researching rare diseases early on and it created a rare disease prevention and control association. At present, the goal of that association is to establish a platform for rare disease diagnosis and information. This project is also supported by the provincial government and has a good research foundation. Shandong has a varying terrain, rich mineral resources, traditional, historical, and cultural backgrounds, both an industrial and an agricultural economy, various occupations and socioeconomic levels, and relatively developed medical technology. It epitomizes China. Data on rare diseases from Shandong Province can be used as a national analogue to a certain extent and will help to study rare diseases and formulate responses nationally. Thus, the current study examined the state of rare disease research and level of awareness of rare diseases among doctors in Shandong. This study also tallied the types of rare diseases and number of cases and analyzed their geographical distribution over the decades.
2. Methods
2.1. Data collection
A questionnaire was sent to 824 doctors in a total of 103 tertiary hospitals and municipal maternity and child care hospitals in 17 cities of Shandong Province. The questionnaire (Table 1) consisted of three parts. The first dealt with background information, including name, specialty, phone number, e-mail, position, title, education, years of experience, and department. The second part was a survey on awareness and recommendations for prevention of rare diseases. The third part dealt with information on which types of rare diseases were present and the number of cases encountered. There were 472 responses from doctors at 59 hospitals (219 doctors at 3 hospitals has never encountered a rare disease and thus did not answer the questionnaire), for a total response rate of 57.28% (472/824). Here, the 253 responses (53.60% of valid responses) from doctors who had encountered a rare disease are analyzed.
Table 1. Portions of the questionnaire.
| Topic | Item | Control |
| Survey on awareness of rare diseases among doctors and recommendations for their prevention | 1. How did you learn of rare diseases? |
|
| 2. If you encounter a rare disease that you are unfamiliar with, what you will do? |
|
|
| 3. What is the best way to improve the diagnosis and treatment of rare diseases? |
|
|
| 4. Which step is the most important in setting up a network to study rare diseases? |
|
|
| 5. Have you ever conducted research on rare diseases? |
|
|
| 6. Have you ever published papers about rare diseases? |
|
|
| 7. Have you ever cared for patients with rare diseases? |
|
|
| Disease informationare |
|
Also studied were articles on rare diseases from the Database of Chinese Biomedicine Literature (http://sinomed.imicams.ac.cn) with a principal author residing in Shandong Province from January 1978 to January 2012. The retrieval strategy used was “all fields: disease and author affiliation: Shandong”. These articles were then narrowed down to eliminate case reports, resulting in 409 papers.
2.2. Data processing and analysis
SPSS 20.0 was used to input and manage data, and then random sampling was used to ensure the accuracy of data. If there were abnormal data or missing values, they were corrected in accordance with the original data.
Descriptive statistical analysis in the form of frequency distribution analysis was used and data were summarized to describe the data characteristics. Charts were then drawn with SPSS and Excel.
Maps with shades of color reflecting the number of cases of rare diseases and number of articles published in each city were created with ArcMap 10.0 and SPSS 20.0.
3. Results
This study found 5,749 cases of 323 different types of rare diseases in Shandong Province. The survey of doctors yielded 293 types and 4,068 cases and the Chinese Biomedical Literature Database yielded 50 types and 1,681 cases. Figure 1 shows data on rare diseases according to descriptive analysis. The ten most prevalent rare diseases were fibrous dysplasia of bone, congenital hypothyroidism, Marfan's syndrome, infectious mononucleosis, hemophilia, osteogenesis imperfecta, multiple osteochondroma, synpolydactyly, phenylketonuria, and neurofibromatosis.
Figure 1.
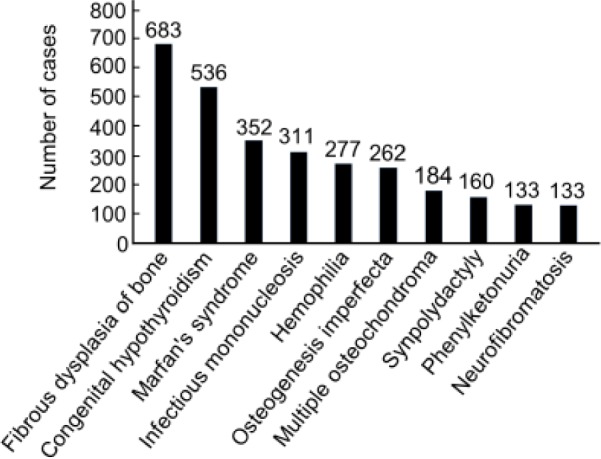
Ten most prevalent rare diseases in terms of the number of cases. Columns represent the number of cases.
Figure 2 displays the geographic distribution of rare disease cases and articles in shades of color. Binzhou, Ji'nan, Qingdao, and Linyi had more cases than other locations (Figure 2A). Cities with the higher number of articles on rare diseases published in the Chinese Biomedical Literature Database were Ji'nan, Qingdao, and Weifang (Figure 2B). Dezhou, Laiwu, and Rizhao had few cases of rare disease and few articles on those diseases in the database.
Figure 2.
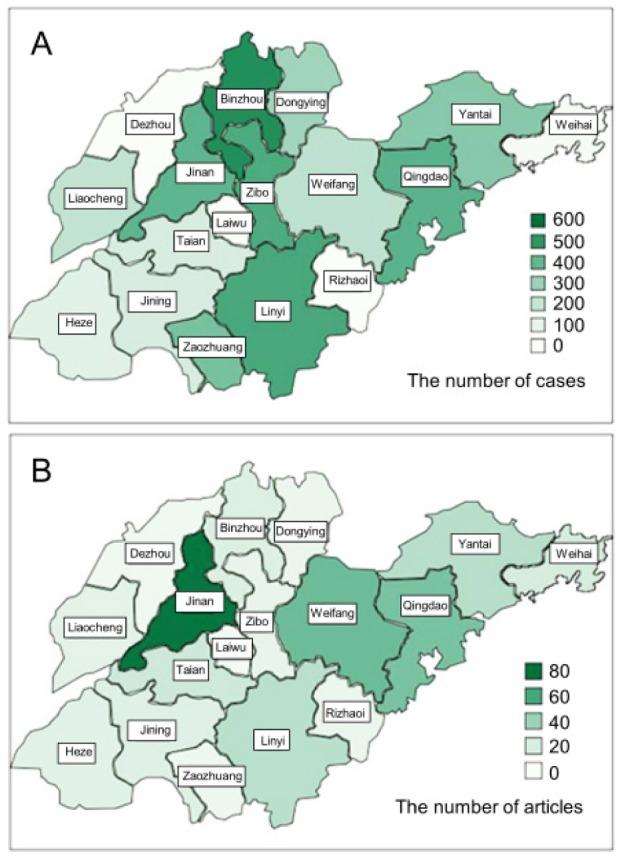
Geographical distribution of rare diseases onthose diseases. (A), Shown is the distribution of cases in each city; (B), Shown are locations where articles on rare diseases are published. Color shades indicate degree.
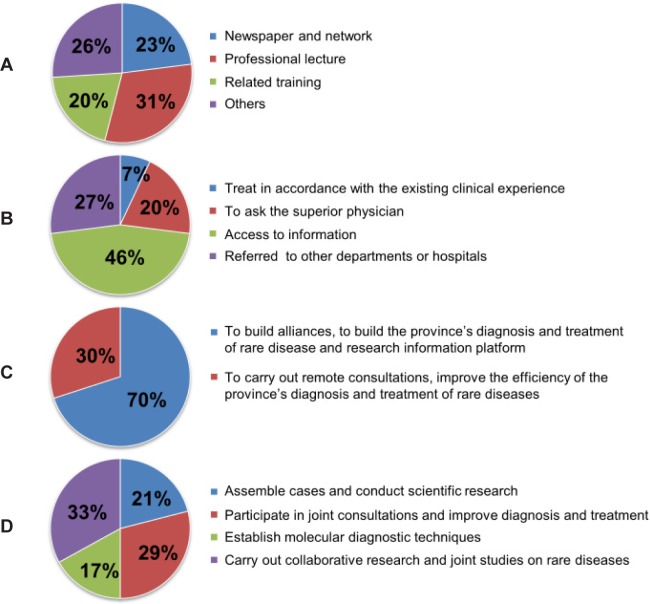
The data in Figure 3 shows the awareness of rare diseases among doctors and the recommendations to prevent those diseases. As shown in the figure, doctors learned about rare diseases through professional seminars, clinical consultation, magazines, newspapers, the Internet, and related training, with professional seminars being the most frequent method of learning (Figure 3A). When doctors encountered a patient with a rare disease, 46% choose to seek more information, 27% referred the patient, 20% consulted a veteran physician and the remaining 7% treated the patient based on their clinical experience (Figure 3B). Seventy percent of respondents felt that the best way to improve the diagnosis and treatment of rare diseases was to establish a network and platform to diagnose, treat, and study rare diseases (Figure 3C). According to respondents, important steps in establishing such a network are assembling cases and conducting scientific research, participating in joint consultations and improving diagnosis and treatment, establishing molecular diagnostic techniques, and carrying out collaborative research and joint studies on rare diseases (Figure 3D).
Figure 3.
Data on awareness of rare diseases among doctors and recommendations. The pie chart shows the proportion of answers. (A), Responses to the question “How did you learn of rare diseases?”; (B), Responses to the question “If you encountera rare disease that you are unfamiliar with, what you will do?”; (C), Responses to the question “What is the best way to improvethe diagnosis and treatment of rare diseases?”; (D), Responses to the question “Which step is the most important in setting up a network to study rare diseases?”.
Figure 4 shows the low degree of emphasis on rare disease research among doctors. Forty-four of 247 doctors had carried out research on rare diseases, 33 of 250 had published an article on a rare disease, and 10 of 249 had participated in a project on a rare disease.
Figure 4.
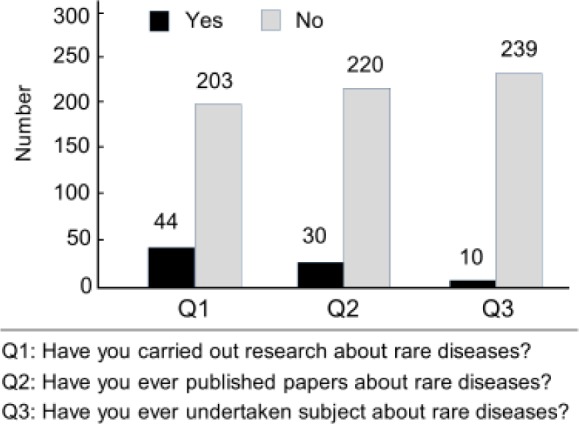
Data on rare disease research. Columns indicate the number of responses.
4. Discussion
As the world's most populous country, China has the world's largest number of rare disease groups in terms of prevalence. However, the country has no system of registering cases of most rare diseases, so there is very little documented information on the epidemiology of those diseases (7,8). The current study tallied the ten most prevalent rare diseases in Shandong Province, but this list did not match the diseases described in other articles. Bibliographic data published in 2011 by Orphanet (http://www.orpha.net) in Europe showed that the ten most prevalent rare diseases were KlippelTrenaunay-Weber syndrome, Whipple disease, incontinentia pigmenti, Aicardi syndrome, CADASIL, Li-Fraumeni syndrome, Silver-Russell syndrome, Castleman disease, cutis marmorata telangiectatica congenital, and Mobius syndrome. The prevalence of rare diseases may differ in different countries or different areas, so a database specific to China must be created for future research.
A survey of rare disease awareness among doctors found that they learn about rare diseases in many ways, though none is authoritative and standards and effective programs for diagnosis and treatment of rare diseases are lacking. A similar survey was conducted in Europe, but patients were surveyed. The survey was part of a long-term study that began in March 2004 by sending a questionnaire to 18,000 patients to ask about experience being diagnosed with a rare disease in 17 countries. The survey results highlighted the dilemma of rare diseases: lack of information, lack of appropriate medical training, difficulties with access to care, and subsequent loss of patient confidence in the health care system and the medical profession. The survey authors put forward solutions including reference centers, databases for the exchange of information, DNA and tissues banks, and networks of professionals (9). The establishment of a rare disease network and information exchange platform is a pressing matter. At the same time, work must also be done to assemble cases and conduct scientific research, participate in joint consultations and improve diagnosis and treatment, establish molecular diagnostic techniques, and carry out collaborative research and joint studies on rare diseases.
The current study noted a very serious problem: the low degree of emphasis on rare disease research among doctors. Rare disease research received little attention and also involved a low degree of cooperation. The current survey had a response rate below 60%, and fewer than 55% of respondents had ever encountered a rare disease. The problem may be due to doctors' specialties, interests, or level of knowledge, but definitive conclusions cannot be reached due to the lack of data. Further study and analysis will be done to determine the factors influencing attention to rare diseases among doctors and increase that attention.
The study of rare diseases in China is still in its infancy. China is also actively promoting regulation of intractable and rare diseases. Some rare disease websites and online databases to register cases have been set up. A number of centers have been established to offering counseling on rare diseases in major Chinese cities like Beijing and Shanghai. Moreover, in Shanghai patients with 12 rare diseases recently became eligible for partial reimbursement, and some special orphan drugs for children are now covered by insurance, but these diseases have not been covered by the national health system and special legislation on orphan drugs was only recently enacted. China still lags far behind the US, EU, Japan, and other countries and regions in terms of orphan drug legislation (6,10,11). Key steps are to examine epidemiological and statistical techniques and then obtain available data to provide a basis for the definition and regulation of rare diseases in China.
The current study has several limitations. There was, for example, substantial bias in the data collection process and the survey was conducted using only descriptive analysis. Rare disease cases and articles have different geographic distributions, but definitive reasons for these differences were not apparent, so data need to be collected and in-depth analysis needs to be performed to determine whether there are significant differences in rare diseases seen in different cities and hospital departments.
5. Conclusion
Shandong Province had at least 5,749 cases of 323 different types of rare diseases, and the distribution of these diseases differed in different cities. Few doctors in the province had ample knowledge about rare diseases and there is insufficient emphasis on the treatment and research of rare diseases. An authoritative and information-rich platform for rare disease research is urgently needed, and the low degree of emphasis on rare disease research among doctors must soon be improved. Key steps are to examine epidemiological and statistical techniques and then obtain available data to provide a basis for the definition and regulation of rare diseases in China.
Acknowledgements
This work was supported by the Health Department of Shandong Province. The authors wish to thank Mrs. Wang Hui and Mrs. Sun Xiaoyun (the chief of the Science and technology education and international cooperation department of Shandong Province) and doctors from all of the tertiary hospitals and municipal maternity and child care hospitals in Shandong Province who participated in this study.
References
- 1. Lavandeira A. Orphan drugs: Legal aspects, current situation. Haemophilia. 2002; 8:194-198 [DOI] [PubMed] [Google Scholar]
- 2. Aronson JK. Rare diseases and orphan drugs. Br J Clin Pharmaco. 2006; 61:243-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villa S, Compagni A, Reich MR. Orphan drug legislation: Lessons for neglected tropical diseases. Int J Health Plann Manage. 2009; 24:27-42 [DOI] [PubMed] [Google Scholar]
- 4. Taruscio D, Capozzoli F, Frank C. Rare diseases and orphan drugs. Ann Ist Super Sanita. 2011; 47:83-93 [DOI] [PubMed] [Google Scholar]
- 5. Rinaldi A. Adopting an orphan. EMBO Rep. 2005; 6:507-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Song PP, Gao JJ, Inagaki Y, Kokudo N, Tang W. Intractable and rare diseases research in Asia. Biosci Trends. 2012; 6:48-51 [PubMed] [Google Scholar]
- 7. Wang JB, Guo JJ, Yang L, Zhang YD, Sun ZQ, Zhang YJ. Rare diseases and legislation in China. Lancet. 2010; 375:708-709 [DOI] [PubMed] [Google Scholar]
- 8. Zhang YJ, Wang YO, Li L, Guo JJ, Wang JB. China's first rare-disease registry is under development. Lancet. 2011; 378:769-770 [DOI] [PubMed] [Google Scholar]
- 9. EURORDIS. Survey of the delay in diagnosis for 8 rare diseases in Europe (Eurordiscare 2). http://archive. eurordis.org/article.php3?id_article=454 (access February 1, 2012)
- 10. Song PP, Gao JJ, Inagaki Y, Kokudo N, Tang W. Rare diseases, orphan drugs, and their regulation in Asia: Current status and future perspectives. Intractable Rare Dis Res. 2012; 1:3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han JX, Cui YZ, Zhou XY. Rare diseases research in China: Opportunities, challenges, and solutions. Intractable Rare Dis Res. 2012; 1:10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]