Summary
Parkinson's disease (PD) is a common, however, intractable neurodegenerative disorder in the aging population. Levodopa (l-dopa) administration is regarded as the most effective strategy in treating PD with prominent motor side-effects after undergoing long-term treatment. Surgical therapies such as deep brain stimulation (DBS) show certain efficacy, yet there are several limitations in adopting such surgical procedures. Therefore, performing electrical stimulation out of the brain, namely peripheral stimulation for PD has been a dream of many clinicians. Recently, the efficacy of dorsal column stimulation was verified in animal PD models; on the other hand, tons of acupunctural studies from East Asia claim good efficacy in treating PD both in bench and clinical studies. This review will introduce the progress of peripheral stimulation for PD, and will discuss the potential mechanisms involved in these strategies.
Keywords: Parkinson's disease; deep brain stimulation; peripheral stimulation; dorsal column, acupuncture; somatosensory system
1. Introduction
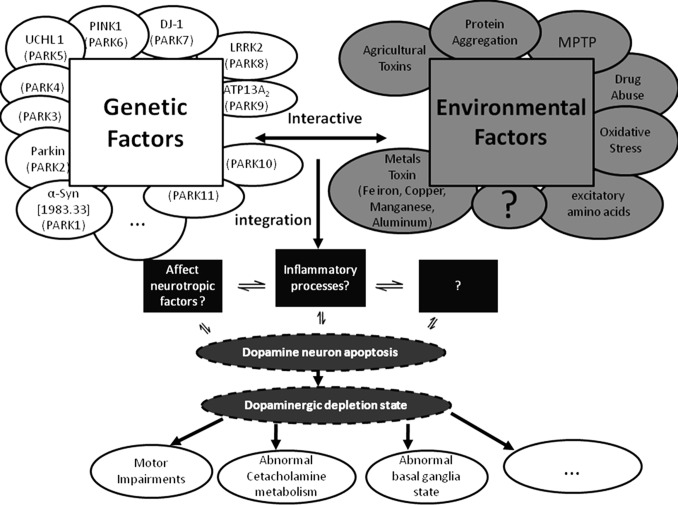
Parkinson's disease (PD), first reported by James Parkinson (1817), is a progressive neurodegenerative disorder which is common in the elder population with an unclear pathogenesis (1). The pathophysiological hallmark is the progressive degeneration of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc), and the main symptoms are static tremor, rigidity, bradykinesia, gait dysfunction and postural instability (2,3). The detailed pathogenesis remains unclear. It is believed that PD is a comprehensive result of genetic factors and environmental toxins (Figure 1) (4).
Figure 1.
The possible mechanisms involved in PD pathogenesis (Asakawa and Xia, 2012).
There is no perfect strategy in treating PD. Medical therapy is still playing the most important role for PD. Currently, levodopa (l-dopa) is certainly the best medicine for idiopathic PD. Other medicines such as dopamine receptor agonists, monoamine oxidase-B (MAO-B) inhibitors, amantadine and anticholinergic medications are also used as adjuvant drugs for l-dopa with functions of reducing the dose of l-dopa, or prolonging l-dopa's effective time, or releasing the side-effects of l-dopa (4). Traditional surgical processes include old surgical ablation (pallidotomy or thalamotomy) and newer high-frequency deep brain stimulation (DBS) of certain structures such as the subthalamic nucleus (STN). STN-DBS has been proved as an effective therapy both in clinical reports (5) and animal studies (6–8). The mechanisms of such traditional surgical therapies are unclear. It is believed that the surgical ablation or STN-DBS breaks the motor controlling circuits concerning the basal ganglia, while DBS corrects the overactive state of STN in the PD state (9–11).
If we define the medical and surgical treatments as “classic treatments”, the next generation treatments for PD should include gene therapy and stem cell transplantation. At present, the main solutions of gene therapy include two directions: one is to improve the cerebral neurotrophic factors, including the brain derived neurotrophic factor (BDNF) (12–14) and glial cell line-derived neurotrophic factor (GDNF) (15,16). However, some clinical trials regarding GDNF produced incompatible results, and thus the efficacy of improving cerebral GDNF needs more evidence (17–19). Another direction of gene therapy is to enhance GABA expression of STN by transfer of the glutamic acid decarboxylase (GAD) gene using adeno-associated virus (AAV) (20). It is hopeful that the method will be acceptable as a new treatment. The most challenging/dramatic next generation therapy is stem cell transplantation. The development of induced pluripotent stem cells (iPSCs) resolved the derivation of stem cells, and has allowed using stem cells to “make” DA neurons which is a promising strategy for DA. However, technical problems such as a low success rate in making DA neurons in vivo and a high cancer rate hold back the clinical application of stem cells.
2. The limitations of the classic therapies currently
All the classic treatments, including medical and surgical, are symptomatic therapies, which contribute little to stop/ameliorate neuron degeneration progression. Such symptomatic therapies have many weaknesses, and are far from satisfactory therapies. In this regard, PD is always thought of as an intractable disease.
l-dopa administration is regarded as the most effective therapy currently. Most of the patients experience a dramatic improvement during the early stage of treatment. Unfortunately, with the progress of PD, the dose of l-dopa has to be enhanced to achieve the same efficacy (wearing off sign). At the advanced stage, the efficacy becomes weaker, and some motor side-effects appear. Such motor side-effects of l-dopa always emerge along with the motor symptoms, which make the patients always suffer from severe motor dysfunction (4).
As to the surgical processes, several limitations are reported in the previous studies: i) The mechanisms of surgical treatments remain unclear, which will influence clinical practice using such therapies. For instance, parameter selection is a tough problem faced by the clinicians and patients undergoing DBS. Albeit the high frequency, experiential pulse (about 60 µs) is accepted by most of the researchers (11,21,22), the stimulation current intensity is a difficult problem. Recently, several animal studies revealed that the best parameters to ameliorate contrasting symptoms are quite different (7,8). The parameter selection is individualized and experience-based in different patients. Moreover, the problem of the battery life of the stimulator embedded under the skin can not be ignored. This problem usually forces the patients to make a tough decision: either to undergo another surgical operation to change the battery, or adopt a palliative pattern by reducing the stimulation current to save the battery (4). ii) DBS is an invasive therapy with a high surgical risk, and the long-term efficacy is also uncertain. Although several clinical studies claimed a good efficacy for DBS after long-term observation (23,24), the significant adverse events reported and the battery life of the stimulator can not be ignored. iii) DBS is an expensive process, which can not be popularized in some developing countries without a good health insurance system.
There are so many limitations of the classic medical and surgical therapies currently, and there is still a long way to go to apply the next generation treatments clinically. To explore a safe (less invasive), low-cost, however effective therapy is a dream of all PD clinicians. In this regard, peripheral stimulation is taken into account.
3. Overview of peripheral stimulation
DBS is an invasion strategy with high surgical risk for PD. If the electrical stimulation can be performed out of the brain, the invasiveness, risk and cost will be profoundly reduced, while the operation process can be dramatically simplified. We defined such performing electrical stimulation at structures out of the brain, such as spinal cord, peripheral nerve, muscle, skin, etc., as peripheral stimulation. Currently, mainly two sorts of peripheral stimulations are reported, namely spinal cord electrical stimulation (25) and acupuncture (4).
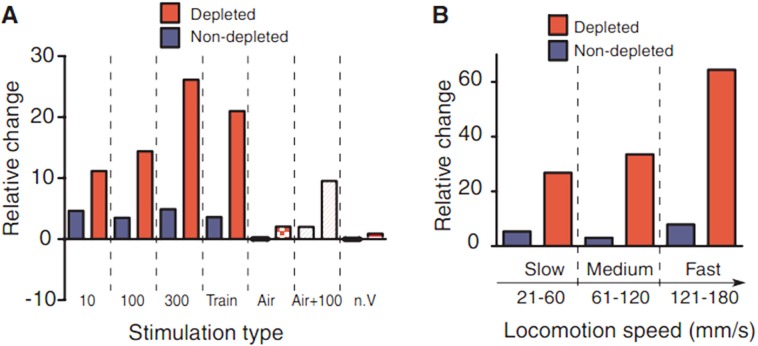
Fuentes in 2009 first reported that epidural electrical stimulation of dorsal columns in the spinal cord improves motor impairments in both rat and mouse PD models (25). They used acute pharmacologically induced DA-depleted mice and chronic 6-hydroxydopamine (6-OHDA)-lesioned rats. Dorsal column stimulation (DCS) was performed and evaluated in these models. They found 300 Hz stimulation dramatically enhanced the amount of locomotion during the stimulation period compared to the control (Figure 2A). DCS also contributed to the alleviation of bradykinesia since fast-movement components are significantly ameliorated (Figure 2B). They also found that DCS affected the firing patterns of individual neurons. When performing DCS in combination with l-dopa administration, they found DCS achieved a 4/5 dose reduction of l-dopa to reach the same efficacy (Figure 3A). Such results were repeated in 6-OHDA-lesioned rat models (Figures 3B and 3C). However, the subsequent clinical study produced incompatible results (26,27). High-frequency epidural cervical spinal cord stimulation was performed for two PD patients using different frequencies and current intensities. Unfortunately, they did not find any significant difference (Table 1). DCS stimulation is a total new approach for PD, and the mechanisms are unknown (we will discuss it in the next section). More bench and clinical studies should be involved since the dorsal column may be a potential target for peripheral stimulation.
Figure 2.
DCS improved the locomotion in DA-depleted mice (Fuentes et al., 2009). (A), Stimulation of dorsal columns enhanced the locomotion in mouse PD models, while 300 Hz stimulation achieved the best efficacy; (B), DCS significantly ameliorates the fraction of faster movement components in mouse PD models.
Figure 3.
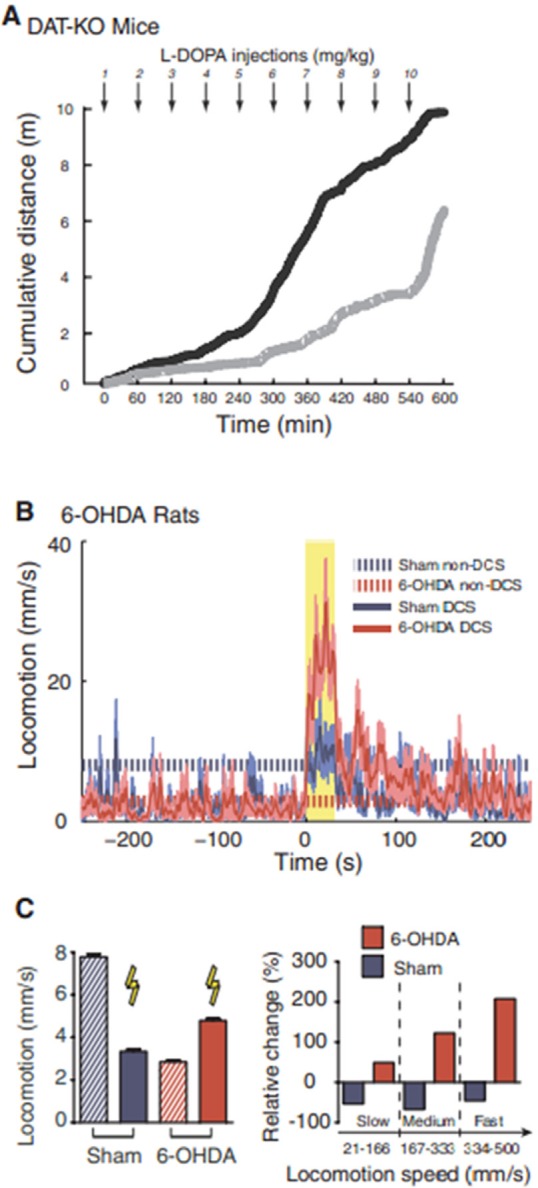
DCS improved locomotion in severely DA-depleted mice and in chronically lesioned rats (Fuentes et al., 2009). (A), DCS established better efficacy in the group undergoing DCS in combination with successive l-dopa injections (black) than the group only receiving l-dopa (gray); (B), DCS (yellow shaded area) caused significant improvements of locomotion in 6-OHDA-lesioned rats (shaded area around trace is SEM); (C), (Left) DCS specifically improved locomotion in 6-OHDA-lesioned rats, (Right) Faster movement components of locomotion were also ameliorated by DCS in the 6-OHDA-lesioned rats.
Table 1. DCS did not conduct any significant amelioration in two PD patients (Thevathasan et al., 2010).
| Motor UPDRS (score/104) | Timed10-meter walk (s) | Timed hand-arm movements (n/30s) | Timed lower limb tapping (n/30s) | |
|---|---|---|---|---|
| baseline (off stimulation) | 37.8 (11.5) | 5.5 (1.2) | 30.3 (15.9) | 54.2 (21.9) |
| subthreshold stimulation | 35.4 (12.5) | 5.4 (0.4) | 32.7 (18.0) | 54.2 (22.8) |
| suprathreshold stimulation | 37.3 (10.5) | 5.6 (1.0) | 31.2 (16.3) | 52.0 (24.7) |
| friedman (p value) | 0.44 | 0.72 | 0.32 | 0.85 |
Acupuncture is another method reported to claim “good efficacy” for treatment of PD by stimulation out of the brain (4). Acupuncture is an alternative therapy which achieves improvement of certain diseases by stimulation of acupoints at the body surface, based on the theories of traditional Chinese medicine (TCM). Acupuncture is popular in east Asia. Tons of papers published in China and Korea claimed good efficacy of acupuncture in treating PD. However, most of these studies are poorly designed, and therefore we can not get rigorous evidence to evaluate the efficacy of acupuncture. Lee in 2008 investigated the acupuncture studies and found only 11 of the 103 studies reached a level of randomized controlled trials (RCTs) with subjective outcome measures, while only 1 study described a double-blind method. This rigorously designed study by Cristian et al. could not find any efficacy for acupuncture in treating PD (28,29). Lam in 2008 evaluated acupuncture studies available in the database, and found only 10 of 784 can be attributed to RCTs, however, there are still flaws in the experimental design in these 10 studies (30). Asakawa in 2012 reviewed 2,354 original studies using acupuncture to treat PD, and could not find even one paper providing believable evidence to prove acupuncture's efficacy (Table 2). He summarized the main flaws involved in the acupuncture studies and aroused large, well-designed and multicenter clinical trials to evaluate the efficacy and safety of acupuncture (4,31).
Table 2. The common flaws in the experimental design of the acupunctural studies. Modified from the chapter of (Asakawa and Xia, 2012).
| Weaknesses in the experimental design | Comments | Solutions |
|---|---|---|
| The undefined sample size | Sample size is the most crucial aspect of formulating an experimental design. |
|
| Lack of “blinding” | Basically, double-blinding is needed; however, single blinding with objective indices is acceptable. | Methods of Allocation concealment should be included in the paper. |
| Insufficient randomization or pseudorandom | Only a small number of these studies used a robust randomization procedure while many of them used insufficient randomization, even pseudo-random in some cases. |
|
| Inappropriate control group setting | There are several flaws in using the popular protocol like C + A vs. C (C = classical therapy; A = acupuncture). |
|
| Lack of objective evaluation standard |
|
The ideas of evidence-based medicine should be set up. Change the traditional experience-based ideas. |
| Weakness in statistical analysis |
|
Consult a statistical expert. Using statistical figures. |
| Neglect in recording adverse events and withdrawals | Without adequate reporting of adverse events and withdrawals, the results from a clinical trial can not be accepted. | Adverse events and withdrawals should be recorded clearly. |
Albeit peripheral stimulation is an attractive and hopeful approach for PD, unfortunately at present there is no powerful evidence to prove efficacy clinically, either in spinal cord stimulation, or in acupuncture. More studies should be engaged in these two directions since peripheral stimulation is a good clue for developing low-invasion PD treatment.
4. The potential mechanisms involved in peripheral stimulation
Until now, we still do not know whether peripheral stimulation can be employed as a candidate new treatment for PD. If the potential mechanisms can be clarified, it will be helpful to develop effective peripheral stimulation.
4.1. The somatosensory system, a bridge between peripheral stimulation and the dopaminergic system?
The essence of this problem is that if stimulation of peripheral structures can affect the cerebral dopaminergic system. Several reports revealed that peripheral electrical stimulation is able to affect cerebral DA release. As far back as 1977, Nieoullon found electrical stimulation of the cats' forepaw resulted in DA release which was reduced in the ipsilateral substantial nigra and enhanced in the caudate nucleus (32). Subsequently, several studies found stimulation of the somatosensory system affects the dopaminergic system, which is related to motor function (Figure 4) (33–35). These findings all indicated a close connection between the somatosensory system and DA system. However, the anatomical structure, distribution and circuits of the somatosensory system are poorly understood. Inoue's 2004 paper deduced the possible anatomical pathways which exist between the mesencephalic DA-ergic nuclei and the sensory system causing the observed modulation of DA release in the basal ganglia. One important plausible connection is from the sensory areas in the contralateral neocortex which projects back into the ipsilateral striatum, and then activates ipsilateral DA release from mesencephalic DA-ergic nuclei. Another possible anatomical connection could be from the projecting fibers between the nuclei intralaminares thalami and the SNpc through the striatum on the contralateral side. Furthermore, there is another potential pathway between the ventral tegmental area and the mesencephalic central gray area, which is innervated collaterally by the spinothalamic tract (4,33).
Figure 4.
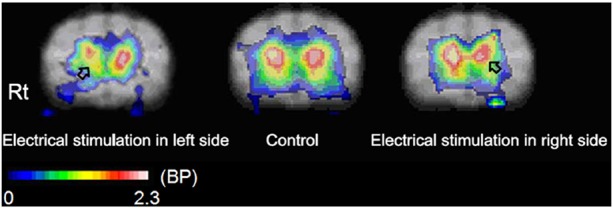
DA-release marked by striatal D2-like receptor activity was affected by forepaw electrical stimulation (Inoue et al., 2004). (Left), The electrical stimulation in the left paw decreased the BP value in the right basal ganglia; (Center), A parametric image of BP without stimulation. (Inoue et al., 2004); (Right), The electrical stimulation in the right paw decreased the BP value in the left basal ganglia. The BP value is shown in the color bar in coregistered MRI. Rt: The right side of the cat brain.
The mysterious somatosensory pathways may play a role in the connection between peripheral stimulation and the DA system. We hypothesize that the dorsal column and the effective acupoints (if the efficacy can be strictly verified) should be the “stations” of the somatosensory pathways. The motor functions related to the dopaminergic system are then affected through the somatosensory pathways when the “stations” are undergoing electrical stimulation.
4.2. DCS unlocks the basal ganglia-cortical circuits?
Besides the dopaminergic system, another important possibility is that DCS (or effective acupuncture) activates the locked basal ganglia-cortical circuits in the PD state. In a later paper to explain the mechanisms of DCS, Fuentes pointed out that the improvement of motor function might be the result of basal ganglia-cortical circuits being unlocked and conducted by synchronous stimulation of a number of tactile afferent fibers terminating in the dorsal column nuclei and ascending through the lemniscal pathway to cortical areas through the thalamus, and the thalamic nuclei most directly activated by DCS differ from those primarily affected by STN-/GPi-DBS. In addition, activating the pedunculopontine nucleus (PPN) through some ascending and descending anatomical tracers from the cervical and thoracic spinal cord dorsal horns projects directly to PPN and may play a role in the mechanisms of DCS (36). It has been well investigated that activation of PPN contributes to improvement of the initiation of movement through a descending drive to locomotor circuits directly; and activation/desynchronization of the motor cortex along with certain structures within the basal ganglia through ascending thalamocortical pathways indirectly (36–38).
No matter what the mechanisms are concerning the dopaminergic system, or concerning the basal ganglia circuits, achieving deeper understanding of the potential anatomical connections is crucial. It may be a key to uncover the secrets of peripheral stimulation.
5. Conclusion
Although only one rigorously designed bench study (25) verified the efficacy of peripheral stimulation in treating PD, we can expect the possibility of treating PD by stimulation outside of the brain. More bench and clinical studies should be designed and verified for peripheral stimulation. Certainly the efficacy of acupuncture should be also strictly verified. The “effective” acupoints may be employed in affording hints of stimulating targets or finding the “stations” of unknown anatomical connections.
Acknowledgements
TA was supported by grants from the Japan Society for the Promotion of Science (Grant-in-Aid for Young Scientists, Type B, No. 20791025 and Grant-in-Aid for Scientific Research C, General, No. 24592157).
References
- 1. Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002; 14:223-236; discussion 222. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988; 334:345-348 [DOI] [PubMed] [Google Scholar]
- 3. Przedborski S. Pathogenesis of nigral cell death in Parkinson's disease. Parkinsonism Relat Disord. 2005; 11 (Suppl 1):S3-S7 [DOI] [PubMed] [Google Scholar]
- 4. Asakawa T, Xia Y. Acupuncture Treatment For Parkinso's Disease, in Current Research in Acupuncture (Xia Y, Ding G, eds.). Springer, New York, USA, 2012; pp. 215-255 [Google Scholar]
- 5. Benabid AL, Koudsié A, Benazzouz A, Fraix V, Ashraf A, Le Bas JF, Chabardes S, Pollak P. Subthalamic stimulation for Parkinson's disease. Arch Med Res. 2000; 31:282-289 [DOI] [PubMed] [Google Scholar]
- 6. Fang X, Sugiyama K, Akamine S, Namba H. Improvements in motor behavioral tests during deep brain stimulation of the subthalamic nucleus in rats with different degrees of unilateral parkinsonism. Brain Res. 2006; 1120:202-210 [DOI] [PubMed] [Google Scholar]
- 7. Asakawa T, Sugiyama K, Akamine S, Yokoyama C, Shukuri M, Mizuma H, Tsukada H, Onoe H, Namba H. The food reaching test: A sensitive test of behavioral improvements by deep brain stimulation in MPTP-treated monkey. Neurosci Res. 2012; 74:122-128 [DOI] [PubMed] [Google Scholar]
- 8. Fang X, Sugiyama K, Akamine S, Sun W, Namba H. The different performance among motor tasks during the increasing current intensity of deep brain stimulation of the subthalamic nucleus in rats with different degrees of the unilateral striatal lesion. Neurosci Lett. 2010; 480:64-68 [DOI] [PubMed] [Google Scholar]
- 9. Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990; 85:119-146 [PubMed] [Google Scholar]
- 10. Alexander GE, Crutcher MD, DeLong MR. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986; 9:357-381 [DOI] [PubMed] [Google Scholar]
- 11. Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002; 43:111-117 [DOI] [PubMed] [Google Scholar]
- 12. Frim DM, Uhler TA, Galpern WR, Beal MF, Breakefield XO, Isacson O. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc Natl Acad Sci U S A. 1994; 91:5104-5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang XB, Liu XY, Li FQ, Luo Y, Lu J, Zhang WM, Wang XM, Han JS. Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates BDNF mRNA in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Res Mol Brain Res. 2002; 108:51-59 [DOI] [PubMed] [Google Scholar]
- 14. Shults CW, Kimber T, Altar CA. BDNF attenuates the effects of intrastriatal injection of 6-hydroxydopamine. Neuroreport. 1995; 6:1109-1112 [DOI] [PubMed] [Google Scholar]
- 15. Burke RE, Antonelli M, Sulzer D. Glial cell line-derived neurotrophic growth factor inhibits apoptotic death of postnatal substantia nigra dopamine neurons in primary culture. J Neurochem. 1998; 71:517-525 [DOI] [PubMed] [Google Scholar]
- 16. Clarkson ED, Edwards-Prasad J, Freed CR, Prasad KN. Immortalized dopamine neurons: A model to study neurotoxicity and neuroprotection. Proc Soc Exp Biol Med. 1999; 222:157-163 [DOI] [PubMed] [Google Scholar]
- 17. Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003; 9:589-595 [DOI] [PubMed] [Google Scholar]
- 18. Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006; 59:459-466 [DOI] [PubMed] [Google Scholar]
- 19. Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005; 102:216-222 [DOI] [PubMed] [Google Scholar]
- 20. Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: An open label, phase I trial. Lancet. 2007; 369:2097-2105 [DOI] [PubMed] [Google Scholar]
- 21. Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003; 23:1916-1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang X, Sugiyama K, Akamine S, Namba H. The stepping test and its learning process in different degrees of unilateral striatal lesions by 6-hydroxydopamine in rats. Neurosci Res. 2006; 55:403-409 [DOI] [PubMed] [Google Scholar]
- 23. Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003; 99:489-495 [DOI] [PubMed] [Google Scholar]
- 24. Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003; 349:1925-1934 [DOI] [PubMed] [Google Scholar]
- 25. Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA. Spinal cord stimulation restores locomotion in animal models of Parkinson's disease. Science. 2009; 323:1578-1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicolelis MA, Fuentes R, Petersson P, Thevathasan W, Brown P. Spinal cord stimulation failed to relieve akinesia or restore locomotion in Parkinson disease. Neurology. 2010; 75:1484-1485 [DOI] [PubMed] [Google Scholar]
- 27. Thevathasan W, Mazzone P, Jha A, Djamshidian A, Dileone M, Di Lazzaro V, Brown P. Spinal cord stimulation failed to relieve akinesia or restore locomotion in Parkinson disease. Neurology. 2010; 74:1325-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cristian A, Katz M, Cutrone E, Walker RH. Evaluation of acupuncture in the treatment of Parkinson's disease: A double-blind pilot study. Mov Disord. 2005; 20:1185-1188 [DOI] [PubMed] [Google Scholar]
- 29. Lee MS, Shin BC, Kong JC, Ernst E. Effectiveness of acupuncture for Parkinson's disease: A systematic review. Mov Disord. 2008; 23:1505-1515 [DOI] [PubMed] [Google Scholar]
- 30. Lam YC, Kum WF, Durairajan SS, Lu JH, Man SC, Xu M, Zhang XF, Huang XZ, Li M. Efficacy and safety of acupuncture for idiopathic Parkinson's disease: A systematic review. J Altern Complement Med. 2008; 14:663-671 [DOI] [PubMed] [Google Scholar]
- 31. Asakawa T, Xia Y. Future Research in Acupuncture - Better design and analysis for novel and valid findings, in Current Research in Acupuncture (Xia Y, Ding G, eds.). Springer, New York, USA, 2012; pp. 687-727 [Google Scholar]
- 32. Nieoullon A, Cheramy A, Glowinski J. Nigral and striatal dopamine release under sensory stimuli. Nature. 1977; 269:340-342 [DOI] [PubMed] [Google Scholar]
- 33. Inoue M, Katsumi Y, Hayashi T, Mukai T, Ishizu K, Hashikawa K, Saji H, Fukuyama H. Sensory stimulation accelerates dopamine release in the basal ganglia. Brain Res. 2004; 1026:179-184 [DOI] [PubMed] [Google Scholar]
- 34. Rothblat DS, Schneider JS. Response of caudate neurons to stimulation of intrinsic and peripheral afferents in normal, symptomatic, and recovered MPTP-treated cats. J Neurosc. 1993; 13:4372-4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J Neurophysiol. 1987; 57: 201-217 [DOI] [PubMed] [Google Scholar]
- 36. Fuentes R, Petersson P, Nicolelis MA. Restoration of locomotive function in Parkinson's disease by spinal cord stimulation: Mechanistic approach. Eur J Neurosci. 2010; 32:1100-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hikosaka O. Basal ganglia - possible role in motor coordination and learning. Curr Opin Neurobiol. 1991; 1:638-643 [DOI] [PubMed] [Google Scholar]
- 38. Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ. Physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord. 2009; 24:319-328 [DOI] [PubMed] [Google Scholar]