Summary
Alzheimer's disease (AD) is a severe condition in aging countries. The currently used drugs including donepezil, rivastigmine, galantamine, and memantine are effective in managing the symptoms. However, they are hardly capable of preventing, halting, or reversing the disease. In the long history of development of traditional Chinese medicine, much experience has accumulated and is summarized in treatment of diseases that correspond to the concept of AD. In recent years, exploration of natural active ingredients from medicinal herbs for treatment of AD has attracted substantial attention. Some flavonoids have been revealed to have a variety of biological actions such as scavenging free radicals, inhibiting neuron apoptosis, and nurturing neuronal cells that constitute the basis for treatment of AD. In this article, we review recent research progress on flavonoids isolated from traditional Chinese medicine against AD and their underlying mechanisms.
Keywords: Ginkgo flavonoids, soy isoflavones, puerarin, total flavonoids of Baical Skullcap stem and leaf, liquiritin, apigenin
1. Introduction
Alzheimer's disease (AD) is characterized by progressive deterioration in intellect including memory and cognitive functions. It is the most common type of dementia among older people, accounting for 50–75% of all dementia cases (1). The number of AD patients was estimated at 36 million in 2010 and will triple in the world by 2050 (2). In China, this figure is estimated at 9 million currently and the prevalence rate of AD in the population over the age of 60 years is 2.43% (3,4). Proportionate increases over the next forty years in the number of people with AD will be much steeper in China since it is witnessing the aging of society in which the population over the age of 60 years will account for approximately 31% (about 400 million calculated on the current population base) of the whole population by the year of 2050 (5). These epidemiological data have painted a less than optimistic outlook in prevention and treatment of this disease in the world, especially in those countries with a rapidly aging society such as China.
The currently approved drugs for treatment of AD, e.g. donepezil, rivastigmine, galantamine, and memantine, aim to either inhibit acetylcholine esterase to increase the levels of the neurotransmitter acetylcholine, or antagonize N-methyl-d-aspartic acid (NMDA)-type glutamate receptors to prevent aberrant neuronal stimulation (6,7). These medicines, however, exhibit modest and transient effects in improving disease manifestation and could hardly prevent, halt, or reverse the disease (2). The typical course of AD lasts for a decade or so, from the mildest stage when the symptoms like memory problems appear to the most severe stage when the patients must depend on others for basic activities of daily living and finally die in a completely helpless state. The long duration of AD and shortage of effective or curative treatments bring an enormous emotional and financial burden on patients, their families and society.
In the past several decades, much research has been done to evaluate the anti-AD effects of natural agents isolated from traditional Chinese medicines from perspectives such as scavenging free radicals, inhibiting lipid peroxidation, suppressing neuronal apoptosis, enhancing the function of cholinergic neurons, and improving behavioral abnormalities in experimental animal models (8–10). Flavonoids are a series of compounds that are spread widely in higher plants and ferns and have attracted much attention due to their various biological actions (11). The characteristic chemical structures of these compounds is two benzene rings with hydroxyl groups linked by a three-carbon chain (11). The most commonly known biological action of flavonoids is their antioxidant activity, which could be understood from the reduction properties of phenol hydroxyls in the chemical structures. That said, compounds of this type exhibit various pharmacological effects and clinical efficacies that may not be solely related to their anti-oxidative activities, such as effects on the vascular system, inflammatory response, and estrogen-like effects (11). These actions of flavonoids constitute the underlying basis for their anti-AD effects. In this article, we review recent research progress on flavonoids isolated from traditional Chinese medicine against AD and their underlying mechanisms.
2. Pathological basis of AD
The presence of extracellular amyloid plaques, intracellular neurofibrillary tangles (NFTs), and loss of neurons and synapses in the cerebral cortex and certain subcortical regions in the brain are the main features of AD (2). A great deal of evidence indicates that the onset of AD is probably the consequences of complex interactions among genetic, environmental, and lifestyle factors (12). The pathogenesis of AD has been revealed to correlate with the following aspects.
2.1. Genetic factors
AD has been demonstrated to be related to mutations or polymorphisms of at least four genes, including amyloid precursor protein (APP), presenilin (PS)-1, PS-2, and apolipoprotein E4 (APOE4) located at chromosomes 21, 14, 1, and 19, respectively (13). Early-onset (< 60 years) familial AD, which probably accounts for less than 1% of AD cases, was found to be caused by mutations in APP, PS-1, and PS-2 genes (14,15). It was demonstrated that genetic abnormality occurred in at least one of these three genes in the early-onset familial AD. Late-onset (> 60 years) familial and sporadic AD, which accounts for most AD cases, has been genetically linked to APOE4 which has a gene-dosage effect on increasing the risk and lowering the age of onset of the disease (16,17). In addition, genetic defects of PS-1 and APOE4 were usually discovered in sporadic AD (12).
2.2. Aggregation and accumulation of amyloid-β (Aβ) in the brain
The amyloid plaques of AD brains largely consist of Aβ protein, which is a 39–42 amino acid protein derived from its parent protein, APP, by proteolytic cleavage at the β- and γ-secretase cleavage sites (12). The amyloid cascade hypothesis suggested Aβ is the pathogenic factor and drives the progression of this disease. The aggregation and accumulation of Aβ, which may result from increased production of Aβ, decreased degradation by Aβ-degrading enzymes, or reduced clearance across the blood-brain barrier, gave rise to plaques which induced neurodegeneration and finally led to the clinical dementia syndrome typical of AD (2). It was found that nonfibrillar assemblies of Aβ such as Aβ dimers, trimers, and larger oligomers are more pathogenic than insoluble Aβ fibrils found in amyloid plaques and monomeric Aβ (2). The neurotoxic activities of Aβ were expressed through a mechanism that induces intracellular generation of reactive oxygen species (ROS), lipid peroxidation, calcium overload, and eventually neuronal death (18–22).
2.3. Formation of NFT in neurons
Besides the abnormal accumulation of amyloid plaques, another pathologic feature of AD is intracellular formation of NFT which are primarily made up of aggregated tau protein bearing abnormal posttranslational modifications, including increased phosphorylation and acetylation (23–25). Tau protein is abundant in neurons with a function of stabilizing microtubules. The progressive accumulation of abnormal tau protein may lead to instability of the microtubular structure and the consequent loss of effective intracellular transport, and ultimately, neuronal death (26,27).
2.4. Disequilibrium of calcium homeostasis
Overload of intracellular Ca2+ concentration ([Ca2+]i) is one of the key factors that leads to neuron damage or death (28). Ca2+ is a major intracellular messenger that mediates many physiological responses of neurons to chemical and electrical stimulation. A regulated rise in [Ca2+]i could trigger many physiological events, while an unregulated elevation in [Ca2+]i can alter cell viability or induce cell apoptosis through activating proteases (i.e. calpains), reinforcing signals leading to caspase activation, or triggering other catabolic processes mediated by lipases and nucleases (29).
2.5. Free radical oxidative damage
Much evidence supported that free radical induced oxidative damage may play a role in the pathogenesis of AD (30,31). Features of brain, including a high content of readily oxidized fatty acids, high use of oxygen, and low levels of antioxidants, make it especially sensitive to oxidative damage. Both postmortem and living patients with AD demonstrated evidence of oxidative damage in brain tissue. Free radicals may attack and damage lipids, proteins, and DNA, lead to change in structure and function of these molecules, and consequently result in cellular damage, dysfunction and cell death (32). Besides, oxidative stress could also enhance Aβ production, which further induces nerve tissue damage (33).
2.6. Mitochondrial impairments
Mitochondrial dysfunction has a certain impact on the pathogenesis of AD as indicated by impaired mitochondrial respiration observed in brain, platelets, and fibroblasts of AD patients (34). Energy failure, increased oxidative stress, and accumulation of Aβ could be caused by dysfunction of mitochondria, which would damage neurons and could explain many of the biochemical, genetic, and pathological features of sporadic AD (35).
3. Flavonoids as anti-AD agents
Thus far, flavonoids including ginkgo flavonoids, soy isoflavones, puerarin, total flavonoids of Baical Skullcap stem and leaf, liquiritin, apigenin, rhodosin, and hyperoside were reported to have potent effects against AD (Table 1).
Table 1. Flavonoids isolated from traditional Chinese medicine in treatment of AD.
| Agents | Structures or contents | Typical origin | Reference |
| Gingko flavonoids | Mixture: mainly including quercetin, kaempferol, isorhamnetin, and biflavonoids like ginkgetin, isoginkgetin, and amentoflavone | Ginkgo biloba L. leaves | 36,37 |
| Soy isoflavones | Mixture: mainly including daidzin, daidzein, genistin, genistein, and glycitin, glycitein | Glycine max | 41,42 |
| Total flavonoids of Baical Skullcap stem and leaf | Mixture: mainly including scutellarin, baicalin, and chrysin | Radix puerariae roots | 64 |
| Puerarin | 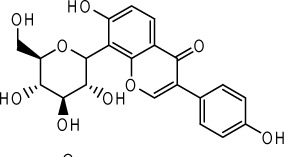 |
Scutellaria baicalensis Georgi stems and leaves | 69 |
| Liquiritin | 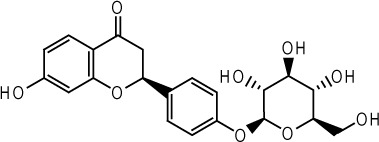 |
Glycyrrhiza uralensis Fisch. roots | 73 |
| Apigenin | 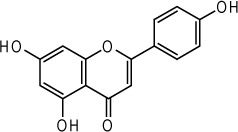 |
Apium graveolens | 76 |
| Hyperin | 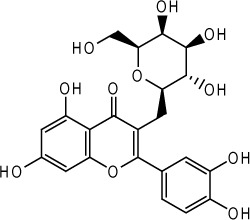 |
Hypericum perforatum L. | 81 |
| Rhodosin | 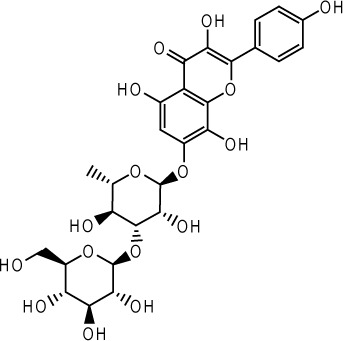 |
Rhodiola rosea | 83 |
3.1. Ginkgo flavonoids
Ginkgo flavonoids are the main constituents in the extract of Ginkgo biloba (EGB). Ginkgo flavonoids consist mainly of flavonols such as quercetin, kaempferol, and isorhamnetin and biflavonoids like ginkgetin, isoginkgetin, and amentoflavone (36,37). These ginkgo flavonoids have free radical scavenging effects and could inhibit lipid peroxidation. Studies demonstrated that mitochondrial DNA from brain of old rats exhibited oxidative damage that is significantly higher than that from young rats (38). In addition, mitochondrial glutathione was more oxidized and peroxide formation in mitochondria was higher in old than in young rats (38). Treatment with EGB could partially prevent the indices of oxidative damage in brain from old animals (38). Other studies demonstrated that ginkgo flavonoids exhibited neuroprotective effects via antioxidant activity in brain damaged mice caused by ischemia-reperfusion (39). One randomized, double-blind, placebo-controlled, and multicenter clinical trial indicated that EGB was safe and capable of stabilizing and improving the cognitive performance and the social functioning of AD patients for 6 months to 1 year (40). Currently, EGB is used in clinics as a medical drug for treatment of AD in China, France, and Germany.
3.2. Soy isoflavones
Soy isoflavones attracted much interests in recent years due to its estrogen-like effects and role in influencing sex hormone metabolism. The main constituents of isoflavones are demonstrated to be daidzin, daidzein, genistin, genistein, glycitin, and glycitein (41,42). It is thought that soy isoflavones intake is a “natural” way to replenish the aging body's declining estrogen levels and thus relieve menopausal symptoms. A previous study demonstrated that postmenopausal women who undertook estrogen-replacement therapy had a significantly lower risk for the onset of AD than women who did not (43). These facts suggested the possible benefits of soy isoflavones in AD prevention and treatment.
Mechanisms of anti-AD effects of estrogen lie in the following aspects (44,45). i) Estrogen reduces the production of Aβ (46). Estrogen is capable of regulating the metabolism of APP to enhance the production of soluble APP and decrease the accumulation of Aβ, thus exerting neuroproductive effects. ii) Estrogen antagonizes the toxicity of Aβ (47). Aβ is capable of promoting lipid peroxidation at the membrane of neuronal cells, leading to production of ROS which further impairs the membrane proteins and breaks the homeostasis of ion balance. The membrane depolarizes and thereby Ca2+ influx occurs via NMDA receptor channels, which enhances the damage of DNA and lipids and finally leads to neuronal death. Studies indicated that estradiol is a natural anti-oxidant for membrane lipid peroxidation, thereby alleviating the toxicity of Aβ to neurons (48). iii) Estrogen promotes Ca2+ outflow (49). Estrogen is capable of releasing intracellular Ca2+ via non-genomic mechanisms, which is not affected by the concentration of extracellular Ca2+. It was found that estrogen could inhibit the elevation of intracellular Ca2+ concentration induced by glutamic acid and antagonize the disequilibrium of calcium homeostasis caused by Aβ. iv) Estrogen inhibits inflammation mediated by the transcription factor nuclear factor κB (NF-κB) which is involved in the pathological process of AD (50). v) Estrogen promotes synaptic growth and expressions of nerve growth factor (NGF) and its receptor (51). NGF was demonstrated to be a cytokine that could increase the mRNA levels of choline acetyltransferase, enhance the activities of choline acetyltransferase, and promote the release of acetylcholine. Thus, estrogen is capable of enhancing the effects of NGF. vi) Estrogen prevents excessive phosphorylation of tau protein (52). Although estrogen exhibits the various above potential actions, its application in clinics for treatment of AD is dismal since it also causes side effects to non-neuronal cells, such as increasing the incidence of breast and endometrial cancer (53–55).
Previous studies found that phytoestrogens such as genistein, one of the main ingredients of soy isoflavones, exerted pharmacological effects in a tissue specific manner (56). They selectively act on non-reproductive tissues to a certain degree and thus reduce the risk of side effects. Animal studies indicated that soy isoflavones were capable of improving learning and memory abilities through influencing the brain cholinergic system and reducing age-related neuron loss especially in female rats (57–59). The underlying mechanisms of favorable effects of soy isoflavones on cognitive function were thought to relate to their potential to mimic the actions and functions of estrogens in the brain (60), and promote the synthesis of acetylcholine and neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and NGF in the hippocampus and frontal cortex (61,62). A randomized, double-blind, cross-over, and placebo-controlled trial revealed that soy isoflavones were safe and had positive effects on cognitive function, especially verbal memory, in postmenopausal women (63). These studies provided evidence of the potential usefulness of soy isoflavones in treatment of AD patients.
3.3. Puerarin
Puerarin is an isoflavanone glycoside extracted from species in the family Leguminosae such as Radix puerariae and is currently used to treat ischemic cerebrovascular disease and other vascular dysfunctions in China (64). Studies found that puerarin had potent effects in improving learning and memory disorders induced by scopolamine or D-galactose in a mouse model (65). Yan et al. reported that puerarin protected neurons against apoptosis in the cortex and hippocampus of AD rats caused by Aβ25–35 through downregulating Aβ1–40 and Bax expression in brain tissues, therefore alleviating the spatial learning and memory impairment of diseased animals (66). The anti-AD effects of puerarin were also suggested to be related to its abilities in decreasing the lipid peroxidase levels and increasing superoxide dismutase levels in brain tissues, enhancing cerebral blood flow, and improving brain microcirculation (67,68).
3.4. Total flavonoids of Baical Skullcap stem and leaf
Baical Skullcap is a frequently used traditional Chinese medicine in China. Studies on its active ingredients revealed that the total flavonoids extracted from the stem and leaf, mainly including scutellarin, baicalin, and chrysin, exhibited a series of pharmacological effects such as anti-inflammation, prevention from myocardial damage induced by ischemia-reperfusion, and improved cerebral ischemia (69,70). Regarding its effects against AD, Zuo et al. found that total flavonoids of Baical Skullcap stem and leaf were capable of protecting hippocampal neurons against damage induced by injection of Aβ25–35 in hippocampus in rat (71). The underlying mechanisms were related to its actions of decreasing the accumulation of lipid peroxide and proliferation of glial cells induced by Aβ25–35 (71). Another study conducted by Ye et al. demonstrated that the total flavonoids alleviated memory and learning injury and protected morphological change of hippocampal neurons in AD rats induced by Aβ25–35 injection (72). These studies suggested the potential efficacies of total flavonoids of Baical Skullcap stem and leaf against AD.
3.5. Liquiritin
Liquiritin is an extract from the root of Glycyrrhiza uralensis Fisch. (73). Yang et al. investigated the protective effects of liquiritin on primary cultured rat hippocampal neurons (74). They found that pretreatment with liquirtin for 6 h decreased the elevated levels of intracellular Ca2+ concentration and neuron apoptosis caused by Aβ25–35. On the other hand, liquirtin is capable of enhancing the effects of nerve growth factor in extending neuraxons (74). It is worth noting that liquirtin could also specifically inhibit the activity of acetylcholinesterase and promote the differentiation of neuronal stem cells into cholinergic neurons (74,75). The neuroprotective and neurotrophic effects make liquirtin a promising agent against AD.
3.6. Apigenin
Apigenin is a flavone usually obtained from Apium graveolens (76). It is a potent chelating agent that could decrease the metal ions participating in radical reactions and therefore reduce the creation of free radicals (77). In addition, apigenin could serve as an anti-oxidant to scavenge free radicals such as oxygen, nitric oxide (NO), and superoxide anion. On the other hand, apigenin possesses estrogen-like effects which are similar to the actions of estradiol (78). Due to these biological actions, apigenin was reported to protect human neuroblastoma cells SH-SY5Y against apoptosis induced by oxidative stress in vitro (79). In vivo, apigenin was found to improve the memory and learning disorders of aging mice induced by D-galactose (80).
3.7. Other flavonoids
Hyperoside is a flavonol isolated from species of Hypericum (81). In the mouse ischemia-reperfusion injury model, hyperoside was capable of inhibiting lactate dehydrogenase activity decline in brain tissues and obviously improve memory and learning disorders of model mice (82). Rhodosin is also a flavonol obtained from the root of Rhodiola rosea (83). Rhodosin functions as an anti-oxidant which scavenges free radicals, reduces the content of lipid peroxide, and inhibits degeneration of mitochondria in cerebrum cells and hippocampal pyramidal cells (68). Administration of rhodosin was reported to be capable of improving the memory and learning abilities of aging or AD mice (84).
4. Conclusion and prospects
AD is a chronic neurodegenerative disease in the central nervous system characterized by progressive memory loss and damage of cognition function. The pathogenesis underlying AD is complicated and not yet well clarified. The currently used medications for treatment of AD are mainly symptom-management drugs. Although they do improve symptoms such as memory disorders and play a key role in treatment of AD at present, these drugs are not capable of reversing the progress of AD. Disease-modifying drugs that aim at root causes of AD are the current research focus and represent the future direction of new drugdevelopment.
In light of the pathogenic complexities of AD, it is probably unlikely that single-target drugs will achieve satisfactory curative effects. The main reasons include the following points. i) The onset of this disease involves abnormalities of multiple genes such as APP, PS-1, PS-2, and/or APOE4. ii) The current targets are multifunctional and strong inhibition or activation of one target may lead to undesired side effects. For example, acetylcholinesterase inhibitors may cause accumulation of peripheral acetylcholine, resulting in peripheral acetylcholine responses such as nausea and vomiting. iii) The single-target theory overlooks possible molecular interactions which may constitute cross-talk. Intervention in one of them may not finally affect cell functions or status due to compensatory mechanisms. Given these considerations, development of multiple-target drugs that have both neuroprotective and neurotrophic efficacies are rational strategies in treatment of AD.
Flavonoids reviewed in this article exhibit a series of biological actions against AD including increasing the functions of cholinergic neurons, suppressing typical pathology changes such as neuronal apoptosis, and/or regulating neurotrophy and regeneration relevant mechanisms. These pharmacological effects suggest that more flavonoids may be translated into a new type of anti-AD drugs in the future.
References
- 1. World Alzheimer Report 2009. Alzheimer's Disease International. http://www.alz.co.uk/research/files/WorldAlzheimerReport.pdf (accessed September 21, 2012). [Google Scholar]
- 2. Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012; 148:1204-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. News. Alzheimer's Disease Chinese (ADC). http://www.adc.org.cn/html/news/qqzx_1268.shtml (accessed September 21, 2012). [Google Scholar]
- 4. Chen CF, He CL, Chen HX, Sun YF, Pan X. A summary of dementia studies in China. Journal of Ningbo University (Educational Science Edition). 2012; 34:45-50 [Google Scholar]
- 5. News. http://news.xinhuanet.com/life/2010-08/20/c_12465497.htm (accessed September 21, 2012).
- 6. Cummings JL. Alzheimer's disease. N Engl J Med. 2004; 351:56-67 [DOI] [PubMed] [Google Scholar]
- 7. Sun XT, Jin L, Ling PX. Review of drugs for Alzheimer's disease. Drug Discov Ther. 2012; 6:285-290 [PubMed] [Google Scholar]
- 8. Okonogi S, Chaiyana W. Enhancement of anticholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discov Ther. 2012; 6:249-255 [PubMed] [Google Scholar]
- 9. Mishra M, Huang J, Lee YY, Chua DS, Lin X, Hu JM, Heese K. Gastrodia elata modulates amyloid precursor protein cleavage and cognitive functions in mice. Biosci Trends. 2011; 5:129-138 [DOI] [PubMed] [Google Scholar]
- 10. Geng HM, Wang YZ, Zhang DQ. Natural medicines in treatment of Alzheimer disease. China Pharmacy. 2006; 17:1019-1021 [Google Scholar]
- 11. Wu LJ. Flavonoids. In: Natural Medicine Chemistry (Wu LJ, ed.). 4th ed., People's Medical Publishing House, Beijing, China, 2003; p. 173 [Google Scholar]
- 12. Swerdlow RH. Pathogenesis of Alzheimer's disease. Clin Interv Aging. 2007; 2:347-359 [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang JT. In: Research Progresses of Neuropharmacology (Zhang JT, ed.). People's Medical Publishing House, Beijing, China, 2002; pp. 16-29 [Google Scholar]
- 14. Campion D, Dumanchin C, Hannequin D, et al. Early-onset autosomal dominant Alzheimer disease: Prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999; 65:664-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: Back to the future. Neuron. 2010; 68:270-281 [DOI] [PubMed] [Google Scholar]
- 16. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993; 261:921-923 [DOI] [PubMed] [Google Scholar]
- 17. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997; 278:1349-1356 [PubMed] [Google Scholar]
- 18. Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994; 77:817-827 [DOI] [PubMed] [Google Scholar]
- 19. Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997; 68:255-264 [DOI] [PubMed] [Google Scholar]
- 20. Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G, Smith MA. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001; 30:447-450 [DOI] [PubMed] [Google Scholar]
- 21. Wei W, Wang X, Kusiak JW. Signaling events in amyloid beta-peptide-induced neuronal death and insulin-like growth factor I protection. J Biol Chem. 2002; 277:17649-17656 [DOI] [PubMed] [Google Scholar]
- 22. Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992; 12:376-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee VM. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011; 2:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010; 7:656-664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010; 67:953-966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U S A. 1997; 94:298-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986; 83:4044-4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium. 2010; 47:183-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu B, Xu ZF, Deng Y. Effect of manganese exposure on intracellular Ca2+ homeostasis and expression of NMDA receptor subunits in primary cultured neurons. Neurotoxicology. 2009; 30:941-949 [DOI] [PubMed] [Google Scholar]
- 30. Pratico D, Delanty N. Oxidative injury in diseases of the central nervous system: Focus on Alzheimer's disease. Am J Med. 2000; 109:577-585 [DOI] [PubMed] [Google Scholar]
- 31. Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J Alzheimers Dis. 2010; 19:341-353 [DOI] [PubMed] [Google Scholar]
- 32. Tuppo EE, Forman LJ. Free radical oxidative damage and Alzheimer's disease. J Am Osteopath Assoc. 2001; 101(12 Suppl Pt 1):S11-S15 [PubMed] [Google Scholar]
- 33. Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M. Oxidative stress activates a positive feedback between the γ-and β-secretase cleavages of the β-amyloid precursor protein. J Neurochem. 2008; 104:683-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mancuso M, Orsucci D, Siciliano G, Murri L. Mitochondria, mitochondrial DNA and Alzheimer's disease. What comes first? Curr Alzheimer Res. 2008; 5:457-468 [DOI] [PubMed] [Google Scholar]
- 35. Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer's disease. Med Hypotheses. 2004; 63:8-20 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Cao J, Weng JH, Zeng S. Simultaneous determination of quercetin, kaempferol and isorhamnetin accumulated human breast cancer cells, by high-performance liquid chromatography. J Pharm Biomed Anal. 2005; 39:328-333 [DOI] [PubMed] [Google Scholar]
- 37. Hyun SK, Kang SS, Son KH, Chung HY, Choi JS. Biflavone glucosides from Ginkgo biloba yellow leaves. Chem Pharm Bull (Tokyo). 2005; 53:1200-1201 [DOI] [PubMed] [Google Scholar]
- 38. Sastre J, Millan A, Garcia de la Asuncion J, Pla R, Juan G, Pallardo, O'Connor E, Martin JA, Droy-Lefaix MT, Vina J. A Ginkgo biloba extract (EGb 761) prevents mitochondrial aging by protecting against oxidative stress. Free Radic Biol Med. 1998; 24:298-304 [DOI] [PubMed] [Google Scholar]
- 39. Zhu JT, Choi RC, Chu GK, Cheung AW, Gao QT, Li J, Jiang ZY, Dong TT, Tsim KW. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: A comparison of different flavonoids in activating estrogenic effect and in preventing β-amyloid-induced cell death. J Agric Food Chem. 2007; 55:2438-2445 [DOI] [PubMed] [Google Scholar]
- 40. Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997; 278:1327-1332 [DOI] [PubMed] [Google Scholar]
- 41. Manjanatha MG, Shelton S, Bishop ME, Lyn-Cook LE, Aidoo A. Dietary effects of soy isoflavones daidzein and genistein on 7,12-dimethylbenz[a]anthracene-induced mammary mutagenesis and carcinogenesis in ovariectomized Big Blue transgenic rats. Carcinogenesis. 2006; 27:2555-2564 [DOI] [PubMed] [Google Scholar]
- 42. Wang CE, Liu SY. The components, contents and characteristics of soy isoflavones. Food Sci. 1998; 19:39-43 [Google Scholar]
- 43. Henderson VW. Estrogen-containing hormone therapy and Alzheimer's disease risk: Understanding discrepant inferences from observational and experimental research. Neuroscience. 2006; 138:1031-1039 [DOI] [PubMed] [Google Scholar]
- 44. Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids. 2007; 72:381-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu RT, Lv QJ. Progress in the research on multitarget-directed drugs against Alzheimer's disease. Acta Pharmaceutica Sinica. 2009; 44:258-263 [PubMed] [Google Scholar]
- 46. Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci U S A. 2005; 102:19198-19203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: Role in protection against β-amyloid peptide-induced neuronal death. J Neurosci. 2007; 27:1422-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keller JN, Germeyer A, Begley JG, Mattson MP. 17β-estradiol attenuates oxidative impairment of synaptic Na+/K+-ATPase activity, glucose transport, and glutamate transport induced by amyloid β-peptide and iron. J Neurosci Res. 1997; 50:522-530 [DOI] [PubMed] [Google Scholar]
- 49. Morley P, Whitfield JF, Vanderhyden BC, Tsang BK, Schwartz JL. A new, nongenomic estrogen action: The rapid release of intracellular calcium. Endocrinology. 1992; 131:1305-1312 [DOI] [PubMed] [Google Scholar]
- 50. Chami L, Buggia-Prevot V, Duplan E, Delprete D, Chami M, Peyron JF, Checler F. Nuclear factor-κB regulates βAPP and β- and γ-secretases differently at physiological and supraphysiological Aβ concentrations. J Biol Chem. 2012; 287:24573-24584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: How do they signal and what are their targets. Physiol Rev. 2007; 87:905-931 [DOI] [PubMed] [Google Scholar]
- 52. Alvarez-de-la-Rosa M, Silva I, Nilsen J, Perez MM, Garcia-Segura LM, Avila J, Naftolin F. Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer's disease. Ann N Y Acad Sci. 2005; 1052:210-224 [DOI] [PubMed] [Google Scholar]
- 53. Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997; 350:1047-1059 [PubMed] [Google Scholar]
- 54. Beresford SA, Weiss NS, Voigt LF, McKnight B. Risk of endometrial cancer in relation to use of oestrogen combined with cyclic progestagen therapy in postmenopausal women. Lancet. 1997; 349:458-461 [DOI] [PubMed] [Google Scholar]
- 55. Ravnikar VA. Compliance with hormone replacement therapy: Are women receiving the full impact of hormone replacement therapy preventive health benefits? Womens Health Issues. 1992; 2:75-80; discussion 80–72. [DOI] [PubMed] [Google Scholar]
- 56. Escande A, Pillon A, Servant N, Cravedi JP, Larrea F, Muhn P, Nicolas JC, Cavailles V, Balaguer P. Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor α or β. Biochem Pharmacol. 2006; 71:1459-1469 [DOI] [PubMed] [Google Scholar]
- 57. Lee YB, Lee HJ, Won MH, Hwang IK, Kang TC, Lee JY, Nam SY, Kim KS, Kim E, Cheon SH, Sohn HS. Soy isoflavones improve spatial delayed matching-to-place performance and reduce cholinergic neuron loss in elderly male rats. J Nutr. 2004; 134:1827-1831 [DOI] [PubMed] [Google Scholar]
- 58. Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KD, Adlercreutz H, Lephart ED. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001; 2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pan Y, Anthony M, Watson S, Clarkson TB. Soy phytoestrogens improve radial arm maze performance in ovariectomized retired breeder rats and do not attenuate benefits of 17β-estradiol treatment. Menopause. 2000; 7:230-235 [DOI] [PubMed] [Google Scholar]
- 60. Birge SJ. Is there a role for estrogen replacement therapy in the prevention and treatment of dementia? J Am Geriatr Soc. 1996; 44:865-870 [DOI] [PubMed] [Google Scholar]
- 61. Pan Y, Anthony M, Clarkson TB. Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Proc Soc Exp Biol Med. 1999; 221:118-125 [DOI] [PubMed] [Google Scholar]
- 62. Pan Y, Anthony M, Clarkson TB. Evidence for up-regulation of brain-derived neurotrophic factor mRNA by soy phytoestrogens in the frontal cortex of retired breeder female rats. Neurosci Lett. 1999; 261:17-20 [DOI] [PubMed] [Google Scholar]
- 63. Casini ML, Marelli G, Papaleo E, Ferrari A, D'Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: A randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. 2006; 85:972-978 [DOI] [PubMed] [Google Scholar]
- 64. Yeung DK, Leung SW, Xu YC, Vanhoutte PM, Man RY. Puerarin, an isoflavonoid derived from Radix puerariae, potentiates endothelium-independent relaxation via the cyclic AMP pathway in porcine coronary artery. Eur J Pharmacol. 2006; 552:105-111 [DOI] [PubMed] [Google Scholar]
- 65. Xu XH. Effects of puerarin on fatty superoxide in aged mice induced by D-galactose. China Journal of Chinese Materia Medica. 2003; 28:66-69 [PubMed] [Google Scholar]
- 66. Yan FL, Lu G, Wang YQ, Hong Z. Effect of puerarin on the expression of Aβ1-40 and Bax in brain of AD rats induced by Aβ25-35. Chin J Neuromed. 2006; 5:158-161 [Google Scholar]
- 67. Jiang B, Liu JH, Bao YM, An LJ. Hydrogen peroxide-induced apoptosis in pc12 cells and the protective effect of puerarin. Cell Biol Int. 2003; 27:1025-1031 [DOI] [PubMed] [Google Scholar]
- 68. Zhang JJ, Zhong XM. Natural drug in senile dementia treatment: Research progress. Journal of Liaoning University of TCM. 2009; 11:47-49 [Google Scholar]
- 69. Li XL, Tong L. Research progresses on chemical components and pharmacological effects of Baical Skullcap stem and leaf. Journal of Chengde Medical College. 2006; 23:284-286 [Google Scholar]
- 70. Zhao SM, Liu S, Yang HG, Kong XY, Song CJ, Liu YP. Protective effect of scutellaria baicalensis stem-leaf total flavonoid on lipid peroxidation induced by myocardial ischemia reperfusion in rats. Chinese Journal of Anatomy. 2006; 29:450-452 [Google Scholar]
- 71. Zuo YZ, Guan LH, Wang RT. Protective effects of SSTF on injury of hippocampus neurons induced by injection Aβ25–35. Journal of Chengde Medical College. 2009; 26:5-7 [Google Scholar]
- 72. Ye H, Wang RT, Zuo YZ, Guan LH, Shen XB. The effect of total flavonoid of Scutellarea Baicalensis stem-leaf against learning and memory deficit induced by Aβ25–35 injection in rat hippocampus. Lishizhen Medicine and Materia Medica Research. 2009; 20:879-880 [Google Scholar]
- 73. Sun YX, Tang Y, Wu AL, Liu T, Dai XL, Zheng QS, Wang ZB. Neuroprotective effect of liquiritin against focal cerebral ischemia/reperfusion in mice via its antioxidant and antiapoptosis properties. J Asian Nat Prod Res. 2010; 12:1051-1060 [DOI] [PubMed] [Google Scholar]
- 74. Yang Y, Bian GX, Lu QJ. Neuroprotection and neurontrophism effects of liquiritin on primary cultured hippocampal cells. Zhongguo Zhong Yao Za Zhi. 2008; 33:931-935 [PubMed] [Google Scholar]
- 75. Liu RT, Bian GX, Zou LB, Huang XW, Lu QJ. Neuroprotective effects of liquiritin and its inhibitory actions on cholinesterase activity. Chinese Journal of New Drugs. 2008; 17:574-581 [Google Scholar]
- 76. Ko FN, Huang TF, Teng CM. Vasodilatory action mechanisms of apigenin isolated from Apium graveolens in rat thoracic aorta. Biochim Biophys Acta. 1991; 1115:69-74 [DOI] [PubMed] [Google Scholar]
- 77. Sugihara N, Arakawa T, Ohnishi M, Furuno K. Anti- and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with α-linolenic acid. Free Radic Biol Med. 1999; 27:1313-1323 [DOI] [PubMed] [Google Scholar]
- 78. Zhao YH, Chen WQ, Luo SH, Yang H. Effect of apigenin on learning and memory behavior in mice with alzheimer's disease induced with D-galactose. Journal of Guangdong College of Pharmacy. 2005; 21:292-294 [Google Scholar]
- 79. Kang SS, Lee JY, Choi YK, Kim GS, Han BH. Neuroprotective effects of flavones on hydrogen peroxide-induced apoptosis in SH-SY5Y neuroblostoma cells. Bioorg Med Chem Lett. 2004; 14:2261-2264 [DOI] [PubMed] [Google Scholar]
- 80. Zhou MM, Xie HS, Zhang J, Zhang Q. The anti-aging effect of apigenin on aging mice induced by D-galactose. Acad J Sec Mil Med Univ. 2007; 28:452-453 [Google Scholar]
- 81. Wu Y, Zhou SD, Li P. Determination of flavonoids in Hypericum perforatum by HPLC analysis. Yao Xue Xue Bao. 2002; 37:280-282 [PubMed] [Google Scholar]
- 82. Liu XH, Lou HX. Natural agents in treatment of dementia. Qilu Pharmaceutical Affairs. 2004; 23:42-43 [Google Scholar]
- 83. Wu L, Cheng SY, Wang Q, Chen YB. Advances in study on the pharmacological effects of active components of Chinese herbs on Alzheimer's disease. Zhongguo Zhong Yao Za Zhi. 2004; 29:387-389 [PubMed] [Google Scholar]
- 84. Zhu AQ, Li QX, Zhang XS, Teng CQ, Chu YD, Masters CL, George A, Cardamong T, Evin G. Effects of Rhodiola on Alzheimer pathology and open field activity in APP-C100 transgenic mice. Chinese Journal of Gerontology. 2004; 24:530-533 [Google Scholar]


