Abstract
Individuals in the developing world live in conditions of intense exposure to enteric pathogens due to suboptimal water and sanitation. These environmental conditions lead to alterations in intestinal structure, function, and local and systemic immune activation that are collectively referred to as environmental enteropathy (EE). This condition, although poorly defined, is likely to be exacerbated by undernutrition as well as being responsible for permanent growth deficits acquired in early childhood, vaccine failure, and loss of human potential. This article addresses the underlying theoretical and analytical frameworks informing the methodology proposed by the Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) cohort study to define and quantify the burden of disease caused by EE within a multisite cohort. Additionally, we will discuss efforts to improve, standardize, and harmonize laboratory practices within the MAL-ED Network. These efforts will address current limitations in the understanding of EE and its burden on children in the developing world.
Keywords: environmental enteropathy, infant growth failure, intestinal infections, lactulose mannitol test, tropical enteropathy
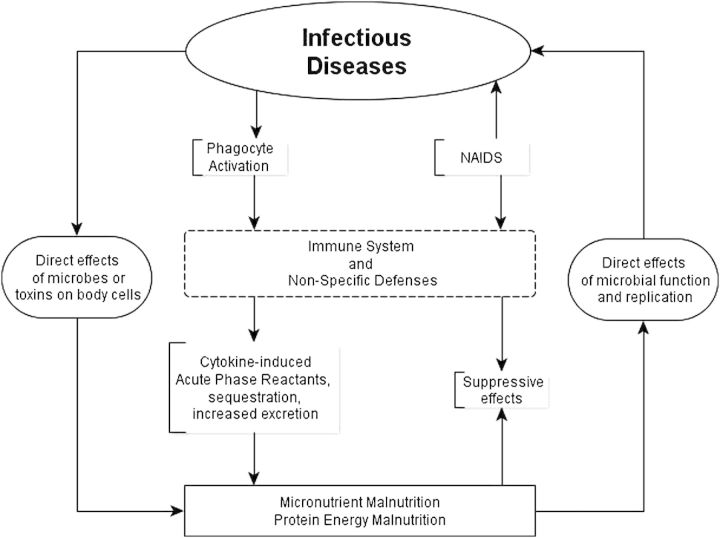
In the last 25 years, many nutritional and disease control interventions have targeted linear growth deficits acquired in early life—a surrogate for both chronic undernutrition and human potential lost among children living in poverty—with disappointing results [1, 2]. The smaller-than-expected effect sizes associated with these interventions have been attributed to the complex relationships between infection and undernutrition [3]. However, the mechanisms and specific mediators of this interaction remain poorly defined and largely theoretical, with limited data to support the relative contribution of major contributing factors (Figure 1). Increasingly, the entity of environmental enteropathy (EE) is identified as a purported key mediator in this relationship [5, 6].
Figure 1.
General overview of interactions between infection and undernutrition. Although it is recognized that this cycle exists for systemic and other nonenteric infections, enteric infections and undernutrition are clearly linked in a way that is clinically evident. Adapted from Mandell et al [4]. Abbreviation: NAIDS, Nutritionally Acquired Immune Deficiency Syndrome.
Environmental or tropical enteropathy has recently been renamed by at least 1 expert committee as environmental enteric dysfunction [7]. Although the condition has periodically been the focus of several expert opinion panels over the last 20 years [8–10] case definitions, indicators of disease severity, and consensus about principal outcomes associated with disease activity remain sparse. At the present time, there is no well-accepted published case definition or known pathognomonic lesion to unequivocally classify individuals as cases or controls, or to attempt to describe the level of disease activity for EE.
Enteropathic lesions among individuals living in the tropics have been described since the 1950s and 1960s. Histopathologic findings of increased crypt depth decrease in villus height and lymphocytic infiltration were found, as was malabsorption [11]. Clinical and anatomic findings and dysfunction improved upon extended stays in the United States or Europe in cases of affected expatriates or migrants from the developing world [12, 13]. Environmental enteropathy is therefore described as the physiologic, anatomic, and functional changes in the gut that are a result of a prolonged and persistent exposure to multiple enteropathogens that both has an adverse effect on host well-being and is fully reversible upon removal from the inducing environmental exposures. The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) study, a birth cohort simultaneously enrolling children in 8 countries (Bangladesh, Brazil, India, Nepal, Pakistan, Peru, South Africa, and Tanzania), has collected data on environmental exposures, gut inflammation and permeability assessments, symptomatic and asymptomatic enteropathogen exposures, nutritional intakes and periodic measures of micronutrient status, and vaccine response and growth on 1600 children under a common protocol. The data accumulated allow for the comparative study of the causes and consequences of EE in a broad set of epidemiologic contexts.
METHODS
Theoretical Framework
As detection of enteropathogens becomes increasingly sensitive and comprehensive, previous categorical definitions relating disease to the presence of a single identified enteropathogen become increasingly problematic. Multiple enteropathogens are frequently identified in asymptomatic as well as symptomatic stools. The approach taken in the MAL-ED cohort study is that EE is nearly universally present in children living in extreme poverty, at least in the setting where growth restriction is documented. Therefore, there is a need to move from a model of disease activity based on specific pathogens and diarrheal illness episodes to an EE-based model that acknowledges an underlying continuous exposure to multiple pathogens.
Disease activity is likely both to change within an individual over time [14] and to have important interindividual differences that are modulated by genetic and epigenetic factors. Based on the existing literature and prior work by members of the MAL-ED network, the following processes were identified as integral to EE assessment: malabsorption, intestinal permeability, local immune activation as assayed by T-helper cell 1 (Th1) activity and neutrophil activity in the intestinal mucosa, the loss or “wasting” of macro- and micronutrients, and systemic immune activation as measured in the plasma. Ideal biomarkers of disease activity would measure these key parameters, and would have minimal age-related effects in the first 2 years of life. Additionally, they would not be affected by diet or breastfeeding to allow for longitudinal disease activity measures that would be meaningful at the level of the individual and in different epidemiologic settings. The assays used should be run on specimens obtained noninvasively or by minimally invasive procedures to allow for frequent sampling, require minimal primary processing to allow for sampling in remote locations, be highly reproducible, and be processed in laboratories in areas where EE is thought to be present.
Analytic Framework
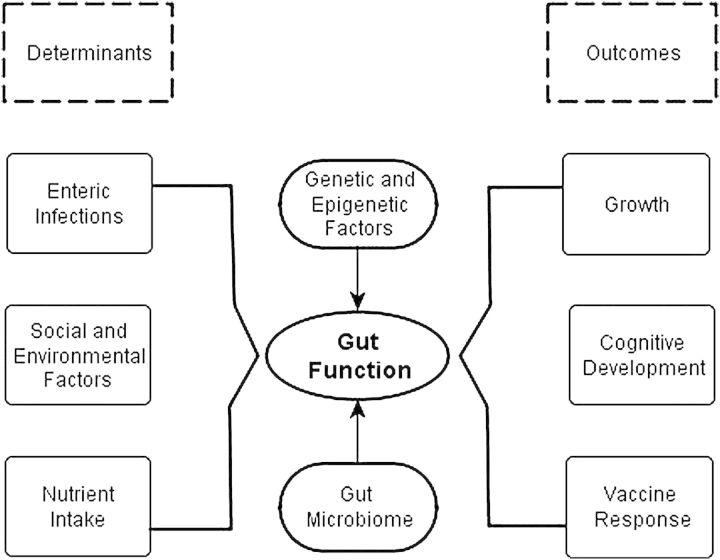
Given that adaptation to environmental context is likely composed of both adaptive responses (ie, acquired immunity) and decompensated injury responses, the selection of outcomes that are reliable and convincing assessments of physiological insults and injury are critical. Key outcomes to measure the impact of EE defined by the MAL-ED study's Gut Function Technical Subcommittee include growth, subsequent risk of diarrhea, vaccine responsiveness, and cognitive development (Figure 2). As it is recognized that the relationship between infection and malnutrition is bidirectional and that children fall into cycles of infection and undernutrition (Figure 3), temporality of determinants and outcomes are essential in drawing meaningful conclusions as to the relative importance of coexisting factors that attenuate optimal growth and development. Therefore, the longitudinal design with age-structured sampling is well suited to this analytic framework, and the timing and nature of assessments done in the MAL-ED study are shown in Table 1. Sampling is more intense in the first 12 to 15 months of life, as this is the period during which growth faltering occurs with minimal capacity for later recovery in most contexts and in which the burden of diarrhea is the greatest.
Figure 2.
It is hypothesized that alterations in gut structure and physiology have diverse but measureable inputs including symptomatic and asymptomatic enteric infections, micronutrient, and macronutrient availability, as well as genetic and epigenetic factors. Alterations in gut function described as environmental enteropathy, in turn, are able to alter mucosal immune responses and macronutrient/micronutrient absorption and availability to have long-term influences on human potential.
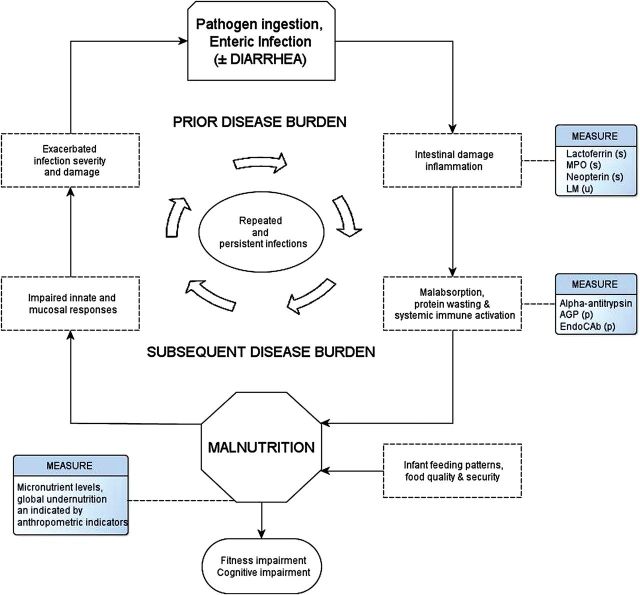
Figure 3.
The relationship between enteric infection and undernutrition has long been noted. However, the relationship is complex and bidirectional, and assessments are rarely made with the temporal definition necessary to determine directionality. Furthermore, they have historically lacked the design completeness that is necessary to determine the relative import of nutritional intake and feeding patterns and intestinal infections in the same individuals or done so in samples large enough to address the recognized complexity of interaction between key variables and outcomes. Measures made on clinical specimens of surveillance monthly stool (s), urine (u), or plasma (p). Adapted from Mata [15] and Guerrant et al [16]. Abbreviations: AGP, α-2-acid glycoprotein; LM, lactulose-mannitol test; MPO, myeloperoxidase.
Table 1.
Temporal Sampling of 1600 Children for Assessment of Levels of Activity for Environmental Enteropathy
| Measure | Assay | Age, mo |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | ||
| Intestinal permeability | LM test | x | x | x | x | ||||||||||||||||||||
| Absorptive capacity | LM test | x | x | x | x | ||||||||||||||||||||
| Mucosal Th1 activity | Fecal neopterin | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||
| Mucosal neutrophil activity | Fecal myeloperoxidase | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||
| Exudation of serum protein from intestinal mucosa | Fecal α-1 anti-trypsin | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||
| Systemic immune activity | α-2-acid glycoprotein | x | x | x | |||||||||||||||||||||
Fecal testing is limited to asymptomatic monthly stools.
Abbreviations: LM, lactulose-mannitol test; Th1, T-helper cell type 1.
Growth is assessed using monthly anthropometric data to derive temporally lagged ponderal (weight) and linear growth velocity. Linear growth deficits are the preferred assessment of physiological insults as they are stable, durable, and better associated with deficits in adult work capacity and cognitive potential. Ponderal velocities over shorter periods will also be examined for biologic consistency. For this reason, interval velocities and growth acceleration rather than attained growth have been selected as the preferred outcomes, although the Gut Function Technical Subcommittee recognized that poor water and sanitation and other environmental exposures experienced indirectly in utero may also have measureable and durable effects on postnatal as well as in utero growth.
Understanding the relationship between markers of EE and suboptimal vaccine response is a primary focus of the MAL-ED study. Programmatic priorities of global immunization strategies have made deficits in protection afforded by oral poliovirus and rotavirus vaccines the most cited examples of this phenomenon. It is also true that the immunogenicity of many other vaccines, particularly products with promising results in phase 1 studies when conducted in experimental populations in the United States and Europe, have failed to prove immunogenic or protective in relevant tropical populations [17–19]. Defining markers of this decreased responsiveness is likely to facilitate the development and evaluation of adjuvant strategies (eg, interval treatment with antibiotics, antihelmintics, probiotics, micronutrients) to optimize immunogenicity in these settings.
Rationale for Tests Selected for the MAL-ED Study
Permeability and Malabsorption
Monosaccharide and disaccharide administration and assessment in urine and/or serum are the most frequently used assays to measure intestinal permeability and malabsorption; therefore, the lactulose:mannitol ratio test was used in MAL-ED as per previously published reports [20–22]. This test serves as a reference standard for disease activity as well as to assess intestinal permeability and malabsorption. However, from a careful assessment in studies in which contributors were analyzed individually, it is clear that mannitol, rather than lactulose, is the primary determinant of the ratio [23, 24]. In the MAL-ED study, the selection of an assay with measurement of the analyte in a urine (as opposed to blood) sample was also an important consideration, as the collection was repeated multiple times (4) per child and the requirement of venipuncture was likely to decrease acceptance among study participants.
Intestinal Inflammation
The measure of the level of immune activity at the level of the intestine and systemic inflammation were evaluated. Stool markers chosen as indicators of gut inflammation were myeloperoxidase, an indicator of increased neutrophil activity, and neopterin, a marker of Th1 immune activation that is produced by macrophages and dendritic cells upon stimulation by activated T lymphocytes. Environmental enteropathy has been characterized in small studies as having a prominent cell-mediated Th1 response [25], and neopterin has been shown to be associated with growth failure children in The Gambia [24] and levels of clinical disease activity in patients with inflammatory bowel disease [26]. With respect to practical features, both myeloperoxidase and neopterin are stable in stool specimens, and have commercially available assay kits that are relatively inexpensive and could be performed at regional laboratories.
Other biomarkers considered, but not used, were calprotectin (because of strong age-related effects and well-documented reports of increased levels in the stool of breastfed children [27]) and lactoferrin. Lactoferrin was assessed at several MAL-ED field sites, but found to be highly elevated in breastfed children. Furthermore, the need to employ multiple dilutions to quantify levels made the lactoferrin assay more cumbersome and expensive than myeloperoxidase to measure the same parameter (ie, neutrophil activation in the intestine). Multianalyte cytokine panels were also considered, but the need for primary specimen processing with protease inhibitors and cost discouraged their use despite promising findings for several analytes, most notably interleukin 8, when evaluated in India, Pakistan, and Peru.
Nutrient Wasting
Related, but possibly distinct from the measure of intestinal inflammation, is the “wasting” of macro- and micronutrients, which has been documented in chronic inflammatory conditions of the gastrointestinal tract and in acute infections, most notably shigellosis [28], an enteric infection with an unusually strong effect on linear growth [29]. The marker chosen to represent this pathophysiologic component was the classical marker for protein-losing enteropathy, α-1-antitrypsin (AAT). Unlike other serum proteins, AAT is not degraded by proteases in the intestinal lumen and can be readily detected in stool.
Systemic Inflammation
The measure of systemic infection most reported in the literature is the test of immunoglobulin G (IgG) directed at lipopolysaccharides derived from several gram-negative bacteria (Escherichia coli, Klebsiella aerogenes, Pseudomonas aeruginosa, and Salmonella typhimurium) [30]. The endotoxin core antibody (EndoCAb) assay is thought to represent the amount of chronic intestinal translocation and immune stimulation. This test has been reported in the literature, first through single laboratories [30] and then transferred to a different company as the Coaset EndoCAb (Chromogenix, Quadritech Diagnostics, Epsom, UK). The test that is now commercially available is available from Hycult Biotech (Uden, the Netherlands). The assay in its current form is conceptually similar to the test initially reported but has undergone technical changes in production, and results from the present commercial assay are not directly comparable to those reported in the early literature [31]. This current form of EndoCAb and another test for serum α-2-acid glycoprotein (AGP) were also chosen for evaluation.
Methods of Assay Performance
Lactulose-Mannitol Test
Children are fasted for 2 hours prior to and 30 minutes following the administration of the disaccharide solution with the exception of breast milk, which is permitted ad libitum. Children are encouraged to void prior to administration of the solution, and a urine collection bag is placed and changed as needed for the following 5-hour collection period. Lactulose is administered at 250 mg/mL and mannitol at 50 mg/mL at a dose of 2 mL/kg to a maximum administered dose of 20 mL at a concentration of 1002 mOsm/L. The MAL-ED field-workers collect and measure urine following voiding, 1–2 drops of chlorhexidine (2.35%) are added, and samples are stored on ice to limit bacterial growth. Urine aliquots are stored at −70°C prior to testing. Concentrations of lactulose and mannitol are measured by high-performance liquid chromatography (HPLC) and either pulsed amperometric detection (PAD) [28] or ion chromatography using a common standard protocol across the 4 laboratories (Brazil, Bangladesh, Pakistan, and India) running the assay and expressed as a molar ratio of lactulose to mannitol present in urine.
Fecal Markers
Stool samples are collected without fixative by field-workers and frozen at −70°C prior to testing. Samples are evaluated in parallel for myeloperoxidase (Alpco, Salem, New Hampshire), neopterin (GenWay Biotech, San Diego, California), and AAT (Biovendor, Chandler, North Carolina). Specimens are processed at dilutions of 1:500 initially for myeloperoxidase, neopterin, and AAT, and samples out of range of the standard curves of any analyte are run at a 2-fold higher or lower dilution as appropriate.
Plasma Markers
Plasma levels of AGP are determined by radial immunodiffusion [32]. EndoCAb testing was evaluated in plasma samples as per kit instructions to assess the amount of IgG to endotoxin.
CHALLENGES ENCOUNTERED
The application of standard tests for EE across multiple sites has revealed suboptimal performance characteristics not reported in small initial studies done at a single site. This was most evident for the EndoCAb test, which is one of the principal tests recently used, in other studies [31, 33], but which failed to meet MAL-ED quality standards for reproducibility. The large scale of the MAL-ED study makes limitations in assay performance, particularly reproducibility, more evident. The use of many sites and the large number of specimens run using clearly defined standard operating procedures at laboratories that are widely known centers of excellence in enteric disease research with a centralized structure for communication and real-time quality assessment allows for the discovery of inconsistent assay performance that would not be recognized during the conduct of small studies done in individual laboratories.
The current EndoCAb assay test failed to display acceptable lot-to-lot reproducibility. This reproducibility was not evident during the initial piloting when done on a smaller number of samples at a single site (on a scale similar to Campbell et al [31] and Mondal et al [33]); however, upon scale-up within the MAL-ED study it became clearly evident. Although a newer-generation commercial assay is under development, the MAL-ED study does not have another to test at this time.
Lactulose-mannitol testing is often referred to as the gold standard noninvasive test to assess EE. However, even with strict protocol adherence and despite within-site quality control standards being met, obtained concentrations in the 4 different study laboratories were not directly comparable, as small differences in assay platforms affected test results. Rank-order correlations for measures were high, demonstrating the ability of laboratories to reach common classification of concentrations as low or high in relative terms, although the exact values of the concentrations differed. To account for this, all 4 platforms were standardized to results obtained by liquid chromatography–tandem mass spectrometry to obtain comparable results across the study sites. This is a highly cumbersome requirement for the assay of a condition that is likely to be of both great importance and nearly global in its use. It was evident from the literature that lactulose-mannitol test values are quite divergent; however, because the age of subjects varied between studies (as did dosing and osmolarity of the administered solutions [20, 24, 34, 35]), because the manner of reporting results differed (eg, geometric vs arithmetic means, percentage of lactulose and percentage of mannitol recovery), and because there were major differences in methods between the older enzymatic assays [36, 37] and the current HPLC-PAD [34] and HPLC mass spectrometry [35, 38] platforms, the reason for these differences were not decipherable. It now appears that there are significant practical challenges in adapting this lactulose-mannitol assay test to different assay platforms even if probe dosing and collection strategies were uniform, thereby greatly limiting the ability to compare lactulose-mannitol test results between studies.
DISCUSSION
The diverse epidemiologic context and sample size of the MAL-ED cohort is ideally poised to address limitations in the current understanding of EE. The study includes data from children with a variable intensity of exposure to enteric pathogens that are being identified systematically with a single protocol, heterogeneous incidence rates of clinically evident diarrhea, and differing timing in onset of growth deficits. Whereas most studies that are frequently referenced include children from a single site and sample sizes of 100–200 individuals, the MAL-ED study will include data from >1600 children who are representative of children living in poverty in Southeast Asia, Africa, and South America. Data will be further enriched by the collection of complementary data on feeding patterns, nutrient intakes, and micronutrient status, a component that is universally absent in the literature regarding manifestations and consequences of EE, but which clearly influences the primary outcome of this and other studies of infant growth.
The classification of a subject at any point in time as having EE or not is simplistic. Most subjects living in poverty will have deviations in measured analytes or in anatomic or pathological findings on biopsy that will differ from nontropical reference populations. For the same reason, heterogeneity among findings within small populations in the absence of clear associations with well defined, time-lagged outcomes marking physiological insults is unlikely to clarify our understanding of the burden of EE or to guide interventions to diminish its impact. In a pilot study using data from all 8 MAL-ED field sites, we have already demonstrated that a multifactorial disease activity score of markers of intestinal inflammation and intestinal permeability is associated with the subsequent development of growth deficits over the subsequent 6 months in children after controlling for intrauterine growth retardation by either the birth weight or length-for-age z score at 1 month of age [39]. This EE activity index, composed of only stool biomarkers that can be done with straightforward, commercially available assays for approximately US$15, is associated with a difference in 6-month growth velocity of as much as 1 cm between children with the highest and lowest index scores. This difference was observed in infants in a susceptible age range (3–12 months) where childhood growth faltering is most concentrated in vulnerable populations worldwide [40]. A similar approach has also been used to evaluate a new fecal marker of intestinal repair in children from Bangladesh and Peru, some of whom were MAL-ED participants [41].
There is a growing movement to use small intervention studies at multiple sites as a way to accelerate discovery. In the MAL-ED study, we uncovered many limitations in the performance of diagnostic tests that had not previously been evident because of the small number of specimens initially processed, differences in probe dosages administered, and analytic platforms that were incompletely detailed and inadequately compared across study sites. The limitations reduce the ability to draw valid comparisons across epidemiologic contexts and even between individual studies, and speaks to the importance of larger multisite studies employing common protocols to improve our understanding of EE.
Enteric infection, diarrhea, and undernutrition are frequently cited as acting in a vicious cycle that affects the most vulnerable children living in conditions of poverty. An intensive diagnostic panel will allow data on individual pathogens to be related to markers of intestinal injury as well as growth [42]. We expect such multiplex diagnostics to be useful in prioritizing the use of disease-specific interventions such as vaccines, rapid diagnostics, and the treatment of priority pathogens. The association of EE activity indexes with vaccine responsiveness is important, both for understanding the suboptimal performance of some vaccines in certain epidemiologic settings, but also as a surrogate marker of the ability of the individual at a given point in time to respond to intercurrent infections, which is an important bioassay in and of itself.
In a subset of children (n = 152) from 5 MAL-ED sites, complementary metabolomic and microbiome analysis are under way to determine the contributions of host flora to the clinical manifestations and long-term outcomes related to exposure to heavy burdens on enteric infection in early life. Metagenomic work in a twin study with time-series analysis has recently shown that the microbiome is associated with the manifestation of kwashiorkor [43]. It will be important to determine if this is also the case in the more common adverse outcomes related to undernutrition, linear growth failure, vaccine response, and small but definable restrictions to neurocognitive potential and physical fitness. If these outcomes can be predicted by assays of gut inflammation and permeability that can be employed routinely in resource-limited settings, then these findings will be of increased value as proximate measures of adverse long-term outcomes that are too expensive to routinely measure in small, short-term intervention projects.
A practical strategy for biomarker development and validation is needed. There is growing evidence to show that EE is an important syndrome, despite numerous challenges and limitations of current assays and limited high-quality existing data. There are a multitude of diverse interventions to be used and evaluated in the context of EE, including water and sanitation interventions, improved feeding practices, cash transfer programs, and new and existing vaccines that would benefit from reliable and affordable standardized tests that are simple to perform and can be done at the local or regional level for comparison across different epidemiologic contexts.
CONCLUSIONS
Ultimately, the impact of early childhood infections on individuals and societies will depend on the durable neurocognitive deficits, rather than attained height or even physical fitness [44]. The MAL-ED cohort study will produce individual EE disease activity scores in early life and cumulative disease activity scores over the first 2 years of life, which will be associated with the best available measures of neurocognitive potential adapted for local contexts.
The detailed longitudinal information on social and environmental factors related to hygiene and caregiving, obtained at multiple points for each enrolled child and across epidemiologic contexts, will be valuable in determining which environmental factors drive key facets of EE. Understanding the influence of these factors should greatly enrich available information with which to guide future interventions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the staff and participants of the MAL-ED Network for their important contributions. The authors also thank Tom Brewer and Phil Tarr for early critical comments in the framework of evaluating and interpreting limitations to current assays of EE.
Financial support. The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health, and the National Institutes of Health, Fogarty International Center.
Supplement sponsorship. This article is published as part of a supplement entitled “The Malnutrition and Enteric Disease Study (MAL-ED): Understanding the Consequences for Child Health and Development,” sponsored by the National Institutes of Health and the Foundation for the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gershoff SN, McGandy RB, Suttapreyasri D, et al. Nutrition studied in Thailand. II. Effects of fortification of rice with lysine, threonine, thiamin, riboflavin, vitamin A, and iron on preschool children. Am J Clin Nutr. 1977;30:1185–95. doi: 10.1093/ajcn/30.7.1185. [DOI] [PubMed] [Google Scholar]
- 2.Gordon JE, Scrimshaw NS. Field trial of a newly developed food for prevention of malnutrition. World Rev Nutr Diet. 1972;15:256–88. doi: 10.1159/000393581. [DOI] [PubMed] [Google Scholar]
- 3.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 4.Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th ed. Philadelphia, PA: Elsevier; 2010. [Google Scholar]
- 5.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–9. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–5. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 7.Keusch GT, Denno DM, Black RE, et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Food Nutr Bull. 2013;34:357–64. doi: 10.1177/156482651303400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salazar-Lindo E, Allen S, Brewster DR, et al. Intestinal infections and environmental enteropathy: working group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39(suppl 2):S662–9. doi: 10.1097/00005176-200406002-00013. [DOI] [PubMed] [Google Scholar]
- 9.Korpe PS, Petri WA., Jr Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328–36. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg. 2012;86:756–63. doi: 10.4269/ajtmh.2012.11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenbaum J, Harmon JW, Gerson CD. Subclinical malabsorption in developing countries. Am J Clin Nutr. 1972;25:1056–61. doi: 10.1093/ajcn/25.10.1056. [DOI] [PubMed] [Google Scholar]
- 12.Gerson CD, Kent TH, Saha JR, Siddiqi N, Lindenbaum J. Recovery of small-intestinal structure and function after residence in the tropics. II. Studies in Indians and Pakistanis living in New York City. Ann Intern Med. 1971;75:41–8. doi: 10.7326/0003-4819-75-1-41. [DOI] [PubMed] [Google Scholar]
- 13.Lindenbaum J, Gerson CD, Kent TH. Recovery of small-intestinal structure and function after residence in the tropics. I. Studies in Peace Corps volunteers. Ann Intern Med. 1971;74:218–22. doi: 10.7326/0003-4819-74-2-218. [DOI] [PubMed] [Google Scholar]
- 14.Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412–9. [PubMed] [Google Scholar]
- 15.Mata L. Diarrheal disease as a cause of malnutrition. Am J Trop Med Hyg. 1992;47:16–27. doi: 10.4269/ajtmh.1992.47.16. [DOI] [PubMed] [Google Scholar]
- 16.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotuzzo E, Butron B, Seas C, et al. Safety, immunogenicity, and excretion pattern of single-dose live oral cholera vaccine CVD 103-HgR in Peruvian adults of high and low socioeconomic levels. Infect Immun. 1993;61:3994–7. doi: 10.1128/iai.61.9.3994-3997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallander HO, Paniagua M, Espinoza F, et al. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine. 2002;21:138–45. doi: 10.1016/s0264-410x(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 19.Rahman KM, Arifeen SE, Zaman K, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine. 2011;29:1347–54. doi: 10.1016/j.vaccine.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–10. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]
- 21.Lunn PG, Northrop-Clewes CA, Downes RM. Recent developments in the nutritional management of diarrhoea. 2. Chronic diarrhoea and malnutrition in The Gambia: studies on intestinal permeability. Trans R Soc Trop Med Hyg. 1991;85:8–11. doi: 10.1016/0035-9203(91)90137-n. [DOI] [PubMed] [Google Scholar]
- 22.Nathavitharana KA, Lloyd DR, Raafat F, Brown GA, McNeish AS. Urinary mannitol: lactulose excretion ratios and jejunal mucosal structure. Arch Dis Child. 1988;63:1054–9. doi: 10.1136/adc.63.9.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bijlsma PB, Peeters RA, Groot JA, Dekker PR, Taminiau JA, Van Der Meer R. Differential in vivo and in vitro intestinal permeability to lactulose and mannitol in animals and humans: a hypothesis. Gastroenterology. 1995;108:687–96. doi: 10.1016/0016-5085(95)90440-9. [DOI] [PubMed] [Google Scholar]
- 24.Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004;39:153–7. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Campbell DI, Murch SH, Elia M, et al. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54:306–11. doi: 10.1203/01.PDR.0000076666.16021.5E. [DOI] [PubMed] [Google Scholar]
- 26.Nancey S, Boschetti G, Moussata D, et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:1043–52. doi: 10.1097/MIB.0b013e3182807577. [DOI] [PubMed] [Google Scholar]
- 27.Dorosko SM, Mackenzie T, Connor RI. Fecal calprotectin concentrations are higher in exclusively breastfed infants compared to those who are mixed-fed. Breastfeed Med. 2008;3:117–9. doi: 10.1089/bfm.2007.0036. [DOI] [PubMed] [Google Scholar]
- 28.Black RE, Levine M. Intestinal protein loss in shigellosis. Nutr Res. 1991;11:1215–20. [Google Scholar]
- 29.Black RE, Brown KH, Becker S, Alim AR, Huq I. Longitudinal studies of infectious diseases and physical growth of children in rural Bangladesh. II. Incidence of diarrhea and association with known pathogens. Am J Epidemiol. 1982;115:315–24. doi: 10.1093/oxfordjournals.aje.a113308. [DOI] [PubMed] [Google Scholar]
- 30.Barclay GR. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263–72. [PubMed] [Google Scholar]
- 31.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:1332–8. doi: 10.1093/jn/133.5.1332. [DOI] [PubMed] [Google Scholar]
- 32.Rosales FJ, Topping JD, Smith JE, Shankar AH, Ross AC. Relation of serum retinol to acute phase proteins and malarial morbidity in Papua New Guinea children. Am J Clin Nutr. 2000;71:1582–8. doi: 10.1093/ajcn/71.6.1582. [DOI] [PubMed] [Google Scholar]
- 33.Mondal D, Minak J, Alam M, et al. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;54:185–92. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barboza Junior MS, Silva TM, Guerrant RL, Lima AA. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz J Med Biol Res. 1999;32:1499–504. doi: 10.1590/s0100-879x1999001200008. [DOI] [PubMed] [Google Scholar]
- 35.Camilleri M, Nadeau A, Lamsam J, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. 2010;22:e15–26. doi: 10.1111/j.1365-2982.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lunn PG, Northrop CA, Northrop AJ. Automated enzymatic assays for the determination of intestinal permeability probes in urine. 2. Mannitol. Clin Chim Acta. 1989;183:163–70. doi: 10.1016/0009-8981(89)90332-x. [DOI] [PubMed] [Google Scholar]
- 37.Northrop CA, Lunn PG, Behrens RH. Automated enzymatic assays for the determination of intestinal permeability probes in urine. 1. Lactulose and lactose. Clin Chim Acta. 1990;187:79–87. doi: 10.1016/0009-8981(90)90333-n. [DOI] [PubMed] [Google Scholar]
- 38.Lostia AM, Lionetto L, Principessa L, et al. A liquid chromatography/mass spectrometry method for the evaluation of intestinal permeability. Clin Biochem. 2008;41:887–92. doi: 10.1016/j.clinbiochem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Kosek M, Haque R, Lima A, et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–6. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–80. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 41.Peterson KM, Buss J, Easley R, et al. REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am J Clin Nutr. 2013;97:1129–33. doi: 10.3945/ajcn.112.048306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houpt E, Gratz J, Kosek M, et al. Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis. 2014;59(suppl 4):S225–32. doi: 10.1093/cid/ciu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MI, Yatsunenko T, Manary MJ, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–54. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.