Summary
This study reported a family with primary hyperparathyroidism due to parathyroid carcinoma and investigated the pathological and genetic features of family members. Three members of the family had clinical manifestation of primary hyperparathyroidism and tumors in the neck. All three patients underwent parathyroidectomy, thyroidectomy and level-VI neck dissection and were definitively diagnosed based on pathology. The index case was a patient that was found to have parathyroid carcinoma on the right side and parathyroid adenoma on the left side. The other two patients had local tumor recurrence and metastasis to distant organs. A germline mutation in the HRPT2 gene (Arg91Pro) was identified in all of the patients in this family. Study of the literature indicated that this is the first report of familial parathyroid carcinomas with an HRPT2 gene missense mutation. Results also indicated that HRPT2 may play an important role in the development of parathyroid carcinoma.
Keywords: Familial, parathyroid tumor, hyperparathyroidism, HRPT2
1. Introduction
Parathyroid carcinoma (PTC) is a rare malignant tumor of the endocrine system and accounts for fewer than 1–3% of all cases of primary hyperparathyroidism (1,2). Primary hyperparathyroidism usually occurs sporadically but may be inherited as an autosomal dominant trait. However, few previous reports have described familial parathyroid carcinoma. The current study reported a family with three parathyroid carcinoma patients, and a germline mutation in the HRPT2 gene was identified in these family members.
2. Materials and Methods
2.1. Clinical data
The index case, a 30-year-old man, complained of pain in the lower extremities for 2 years and headache, fatigue, nausea and vomiting for 1 month when seen on November 14, 2008. A computed tomography (CT) scan revealed two tumors with distinct margins (17 × 17 × 25 mm and 15 × 14 × 25 mm) in the posterior and lower portions of both lobes of the thyroid. No significant increase in shadows from neck lymph node shadows on either side was noted (Figure 1A). An emission CT scan revealed nodules in the right lower lobe that had radioactive distribution equal to the surrounding thyroid tissue, and the lower pole of the left lobe had limited radiation uptake (Figure 1B). The serum levels of parathyroid hormone (PTH), alkaline phosphatase (ALP), calcium, phosphorus, and potassium were 1,367 pg/mL, 392 U/L, 4.03 mmol/L, 0.56 mmol/L, and 3.43 mmol/L, respectively. The patient in the index case underwent parathyroidectomy, thyroidectomy, and level-VI neck dissection. Postoperative pathology confirmed parathyroid carcinoma on the right side (Figure 2A) and parathyroid adenoma on the left side, and no lymph node metastasis was found.
Figure 1.
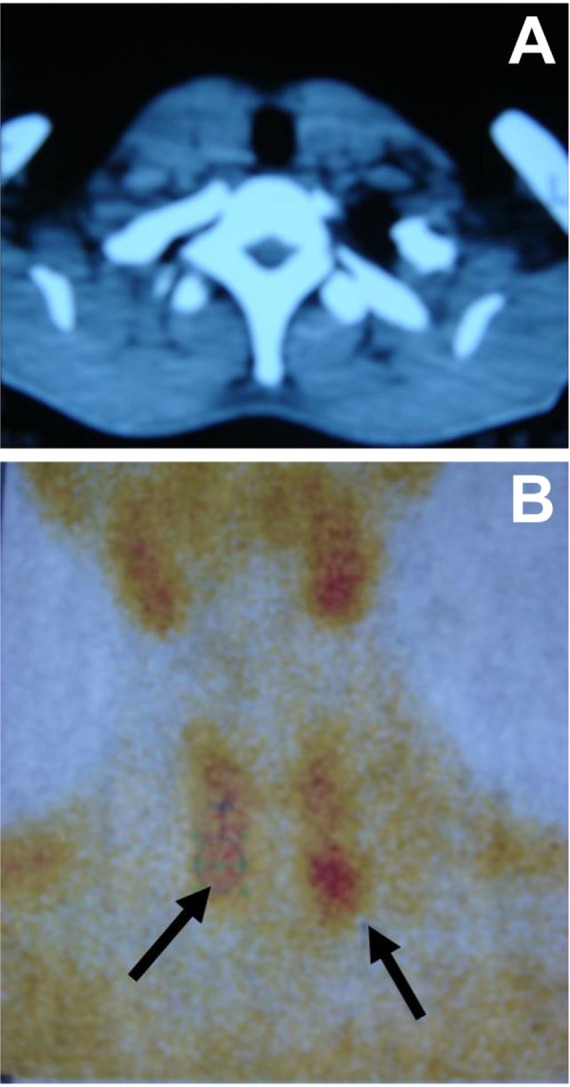
Images of the parathyroid tumors in the index case. (A), Computed tomography. (B) Emission computed tomography.
Figure 2.
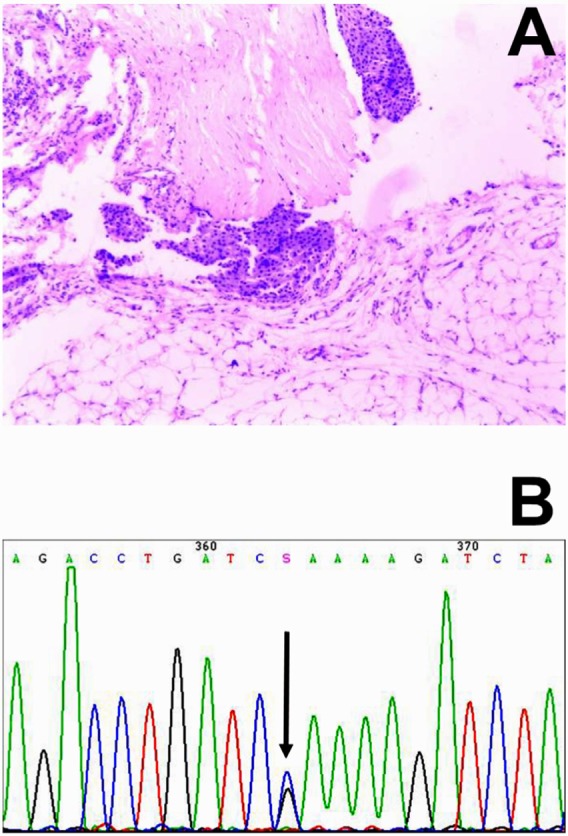
Pathological images of the parathyroid carcinoma in the index case (A, HE staining, Original magnification 100×), and a HRPT2 gene missense mutation at exon 3 (Arg91Pro, B).
The father and sister of the patient in index case underwent surgery at other hospitals and were confirmed to have parathyroid carcinoma by a pathological examination. Furthermore, both had distant bone metastasis, and the sister had already died of multiple bone metastases.
2.2. Immunohistochemical staining
Immunohistochemistry was performed on the tissues of the parathyroid carcinoma and the adenoma from the index case. Immunohistochemical staining was performed with commercially available monoclonal antibodies against human Syn, CgA, Calcitonin, TG, TTF-1 and Ki-67 as described previously (2).
2.3. HRPT2 gene mutation analysis
Peripheral blood was obtained from all family members (3 patients and 3 asymptomatic relatives) with their informed consent to identify germline HRPT2 mutations. This was approved by the local ethics committee. Extracted DNA was amplified in 15 segments (from 226 to 599 bp) to allow mutation screening of the 17 translated HRPT2 exons and their exon-intron boundaries. The primers used for PCR amplification are described in supplemental data. A 20-µL mixture was prepared for each reaction and included 1× HotStarTaq buffer, 2.0 mM Mg2+, 0.2 mM dNTP, 0.2 µM of each primer, 1 U HotStarTaq polymerase (Qiagen Inc. CA, USA), and 1 µL template DNA. The cycling program was 95°C for 15 min; 11 cycles of 94°C for 15 sec, 62°C–0.5°C per cycle for 40 sec, 72°C for 1 min; 24 cycles of 94°C for 15 sec, 56°C for 30 sec, 72°C for 1 min; 72°C for 2 min. PCR products were purified using SAP and Exo I and then sequenced on an ABI 3130XL sequencing system (Applied Biosystems, CA, USA).
3. Results and Discussion
Mutations of the HRPT2 gene have recently been implicated in the development of parathyroid carcinoma (3–5). Familial isolated hyperparathyroidism with the HRPT2 mutation may represent a risk factor for developing parathyroid carcinoma. However, familial parathyroid with primary hyperparathyroidism has rarely been reported.
The current study is the first to report a family with 3 cases of parathyroid carcinoma with the HRPT2 gene mutation. A 3-generation pedigree of this family revealed that 3 members had parathyroid carcinoma with a HRPT2 gene missense mutation at exon 3 (Arg91Pro) (Figure 2B), and asymptomatic relatives were negative for the Arg91Pro germline mutation. Furthermore, the index case had both benign and malignant parathyroid tumors, a finding that has been reported in previous studies (6). These findings suggest that the Arg91Pro mutation of HEPT2 gene might be required for multistage development of parathyroid carcinoma. The molecular characteristics of benign and malignant tumors were also compared based on immunohistochemistry. Immunohistochemistry results for the parathyroid carcinoma tissues (right side) were follows: Syn (weak positive), CgA (weak positive), Calcitonin (−), TG (−), TTF-1 (−), and Ki-67 (positive cells 5–10%). Immunohistochemistry results for the left parathyroid adenoma were as follows: Syn (weak positive), CgA (weak positive), Calcitonin (−), TG (−), TTF-1 (−), and Ki-67 (positive cells < 2%). Molecular characteristics of the parathyroid adenoma and carcinoma were similar, indicating that the parathyroid carcinoma may have originated from the adenoma, which was induced by the HEPT2 gene mutation at Arg91Pro.
In a previous study, Cetani et al. (7) identified the Arg91Pro mutation in patients with early onset of primary hyperparathyroidism (PHPT), and they suggested this HRPT2 mutation may be ascribed to modification of parafibromin conformation and/or stability. The Arg91Pro mutation is also associated with different HRPT2 somatic alterations in parathyroid tumors, which is consistent with the “two hit” concept of biallelic inactivation of classical tumor suppressor genes.
In conclusion, this report is the first to identify the pedigree of parathyroid carcinomas with the HRPT2 gene missense mutation. Together with previous findings, the current results suggest that the Arg91Pro mutation of the HRPT2 gene might be associated with early onset of PHPT and a high risk of developing a parathyroid carcinoma. Results also suggest that HRPT2 may serve as an important suppressor gene in the development of parathyroid carcinoma.
Supplemental data
PCR Primers
P2F GGGGCGCAGTCAGGCGTTCTC
P2R CCCGAACACCCGTTTTATCCCATCC
P3F TCATTGTTGTTAGCAAAGTTGTTT
P3R ATGAATTATTGGCCAGGCACA
P4F CGCCCAGCCAAGAAAATTA
P4R CCATAAGGGCAAAGACAGTGC
P5F TGCAGAGCTGCTTTAAAACTGAA
P5R CCTTGAGCCAATAGGTTCATCC
P6F TTGACTCTGGTGAAGGCTTGTC
P6R CCAATCCCCACACATGTTCTT
P7F TTGCCATGTAAGTGTTTTTACCAGA
P7R AACAGGAAAACTGGGCCATTC
P8F GCCTCCCGAATGTAGCAGTTT
P8R TTTAATGTTGGGGAGGGCTTT
P9F TGAGCCATGGTCATGCTACTG
P9R CCCTTAAACATCAGGCCACAC
P10F TGCCTTATGACTGAAACTTTGGA
P10R CCACACAGATCAATCAGCACAA
P11F GGTTTTTCCAACAGGAGGGTA
P11R TCGACAGTCTTCAAAGAAACATGA
P12F TTTCCTTTTGACTACAGATTGTTGAA
P12R GCCTATAGCACAGAAACCGAAA
P13F TTGAAACCAGAAGGTGGAGGT
P13R CAACGTCATCAACGGCAATAA
P14F AAGGGAACAGAAAGGGCAAAC
P14R TGTTGTGGGATAGGCAATATTCA
P15F TTATAATACGGCTTCAGTTGGTGGA
P15R TTGCAAAAAACACAGGGTTCTC
P16F CGGATGGTTCACAAAAGGAAA
P16R CTTGAAGCACAAAGCATCAAA
References
- 1. Fang SH, Lal G. Parathyroid cancer. Endocr Pract. 2011; 17:36-43 [DOI] [PubMed] [Google Scholar]
- 2. Sharretts JM, Simonds WF. Clinical and molecular genetics of parathyroid neoplasms. Best Pract Res Clin Endocrinol Metab. 2010; 24:491-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim HK, Oh YL, Kim SH, Lee DY, Kang HC, Lee JI, Jang HW, Hur KY, Kim JH, Min YK, Chung JH, Kim SW. Parafibromin immunohistochemical staining to differentiate parathyroid carcinoma from parathyroid adenoma. Head Neck. 2012; 34:201-206 [DOI] [PubMed] [Google Scholar]
- 4. Carlson D. Parathyroid pathology: Hyperparathyroidism and parathyroid tumors. Arch Pathol Lab Med. 2010; 134:1639-1644 [DOI] [PubMed] [Google Scholar]
- 5. Okamoto T, Iihara M, Obara T, Tsukada T. Parathyroid carcinoma: Etiology, diagnosis, and treatment. World J Surg. 2009; 33:2343-2354 [DOI] [PubMed] [Google Scholar]
- 6. Lee YS, Nam KH, Chung WY, Chang HS, Park CS. Coexistence of parathyroid adenoma and papillary thyroid carcinoma. J Korean Surg Soc. 2011; 81:316-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cetani F, Pardi E, Ambrogini E, Viacava P, Borsari S, Lemmi M, Cianferotti L, Miccoli P, Pinchera A, Arnold A, Marcocci C. Different somatic alterations of the HRPT2 gene in a patient with recurrent sporadic primary hyperparathyroidism carrying an HRPT2 germline mutation. Endocr Relat Cancer. 2007; 14:493-499 [DOI] [PubMed] [Google Scholar]


