Summary
Age-related macular degeneration (AMD) is a leading cause of severe visual impairment and disability in older people worldwide. Although considerable advances in the management of the neovascular form of AMD have been made in the last decade, no therapy is yet available for the advanced dry form of AMD (geographic atrophy). This review focuses on current trends in the development of new therapies targeting specific pathophysiological pathways of dry AMD. Increased understanding of the complex mechanisms that underlie dry AMD will help to address this largely unmet clinical need.
Keywords: Age-related macular degeneration, dry age-related macular, degeneration, geographic atrophy, treatment, clinical trials
1. Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness and visual disability in the elderly. AMD prevalence substantially increases with increasing age and up to one third of individuals aged 75 and older suffer from a form of AMD (1). According to the European Eye Study (EUREYE), an estimated 2.5 million of the European population 65 years and older have AMD and more than 1.1 million have significantly impaired vision due to bilateral AMD (2). A meta-analysis of population-based data estimated that AMD affects 1.75 million individuals aged 40 years and older in the United States, and owing to the rapidly aging population, this figure is projected to rise to almost 3 million by 2020 (3). Although some early data in the past suggested that AMD in non-Caucasian populations may be less common, recent population-based studies indicate that an increasing prevalence of AMD may be occurring elsewhere (4–6).
AMD is a gradually progressive disease that evolves through stages into severe central vision loss (Figures 1A and 1B). The earliest signs of AMD designated as age-related maculopathy (ARM) involve the appearance of extracellular deposits (drusen), subretinal deposits of oxidized lipids and proteins beneath the retinal pigment epithelium (RPE), and variable amounts of visible clumps of pigment in the macula. In the intermediate stages of AMD, drusen become larger and pigmentary changes are more severe. In the advanced stages, patients develop either subretinal choroidal neovascularization (the exudative or “wet” form of AMD) or the non-neovascular “dry” form of AMD. The dry form of AMD is characterized by sharply demarcated uni- or multi-focal regions of dysfunctional macula, termed geographic atrophy (GA) (Figure 2). The GA patches gradually enlarge to involve the RPE and the corresponding neurosensory retina and choriocapillaris layer of the choroid. These progressive and irreversible changes ultimately cause permanent loss of central (macular) vision. Areas of GA therefore correspond to absolute scotomas. GA occurs bilaterally in over 50% of patients, as reviewed (7). It represents the slower and more insidious form of the disease, and accounts for approximately 20–25% of patients with severe visual loss secondary to AMD (legal blindness related to AMD) (8) and for a much larger percentage of moderate visual loss of the elderly (9).
Figure 1.

A scene viewed by a person with normal vision and age-related macular degeneration (AMD). (A) A scene as it might be viewed by a person with normal vision; (B) The same scene viewed by a person with age-related macular degeneration. (Image courtesy of Dr. Frangov)
Figure 2.
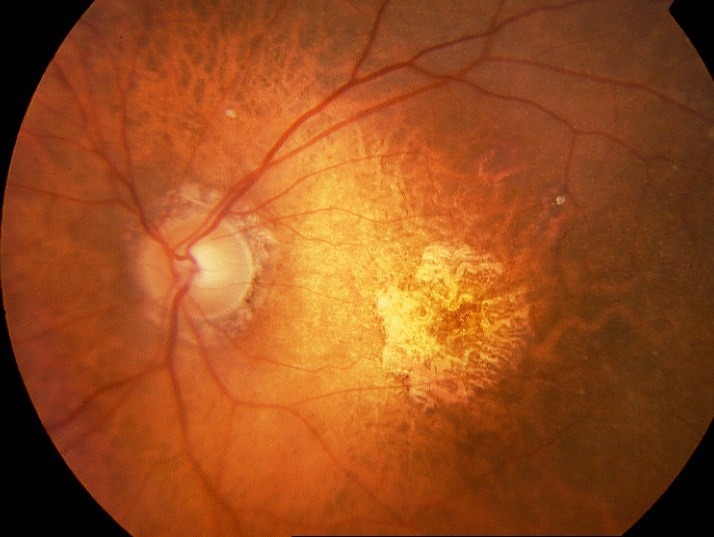
Fundus photograph showing geographic atrophy due to age-related macular degeneration. (Image courtesyof Dr. J-F Girmens)
In recent years, significant progress has been made towards developing treatments for the wet form of AMD. Some of these treatment options demonstrated clear therapeutic success, including laser photocoagulation, photodynamic therapy, and most recently, intraocular drug therapy with anti-VEGF agents, such as ranibizumab (Lucentis®) or pegaptanib sodium (Macugen®). However, development of treatments for advanced dry AMD has not yet progressed either as quickly or to an extent comparable to those for the wet form of AMD. So far, no treatments have been proven to effectively prevent the onset of GA (10) or to halt lesion enlargement and/or retard vision loss (11,12). Taking into consideration the rapidly aging population throughout the world, the morbidity resulting from AMD becomes increasingly significant and dry AMD remains a large unmet clinical need. Its prevention would be of paramount socioeconomic importance for the individual patients and for the healthcare system. Improved understanding of the pathogenesis of dry AMD would certainly provide a powerful basis for development of novel therapeutic strategies.
2. Pathophysiology and risk factors
AMD is a multifactorial disease with largely unknown pathophysiology. It is believed to be caused by cumulative damage over a lifetime leading to progressive deterioration of the RPE, Bruch's membrane and choriocapillaris-choroid complex (key players in maintaining retinal function), followed by photoreceptor cells damage. However, the locus of primary damage still remains unclear. Identified pathogenic mechanisms to date include a range of genetic and environmental factors related to primary RPE senescence, oxidative stress, alterations in the complement pathway, increased inflammation, changes in the balance of growth factors, and excessive lipofuscin accumulation (1,13).
Drusen, deposits of extracellular material beneath the RPE and within Bruch's membrane, are considered the hallmark of the disease. Factors that were correlated with increased risk of progression to advanced AMD include large initial soft drusen and large numbers of small hard drusen (14). As supported by epidemiological data, aging is considered to play a major role in AMD development. Only 2% of 50–60 year-old individuals have AMD, while the disease prevalence rises exponentially with increasing age so that over 30% of individuals over age 75 suffer some form of AMD (15). A key component of drusen is amyloid beta, a waste product which accumulates in the central nervous system with aging and in several age-related diseases, such as Alzheimer's disease. Age related accumulation of amyloid beta is documented along Bruch's membrane and blood vessels, and also in the photoreceptor outer segment (16). In AMD and aging, an increased oxidative stress induced by various intracellular photochemical reactions or normal metabolism is present in the retinal and choroidal tissue. Oxidative stress can result in blood-retinal barrier breakdown allowing plasma proteins and platelets to invade retinal tissue. Oxidative stress itself may contribute to further inflammatory responses, including complement activation and pro-inflammatory cytokine production. A potential role for lipofuscin in drusen biogenesis has also been proposed, even though the reasons for lipofuscin accumulation remain insufficiently understood.
Recently, inflammatory etiology of AMD (inflammation, immune system and autophagy) has been increasingly recognized and strengthened with the identification of complement factor H mutations being associated with over 40% of AMD cases (17,18). Complement is constitutively present in normal retina (and active at low levels) where it plays a vital defensive role against pathogens and helps to maintain immune privilege. In case of excessive activation, however, it may have a potentially harmful action. Since drusen include elements of the complement system belonging to all pathways, such as C1q, mannose binding lectin, factor B (CFB), factor I (CFI), factor H (CFH), C3 and its fragments - C5, many studies support the idea that the complement cascade plays an important role in the pathogeneses of AMD. It may act both as a trigger and progression factor, alone or together with other pathogenetic mechanisms, such as oxidative stress, although the molecular basis of these interactions remains to be determined. Other potential mediators of inflammation in AMD are the pro-inflammatory cytokines IL-1, IL-6, and TNFα, which are released from the choroid of patients with AMD.
In recent years, the whole genome screening allowed identification of mutations and polymorphisms in protein sequences of key regulators of the complement system associated with AMD. Thanks to this technological progress, heredity is now recognized to play an important role as an AMD risk factor, with major risk loci on chromosome 1 in the complement regulatory genes (17) and on chromosome 10 in the promoter region of the HTRA1 gene (19). Genetic studies reported multiple polymorphisms in complement genes (including CFH, complement factor H-related (CFHR) 1 and 3, CFB, C2, C3, and CFI) as risk factors for AMD (20). Three initial studies have found an association between AMD and polymorphism of the CFH gene (Y402H) (18,21,22). Another member of the complement system, factor B, has also been shown to have both high-risk and protective variants associated with the development of AMD (23). A hypothetical gene LOC387715, residing in chromosome 10 in a region associated with susceptibility to AMD (subsequently reinstated with the symbol ARMS2), has been demonstrated to have an independent effect on AMD that is almost as strong as that of the CFH gene (24,25). A recent study (26) discovered that genetic variants near TIMP3 (a metalloproteinase involved in degradation of the extracellular matrix, previously implicated in early-onset maculopathy) and high-density lipoprotein (HDL)-associated loci (human hepatic lipase, LIPC, and cholesterol ester transfer protein, CETP) modify susceptibility to AMD. Consistent with the hypothesis that the HDL pathway is associated with AMD pathogenesis, these investigators identified two additional genes, lipoprotein lipase (LPL) and ATP-binding cassette transporter 1 (ABCA1). The genetic variants found in the cholesterol pathway are believed to impact the retina and may be a target for a future AMD therapeutic strategy. CFH and HTRA1 variants appear to predispose to both atrophic and neovascular AMD (27,28); however, the mechanisms that are specific to GA are still largely unknown. Recently, toll-like receptor-3 (TLR3) activation (enhanced with the 412L variant) has been proposed to specifically promote progression of the disease to the GA phenotype (29), but this association still remains controversial and needs further elucidation. Also, bone morphogenetic protein-4 (BMP4) has been found prominently and specifically expressed only in the RPE and adjacent extracellular matrix of patients with dry AMD, while almost no expression was observed in the same tissues of patients with wet AMD (30). These findings opened a new hope towards novel potential therapeutic solutions specifically targeting the dry form of AMD.
Environmental and demographic factors seem to be involved in the pathogenesis of AMD. Large population-based studies indicated that cigarette smoking and in particular, prior and current smokers are at increased risk for developing AMD, female smokers being at higher risk for progression to advanced AMD, while male smokers may be at higher risk for dry AMD (31,32). Obese individuals appear at higher risk for dry and neovascular AMD, while very lean individuals may be at higher risk for dry AMD (33). Alcohol consumption was not found to be associated with AMD, as reviewed (34). AMD appears to be more prevalent in the white population (35). Among demographic factors, the female sex, light skin pigmentation and light iris pigmentation may represent risk factors for AMD. Among environmental factors, excessive visible light exposure may be linked to the risk of developing AMD. Improving knowledge of the multiple genetic, environmental and demographic factors involved in the pathogenesis of the dry AMD is expected to enable development of targeted drugs to specifically treat and possibly even prevent the disease.
The contribution of epigenetic factors in the pathophysiology of AMD remains largely unknown. Recent investigations point at the unknown portion of genetic risk in AMD and the relevance of epigenetics in this respect (36,37). Genome-wide association studies would certainly help researchers enhance the global knowledge on the genetics of complex diseases such as AMD and its potential to propose targeted disease prevention or treatment. Future research is required to obtain valuable information on epigenetic regulation of AMD.
3. Current therapeutic strategies
No therapy is currently available for the dry slowly progressing atrophic form of AMD. Treatment directed against the cause of the disease yet remains difficult to accomplish, as the underlying etiology is very complex and remains elusive. This review focuses on recent trends in the development of new therapies targeting specific pathophysiological mechanisms of dry AMD. Special attention was paid to advanced treatments under evaluation in ongoing clinical investigations. The information on registered clinical trials and observational studies cited in this paper was obtained from the ClinicalTrials.gov Results Database assessed in May 2012.
3.1. Supportive measures
Without proven treatment to stop or prevent progression of the disease, patients should be offered supportive measures and appropriate advice on adequate lighting for near-vision tasks. Common low vision aids include low vision filters (including red or amber filters to reduce the severe photophobia, colored translucent acetate sheets to enhance contrast between the print and background), stand-mounted or handheld video magnifiers (e.g. closed circuit video magnification systems), electronic aids (e.g. autofocus systems, electronic visual enhancement systems, prismatic eye glasses to refract images from a field outside the normal central field), and low vision rehabilitation (e.g. eccentric fixation technique, which teaches patient to look just to one side of the object of interest rather than directly at the object). A newer approach to visual rehabilitation includes the implantable miniature telescope, a monocular-fixed focus telescopic device. It is implanted into the patient eye during surgery (e.g. cataract surgery) and projects images over healthy areas of the retina; after surgery, a vision rehabilitation program should be proposed (38). Excessive visible light exposure should be avoided and sunglasses should be advised (absorptive sunlenses/sunglasses). Cessation of smoking should be vigorously advised as it believed that this measure may reduce the risk of AMD progression.
3.2. Drugs to prevent injury from micronutrient depletion and oxidative stress (micronutrient supplements and antioxidants)
A variety of nutrients has been epidemiologically linked with decreased risk of AMD, including the antioxidant vitamins C, E, and A, and the zinc and selenium minerals that may act as co-factors for a number of endogenous antioxidant enzymes. Patients should be advised of the importance of a well-balanced diet including omega-3 fatty acids, which can be obtained from fish (salmon, tuna, mackerel, herring, and sardines), vegetable oils (soybean, rapeseed, linseed, and walnuts) or some green vegetables (Brussels sprouts, kale, spinach, and salad greens). Several epidemiological studies have indicated that diets rich in these particular lipids are associated with decreased risk of AMD. The Age-Related Eye Disease Study (AREDS) is the major clinical trial that was designed to obtain knowledge about the natural history and risk factors of AMD. This multi-center, controlled randomized study followed 4757 subjects, 55–80 years old, for at least five years. AREDS formulation included 500 mg of vitamin C, 400 IU of vitamin E, 15 mg of beta-carotene (often labeled as equivalent to 25,000 IU of vitamin A), 80 mg of zinc as zinc oxide and 2 mg of copper as cupric oxide (these high doses of zinc and anti-oxidants cannot be achieved from diet alone). Participants received daily oral tablets of either: 1) zinc alone; 2) antioxidants alone; 3) a combination of antioxidants and zinc; or 4) a placebo (39–41). The results of this study showed that a high-dose combination of vitamins and zinc significantly reduced (by about 25%) the risk of advanced AMD and associated vision loss in the high risk group of people with intermediate or advanced (late) AMD in one eye. There was no benefit in patients with no signs of AMD or early AMD, or those with bilateral advanced AMD. A few adverse effects have been registered in this study, e.g. yellowing of the skin and genitourinary complications (mostly due to antioxidant and zinc ingestion, respectively). Other studies, however, demonstrated that the beta-carotene component increases the risk of lung cancer in smokers, and this treatment should not be recommended in smokers or ex-smokers (42). The reduction of AMD progression risk upon using the AREDS therapeutic regimen can be considered as modest, but in view of the rapidly increasing aging of the population, the public health implications are enormous. This is why the recommendation of AREDS compliant formulas to at risk patients are considered as standard care in ophthalmology (15).
Among the large number of antioxidants, the dietary xanthophyll carotenoids lutein and zeaxanthin are of particular interest because they are specifically concentrated in the human macula (15). The first epidemiological evidence that lutein and zeaxanthin may be protective against AMD was published in the 1990s by the Eye Disease Case-Control (EDCC) Study Group (43). The initial findings of this study demonstrated in 421 cases and 615 controls that there was an inverse correlation between serum carotenoid levels and risk of exudative AMD. In a follow-up study of a subset of these same patients, it was found that dietary consumption of fruits and vegetables rich in lutein and zeaxanthin was associated with a 43% decrease in risk of AMD, while diets rich in beta-carotene (which is not found in the retina and which cannot be converted to xanthophylls) were not protective (43).
The Lutein Antioxidant Supplementation Trial (LAST) reported that purified lutein (a naturally occurring molecule found in dark green leafy vegetables such as spinach, kale and collard greens) or a supplement mix of lutein and other antioxidants such as vitamin A, vitamin C, vitamin E, and beta carotene led to significant improvements in several objective measurements of visual function including glare recovery, contrast sensitivity, and visual acuity vs. placebo (44). The LAST trial studied 90 AMD patients supplemented daily with an OcuPower supplement capsule containing 10 mg of crystalline FloraGLO lutein (the average daily ingest is ∼1–2 mg of lutein), 10 mg lutein plus a mixed antioxidant formula, or placebo for 12 months.
The AREDS 2 study (NCT00345176, Phase 3 clinical study, currently ongoing) was initiated in 2006 to study the effects of two dietary xanthophylls (lutein and zeaxanthin) and two omega-3 long-chain polyunsaturated fatty acids (LCPUFAs) - docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), on progression to advanced AMD and/or moderate vision loss. AREDS 2 is similar in scale to the original AREDS study with 4,000 subjects (age 50–85 with a high risk for AMD progression: large drusen in both eyes or advanced AMD in one eye and large drusen or non-central geographic atrophy in the fellow eye) who will be followed for five years at 100 sites. AREDS 2 formulation includes lutein 10 mg/zeaxanthin 2 mg, LCPUFA ∼ 1 g (350 mg DHA, 650 mg EPA). These micronutrients are believed to function not only as antioxidants, but also as anti-inflammatory and antiangiogenic agents.
The etiologic relevance of oxidative stress indicates a possible benefit in reducing free radicals in the AMD retina. OT-551 (Othera Pharmaceuticals) is one of the agents with antioxidant properties that are under investigation in dry AMD. It is a small lipophilic molecule, a disubstituted hydroxylamine, that readily penetrates the cornea. Converted by ocular esterases, the active metabolite has potent free-radical scavenger and antioxidant activities and, possibly, anti-inflammatory and antiangiogenic effects. OT-551 was studied in a single-center, open-label phase 2 trial, enrolling 10 participants with bilateral GA (topical 0.45% application, three times daily for up to three years) (45). Although well tolerated, OT-551 had no significant effect on lesion enlargement, retinal sensitivity or total drusen area compared to control fellow eye; a possible effect in maintaining visual acuity was suggested. It was concluded that the current concentration and mode of delivery have limited or no benefit as a treatment for GA (NCT00306488, completed).
3.3. Drugs to preserve photoreceptors and RPE through neuroprotection, improved blood supply and metabolic modulation
Ciliary neurotrophic factor (CNTF) is a cytokine member of the IL-6 family and a potent neuroprotective agent shown to rescue photoreceptors from degeneration in a number of preclinical studies, as reviewed (46). Intravitreal encapsulated CNTF sustained-release platform (CNTF/NT-501) that produces CNTF for a year or longer was developed by Neurotech Pharmaceuticals. It is known as an encapsulated cell technology (ECT) implant. A Phase 1 trial carried out in ten patients with advanced retinitis pigmentosa for a period of six months demonstrated that CNTF delivered by ECT implant could safely be implanted leading to subjective and objective visual acuity improvement (47,48). A randomized Phase 2 trial of a NT-501 implant for patients with atrophic macular degeneration was recently completed (NCT00447954). It showed that both the implant and the implant procedure were well-tolerated. CNTF treatment resulted in a dose-dependent increase in retinal thickness and apparent stabilization of visual acuity (49). These investigators concluded that the CNTF-ECT implant appears to slow the progression of vision loss in GA, especially in eyes with 20/63 or better vision at baseline. Further studies are warranted to establish this benefit.
Brimonidine (Allergan Inc., Irvine, CA, USA) is believed to have neuroprotective properties (based on animal studies) and beneficial effects in the treatment of glaucoma. It has also been shown to release various neutrophins, including BDNF, CNTF and b-FGF (50). Brimonidine belongs to the α-2 adrenergic receptor agonists. It is currently available as an ophthalmic solution (Alphagan-P, Allergan) for lowering the intraocular pressure in patients with open-angle glaucoma or ocular hypertension. A brimonidine tartrate intravitreal implant using the Allergan Novadur™ posterior segment drug delivery system is currently under evaluation in a Phase 2 study in regard to its efficacy and safety, and its possible effect on the progression of GA due to AMD (NCT00658619, ongoing, but not recruiting: 200 µg and 400 µg brimonidine tartrate posterior segment drug delivery system at day 1 and month 6). The implant delivers the drug to the retinal tissue over a period of 3 months.
AL-8309B (Tandospirone, Alcon Laboratories) (1.0% and 1.75% ophthalmic solution) is a selective agonist of the serotonin receptor (5HT1A) that has been shown to protect the retina from severe photo-oxidative stress (51). AL-8309 was previously reported to upregulate antioxidant defense mechanisms in the retina (52) and to interfere with the complement pathway, preventing the deposition of complement C3, factor B, factor H, and membrane attack complex (MAC) (53). AL8309B was evaluated in a randomized, double-masked, multicenter, placebo-controlled GATE clinical study, as a topical ocular treatment for GA secondary to AMD. No positive results have been reported up until now.
The recently discovered rod-derived cone viability factor (RdCVF) which has been shown to induce cone survival and prevent secondary degeneration in cone photoreceptors in retinitis pigmentosa could also hold therapeutic promise in dry AMD (54,55).
MC-1101 (MacuCLEAR, Inc., TX, USA) is a novel, topically administered (eye drops, 1.0% migrating to the back of the eye) compound which increases ocular blood flow in the choroidal vessels and prevents progression of AMD from the early-stage dry AMD to the late-stage wet AMD. It is also believed to possess anti-inflammatory and antioxidative properties, and to reduce accumulation of retinal related waste by-products. The active ingredient of MC-1101 has been previously approved by the FDA as an oral antihypertensive drug with a well-characterized safety and tolerability profile. In April 2012, MacuCLEAR, Inc. announced that it is beginning Phase 3 studies for MC-1101 for early-stage AMD (60 patients) based on a successfully completed Phase 1b/proof of concept human clinical trial.
3.4. Visual cycle modulators and drugs reducing the accumulation of toxic waste products
The regeneration of the visual pigment 11-cis-retinaldehyde (11-cis-RAL, isoretinoin) from all-trans-RAL constitutes an important pathway in the visual cycle. Agents that slow this regeneration process (termed visual cycle modulators) decrease the accumulation of the toxic metabolites A2E and lipofuscin and may find therapeutic application in GA and other forms of macular degeneration.
Fenretinide (RT-101, ReVision Therapeutics, Inc., CA, USA) [4-hydroxy(phenyl) retinamide] is an oral synthetic retinoid derivative which down-regulates photoreceptor metabolism. It binds retinol-binding protein (RBP) in the circulation, blocks association between retinol and RBP, and prevents transport of retinol to the RPE. In 2007, Sirion initiated a randomized double-masked, placebo-controlled, dose ranging (100 and 300 mg/day) Phase 2 study (NCT00429936, completed) to evaluate fenretinide efficacy in patients with GA associated with dry AMD. A reduction in the incidence of wet AMD in patients with GA and slowing the growth of the GA lesions were communicated, but data were not accepted for review by the FDA and further research seems to be halted.
Another visual cycle modulator is the oral agent ACU-4429 (Acucela). ACU-4429 is a small nonretinoid molecule that functions as a modulator of the isomerase (RPE65) required for conversion of all-trans-retinol to 11-cis-RAL in the RPE (56). By modulating isomerization, ACU-4429 slows the visual cycle in rod photoreceptors and decreases accumulation of toxic fluorophores (A2E) and lipofuscin. It was demonstrated to completely prevent light-induced acute retinal degeneration in mice (57) and atrophic changes in the Rdh8(-/-)Abca4(-/-) retina (58). A single orally administered dose of ACU-4429 in healthy subjects produced a dose-dependent inhibition of the b-wave of the electroretinograms and was well tolerated up to 75 mg (59). A safety and tolerability clinical study of repeat doses (orally once a day for 14 days) of ACU-4429 in healthy subjects has been successfully completed demonstrating that this agent effectively targeted the visual cycle in a dose-dependent manner. An ongoing NCT01002950 Phase 2 study of the safety, tolerability, pharmacokinetics and pharmacodynamics of ACU-4429 in subjects with GA is underway. This is a multicenter, randomized, double-masked, placebo-controlled, dose escalation, multiple-dose study in which tablets (2, 5, 7, 10 or 20 mg) are taken orally once daily for 90 days. Because the drug is a non-retinoid, it may be potentially safe for a wide range of people, including young patients and women of child-bearing age. ACU-4429 may represent a new approach to treating dry AMD and other degenerative eye diseases, e.g. Stargardt disease.
RN6G (PF-4382923, Pfizer, New York, USA) is a humanized monoclonal antibody that targets the C-termini of amyloid beta-40 and amyloid beta-42. A safety and tolerability study of RN6G of single escalating doses (ranging from 0.3 mg/kg up to a maximum of 40 mg/kg intravenously) was completed in 2011 (NCT00877032). A multiple escalating dosages study (NCT01003691) is currently recruiting participants. GSK933776 (GlaxoSmithKline) is another humanized monoclonal antibody that decreases the levels of amyloid beta. It is currently under clinical investigation in a Phase 2 study for safety and efficacy in adult patients with GA secondary to AMD (CT01342926). Evaluation of this compound in patients with Alzheimer's disease is also underway.
3.5. Drugs to suppress inflammation (complement inhibitors, immunomodulators and anti-inflammatory drugs)
As mentioned above, the complement system is at the core of many inflammatory processes. The presence of complement factor proteins in drusen in AMD eyes and genetic variation in several complement factor genes in individuals with AMD strongly support the involvement of the complement system in AMD predisposition and progression (60–62). To explore this etiopathological pathway towards development of therapeutic options for AMD, complement inhibitors, immunosuppressive agents and glucocorticoids are under extensive investigation.
3.6. Complement inhibitors
POT-4 (Potentia Pharmaceuticals), a synthetic 13 amino acid cyclic peptide, is a compstatin derivative that effectively inhibits the complement cascade by preventing cleavage of C3 (a central component of all three known complement activation pathways) to its active fragments C3a and C3b. Inhibition of the complement cascade at this level, including the C3 convertases, is of particular interest. This is because both amplification of all initiation pathways and generation of anaphylatoxins (C3a, C5a) and the membrane attack complex (MAC) are affected. This results in prevention of local inflammation, tissue damage and upregulation of angiogenic factors, such as vascular endothelial growth factor (VEGF) in the eye. In experimental models, compstatin has demonstrated effective complement inhibition with negligible toxicity (63). Suppression and reversal of drusen formation in monkeys with early-onset macular degeneration after 6 months of intravitreal injection of compstatin was reported (64). POT-4 recently completed a Phase 1 (ASaP) clinical trial (NCT00473928, safety of intravitreal POT-4 therapy for patients with neovascular AMD, single intravitreal injection). It was found to be safe and demonstrated definite biologic activity at the higher doses.
Another complement component inhibitor of possible therapeutic interest in dry AMD is the pegylated, aptamer-based anti-C5 agent ARC1905 (Archemix Copm.). ARC1905 inhibits cleavage of C5 into C5a and C5b and prevents formation of the key terminal fragments responsible for tissue pathology (65). These include the pro-inflammatory C5a and the membrane attack complex (MAC, C5b-9) which initiates cell lysis and releases proangiogenic molecules (e.g. PDGF and VEGF). MAC has been documented on the RPE, choroidal blood vessels, and drusen of AMD eyes. By inhibiting C5-mediated inflammatory and MAC activities, therapeutic benefit may be achieved in both dry and wet AMD. A randomized Phase 1 safety study (NCT00950638) of ARC1905 given as intravitreal injection is currently ongoing in subjects with dry AMD.
Eculizumab (Soliris, Alexion) is another anti-C5 drug. It represents a humanized monoclonal antibody derived from a murine anti-human C5 antibody. Eculizumab is the first FDA-approved complement inhibitor for the treatment of paroxysmal nocturnal hemoglobinuria. A Phase 2 randomized double blind clinical study “Complement Inhibition With Eculizumab for the Treatment of Non-Exudative Macular Degeneration” is underway (NCT00935883). The protocol aims at evaluating the change in drusen volume and area of GA. In the induction period, 600 mg or 900 mg eculizumab will be administered via intravenous infusion over approximately 30 min, once a week (7 ± 2 days), for 4 weeks. This will be followed by 900 mg or 1,200 mg eculizumab for the fifth dose 7 days later (7 ± 2 days). In the maintenance period patients will receive eculizumab 900 mg or 1,200 mg (intravenous infusion) over approximately 30 min every 2 weeks (14 ± 2 days) until week 24. Patients will further be observed for 6 months off treatment with follow-up visits scheduled for 9 months and 12 months.
TT30 (Taligen Therapeutics) is a new fusion protein coupling domains from complement receptor 2 (CR2) with the alternative pathway inhibitor factor H. It selectively binds to complement activated cells to locally regulate the complement system. Factor H defects or deficiencies can result in aberrant complement system activation and are associated with diseases such as atypical hemolytic uremic syndrome (aHUS) and AMD. It has been demonstrated that intravenously administered TT30 localized to the neovascularization lesion sites in mouse RPE-choroid, prevented the progression of choroidal neovascularization and ameliorated the diminished retinal function (66). TT30 is currently in a Phase 1 clinical trial “Safety and Pharmacokinetics of TT30 in Subjects With Paroxysmal Nocturnal Hemoglobinuria (PNH)” (NCT01335165). Taligen is also developing an intraocular injectable formulation of TA106 (a humanized anti-CFB monoclonal antibody fragment) that could be positioned for the AMD market. Another complement pathway-modulating compound currently being considered for possible use in AMD includes FCFD4514S, an anti-factor D (NCT01229215, Phase 2 ongoing).
Receptor antagonists comprise a new class of promising small molecules in the management of dry AMD. Compared with complement inhibitors that prevent the formation of the pro-inflammatory C5a fragment, these compounds competitively bind to the C5a receptor. Therefore, they have the potential to suppress the inflammatory response without affecting the protective complement-related immune responses. JSM-7717 and JPE-1375 (developing products of Jerini AG) are two peptidomimetic C5a receptor antagonists currently in preclinical assessment for AMD (67). Preclinical studies have suggested that JSM-6427, a potent and highly specific integrin α5β1 antagonist, may prevent conversion of dry AMD to wet AMD and a dose-dependent inhibition of choroidal neovascularization in monkey and rabbit experimental models has been reported (68). A Phase 1 study was designed to evaluate safety, tolerability and pharmacokinetic profile of single and repeated doses of JSM-6427 in weekly intravitreal injections for up to 4 weeks to treat AMD (NCT00536016, completed, clinical results are still not published). Several additional complement pathway-modulating drugs are currently under evaluation in AMD, including CR2-CFH hybrid proteins, antiproperdin antibodies (thought to destabilize the critical C3 convertase), C1-INH and neutrazimab (classical pathway inhibitors), sCR1 (a soluble form of endogenous complement receptor which promotes the degradation of active C3bBb) (65).
3.7. Immunosuppressive agents and steroids
Copaxone (glatiramer acetate), a mix of four naturally occurring amino acids, l-glutamic acid, l-alanine, l-tyrosine, and l-lysine, is a synthetic protein that blocks the T-cell associated autoimmune responses and is approved for the treatment of multiple sclerosis. In a prospective interventional clinical trial of patients with dry AMD, treatment with copaxone for 12 weeks reduced drusen; this effect was confirmed by high-resolution spectral domain OCT/SLO (69). Copaxione is currently in Phase 2/3 clinical trials for dry AMD (NCT00466076). Neurodegenerative and inflammatory conditions, such as Alzheimer's disease, Crohn's disease, and acute optic neuritis are also in the spectrum of potential therapeutic indications for this compound. Sirolimus (also known as rapamycin, Macusight/Santen) was originally developed as a macrolide antifungal agent. It inhibits T-lymphocyte activation and proliferation in response to both antigenic and cytokine stimulation, as reviewed (70). Due to its impressive anti-tumor, immunosuppressive and anti-angiogenic properties, sirolimus was approved for prophylaxis of organ rejection in renal transplants. The mechanisms of action of sirolimus imply inhibition of the mTOR-mediated signal-transduction pathways and associated modulation of cell growth, proliferation, motility, survival, protein synthesis, and transcription. It also modulates the activity of numerous survival proteins involved in angiogenesis and hyperpermeability, including VEGF. A Phase 1/2 randomized trial (NCT00766649) is currently ongoing to evaluate safety/efficacy of sirolimus to treat GA associated with AMD. Steroids are used in single or combination therapy for AMD and other ocular diseases, such as diabetic macular edema, uveitis, and retinal vein occlusion. Currently, intravitreal administration of 0.2 and 0.5 µg/day fluocinolone acetonid (Iluvien, Alimera Sciences) is an object of a Phase 2 randomized, double-masked, fellow-eye comparison study (NCT00695318). It will evaluate the safety of fluocinolone and its efficacy to slow progression of GA in subjects with bilateral GA.
A newly emerging mechanism that has recently been linked to RPE degeneration and GA involves the decreased activity of DICER1, a microRNA-processing enzyme (71). DICER1 activates the NLRP3 inflammasome which was originally proposed to be a sensor of external danger signals, e.g. microbial toxins, and one of the key components of innate immunity. It was later found to be activated in diseases such as gout, atherogenesis, Alzheimer's disease, and type 2 diabetes. Targeting this pathway in GA via overexpression of DICER1 e.g. a vector-based approach to localized delivery of the DICER1 gene and/or antisense oligonucleotides against Alu RNA to regions of GA may ameliorate the disease and be a future therapeutic approach in AMD (29).
3.8. Emerging approaches
Cellular replacement strategies are believed to have the potential for restorative therapy for retinal degenerative diseases and AMD, and a considerable number of experimental studies in this direction has been undertaken. At least two mechanisms are expected to enable the visual improvements potentially associated with this therapeutic approach: a trophic effect of the implant on host cones and/or diffusion of soluble factors produced by healthy transplanted RPE cells to prevent progression of the disease (7,72) and local synaptic connections between the implant and host retina (73). RPE transplantation in human eyes with terminal AMD was performed initially in 1991 (74). So far, limited clinical trials of retinal implantation have been set.
Cell-based therapies represent a regenerative therapeutic approach that consists of introducing new cells to treat a disease. Both embryonic stem cell and induced pluripotent stem (iPS) cells are under extensive evaluation in retinal degenerative disorders. Differentiation of human iPS cells into RPE (75) has already been demonstrated and confirmed that iPS cells can generate functional iPS-RPE. Transplantation of these cells was shown to facilitate the short-term maintenance of photoreceptors through phagocytosis of the photoreceptor outer segments and to support long-term visual function. A secondary protective host cellular response has been suggested. Transplantation of iPS cell-derived RPE into rat models of retinitis pigmentosa has been shown to maintain visual acuity (75). So far, functional results with RPE transplantation techniques do not approach the levels of outcome seen with anti-VEGF treatment; in addition, there is a risk for nonterminally differentiated cells in the stem cell-derived RPE transplant to become tumorigenic (76). The value of stem cell-derived RPE transplantation is currently under evaluation: a Phase1/2 ongoing clinical trial (NCT01344993) assesses the effect of transplantation of human embryonic stem cell (hESC)-derived RPE cells (Advanced Cell Technology) in retinal degeneration. Transplantation of fetal stem cells may have certain advantages (e.g. high immunologic tolerance and high capacity to produce trophic substances enabling the retinal connections) but it is also associated with some unfavorable conditions and ethical concerns. A Phase 1 clinical study (NCT01226628) of the safety and efficacy of the subretinal administration of human umbilical tissue-derived cells (hUTC; CNTO2476) in patients with GA has recently been initiated. The primary outcome measure will be the proportion of eyes with serious ocular adverse events occurring over the first 12 months of the trial. Secondary endpoints include evaluation of the clinical response (e.g. visual acuity) and the findings of OCT and fluorescein angiography (e.g. evolution of GA lesion size).
Optogenetics represents one of the newest strategies to restore vision and is currently under pre-clinical evaluation. Advantages of this therapeutic approach and the possible combination with other vision restoration methods were recently reviewed by Busskamp V et al. 2011 (77). Strategies that are practically feasible today are those using ubiquitous promoters to express optogenetic tools in retinal ganglion cells and those targeting the remaining degenerated cones using photoreceptor-specific promoters. The main advance of the optogenetic approach is that it may provide artificially stimulated retinal activity closer to the normal activity of retinal circuits.
The concept of retinal prostheses has been developed to restore useful vision in blind patients by activating the remaining inner retinal network (78). Many groups worldwide are working today on different types of retinal implant devices. At present, the US company Second Sight Medical Products has the longest and largest clinical follow-up with the epiretinal implant Argus™ designed to stimulate the underlying retinal ganglion cells. The first clinical trial with a 16-electrode device (Argus I) began in 2002 in 6 volunteers with advanced retinitis pigmentosa and reported encouraging results (79). A large-scale multicenter Phase 2/3 clinical trial is currently underway to evaluate a second generation implant consisting of 60 electrodes (Argus II) in 30 patients with profound visual loss due to retinitis pigmentosa. In the future, this device could possibly also be proposed as a therapeutic approach for patients with dry AMD.
4. Conclusion
The prevalence of AMD is expected to double in the next 20 years as the population ages. Given that there is no effective or approved treatment for dry AMD and no means to reduce the risk of a switch from slowly progressive dry AMD to an advanced and rapidly blinding wet AMD, developing new agents and treatment strategies should play a prominent role in the prevention and treatment of this blinding disease. Any advancement in this respect would bring enormous benefit to the patients that without treatment will gradually loose their vision, and with this, their independence to perform everyday tasks. Thus saving sight in an aging population is of major health, social and economic impact.
Although not exhaustive, the current review clearly shows that dry AMD has become a focus of the pharmaceutical industry for intervention and innovations, and that the pathway-based therapy holds a great therapeutic promise. Any of the complex mechanisms implicated in the etiopathological pathway of AMD could serve as a potential target for treatment. Regardless of the fact that at each step in AMD pathogenesis a new treatment could be developed (targeting oxidative stress, inflammation, impaired perfusion, cellular degeneration, accumulation of toxic metabolites, and so on), a complete inhibition of disease progression most likely will require a combination of different treatments and various approaches (80). Most of the treatment approaches and therapeutic agents described here are still under development and investigation, but the future holds a clear therapeutic promise for new therapies that may prevent or retard AMD progression and restore vision.
References
- 1. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004; 122:598-614 [DOI] [PubMed] [Google Scholar]
- 2. Augood CA, Vingerling JR, de Jong PT, Chakravarthy U, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Bentham G, Rahu M, Vioque J, Young IS, Fletcher AE. Prevalence of age-related maculopathy in older Europeans: The European Eye Study (EUREYE). Arch Ophthalmol. 2006; 124:529-535 [DOI] [PubMed] [Google Scholar]
- 3. Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564-572 [DOI] [PubMed] [Google Scholar]
- 4. Bird AC. The Bowman lecture. Towards an understanding of age-related macular disease. Eye (Lond). 2003; 17:457-466 [DOI] [PubMed] [Google Scholar]
- 5. Kawasaki R, Yasuda M, Song SJ, Chen SJ, Jonas JB, Wang JJ, Mitchell P, Wong TY. The prevalence of age-related macular degeneration in Asians: A systematic review and meta-analysis. Ophthalmology. 2010; 117:921-927 [DOI] [PubMed] [Google Scholar]
- 6. Cheung CM, Tai ES, Kawasaki R, Tay WT, Lee JL, Hamzah H, Wong TY. Prevalence of and risk factors for age-related macular degeneration in a multiethnic Asian cohort. Arch Ophthalmol. 2012; 130:480-486 [DOI] [PubMed] [Google Scholar]
- 7. Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007; 26:516-554 [DOI] [PubMed] [Google Scholar]
- 8. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 1997; 104:7-21 [DOI] [PubMed] [Google Scholar]
- 9. Sunness JS. Geographic atrophy. In: Age Related Macular Degeneration (Berger JW, Fine SL, Maguire MG, eds.). Mosby, St. Louis, USA, 1999; p. 155 [Google Scholar]
- 10. Group AREDSR. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001; 119(10):1439-1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindblad AS, Lloyd PC, Clemons TE, Gensler GR, Ferris FL, 3rd, Klein ML, Armstrong JR. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009; 127:1168-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prahs P, Walter A, Regler R, Theisen-Kunde D, Birngruber R, Brinkmann R, Framme C. Selective retina therapy (SRT) in patients with geographic atrophy due to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2010; 248:651-658 [DOI] [PubMed] [Google Scholar]
- 13. Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009; 28:1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004; 137:486-495 [DOI] [PubMed] [Google Scholar]
- 15. Bernstein PS. Nutritional Interventions against Age-Related Macular Degeneration. Acta Hortic. 2009; 841:103-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoh Kam J, Lenassi E, Jeffery G. Viewing ageing eyes: Diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages. PLoS One. 2010; 5:e13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005; 102:7227-7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308:385-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006; 314:992-993 [DOI] [PubMed] [Google Scholar]
- 20. Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: A review of progress to date. Surv Ophthalmol. 2006; 51:316-363 [DOI] [PubMed] [Google Scholar]
- 21. Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308:421-424 [DOI] [PubMed] [Google Scholar]
- 22. Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308:419-421 [DOI] [PubMed] [Google Scholar]
- 23. Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006; 38:458-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005; 77:389-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, Weber BH. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005; 14:3227-3236 [DOI] [PubMed] [Google Scholar]
- 26. Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010; 107:7401-7406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cameron DJ, Yang Z, Gibbs D, et al. HTRA1 variant confers similar risks to geographic atrophy and neovascular age-related macular degeneration. Cell Cycle. 2007; 6:1122-1125 [DOI] [PubMed] [Google Scholar]
- 28. Sepp T, Khan JC, Thurlby DA, Shahid H, Clayton DG, Moore AT, Bird AC, Yates JR. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest Ophthalmol Vis Sci. 2006; 47:536-540 [DOI] [PubMed] [Google Scholar]
- 29. Yang Z, Stratton C, Francis PJ, et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N Engl J Med. 2008; 359:1456-1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu D, Deng X, Xu J, Hinton DR. What determines the switch between atrophic and neovascular forms of age related macular degeneration? - the role of BMP4 induced senescence. Aging (Albany NY). 2009; 1:740-745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitchell P, Wang JJ, Smith W, Leeder SR. Smoking and the 5-year incidence of age-related maculopathy: The Blue Mountains Eye Study. Arch Ophthalmol. 2002; 120:1357-1363 [DOI] [PubMed] [Google Scholar]
- 32. Fujihara M, Nagai N, Sussan TE, Biswal S, Handa JT. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS One. 2008; 3:e3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schaumberg DA, Christen WG, Hankinson SE, Glynn RJ. Body mass index and the incidence of visually significant age-related maculopathy in men. Arch Ophthalmol. 2001; 119:1259-1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boekhoorn SS, Vingerling JR, Hofman A, de Jong PT. Alcohol consumption and risk of aging macula disorder in a general population: The Rotterdam Study. Arch Ophthalmol. 2008; 126:834-839 [DOI] [PubMed] [Google Scholar]
- 35. Klein R, Klein BE, Marino EK, Kuller LH, Furberg C, Burke GL, Hubbard LD. Early age-related maculopathy in the cardiovascular health study. Ophthalmology. 2003; 110:25-33 [DOI] [PubMed] [Google Scholar]
- 36. Hjelmeland LM. Dark matters in AMD genetics: Epigenetics and stochasticity. Invest Ophthalmol Vis Sci. 2011; 52:1622-1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hunter A SP, Cwanger A, Song Y, Zhang Z, Ying GS, Hunter AK, Dezoeten E, Dunaief JL. DNA Methylation Is Associated with Altered Gene Expression in AMD. Invest Ophthalmol Vis Sci. 2012; 53:2089-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hudson HL, Lane SS, Heier JS, Stulting RD, Singerman L, Lichter PR, Sternberg P, Chang DF. Implantable miniature telescope for the treatment of visual acuity loss resulting from end-stage age-related macular degeneration: 1-year results. Ophthalmology. 2006; 113:1987-2001 [DOI] [PubMed] [Google Scholar]
- 39. AREDS Home Page. Age-related eye disease study. https://web.emmes.com/study/areds/ (accessed May 2012).
- 40. Group AREDSR. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000; 107:2224-2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Group AREDSR. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119:1417-1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996; 334:1150-1155 [DOI] [PubMed] [Google Scholar]
- 43. Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, Lawrence A, Walter W. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. Jama. 1994; 272:1413-1420 [PubMed] [Google Scholar]
- 44. Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004; 75:216-230 [DOI] [PubMed] [Google Scholar]
- 45. Wong WT, Kam W, Cunningham D, Harrington M, Hammel K, Meyerle CB, Cukras C, Chew EY, Sadda SR, Ferris FL. Treatment of geographic atrophy by the topical administration of OT-551: Results of a phase II clinical trial. Invest Ophthalmol Vis Sci. 2010; 51:6131-6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012; 31:136-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006; 103:3896-3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Emerich DF, Thanos CG. NT-501: An ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr Opin Mol Ther. 2008; 10:506-515 [PubMed] [Google Scholar]
- 49. Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, Brown DM, Jaffe GJ, Tao W, Williams GA. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011; 108:6241-6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lonngren U, Napankangas U, Lafuente M, Mayor S, Lindqvist N, Vidal-Sanz M, Hallbook F. The growth factor response in ischemic rat retina and superior colliculus after brimonidine pre-treatment. Brain Res Bull. 2006; 71:208-218 [DOI] [PubMed] [Google Scholar]
- 51. Collier RJ, Patel Y, Martin EA, Dembinska O, Hellberg M, Krueger DS, Kapin MA, Romano C. Agonists at the serotonin receptor (5-HT(1A)) protect the retina from severe photo-oxidative stress. Invest Ophthalmol Vis Sci. 2011; 52:2118-2126 [DOI] [PubMed] [Google Scholar]
- 52. Rhoades KL PY, Collier RJ, Romano C. AL-8309, A Serotonin 5-HT1A Agonist, Protects RPE Cells From Oxidative Damage in ARVO E-Abstract 677. 2009, IVOS [Google Scholar]
- 53. Wang Y ME, Hoang H, Rector R, Morgan S, Romano C CR. Inhibition of Complement Deposition by AL-8309A, a 5-HT1a Agonist, in the Rat Photic-Induced Retinopathy Model. IVOS. 2009; 50:ARVO E-Abstract 685. [Google Scholar]
- 54. Leveillard T, Mohand-Said S, Lorentz O, Hicks D, Fintz AC, Clerin E, Simonutti M, Forster V, Cavusoglu N, Chalmel F, Dolle P, Poch O, Lambrou G, Sahel JA. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004; 36:755-759 [DOI] [PubMed] [Google Scholar]
- 55. Leveillard T, Sahel JA. Rod-derived cone viability factor for treating blinding diseases: From clinic to redox signaling. Sci Transl Med. 2010; 2:26ps16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Golczak M, Kuksa V, Maeda T, Moise AR, Palczewski K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci U S A. 2005; 102:8162-8167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maeda A, Maeda T, Golczak M, Imanishi Y, Leahy P, Kubota R, Palczewski K. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol Pharmacol. 2006; 70:1220-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008; 283:26684-26693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kubota R, Boman NL, David R, Mallikaarjun S, Patil S, Birch D. Safety and effect on rod function of ACU-4429, a novel small-molecule visual cycle modulator. Retina. 2012; 32:183-188 [DOI] [PubMed] [Google Scholar]
- 60. Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002; 99:14682-14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20:705-732 [DOI] [PubMed] [Google Scholar]
- 62. Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134:411-431 [DOI] [PubMed] [Google Scholar]
- 63. Holland MC, Morikis D, Lambris JD. Synthetic small-molecule complement inhibitors. Curr Opin Investig Drugs. 2004; 5:1164-1173 [PubMed] [Google Scholar]
- 64. Chi ZL, Yoshida T, Lambris JD, Iwata T. Suppression of drusen formation by compstatin, a peptide inhibitor of complement C3 activation, on cynomolgus monkey with early-onset macular degeneration. Adv Exp Med Biol. 2010; 703:127-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010; 128:349-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rohrer B, Coughlin B, Bandyopadhyay M, Holers VM. Systemic human CR2-targeted complement alternative pathway inhibitor ameliorates mouse laser-induced choroidal neovascularization. J Ocul Pharmacol Ther. 2012; 28:402-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Allegretti M, Moriconi A, Beccari AR, Di Bitondo R, Bizzarri C, Bertini R, Colotta F. Targeting C5a: Recent advances in drug discovery. Curr Med Chem. 2005; 12:217-236 [DOI] [PubMed] [Google Scholar]
- 68. Zahn G, Vossmeyer D, Stragies R, Wills M, Wong CG, Loffler KU, Adamis AP, Knolle J. Preclinical evaluation of the novel small-molecule integrin alpha5beta1 inhibitor JSM6427 in monkey and rabbit models of choroidal neovascularization. Arch Ophthalmol. 2009; 127:1329-1335 [DOI] [PubMed] [Google Scholar]
- 69. Landa G, Rosen RB, Patel A, Lima VC, Tai KW, Perez VR, Aizman A, Garcia PM. Qualitative spectral OCT/SLO analysis of drusen change in dry age-related macular degeneration patients treated with Copaxone. J Ocul Pharmacol Ther. 2011; 27:77-82 [DOI] [PubMed] [Google Scholar]
- 70. Sehgal SN. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant Proc. 2003; 35:7S-14S [DOI] [PubMed] [Google Scholar]
- 71. Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011; 471:325-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mohand-Said S, Hicks D, Leveillard T, Picaud S, Porto F, Sahel JA. Rod-cone interactions: Developmental and clinical significance. Prog Retin Eye Res. 2001; 20:451-467 [DOI] [PubMed] [Google Scholar]
- 73. Seiler MJ, Sagdullaev BT, Woch G, Thomas BB, Aramant RB. Transsynaptic virus tracing from host brain to subretinal transplants. Eur J Neurosci. 2005; 21:161-172 [DOI] [PubMed] [Google Scholar]
- 74. Peyman GA, Blinder KJ, Paris CL, Alturki W, Nelson NC, Jr, Desai U. A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg. 1991; 22:102-108 [PubMed] [Google Scholar]
- 75. Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, da Cruz L, Johnson LV, Clegg DO, Coffey PJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009; 4:e8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Falkner-Radler CI, Krebs I, Glittenberg C, Povazay B, Drexler W, Graf A, Binder S. Human retinal pigment epithelium (RPE) transplantation: Outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br J Ophthalmol. 2011; 95:370-375 [DOI] [PubMed] [Google Scholar]
- 77. Busskamp V, Picaud S, Sahel JA, Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012; 19:169-175 [DOI] [PubMed] [Google Scholar]
- 78. Shannon RV. A model of safe levels for electrical stimulation. IEEE Trans Biomed Eng. 1992; 39:424-426 [DOI] [PubMed] [Google Scholar]
- 79. Humayun MS, Weiland JD, Fujii GY, Greenberg R, Williamson R, Little J, Mech B, Cimmarusti V, Van Boemel G, Dagnelie G, de Juan E. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 2003; 43:2573-2581 [DOI] [PubMed] [Google Scholar]
- 80. Zarbin MA, Rosenfeld PJ. Pathway-based therapies for age-related macular degeneration: An integrated survey of emerging treatment alternatives. Retina. 2010; 30:1350-1367 [DOI] [PubMed] [Google Scholar]


