Abstract
Context
Strategies to improve the course of recurrent major depressive disorder (MDD) have great public health relevance. To reduce the risk of relapse/recurrence after acute phase Cognitive Therapy (CT), a continuation phase model of therapy (C-CT) may improve outcomes.
Objectives
To test the efficacy of C-CT and fluoxetine (FLX) for relapse prevention in a placebo (PBO) controlled randomized trial and compare the durability of prophylaxis after discontinuation of treatments.
Design
A sequential, three stage design with: acute phase (all patients received 12 weeks of CT), 8 month experimental phase (responders at higher risk were randomized to C-CT, FLX, or PBO), and 24 months of longitudinal, post-treatment follow-up.
Setting
Two university-based specialty clinics.
Patients
523 adults with recurrent MDD began acute phase CT, of which 241 “higher risk” responders were randomized and 181 subsequently entered the follow-up.
Interventions
CT responders at higher risk for relapse were randomized to receive 8 months of C-CT (n = 86), FLX (n = 86) or PBO (n = 69).
Main Outcome Measures
Survival analyses of relapse/recurrence rates, as determined by “blinded” evaluators using DSM-IV criteria and the LIFE interview.
Results
As predicted, the C-CT or FLX groups were significantly less likely to relapse than the PBO group across 8 months. Relapse/recurrence rates for C-CT and FLX were nearly identical during the 8 months of treatment, although C-CT patients were more likely to accept randomization, stayed in treatment longer, and attended more sessions than those in FLX/PBO. Contrary to prediction, relapse/recurrence rates following the discontinuation of C-CT and FLX did not differ.
Conclusions
Relapse risk was reduced by both C-CT and FLX in an “enriched” randomization sampling only CT responders. The preventive effects of C-CT were not significantly more ‘durable’ than those of FLX after treatment was stopped, suggesting that some higher risk patients may require alternate longer-term interventions.
Keywords: randomized clinical trial, recurrent depression, cognitive therapy, fluoxetine, placebo, continuation phase, relapse, recurrence
Major depressive disorder is a recurrent, disabling, and potentially lethal illness, with high rates of residual symptomatology and persistent psychosocial impairment even among a large percentage of those who respond to treatment.1–4 Antidepressants have long been a cornerstone of treatment of recurrent depression, and continuation phase pharmacotherapy has become the standard of care to offset a high risk of relapse;5,6 indefinite or even life-long courses of maintenance pharmacotherapy are recommended for prophylaxis against highly recurrent episodes.6,7 However, despite the established efficacy of longer-term pharmacotherapy, treatment utilization data indicate that many patients receive only a few months of treatment with antidepressants.8,9 Identification of alternate therapies that reduce the risks of relapse and recurrence thus has great public health significance and is the focus of intensive study.10–15
Time-limited psychotherapies, such as Cognitive Therapy16 (CT), have emerged over the past 30+ years as viable alternatives to antidepressant medications and, in controlled trials, CT has been found to have comparable efficacy to pharmacotherapy across 12–16 weeks of treatment.6,17–19 Moreover, the relapse risk after completing a 12–16 week course of CT is lower than after stopping a similar course of pharmacotherapy, which suggests that the benefits of acute phase CT may be more durable than those of pharmacotherapy after treatment is stopped.11,13,20 Nevertheless, the hypothesis that CT will significantly increase the likelihood of sustained recovery and/or reduce the risk of recurrent depression over a number of years needs more systematic evaluation.11,18 Indeed, in two of the longest follow-up studies of CT responders, relapse/recurrence rates of 60%21 and 74%22 were observed across 24 months.
One factor that may moderate the durability of CT response in depression is the quality of the response to acute phase therapy. Specifically, in independent studies conducted in Pittsburgh and Dallas, CT-treated patients who had not fully remitted by about the seventh week of therapy were found to have 3–4 times the risk of relapse/recurrence than those with more rapid and complete remissions.12,21–23 In fact, about 90% of the patients who remitted rapidly and fully with CT remained well for at least one year after acute phase therapy.12,23 Thus, the effects of CT may be particularly durable for those who obtain rapid, complete and stable remissions. Conversely, CT responders who experience slower or incomplete remissions may warrant additional intervention to reduce the risk of relapse/recurrence.
To address the problem of relapse and recurrence following acute phase therapy, Jarrett and colleagues developed and refined a model of continuation phase CT (C-CT).24–26 In the first controlled trial of this intervention, Jarrett et al.12 found that C-CT effectively reduced the risk of relapse/recurrence across 8 months of therapy compared to an assessment only control. Moreover, the protective effect of C-CT across 24 months (including 16 months post-treatment) was strongest among patients with early onset depression and those who had slow or incomplete remissions.
The current study was undertaken to test prospectively the relative merits of C-CT among patients with recurrent MDD who, despite responding to a 12 week course of acute phase CT were classified to be at an increased risk of relapse/recurrence. Relative efficacy was assessed by randomizing patients to C-CT or clinical management and either pill placebo (PBO) or fluoxetine (FLX). Outcomes were assessed across 8 months of double blind therapy and, after discontinuation of treatments, across 24 months of follow-up. Predictions were: 1) both C-CT and FLX would significantly reduce the rate of relapse across 8 months compared to PBO; and 2) across 20 months C-CT would have significantly less relapse/recurrence than FLX. Thus, we predicted that whereas both active therapies could suppress the risk of relapse, C-CT would have a more durable benefit after treatment was stopped than FLX.
PATIENTS AND METHODS
A detailed description of methods is available,27 as are acute phase reports.28–33 The methods are briefly summarized below.
PATIENTS
Study procedures and protocol were approved annually by the Institutional Review Boards at The University of Texas Southwestern Medical Center and at The University of Pittsburgh Medical Center. Patients provided written HIPAA authorization and informed consent for evaluation and treatment.
Outpatients were recruited from both clinical referrals and advertisements between March 30, 2000 and July 9, 2008 and were eligible if they presented with a principal diagnosis of recurrent MDD, as diagnosed by the Structured Clinical Interview for the DSM-IV (SCID-I),34 either remitted between depressive episodes or had antecedent dysthymic disorder, and scored 14 or more on the 17-item Hamilton Rating Scale for Depression (HRSD17) at both an initial diagnostic evaluation and a second, confirmatory interview.a Exclusion criteria included unstable medical illnesses and other principal psychiatric conditions that warranted separate treatment (e.g., substance dependence or obsessive compulsive disorder; see Jarrett and Thase).27 Of the 1359 outpatients who began a two-step clinical evaluation, 523 provided informed consent, were fully eligible for study participation, and began acute phase CT.
STUDY PROCEDURES
Psychotropic medications and other psychosocial interventions were prescribed during the study.
Acute Phase Cognitive Therapy (CT)
Experienced therapists delivered acute phase CT as described by Beck et al.16 The 12 week protocol consisted of 16–20 individual sessions, each lasting 50–60 minutes; up to 2 additional weeks were permitted to accommodate scheduling needs. Sessions were twice a week for 4 weeks. Thereafter, patients who had obtained ≥ 40% reduction in the HRSD17 began weekly sessions, whereas the remainder continued twice weekly sessions for 4 more weeks before beginning weekly sessions.
The 16 therapists had completed at least one year of supervised CT training and demonstrated competence as documented by Cognitive Therapy Scale (CTS)35 scores of ≥ 40. Throughout the study, therapists received ongoing supervision or consultation; CTS ratings were made from randomly selected videotaped sessions.
During the course of acute phase therapy, patients also attended two psychoeducational sessions on relapse/recurrence risks and study requirements.27
Acute Phase Treatment Response and Stratification
Therapists completed the HRSD17 weekly; an evaluator without knowledge of cell assignment completed final “blinded” clinician ratings at the end of CT or exit. Response, based on the independent evaluator ratings, was defined as: (a) no DSM-IV MDE and (b) a 17-item HRSD17 ≤ 12. The rationale for this liberal threshold was to include patients who had benefited from treatment but who were at increased relapse risk. Responders were prospectively stratified according a classification of relapse/recurrence risk derived from earlier research.12 Higher Risk patients had at least one HRSD17 score of 7 or higher during the final 7 acute phase assessments, including the blinded evaluation; these patients were eligible for the randomized continuation phase protocol. Lower Risk patients, defined by HRSD17 scores ≤ 6 during the final 7 assessments, received no further protocol treatment and entered the follow-up phase.
Inter-rater reliability for diagnoses of major depressive episodes (MDE) was moderate. Based on a sample of 41 patients rated by 3 to 21 clinicians each, the median kappa of all pairwise comparisons was .48. However, uncorrected percent agreement among raters was 91%.
Randomization to 8 month Experimental Phase Treatments
The study statistician used a computer program to randomize patients within strata (site, number of depressive episodes, and presence/absence of dysthymia); assignments were implemented by the study coordinators and research pharmacists.b,c Only dispensing pharmacists knew assignment to FLX or PBO and could break the blind during a clinical emergency. The integrity of the randomization was confirmed by the study statistician.
During the first 6 years of the study, the primary goal was to test the efficacy of C-CT and FLX versus PBO across the 8 month experimental phase, which necessitated allocating 180 patients evenly to the three arms. Thereafter, the primary goal was to compare the durability of C-CT and FLX across 20 months post-randomization (i.e., one year after continuation treatments were discontinued). To maximize the number of patients assigned to the two active arms, the proportion allocated to the PBO was reduced to less than 10%. This change in the randomization proportions, which was approved by the funding organization and the study’s Data Monitoring and Safety Board, was not revealed to patients, therapists, or research staff.
8 Month Continuation Phase Cognitive Therapy (C-CT)
Continuation phase CT (C-CT)24,26 aimed to prevent relapse and to promote remission and recovery. Patients practiced applying compensatory skills in response to emotional distress, residual, and emerging depressive symptoms. Therapists focused on generalizing the skills across problems, situations, and time. Preemptive coping strategies were practiced in relation to previously identified cognitive and behavioral vulnerabilities. The first four (60 minutes) sessions occurred biweekly and the last 6 occurred monthly.
8 Month Clinical Management Plus FLX/PBO
Fluoxetine was chosen because of its established efficacy36,37 and a low incidence of discontinuation-emergent symptoms.38 Experienced pharmacotherapists provided clinical management according to the methods of Fawcett et al.39 Visits occurred at the same frequency as C-CT. The initial session lasted up to 45 minutes; thereafter sessions lasted up to 30 minutes. Pharmacotherapists evaluated symptoms and side effects and could provide support, but were not permitted to use the specific methods of C-CT. Side effects were rated using a three point scale (0 = absent, 1 = mild, 2 = severe).
Research pharmacies at each site packaged and dispensed active FLX or identical PBO capsules in 10 or 20 mg units. Adherence was estimated by pills counts at each visit.
Study medications were titrated upwards using a “fixed-flexible” protocol: 10 mg/day for two weeks, 20 mg/day for two weeks, and 40 mg/day thereafter. The dose could be decreased to a minimum of 10 mg/day to lessen side effects. Patients who could not tolerate any dose could be followed for clinical management alone. From week 8 onward, the modal doses of FLX and PBO were 40 mg/day. At the end of the experimental phase, study medication was stopped, without a downward taper schedule.
OUTCOME ASSESSMENTS
At the end of months 4 and 8 of the experimental phase, an independent evaluator assessed DSM-IV criteria for MDD using the SCID and Psychiatric Status Ratings (PSR)40 from the Longitudinal Interval Follow-up (LIFE). Interim or emergency evaluations were also performed if a relapse/recurrence was suspected. Assessments were conducted without knowledge of treatment assignment. Infrequently, telephone assessments were performed when the patients was not available for in-clinic assessments.
24 Month Follow-up Phase
During the longitudinal follow-up all protocol treatments were discontinued and independent evaluators used the same methods to evaluate patients every four months (i.e., 12, 16, 20, 24, 28, and 32 months after randomization). Patients were encouraged to contact study staff if they were experiencing depressive symptoms or worsening in some other way so that an interim blinded evaluation could be completed. The last patient completed the follow-up phase in May 2011.
Across study phases, patients who experienced a relapse/recurrence were immediately referred for non-research treatment.
PRIMARY OUTCOMES: RELAPSE/RECURRENCE
Relapse, which designates an exacerbation of the presenting episode after a response but before recovery,41 was defined by DSM-IV criteria for MDD (i.e., LIFE PSR score of 5 or 6 for 2 consecutive weeks).
Stable Remission is synonymous with lower risk and includes: (a) the last seven consecutive HRSD17 scores < 7 during the acute phase or (b) return to “usual self” according to the LIFE (i.e., six Psychiatric Status Ratings (PSR) ≤ 2 over 6 weeks after randomization).
Recovery, the end of an episode, is a remission lasting ≥ 8 consecutive months.
Recurrence, a new episode, is meeting DSM-IV criteria for MDD (i.e., LIFE PSR score of 5 or 6 for 2 consecutive weeks)after recovery.41 Time to relapse/recurrence was computed in weeks, where a week was 7 days and a year was 52 weeks long resulting in a month being 4.33 weeks long. Thus, the continuation phase is 8 months or 35 weeks long, followed by 2 years or 104 weeks of follow-up.
STATISTICAL ANALYSES
Sample Size and power
Sample size was based on a predicted 30% difference in relapse/recurrence rates between C-CT and FLX (i.e., 30% vs. 60%) across both the experimental phase and the first 12 months of follow-up.27 With these assumptions, 180 randomized patients (60 per cell) were required to detect a statistically significant difference using a log-rank test with one-sided α = 0.05 and 80% power. The study did not have adequate power to detect smaller, but potentially clinically meaningful differences between the C-CT and FLX groups across the full 32 month study period. Patient entry ended when the sample size was sufficient to test the primary hypotheses.
All analyses used the intention-to-treat sample, with cumulative relapse/recurrence as the primary outcomes for the survival analysis. Patients who dropped prior to experiencing an ‘event’ were censored at their last available evaluation. Survival curves were estimated using Kaplan-Meier product limit method and the relapse/recurrence rates were compared using a log-rank test. Cox proportional hazard regression analysis was used to assess the effect of covariates on the relapse/recurrence rates. Covariates were site, number of prior depressive episodes, early or late study cohort, length of current episode, and interactions between treatment groups and site, as well as treatment groups and study cohort. Presence/absence of dysthymia was omitted as a covariate because of its low rate. All comparisons were one-sided with a type I error rate of α = 0.05.
No planned, unmasked, interim analyses of the primary hypothesis were done.
RESULTS
SAMPLE DESCRIPTION
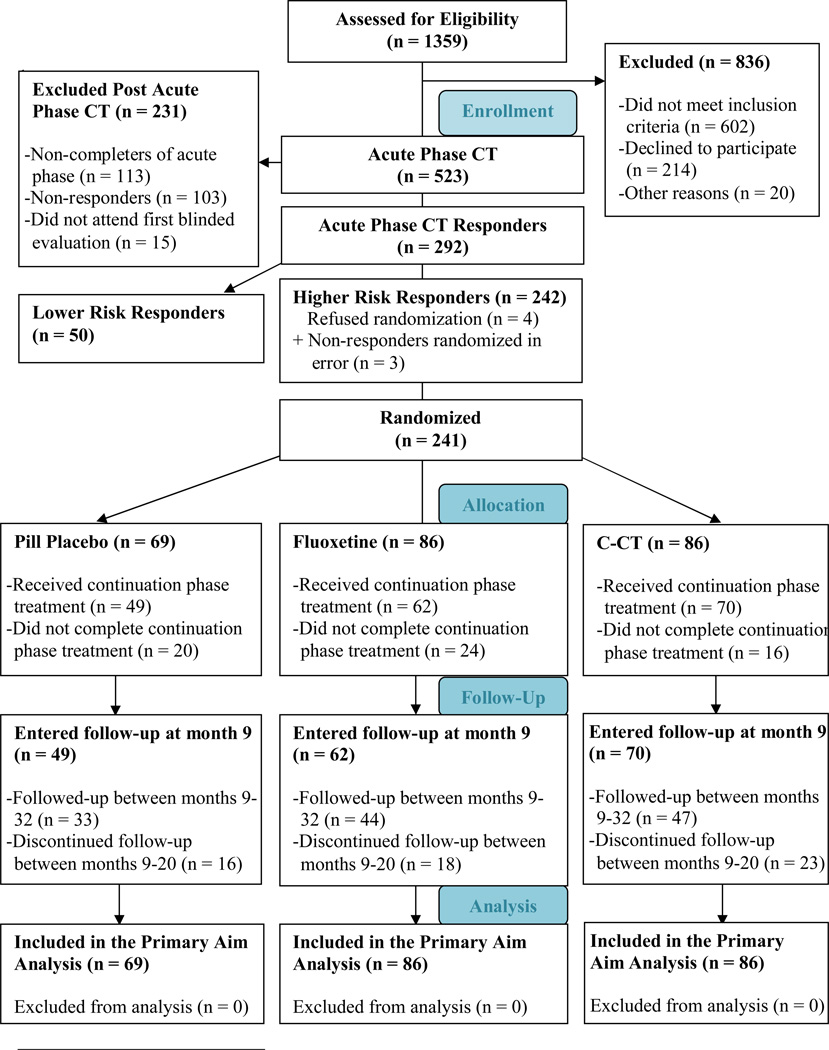
Figure 1 displays the sample composition. A total of 523 outpatients entered acute phase CT and 241 higher risk responders were randomized to C-CT, FLX, or PBO; 50 lower risk responders were eligible for longitudinal follow-up. Demographic and clinical characteristics of the randomized patients are reported in Table 1. Race was self-reported by patients in order to complete the target sample requirements of the sponsor and to comment on generalizability.
Figure 1.
Consort Flow Diagram
Table 1.
Pretreatment Demographic and Clinical Characteristics of Cognitive Therapy Responders Presenting with Recurrent Major Depressive Disorders
| Domain | Characteristics | Total (n=241) |
PBO (n=69) |
FLX (n=86) |
C-CT (n=86) |
|---|---|---|---|---|---|
| Demographic | Sex, No (%) female | 162 (67.2) | 42 (60.9) | 57 (66.3) | 63 (73.3) |
| Race, No (%) white | 205 (85.1) | 62 (89.9) | 72 (83.7) | 71 (82.6) | |
| Age, mean (SD) | 42.7 (11.8) | 43.6 (12.3) | 41.6(11.8) | 43.1 (11.5) | |
| Marital status, No (%) | |||||
| Single | 138 (57.3) | 36 (52.2) | 56 (65.1) | 46 (53.5) | |
| Partnered | 103 (42.7) | 33 (47.8) | 30 (34.9) | 40 (46.5) | |
| Education, mean (SD) | 15.7 (2.9) | 15.3 (3.1) | 16.0(3.0) | 15.7 (2.7) | |
| Employment, No (%) | |||||
| Full time | 120 (49.8) | 35 (50.7) | 44 (51.2) | 41 (47.7) | |
| Part time | 28 (11.6) | 9 (13.0) | 12 (14.0) | 7 (8.1) | |
| Homemaker/Caregiver | 18 (7.5) | 5 (7.3) | 4 (4.7) | 9 (10.5) | |
| Student | 14 (5.8) | 2 (2.9) | 6 (7.0) | 6 (7.0) | |
| Retired | 9 (3.7) | 2 (2.9) | 5 (5.8) | 2 (2.3) | |
| Other | 13 (5.4) | 4 (5.8) | 3 (3.5) | 6 (7.0) | |
| Unemployed | 39 (16.2) | 12 (17.4) | 12 (14.0) | 15 (17.4) | |
| Severity and Clinical | HRSD17 Depression Severity Scores, mean (SD) | ||||
| At final pre-treatment follow-up | 20.0 (3.9) | 20.4 (3.9) | 19.9(3.9) | 19.9(4.0) | |
| At randomization | 7.5 (3.4) | 7.4 (3.0) | 7.5(3.6) | 7.5(3.4) | |
| Age at onset, in years, Median | 18.0 | 19.0 | 17.0 | 19.5 | |
| Length of current episode in months, Median, ¥, †, § | 9.0 | 9.0 | 6.0 | 12.5 | |
| Length of illness in years, Median | 20.0 | 19.0 | 20.5 | 21.0 | |
| No. of episodes, median | 4.0 | 3.0 | 4.0 | 4.0 | |
| Comorbid DSM-IV diagnoses, No (%) | |||||
| Current | 96 (39.8) | 26 (37.7) | 37 (43.0) | 33 (38.4) | |
| Lifetime | 181 (75.1) | 51 (73.9) | 67 (77.9) | 63 (73.3) | |
| Depressive subtype – N (%) | 11 (4.6) | 2 (2.9) | 5 (5.8) | 4 (4.7) | |
| RDC endogenous, definite | 84 (35.2) | 26 (38.2) | 34 (40.0) | 24 (27.9) | |
| DSM-IV melancholia | 85 (35.6) | 22 (32.8) | 37 (43.0) | 26 (30.2) | |
: p ≤ .05 for comparing the patients in the three groups (PBO, FLX, and C-CT).
: p ≤ .05 for comparing the patients in the PBO and FLX groups.
: p ≤ .05 for comparing the patients in the PBO and C-CT groups.
: p ≤ .05 for comparing the patients in the FLX and C-CT groups.
PATIENT DISPOSITION
Attrition
Acute phase CT
Of the 523 patients who began CT, 113 (21.6%) dropped out. Of the 410 completers, 193 had at least 40% reduction in HRSD17 score by session 9 and attended at least 14 of 16 planned sessions. Among the remainder, 217 completed at least 18 of 20 planned sessions. A total of 395 (96.3%) of the completers attended the blinded evaluation, of which 292 (71.2%) met criteria for response. Of these, 242 (59%) were classified as higher risk and 241 were randomized; 4 patients withdrew consent to be randomized and 3 patients were randomized in error. See footnotes b and c in Supplementary Material for further details.
Experimental Phase
Randomized assignments were: C-CT, n = 86; clinical management plus FLX, n = 86; and clinical management plus PBO, n = 69. Sixty patients (25%) withdrew: 16 [19%] in C-CT; 24 [28%] in FLX and 20 [29%] in PBO, χ2 = 2.9, df=2, p = .24). Attrition during the experimental phase was greater at Pittsburgh (32.1%) than at Dallas (18.9%); χ2 = 5.5, df=1, p = .02). Although attrition did not differ by treatment, significantly more patients in the medication clinic (combined FLX and PBO, n = 13) dropped out before the first continuation phase session compared to C-CT (n = 1). Patients who dropped out of the experimental phase were significantly more likely to be single, younger, have antecedent dysthymia, and a shorter illness duration. Pretreatment demographic, clinical, interpersonal, and cognitive characteristics of the drop-outs did not differ across cells.
Follow-up
Of the 181 who entered follow-up (70 in C-CT, 62 in FLX, and 49 in PBO), 145 (80.1%) completed at least 12 months (56 in C-CT, 52 in FLX, and 37 in PBO) and 124 (n=68.5%) completed 24 months (47 in C-CT, 44 in FLX, and 33 in PBO). The percentage of patients who completed longitudinal follow-up did not differ significantly as a function of previous treatments or between sites.
ADHERENCE TO STUDY PROCEDURES
Sessions Completed
Of the 241 randomized patients, 147 (61.0%) completed all 10 sessions (60 in C-CT, 48 in FLX, and 39 in PBO). Patients in C-CT attended significantly more sessions than those in the pharmacotherapy arms [C-CT: 8.9 (2.4) sessions; PBO: 7.1 (3.9) sessions; FLX: 7.5 (3.6) sessions; F2,238 = 6.41, p < .01]. There also was a significant difference in mean length of the continuation phase (F2, 238 = 6.74, p < .01), with C-CT patients staying significantly longer compared to those in the pharmacotherapy arms.
Medication Dosage
Of the 151 patients with available dosage data, 110 (72.8%) achieved the target medication dosage (i.e., either 40 mg/day of FLX or PBO equivalent).
Use of Concomitant Nonprotocol Therapies
During the experimental phase, 13 randomized patients reported using non-study medications that might have had psychoactive effects (C-CT n=7; FLX n=2; PBO n=5). In the majority of cases (n=8) the medication was an over-the-counter sleeping pill; one patient in the C-CT arm deviated from the protocol by taking an antidepressant prescribed by a primary care physician. Two patients (one each in FLX and PBO) deviated from the protocol by attending self-help support groups.. No relationships were evident between usage and treatment cell (according to chi square tests) or between usage and relapse/recurrence (according to Cox regression).
Therapist Competence
Randomly selected sessions were rated using the Cognitive Therapy Scale (CTS)35 [n = 368; (334 acute phase, 34 continuation phase)]; only 27 scores (7.3%) fell below 40. Analyses of variance (ANOVA) showed that mean (±SD) CTS ratings did not differ by study phase, site, or entry cohort.
RISKS OF TREATMENT
Severe Adverse Events
Two patients were hospitalized during acute phase CT for worsening depression and/or suicidal ideation; they were withdrawn from the study and treated as appropriate. During the continuation phase, 1 patient randomized to PBO was hospitalized for suicidal ideation and was withdrawn from the study. Three patients (one from each cell) were hospitalized during the follow-up due to worsening depression and/or suicidal ideation.
Side Effects Analysis
The only side effect that was significantly greater in FLX than PBO was tremors (19.8% vs. 5.8%; χ2 = 6.4, df=1, p = .01).
COMPARISONS OF RELAPSE/RECURRENCE RATES
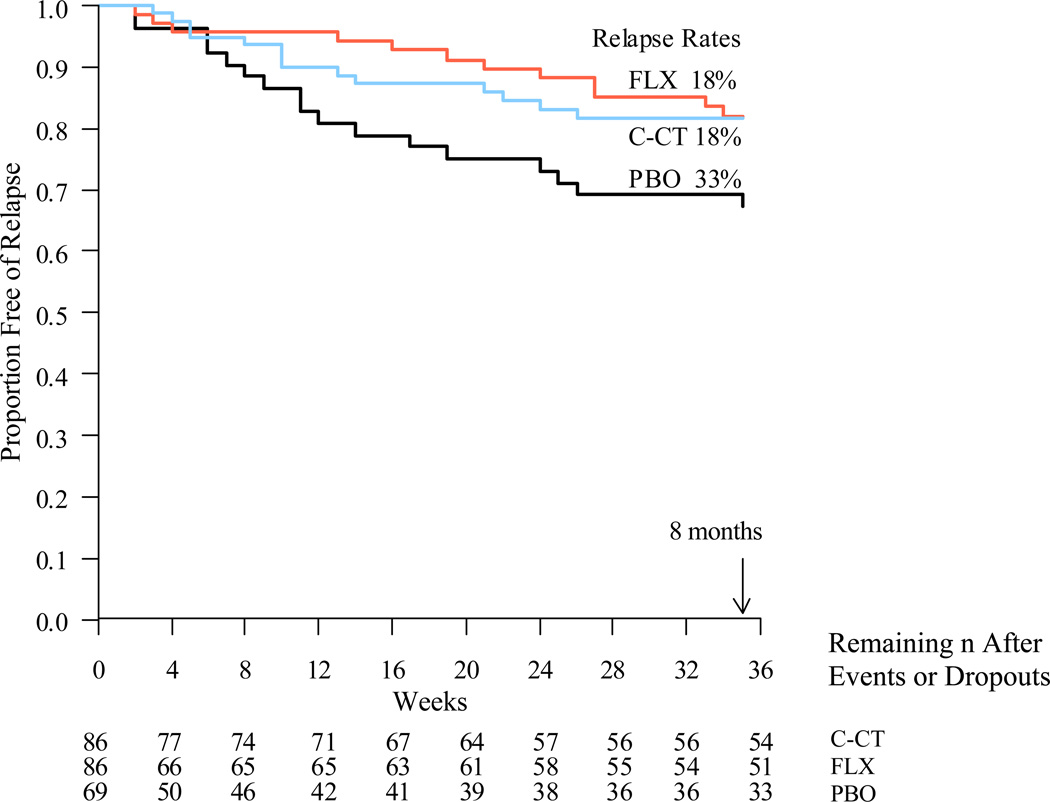
The relapse rate for PBO during the experimental phase was estimated using Kaplan-Meier as 32.7% (17/69), which was significantly higher than the 18.3% estimated relapse rate in the two active treatment arms (see Figure 2; 26/172; log-rank χ2 = 5.06, df=1, p ≤ .01). The relapse rates in the FLX and C-CT arms were nearly identical over 8 months [FLX: 18.0% (12/86 relapsed) and C-CT: 18.3% (14/86 relapsed); log-rank χ2 = 0.038, df=1, p-value ≤ 0.42] and both FLX (log-rank χ2 = 3.92, df=1, p ≤ .02) and C-CT (log-rank χ2 = 3.39, df=1, p ≤ .03) reduced relapse significantly more than PBO. As none of the covariates tested in Cox regression models (including the one significant difference across cells in length of episode) were significantly associated with relapse risk, the log rank tests was interpreted.
Figure 2.
-
-FLX vs. PBO (χ12 = 3.92, p-value = .02)
-
-C-CT vs. PBO (χ12 = 3.39, p-value = .03)
-
-C-CT vs. FLX (χ12 = 0.04, p-value = .42)
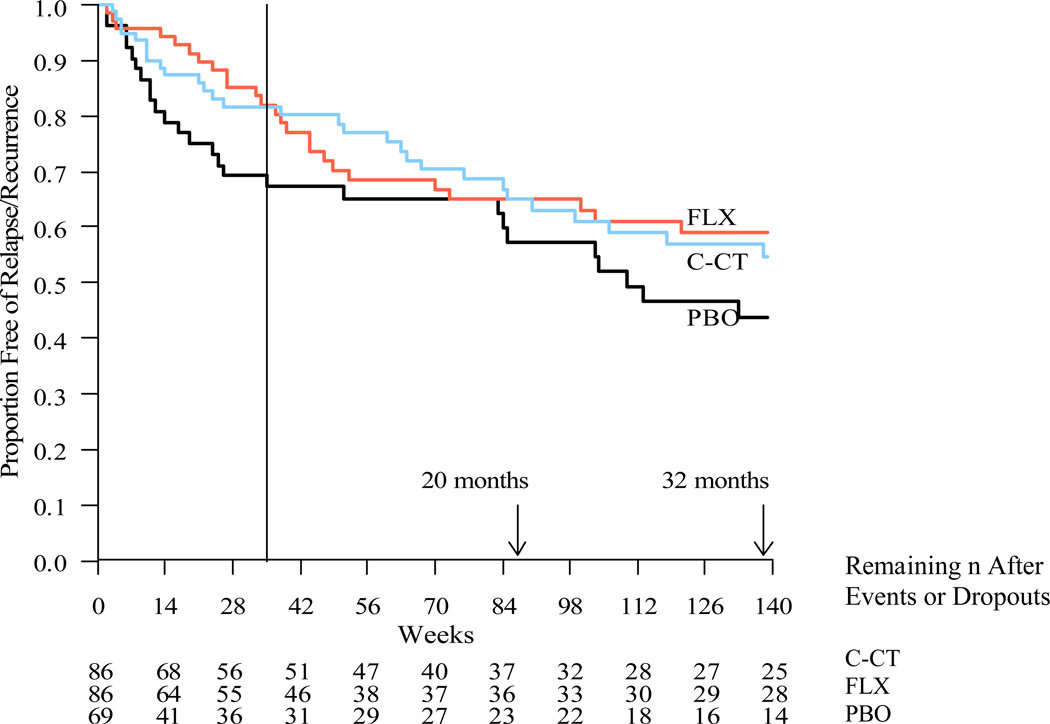
Kaplan-Meier estimates of relapse/recurrence rates across the follow-up are summarized in Table 2 and Figure 3. At follow-up month 12, the relapse/recurrence rates for C-CT (35%; 24/86) and FLX (35%; 22/86) did not differ significantly (log-rank χ2 = 0.002, df=1, p-value ≤ .48). Across the full 32 month protocol, the relapse/recurrence rates were again comparable (FLX: 41.1%; C-CT: 45.2%). Across these same intervals, relapse/recurrence rates for the PBO group were 42.7% and 56.3%, respectively. None of the pair-wise comparisons were significantly different. The Cox proportional hazard regression models including covariates yielded similar results.
Table 2.
Relapse and/or recurrence rates in the three treatment arms over 8, 20, and 32 months post-randomization.
| Time since randomization |
Rate of Relapse/Recurrence | Log- rank |
p- value |
Hazard Ratio † (95% CI) |
|||
|---|---|---|---|---|---|---|---|
| PBO | FLX | C-CT | FLX or CCT |
||||
| 8 Months | 32.7% | 18.0% | 18.3% | 18.3% | |||
| FLX vs. C-CT | 0.038 | .42 | 1.079 (0.50, 2.34) | ||||
| PBO vs. FLX | 3.916 | .02 | 0.481 (0.23, 1.01) | ||||
| PBO vs. C-CT | 3.391 | .03 | 0.519 (0.26, 1.06) | ||||
| PBO vs. FLX or C-CT | 5.057 | .01 | 0.501 (0.27, 0.93) | ||||
| 20 Months | 42.7% | 35.1% | 35.0% | 35.1% | |||
| FLX vs. C-CT | 0.002 | .48 | 0.988 (0.55, 1.76) | ||||
| PBO vs. FLX | 1.190 | .14 | 0.717 (0.39, 1.31) | ||||
| PBO vs. C-CT | 1.262 | .13 | 0.715 (0.40, 1.29) | ||||
| PBO vs. FLX or C-CT | 1.595 | .10 | 0.717 (0.43, 1.20) | ||||
| 32 Months | 56.3% | 41.1% | 45.2% | 43.2% | |||
| FLX vs. C-CT | 0.070 | .40 | 1.075 (0.63, 1.84) | ||||
| PBO vs. FLX | 2.407 | .61 | 0.649 (0.37, 1.13) | ||||
| PBO vs. C-CT | 1.731 | .09 | 0.701 (0.41, 1.19) | ||||
| PBO vs. FLX or C-CT | 2.705 | .05 | 0.676 (0.42, 1.08) | ||||
: Hazard ratios for the category listed second; CI: Confidence interval.
Figure 3.
Kaplan-Meier survival curves estimating time until relapse/recurrence (DSM-IV major depressive disorder diagnosed by blind evaluator) over 20 months (139 weeks) post-randomization. Log rank tests with pairwise comparisons showed no differences among FLX (35.1%; n = 86), (C-CT, 35.0%; n = 86) or PBO (42.7%; n = 69) at α = 0.05. Comparisons at 32 months were also null.
As between-group differences in outcomes during the experimental phase may have influenced effects during the subsequent follow-up, a frailty analysis was conducted as an additional post hoc test.42,43, e Results of this analysis confirmed those of the survival analyses.
COMMENT
The primary goals of this study were: 1) to determine the efficacy of 8 months of C-CT and FLX compared to PBO in patients with recurrent MDD predicted to be at higher risk for relapse/recurrence despite responding to CT, and 2) to compare the durability of outcomes of C-CT and FLX after therapies were stopped. The assessment of risk was based on unstable or partial remission using a prospectively applied algorithm based on earlier research.12,22,23,26 A prospective comparison of the higher and lower risk strata confirmed the validity of this classification; details are the subject of a separate paper.
As predicted, the patients who received either active therapy had a significantly lower risk of relapse/recurrence (about 18%) than did those who received PBO (about 33%) over 8 months. Contrary to prediction, we found no evidence to support the hypothesis that C-CT conveys more enduring prophylaxis in acute phase CT responders than FLX after continuation phase treatments are stopped.
This research has several implications. First, patients who respond to CT but who remain at higher risk because of slow, unstable, or partial remissions not only benefit from C-CT, but obtained comparable prophylaxis from FLX. With respect to the efficacy of C-CT, this finding replicates the earlier RCT of Jarrett et al.12 and extends the research by using a more rigorous control condition (i.e., clinical management and PBO rather than assessment only). In addition, the current findings show that C-CT’s preventive effects can be generalized to a site that was not involved in its development.
Second, the findings indicate that continuation phase FLX alone also can be used to reduce the risk of relapse after an initial course of CT. This is, to our knowledge, the first time that an antidepressant medication has been shown to significantly reduce the risk of relapse/recurrence for patients who first received psychotherapy. We note that the converse (i.e., the use of CT and related therapies in sequence to reduce the risk of relapse/recurrence after antidepressant therapy) has been demonstrated by other groups.44–46
Third, although FLX was effective, patients in the pharmacotherapy arms were more likely to drop out during the first month of the experimental phase and they attended fewer treatment sessions than did those in the C-CT arm. We therefore suspect that, in practice, offering a continuation phase of CT will be preferred by most patients who received psychotherapy alone as the initial intervention. Nevertheless, these results suggest that antidepressant medication can provide a preventive effect for acute phase CT responders when C-CT is either not feasible or not preferred.
Fourth, and contrary to prediction, we found no evidence that C-CT conveyed more durable prophylaxis in acute phase CT responders after treatment was stopped than FLX. Moreover, although our study did not have adequate statistical power to reliably detect modest differences between the active treatments, the groups had comparable survival rates across the full 32 month study period. It is therefore possible that such higher risk patients may require more than 8 months of continuation phase treatment (e.g., ongoing maintenance phase therapy) or some alternate intervention to reduce the risk of recurrent depression. The timing, amount, and duration of such treatment with the aims of promoting recovery and reducing recurrence of MDD will require additional study among higher risk CT responders.
Fifth, the null comparisons with the PBO-treated group after the end of the experimental phase cannot be interpreted with confidence because of the different time courses of relapse (i.e., those assigned to PBO were most likely to relapse during the experimental phase) and because the study was not designed or powered to make such sequential comparisons.27
Finally, the cumulative relapse/recurrence rates for the sample show that even patients judged to be at high risk for relapse/recurrence after CT have a relatively low risk of relapse/recurrence after continuation phase therapies were stopped as compared to both: a) the natural history of recurrent depression or b) the documented course of patients switched to placebo after responding to acute phase pharmacotherapy. Specifically, whereas recurrence rates as high as 80% might be expected among patients with either highly recurrent MDD3,44,48,49 or incomplete remission despite adequate pharmacotherapy45,46 across one to two years, only 56% of our ‘at higher risk’ sample treated with placebo during the continuation phase had suffered a relapse/recurrence 32 months after completion of acute phase therapy. In summary, these results are consistent with the hypothesis that acute phase CT does convey some degree of enduring prophylaxis and emphasized in the lower than expected relapse/recurrence rates in PBO.
This study has a number of strengths. To our knowledge, it is the largest, well-characterized sample of CT responders ever followed.27 It is also the largest study of relapse prevention strategies after acute phase CT ever undertaken. The study “medication” consisted of an identical appearing pill placebo matched to FLX. The therapists were quite proficient; their competence was monitored and documented longitudinally. The longitudinal evaluation of relapse and recurrence was conducted across 32 months post-randomization by evaluators without knowledge of treatment assignment and was longitudinal over 32 months post randomization. Patient drop-outs rates across all three phases of study participation were typical and acceptable. Site and cohort effects over 11 years of data collection were either absent or relatively minor. Effects of tested covariates were null.
The generalizability of the findings is limited by the inclusion/exclusion criteria, particularly the fact that our study involved unmedicated adults with recurrent MDD who responded to a 12 week course of CT but showed a slow or unstable remission. We also note that our definition of ‘lower risk’ was very rigorous, as only 17% of CT responders met this definition for stable remission. Attrition across a 3 stage longitudinal study, which for some patients amounted to almost 3 years of research participation, also can limit interpretations. We note that patients who dropped out of the experimental phase were more likely to be single, younger with a shorter duration of illness, and antecedent dysthymia. Such attrition limits generalizability. The patients and therapists over-represented white persons. The integrity and quality of the therapy delivered in this study may also differ from that available in the community, as all therapists were well trained, proficient in CT, and continued to receive both regular and ‘as needed’ consultation throughout the study.
In conclusion, CT responders at higher risk for relapse/recurrence due to slow or incomplete remission can be safely and effectively treated with either continuation phase CT or switching modalities to FLX. Although the two treatments were comparably effective, continuing CT was the more acceptable strategy, which is perhaps not surprising since they had benefitted from acute phase therapy and were able to continue working with the same clinician. After active therapies were discontinued, the preventive effects of both treatments dissipated, suggesting that some higher risk patients may benefit from additional continuation/maintenance therapies. The parameters of such continuation/maintenance therapy warrant further study.
Supplementary Material
ACKNOWLEDGMENTS
This report was supported by Grant Numbers K24 MH001571, R01 MH058397, R01 MH069619 (Robin B. Jarrett, Ph.D.) and R01 MH058356, R01 MH069618 (Michael E. Thase, M.D.) from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health. The National Institute of Mental Health had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
We also wish to acknowledge the unrestricted support of Eli Lilly and Company, who provided fluoxetine and matched pill placebo for the first 6 years of the study. Thereafter, study materials were purchased and prepared to appear identical for both sites by the pharmacy at The University of Texas Southwestern Medical Center.
We are grateful to our patients, research teams, and colleagues at The University of Texas Southwestern Medical Center, The University of Pittsburgh, and The University of Pennsylvania who made this trial possible and are named below.d
This report was supported by Grant Numbers K24 MH001571, R01 MH058397, and R01 MH069619 (Robin B. Jarrett, Ph.D.) and by R01 MH058356 and R01 MH069618 (Michael E. Thase, M.D.) from the National Institute of Mental Health (NIMH).
Financial Disclosure:
During the past 5 years, Dr. Thase has consulted with, served on advisory boards for, or received honoraria for talks from Alkermes, Allergan, AstraZeneca, Bristol-Myer Squibb, Eli Lilly and Co, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceuitica, Lundbeck, MedAvante Inc, Merck, Neuronetics Inc, Novartis, Otsuka, Pamlab, Pfizer Pharmaceuiticals, Pharmaneuroboost, Shire US Inc., Sunovion, Takeda, Teva, and Transcept Pharmaceuticals. And he has received grant support from Alkermes, AstraZeneca, Eli Lilly and Co, Forest Laboratories, GlaxoSmithKline, Neosync, Otsuka, Pharmaneuroboost, and Roche, in addition to funding from the National Institute of Mental Health and the Agency for Healthcare Research and Quality. He has equity holding for MedAvante Inc and has received royalties from American Psychiatric Publishing Inc. (APPI), Guilford Publications, Herald House, and W.W. Norton & Co Inc. Two books currently promoted by the APPI specifically pertain to cognitive therapy. Dr. Thase also discloses that his spouse is an employee of Peloton Advantage, which does business with several pharmaceutical companies that market medications used to treat depression.
Dr. Jarrett’s medical center collects the payments from the cognitive therapy she personally provides to patients. Dr. Jarrett is a paid consultant to the NIMH.
Dr. Friedman has received grant support from the National Institute of Mental Health and Agency for Healthcare Research and Quality. He has served as an expert forensic psychiatrist for Thompson Rhodes & Cowie PC and Berger and Zavesky Co LLP. He receives royalties from Springer. He has been a member of speaker bureaus or advisory boards for AstraZeneca, Eli Lilly, GlaxoSmithKline, Pfizer, Weth-Ayers, and Pamlab, He has received grant or research support from Aspect Medical Systems, Indevus, AstraZeneca, Bristol-Myers Squibb, Pfizer, Sanofi-Aventis, Wyeth-Ayerst, Cyberonics, Novartis, Northstar, and Medtronic.
Drs. Minhajuddin and Gershenfeld report no related financial interests.
Footnotes
All authors take responsibility for the integrity and interpretation of the data. All authors had full access to the data during their study involvement.
Study concept and design: Drs. Jarrett and Thase
Acquisition of data: Drs. Jarrett, Thase, Gershenfeld, Friedman, and all study team members
Drafting manuscript: Drs. Jarrett, Thase, and Minhajuddin
Critical review of manuscript for important intellectual content: All authors
Statistical analysis: Dr. Minhajuddin
Obtained funding: Drs. Jarrett and Thase
Administrative technical or material support: All authors and their research teams; as well as the Data Safety and Monitoring Board
Study supervision: Drs. Jarrett, Thase, Gershenfeld, and Friedman
Contributor Information
Robin B. Jarrett, Department of Psychiatry, The University of Texas Southwestern Medical Center
Abu Minhajuddin, Department of Clinical Sciences, The University of Texas Southwestern Medical Center
Howard Gershenfeld, Department of Psychiatry, The University of Texas Southwestern Medical Center
Edward S. Friedman, Department of Psychiatry, The University of Pittsburgh Medical Center
Michael E. Thase, Departments of Psychiatry, Perelman School of Medicine of the University of Pennsylvania, Philadelphia Veterans Affairs Medical Center and the University of Pittsburgh Medical Center
References
- 1.Coryell W, Scheftner W, Keller M, Endicott J, et al. The enduring psychosocial consequences of mania and depression. Am. J. Psychiatry. 1993 May;150(5):720–727. doi: 10.1176/ajp.150.5.720. [DOI] [PubMed] [Google Scholar]
- 2.Angst J. Fortnightly review: A regular review of the long term follow up of depression. BMJ. 1997;315(7116):1143–1146. doi: 10.1136/bmj.315.7116.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: A prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J. Affect. Disord. 1998 Sep;50(2–3):97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 4.Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003 Jun 18;289(23):3152–3160. doi: 10.1001/jama.289.23.3152. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Practice Guideline for the treatment of patients with Major Depressive Disorder in Adults. American Psychiatric Association; 1993. p. 51. [Google Scholar]
- 6.American Psychiatric Association. Practice guideline for the treatment of patients with Major Depressive Disorder. 3rd. ed. ed. Arlington, VA: American Psychiatric Association; 2010. [Google Scholar]
- 7. Thase ME. Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr. 2006 Dec;11(12) Suppl 15:12–21. doi: 10.1017/s1092852900015212. [DOI] [PubMed] [Google Scholar]
- 8.Lin EHB, Von Korff M, Katon W, et al. The role of the primary care physician in patients' adherence to antidepressant therapy. Med. Care. 1995 Jan;33(1):67–74. doi: 10.1097/00005650-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Bull SA, Hu XH, Hunkeler EM, et al. Discontinuation of use and switching of antidepressants: Influence of patient-physician communication. JAMA. 2002;288(11):1403–1409. doi: 10.1001/jama.288.11.1403. [DOI] [PubMed] [Google Scholar]
- 10.Biesheuvel-Leliefeld KE, Kersten SM, van der Horst HE, et al. Cost-effectiveness of nurse-led self-help for recurrent depression in the primary care setting: design of a pragmatic randomised controlled trial. BMC Psychiatry. 2012 Jun;12:59–68. doi: 10.1186/1471-244X-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy's effects. J Consult Clin Psychol. 2007;75:475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves G, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase. Arch. Gen. Psychiatry. 2001 Apr;58(4):381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch. Gen. Psychiatry. 2005 Apr;62(4):417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 14.Bockting CLH, Elgersma HJ, van Rijsbergen GD, et al. Disrupting the rhythm of depression: design and protocol of a randomized controlled trial on preventing relapse using brief cognitive therapy with or without antidepressants. BMC Psychiatry. 2011;11(8):1–9. doi: 10.1186/1471-244X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J. Consult. Clin. Psychol. 2008;76(6):966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- 17.DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch. Gen. Psychiatry. 2005 Apr;62(4):409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 18.Hollon SD, Thase ME, Markowitz JC. Treatment and prevention of depression. Psychological Science in the Public Interest. 2002;3(2):39–77. doi: 10.1111/1529-1006.00008. [DOI] [PubMed] [Google Scholar]
- 19.Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J. Consult. Clin. Psychol. 2006 Aug;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- 20.Dobson KS, Hollon SD, Dimidjian S, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J. Consult. Clin. Psychol. 2008 Jun;76(3):468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thase ME, Simons AD, Reynolds CF. Abnormal electroencephalographic sleep profiles in major depression. Association with response to cognitive behavior therapy. Arch. Gen. Psychiatry. 1996;53:99–108. doi: 10.1001/archpsyc.1996.01830020013003. [DOI] [PubMed] [Google Scholar]
- 22.Jarrett RB, Basco MR, Risser R, et al. Is there a role for continuation phase cognitive therapy for depressed outpatients? J. Consult. Clin. Psychol. 1998 Dec;66(6):1036–1040. doi: 10.1037//0022-006x.66.6.1036. [DOI] [PubMed] [Google Scholar]
- 23.Thase ME, Simons AD, McGeary J, et al. Relapse after cognitive behavior therapy of depression: Potential implications for longer courses of treatment. Am. J. Psychiatry. 1992 Aug;149(8):1046–1052. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- 24.Jarrett RB. Cognitive therapy for recurrent unipolar major depressive disorder. The continuation/maintenance phase. Unpublished treatment manuals. 1989;1992 [Google Scholar]
- 25.Jarrett RB, Kraft D. Prophylactic cognitive therapy for major depressive disorder. In Session. 1997;3:65–79. [Google Scholar]
- 26.Jarrett RB, Vittengl JR, Clark LA. How much cognitive therapy, for which patients will prevent depressive relapse? J. Affect. Disord. 2008;111:185–192. doi: 10.1016/j.jad.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarrett RB, Thase ME. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: Design of a double-blinded, Fluoxetine- and pill-placebo–controlled, randomized trial with 2-Year follow-up. Contemporary Clinical Trials. 2010;31:355–377. doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renner F, Jarrett RB, Vittengl JR, Barrett MS, Clark LA, Thase ME. Interpersonal problems as predictors of therapeutic alliance and symptom improvement in cognitive therapy for depression. J. Affect. Disord. 2012;138:458–467. doi: 10.1016/j.jad.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarrett RB, Vittengl JR, Thase ME, Clark LA. Skills of Cognitive Therapy (SoCT): A new measure of patients’ comprehension and use. Psychol Assess. 2011;23(3):578–586. doi: 10.1037/a0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarrett RB, Minhajuddin A, Borman PD, et al. Cognitive reactivity, dysfunctional attitudes, and depressive relapse and recurrence in cognitive therapy responders. Behav. Res. Ther. 2012;50:280–286. doi: 10.1016/j.brat.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn TW, Vittengl JR, Clark LA, Carmody T, Thase ME, Jarrett RB. Change in psychosocial functioning and in depressive symptoms during acute phase cognitive therapy for depression. Psychol. Med. 2012;42:317–326. doi: 10.1017/S0033291711001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smits JAJ, Minhajuddin A, Thase ME, Jarrett RB. Outcomes of acute phase cognitive therapy in outpatients with anxious versus nonanxious depression. Psychother. Psychosom. 2012;81:153–160. doi: 10.1159/000334909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarrett RB, Minhajuddin A, Kangas JL, et al. Acute phase cognitive therapy for recurrent major depressive disorder: Who drops out and ho wmuch do patient skills influence response? Behav Res Ther. doi: 10.1016/j.brat.2013.01.006. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- 35.Young J, Beck AT. Cognitive Therapy Scale: Rating manual: Center for Cognitive Therapy. 1980 [Google Scholar]
- 36.Montgomery SA, Dufour H, Brion S, et al. The prophylactic efficacy of fluoxetine in unipolar depression. Br. J. Psychiatry. Suppl. 1988 Sep;(3):69–76. [PubMed] [Google Scholar]
- 37.Wernicke JF, Dunlop SR, Dornseif BE. Low-dose fluoxetine therapy for depression. Psychopharmacol. Bull. 1988;24:183–188. [PubMed] [Google Scholar]
- 38.Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J. Clin. Psychopharmacol. 1996 Oct;16(5):356–362. doi: 10.1097/00004714-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Fawcett J, Epstein P, Fiester S, Elkin I, Autry J. Clinical management-imipramine/placebo administration manual. Psychopharmacol. Bull. 1987;23(2):309–324. [PubMed] [Google Scholar]
- 40.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Arch. Gen. Psychiatry. 1987 Jun;44(6):540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 41.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 42.Wienke A. Frailty models. [Last visited 01/16/2013];MPIDR working paper WP 2002–2003. 2003 Sep; Available at: http://www.ressources-actuarielles.net/ext/isfa/1226.nsf/769998e0a65ea348c1257052003eb94f/37d8815030298678c125737b002e1b13/ [Google Scholar]
- 43.Wienke A. Frailty models in survival analysis. [Last visited: 01/16/2013]; Available at: http://sundoc.bibliothek.uni-halle.de/habil-online/07/07H056/habil.pdf. [Google Scholar]
- 44.Fava GA, Ruini C, Rafanelli C, Finos L, Conti S, Grandli S. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am. J. Psychiatry. 2004;161:1872–1876. doi: 10.1176/ajp.161.10.1872. [DOI] [PubMed] [Google Scholar]
- 45.Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by 5ognitive therapy: A controlled trial. Arch. Gen. Psychiatry. 1999 Sep;56(9):829–835. doi: 10.1001/archpsyc.56.9.829. [DOI] [PubMed] [Google Scholar]
- 46.Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Archives of General Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frank E, Kupfer DJ, Perel JM, et al. Three-year outcomes for maintenance therapies in recurrent depression. Arch. Gen. Psychiatry. 1990 Dec;47(12):1093–1099. doi: 10.1001/archpsyc.1990.01810240013002. [DOI] [PubMed] [Google Scholar]
- 48.Keller MB, Boland RJ. Implications of failing to achieve successful long-term maintenance treatment of recurrent unipolar major depression. Biol. Psychiatry. 1998 Sep 1;44(5):348–360. doi: 10.1016/s0006-3223(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 49.Rush AJ, Kraemer HC, Sackeim HA, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006 Sep;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.