Summary
Biliary cystic tumors are rare hepatic neoplasms, and knowledge regarding the origin and pathology of these tumors remains vague. They should be analyzed in more detail. In our institution, 4 biliary cystic tumor surgeries were performed between December 1999 and March 2010. Pathological evaluation of resected specimens was performed to evaluate the characteristics of the intracystic epithelium and to determine the presence or absence of interstitial infiltrate, ovarian mesenchymal stroma (OMS), luminal communication between the cystic tumor and the bile duct, and mucin (MUC) protein expression. We evaluated the following 4 cases: case 1, a 21-year-old woman with a biliary cystadenoma who underwent extended right hepatectomy; case 2, a 39-year-old woman with a biliary cystadenoma who underwent left hepatectomy; case 3, an 80-year-old man with a biliary cystadenoma who underwent left hepatectomy; and case 4, a 61-year-old man with a biliary cystadenocarcinoma revealing papillary proliferation of atypical epithelium and interstitial infiltrates who underwent left hepatectomy. Case 3 had papillary proliferation of the intracystic atypical epithelium but showed interstitial infiltrates. Luminal communication with the bile duct, centrally or peripherally, was found in all 4 cases. Only case 2 showed OMS. Immunohistochemical staining revealed the following findings: cases 1 and 2, MUC1−/MUC2−; case 3, MUC1+/MUC2−; and case 4, MUC1+/MUC2+. It is important to gather information on more cases of biliary cystic tumors because atypical cases were observed, where both OMS and luminal communication with the bile duct were present or absent.
Keywords: Biliary cystic tumors, ovarian mesenchymal stroma, luminal communication with bile duct
1. Introduction
Biliary cystic tumors, namely biliary cystadenomas or cystadenocarcinomas, form a unilocular or septated multilocular cystic cavity containing mucin. Mural nodules or excrescences may also be observed along the capsular wall (1–3). The postulated origin of these lesions is proliferation of the ectopic embryonic tissues that otherwise aid in the development of the adult gallbladder (4). The gross and microscopic characteristics of biliary cystadenoma and cystadenocarcinoma distinguish these entities from other hepatic-based cystic lesions, including simple cysts, degenerating metastatic tumors, bilomas, hematomas, abscesses, parasitic diseases, polycystic liver disease, and Caroli disease. Recently, biliary cystic tumors, which are rare hepatic neoplasms, have been divided into 2 clinicopathological groups, based on the presence or absence of ovarian mesenchymal stroma (OMS) and luminal communication with the bile duct. Cystic tumors showing OMS in the cyst wall have been recently classified as mucinous cystic neoplasms (MCNs), according to the World Health Organization (WHO) classification of biliary tumors (5). Intraductal papillary neoplasm (IPN) of the bile duct is the proposed term for describing biliary cystic tumors characterized by the presence of luminal communication with the bile duct. Excessive mucin is frequently produced by the neoplastic cells and the affected bile duct is filled with mucin, showing tubular or cystic luminal dilatation (6). This disease, which is distinctly different from MCN, has been recently recognized as IPN of the bile duct according to the WHO classification of biliary neoplasms (5).
Each entity resembles MCN and intraductal papillary mucinous neoplasms (IPMNs) of the pancreas, respectively. These entities can be regarded as the biliary counterparts of pancreatic entities (7–9). The biologic similarities between the biliary and pancreatic ductal systems have attracted attention because both organs are derived from the ventral endoderm of the foregut (10). Similar tumors such as IPNs, intraepithelial neoplasms, and MCNs can arise in biliary and pancreatic ducts (11–13).
We have 4 recorded cases of biliary cystic tumors over a 10-year period. In this study, our cases were reclassified and elucidated according to their clinicopathological features. In addition, we performed a search of the Japana Centra Revuo Medicina database and reviewed articles on biliary cystic tumors from 1983 to 2010, with special attention to the presence or absence of OMS and luminal communication of the bile duct. The efficacy and relevance of the subclassifications were analyzed.
2. Case report and review of the literature
2.1. Report of 4 cases
Case 1. The patient was a 21-year-old woman. During examination for cholangitis, a multilocular cyst was noted in the right lobe of the liver, and bile duct cystadenoma was suspected based on the Magnetic Resonance Imaging findings. In accordance with the patient's wishes, a wait-and-see approach was adopted; however, the cyst showed a tendency to increase in size, and thus, the possibility of a malignant transformation could not be ruled out. Therefore, an extended resection of the right lobe of the liver was performed. The resected specimen showed a multilocular cyst with a maximum diameter of 11 cm. Further, the cyst contained a viscous, bile-like substance. Histopathological analysis results showed that the cyst was lined with simple cuboidal epithelium showing no atypia, thus establishing the diagnosis of biliary cystadenoma. No ovarian-like stroma was observed. Gallstones were found inside the cyst, suggesting possible communication between the cyst lumen and peripheral bile ducts. Immunostaining results showed that both MUC1 and MUC2 were negative.
Case 2. The patient was a 39-year-old woman. A hepatic cyst was noted as the patient underwent detailed physical and laboratory examination for abdominal pain. The findings showed that the cyst tended to grow in size and that its inner cavity was divided by an emerging septum. Bile duct cystadenoma was suspected, and the left liver lobe was resected. A multilocular cyst with a maximum diameter of 8 cm was found in the resected specimen. Further, a communication between the cyst and bile duct (B4) was macroscopically visible. Histopathological examination results revealed that the cyst was lined with a single layer of cubic columnar epithelium, and an ovarian-like stroma with high cell density was found beneath the epithelium (Figure 1); thus the condition was diagnosed as bile duct cystadenoma. Immunostaining results showed that both MUC1 and MUC2 were negative.
Figure 1.
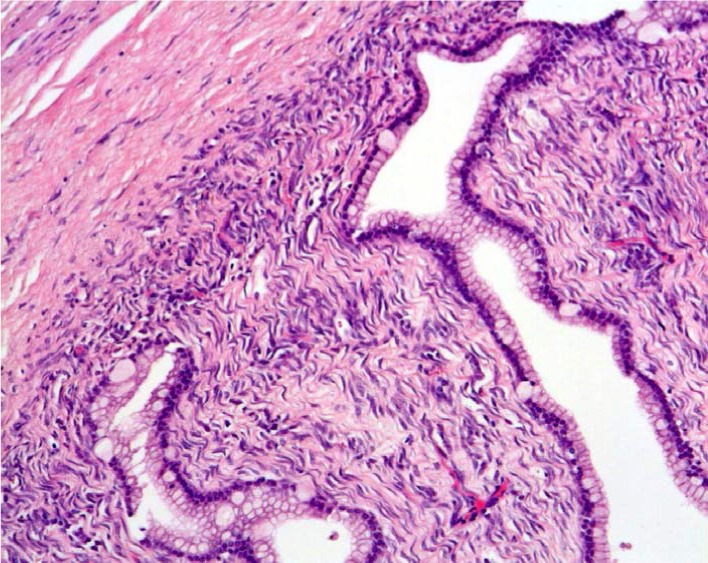
Histological findings revealed the cyst wall consisted of a single layer of cuboidal epithelial cells with ovarian mesenchymal stroma (H & E, ×200).
Case 3. The patient was an 80-year-old man. A computed tomography (CT) scan showed the presence of a hepatic cyst during follow-up examination for another disease. Although a wait-and-see approach was adopted, a solid tumor with a tendency to increase in size was detected inside the cyst, and the possibility of bile duct cystadenocarcinoma could not be ruled out. Therefore, a resection of the left lobe was performed. Macroscopic examination of the resected specimen revealed a communication between the cyst and central bile duct. Further, microscopic findings aided in identifying a region showing the transition from a normal bile duct epithelium to an atypical epithelium (Figure 2). No ovarian-like stroma and stromal invasion were observed. Thus, histological diagnosis of bile duct cystadenoma was established. However, the finding of an atypical epithelium inside the cyst suggested that unlike the cases of patients 1 and 2, the malignant potential was high in this patient. Immunostaining showed positive results only for MUC1 (Figure 3).
Figure 2.
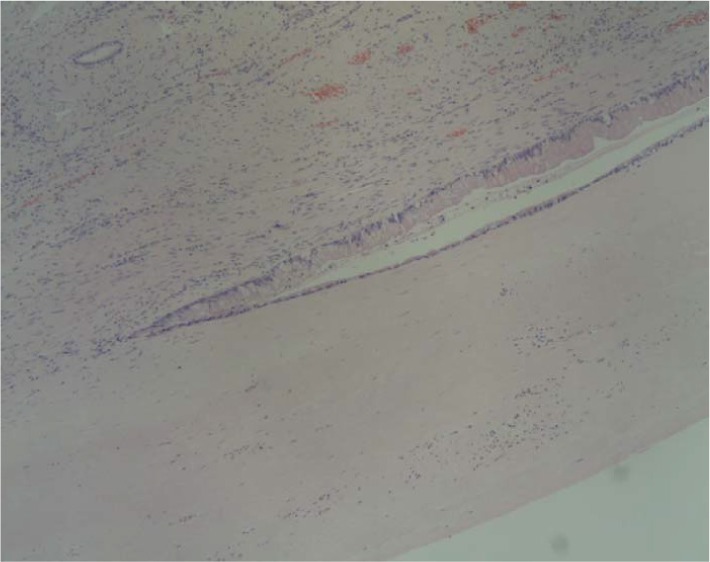
The site of transition from normal biliary epithelium to the atypical epithelium is shown by the histological finding (H & E, ×200).
Figure 3.
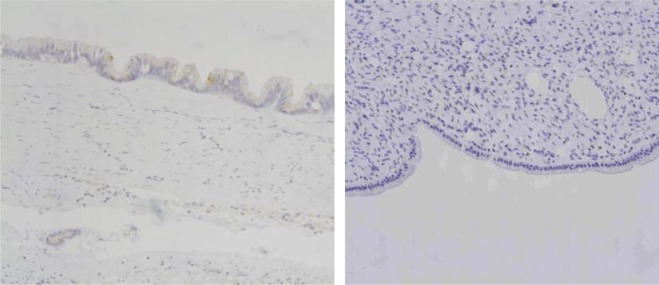
Immunohistochemical examination revealed MUC1+ (left side)/MUC2− (right side) (×40).
Case 4. The patient was a 61-year-old man. CT scan showed a cystic lesion in the hepatic portal region during follow-up for another disease conducted at our hospital's Department of Internal Medicine. Progressive dilation of the intrahepatic bile ducts was confirmed. Magnetic resonance cholangiopancreatography showed that the dilation started from the B2 bile duct to the left hepatic duct. A solid component was detected inside the B2 bile duct. Mucin-producing cholangiocarcinoma was suspected; therefore, resection of the left lobe of the liver was performed. The resected specimen showed cystic dilation of the intrahepatic bile ducts and contained a tumor with a maximum diameter of 5 cm. Histopathological analysis of the epithelium inside the cyst showed a transition from simple cuboidal epithelium to atypical epithelium with papillary growth (Figure 4). Stromal invasion was observed at the site of the solid tumor, leading to the diagnosis of bile duct cystadenocarcinoma. No ovarian-like stroma was found. Immunostaining results showed that both MUC1 and MUC2 were positive (Figure 5).
Figure 4.
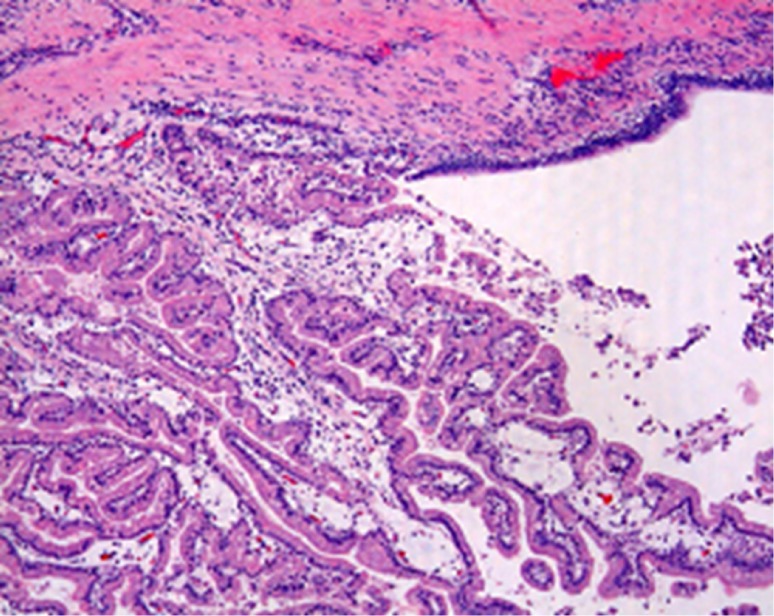
Papillary proliferation of intracystic atypical epithelium and its interstitial infiltration observed (H & E, ×200).
Figure 5.
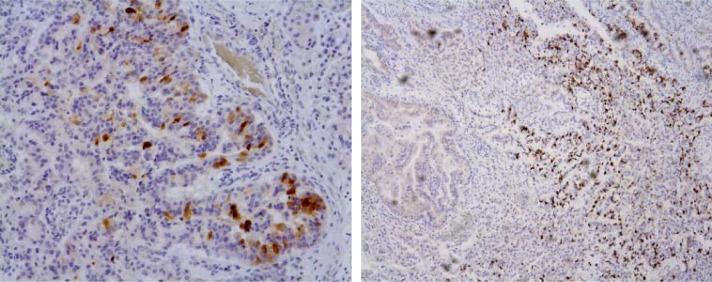
Immunohistochemical examination reveals MUC1+ (left side)/MUC2+ (right side) (×100 and ×40, respectively).
Preoperative characteristics, treatment procedures, and histopathological and immunohistochemical findings of the 4 patients are summarized in Table 1. All 4 patients showed a good postoperative course, were discharged after the hospital stay with no complications, and are currently alive with no recurrence.
Table 1. Characteristics of our cases.
| Items | Case 1 | Case 2 | Case 3 | Case 4 |
| Gender | Female | Female | Male | Male |
| Age(y) | 21 | 39 | 80 | 61 |
| Clinical Findings | None | Abdominal pain | None | None |
| Clinical Diagnosis | Biliary cystadenoma | Biliary cystadenoma | Biliary cystadenoma | Mucus-producing cholangio-carcinoma |
| Operative Procedure | Extended right hepatectomy | Left hepatectomy | Left hepatectomy | Left hepatectomy |
| OMS | Absence | Presence | Absence | Absence |
| Luminal communication with bile duct | Presence | Presence | Presence | Presence |
| Atypicality of intracystic epithelium | Absence | Absence | Presence | Presence |
| Interstitial infiltration | Absence | Absence | Absence | Presence |
| Expression of mucin protein | MUC1−/MUC2− | MUC1−/MUC2− | MUC1+/MUC2− | MUC1+/MUC2+ |
| Traditional histopathological diagnosis | Biliary cystadenoma | Biliary cystadenoma | Biliary cystadenoma | Biliary cystadenocarcinoma |
| New histopathological diagnosis | IPN of the bile duct | MCN and IPN of the bile duct | IPN of the bile duct | IPN of the bile duct |
OMS, ovarian mesenchymal stroma; MUC, mucin; IPN, intraductal papillary neoplasm; MCN, mucinous cystic neoplasm.
2.2. Review of the literature (Table 2)
Table 2. Characteristics of the reported cases in our review.
|
(a) | |||
| Items | OMS (−) | OMS (+) | Total |
| Luminal communication with bile duct (−) | 46 (39.3) | 28 (23.9) | 74 (63.2) |
| Luminal communication with bile duct (+) (main duct type) | 33 (28.2) | 2 (1.7) | 35 (29.9) |
| Luminal communication with bile duct (+) (branch duct type) | 6 (5.1) | 2 (1.7) | 8 (6.8) |
| Total | 85 (72.7) | 32 (27.4) | 117 (100) |
|
(b) | ||
| Items | OMS (−) | OMS (+) |
| Luminal communication with bile duct (−) | ||
| Female | 31/46 (67.4) | 28/28 (100) |
| Male | 15/46 (32.6) | 0/28 (0) |
| Adenocarcinoma | 29/46 (63.0) | 2/28 (7.1) |
| Adenoma | 17/46 (37.0) | 26/28 (92.9) |
| Luminal communication with bile duct (+) | ||
| Female | 21/39 (53.8) | 4/4 (100) |
| Male | 18/39 (46.2) | 0/4 (0) |
| Adenocarcinoma | 28/39 (71.8) | 1/4 (25) |
| Adenoma | 11/39 (28.2) | 3/4 (75) |
Values in parentheses are percentages. OMS, ovarian mesenchymal stroma. The main biliary duct was defined as the primary and secondary branches of the biliary tree.
In the 117 biliary cystic tumors reported in the Japana Centra Revuo Medicina database between 1983 and 2010, the subtype without OMS and luminal communication with the bile duct occurred at the highest frequency [39.3% (n = 46)]. On the other hand, only 4 tumors (3.4%) were found to have both OMS and luminal communication with the bile duct. Among 43 tumors with luminal communication with the bile duct, 35 tumors (81.4%) showed communication between the main biliary duct and the cystic tumor. The main biliary duct was defined as the primary and secondary branches of the biliary tree. As for gender distribution, the tumors with OMS occurred in all 32 women. Although adenocarcinoma was seen in 2 out of 28 tumors (7.1%) that showed OMS rather than luminal communication with the bile duct, as many as 28 of the 39 patients (71.8%) with luminal communication with the bile duct rather than OMS had adenocarcinoma.
3. Discussion
First, it seems reasonable to divide biliary cystic tumors that originally included biliary cystadenoma and biliary cystadenocarcinoma into IPN of the bile duct and hepatic MCN, as is the case for the pancreas. The existence of OMS contributed to the subclassification of biliary cystic tumors in epithelial tumors of the liver, analogous to IPMN and MCN in pancreatic epithelial tumors, according to the WHO classification. As seen in case 4 from our institution, a mucus-producing cholangiocarcinoma or an intra-bile duct tumor growth type of intrahepatic cholangiocarcinoma, which showed a poor tendency for interstitial infiltration, might be involved in IPN of the bile duct. In contrast to cases 1, 3, and 4, which were considered typical IPN of the bile duct because they lacked OMS but showed luminal communication with the bile duct, case 2 was an atypical form because it showed both luminal communication with the main biliary duct and OMS. Occasionally, we have seen atypical forms of pancreatic mucin-producing pancreatic neoplasms, including IPMN and MCN (14).
Second, the presence or absence of MUC1 and MUC2 expression could be an indicator of the malignant grade of the tumor. During the past several years, a number of human mucins (MUC1-MUC9) have been identified (15–23). MUC1 is a membrane-associated glycoprotein with an extracellular domain consisting of a variable number of highly conserved tandem repeats of 20 amino acids, a transmembrane domain, and a cytoplasmic tail of 69 amino acids (24,25). MUC2-MUC7 are expressed by secretory cell types (26,27), whereas MUC8 and MUC9 are expressed in the reproductive tract tissues (22,23). MUC2 expression has been frequently observed in biliary papillary tumors, including non-invasive tumors and invasive lesions (tubular adenocarcinoma and mucinous carcinoma), whereas MUC1 expression is commonly found in tubular adenocarcinoma cases but rarely in non-invasive tumors and mucinous carcinoma (28). Pancreatic IPMN is commonly divided into 4 histopathological subtypes: pancreatobiliary, intestinal, gastric, and oncocytic subtypes. These subtypes correlate well with tumor cellular atypism (28). The pancreatobiliary, intestinal, and oncocytic subtypes tend to occur in the main pancreatic duct-type IPMN and are frequently associated with invasive carcinoma. However, the gastric type tends to occur in branched-type IPMN and appears to be benign (29). Furthermore, these subtypes show differences in MUC expression. The pancreatobiliary, intestinal, oncocytic, and gastric subtypes are often MUC1+/MUC2±, MUC1−/MUC2+, MUC1+/MUC2±, and MUC1−/MUC2−, respectively. Thus, either MUC1+ or MUC2+ seems to be associated with an increased risk of malignancy. In the above mentioned cases, case 3 showed atypical epithelial cells and was MUC1+/MUC2−, and case 4, which included papillary proliferation of atypical epithelium and interstitial infiltration, was MUC1+/MUC2+. In contrast, cases 1 and 2, which had normal intracystic simple cuboidal epithelium, were MUC1−/MUC2−. Furthermore, Zen et al. revealed that IPN of the bile duct commonly was MUC1+/MUC2±, whereas pancreatic IPMN was often MUC1−/MUC2± (30). Therefore, although IPN of the bile duct and pancreatic IPMN are thought to be related diseases, the former should be given more attention because it might have a higher malignant potential. MUC immunohistochemistry, which is the prevalent technique in pancreatic IPMN, could provide a good indication of the malignancy grade of IPN in the bile duct.
Interestingly, our analysis of these past cases revealed that biliary cystic tumors with OMS all occurred in women as equally as the findings in pancreatic IPMN. Similarly, the cases without OMS had a higher tendency to be adenocarcinoma than cases with OMS. Thus, clinicopathological subclassification of biliary cystic tumors, according to the presence or absence of OMS and luminal communication with the bile duct, is required for further recognition of the pathophysiology and prognosis. However, since several intermediate or transitional types have been detected, with the exception of the typical type, further accumulation and elucidation of clinical cases are required.
Since many cases with malignant potential or malignancy itself were observed, such as our case series, radical hepatic resection is the recommended treatment for IPN of the bile duct and hepatic MCN as long as the patient is operable. All 4 of our patients survived and showed no recurrence.
In conclusion, research on more cases of biliary cystic tumors must be conducted and it is reasonable and appropriate to subclassify biliary cystic tumors according to the presence or absence of OMS and luminal communication with the bile duct. Hepatic resection is recommended as the curative treatment for biliary cystic tumors because they reveal various grades of malignancy.
References
- 1. Korobkin M, Stephens DH, Lee JK, Stanley RJ, Fishman EK, Francis IR, Alpern MB, Rynties M. Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. AJR Am J Roentgenol. 1989; 153:507-511 [DOI] [PubMed] [Google Scholar]
- 2. Buetow PC, Buck JL, Pantongrag-Brown L, Ros PR, Devaney K, Goodman ZD, Cruess DF. Biliary cystadenoma and cystadenocarcinoma: Clinical-imaging-pathologic correlations with emphasis on the importance of ovarian stroma. Radiology. 1995; 196:805-810 [DOI] [PubMed] [Google Scholar]
- 3. Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: Differential CT and MR imaging features. Radiographics. 2001; 21:895-910 [DOI] [PubMed] [Google Scholar]
- 4. Subramony C, Herrera GA, Turbat-Herrera EA. Hepatobiliary cystadenoma: A study of five cases with reference to histogenesis. Arch Pathol Lab Med. 1993; 117:1036-1042 [PubMed] [Google Scholar]
- 5. Bosman FT, Carneiro F, Hurban RH, Thiese ND. WHO classification of tumours of digestive system. International Agency for Research on Cancer Press, Lyon, France. 2010: 222-238 [Google Scholar]
- 6. Lim JH, Yoon KH, Kim SH, Kim HY, Lim HK, Song SY, Nam KJ. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004; 24:53-66 [DOI] [PubMed] [Google Scholar]
- 7. Augustin T, Vandermeer TJ. Intraductal papillary mucinous neoplasm: A clinicopathologic review. Surg Clin North Am. 2010; 90:377-398 [DOI] [PubMed] [Google Scholar]
- 8. Basturk O, Coban I, Adsay NV. Pancreatic cysts: Pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009; 133:423-438 [DOI] [PubMed] [Google Scholar]
- 9. Procacci C, Carbognin G, Biasiutti C, Guarise A, Ghirardi C, Schenal G. Intraductal papillary mucinous tumors of the pancreas: Spectrum of CT and MR findings with pathologic correlation. Eur Radiol. 2001; 11:1939-1951 [DOI] [PubMed] [Google Scholar]
- 10. Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: Is the biliary tract an incomplete pancreas? Pathol Int. 2010; 60:419-429 [DOI] [PubMed] [Google Scholar]
- 11. Oshikiri T, Kashimura N, Katanuma A, Maguchi H, Shinohara T, Shimizu M, Kondo S, Katoh H. Mucin-secreting bile duct adenoma: Clinicopathological resemblance to intraductal papillary mucinous tumor of the pancreas. Dig Surg. 2002; 19:324-327 [DOI] [PubMed] [Google Scholar]
- 12. Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y, Yonezawa S. Pathologic features of mucin-producing bile duct tumors: Two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004; 28:327-338 [DOI] [PubMed] [Google Scholar]
- 13. Kloppel G, Kosmahl M. Is the intraductal papillary mucinous neoplasia of the biliary tract a counterpart of pancreatic papillary mucinous neoplasm? J Hepatol. 2006; 44:249-250 [DOI] [PubMed] [Google Scholar]
- 14. Furukawa T, Kloppel G, Volkan Adsav N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. 2005; 447:794-799 [DOI] [PubMed] [Google Scholar]
- 15. Abe M, Kufe D. Characterization of cis-acting elements regulating transcription of the human DF3 breast carcinoma-associated antigen (MUC1) gene. Proc Natl Acad Sci U S A. 1993; 90:282-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gum JR, Byrd JC, Hicks JW, Toribara NW, Lamport DT, Kim YS. Molecular cloning of human intestinal mucin cDNAs: Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989; 264:6480-6487 [PubMed] [Google Scholar]
- 17. Gum JR, Hicks JW, Swallow DM, Lagace RL, Byrd JC, Lamport DT, Siddiki B, Kim YS. Molecular cloning of cDNA derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990; 171:407-415 [DOI] [PubMed] [Google Scholar]
- 18. Porchet N, Nguyen VC, Dufosse J, Audie JP, Guyonnet-Duperat V, Gross MS, Denis C, Degand P, Bernheim A, Aubert JP. Molecular cloning and chromosomal localization of novel human tracheobronchial mucin cDNA containing tandemly repeated sequence of 48 base pairs. Biochem Biophys Res Commun. 1991; 175:414-422 [DOI] [PubMed] [Google Scholar]
- 19. Meerzaman D, Charles P, Daskal E, Polymeropoulos MH, Martin BM, Rose MC. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5). J Biol Chem. 1994; 269:12932-12939 [PubMed] [Google Scholar]
- 20. Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW, Gum JR, Jr, Byrd JC, Siddiki B, Kim YS. Human gastric mucin: Identification of a unique species by expression cloning. J Biol Chem. 1993; 268:5879-5885 [PubMed] [Google Scholar]
- 21. Bobek LA, Tasai H, Biesbrock AR, Levine MJ. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivarymucin (MUC7). J Biol Chem. 1993; 268:20563-20569 [PubMed] [Google Scholar]
- 22. D'Cruz OJ, Haas GG, Dunn TS, Hass GG, Jr, Sachdev GP. Antigenic cross-reactivity of human tracheal mucin with human sperm and trophoblasts correlates with the expression of mucin 8 gene messenger ribonucleic acid in reproductive tract tissues. Fertil Steril. 1996; 66:316-326 [DOI] [PubMed] [Google Scholar]
- 23. Lapensee L, Paquette Y, Bleau G. Alleric polymorphism and chromosomal localization of the human oviductin gene (MUC9). Fertil Steril. 1997; 68:702-708 [DOI] [PubMed] [Google Scholar]
- 24. Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990; 265:15286-15293 [PubMed] [Google Scholar]
- 25. Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA. Cloning and sequencing of a human pancreatic mucin cDNA. J Biol Chem. 1990; 265:15294-15299 [PubMed] [Google Scholar]
- 26. Carraway KL, Fregien N. Mucin Structure and Function: Insights from Molecular Biology. Trends in Glycoscience and Glycotechnology. 1995; 33:31-44 [Google Scholar]
- 27. Kim YS, Gum JR, Jr. Diversity of mucin genes, structure, function, and expression. Gastroenterology. 1995; 109:999-1013 [DOI] [PubMed] [Google Scholar]
- 28. Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct-an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006; 44:350-358 [DOI] [PubMed] [Google Scholar]
- 29. Ban S, Naitoh Y, Mino-Kenudson M, Sakurai T, Kuroda M, Koyama I, Lauwers GY, Shimizu M. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Its histopathologic difference between 2 major types. Am J Surg Pathol. 2006; 30:1561-1569 [DOI] [PubMed] [Google Scholar]
- 30. Zen Y, Fujii T, Itatsu K, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006; 44:1333-1343 [DOI] [PubMed] [Google Scholar]


