Summary
The purpose of this study was to investigate optimal concentrations of zoledronic acid (ZA) in terms of their effect on the proliferation, differentiation, and mineralization of primary osteoblasts (OBs) and fibroblasts (FBs). Primary OBs and FBs isolated from patients with clinical osteogenesis imperfecta (OI) and developmental dysplasia of the hip (DDH) were treated in vitro with serial concentrations of ZA ranging from 10−3 M to 10−13 M. An MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)) colorimetric assay, flow cytometry, alkaline phosphatase (ALP) determination activity, and alizarin red staining were used to measure the proliferation, differentiation, and mineralization of cells. The MTT assay indicated that high concentrations of ZA may be toxic to cultured cells. No obvious inhibition was observed with a ZA concentration of 10−7 M to 10−10 M. Proliferation was evident with a ZA concentration below 10−11 M (p < 0.05). Flow cytometry analysis revealed that cell cycle was arrested at G1/G0 stage with a ZA concentration ranging from 10−10 M to 10−8 M. ZA did not enhance ALP activity at a concentration of 10−8 M or 10−10 M. Alizarin red staining indicated the mineralization of primary OBs with a low concentration of ZA (10−12 M). In conclusion, this in vitro study indicated that ZA-mediated cell proliferation was dose-dependent and that ZA did not inhibit cell proliferation at concentrations below 10−8 M. These findings suggest low concentrations of ZA have more of an effect on cell differentiation and mineralization, so low concentrations are better at regulating bone formation and repair.
Keywords: Osteogenesis imperfecta, proliferation, differentiation, mineralization
1. Introduction
Zoledronic acid (ZA) is the most potent bisphosphonate (BPs) in clinical use and has been used as an anti-resorptive agent to prevent bone resorption in the treatment of metabolic bone diseases like osteoporosis, Paget's disease, osteolytic disease, hypercalcemia of malignancy, and cancer-related osteolytic lesions (1–3). ZA regulates the bone balance by inhibiting bone resorptive activity of osteoclasts (OCs), thus effectively reducing bone loss and bone turnover (4,5). Recent evidence supports the notion that osteoblasts (OBs) could be target cells for ZA and that this action by ZA may in turn contribute to a decrease in OC formation and activity (6) but the mechanism for this action is less clear. Osteogenesis imperfecta (OI) is a heterogeneous group of genetic disorders characterized by low bone mass, increased bone fragility, and susceptibility to bone fractures with variable severity. Its main clinical symptoms include increased bone fragility, osteoporosis, susceptibility to fractures, and bone anisotropy, and OI is accompanied by blue sclerae, dentinogenesis imperfecta, hearing loss, excessive joint laxity, and muscle weakness (7–10). At present, ZA is the most promising therapy to treat OI, and this is especially true for its intravenous administration. ZA has become the accepted treatment for both adults and children who suffer from OI. Developmental dysplasia of the hip (DDH) is an abnormal alignment of the femoral head and acetabulum caused by unilateral or bilateral hip instability; DDH is due to genetics, breech birth, swaddling that dislocates the hips, and other factors (11,12). The clinical features of DDH differ vastly from those of OI (e.g. no osteoporosis), so in the current study DDH served as the control.
The current study used OBs and fibroblasts (FBs) from children with OI and DDH to investigate the effect of ZA on the biological function of OBs and FBs at the cellular level. This was done to determine the optimal concentration of ZA in terms of its effect on the proliferation, differentiation, and mineralization of primary OBs and FBs and to provide a theoretical basis for clinical use of ZA. This study explored the feasibility of ZA because of its role in bone formation and explored its use to treat bone-related diseases.
2. Materials and Methods
2.1. Materials
Bone and skin tissue were collected from children with OI and DDH. The study protocol was approved by the Ethics Committee of Shandong Medical Biotechnology Center, Ji'nan, Shandong, China and written informed consent was obtained.
2.2. Cell lines and cell culture
Primary OBs and FBs were obtained by collagenase digestion of bone and skin tissue from children with OI and DDH. Tissue was washed and placed in culture dishes with preheated phosphate buffered saline (PBS) (Gibco, NY, USA). Tissue was cultured in 5 mL serum-free Dulbecco's modified eagle medium (DMEM) (Gibco), and 12.5 U/µL collagenase type I (Sigma, NY, USA) was added for digestion at 37°C for 3 h. Cells were centrifuged and resuspended in growth medium. Cells were cultured in DMEM (Gibco) containing 10% fetal bovine serum (FBS) (Gibco) and 1× penicillin-streptomycin (Beyotime, Shanghai, China). Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C in 75 cm2 plates. Medium was changed every 3 days until cell density reached 90%. Cells were then serially passaged and digested at 37°C for 3 min with 1 mL 0. 25% trypsin (Beyotime).
2.3. Assay of alkaline phosphatase (ALP) activity (histochemistry)
Four types of cells were cultured for 9 days in 6-well culture plates at a density of 1 × 104 cells/well. Cells were washed with 4°C precooled PBS (Gibco) and then fixed with 4% paraformaldehyde (PFA) (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) for 10 min before they were washed with PBS (Gibco) (4°C precooling). The samples were then incubated for 30 min with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) liquid substrate (Beyotime) at 37°C. The reaction was terminated by removing the substrate solution and washing with distilled water. ALP-positive cells appeared dark blue.
2.4. Cell viability assay
Four types of cells were cultured for 24 h in 96-well culture plates at a density of 2 × 103 cells/well and then growth medium was replaced. Cells were treated with ZA (SELLECK, Houston, USA) at concentrations of 10−3 M to 10−13 M at various times (2, 4, and 6 days). At the indicated times, a 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA) assay was performed by incubating cells with 20 µL 5 mg/mL MTT solution/well at 37°C for 4 h. The reaction was then stopped with 150 µL dimethyl sulfoxide (DMSO) (Amresco, OH, USA)/well. Color development was then analyzed by measuring absorbance at 490 nm (A490), and A490 thus corresponded to the viability of cells.
2.5. Cell cycle analysis (flow cytometry)
OBs and FBs were cultured in 6-well culture plates at a final density of 6.5 × 104 cells/well with or without 10−8 M, 10−9 M, or 10−10 M ZA for 2 days and 4 days, respectively. After culturing, cells were digested at 37°C for 3 min with 0.3 mL 0.25% trypsin. Cells were collected in 1.5 mL tubes and washed and they were then fixed with 4% PFA (Sinopharm Chemical Reagent Co. Ltd., Beijing, China) for 12 h in 4°C. Cells were washed with precooled PBS and stained with propidium (PI) (Beyotime) at 37°C for 30 min away from light. Flow cytometry was performed as described in the instructions to the Cell Cycle and Apoptosis Analysis Kit (Beyotime).
2.6. Assay of ALP activity (biochemistry)
Four types of cells were cultured as previously described in 6-well culture plates at a final density of 6.5 × 104 cells/well. They were treated with ZA for 6 days at a final concentration of 10−8 M, 10−10 M, and 10−8 M with dexamethasone (Sigma-Aldrich) serving as a positive control and growth medium without ZA serving as a normal control. Medium was replaced every 3 days. Samples were washed with PBS (Gibco) and digested and then cells were collected. One percent SDS cell lysis solution was added to each sample to lyse cells at 4°C for 2 h. Cell lysates were then obtained for analysis. The moieties of cell lysates were used to analyze protein content using the BCA Protein Assay Kit (Beyotime), with 5 µL/well cell lysates in 96-well plates. Color development was then analyzed by measuring the optical density (OD) at 495 nm (OD495). The moieties of cell lysates were analyzed to detect ALP activity by adding 80 µL p-nitrophenylphosphate (p-NPP) (Sigma-Aldrich) and incubating 40 µL/well cell lysates in 96-well plates at 37°C for 20 min. The reaction was then stopped with 3 N NaOH. The amount of p-nitrophenol (p-NP) product, corresponding to ALP activity, was measured at 405 nm. Activity was calculated using the formula [ALP activity = A/(X/5 × 40)].
2.7. Mineralized matrix formation
OBs from patients with OI were cultured in 24-well culture plates at a density of 8 × 103 cells/well. When the cells reached confluence, the medium was changed to induction medium (10% FBS (Gibco) and 1× penicillin-streptomycin (Beyotime), 500 µg/mL l-ascorbic acid (Sigma-Aldrich), and 10−2 mol/L β-glycerophosphate disodium salt hydrate (Sigma-Aldrich) for control cells. For treated cells, the medium was changed to induction medium with ZA at a final concentration of 10−6 M, 10−8 M, 10−10 M, and 10−12 M. Medium was replaced every 3 days. Matrix formation was detected at 18 and 21 days by washing cell matrix layers three times with PBS, fixing them with ice-cold 4% PFA (Sinopharm Chemical Reagent Co. Ltd.) for 10 min, and then washing them with distilled water. Matrix layers were then thoroughly stained with 1% alizarin red (Sinopharm Chemical Reagent Co. Ltd.) for 10 min, and excess stain was removed with distilled water. Mineralized matrix formation appeared in red.
2.8. Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). One-way ANOVA was used to determine the statistical significance of differences between the means of experiments if data had a normal distribution. A Wilcoxon test was used for non-parametric data, and p < 0.05 was considered to be statistically significant.
3. Results
3.1. Assay of ALP activity (histochemistry)
After plates were stained, microscopic images revealed OBs with a long, fusiform shape, elongation, abundant cytoplasm, and a clear nucleus. Cells grew in clumps and overlapped. FBs also had a long, fusiform shape with a round or elliptical nucleus, and FBs were larger than OBs. All of the four cell types were dyed dark bluish-purple, consistent with the characteristics of OBs and FBs. More OBs were stained and they had a deeper color than FBs. OBs had greater ALP activity than did FBs (Figure 1).
Figure 1.
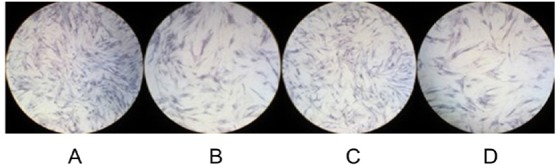
Microscopic images of four types of cells after histochemical staining. (A), OBs from patients with DDH; (B), FBs from patients with DDH; (C), OBs from patients with OI; (D), FBs from patients with OI. All four types of cells were stained deep bluish-purple.
3.2. Cell viability assay
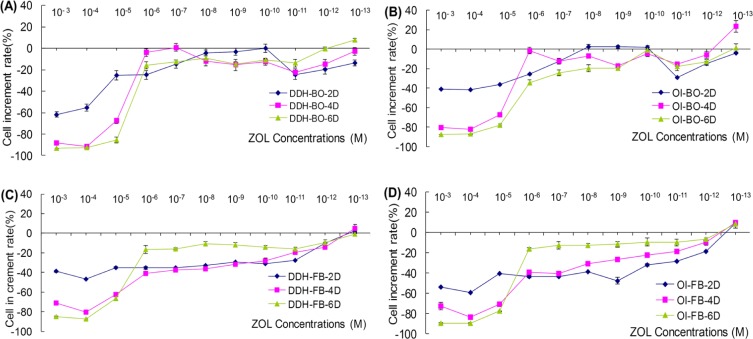
Action of ZA was detected at three times (2, 4, and 6 days). ZA at a concentration of 10−6 M and higher inhibited the proliferation of OBs from patients with OI and DDH (p < 0.05). A ZA concentration between 10−7 M to 10−10 M slightly inhibited the proliferation of cells. ZA concentrations below 10−11 M tended to promote cell proliferation (p < 0.05). All of the ZA concentrations tested had little effect on FBs viability at the observed times. In addition, cell proliferation decreased most with ZA concentrations greater than 10−6 M (cell viability was approximately 90% of the control), but the rate of inhibition increased over time. There was a dose-dependent change in cell viability with a ZA concentration of 10−6 M or lower. Inhibition diminished with a lower ZA concentration as time passed. A ZA concentration below 10−11 M tended to promote cell proliferation. Proliferation of cells from patients with OI was promoted more than was the proliferation of cells from patients with DDH (Figure 2). Note: cell proliferation rate (%) = (OD of treated cells − OD of control cells)/OD of control cells × 100%.
Figure 2.
OBs and FBs from patients with OI and DDH cultured in decreasing concentrations of ZA and assessment of the cell count and viability at 2, 4, and 6 days using MTT. (A), Effect on the proliferation of OBs from patients with DDH. At a concentration of 10−6 M and higher, ZA inhibited the proliferation of OBs from patients with OI and DDH (p < 0.05) after 2, 4, and 6 days of treatment. A ZA concentration between 10−7 M to 10−10 M slightly inhibited the proliferation of cells. At concentrations below 10−11 M, ZA tended to promote cell proliferation (p < 0.05), and this trend was more apparent with longer treatment; (B), Effect on the proliferation of OBs from patients with OI (same as in (A)); (C), Effect on the proliferation of FBs from patients with DDH. Generally, ZA inhibits FBs. ZA at concentrations greater than 10−6 M (p < 0.05) resulted in a significant decrease in the cell count compared to untreated control cells as time passed. At concentrations greater than 10−6 M, inhibition of cell proliferation diminished as time passed (p < 0.05). At concentrations below 10−11 M, cell proliferation tended to be promoted (p < 0.05); (D), Effect on the proliferation of FBs from patients with OI (same as in (C)). Clearly, ZA acted on cells from patients with OI more than it did on cells from patients with DDH.
3.3. Cell cycle analysis
Treatment with ZA concentrations of 10−8 M, 10−9 M, or 10−10 M arrested both OBs and FBs from patients with OI and DDH in the G1/G0 phase. Cell cycle arrest was more obvious in OBs than in FBs. ZA had more of an effect after 4 days than it did after 2 days, and it had more of an effect on cells from patients with DDH than it did on cells from patients with OI (Figure 3). Note: cell arrest rate = (percentage of ZA-treated cells in G1 phase − percentage of control cells in G1 phase)/percentage of control cells in G1 phase × 100%.
Figure 3.
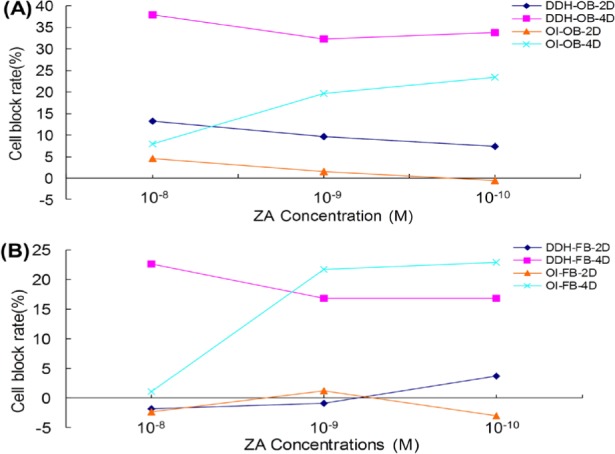
Effect of ZA on cell cycle arrest. (A), ZA arrested the cell cycle of OBs in 2 days. This was true for OBs from patients with OI and those with DDH. After 4 days, the percentage of cells with a cycle arrested at G1/G0 decreased to 38%. A greater percentage of cells from patients with DDH than from patients with OI had their cycle arrested; (B), After 2 days, ZA had no effect on the cell cycle of FBs in the G1/G0 phase. After 4 days, a 10−8 M concentration of ZA inhibited cells from patients with DDH at a rate of 23%; this was higher than the rate of inhibition of cells from patients with OI.
3.4. Assay of ALP activity (biochemistry)
ALP activity is a well-known marker of OBs differentiation. After 6 days of culturing, ALP activity was measured. Compared to the positive control (with 10−8 M dexamethasone) and normal control, 10−8 M and 10−10 M had no effect on ALP (Figure 4).
Figure 4.
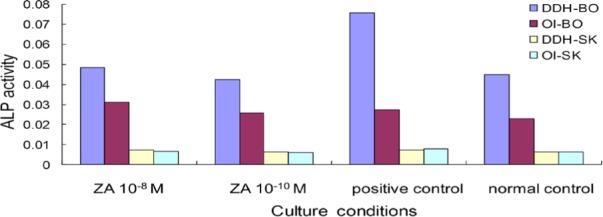
Absence of ZA's effect on ALP activity. Two concentrations of ZA had no effect on ALP, and OBs had greater ALP activity than did FBs.
3.5. Mineralized matrix formation
In the absence of ZA, OBs stained positive with alizarin red in induction medium after 18 days, as did cells treated with 10−12 M ZA. A ZA concentration of 10−12 M promoted mineralization (Figure 5).
Figure 5.
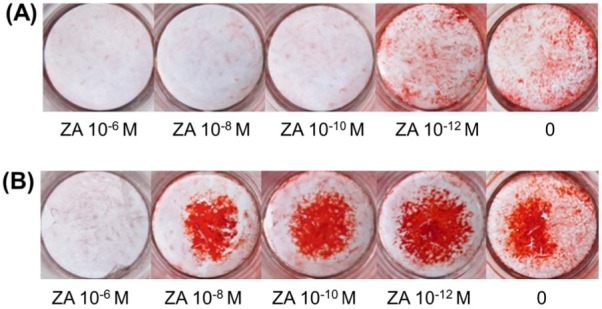
Mineralization staining. (A), After 18 days of culturing, bone nodules were stained with alizarin red. Cultured control cells and cultured cells treated with 10−12 M ZA had mineralized matrix deposition in red; staining was more evident in cells treated with 10−12 M ZA than in control cells; (B), After 21 days of culturing, all plates had mineralized matrix deposition in red except cells treated with 10−6 M ZA. Staining was more evident at a lower ZA concentration, and cells treated with 10−12 M ZA had more evident staining than control cells did.
4. Discussion
The first BP was approved in the US in 1977, and BPs are now widely used for the treatment of osteoporosis, hypercalcemia of malignancy, and Paget's disease. Clinical studies have revealed that third-generation BPs have greater efficacy and are more effective at preventing bone-related events. If treatment with other BPs fails, ZA is available. ZA is currently the only BP approved for use in treating a variety of bone diseases. Numerous reports have described the action of BPs on OCs. BPs can have a negative impact on OCs, inhibiting their formation and recruitment (13,14), inhibiting the activation of OCs by OBs (15–18), inhibiting the maturation and activity of OCs (19,20), and promoting the apoptosis of OCs (21,22). However, there is no experimental evidence that BPs can reduce the number of mature OCs (23). Nitrogen-containing BPs can act by inhibiting the mevalonate pathway and nitrogen-free BPs can act by interfering with the process of energy conversion inside a cell (24,25).
Numerous reports have described the role of BPs and their action on OBs in terms of their effect on the proliferation, differentiation, and mineralization of OBs, but the conclusions of these reports differ (26–29). The current study was an in vitro study. Findings indicated that ZA affected the proliferation of primary OBs in a dose-dependent manner. ZA is also toxic to human OBs at concentrations below 10−6 M, since ZA inhibited OBs and FBs proliferation. This inhibition was more evident over time, and the rate of inhibition was about 90% after 6 days of culturing. ZA concentrations from 10−7 M to 10−10 M slightly inhibited cell proliferation. ZA concentrations greater than 10−10 M tended to promote cell proliferation, and this trend was more evident over time. Second-generation BPs can promote the proliferation, differentiation, and mineralization of OBs, and a concentration of 10−8 M is optimal (30,31). ZA has little effect on cell proliferation (32), which is true according to the current results as well. The current results agree with the conclusions of Orriss et al. (33) and the hypothesis of Maruotti et al. (34).
ALP is an essential enzyme for bone formation, and ALP is an early indicator to identify and evaluate the degree of differentiation of OBs (35). ALP also reflects the activity of OBs. In the current study, treatment with ZA (10−8 M and 10−10 M) led to changes in ALP activity. Alizarin red staining showed that mineralization/nodule formation increased as the ZA concentration decreased, which is consistent with the findings regarding cell proliferation.
In this study, different concentrations of ZA affected OBs proliferation differently. ZA may affect OI more during mineralization rather than during differentiation. Lower concentrations of ZA may be able to inhibit OCs but also promote OBs and FBs proliferation and differentiation. Selecting an appropriate dose and dosing regimen of ZA may help facilitate bone formation.
References
- 1. Russell RG, Rogers MJ. Bisphosphonates: From the laboratory to the clinic and back again. Bone. 1999; 25:97-106 [DOI] [PubMed] [Google Scholar]
- 2. Brandi ML. Current treatment approaches for Paget's disease of bone. Discov Med. 2010; 10:209-212 [PubMed] [Google Scholar]
- 3. Mahtani R, Jahanzeb M. Bisphosphonates as anticancer therapy for early breast cancer. Clin Breast Cancer. 2010; 10:359-366 [DOI] [PubMed] [Google Scholar]
- 4. Fleisch H. Bisphosphonates: Mechanism of action. Endocr Rev. 1998; 19:80-100 [DOI] [PubMed] [Google Scholar]
- 5. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000; 88:2961-2978 [DOI] [PubMed] [Google Scholar]
- 6. Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone. 2011; 49:50-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glorieux FH. Osteogenesis imperfecta. Best Pract Clin Rheumatol. 2008; 22:85-100 [DOI] [PubMed] [Google Scholar]
- 8. Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011; 7:540-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kataoka K, Ogura E, Hasegawa K, Inoue M, Seino Y, Morishima T, Tanaka H. Mutations in type I collagen genes in Japanese osteogenesis imperfecta patients. Pediatr Int. 2007; 49:564-569 [DOI] [PubMed] [Google Scholar]
- 10. Liu W, Gu F, Ji J, Lu D, Li X, Ma X. A novel COL1A1 nonsense mutation causing osteogenesis imperfecta in a Chinese family. Mol Vis. 2007; 13:360-365 [PMC free article] [PubMed] [Google Scholar]
- 11. Kokavec M, Bialik V. Developmental dysplasia of the hip. Prevention and real incidence. Bratisl Lek Listy. 2007; 108:251-254 [PubMed] [Google Scholar]
- 12. Nemeth BA, Narotam V. Developmental dysplasia of the hip. Pediatr Rev. 2012; 33:553-561 [DOI] [PubMed] [Google Scholar]
- 13. Hughes DE, MacDonald BR, Russell RG, Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest. 1989; 83:1930-1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boonekamp PM, van der Wee-Pals LJ, van Wijk-van Lennep MM, Thesing CW, Bijvoet OL. Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner. 1986; 1:27-39 [PubMed] [Google Scholar]
- 15. Wesolowski G, Duong LT, Lakkakorpi PT, Nagy RM, Tezuka K, Tanaka H, Rodan GA, Rodan SB. Isolation and characterization of highly enriched, prefusion mouse osteoclastic cells. Exp Cell Res. 1995; 219:679-686 [DOI] [PubMed] [Google Scholar]
- 16. Breuil V, Cosman F, Stein L, Horbert W, Nieves J, Shen V, Lindsay R, Dempster DW. Human osteoclast formation and activity in vitro: Effects of alendronate. J Bone Miner Res. 1998; 13:1721-1729 [DOI] [PubMed] [Google Scholar]
- 17. Jimi E, Nakamura I, Amano H, Taguchi Y, Tsurukai T, Tamura M, Takahashi N, Suda T. Osteoclast function is activated by osteoblastic cells through a mechanism involving cell-to-cell contact. Endocrinology. 1996; 137:2187-2190 [DOI] [PubMed] [Google Scholar]
- 18. Vitté C, Fleisch H, Guenther HL. Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology. 1996; 137:2324-2333 [DOI] [PubMed] [Google Scholar]
- 19. Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991; 88:2095-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plasmans CM, Jap PH, Kuijpers W, Slooff TJ. Influence of a diphosphonate on the cellular aspect of young bone tissue. Calcif Tissue Int. 1980; 32:247-266 [DOI] [PubMed] [Google Scholar]
- 21. Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995; 10:1478-1487 [DOI] [PubMed] [Google Scholar]
- 22. Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996; 2:1132-1136 [DOI] [PubMed] [Google Scholar]
- 23. Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009; 360:53-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ebetino FH, Hogan AM, Sun S, Tsoumpra MK, Duan X, Triffitt JT, Kwaasi AA, Dunford JE, Barnett BL, Oppermann U, Lundy MW, Boyde A, Kashemirov BA, McKenna CE, Russell RG. The relationship between the chemistry and biological activity of the bisphosphonates. Bone. 2011; 49:20-33 [DOI] [PubMed] [Google Scholar]
- 25. Lehenkari PP, Kellinsalmi M, Näpänkangas JP, Ylitalo KV, Mönkkönen J, Rogers MJ, Azhayev A, Väänänen HK, Hassinen IE. Further insight into mechanism of action of clodronate: Inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002; 61:1255-1262 [DOI] [PubMed] [Google Scholar]
- 26. Fromigue O, Body JJ. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest. 2002; 25:539-546 [DOI] [PubMed] [Google Scholar]
- 27. Koch FP, Merkel C, Al-Nawas B, Smeets R, Ziebart T, Walter C, Wagner W. Zoledronate, ibandronate and clodronate enhance osteoblast differentiation in a dose dependent manner - a quantitative in vitro gene expression analysis of Dlx5, Runx2, OCN, MSX1 and MSX2. J Craniomaxillofac Surg. 2011; 39:562-569 [DOI] [PubMed] [Google Scholar]
- 28. Naidu A, Dechow PC, Spears R, Wright JM, Kessler HP, Opperman LA. The effects of bisphosphonates on osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:5-13 [DOI] [PubMed] [Google Scholar]
- 29. Giuliani N, Pedrazzoni M, Passeri G, Negri G, Impicciatore M, Girasole G. Bisphosphonates stimulate the production of basic fibroblast growth factor and the formation of bone marrow precursors of osteoblasts. New findings about their mechanism of action. Minerva Med. 1998; 89:249-258 [PubMed] [Google Scholar]
- 30. Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000; 60:6001-6007 [PubMed] [Google Scholar]
- 31. Ebert R, Zeck S, Krug R, Meissner-Weigl J, Schneider D, Seefried L, Eulert J, Jakob F. Pulse treatment with zoledronic acid causes sustained commitment of bone marrow derived mesenchymal stem cells for osteogenic differentiation. Bone. 2009; 44:858-864 [DOI] [PubMed] [Google Scholar]
- 32. Patntirapong S, Singhatanadgit W, Chanruangvanit C, Lavanrattanakul K, Satravaha Y. Zoledronic acid suppresses mineralization through direct cytotoxicity and osteoblast differentiation inhibition. J Oral Pathol Med. 2012; 41:713-720 [DOI] [PubMed] [Google Scholar]
- 33. Orriss IR, Key ML, Colston KW, Arnett TR. Inhibition of osteoblast function in vitro by aminobisphosphonates. J Cell Biochem. 2009; 106:109-118 [DOI] [PubMed] [Google Scholar]
- 34. Maruotti N, Corrado A, Neve A, Cantatore FP. Bisphosphonates: Effects on osteoblast. Eur J Clin Pharmacol. 2012; 68:1013-1018 [DOI] [PubMed] [Google Scholar]
- 35. Chaudhary LR, Hofmeister AM, Hruska KA. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone. 2004; 34:402-411 [DOI] [PubMed] [Google Scholar]