Abstract
Background
Recent US and UK clinical practice guidelines recommend that second‐generation antidepressants should be considered amongst the best first‐line options when drug therapy is indicated for a depressive episode. Systematic reviews have already highlighted some differences in efficacy between second‐generation antidepressants. Citalopram, one of the first selective serotonin reuptake inhibitors (SSRI) introduced in the market, is one of these antidepressant drugs that clinicians use for routine depression care.
Objectives
To assess the evidence for the efficacy, acceptability and tolerability of citalopram in comparison with tricyclics, heterocyclics, other SSRIs and other conventional and non‐conventional antidepressants in the acute‐phase treatment of major depression.
Search methods
We searched The Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register and the Cochrane Central Register of Controlled Trials up to February 2012. No language restriction was applied. We contacted pharmaceutical companies and experts in this field for supplemental data.
Selection criteria
Randomised controlled trials allocating patients with major depression to citalopram versus any other antidepressants.
Data collection and analysis
Two reviewers independently extracted data. Information extracted included study characteristics, participant characteristics, intervention details and outcome measures in terms of efficacy (the number of patients who responded or remitted), patient acceptability (the number of patients who failed to complete the study) and tolerability (side‐effects).
Main results
Thirty‐seven trials compared citalopram with other antidepressants (such as tricyclics, heterocyclics, SSRIs and other antidepressants, either conventional ones, such as mirtazapine, venlafaxine and reboxetine, or non‐conventional, like hypericum). Citalopram was shown to be significantly less effective than escitalopram in achieving acute response (odds ratio (OR) 1.47, 95% confidence interval (CI) 1.08 to 2.02), but more effective than paroxetine (OR 0.65, 95% CI 0.44 to 0.96) and reboxetine (OR 0.63, 95% CI 0.43 to 0.91). Significantly fewer patients allocated to citalopram withdrew from trials due to adverse events compared with patients allocated to tricyclics (OR 0.54, 95% CI 0.38 to 0.78) and fewer patients allocated to citalopram reported at least one side effect than reboxetine or venlafaxine (OR 0.64, 95% CI 0.42 to 0.97 and OR 0.46, 95% CI 0.24 to 0.88, respectively).
Authors' conclusions
Some statistically significant differences between citalopram and other antidepressants for the acute phase treatment of major depression were found in terms of efficacy, tolerability and acceptability. Citalopram was more efficacious than paroxetine and reboxetine and more acceptable than tricyclics, reboxetine and venlafaxine, however, it seemed to be less efficacious than escitalopram. As with most systematic reviews in psychopharmacology, the potential for overestimation of treatment effect due to sponsorship bias and publication bias should be borne in mind when interpreting review findings. Economic analyses were not reported in the included studies, however, cost effectiveness information is needed in the field of antidepressant trials.
Keywords: Humans; Antidepressive Agents; Antidepressive Agents/therapeutic use; Antidepressive Agents, Second‐Generation; Antidepressive Agents, Second‐Generation/therapeutic use; Citalopram; Citalopram/therapeutic use; Cyclohexanols; Cyclohexanols/therapeutic use; Depression; Depression/drug therapy; Morpholines; Morpholines/therapeutic use; Paroxetine; Paroxetine/therapeutic use; Reboxetine; Selective Serotonin Reuptake Inhibitors; Selective Serotonin Reuptake Inhibitors/therapeutic use; Venlafaxine Hydrochloride
Plain language summary
Citalopram versus other antidepressants for depression
Major depression is a severe mental illness characterised by a persistent and unreactive low mood and loss of all interest and pleasure, usually accompanied by a range of symptoms including appetite change, sleep disturbance, fatigue, loss of energy, poor concentration, psychomotor symptoms, inappropriate guilt and morbid thoughts of death. Antidepressant drugs remain the mainstay of treatment in moderate‐to‐severe major depression. During the last 20 years, selective serotonin reuptake inhibitors (SSRIs) have progressively become the most commonly prescribed antidepressants. Citalopram, one of the first SSRIs introduced in the market, is the racemic mixture of S‐ and R‐enantiomer. In the present review we assessed the evidence for the efficacy, acceptability and tolerability of citalopram in comparison with all other antidepressants in the acute‐phase treatment of major depression. Thirty‐seven randomised controlled trials (more than 6000 participants) were included in the present review. In terms of efficacy, citalopram was more efficacious than other reference compounds like paroxetine or reboxetine, but worse than escitalopram. In terms of side effects, citalopram was more acceptable than older antidepressants, like tricyclics. Based on these findings, we conclude that clinicians should focus on practical or clinically relevant considerations including differences in efficacy and side‐effect profiles.
Background
Description of the condition
Major depression is generally diagnosed when a persistent and unreactive low mood and/or loss of interest and pleasure are accompanied by a range of symptoms including appetite loss, insomnia, fatigue, loss of energy, poor concentration, psychomotor symptoms, inappropriate guilt and morbid thoughts of death (APA 1994). It was the third leading cause of burden among all diseases in the year 2004 and it is expected to be the greatest cause in 2030 (WHO 2006). This condition is associated with marked personal, social and economic morbidity, loss of functioning and productivity, and creates significant demands on service providers in terms of workload (APA 2000; NICE 2010). Although pharmacological and psychological interventions are both effective for major depression, in primary and secondary care settings antidepressant (AD) drugs remain the mainstay of treatment in moderate to severe major depression (APA 2006; NICE 2010). Amongst ADs many different agents are available, including tricyclics (TCAs), monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin‐noradrenaline reuptake inhibitors (SNRIs, such as venlafaxine, duloxetine and milnacipran), and other agents (mirtazapine, reboxetine, bupropion). During the last 20 years, ADs prescription has dramatically risen in western countries, mainly because of the increasing prescription of SSRIs which have progressively become the most commonly prescribed ADs (Ciuna 2004). SSRIs are generally more acceptable than TCAs, and there is evidence of similar efficacy (NICE 2010). However, head‐to‐head comparisons have provided contrasting findings (Cipriani 2006).
Description of the intervention
Citalopram hydrobromide is a selective serotonin reuptake inhibitor (SSRI) that has been available as an antidepressant since the 1980s in US and Europe. It is also available in many countries for anxiety disorders, including obsessive‐compulsive disorder and social anxiety disorder. Citalopram is a racemic dicyclic phthalane derivative designated (±)‐1‐(3‐dimethylaminopropyl)‐1‐(4‐fluorophenyl)‐1,3‐dihydroisobenzofuran‐5carbonitrile (www.fda.gov). Citalopram has a chemical structure unrelated to that of other SSRIs or of tricyclic, tetracyclic, or other available antidepressant agents. Therefore, some differential clinical potency may be expected, not only between the drugs classes but also among the SSRIs.
How the intervention might work
Inhibition of the neuronal transporter for serotonin has long been established as one of the mechanisms of action of numerous antidepressants (Barker 1995). Citalopram is a dicyclic phthalide derivative and its effect is due to a specific inhibition of the re‐uptake of serotonin in the brain (Stahl 1994). Citalopram is a highly selective and potent SSRI with minimal effects on the neuronal reuptake of norepinephrine (NE) and dopamine (DA). Citalopram has no or very low affinity for a series of receptors including serotonin 5‐HT1A, 5‐HT2, dopamine D1, and D2, a1‐, a2‐, b‐adrenergic, histamine H1, muscarinic cholinergic, benzodiazepine, gamma aminobutyric acid (GABA) and opioid receptors (Stahl 1998). Citalopram has a pronounced tissue distribution and its binding to human plasma proteins is about 80%. Maximum concentration in blood is reached after one to six hours and the steady state concentration in blood is reached after one to two weeks. Protein binding is about 14L/k and the half‐life is about 36 hours, (possibly longer for the elderly). The drug is metabolized before it is excreted. Citalopram is metabolized in the liver and the biotransformation of citalopram to its demethyl metabolites depends on both CYP2C19 and CYP3A4, with a small contribution from CYP2D6.
Why it is important to do this review
To shed light on the field of antidepressant trials and the treatment of major depression, a group of researchers agreed to join forces under the rubric of the Meta‐Analyses of New Generation Antidepressants Study Group (MANGA Study Group) to systematically review all available evidence for each specific newer antidepressant. We have up to now completed some individual reviews about fluoxetine (Cipriani 2005a), sertraline (Cipriani 2009b), escitalopram (Cipriani 2009c), milnacipran (Nakagawa 2009), fluvoxamine (Omori 2010), and a number of other reviews are now underway. Thus, the aim of the present review is to assess the evidence for the efficacy and tolerability of citalopram in comparison with TCAs, heterocyclics, MAOIs, SSRIs, SNRIs and other antidepressants in the acute‐phase treatment of major depression.
Objectives
(1) To determine the efficacy of citalopram in comparison with other antidepressants in alleviating the acute symptoms of major depressive disorder. (2) To review acceptability of treatment with citalopram in comparison with other antidepressants. (3) To investigate the adverse effects of citalopram in comparison with other antidepressants.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials that compared citalopram with all other active antidepressants as monotherapy in the acute phase treatment of depression. Quasi‐randomised trials, such as those allocating by using alternate days of the week, were excluded. For trials which have a cross‐over design, we only considered results from the first randomisation period.
Types of participants
The review included trials of patients 18 years or older, of both sexes, with a primary diagnosis of depression and studies adopting standardised criteria (DSM‐III / DSM‐III‐R, DSM‐IV (APA 2000), ICD‐10 (WHO 1992), Feighner criteria (Feighner 1972) or Research Diagnostic Criteria (Spitzer 1972) to define patients suffering from unipolar major depression. We excluded studies using ICD‐9, as it has only disease names and no diagnostic criteria. We included the following subtypes of depression: chronic, with catatonic features, with melancholic features, with atypical features, with postpartum onset, and with seasonal pattern. We also included studies in which up to 20% of patients presented depressive episodes in bipolar affective disorder. A concurrent secondary diagnosis of another psychiatric disorder was not considered an exclusion criterion. A concurrent primary diagnosis of Axis I or II disorders was an exclusion criterion. AD trials in depressive patients with a serious concomitant medical illness were excluded.
Types of interventions
We examined citalopram intervention in comparison with conventional treatment of acute depression. We also examined citalopram intervention in comparison with non‐conventional antidepressants (herbal products or other non‐conventional antidepressants. We excluded trials in which citalopram was compared with another type of psychopharmacological agent (i.e., anxiolytics, anticonvulsants, antipsychotics or mood‐stabilizers). We also excluded trials in which citalopram was used as an augmentation strategy.
Eligible intervention:
1. Citalopram: any dose and pattern of administration.
Eligible comparators:
2. Conventional antidepressants: any dose and mode or pattern of administration. 2.1 TCAs 2.2 Heterocyclics 2.3 SSRIs 2.4 SNRIs 2.5 MAOIs or newer ADs 2.6 Other conventional psychotropic drugs
3. Non‐conventional antidepressants 3.1 Herbal products 3.2 Other non‐conventional antidepressants
Types of outcome measures
Primary outcomes
1. Response ‐ acute phase
We examined trials regarding the number of patients (1) who responded to treatment by showing a reduction of at least 50% on the Hamilton Rating Scale for depression (HRSD) (Hamilton 1960), Montgomery Åsberg Depression Rating Scale (MADRS) (Montgomery 1979), or any other depression scale, depending on the study authors' definition or (2) who were "much or very much improved" (score 1 or 2) on the CGI‐Improvement scale (Guy 1976) out of the total number of randomised patients. Where both were provided, we preferred the former criteria for judging response. The original authors' definitions of response and remission were not used in this review, to avoid possible outcome reporting bias (Furukawa 2007).
As studies report response rates at various time points throughout the trial period, we had determined a priori to subdivide the treatment indices ‐ since one systematic review suggested that SSRIs begin to have observable beneficial effects in depression during the first week of treatment ‐ as follows (Taylor 2006):
(i) Response ‐ early phase: between one and four weeks, with the time point closest to two weeks given preference. (ii) Response ‐ acute phase: between six and 12 weeks, with preference given to the time point given in the original study as the study endpoint. (iii) Response ‐ follow‐up phase: between four and six months, with the time point closest to 24 weeks given preference.
The acute phase treatment response rates were our primary outcome of interest.
Secondary outcomes
1. Response ‐ early phase, and follow‐up phase
2. Remission ‐ early phase, acute phase, and follow‐up phase
We were interested in the number of patients who achieved remission, (1) showing =< 7 on HRSD‐17, =< 8 on for all the other longer versions of HRSD, and =< 11 on MADRS or (2) who were "not ill or borderline mentally ill" (score 1 or 2) on the CGI‐Severity score out of the total number of randomised patients. Where both were provided, we preferred the former criterion for judging remission.
3. Group mean scores at the end of the trial and change score on depression scale
4. Social adjustment, social functioning, including the Global Assessment of Function (GAF) scores
5. Health‐related quality of life (QOL)
We limited ourselves to SF‐12 (Ware 1998), SF‐36 (Ware 1992), HoNOS (Wing 1998) and the WHO 2009‐QOL (WHOQOL Group 1998).
6. Costs to healthcare services
7. Acceptability
7.1 Total dropout
Number of patients who dropped out during the trial as a proportion of the total number of randomised patients.
7.2 Dropout due to inefficacy
Number of patients who dropped out during the trial because the fluvoxamine was ineffective as a proportion of the total number of randomised patients.
7.3 Dropout due to side effects
Number of patients who dropped out during the trial due to side effects, as a proportion of the total number of randomised patients.
7.4 Number of patients experiencing at least one side effect
7.5 Number of patients experiencing the following specific side effects was sought:
sleepiness/drowsiness
insomnia
dry mouth
constipation
problems urinating
hypotension
agitation/anxiety
suicide wishes/gestures/attempts
completed suicide
vomiting/nausea
diarrhoea
To avoid missing any relatively rare or unexpected side effects in the data extraction phase, we collected all side effect data reported in the literature and discussed ways to summarize them post hoc. Descriptive data regarding side‐effect profiles were extracted from all available studies. Only studies reporting the number of patients experiencing individual side effects were retained. Due to a lack of consistent reporting of side effects, which came primarily from the study authors' descriptions, we combined terms describing similar side effects; for example, we combined "dry mouth", "reduced salivation" and "thirst" into "dry mouth". All side‐effect categories were then grouped by organ system, such as neuropsychiatric, gastrointestinal, respiratory, sensory, genitourinary, dermatological and cardiovascular, in accordance with the advice of a previous study (Mottram 2006).
Search methods for identification of studies
Electronic searches
We searched The Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CCDANCTR) up to February 2012, MEDLINE (1966 to 2012), EMBASE (1974 to 2012). We also searched trial databases of the following drug‐approving agencies for published, unpublished and ongoing controlled trials: the Food and Drug Administration (FDA) in the USA, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, the European Medicines Agency (EMA) in the EU, the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan and the Therapeutic Goods Administration (TGA) in Australia. In addition, we searched ongoing trial registers such as clinicaltrials.gov in the USA, International Standard Randomised Controlled Trial Number Register (ISRCTN) and the National Research Register in the UK, Nederland's Trial Register in the Netherlands, European Union Drug Regulating Authorities Clinical Trials (EudraCT) in the EU, UMIN‐CTR in Japan, the Australian Clinical Trials Registry in Australia and the clinical trial register of Lundbeck and Forest (citalopram manufacturer): http://www.lundbecktrials.com/ and http://www.forestclinicaltrials.com/CTR/CTRController/CTRHome, respectively These searches were undertaken in November 2010 and replicated in February 2012. No language restriction was applied.
CCDANCTR‐Studies were searched using the following search strategy: Diagnosis = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Intervention = Citalopram
CCDANCTR‐References were searched using the following search strategy: Keyword = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Free‐Text = Citalopram
Searching other resources
1. Handsearches Appropriate journals and conference proceedings relating to citalopram treatment for depression have already been handsearched and incorporated into the CCDANCTR databases.
2. Personal communication
We asked pharmaceutical companies and experts in this field if they knew of any study that met the inclusion criteria of this review.
3. Reference checking
We checked reference lists of the included studies, previous systematic reviews and major textbooks of affective disorder written in English for published reports and citations of unpublished research (Trespidi 2011).
Data collection and analysis
Selection of studies
Two review authors independently checked to ensure that studies relating to duloxetine generated by the search strategies of the CCDANCTR‐References and the other complementary searches met the rough inclusion criteria, firstly based on the title and abstracts. All of the studies that were rated as possible candidates by either of the two review authors were added to the preliminary list, and their full texts were retrieved. Review authors AC, GI, MP, AS and CT then assessed all of the full text articles in this preliminary list to see if they met the strict inclusion criteria. If the raters disagreed, the final rating was made by consensus with the involvement ‐ if necessary ‐ of another member of the review group (CB, NW or TAF). Considerable care was taken to exclude duplicate publications.
Data extraction and management
AC, GI, MP, AS and CT extracted data from the included studies. Again, any disagreement was discussed, and decisions were documented. If necessary, we contacted authors of studies for clarification. We extracted the following data:
(i) participant characteristics (age, sex, depression diagnosis, comorbidity, depression severity, antidepressant treatment history for the index episode, study setting); (ii) intervention details (intended dosage range, mean daily dosage actually prescribed, co‐intervention if any, duloxetine as investigational drug or as comparator drug, sponsorship); (iii) outcome measures of interest from the included studies.
The results were compared with those in the completed reviews of individual antidepressants in The Cochrane Library. If the trial was a three (or more)‐armed trial involving a placebo arm, the data were extracted from the placebo arm as well.
Assessment of risk of bias in included studies
Two review authors independently assessed trial quality in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This set of criteria is based on evidence of associations between effect overestimation and a high risk of bias in an article, such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting. The categories are defined as:
low risk of bias;
high risk of bias;
unclear risk of bias.
If the raters disagreed, the final rating was made by consensus with the involvement (if necessary) of another member of the review group. Non‐congruence in quality assessment was reported as percentage disagreement. The ratings were also compared with those in the completed reviews of individual antidepressants in The Cochrane Library. If there were any discrepancies, these were fed back to the authors of the completed reviews.
Measures of treatment effect
All comparisons were performed between citalopram and comparator ADs as individual ADs. Citalopram was also compared with TCAs and heterocyclics as a class.
1. Dichotomous data
For dichotomous, or event‐like, data, odds ratios (ORs) were calculated with 95% confidence intervals (CIs). For statistically significant results, we calculated the number needed to treat to provide benefit (NNTB) and the number needed to treat to induce harm (NNTH) as the inverse of the risk difference.
2. Continuous data
For continuous data, we calculated mean differences (MD), or standardised mean differences (SMD) where different measurement scales were used, with 95% CIs.
Unit of analysis issues
1. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g., pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, the participants can differ systematically from their initial state, despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in major depression, we only used data from the first phase of the cross‐over studies.
2. Cluster‐randomised trials
No cluster‐randomised trials were identified for this version of the review. Should they be identified in a future update, we plan to use the generic inverse variance technique, if such trials have been appropriately analysed taking into account intraclass correlation coefficients to adjust for cluster effects.
3. Multiple intervention groups
Studies that compared more than two intervention groups were included in meta‐analysis by combining all relevant experimental intervention groups of the study into a single group, and all relevant control intervention groups into a single control group, as recommended in section 16.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
1. Dichotomous data
Responders and remitters to treatment were calculated on the strict intention‐to‐treat (ITT) basis: dropouts were included in this analysis. Where participants had been excluded from the trial before the endpoint, we assumed that they experienced a negative outcome by the end of the trial (e.g., failure to respond to treatment). We examined the validity of this decision in sensitivity analyses by applying worst‐ and best‐case scenarios. We applied the loose ITT analyses for continuous variables, whereby all the patients with at least one post‐baseline measurement were represented by their last observations carried forward (LOCF), with due consideration of the potential bias and uncertainty introduced.
When dichotomous outcomes were not reported but baseline mean, endpoint mean and the standard deviation (SD) of the HRSD (or other depression scale) were provided, we converted continuous outcome data expressed as mean and SD into the number of responding and remitted patients, according to the validated imputation method (Furukawa 2005). We examined the validity of this imputation in the sensitivity analyses. Where SDs were not reported, authors were asked to supply the data. When only the standard error (SE) or t‐statistics or P values were reported, SDs were calculated according to Altman (Altman 1996). In the absence of data from the authors, we substituted SDs by those reported in other studies in the review (Furukawa 2006).
2. Continuous data
When there were missing data and the method of LOCF had been used to do an ITT analysis, then the LOCF data were used. When SDs were missing, we presented data descriptively.
Assessment of heterogeneity
Skewed data and non‐quantitative data were presented descriptively. An outcome whose minimum score is zero could be considered skewed when the mean was smaller than twice the SD. Heterogeneity between studies was investigated by the I2 statistic (Higgins 2003) (an I2 equal to or more than 50% was considered indicative of heterogeneity) and by visual inspection of the forest plots. We performed subgroup analyses to investigate heterogeneity (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Data from included studies were entered into a funnel plot (trial effect against trial variance) to investigate small‐study effects (Sterne 2000). We used the tests for funnel plot asymmetry only when there were at least 10 studies included in the meta‐analysis, and results were interpreted cautiously, with visual inspection of the funnel plots (Higgins 2011). When evidence of small‐study effects was identified, we investigated possible reasons for funnel plot asymmetry, including publication bias.
Data synthesis
For the primary analysis we used a random‐effects model OR, which had the highest generalisability in our empirical examination of summary effect measures for meta‐analyses (Furukawa 2002a). The robustness of this summary measure was routinely examined by checking the fixed‐effect model OR and the random‐effects model risk ratio (RR). Material differences between the models were reported. A P value of less than 0.05 and a 95% CI were considered statistically significant. Fixed‐effect analyses were performed routinely for the continuous outcomes as well, to investigate the effect of the choice of method on the estimates. Material differences between the models were reported. Skewed data and non‐quantitative data were presented descriptively. An outcome was considered skewed when the mean was smaller than twice the SD. In terms of change score, data were difficult to depict as skewed or not, as the possibility existed for negative values; therefore, we entered all of the results of this outcome into a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses for the primary outcome where possible, for the following a priori reasons. Results were interpreted with caution, since multiple comparisons could lead to false positive conclusions (Oxman 1992).
1. Citalopram dosing (fixed low dosage, fixed standard dosage, fixed high dosage; flexible low dosage, flexible standard dosage, flexible high dosage) Existing evidence implies that low dosage antidepressants may be associated with better outcomes ‐ both in terms of efficacy and side effects ‐ than standard or high dosage antidepressants (Bollini 1999; Furukawa 2002b). In addition, a fixed versus flexible dosing schedule may affect estimates of treatment effectiveness (Khan 2003). In the case of citalopram, based on the Defined Daily Dosage (DDD) by WHO (WHO 2009a), low dosage is referred to as < 20, standard dosage to >= 20 but < 40, and high dosage to >= 40 mg/day. We categorised studies by intended maximum dosage of citalopram.
2. Comparator dosing (low dosage, standard dosage, and high dosage) It is easy to imagine that people taking a comparator drug are less likely to complete a study if they are taking a high dosage of the comparator drug. We categorised studies by the intended maximum dose of the comparator based on the DDD.
3. Depression severity (severe major depression, moderate/mild major depression) "Severe major depression" was defined by a threshold baseline severity score for entry of 25 or more for HRSD and 31 or more for MADRS (Dozois 2004; Müller 2003).
4. Treatment settings (psychiatric in‐patients, psychiatric outpatients, primary care) Because depressive disorder in primary care has a different profile than that of psychiatric in‐patients or outpatients (Suh 1997), it is possible that results obtained from either of these settings may not be applicable to the other settings (Depression Guideline Panel 1993).
5. Elderly patients (>= 65 years of age), separately from other adult patients Older people may be more vulnerable to side effects associated with antidepressants and decreased dosage is often recommended for them (Depression Guideline Panel 1993).Because the number of a priori planned subgroup analyses now appears excessive in comparison with the identified studies, we will consider reducing the number of subgroup analyses or adjusting the level of significance to account for making multiple comparisons in the next update.
Sensitivity analysis
The following sensitivity analyses for primary outcome were planned a priori. By limiting the included studies to those with higher quality (analyses one to five) or to those free from some "bias" (analyses six to nine), we examined whether the results changed and we intended to check for the robustness of the observed findings.
We excluded trials with unclear concealment of random allocation and/or unclear double blinding.
We excluded trials with a dropout rate greater than 20%.
We performed the worst‐case scenario ITT: that all patients in the experimental group experienced the negative outcome and all those in the comparison group experienced the positive outcome.
We performed the best‐case scenario ITT: that all patients in the experimental group experienced the positive outcome and all those in the comparison group experienced the negative outcome.
We excluded trials for which the response rates had to be calculated based on the imputation method (Furukawa 2005) and for which the SD had to be borrowed from other trials (Furukawa 2006).
We examined a "wish bias" by comparing the trials where citalopram was used as an investigational drug, the drug that was used as a new compound, to the trials where citalopram was used as a comparator, since some evidence suggests that a new antidepressant might perform worse when used as a comparator than when used as an investigational agent (Barbui 2004).
We excluded trials funded by, or with at least one author affiliated with, a pharmaceutical company marketing citalopram. This sensitivity analysis is particularly important in light of the recent repeated findings that funding strongly affects outcomes of research studies (Als‐Nielsen 2003; Bhandari 2004; Lexchin 2003; Montgomery 2004; Perlis 2005; Procyshyn 2004) and because industry sponsorship and authorship of clinical trials have increased over the past 20 years (Buchkowsky 2004).
We excluded studies that included patients with bipolar depression.
We excluded trials that included patients with psychotic features.
Our routine application of random‐effects and fixed‐effect models, as well as our secondary outcomes of remission rates and continuous severity measures, may be considered additional forms of sensitivity analyses.
If the CIs of ORs in the groups did not overlap, potential sources of heterogeneity were investigated.
Results
Description of studies
See:Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
Initially, we identified 303 references. After reading the abstracts, 265 references were considered relevant for our review and retrieved for more detailed evaluation. The search found 37 additional studies written in Chinese. We commissioned a professional translator for the full translation of these papers. The translation process is still ongoing, so in the present review we considered all Chinese studies as awaiting assessment studies (we will include them in the next update of the review, which is expected to be in a two years time). An additional four studies were considered as awaiting assessment because the papers reported insufficient information to decide about inclusion or exclusion (Ahlfors 1988; Galecki 2004; Moeller 1986; Thomas 2008). We contacted corresponding authors and at the time the review has been submitted we are still waiting for their reply and further information. We identified two ongoing studies. Although the search was thorough, it is still possible that there are still unpublished studies which have not been identified.
Included studies
A total of 37 studies were included in this systematic review. Of these, four trials were unpublished (29060/785; Lu 10‐171, 83‐01; Lu 10‐171,79‐01; SCT‐MD‐02). Attempts to contact authors for additional information were successful in seven cases (with additional data provided by authors) and unsuccessful in 13.
Sample Size
The mean sample size per arm was 107 participants (range 17‐303). Sixteen studies recruited fewer than 100 participants overall.
Study design
The great majority of included studies were reported to be double‐blind (28 out of 37 RCTs, that is 75.6%).
Country
The great majority of included studies had been carried out in Europe or in the US (29 out of 37 RCTs, that is 78.4%). Two studies randomised patients in China (Hsu 2011; Ou 2010), three in India (Khanzode 2003; Lalit 2004; Matreja 2007) and one in Russia (Yevtushenko 2007).
Age
Four studies randomised only elderly patients (Allard 2004; Karlsson 2000; Kyle 1998; Navarro 2001) and 22 studies only patients aged between 18 and 65 years (59.4%). The remaining studies randomised both adult and elderly patients or it was unclear.
Diagnosis
Only three studies (8.1%) included patients with bipolar disorder (Bougerol 1997a; Hosak 1999; Timmerman 1993). As per protocol, RCTs were included in the present review only if patients with bipolar disorder were less than 20% in each study.
Setting/participants
Twenty trials enrolled only out‐patients, four studies only in‐patients (Andersen 1986; de Wilde 1985; Hosak 1999; Lu 10‐171,79‐01), seven recruited both in‐ and out‐patients (Bougerol 1997a; Gravem 1987; Karlsson 2000; Lu 10‐171, 83‐01; Navarro 2001; Ou 2010; Shaw 1986), three studies enrolled patients from general practice (Bougerol 1997b; Ekselius 1997; Lewis 2011). In the remaining three studies the setting was unclear. About two thirds of the participants were women. In 31 RCTs patients had a formal diagnosis of major depression (or major depressive disorder) according to DSM‐III, DSM‐III‐R, DSM‐IV or ICD‐10 criteria. In six studies the diagnosis was based on different standardized research criteria (i.e., Feighner criteria).
Interventions and comparators
We found RCTs comparing citalopram with TCAs (amitriptyline, imipramine and nortriptyline), tetracycles (mianserin and maprotiline), other SSRIs (escitalopram, fluoxetine, sertraline, fluvoxamine and paroxetine), one SNRI (namely, venlafaxine), one MAOI (moclobemide), other conventional ADs (mirtazapine and reboxetine) and also only one non‐conventional ADs (St John's wort, or hypericum). Hypericum, a member of the Hypericaceae family, has been used in folk medicine for a long time for a range of indications including depressive disorders. It is licensed and widely used in Germany for the treatment of depressive, anxiety and sleep disorders and in recent years it has also become increasingly popular in other European and non‐European countries (Linde 2008).
Details on the included studies are as follows: nine studies (overall 1277 participants) comparing citalopram with TCAs (four studies versus amitriptyline, two versus imipramine and two studies versus nortriptyline and one study versus clomipramine, respectively); three studies (overall 477 participants) comparing citalopram with tetracyclics (two studies versus mianserin and one study versus maprotiline); 18 studies (overall 4200 participants) comparing citalopram with SSRIs (seven studies versus escitalopram, four studies versus fluoxetine), four studies versus sertraline, one study versus fluvoxamine, one study versus paroxetine and one study versus either escitalopram or sertraline); six studies (overall 1137 participants) comparing citalopram with SNRIs (one study versus each of the following drugs: venlafaxine and mirtazapine), comparing citalopram with MAOI (one study versus moclobemide), comparing citalopram with other conventional psychotropic drugs (two studies versus reboxetine), comparing citalopram with non‐conventional antidepressants (one study versus hypericum).
There were four three‐arm trials: one study comparing citalopram (20 mg/day) with escitalopram 20 mg/day or escitalopram 10 mg/day; one study comparing citalopram (20‐60 mg/day) with amitriptyline (150‐300 mg/day) or fluoxetine (20‐60 mg/day); one study comparing citalopram 10‐30 mg/day with citalopram 20‐60 mg/day or imipramine (50‐150 mg/day); one study compared citalopram (20 mg/day) with escitalopram 10 mg/day or citalopram 10 mg/day. One four‐arm trial compared citalopram 20 mg/day with citalopram 40 mg/day or paroxetine controlled‐release 12.5 mg/day or paroxetine controlled‐release 25 mg/day.
Outcomes
Of the included 37 studies, one study (Andersen 1986) did not report efficacy data and one study reported split data according to different genotypes (Lewis 2011). We were not able to obtain further data for these trials because we could not contact the authors by any means and therefore, could not obtain extra information from these authors. By contrast, all 37 studies did report tolerability/acceptability data that could be entered into a meta‐analysis The great majority of the identified studies (34 out of 37 RCTs) used the MADRS or HRSD as the rating scale of choice for primary or secondary outcome measures. Among the 35 studies reporting dropouts due to any reason, 31 reported dropouts due to side effects. Twenty‐eight studies reported the number of patients experiencing individual side effects.
Excluded studies
Of the 265 references retrieved for more detailed evaluation, 214 articles did not meet our inclusion criteria and were excluded because of one of the following reasons: duplicate publications (eight articles), wrong diagnosis (24 articles), wrong population (51 articles), wrong comparison or intervention (63 articles) and non‐randomised or wrong design (68 articles). Fourteen additional studies were considered as awaiting assessment (overall we found 51 awaiting assessment studies ‐ see above).
Risk of bias in included studies
See: Included studies, Figure 1, Figure 2.
1.
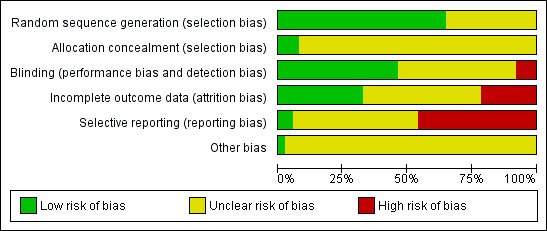
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
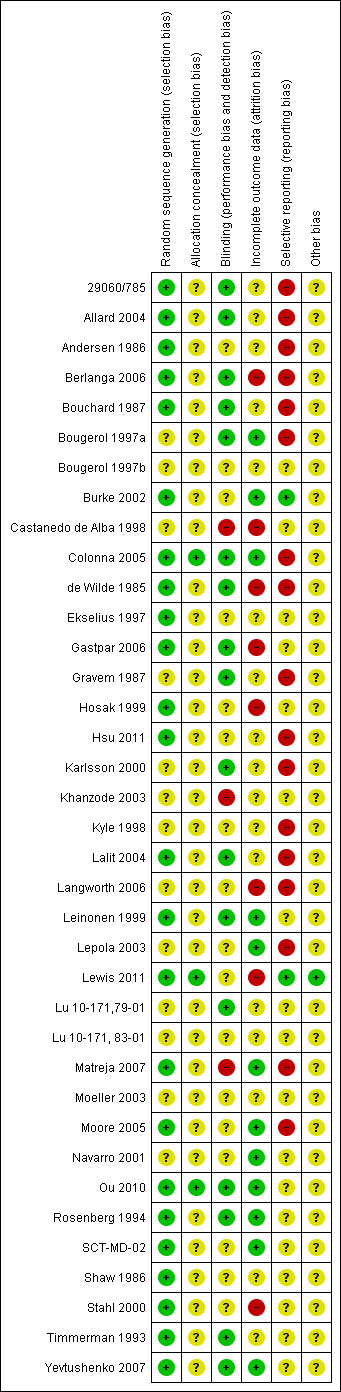
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Our judgment about the overall risk of bias in the individual studies is illustrated in Figure 1 and Figure 2. The methodological quality of these included studies was judged as poor, although judging articles from some time ago by today’s standard might be problematic (Begg 1996). Nevertheless, the reporting in these studies overall was not good. This type of reporting has been associated with an overestimate of the estimate of effect (Schulz 1995) and this should be considered when interpreting the results.
Allocation
The majority of studies reported the methods of generating random sequence, in which “a computer originated schedule” was used, however, only three studies reported enough details on allocation concealment (Colonna 2005; Lewis 2011; Ou 2010). We were not assured that bias was minimised during the allocation procedure in the other studies, yet the great majority of them reported that the participants allocated to each treatment group were “similar”, “the same”, “not significantly different”, “comparable” or “matched”.
Blinding
Thirty out of 37 RCTs (81.1%) described their design as “double‐blind”; however, no tests were conducted to ensure successful blinding. In the review we have included one “single‐blind” trial (Navarro 2001) which was rated as having a “high risk of bias” because it was unclear whether its outcome assessment was blinded to the medication. Four trials were open trials that did not seek blinding (Castanedo de Alba 1998; Hosak 1999; Lewis 2011; Matreja 2007) and in two studies the blinding was unclear (Moeller 2003; Ou 2010).
Incomplete outcome data
Total dropout rate was overall relatively high, ranging from 2% (Matreja 2007) to 56% (Stahl 2000). There were 23 studies (62.2%) where the total dropout rates were more than 20%.
Selective reporting
The study protocol was not available for almost all studies. Only six studies reported SDs of change scores (Burke 2002; Langworth 2006; Lepola 2003; Ou 2010; SCT‐MD‐02; Yevtushenko 2007); 10 studies (Allard 2004; Bouchard 1987; de Wilde 1985; Bougerol 1997a; Bougerol 1997b; Khanzode 2003; Lu 10‐171, 83‐01; Lu 10‐171,79‐01; Shaw 1986; Timmerman 1993) reported SDs of endpoint score of continuous efficacy variables.
Other potential sources of bias
Most of the included studies were funded by industry and only one study was clearly not funded by industry sponsor (Castanedo de Alba 1998). Among the trials comparing citalopram to TCAs or heterocyclics, the great majority (nine out of 11) were sponsored by, or had at least one author affiliated with, the pharmaceutical company marketing citalopram. Most of the studies comparing citalopram with other SSRIs (11 out of 16) were sponsored by the citalopram manufacturer, however, all the studies comparing citalopram with escitalopram (seven RCTs) were sponsored by their mutual manufacturer and in these studies citalopram was always considered as the reference drug. Among the six studies comparing citalopram with other ADs or non‐conventional antidepressant agents, only one was sponsored by the citalopram manufacturer (Berlanga 2006).
Effects of interventions
The included studies did not report on all the outcomes that were pre‐specified in the protocol of this review. Outcomes of clear relevance to patients and clinicians, in particular, patient's and their relatives' attitudes to treatment, their ability to return to work and resume normal social functioning, health‐related quality of life measures and costs to healthcare services were not reported in the included studies. Overall, 6147 patients were available for assessing efficacy (3183 participants randomised to citalopram and 3023 to another antidepressant) and 6960 for examining acceptability of treatments (3538 participants allocated to citalopram and 3378 to another antidepressant). Evidence of differences in efficacy, acceptability and tolerability was found and details are listed below. To obtain missing response rates and remission, we used validated imputation methods from continuous outcomes. We imputed SDs for some continuous outcomes of the following studies: Castanedo de Alba 1998; Colonna 2005; Ekselius 1997; Hosak 1999; Leinonen 1999; Moore 2005; Rosenberg 1994; Stahl 2000.
The results of the present systematic review were reported comparison by comparison (grouping them into different drug classes according to review protocol, see Methods section ‐ Types of interventions) and by outcome (following the review protocol ‐ for details see Imperadore 2007). The forest plots were organised according to the relevance of outcomes, as reported in the review protocol. For adverse events, all the retrieved information about the adverse events specified in the review protocol were reported (either statistically or non‐statistically significant). Remaining adverse events were only reported when statistically significant (non‐statistically significant results about adverse events are presented in Table 1).
1. Adverse events.
| Adverse event | Study | CItalopram | Comparator | Odds Ratio, Random [95% CI] | ||
| Events | Total | Events | Total | |||
| Citalopram versus TCAs | ||||||
| Citalopram vs amitriptyline | ||||||
| Asthenia | Shaw 1986 | 3 | 27 | 5 | 25 | 0.50 [0.11, 2.35] |
| Confusion | Shaw 1986 | 2 | 27 | 5 | 25 | 0.32 [0.06, 1.83] |
| Conjunctivitis | Gravem 1987 | 1 | 27 | 0 | 24 | 2.77 [0.11, 71.35] |
| Dermatological problems | Gravem 1987 | 1 | 27 | 1 | 24 | 0.88 [0.05, 14.96] |
| Dizziness | Gravem 1987; Kyle 1998; Shaw 1986 | 16 | 233 | 28 | 235 | 0.47 [0.15, 1.44] |
| Fatigue | Kyle 1998 | 6 | 179 | 11 | 186 | 0.55 [0.20, 1.53] |
| Gastrointestinal | Gravem 1987; Shaw 1986 | 3 | 54 | 6 | 49 | 0.45 [0.10, 2.07] |
| Headache | Gravem 1987; Hosak 1999; Kyle 1998; Shaw 1986 | 22 | 262 | 18 | 266 | 1.25 [0.65, 2.42] |
| Loss of hair | Gravem 1987 | 1 | 27 | 0 | 24 | 2.77 [0.11, 71.35] |
| Meteorism | Gravem 1987 | 0 | 27 | 1 | 24 | 0.28 [0.01, 7.33] |
| Palpitations | Gravem 1987; Shaw 1986 | 4 | 54 | 9 | 49 | 0.36 [0.10, 1.24] |
| Rash | Shaw 1986 | 1 | 27 | 1 | 25 | 0.92 [0.05, 15.59] |
| Restlessness | Gravem 1987; Shaw 1986 | 4 | 54 | 5 | 49 | 0.71 [0.18, 2.82] |
| Sweating | Gravem 1987 | 2 | 27 | 3 | 24 | 0.56 [0.09, 3.67] |
| Syncope | Gravem 1987 | 0 | 27 | 1 | 24 | 0.28 [0.01, 7.33] |
| Taste abnormalities | Gravem 1987 | 0 | 27 | 1 | 24 | 0.28 [0.01, 7.33] |
| Tremor | Gravem 1987 | 1 | 27 | 1 | 24 | 0.88 [0.05, 14.96] |
| Visual problems | Gravem 1987 | 0 | 27 | 3 | 24 | 0.11 [0.01, 2.28] |
| Citalopram vs imipramine | ||||||
| Asthenia | Lu 10‐171, 83‐01 | 2 | 22 | 3 | 21 | 0.60 [0.09, 4.01] |
| Dizziness | Lu 10‐171, 83‐01 | 7 | 22 | 12 | 21 | 0.35 [0.10, 1.22] |
| Gastrointestinal | Lu 10‐171, 83‐01 | 6 | 22 | 5 | 21 | 1.20 [0.30, 4.74] |
| Headache | Lu 10‐171, 83‐01 | 6 | 22 | 2 | 21 | 3.56 [0.63, 20.15] |
| Irritability | Rosenberg 1994 | 28 | 380 | 12 | 92 | 0.53 [0.26, 1.09] |
| Restlessness | Lu 10‐171, 83‐01 | 3 | 22 | 4 | 21 | 0.67 [0.13, 3.44] |
| Citalopram vs maprotiline | ||||||
| Appetite increased | Bouchard 1987 | 1 | 48 | 1 | 48 | 1.00 [0.06, 16.46] |
| Concentration decrease | Bouchard 1987 | 1 | 48 | 0 | 48 | 3.06 [0.12, 77.09] |
| Craving for sweets | Bouchard 1987 | 2 | 48 | 0 | 48 | 5.22 [0.24, 111.55] |
| Dermatological problems | Bouchard 1987 | 1 | 48 | 1 | 48 | 1.00 [0.06, 16.46] |
| Dizziness | Bouchard 1987 | 7 | 48 | 5 | 48 | 1.47 [0.43, 5.00] |
| Dyspepsia | Bouchard 1987 | 2 | 48 | 1 | 48 | 2.04 [0.18, 23.32] |
| Dyspnea | Bouchard 1987 | 0 | 48 | 1 | 48 | 0.33 [0.01, 8.22] |
| Feeling of numbness | Bouchard 1987 | 2 | 48 | 0 | 48 | 5.22 [0.24, 111.55] |
| Headache | Bouchard 1987 | 6 | 48 | 3 | 48 | 2.14 [0.50, 9.12] |
| Hypertonia | Bouchard 1987 | 1 | 48 | 1 | 48 | 1.00 [0.06, 16.46] |
| Increased salivation | Bouchard 1987 | 1 | 48 | 0 | 48 | 3.06 [0.12, 77.09] |
| Nasal congestion | Bouchard 1987 | 1 | 48 | 1 | 48 | 1.00 [0.06, 16.46] |
| Orthostatic symptoms | Bouchard 1987 | 3 | 48 | 3 | 48 | 1.00 [0.19, 5.22] |
| Restlessness | Bouchard 1987 | 1 | 48 | 5 | 48 | 0.18 [0.02, 1.63] |
| Sweating | Bouchard 1987 | 8 | 48 | 4 | 48 | 2.20 [0.62, 7.87] |
| Tachycardia | Bouchard 1987 | 3 | 48 | 5 | 48 | 0.57 [0.13, 2.55] |
| Taste abnormalities | Bouchard 1987 | 1 | 48 | 0 | 48 | 3.06 [0.12, 77.09] |
| Tremor | Bouchard 1987 | 5 | 48 | 8 | 48 | 0.58 [0.18, 1.93] |
| Visual problems | Bouchard 1987 | 1 | 48 | 0 | 48 | 3.06 [0.12, 77.09] |
| Yawning | Bouchard 1987 | 1 | 48 | 0 | 48 | 3.06 [0.12, 77.09] |
| Citalopram vs nortriptyline | ||||||
| Confusion | Lu 10‐171,79‐01 | 1 | 17 | 0 | 18 | 3.36 [0.13, 88.39] |
| Headache | Lu 10‐171,79‐01 | 5 | 17 | 5 | 18 | 1.08 [0.25, 4.70] |
| Palpitations | Lu 10‐171,79‐01 | 4 | 17 | 4 | 18 | 1.08 [0.22, 5.22] |
| Pruritus | Lu 10‐171,79‐01 | 5 | 17 | 3 | 18 | 2.08 [0.41, 10.53] |
| Citalopram versus heterocyclics | ||||||
| Citalopram vs mianserin | ||||||
| Back pain | Karlsson 2000 | 6 | 163 | 10 | 173 | 0.62 [0.22, 1.75] |
| Dizziness | Karlsson 2000 | 4 | 163 | 10 | 173 | 0.41 [0.13, 1.33] |
| Headache | Karlsson 2000 | 12 | 163 | 12 | 173 | 1.07 [0.46, 2.45] |
| Pain (general) | Karlsson 2000 | 6 | 163 | 9 | 173 | 0.70 [0.24, 2.00] |
| Citalopram versus other SSRIs | ||||||
| Citalopram vs escitalopram | ||||||
| Abdominal pain | Moore 2005 | 1 | 152 | 0 | 142 | 2.82 [0.11, 69.84] |
| Accidental injury | Colonna 2005 | 4 | 182 | 10 | 175 | 0.37 [0.11, 1.21] |
| Aggressive behaviour | Moore 2005 | 0 | 152 | 1 | 142 | 0.31 [0.01, 7.65] |
| Anorexia | Ou 2010; Yevtushenko 2007 | 2 | 225 | 4 | 223 | 0.64 [0.06, 7.29] |
| Asthenia | Moore 2005 | 2 | 152 | 2 | 142 | 0.93 [0.13, 6.72] |
| Back pain | Colonna 2005; SCT‐MD‐02 | 14 | 305 | 12 | 300 | 1.36 [0.34, 5.51] |
| Breast surgery | Moore 2005 | 1 | 152 | 0 | 142 | 2.82 [0.11, 69.84] |
| Bronchitis | Colonna 2005 | 3 | 182 | 10 | 175 | 0.28 [0.07, 1.02] |
| Chest pain | Moore 2005 | 1 | 152 | 0 | 142 | 2.82 [0.11, 69.84] |
| Chicken pox | Moore 2005 | 0 | 152 | 1 | 142 | 0.31 [0.01, 7.65] |
| Dermatological problems | Yevtushenko 2007 | 2 | 110 | 1 | 109 | 2.00 [0.18, 22.38] |
| Dizziness | Moore 2005; Ou 2010; SCT‐MD‐02; Yevtushenko 2007 | 11 | 502 | 17 | 491 | 0.69 [0.28, 1.71] |
| Dyspepsia | Yevtushenko 2007 | 1 | 110 | 0 | 109 | 3.00 [0.12, 74.45] |
| Enuresis | Moore 2005 | 0 | 152 | 1 | 142 | 0.31 [0.01, 7.65] |
| Exacerbation of depression | Moore 2005 | 1 | 152 | 0 | 142 | 2.82 [0.11, 69.84] |
| Gastrointestinal | Ou 2010 | 14 | 117 | 16 | 115 | 0.84 [0.39, 1.81] |
| Headache | Colonna 2005; Moore 2005; SCT‐MD‐02; Yevtushenko 2007 | 45 | 567 | 46 | 551 | 0.96 [0.49, 1.88] |
| Hot flash | Moore 2005 | 0 | 152 | 1 | 142 | 0.31 [0.01, 7.65] |
| Memory impairment | Moore 2005 | 2 | 152 | 0 | 142 | 4.73 [0.23, 99.47] |
| Palpitations | Moore 2005 | 0 | 152 | 1 | 142 | 0.31 [0.01, 7.65] |
| Panic attack | Moore 2005 | 1 | 152 | 0 | 142 | 2.82 [0.11, 69.84] |
| Pharyngitis | Moore 2005 | 0 | 152 | 1 | 142 | 0.31 [0.01, 7.65] |
| Pruritus | Moore 2005 | 1 | 152 | 0 | 142 | 2.82 [0.11, 69.84] |
| Rash | Yevtushenko 2007 | 1 | 110 | 0 | 109 | 3.00 [0.12, 74.45] |
| Rhinitis | Colonna 2005; Lepola 2003; SCT‐MD‐02 | 24 | 466 | 28 | 456 | 0.87 [0.40, 1.87] |
| Sexual problems: erectile dysfunction | Lepola 2003 | 0 | 161 | 2 | 156 | 0.19 [0.01, 4.02] |
| Sexual problems: increased sexual desire | SCT‐MD‐02 | 9 | 123 | 8 | 125 | 1.15 [0.43, 3.10] |
| Sexual problems: other | Burke 2002; Moore 2005; SCT‐MD‐02; Yevtushenko 2007 | 16 | 452 | 31 | 563 | 0.72 [0.36, 1.43] |
| Sweating | Lepola 2003; Moore 2005; SCT‐MD‐02 | 13 | 436 | 15 | 423 | 0.83 [0.39, 1.78] |
| Tachycardia | SCT‐MD‐02 | 0 | 123 | 1 | 125 | 0.34 [0.01, 8.33] |
| Tremor | Moore 2005 | 0 | 152 | 4 | 142 | 0.10 [0.01, 1.89] |
| Upper respiratory tract infection | SCT‐MD‐02 | 8 | 123 | 12 | 125 | 0.66 [0.26, 1.66] |
| Visual problems | Moore 2005 | 1 | 152 | 0 | 142 | 2.82 [0.11, 69.84] |
| Weight gain | Colonna 2005; Moore 2005 | 15 | 334 | 12 | 317 | 1.21 [0.55, 2.64] |
| Citalopram vs fluoxetine | ||||||
| Abdominal pain | Bougerol 1997a; Bougerol 1997b | 16 | 331 | 10 | 342 | 1.57 [0.55, 4.53] |
| Back pain | Bougerol 1997b | 5 | 173 | 0 | 184 | 12.04 [0.66, 219.46] |
| Bronchitis | Bougerol 1997b | 5 | 173 | 7 | 184 | 0.75 [0.23, 2.42] |
| Decreased weight | Bougerol 1997a; Bougerol 1997b | 13 | 331 | 22 | 342 | 0.62 [0.25, 1.50] |
| Headache | Bougerol 1997a; Bougerol 1997b; Hosak 1999 | 25 | 360 | 28 | 372 | 0.90 [0.51, 1.60] |
| Influenza‐like symptoms | Bougerol 1997b | 2 | 173 | 6 | 184 | 0.35 [0.07, 1.74] |
| Nervousness | Bougerol 1997a | 6 | 158 | 5 | 158 | 1.21 [0.36, 4.04] |
| Pruritus | Bougerol 1997a | 2 | 158 | 5 | 158 | 0.39 [0.07, 2.05] |
| Sweating | Bougerol 1997a | 6 | 158 | 2 | 158 | 3.08 [0.61, 15.49] |
| Tension | Bougerol 1997a | 6 | 158 | 6 | 158 | 1.00 [0.32, 3.17] |
| Vertigo | Bougerol 1997a | 7 | 158 | 3 | 158 | 2.40 [0.61, 9.43] |
| Citalopram vs paroxetine | ||||||
| Asthenia | 29060/785 | 36 | 207 | 22 | 199 | 1.69 [0.96, 3.00] |
| Headache | 29060/785 | 54 | 207 | 44 | 199 | 1.24 [0.79, 1.96] |
| Sexual problems: other | 29060/785 | 13 | 207 | 11 | 199 | 1.15 [0.50, 2.62] |
| Syncope | 29060/785 | 1 | 207 | 0 | 199 | 2.90 [0.12, 71.57] |
| Citalopram vs sertraline | ||||||
| Asthenia | Ekselius 1997 | 3 | 200 | 6 | 200 | 0.49 [0.12, 2.00] |
| Concentration decrease | Ekselius 1997 | 1 | 200 | 2 | 200 | 0.50 [0.04, 5.53] |
| Decreased weight | Ekselius 1997 | 19 | 200 | 9 | 200 | 2.23 [0.98, 5.05] |
| Dermatological problems | Ekselius 1997 | 6 | 200 | 5 | 200 | 1.21 [0.36, 4.02] |
| Dizziness | Ekselius 1997 | 14 | 200 | 14 | 200 | 1.00 [0.46, 2.16] |
| Emotional indifference | Ekselius 1997 | 2 | 200 | 1 | 200 | 2.01 [0.18, 22.35] |
| Forgetfulness | Ekselius 1997 | 7 | 200 | 4 | 200 | 1.78 [0.51, 6.17] |
| Gastrointestinal | Ekselius 1997 | 5 | 200 | 12 | 200 | 0.40 [0.14, 1.16] |
| Headache | Ekselius 1997 | 13 | 200 | 18 | 200 | 0.70 [0.33, 1.48] |
| Increased salivation | Ekselius 1997 | 1 | 200 | 0 | 200 | 3.02 [0.12, 74.46] |
| Palpitations | Ekselius 1997 | 8 | 200 | 6 | 200 | 1.35 [0.46, 3.96] |
| Sexual problems: anorgasmia | Ekselius 1997 | 24 | 200 | 13 | 200 | 1.96 [0.97, 3.97] |
| Sexual problems: erectile dysfunction | Ekselius 1997 | 7 | 200 | 3 | 200 | 2.38 [0.61, 9.34] |
| Sexual problems: increased sexual desire | Ekselius 1997 | 14 | 200 | 7 | 200 | 2.08 [0.82, 5.26] |
| Sexual problems: loss of sexual interest | Ekselius 1997 | 16 | 200 | 19 | 200 | 0.83 [0.41, 1.66] |
| Sexual problems: other | Ekselius 1997 | 13 | 200 | 8 | 200 | 1.67 [0.68, 4.12] |
| Sweating | Ekselius 1997 | 34 | 200 | 26 | 200 | 1.37 [0.79, 2.38] |
| Tension | Ekselius 1997 | 7 | 200 | 6 | 200 | 1.17 [0.39, 3.55] |
| Visual problems | Ekselius 1997 | 6 | 200 | 11 | 200 | 0.53 [0.19, 1.47] |
| Weight gain | Ekselius 1997 | 26 | 200 | 30 | 200 | 0.85 [0.48, 1.49] |
| Citalopram versus other antidepressants | ||||||
| Citalopram vs mirtazapine | ||||||
| Dizziness | Leinonen 1999 | 6 | 133 | 12 | 137 | 0.49 [0.18, 1.35] |
| Fatigue | Leinonen 1999 | 18 | 133 | 17 | 137 | 1.10 [0.54, 2.25] |
| Headache | Leinonen 1999 | 19 | 133 | 13 | 137 | 1.59 [0.75, 3.37] |
| Influenza‐like symptoms | Leinonen 1999 | 3 | 133 | 7 | 137 | 0.43 [0.11, 1.69] |
| Citalopram vs moclobemide | ||||||
| Gastrointestinal | Castanedo de Alba 1998 | 6 | 22 | 5 | 20 | 1.13 [0.28, 4.47] |
| Headache | Castanedo de Alba 1998 | 0 | 22 | 2 | 20 | 0.16 [0.01, 3.64] |
| Sexual problems: loss of sexual interest | Castanedo de Alba 1998 | 0 | 22 | 1 | 20 | 0.29 [0.01, 7.51] |
| Tremor | Castanedo de Alba 1998 | 2 | 22 | 1 | 20 | 1.90 [0.16, 22.72] |
| Citalopram vs reboxetine | ||||||
| Concentration decrease | Langworth 2006 | 2 | 176 | 3 | 181 | 0.68 [0.11, 4.13] |
| Confusion | Langworth 2006 | 1 | 176 | 2 | 181 | 0.51 [0.05, 5.69] |
| Decreased weight | Langworth 2006 | 1 | 176 | 8 | 181 | 0.12 [0.02, 1.00] |
| Dizziness | Berlanga 2006 | 13 | 54 | 14 | 47 | 0.75 [0.31, 1.81] |
| Emotional indifference | Langworth 2006 | 1 | 176 | 4 | 181 | 0.25 [0.03, 2.28] |
| Headache | Berlanga 2006; Langworth 2006 | 17 | 230 | 27 | 228 | 0.50 [0.25, 1.00] |
| Increased dream activity | Langworth 2006 | 5 | 176 | 10 | 181 | 0.50 [0.17, 1.49] |
| Increased salivation | Langworth 2006 | 0 | 176 | 2 | 181 | 0.20 [0.01, 4.27] |
| Influenza‐like symptoms | Langworth 2006 | 9 | 176 | 8 | 181 | 1.17 [0.44, 3.09] |
| Memory impairment | Langworth 2006 | 2 | 176 | 3 | 181 | 0.68 [0.11, 4.13] |
| Orthostatic symptoms | Langworth 2006 | 4 | 176 | 9 | 181 | 0.44 [0.13, 1.47] |
| Paraesthesia | Langworth 2006 | 5 | 176 | 5 | 181 | 1.03 [0.29, 3.62] |
| Rash | Langworth 2006 | 2 | 176 | 4 | 181 | 0.51 [0.09, 2.81] |
| Sexual problems: loss of sexual interest | Langworth 2006 | 5 | 176 | 3 | 181 | 1.73 [0.41, 7.37] |
| Tachycardia | Langworth 2006 | 3 | 176 | 3 | 181 | 1.03 [0.20, 5.17] |
| Tension | Langworth 2006 | 4 | 176 | 3 | 181 | 1.38 [0.30, 6.26] |
| Tremor | Langworth 2006 | 2 | 176 | 5 | 181 | 0.40 [0.08, 2.11] |
| Upper respiratory tract infection | Langworth 2006 | 8 | 176 | 5 | 181 | 1.68 [0.54, 5.23] |
| Vertigo | Langworth 2006 | 10 | 176 | 8 | 181 | 1.30 [0.50, 3.38] |
| Visual problems | Langworth 2006 | 1 | 176 | 4 | 181 | 0.25 [0.03, 2.28] |
| Weight gain | Berlanga 2006; Langworth 2006 | 21 | 230 | 8 | 228 | 2.37 [0.61, 9.19] |
| Citalopram vs venlafaxine XR | ||||||
| Common cold | Allard 2004 | 2 | 75 | 3 | 76 | 0.67 [0.11, 4.11] |
| Dizziness | Allard 2004 | 3 | 75 | 4 | 76 | 0.75 [0.16, 3.47] |
| Citalopram vs hypericum (St. John's wort) | ||||||
| Dermatological problems | Gastpar 2006 | 6 | 127 | 4 | 131 | 1.57 [0.43, 5.72] |
| Infection | Gastpar 2006 | 17 | 127 | 20 | 131 | 0.86 [0.43, 1.72] |
| Musculoskeletal and connective tissue disorders | Gastpar 2006 | 5 | 127 | 6 | 131 | 0.85 [0.25, 2.87] |
1. CITALOPRAM versus TCAs
PRIMARY OUTCOME
EFFICACY ‐ Number of patients who responded to treatment (six to 12 weeks)
The analysis found no difference in terms of efficacy between citalopram and TCAs in total (OR 1.10, 95% CI 0.75 to 1.63, P = 0.62; 3 trials, 888 participants) nor in head‐to‐head comparisons (Analysis 1.1).
1.1. Analysis.
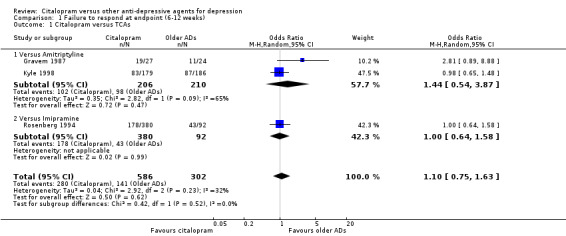
Comparison 1 Failure to respond at endpoint (6‐12 weeks), Outcome 1 Citalopram versus TCAs.
SECONDARY OUTCOMES
1) EFFICACY ‐ Number of patients who responded to treatment
a) Early response (one to four weeks)
There was no evidence that citalopram was more effective than TCAs in total in terms of early response (OR 0.95, 95% CI 0.46 to 1.98, P = 0.90; 4 trials, 751 participants) (Analysis 2.1). In head‐to‐head comparisons citalopram was more efficacious than imipramine (OR 0.45, 95% CI 0.24 to 0.86, P = 0.01; one trial, 275 participants; NNTB 4, 95% CI 4 to 25) (Analysis 2.1).
2.1. Analysis.
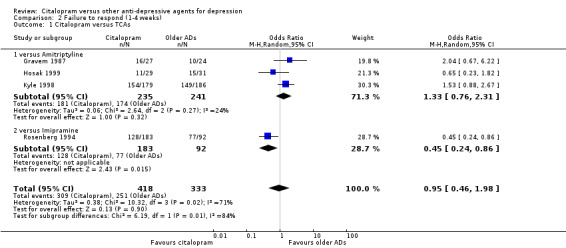
Comparison 2 Failure to respond (1‐4 weeks), Outcome 1 Citalopram versus TCAs.
b) Follow‐up response (16 to 24 weeks)
There was no evidence that citalopram was more effective than imipramine (Analysis 3.1).
3.1. Analysis.
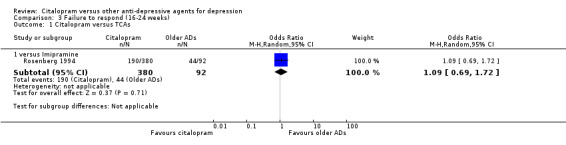
Comparison 3 Failure to respond (16‐24 weeks), Outcome 1 Citalopram versus TCAs.
2) EFFICACY ‐ Number of patients who remitted
a) Acute phase treatment (six to 12 weeks)
There was no difference between citalopram and TCAs, neither as a group (5 trials, 256 participants) nor as individual drugs in terms of remission (Analysis 5.1).
5.1. Analysis.
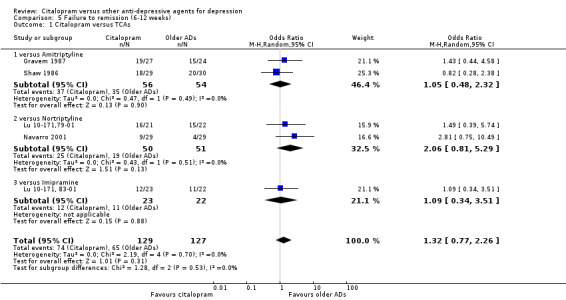
Comparison 5 Failure to remission (6‐12 weeks), Outcome 1 Citalopram versus TCAs.
b) Early remission (one to four weeks)
There was no difference between citalopram and TCAs, neither as a group (3 trials, 225 participants) nor as individual drugs (see Analysis 4.1).
4.1. Analysis.
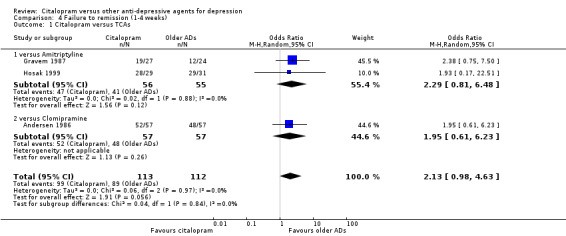
Comparison 4 Failure to remission (1‐4 weeks), Outcome 1 Citalopram versus TCAs.
c) Follow‐up remission (16 to 24 weeks)
No data available.
3) EFFICACY ‐ Mean change from baseline
a) Acute phase treatment: between six and 12 weeks
Using rating scale scores, there was no evidence that citalopram was different from TCAs, neither as a group (5 trials, 402 participants) nor as individual drugs (see Analysis 8.1).
8.1. Analysis.
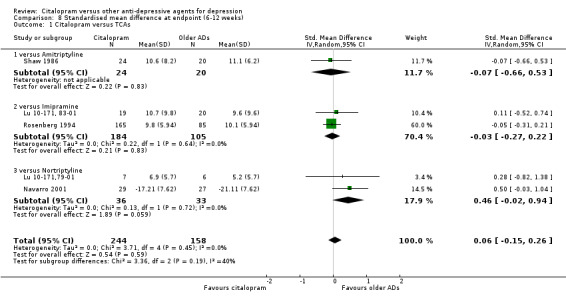
Comparison 8 Standardised mean difference at endpoint (6‐12 weeks), Outcome 1 Citalopram versus TCAs.
b) Early response (one to four weeks)
There was no difference between citalopram and TCAs neither individually nor as a class (see Analysis 7.1).
7.1. Analysis.
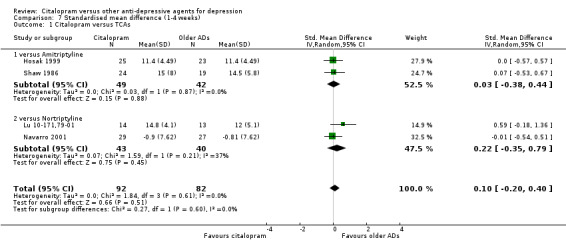
Comparison 7 Standardised mean difference (1‐4 weeks), Outcome 1 Citalopram versus TCAs.
c) Follow‐up response (16 to 24 weeks)
There was no evidence that citalopram was less effective than imipramine (Analysis 9.1).
9.1. Analysis.
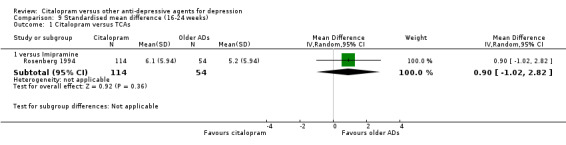
Comparison 9 Standardised mean difference (16‐24 weeks), Outcome 1 Citalopram versus TCAs.
4) EFFICACY‐ Social adjustment, social functioning, health‐related quality of life, costs to healthcare services
No data available.
5) ACCEPTABILITY ‐ Dropout rate
a) No statistically significant difference was found between citalopram and TCAs in terms of discontinuation due to any cause. However, even though not significant, we observed a trend in favour of citalopram (OR 0.81 95% CI 0.61 to 1.07, P = 0.14; 8 studies, 1209 participants) (Analysis 10.1).
10.1. Analysis.
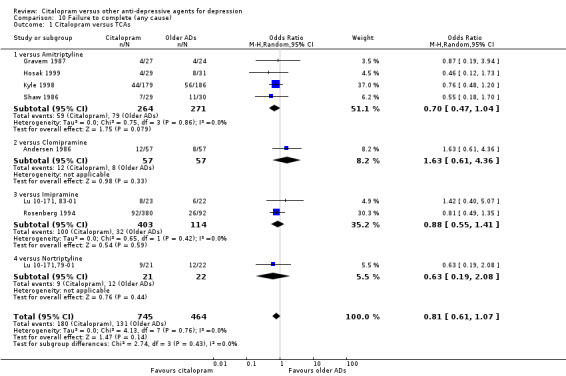
Comparison 10 Failure to complete (any cause), Outcome 1 Citalopram versus TCAs.
b) No differences were found in terms of discontinuation due to inefficacy (Analysis 12.1).
12.1. Analysis.
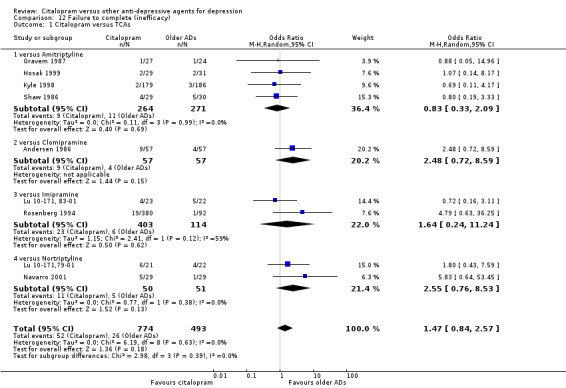
Comparison 12 Failure to complete (inefficacy), Outcome 1 Citalopram versus TCAs.
c) Differences were found in terms of discontinuation due to side effects: patients allocated to citalopram were less likely to withdraw than patients allocated to amitriptyline (OR 0.54, 95% CI 0.34 to 0.87, P = 0.01; 3 studies, 484 participants; NNTH 10, 95% CI 6 to 34) and to TCAs as a group (OR 0.54, 95% CI 0.38 to 0.78, P = 0.001; 8 studies, 1216 participants; NNTH 15, 95% CI 9 to 25) (Analysis 11.1; Figure 3)
11.1. Analysis.
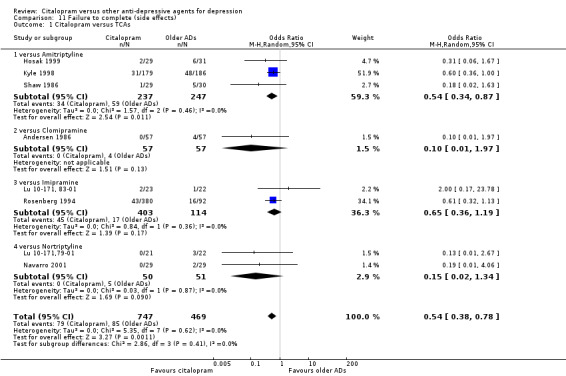
Comparison 11 Failure to complete (side effects), Outcome 1 Citalopram versus TCAs.
3.
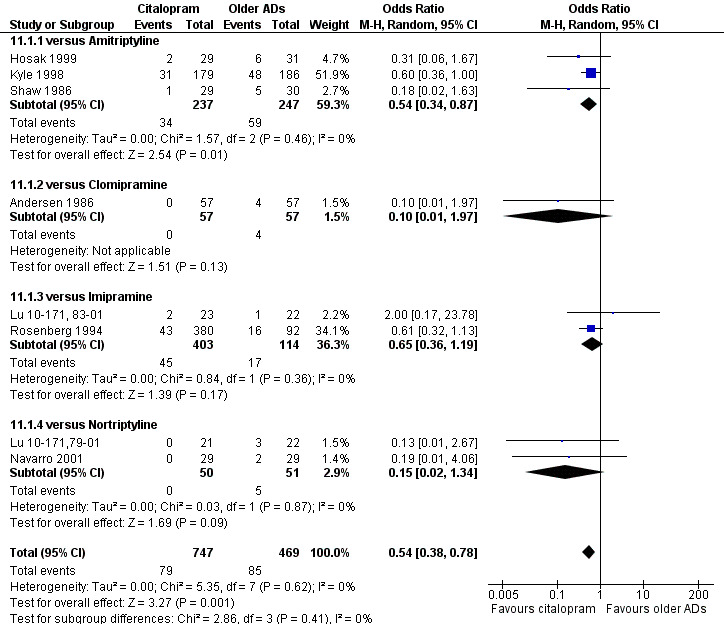
Forest plot of comparison: 11 Failure to complete (side effects), outcome: 11.1 Citalopram versus TCAs.
6) TOLERABILITY
Total number of patients experiencing at least some side effects.
There was evidence that citalopram was associated with a lower rate of adverse events than amitriptyline (OR 0.43, 95% CI 0.28 to 0.65, P < 0.0001; 4 studies, 528 participants; NNTH 8, 95% CI 5 to 15 ‐ Analysis 13.1) and with a higher rate of adverse events than imipramine (OR 1.82, 95% CI 1.14 to 2.89, P = 0.01; 2 studies 517 participants ‐ Analysis 13.1). By contrast, there was no evidence that citalopram was associated with a smaller or higher rate of adverse events than nortriptyline (OR 0.94, 95% CI 0.20 to 4.39; 1 study 43 participants ‐ Analysis 13.1).
13.1. Analysis.
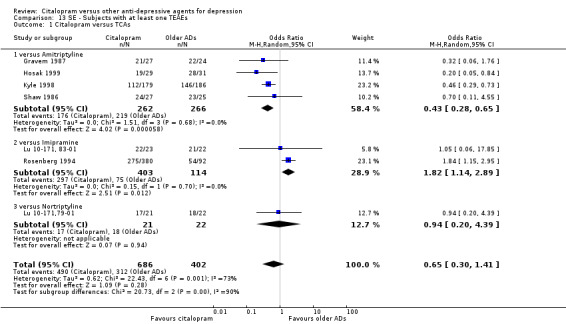
Comparison 13 SE ‐ Subjects with at least one TEAEs, Outcome 1 Citalopram versus TCAs.
Number of patients experiencing specific side effects (only figures for statistically significant differences were reported in the text)
a) Anxiety/agitation
There was no evidence that citalopram was associated with a lower rate of participants experiencing agitation/anxiety than nortriptyline (Analysis 18.1).
18.1. Analysis.
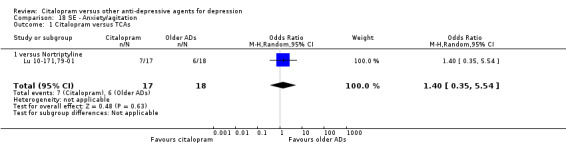
Comparison 18 SE ‐ Anxiety/agitation, Outcome 1 Citalopram versus TCAs.
b) Constipation
There was evidence that citalopram was associated with a lower rate of participants experiencing constipation than TCAs (OR 0.36, 95% CI 0.24 to 0.55, P < 0.00001; 6 trials, 1018 participants; NNTH 10, 95% CI 6 to 34 ‐ Analysis 30.1). In head‐to‐head comparison, the difference was statistically significant in favour of citalopram when compared with amitriptyline (OR 0.46, 95% CI 0.23 to 0.90, P = 0.02; 3 studies, 468 participants ‐ Analysis 30.1) and imipramine (OR 0.31, 95% CI 0.18 to 0.53, P < 0.0001; 2 studies, 515 participants; NNTH 7, 95% CI 4 to 15 ‐ Analysis 30.1), respectively.
30.1. Analysis.
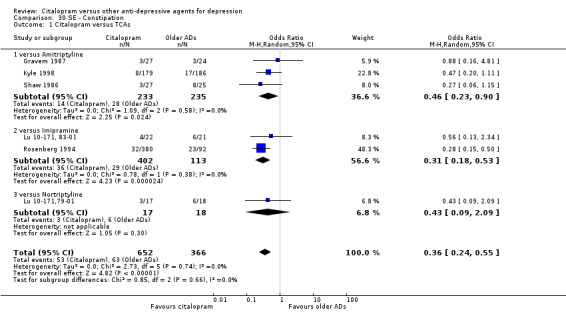
Comparison 30 SE ‐ Constipation, Outcome 1 Citalopram versus TCAs.
c) Diarrohea
There was no evidence that citalopram was associated with a different rate of participants experiencing diarrhoea than amitriptyline or imipramine (Analysis 34.1).
34.1. Analysis.
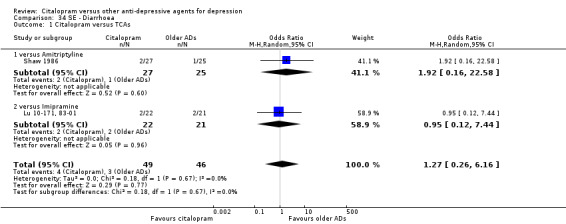
Comparison 34 SE ‐ Diarrhoea, Outcome 1 Citalopram versus TCAs.
d) Dry mouth
There was evidence that citalopram was associated with a lower rate of participants experiencing dry mouth than TCAs (OR 0.25, 95% CI 0.18 to 0.35, P < 0.00001; 7 trials, 1078 participants; NNTH 4, 95% CI 3 to 5 ‐ Analysis 36.1). In head‐to‐head comparisons, the difference between citalopram and imipramine was statistically significant in favour of citalopram (OR 0.32, 95% CI 0.21 to 0.50, P < 0.00001; 2 trials, 515 participants; NNTH 4, 95% CI 3 to 7); furthermore, citalopram was associated with a lower rate of patients experiencing dry mouth than amitriptyline (OR 0.17, 95% CI 0.10 to 0.28, P < 0.00001; 4 trials, 528 participants; NNTH 4, 95% CI 3 to 5 ‐ Analysis 36.1).
36.1. Analysis.
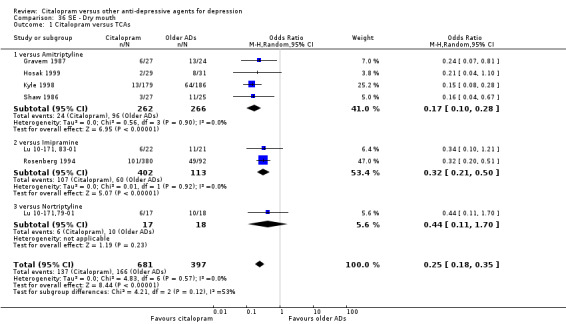
Comparison 36 SE ‐ Dry mouth, Outcome 1 Citalopram versus TCAs.
e) Hypotension
Citalopram was associated with lower rate of patients experiencing hypotension than imipramine (OR 0.38, 95% CI 0.19 to 0.75, P = 0.005; 1 trial, 472 participants ‐ Analysis 49.1).
49.1. Analysis.
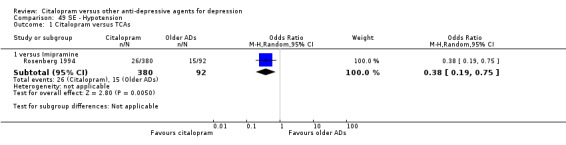
Comparison 49 SE ‐ Hypotension, Outcome 1 Citalopram versus TCAs.
f) Insomnia
There was no evidence that citalopram was associated with a higher rate of participants experiencing insomnia than TCAs (Analysis 54.1).
54.1. Analysis.
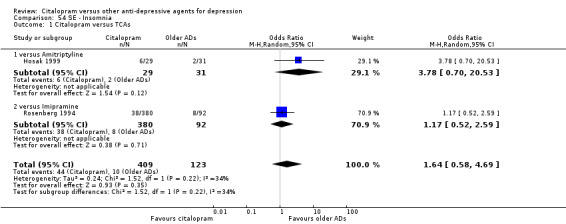
Comparison 54 SE ‐ Insomnia, Outcome 1 Citalopram versus TCAs.
g) Nausea/vomiting
There was evidence that citalopram was associated with a higher rate of participants experiencing nausea than amitriptyline (OR 2.44, 95% CI 1.27 to 4.66, P = 0.007; 3 trials, 477 participants ‐ Analysis 61.1) and nortriptyline (OR 7.11, 95% CI 1.23 to 40.98; 1 trial, 35 participants ‐ Analysis 61.1 ).
61.1. Analysis.
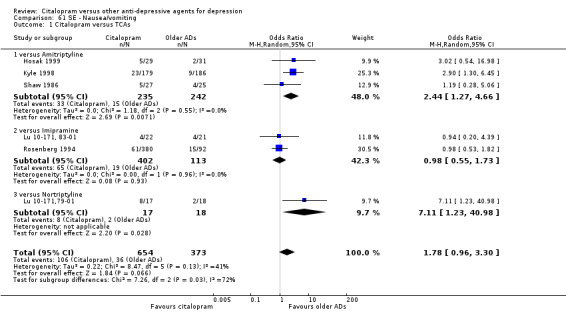
Comparison 61 SE ‐ Nausea/vomiting, Outcome 1 Citalopram versus TCAs.
h) Sedation/drowsiness
In head‐to‐head comparisons, citalopram was associated with a lower rate of patients experiencing sedation/drowsiness than amitriptyline (OR 0.25, 95% CI 0.09 to 0.70, P = 0.009; 2 studies, 112 participants ‐ Analysis 72.1).
72.1. Analysis.
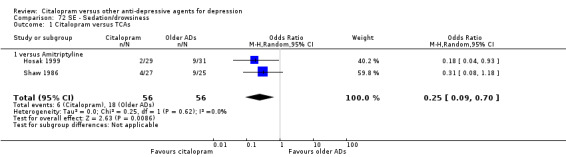
Comparison 72 SE ‐ Sedation/drowsiness, Outcome 1 Citalopram versus TCAs.
i) Sleepiness/somnolence
There was evidence that citalopram was associated with a lower rate of participants experiencing sleepiness/somnolence than TCAs (OR 0.49, 95% CI 0.33 to 0.74, P = 0.0006; 5 trials, 966 participants ‐ Analysis 76.1). In head‐to‐head comparisons, the difference between citalopram and amitriptyline was statistically significant in favour of citalopram (OR 0.45, 95% CI 0.24 to 0.85, P < 0.00001; 2 trials, 416 participants); furthermore, citalopram was associated with a lower rate of patients experiencing sleepiness than imipramine (OR 0.48, 95% CI 0.27 to 0.83, P = 0.009; 2 studies, 515 participants ‐ Analysis 76.1).
76.1. Analysis.
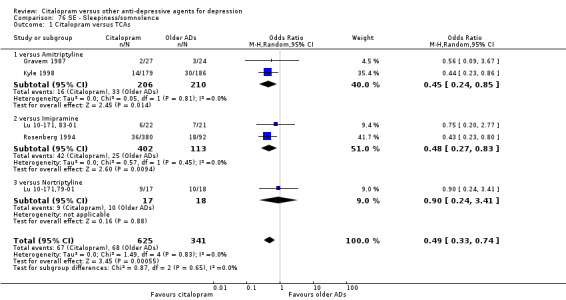
Comparison 76 SE ‐ Sleepiness/somnolence, Outcome 1 Citalopram versus TCAs.
j) Urination problems
There was no evidence that citalopram was associated with a lower rate of participants experiencing urination problems than TCAs (Analysis 83.1).
83.1. Analysis.
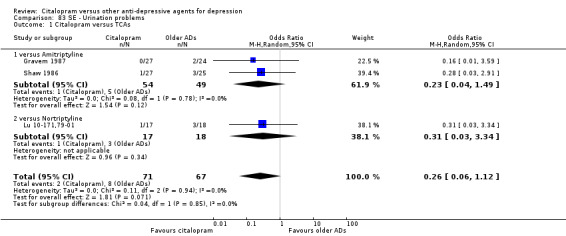
Comparison 83 SE ‐ Urination problems, Outcome 1 Citalopram versus TCAs.
k) Suicide wishes/gestures/attempts
There was no difference between citalopram and TCAs, neither as a group nor as individual drugs (Analysis 89.1).
89.1. Analysis.
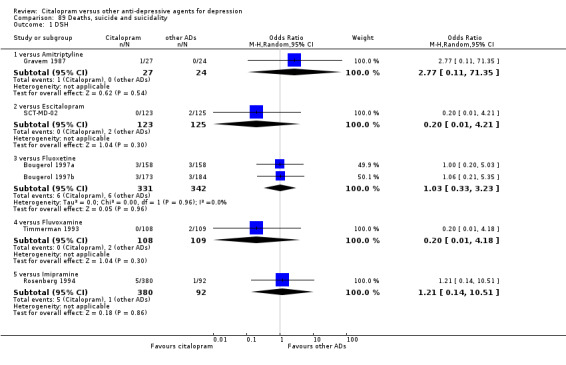
Comparison 89 Deaths, suicide and suicidality, Outcome 1 DSH.
l) Deaths (all cause)/Completed suicide
There was no difference between citalopram and imipramine (Analysis 89.3; Analysis 89.4).
89.3. Analysis.
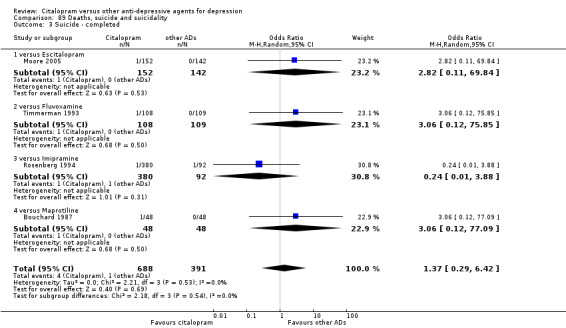
Comparison 89 Deaths, suicide and suicidality, Outcome 3 Suicide ‐ completed.
89.4. Analysis.
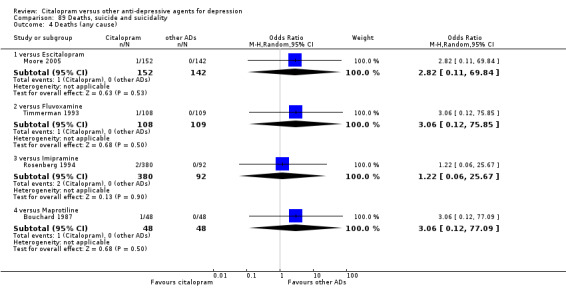
Comparison 89 Deaths, suicide and suicidality, Outcome 4 Deaths (any cause).
m) Other adverse events
Citalopram was associated with a lower rate of participants experiencing sweating (OR 0.50, 95% CI 0.30 to 0.83, P = 0.007; two studies, 515 participants ‐ Analysis 77.1), tachycardia (OR 0.36, 95% CI 0.13 to 0.99, P = 0.05; 2 trials, 515 participants ‐ Analysis 79.1), tremor (OR 0.45, 95% CI 0.25 to 0.80, P = 0.007; 2 studies, 515 participants ‐ Analysis 82.1) and visual problems (OR 0.23, 95% CI 0.06 to 0.84, P = 0.03; 1 study, 43 participants ‐ Analysis 86.1) than imipramine. Citalopram was associated with a lower rate of participants experiencing visual problems (OR 0.14, 95% CI 0.02 to 0.82, P = 0.03; 2 studies, 103 participants ‐ Analysis 86.1) than amitriptyline.
77.1. Analysis.
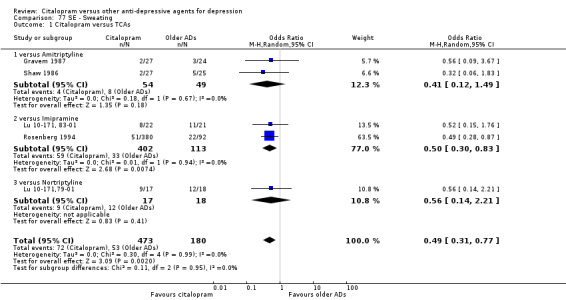
Comparison 77 SE ‐ Sweating, Outcome 1 Citalopram versus TCAs.
79.1. Analysis.
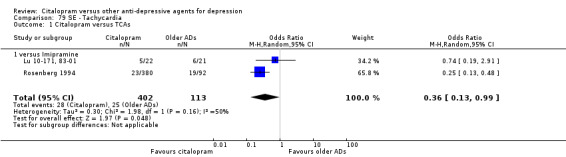
Comparison 79 SE ‐ Tachycardia, Outcome 1 Citalopram versus TCAs.
82.1. Analysis.
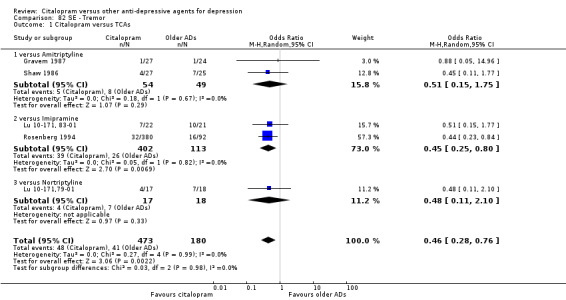
Comparison 82 SE ‐ Tremor, Outcome 1 Citalopram versus TCAs.
86.1. Analysis.
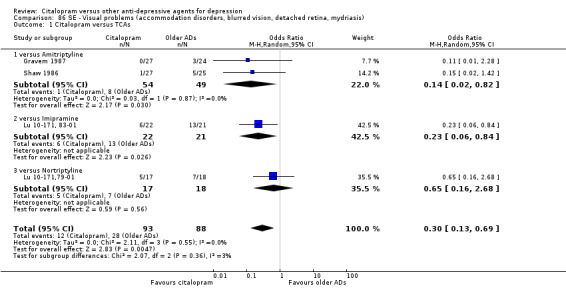
Comparison 86 SE ‐ Visual problems (accommodation disorders, blurred vision, detached retina, mydriasis), Outcome 1 Citalopram versus TCAs.
2. CITALOPRAM versus HETEROCYCLICS
PRIMARY OUTCOME
EFFICACY ‐ Number of patients who responded to treatment (six to 12 weeks)
The analysis found no difference in terms of efficacy between citalopram and heterocyclics in total (OR 1.05, 95% CI 0.56 to 1.96, P = 0.88; 2 trials, 432 participants) nor in head‐to‐head comparisons (Analysis 1.2).
1.2. Analysis.
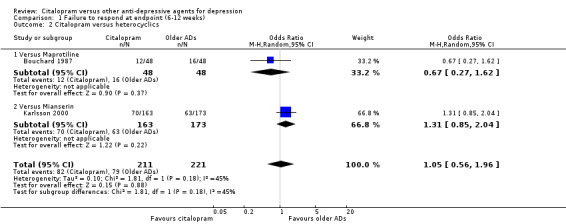
Comparison 1 Failure to respond at endpoint (6‐12 weeks), Outcome 2 Citalopram versus heterocyclics.
SECONDARY OUTCOMES
1) EFFICACY ‐ Number of patients who responded to treatment
a) Early response (one to four weeks)
No data available.
b) Follow‐up response (16 to 24 weeks)
No data available.
2) EFFICACY ‐ Number of patients who remitted
a) Acute phase treatment (six to 12 weeks)
There was no difference between citalopram and heterocyclics, neither as a group (5 trials, 256 participants) nor as individual drugs in terms of remission (Analysis 5.2).
5.2. Analysis.
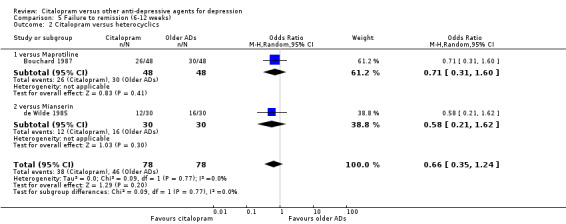
Comparison 5 Failure to remission (6‐12 weeks), Outcome 2 Citalopram versus heterocyclics.
b) Early remission (one to four weeks)
No data available.
c) Follow‐up remission (16 to 24 weeks)
No data available.
3) EFFICACY ‐ Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
Using rating scale scores, there was no evidence that citalopram was different from heterocyclics, neither as a group (2 trials, 131 participants) nor as individual drugs (Analysis 8.2).
8.2. Analysis.
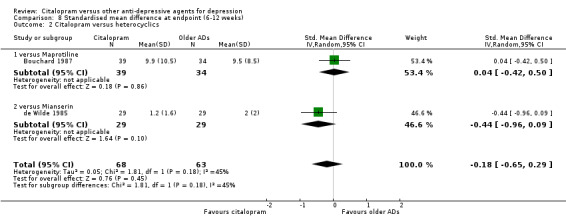
Comparison 8 Standardised mean difference at endpoint (6‐12 weeks), Outcome 2 Citalopram versus heterocyclics.
b) Early response (1 to 4 weeks)
There was evidence that citalopram was more effective than mianserin (SMD ‐0.55, 95% CI ‐1.07 to ‐0.02, P = 0.04, 1 trial, 58 participants) (see Analysis 7.2). There was no difference between citalopram and heterocyclics as a class.
7.2. Analysis.
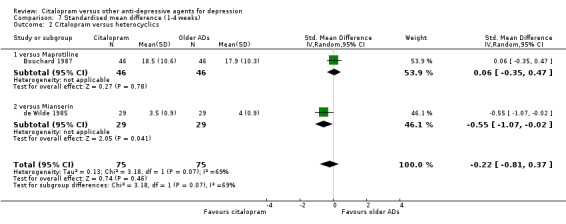
Comparison 7 Standardised mean difference (1‐4 weeks), Outcome 2 Citalopram versus heterocyclics.
c) Follow‐up response (16 to 24 weeks)
No data available.
4) EFFICACY‐ Social adjustment, social functioning, health‐related quality of life, costs to healthcare services
No data available.
5) ACCEPTABILITY ‐ Dropout rate
a) No statistically significant difference was found between citalopram and heterocyclics in terms of discontinuation due to any cause (Analysis 10.2), due to inefficacy (Analysis 12.2) or due to side effects (Analysis 11.2)
10.2. Analysis.
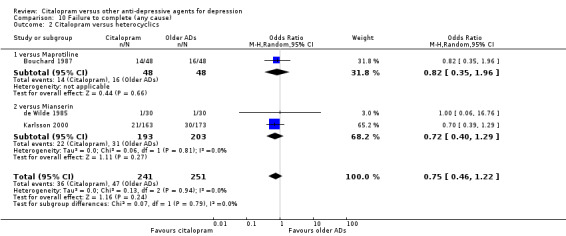
Comparison 10 Failure to complete (any cause), Outcome 2 Citalopram versus heterocyclics.
12.2. Analysis.
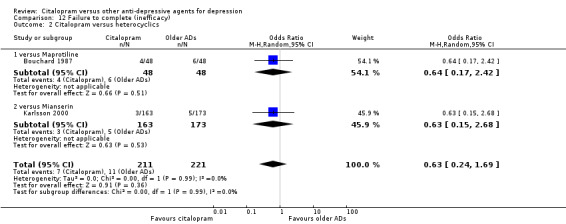
Comparison 12 Failure to complete (inefficacy), Outcome 2 Citalopram versus heterocyclics.
11.2. Analysis.
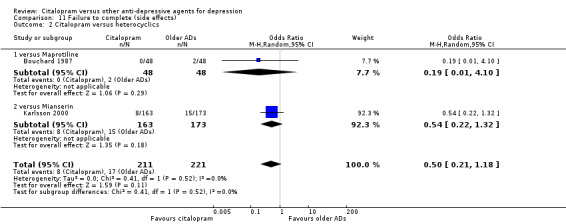
Comparison 11 Failure to complete (side effects), Outcome 2 Citalopram versus heterocyclics.
6) TOLERABILITY
Total number of patients experiencing at least some side effects.
There was no evidence that citalopram was associated with a smaller or higher rate of adverse events than mianserin (OR 0.84, 95% CI 0.52 to 1.37; 1 study, 336 participants ‐ Analysis 13.2).
13.2. Analysis.
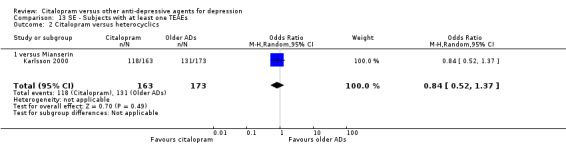
Comparison 13 SE ‐ Subjects with at least one TEAEs, Outcome 2 Citalopram versus heterocyclics.
Number of patients experiencing specific side effects (only figures for statistically significant differences were reported in the text)
a) Anxiety/agitation
There was no evidence that citalopram was associated with a lower rate of participants experiencing agitation/anxiety than heterocyclics (Analysis 18.2).
18.2. Analysis.
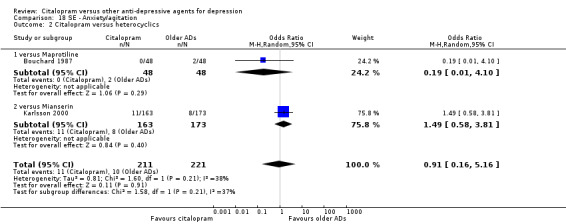
Comparison 18 SE ‐ Anxiety/agitation, Outcome 2 Citalopram versus heterocyclics.
b) Constipation
There was no evidence that citalopram was associated with a lower rate of participants experiencing constipation than mianserin (Analysis 30.2)
30.2. Analysis.
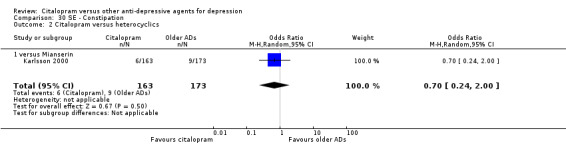
Comparison 30 SE ‐ Constipation, Outcome 2 Citalopram versus heterocyclics.
c) Diarrohea
There was no evidence that citalopram was associated with a lower rate of participants experiencing diarrhoea than maprotiline (Analysis 34.1).
d) Dry mouth
There was no evidence that citalopram was associated with a lower rate of participants experiencing diarrhoea than maprotiline (Analysis 36.2).
36.2. Analysis.
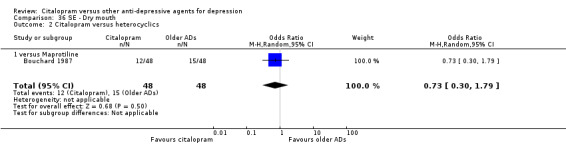
Comparison 36 SE ‐ Dry mouth, Outcome 2 Citalopram versus heterocyclics.
e) Hypotension
No data available.
f) Insomnia
Citalopram was associated with higher rate of patients experiencing insomnia than mianserin (OR 2.94, 95% CI 1.20 to 7.25; 1 trial, 336 participants ‐ Analysis 54.2).
54.2. Analysis.
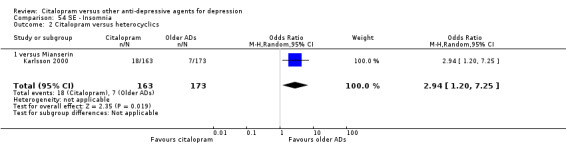
Comparison 54 SE ‐ Insomnia, Outcome 2 Citalopram versus heterocyclics.
g) Nausea/vomiting
There was no evidence that citalopram was associated with a higher rate of participants experiencing nausea than heterocyclics (Analysis 61.2).
61.2. Analysis.
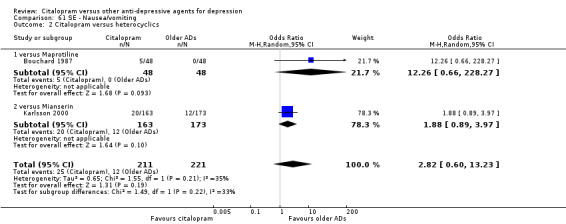
Comparison 61 SE ‐ Nausea/vomiting, Outcome 2 Citalopram versus heterocyclics.
h) Sedation/drowsiness
There was no evidence that citalopram was associated with a higher rate of participants experiencing nausea than maprotiline (Analysis 72.2).
72.2. Analysis.
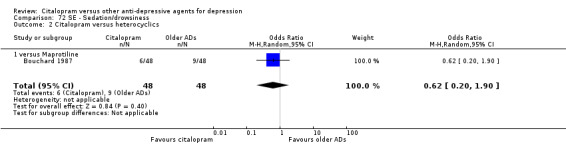
Comparison 72 SE ‐ Sedation/drowsiness, Outcome 2 Citalopram versus heterocyclics.
i) Sleepiness/somnolence
Citalopram was associated with a lower rate of patients experiencing sleepiness than mianserin (OR 0.20, 95% CI 0.04 to 0.94; 1 trial, 336 participants ‐ Analysis 76.2).
76.2. Analysis.
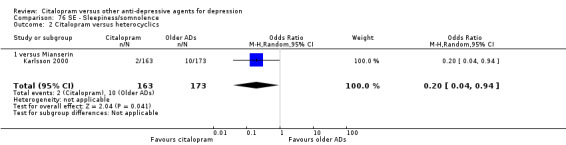
Comparison 76 SE ‐ Sleepiness/somnolence, Outcome 2 Citalopram versus heterocyclics.
j) Urination problems
There was no evidence that citalopram was associated with a higher rate of participants experiencing urination problems than maprotiline (Analysis 83.2).
83.2. Analysis.
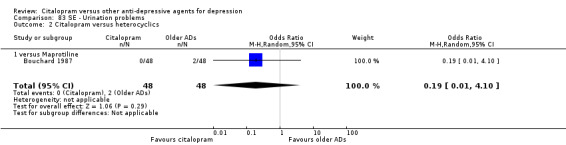
Comparison 83 SE ‐ Urination problems, Outcome 2 Citalopram versus heterocyclics.
k) Suicide wishes/gestures/attempts
No data available
l) Deaths (all cause)/Completed suicide
There was no difference between citalopram and maprotiline (Analysis 89.3; Analysis 89.4).
m) Other adverse events
Citalopram was associated with a lower rate of participants experiencing fatigue than mianserin (OR 0.21, 95% CI 0.06 to 0.76, P = 0.02; 1 trial, 336 participants ‐ Analysis 42.2).
42.2. Analysis.
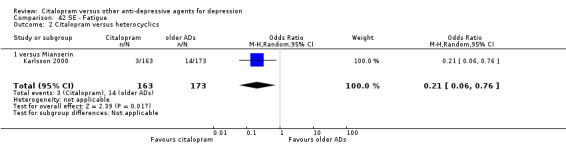
Comparison 42 SE ‐ Fatigue, Outcome 2 Citalopram versus heterocyclics.
3. CITALOPRAM versus other SSRIs
PRIMARY OUTCOME
EFFICACY ‐ Number of patients who responded to treatment (six to 12 weeks)
The analysis found that citalopram was less effective than escitalopram (OR 1.47, 95% CI 1.08 to 2.02, P = 0.02, six trials, 1806 participants; NNTB 13, 95% CI 8 to 34) but more effective than paroxetine (OR 0.65, 95% CI 0.44 to 0.96, P = 0.03, 1 trial, 406 participants; NNTB 9, 95% CI 5 to 100) ( Analysis 1.3; Figure 4).
1.3. Analysis.
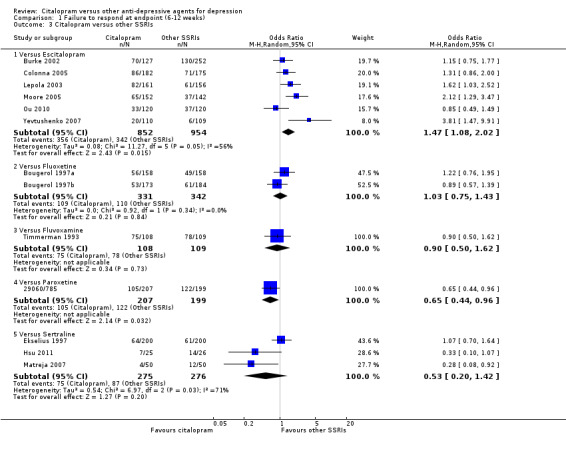
Comparison 1 Failure to respond at endpoint (6‐12 weeks), Outcome 3 Citalopram versus other SSRIs.
4.
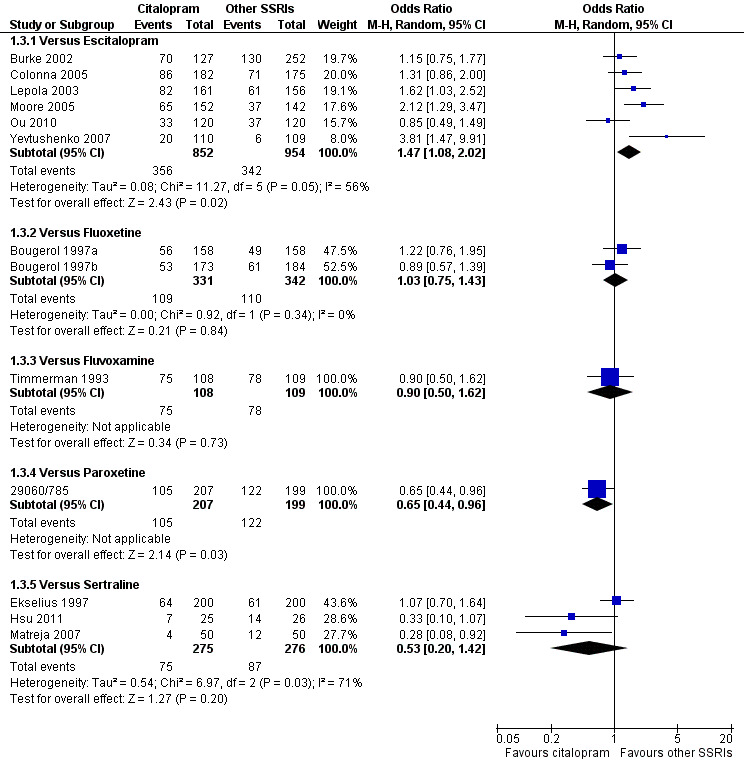
Forest plot of comparison: 1 Failure to respond at endpoint (6‐12 weeks), outcome: 1.3 Citalopram versus other SSRIs.
SECONDARY OUTCOMES
1) EFFICACY ‐ Number of patients who responded to treatment
a) Early response (one to four weeks)
There was no evidence that citalopram was more effective than other SSRIs (Analysis 2.2).
2.2. Analysis.
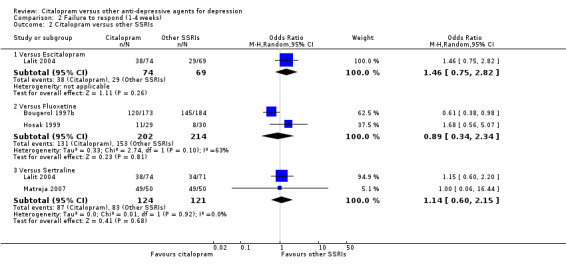
Comparison 2 Failure to respond (1‐4 weeks), Outcome 2 Citalopram versus other SSRIs.
b) Follow‐up response (16 to 24 weeks)
There was no evidence that citalopram was more effective than other SSRIs (Analysis 3.2).
3.2. Analysis.
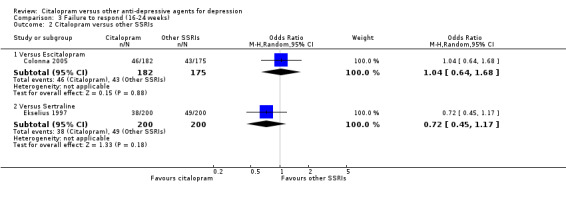
Comparison 3 Failure to respond (16‐24 weeks), Outcome 2 Citalopram versus other SSRIs.
2) EFFICACY ‐ Number of patients who remitted
a) Acute phase treatment (six to 12 weeks)
There was evidence that citalopram was less effective than escitalopram (OR 1.94, 95% CI 1.16 to 3.26, P = 0.01, 5 trials, 1427 participants) (Analysis 5.3).
5.3. Analysis.
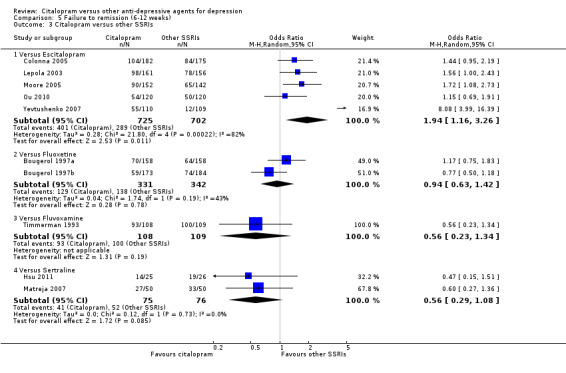
Comparison 5 Failure to remission (6‐12 weeks), Outcome 3 Citalopram versus other SSRIs.
b) Early remission (one to four weeks)
There was no evidence that citalopram was more effective than other SSRIs (Analysis 4.2).
4.2. Analysis.
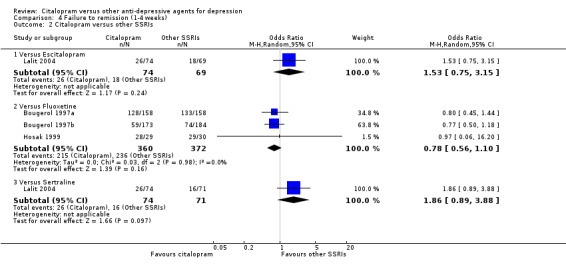
Comparison 4 Failure to remission (1‐4 weeks), Outcome 2 Citalopram versus other SSRIs.
c) Follow‐up remission (16 to 24 weeks)
There was no evidence that citalopram was more effective than other SSRIs (Analysis 6.1).
6.1. Analysis.
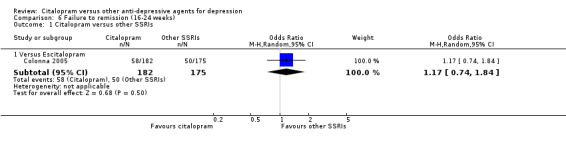
Comparison 6 Failure to remission (16‐24 weeks), Outcome 1 Citalopram versus other SSRIs.
3) EFFICACY ‐ Mean change from baseline
a) Acute phase treatment: between six and 12 weeks
There was evidence that citalopram was less effective than escitalopram (SMD 0.16, 95% CI 0.05 to 0.27, P = 0.006, 7 trials, 1874 participants) (Analysis 8.3).
8.3. Analysis.
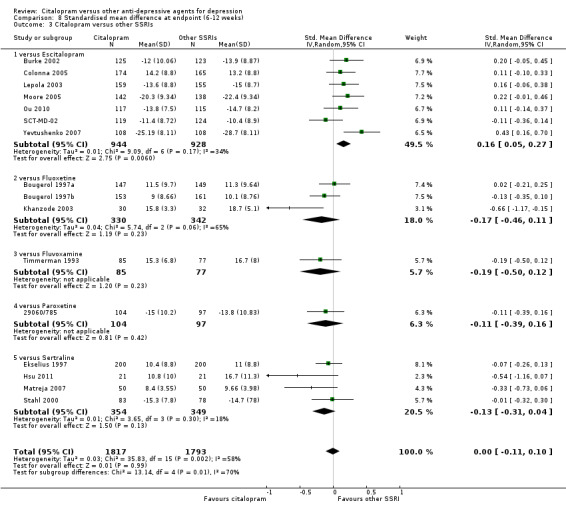
Comparison 8 Standardised mean difference at endpoint (6‐12 weeks), Outcome 3 Citalopram versus other SSRIs.
b) Early response (one to four weeks)
There was evidence that citalopram was more effective than fluoxetine (SMD ‐0.15, 95% CI ‐0.30 to ‐0.01, P = 0.04, 4 trials, 723 participants) (Analysis 7.3).
7.3. Analysis.
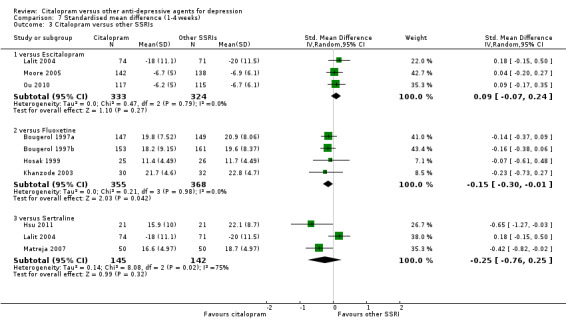
Comparison 7 Standardised mean difference (1‐4 weeks), Outcome 3 Citalopram versus other SSRIs.
c) Follow‐up response (16 to 24 weeks)
No data available.
4) EFFICACY‐ Social adjustment, social functioning, health‐related quality of life, costs to healthcare services
No data available.
5) ACCEPTABILITY ‐ Dropout rate
a) There was no difference between patients allocated to citalopram withdrawing from studies than those allocated to other SSRIs for discontinuation due to any cause (Analysis 10.3;).
10.3. Analysis.
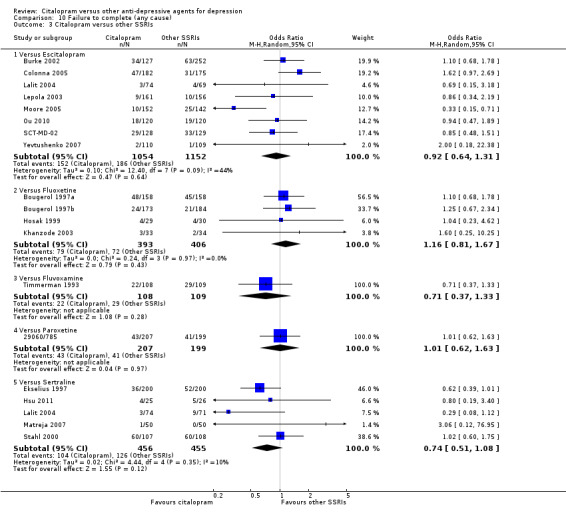
Comparison 10 Failure to complete (any cause), Outcome 3 Citalopram versus other SSRIs.
b) No differences were found in terms of discontinuation due to inefficacy (Analysis 12.3).
12.3. Analysis.
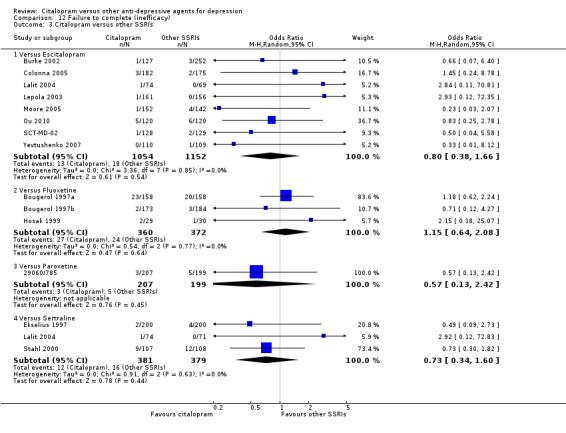
Comparison 12 Failure to complete (inefficacy), Outcome 3 Citalopram versus other SSRIs.
c) No differences were found in terms of discontinuation due to side effects (Analysis 11.3).
11.3. Analysis.
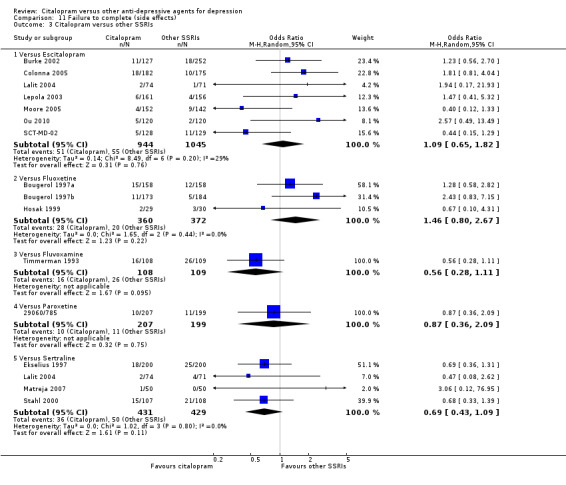
Comparison 11 Failure to complete (side effects), Outcome 3 Citalopram versus other SSRIs.
6) TOLERABILITY
Total number of patients experiencing at least some side effects.
There was no evidence that citalopram was associated with a smaller or higher rate of adverse events than other SSRIs (Analysis 13.3).
13.3. Analysis.
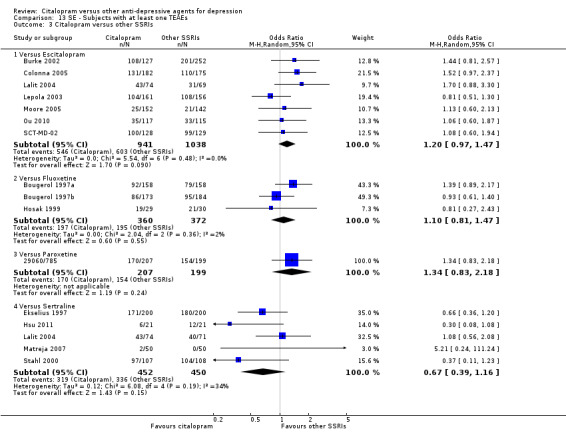
Comparison 13 SE ‐ Subjects with at least one TEAEs, Outcome 3 Citalopram versus other SSRIs.
Number of patients experiencing specific side effects is reported below.
a) Anxiety/agitation
There was no evidence that citalopram was associated with a lower rate of participants experiencing anxiety/agitation than other SSRIs (Analysis 18.3).
18.3. Analysis.
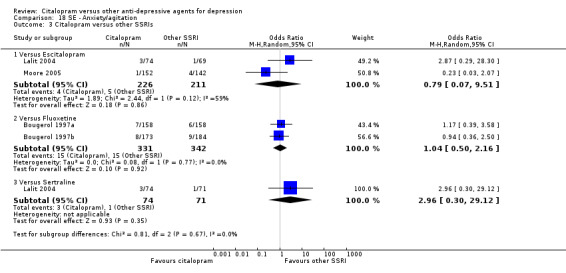
Comparison 18 SE ‐ Anxiety/agitation, Outcome 3 Citalopram versus other SSRIs.
b) Constipation
There was no evidence that citalopram was associated with a lower rate of participants experiencing diarrhoea than other SSRIs (Analysis 30.3).
30.3. Analysis.
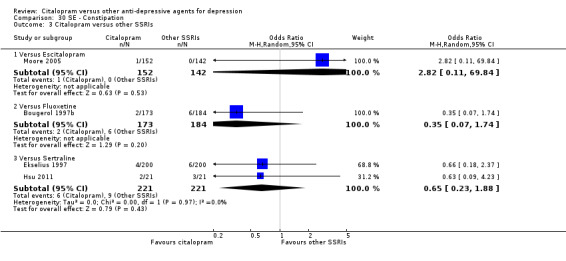
Comparison 30 SE ‐ Constipation, Outcome 3 Citalopram versus other SSRIs.
c) Diarrohea
There was no evidence that citalopram was associated with a lower rate of participants experiencing diarrhoea than other SSRIs (Analysis 34.3).
34.3. Analysis.
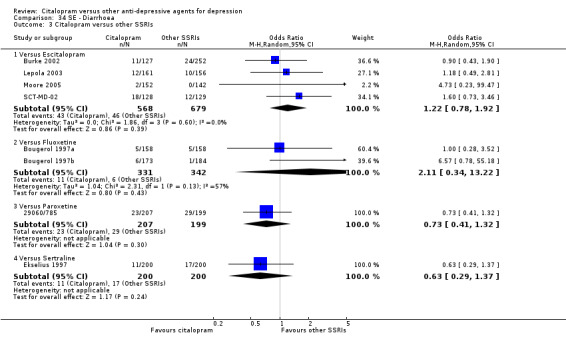
Comparison 34 SE ‐ Diarrhoea, Outcome 3 Citalopram versus other SSRIs.
d) Dry mouth
There was no evidence that citalopram was associated with a lower rate of participants experiencing dry mouth than other SSRIs (Analysis 36.3).
36.3. Analysis.
Comparison 36 SE ‐ Dry mouth, Outcome 3 Citalopram versus other SSRIs.
e) Hypotension
There was no evidence that citalopram was associated with a lower rate of participants experiencing hypotension than escitalopram (OR 0.31, 95% CI 0.01 to 7.65; 1 trial, 294 participants) (Analysis 49.2).
49.2. Analysis.
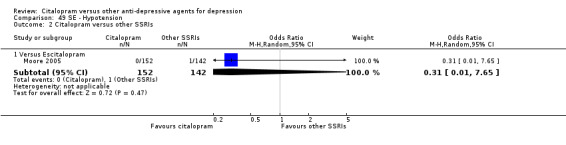
Comparison 49 SE ‐ Hypotension, Outcome 2 Citalopram versus other SSRIs.
f) Insomnia
There was no evidence that citalopram was associated with a lower rate of participants experiencing insomnia than other SSRIs (Analysis 54.3).
54.3. Analysis.
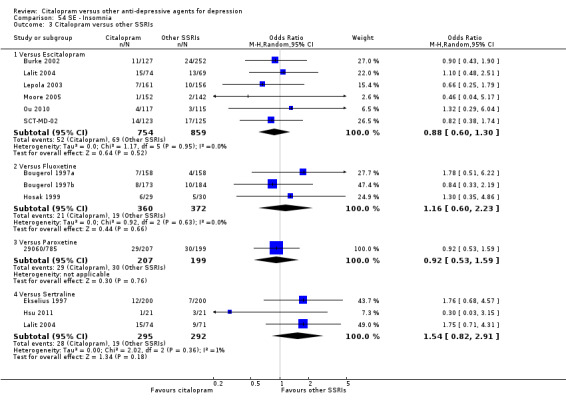
Comparison 54 SE ‐ Insomnia, Outcome 3 Citalopram versus other SSRIs.
g) Nausea/vomiting
There was no evidence that citalopram was associated with a lower rate of participants experiencing nausea or vomiting than other SSRIs (Analysis 61.3).
61.3. Analysis.
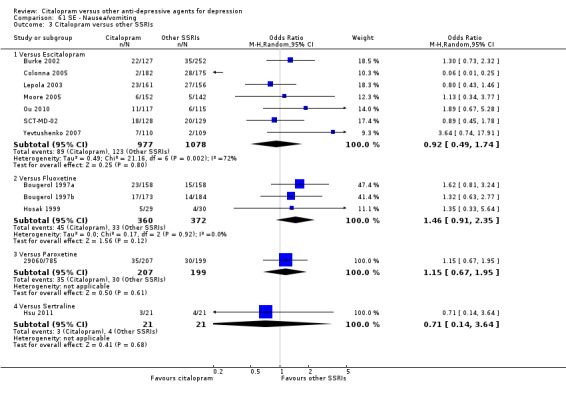
Comparison 61 SE ‐ Nausea/vomiting, Outcome 3 Citalopram versus other SSRIs.
h) Sedation/drowsiness
There was no evidence that citalopram was associated with a lower rate of participants experiencing sedation/drowsiness than other SSRIs (Analysis 72.3).
72.3. Analysis.
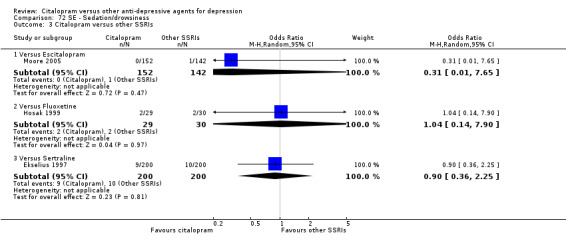
Comparison 72 SE ‐ Sedation/drowsiness, Outcome 3 Citalopram versus other SSRIs.
i) Sleepiness/somnolence
There was no evidence that citalopram was associated with a lower rate of participants experiencing somnolence than other SSRIs (Analysis 76.3).
76.3. Analysis.
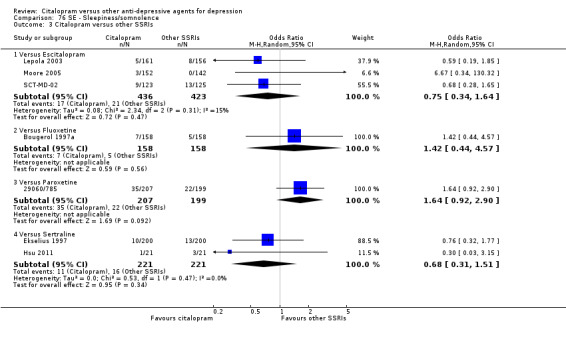
Comparison 76 SE ‐ Sleepiness/somnolence, Outcome 3 Citalopram versus other SSRIs.
j) Urination problems
There was no evidence that citalopram was associated with a higher rate of participants experiencing hypotension than sertraline (OR 1.52, 95% CI 0.42 to 5.45; 1 trial, 400 participants) (Analysis 83.3).
83.3. Analysis.
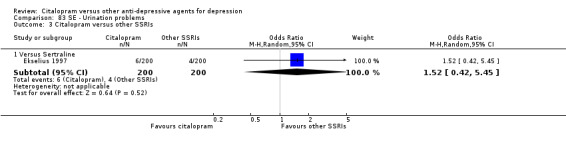
Comparison 83 SE ‐ Urination problems, Outcome 3 Citalopram versus other SSRIs.
k) Suicide wishes/gestures/attempts
There was no difference between citalopram and other SSRIs (Analysis 89.1; Analysis 89.2).
89.2. Analysis.
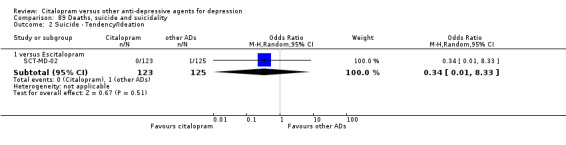
Comparison 89 Deaths, suicide and suicidality, Outcome 2 Suicide ‐ Tendency/Ideation.
l) Deaths (all cause)/Completed suicide
There was no difference in suicide rate between citalopram and other SSRIs (two patients committed suicide and both were in the citalopram group: one in a study that compared citalopram with fluvoxamine (Timmerman 1993) and one in a study comparing citalopram with escitalopram (Moore 2005) (Analysis 89.3; Analysis 89.4).
m) Other adverse events
Citalopram was associated with a lower rate of participants experiencing fatigue than escitalopram (OR 0.31, 95% CI 0.12 to 0.84, P = 0.02; 2 trials, 467 participants ‐ Analysis 42.3) and a lower rate of participants experiencing headache than sertraline (OR 0.55, 95% CI 0.33 to 0.91, P = 0.02; 3 trials, 587 participants ‐ Analysis 46.3)
42.3. Analysis.
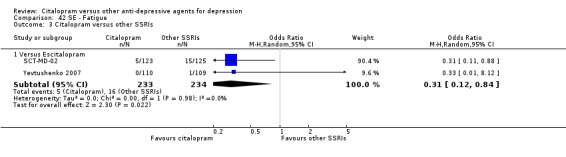
Comparison 42 SE ‐ Fatigue, Outcome 3 Citalopram versus other SSRIs.
46.3. Analysis.
Comparison 46 SE ‐ Headache, Outcome 3 Citalopram versus other SSRIs.
4. CITALOPRAM versus SNRIs, MOAIs, other conventional ADs and non‐conventional ADs
PRIMARY OUTCOME
EFFICACY ‐ Number of patients who responded to treatment (six to 12 weeks)
The analysis of primary outcome found that citalopram is more effective than reboxetine (OR 0.63, 95% CI 0.43 to 0.91, P = 0.01, 2 trials, 458 participants; NNTB 9, 95% CI 5 to 50) (Analysis 1.5; Figure 5). No differences were found between citalopram and mirtazapine (Analysis 1.5), venlafaxine (Analysis 1.4) or hypericum (Analysis 1.6)
1.5. Analysis.
Comparison 1 Failure to respond at endpoint (6‐12 weeks), Outcome 5 Citalopram versus other conventional ADs.
5.
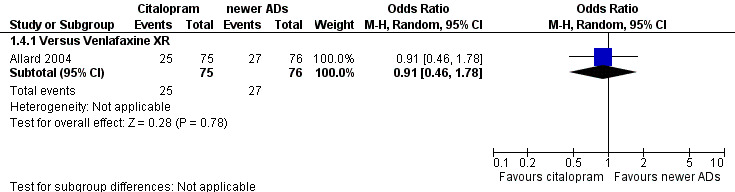
Forest plot of comparison: 1 Failure to respond at endpoint (6‐12 weeks), outcome: 1.4 Citalopram versus SNRI.
1.4. Analysis.
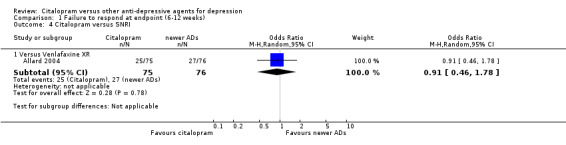
Comparison 1 Failure to respond at endpoint (6‐12 weeks), Outcome 4 Citalopram versus SNRI.
1.6. Analysis.
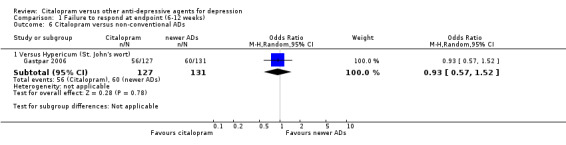
Comparison 1 Failure to respond at endpoint (6‐12 weeks), Outcome 6 Citalopram versus non‐conventional ADs.
SECONDARY OUTCOMES
1) EFFICACY ‐ Number of patients who responded to treatment
a) Early response (one to four weeks)
There was no evidence that citalopram is more effective than reboxetine (Analysis 2.3).
2.3. Analysis.
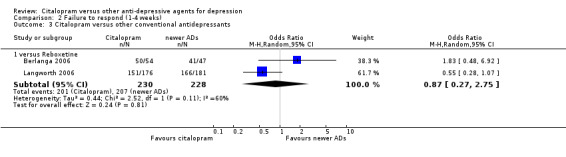
Comparison 2 Failure to respond (1‐4 weeks), Outcome 3 Citalopram versus other conventional antidepressants.
b) Follow‐up response (16 to 24 weeks)
Citalopram is more effective than reboxetine (OR 0.46, 95% CI 0.30 to 0.70, P = 0.0003, 1 trial, 357 participants) (Analysis 3.4).
3.4. Analysis.
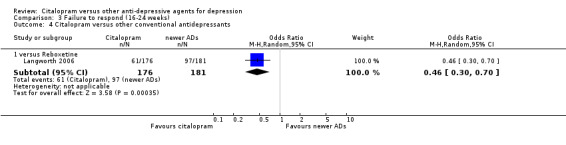
Comparison 3 Failure to respond (16‐24 weeks), Outcome 4 Citalopram versus other conventional antidepressants.
2) EFFICACY ‐ Number of patients who remitted
a) Acute phase treatment (six to 12 weeks)
Citalopram was more effective than reboxetine (OR 0.59, 95% CI 0.38 to 0.92, P = 0.02, 1 trial, 357 participants; NNTB 9, 95% CI 5 to 50) (Analysis 5.5), but not than venlafaxine (Analysis 5.4).
5.5. Analysis.
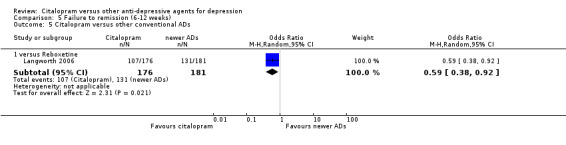
Comparison 5 Failure to remission (6‐12 weeks), Outcome 5 Citalopram versus other conventional ADs.
5.4. Analysis.
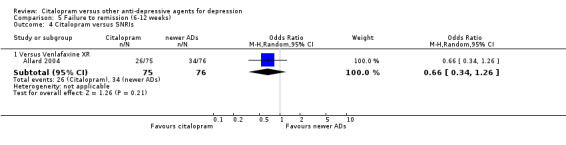
Comparison 5 Failure to remission (6‐12 weeks), Outcome 4 Citalopram versus SNRIs.
b) Early remission (one to four weeks)
There was no evidence that citalopram was more effective than reboxetine (Analysis 4.3).
4.3. Analysis.
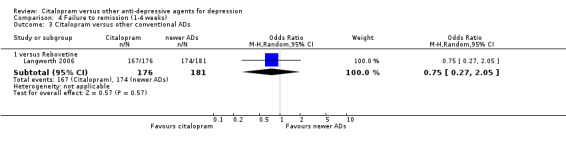
Comparison 4 Failure to remission (1‐4 weeks), Outcome 3 Citalopram versus other conventional ADs.
c) Follow‐up remission (16 to 24 weeks)
Citalopram was more effective than reboxetine (OR 0.43, 95% CI 0.28 to 0.65, P < 0.0001, 1 trial, 357 participants) (Analysis 6.3), but not than venlafaxine (Analysis 6.2).
6.3. Analysis.
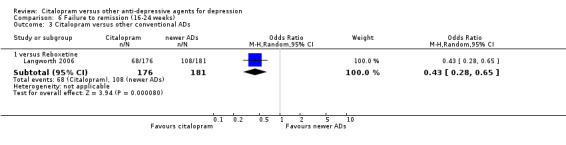
Comparison 6 Failure to remission (16‐24 weeks), Outcome 3 Citalopram versus other conventional ADs.
6.2. Analysis.
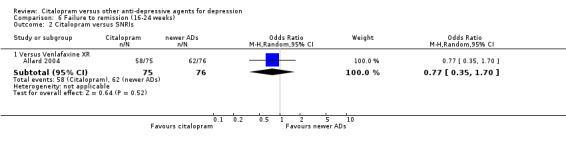
Comparison 6 Failure to remission (16‐24 weeks), Outcome 2 Citalopram versus SNRIs.
3) EFFICACY ‐ Mean change from baseline
a) Acute phase treatment: between six and 12 weeks
There was evidence that citalopram was more efficacious than moclobemide (MD ‐4.60, 95% CI ‐8.28 to ‐0.92, P = 0.01, 1 trial, 40 participants) (Analysis 8.5). In term of efficacy, no difference was found between citalopram and venlafaxine (Analysis 8.4), and citalopram and reboxetine or mirtazapine (Analysis 8.6).
8.5. Analysis.
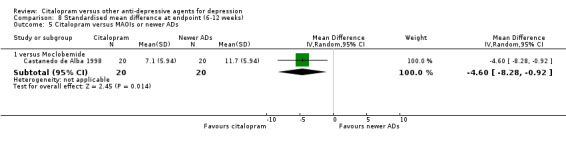
Comparison 8 Standardised mean difference at endpoint (6‐12 weeks), Outcome 5 Citalopram versus MAOIs or newer ADs.
8.4. Analysis.
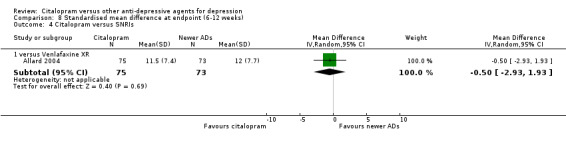
Comparison 8 Standardised mean difference at endpoint (6‐12 weeks), Outcome 4 Citalopram versus SNRIs.
8.6. Analysis.
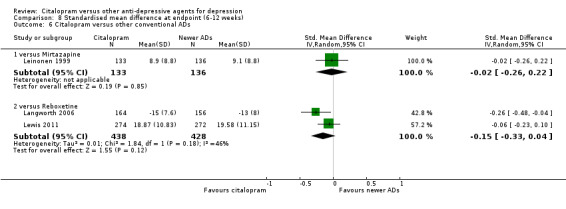
Comparison 8 Standardised mean difference at endpoint (6‐12 weeks), Outcome 6 Citalopram versus other conventional ADs.
b) Early response (one to four weeks)
No data available.
c) Follow‐up response (16 to 24 weeks)
We observed a trend in favour of citalopram compared with reboxetine in term of efficacy, although not statistically significant (MD ‐1.80, 95% CI ‐3.62 to 0.02, P < 0.05, 1 trial, 320 participants) (Analysis 9.3).
9.3. Analysis.
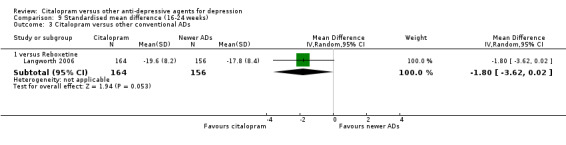
Comparison 9 Standardised mean difference (16‐24 weeks), Outcome 3 Citalopram versus other conventional ADs.
4) EFFICACY‐ Social adjustment, social functioning, health‐related quality of life, costs to healthcare services
No data available.
5) ACCEPTABILITY ‐ Dropout rate
a) There was no statistically significant difference between patients allocated to citalopram withdrawing from studies than those allocated to reboxetine or hypericum for discontinuation due to any cause (Analysis 10.4; Analysis 10.5). However, even though not significant, we observed a trend in favour of citalopram compared with mirtazapine (OR 0.42, 95% CI 0.18 to 1.01, P = 0.05; 1 study, 270 participants) (Analysis 10.4).
10.4. Analysis.
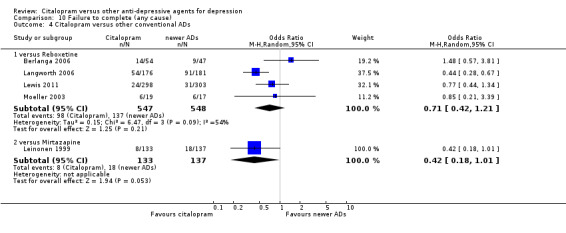
Comparison 10 Failure to complete (any cause), Outcome 4 Citalopram versus other conventional ADs.
10.5. Analysis.
Comparison 10 Failure to complete (any cause), Outcome 5 Citalopram versus non‐conventional ADs.
b) No differences were found in terms of discontinuation due to inefficacy between citalopram and mirtazapine or reboxetine (Analysis 12.4).
12.4. Analysis.
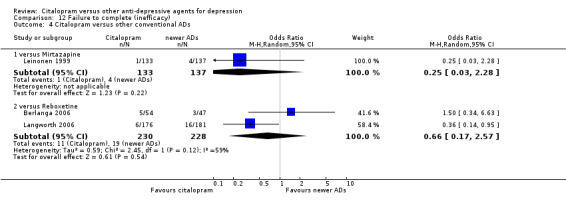
Comparison 12 Failure to complete (inefficacy), Outcome 4 Citalopram versus other conventional ADs.
c) No differences were found in terms of discontinuation due to side effects between citalopram and venlafaxine (Analysis 11.4), mirtazapine or reboxetine (Analysis 11.5).
11.4. Analysis.
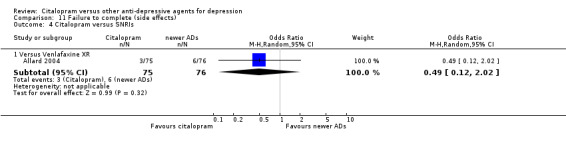
Comparison 11 Failure to complete (side effects), Outcome 4 Citalopram versus SNRIs.
11.5. Analysis.
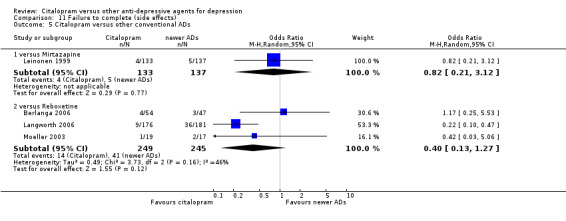
Comparison 11 Failure to complete (side effects), Outcome 5 Citalopram versus other conventional ADs.
6) TOLERABILITY
Total number of patients experiencing at least some side effects.
We found that citalopram was associated with a lower rate of patients experiencing side effects than reboxetine (OR 0.64, 95% CI 0.42 to 0.97, P < 0.04; 1 trial, 357 participants) (Analysis 13.6) and than venlafaxine XR (OR 0.46, 95% CI 0.24 to 0.88, P < 0.02; 1 trial, 151 participants) (Analysis 13.4). By contrast, we found that citalopram was associated with a higher rate of patients experiencing side effects than hypericum (OR 1.69, 95% CI 1.01 to 2.83; 1 trial, 258 participants) (Analysis 13.7). No differences were found between citalopram and moclobemide (Analysis 13.5) or mirtazapine (Analysis 13.6).
13.6. Analysis.
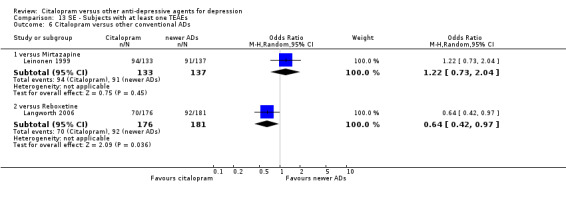
Comparison 13 SE ‐ Subjects with at least one TEAEs, Outcome 6 Citalopram versus other conventional ADs.
13.4. Analysis.
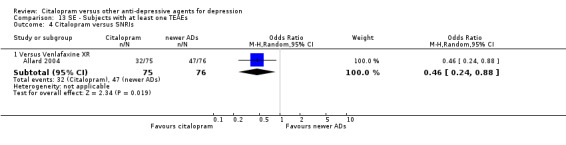
Comparison 13 SE ‐ Subjects with at least one TEAEs, Outcome 4 Citalopram versus SNRIs.
13.7. Analysis.
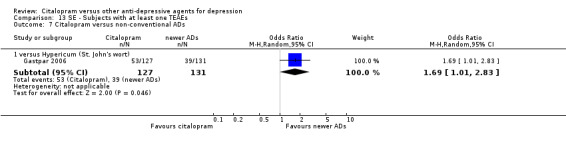
Comparison 13 SE ‐ Subjects with at least one TEAEs, Outcome 7 Citalopram versus non‐conventional ADs.
13.5. Analysis.
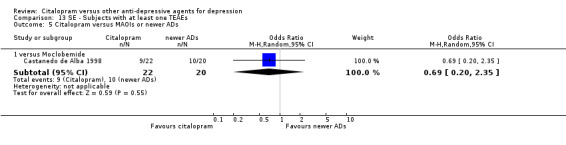
Comparison 13 SE ‐ Subjects with at least one TEAEs, Outcome 5 Citalopram versus MAOIs or newer ADs.
Number of patients experiencing specific side effects is reported below.
a) Anxiety/agitation
No data available.
b) Constipation
There was evidence that citalopram was associated with a lower rate of participants experiencing constipation than reboxetine (OR 0.06, 95% CI 0.00 to 0.90, P < 0.04; 2 trials, 458 participants) (Analysis 30.5).
30.5. Analysis.
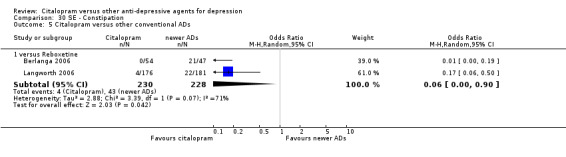
Comparison 30 SE ‐ Constipation, Outcome 5 Citalopram versus other conventional ADs.
c) Diarrohea
There was no evidence that citalopram was associated with a lower rate of participants experiencing diarrhoea than mirtazapine or reboxetine (Analysis 34.4).
34.4. Analysis.
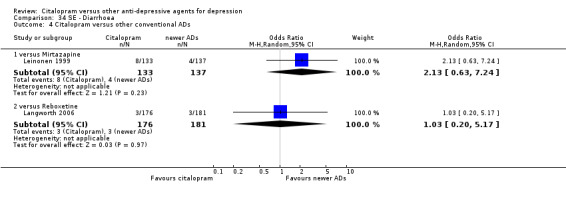
Comparison 34 SE ‐ Diarrhoea, Outcome 4 Citalopram versus other conventional ADs.
d) Dry mouth
There was no evidence that citalopram was associated with a lower rate of participants experiencing dry mouth than venlafaxine (Analysis 36.4) or mirtazapine (Analysis 36.5).
36.4. Analysis.
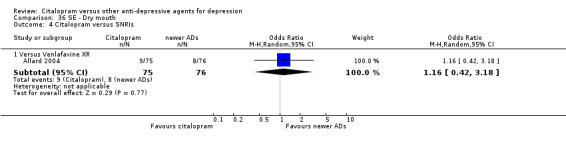
Comparison 36 SE ‐ Dry mouth, Outcome 4 Citalopram versus SNRIs.
36.5. Analysis.
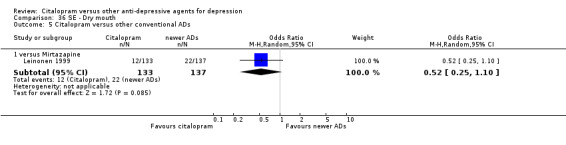
Comparison 36 SE ‐ Dry mouth, Outcome 5 Citalopram versus other conventional ADs.
e) Hypotension
No data available.
f) Insomnia
There was no evidence that citalopram was associated with a lower rate of participants experiencing insomnia than moclobemide (Analysis 54.4) or reboxetine (Analysis 54.5).
54.4. Analysis.
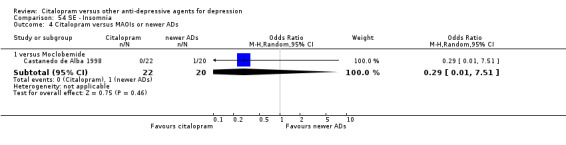
Comparison 54 SE ‐ Insomnia, Outcome 4 Citalopram versus MAOIs or newer ADs.
54.5. Analysis.
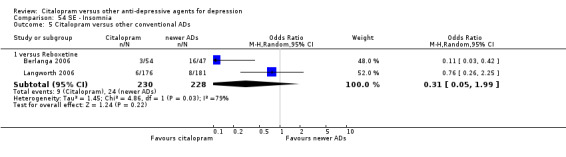
Comparison 54 SE ‐ Insomnia, Outcome 5 Citalopram versus other conventional ADs.
g) Nausea/vomiting
There was evidence that citalopram was associated with a higher rate of participants experiencing nausea than mirtazapine (OR 2.24, 95% CI 1.12 to 4.49, P = 0.02; 1 trial, 270 participants), but not than reboxetine (Analysis 61.4).
61.4. Analysis.
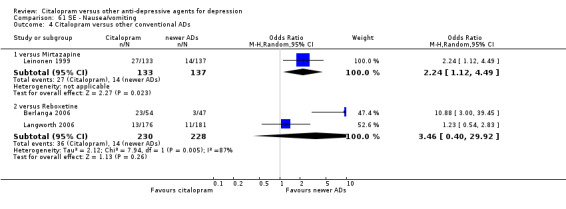
Comparison 61 SE ‐ Nausea/vomiting, Outcome 4 Citalopram versus other conventional ADs.
h) Sedation/drowsiness
There was no evidence that citalopram was associated with a lower rate of participants experiencing somnolence than mirtazapine or reboxetine (Analysis 72.4).
72.4. Analysis.
Comparison 72 SE ‐ Sedation/drowsiness, Outcome 4 Citalopram versus other conventional ADs.
i) Sleepiness/somnolence
There was no evidence that citalopram was associated with a lower rate of participants experiencing sedation/drowsiness than moclobemide (Analysis 76.4) or reboxetine (Analysis 76.5).
76.4. Analysis.
Comparison 76 SE ‐ Sleepiness/somnolence, Outcome 4 Citalopram versus MAOIs or newer ADs.
76.5. Analysis.
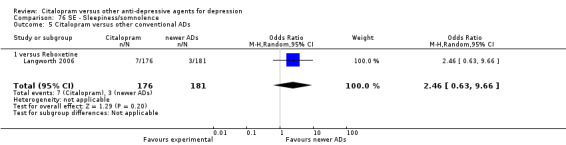
Comparison 76 SE ‐ Sleepiness/somnolence, Outcome 5 Citalopram versus other conventional ADs.
j) Urination problems
There was no evidence that citalopram was associated with a lower rate of subjects experiencing urination problems than reboxetine (Analysis 83.4.
83.4. Analysis.
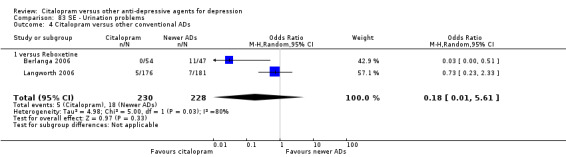
Comparison 83 SE ‐ Urination problems, Outcome 4 Citalopram versus other conventional ADs.
k) Suicide wishes/gestures/attempts
No data available.
l) Deaths (all cause)/Completed suicide
No data available.
l) Other adverse events
In comparison with hypericum, citalopram was associated with a higher rate of patients experiencing gastrointestinal problems (OR 2.41, 95% CI 1.12 to 5.18, P = 0.02; 1 trial, 258 participants) (Analysis 45.4) and vertigo (OR 6.12, 95% CI 1.33 to 28.17, P = 0.02; 1 trial, 258 participants) (Analysis 85.3). Citalopram was associated with a lower rate of participants experiencing appetite increase (OR 0.16, 95% CI 0.03 to 0.72, P = 0.02; 1 trial, 270 participants) (Analysis 19.2) and weight gain (OR 0.26, 95% CI 0.10 to 0.67, P = 0.005; 1 trial, 270 participants) (Analysis 87.2) than mirtazapine, but it was associated with a higher rate of participants experiencing sweating (OR 7.91, 95% CI 2.29 to 27.29, P = 0.001; 1 trial, 270 participants) (Analysis 77.4). Citalopram was associated with a lower rate of participants experiencing reduced salivation (OR 0.31, 95% CI 0.14 to 0.67, P = 0.003; 1 trial, 357 participants) (Analysis 71.1) and sweating (OR 0.38, 95% CI 0.16 to 0.90, P = 0.03; 1 trial, 357 participants) (Analysis 77.4) than reboxetine, but it was associated with a higher rate of participants with orgastic dysfunction (OR 3.74, 95% CI 1.56 to 8.95, P = 0.003; 1 trial, 357 participants) (Analysis 75.5), and with other sexual problems (OR 8.65, 95% CI 1.86 to 40.22, P = 0.006; 1 trial, 101 participants) (Analysis 75.6).
45.4. Analysis.
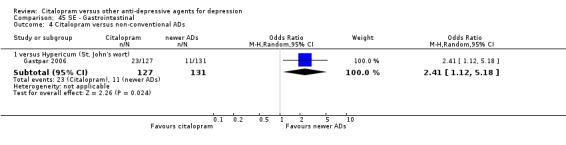
Comparison 45 SE ‐ Gastrointestinal, Outcome 4 Citalopram versus non‐conventional ADs.
85.3. Analysis.
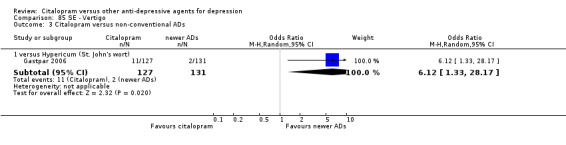
Comparison 85 SE ‐ Vertigo, Outcome 3 Citalopram versus non‐conventional ADs.
19.2. Analysis.
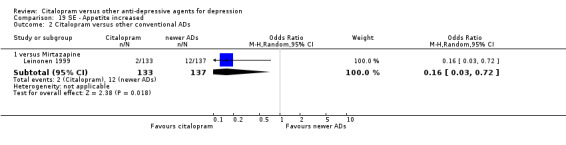
Comparison 19 SE ‐ Appetite increased, Outcome 2 Citalopram versus other conventional ADs.
87.2. Analysis.
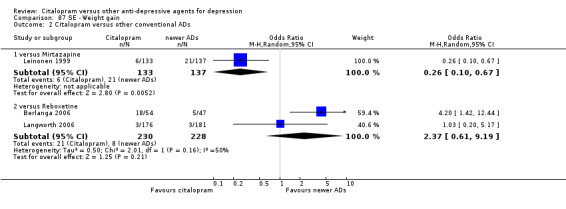
Comparison 87 SE ‐ Weight gain, Outcome 2 Citalopram versus other conventional ADs.
77.4. Analysis.
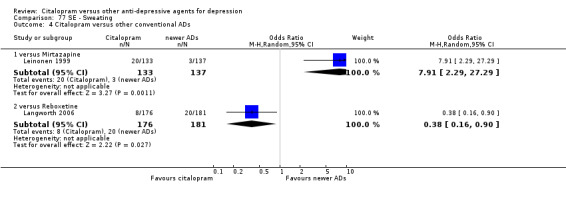
Comparison 77 SE ‐ Sweating, Outcome 4 Citalopram versus other conventional ADs.
71.1. Analysis.
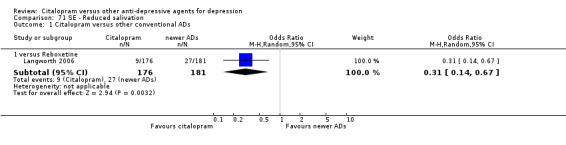
Comparison 71 SE ‐ Reduced salivation, Outcome 1 Citalopram versus other conventional ADs.
75.5. Analysis.
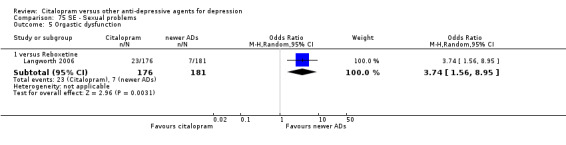
Comparison 75 SE ‐ Sexual problems, Outcome 5 Orgastic dysfunction.
75.6. Analysis.
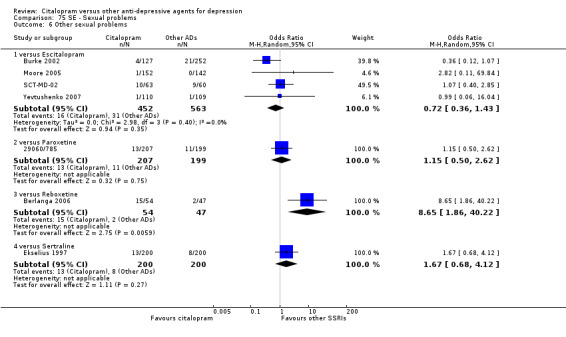
Comparison 75 SE ‐ Sexual problems, Outcome 6 Other sexual problems.
SUBGROUP ANALYSES
1) Citalopram dosing All studies used citalopram within the standard therapeutic range (20 to 60 mg/day). Only in one study were investigators allowed to use citalopram up to 80 mg/day, but the mean dose was below 60 mg/day (de Wilde 1985). Therefore, it was not meaningful to carry out this pre‐planned subgroup analysis.
2) Comparator dosing All comparator doses were within the therapeutic range. Due to the small number of trials outside the therapeutic range, it was not considered meaningful to carry out this pre‐planned subgroup analysis.
3) Depression severity The great majority of studies reported a mean baseline score corresponding to moderate to severe major depression. Therefore, it was not meaningful to carry out this pre‐planned subgroup analysis.
4) Treatment settings Only three studies selectively recruited patients in general practice (Bougerol 1997b; Ekselius 1997; Lewis 2011) and only three studies enrolled only in‐patients (Andersen 1986; de Wilde 1985; Hosak 1999), therefore, it was not considered meaningful to carry out this pre‐planned subgroup analysis.
5) Elderly patients As only three studies specifically recruited elderly patients (Karlsson 2000; Kyle 1998; Navarro 2001), it was not meaningful to carry out this pre‐planned subgroup analysis.
FUNNEL PLOT ANALYSIS
Where available, the funnel plot analyses did not suggest evidence of publication bias, however, for many comparisons the presence of publication bias was not examined because there were insufficient trials to allow meaningful formal assessment using funnel plots.
SENSITIVITY ANALYSES
1) Excluding trials with unclear concealment of random allocation and/or unclear double blinding Although technically possible to carry out these sensitivity analyses, they were not performed, because they would not have contributed useful information due to the small number of studies (only two trials) reporting clear details on concealment of random allocation (Colonna 2005; Ou 2010). About 20% of studies were not double‐blind (about one fifth), however they compared many different compounds with citalopram, so a sensitivity analysis excluding those studies from the analysis was not meaningful because it would not have been informative.
2) Excluding trials whose dropout rate was greater than 20% Overall, in 16 studies dropout rate was less than 20% in each arm (Bougerol 1997b; de Wilde 1985; Gastpar 2006; Gravem 1987; Hosak 1999; Hsu 2011; Karlsson 2000; Khanzode 2003; Lalit 2004; Leinonen 1999; Lepola 2003; Lewis 2011; Matreja 2007; Moore 2005; Ou 2010; Yevtushenko 2007). However, excluding trials whose dropout rate was greater than 20% from the analysis did not materially change the results.
3) Performing the worst‐ and best‐case scenario analysis Results from these sensitivity analyses did not materially change the main findings (full details available on request from authors).
4) Excluding trials for which imputation methods were used a) Imputed response rate Excluding trials for which the response rate had to be calculated based on the imputation method, results for all comparisons did not materially change. b) Imputed remission rate We did not impute remission rates. c) Borrowed SDs Excluding trials for which the SDs had to be borrowed from other trials, results for all comparisons did not materially change.
5) Examination of “wish bias” and exclusion of studies funded by the pharmaceutical company marketing citalopram These pre‐planned sensitivity analyses were not carried out because we found only a few studies per comparison.
6) Excluding studies that included patients with bipolar depression or psychotic features
After discussion within the review group, we decided not to carry out these two pre‐planned subgroup analyses, because only three studies included bipolar patients (Bougerol 1997a; Hosak 1999; Timmerman 1993) and only one study patients with psychotic symptoms (Navarro 2001).
Discussion
Summary of main results
This systematic review and meta‐analysis included 37 trials that compared citalopram versus other antidepressants in terms of efficacy and tolerability. The included studies did not report on all the outcomes that were pre‐specified in the protocol of this review and only a small number of trials per comparison was found for most ADs (with the exception of escitalopram). The present review showed that citalopram should be considered for treating depression because it was significantly more effective than other ADs (reboxetine and paroxetine) and appeared to be more acceptable than other AD, like tricyclics. The finding that citalopram was less effective than escitalopram should be carefully interpreted considering that all trials included in this comparison were sponsored by the manufacturer of both drugs, and therefore, the possibility of wish bias cannot be ruled out (Barbui 2004). The dataset of the present review collected insufficient randomised evidence to detect a difference in early response to treatment (within four weeks of treatment). Looking at the data reported in the trials included in this systematic review, the question on comparative efficacy of early onset response has yet to be proven and remains a matter of ongoing debate (Gourion 2008).
Overall completeness and applicability of evidence
It has long been argued that placebo controlled trials are required to adequately demonstrate the efficacy of novel antidepressant drugs (Cipriani 2009a), however, in the present review we focused only on the comparison between citalopram and other active treatments. Retrieved randomised evidence compared citalopram with a selection of possible comparator antidepressants but only a few studies per comparison were found. Although the search was thorough, it is still possible that there are unpublished studies that have not been identified but the small number of trials identified per comparison hinders the detection of any publication bias. Although we did our very best to retrieve as much data as possible, through asking pharmaceutical companies and study authors to supply all available information, we can assume that data from some trials are still lacking, most of which are likely to be studies with negative findings. We are also aware of the possibility that a number of additional randomised controlled trials (RCTs) comparing citalopram with other antidepressant drugs are currently being conducted and will be included in future updates of the review.
Quality of the evidence
All included studies were RCTs and were very similar in design and conduct. Using high‐quality research evidence is relevant to review results and to speed translation of research in a way that really responds to clinically relevant questions. However, the quality of RCTs is not easy to assess and the problem of study quality is relevant for interpreting results and for usefulness of results in practice. Despite the fact that RCTs are the best methodological standards for clinical research, included studies failed to report key methodological issues. For example, the majority of trials still do not report adequate information about methods of randomisation and allocation concealment. The reporting of the outcomes in the included studies was often unclear or incomplete and the figures used for the analyses not immediately understandable. The scant information about randomisation and allocation concealment may be a matter of reporting in the text rather than real defects in study design. However, sometimes there were some discrepancies between published reports and unpublished data available on the websites of the pharmaceutical industries. When dealing with summary statistics, the quality of information is important. Meta‐analyses of poor quality studies may be seriously misleading (Ioannidis 2005), because the bias associated with defects in the conduct of primary studies (randomised trials) can seriously affect overall estimates of intervention. Systematic reviewers (not only within The Cochrane Collaboration) should routinely assess the risk of bias in the results of trials, and should report meta‐analyses restricted to trials at low risk of bias (Wood 2008).
Potential biases in the review process
Some possible limitations of this review should be noted.
We had to impute the response rate, our primary outcome, for some of the included trials. However, we consider that imputation of response and remission rates by a validated statistical method (Furukawa 2006) in our review should minimize the risk of bias. Nevertheless, we regret that we were unable to do a sensitivity analysis excluding trials with imputed response rates. As we update this review and assemble more trials involving citalopram, we hope to conduct such a sensitivity analysis and be able to examine if our conclusions are robust.
By making multiple comparisons we might have committed a type 1 error, that is, identifying and reporting a spurious association. As stated in the review protocol, we did not carry out a Bonferroni correction. As many statistical tests have been used in the review, the findings from this review are better thought of as hypothesis forming rather than hypothesis testing and it would be very comforting to see the conclusions replicated in future trials.
Pharmaceutical industry sponsor. Most of included studies were sponsored by the drug industry, and these have been shown to be more than four times likely to demonstrate positive effects of the sponsors' drug as independent studies (Lexchin 2003). The sponsorship bias may play a role also in the issue related to the comparison between citalopram and escitalopram (Leonard 2010). Citalopram is the racemic mixture of S‐citalopram and R‐citalopram and escitalopram is the S‐enantiomer of the racemate citalopram (Sanchez 2004). As for all other new investigational compounds, the potential for overestimation of treatment effect due to sponsorship bias should be borne in mind, as we found marked heterogeneity for the escitalopram comparisons. So, results reported for comparative efficacy favouring escitalopram have therefore to be viewed with caution because a possible inflation of efficacy in favour of escitalopram cannot be ruled out. We asked Lundbeck to have access to individual patient data and we are still waiting for a reply (last contact via e‐mail correspondence: June 2010)
Economic evaluation. In the present review only one RCT reported economic outcomes (Hosak 1999). The authors concluded that limitation of prescription of SSRIs in Czech Republic by health insurance companies did not appear to lead to cost savings, while it may have led to unnecessary patients' suffering due to adverse events of TCAs. Given that several SSRIs and the great majority of antidepressants are now available as generic formulation (only escitalopram and duloxetine are still on patent), more comprehensive economic estimates of antidepressant treatment effect should be considered to better inform healthcare policy.
In this review we decided to focus on treatment response because it is one of the main goals for the treatment of major depressive disorder. The term “treatment response” describes a state of improvement in the patient’s condition of sufficient quality to result in treating the physician’s impression of at least a moderate degree of global improvement, conventionally defined as a reduction of at least 50% in depressive symptomatology. However, from a clinical point of view, the ultimate goal of the acute treatment phase of major depressive disorder may well be to achieve remission. Full remission from depression correlates with better longer‐term functional recovery, lower risk of relapse and higher level of patients satisfaction than partial response (without remission). Thus, one important limitation of the included trials (and consequently of the present review) is that only a few studies reported remission rates, underpowering the analysis and undermining the possibility to find significant differences between comparisons. Moreover, outcomes of clear relevance to patients and clinicians, in particular, patients’ and their carers’ attitudes to treatment, their ability to return to work and resume normal social functioning, were not reported in the included studies.
In this review we included only RCTs. As debate in the scientific literature, one of the main limitations of efficacy trials is to include patients far from “real world” (Rothwell 2005). When drafting the systematic review protocol, we did our best to include as much evidence as possible to inform clinical practice, balancing internal with external validity (Cipriani 2009d). This is the reason why we included single‐blind or non‐blind randomised studies, but on the other hand, decided to exclude patients with medical comorbidity.
As expected, in this review only a few studies reported data about suicide and deliberate self‐harm (Geddes 2004). Deliberate self‐harm, particularly suicide, is often thought to be a relatively “hard” outcome in studies of antidepressants, but enormous scope exists for ascertainment bias. Observational evidence offers insights into long‐term and real‐world outcomes for large groups of people, but it can rarely show a convincing causal relation between two events (Cipriani 2007). Systematic reviews of randomised controlled trials may increase statistical power, but absolute numbers of patients having rare adverse events such as completed or attempted suicide are low. Thus, reporting or not reporting a few cases can completely change the overall outcome (Cipriani 2005b).
Agreements and disagreements with other studies or reviews
Even though it is matter of ongoing discussion in the scientific literature (Gartlehner 2010; Gartlehner 2011), there is now robust evidence that there are statistically and clinically significant differences among antidepressants (Cipriani 2009a). Results from this review are consistent with this interpretation and might contribute to developing and keeping up to date an evidence‐based hierarchy of antidepressants to be used by clinicians (both specialists and general practitioners) (Barbui 2011). Even though citalopram was not among the best treatments in terms of efficacy, it scored well in terms of acceptability and remains an important option for physicians when an AD is to be prescribed for moderate‐to‐severe major depression.
Authors' conclusions
Implications for practice.
Citalopram appears to be a suitable option to be used for moderate‐to‐severe acute major depression because it showed to be more effective than other antidepressants (namely, paroxetine and reboxetine) and it was overall well tolerated.
Implications for research.
Results described in this systematic review come from a set of randomised studies that are in many cases financially supported by pharmaceutical industries. Industry‐sponsored trials tend to follow a standard design which involves short‐term, double‐blind, parallel‐group studies of patients with acute episodes or exacerbations of chronic illness. Moreover, it is known that economic support by drug manufacturer can strongly influence progress of research and its results. Consequently, there is a risk that these studies do not provide sufficient and adequate information to clinicians in real‐world settings. Studies should be conducted with the intent of provide clinicians with useful practical data regarding the comparative effectiveness of marketed medications, and consider rating scale but also pragmatic outcome measures (for example hospitalisations, return to work, social functioning and so on). Considering the methodological limitation of standard systematic reviews that rely only on evidence from direct comparisons and given the wide spectrum of available comparisons for the treatment of major depression, the use of the methodology of multiple treatments meta‐analysis (MTM) may provide a more informative and clinically useful summary of the results that can be used to guide treatment decisions.
Notes
This review is one of a number of separate reviews examining head‐to‐head comparisons as part of the multiple Meta‐Analyses of New Generation Antidepressants (MANGA) Study. These individual reviews have been then combined in a multiple treatments meta‐analysis (Cipriani 2009a).
Acknowledgements
The authors would like to thank Julian Higgins and Georgia Salanti for their helpful comments and feedback on the review protocol. We also would like to thank all authors that provided additional data to be used in the present report and especially Drs. Ladislav Hosak, Sidney Kennedy, Sven Langworth, Glyn Lewis, Stephen Stahl and Thomas Werge. We are grateful to the Fondazione Cariverona, who provided a three‐year Grant to the WHO Collaborating Centre for Research and Training in Mental Health and Service Organisation at the University of Verona, directed by Professor Michele Tansella. The authors would also like to acknowledge and thank Hugh McGuire for his excellent editorial input on this and other MANGA reviews.
Data and analyses
Comparison 1. Failure to respond at endpoint (6‐12 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 3 | 888 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.75, 1.63] |
| 1.1 Versus Amitriptyline | 2 | 416 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.54, 3.87] |
| 1.2 Versus Imipramine | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.58] |
| 2 Citalopram versus heterocyclics | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.56, 1.96] |
| 2.1 Versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.27, 1.62] |
| 2.2 Versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.85, 2.04] |
| 3 Citalopram versus other SSRIs | 13 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 6 | 1806 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [1.08, 2.02] |
| 3.2 Versus Fluoxetine | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.75, 1.43] |
| 3.3 Versus Fluvoxamine | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 3.4 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.96] |
| 3.5 Versus Sertraline | 3 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.20, 1.42] |
| 4 Citalopram versus SNRI | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.46, 1.78] |
| 5 Citalopram versus other conventional ADs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.38, 1.52] |
| 5.2 Versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 6 Citalopram versus non‐conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Versus Hypericum (St. John's wort) | 1 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.57, 1.52] |
Comparison 2. Failure to respond (1‐4 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 4 | 751 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.46, 1.98] |
| 1.1 versus Amitriptyline | 3 | 476 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.76, 2.31] |
| 1.2 versus Imipramine | 1 | 275 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.86] |
| 2 Citalopram versus other SSRIs | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.75, 2.82] |
| 2.2 Versus Fluoxetine | 2 | 416 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.34, 2.34] |
| 2.3 Versus Sertraline | 2 | 245 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.60, 2.15] |
| 3 Citalopram versus other conventional antidepressants | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.75] |
Comparison 3. Failure to respond (16‐24 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Imipramine | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.69, 1.72] |
| 2 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.64, 1.68] |
| 2.2 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.45, 1.17] |
| 3 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.44, 1.82] |
| 4 Citalopram versus other conventional antidepressants | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.30, 0.70] |
3.3. Analysis.
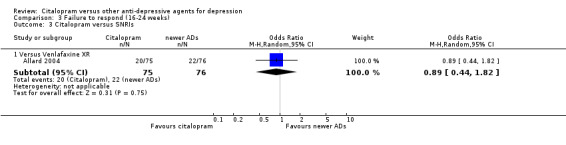
Comparison 3 Failure to respond (16‐24 weeks), Outcome 3 Citalopram versus SNRIs.
Comparison 4. Failure to remission (1‐4 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 3 | 225 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [0.98, 4.63] |
| 1.1 versus Amitriptyline | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 2.29 [0.81, 6.48] |
| 1.2 versus Clomipramine | 1 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 1.95 [0.61, 6.23] |
| 2 Citalopram versus other SSRIs | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.75, 3.15] |
| 2.2 Versus Fluoxetine | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.56, 1.10] |
| 2.3 Versus Sertraline | 1 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 1.86 [0.89, 3.88] |
| 3 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.27, 2.05] |
Comparison 5. Failure to remission (6‐12 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 5 | 256 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.77, 2.26] |
| 1.1 versus Amitriptyline | 2 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.48, 2.32] |
| 1.2 versus Nortriptyline | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.81, 5.29] |
| 1.3 versus Imipramine | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.34, 3.51] |
| 2 Citalopram versus heterocyclics | 2 | 156 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.35, 1.24] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.31, 1.60] |
| 2.2 versus Mianserin | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.21, 1.62] |
| 3 Citalopram versus other SSRIs | 10 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 5 | 1427 | Odds Ratio (M‐H, Random, 95% CI) | 1.94 [1.16, 3.26] |
| 3.2 Versus Fluoxetine | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.42] |
| 3.3 Versus Fluvoxamine | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.23, 1.34] |
| 3.4 Versus Sertraline | 2 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.29, 1.08] |
| 4 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.26] |
| 5 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.92] |
Comparison 6. Failure to remission (16‐24 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.74, 1.84] |
| 2 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.35, 1.70] |
| 3 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.28, 0.65] |
Comparison 7. Standardised mean difference (1‐4 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 4 | 174 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.20, 0.40] |
| 1.1 versus Amitriptyline | 2 | 91 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.38, 0.44] |
| 1.2 versus Nortriptyline | 2 | 83 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.35, 0.79] |
| 2 Citalopram versus heterocyclics | 2 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.81, 0.37] |
| 2.1 versus Maprotiline | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.35, 0.47] |
| 2.2 versus Mianserin | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.07, ‐0.02] |
| 3 Citalopram versus other SSRIs | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 versus Escitalopram | 3 | 657 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.07, 0.24] |
| 3.2 versus Fluoxetine | 4 | 723 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.30, ‐0.01] |
| 3.3 versus Sertraline | 3 | 287 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.76, 0.25] |
| 4 Citalopram versus other conventional ADs | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 versus Reboxetine | 1 | 317 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐2.76, ‐0.24] |
7.4. Analysis.
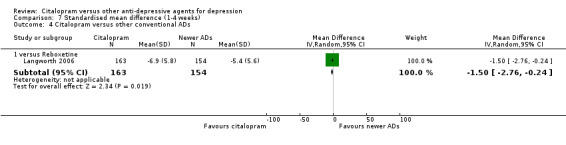
Comparison 7 Standardised mean difference (1‐4 weeks), Outcome 4 Citalopram versus other conventional ADs.
Comparison 8. Standardised mean difference at endpoint (6‐12 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 5 | 402 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.15, 0.26] |
| 1.1 versus Amitriptyline | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.66, 0.53] |
| 1.2 versus Imipramine | 2 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.27, 0.22] |
| 1.3 versus Nortriptyline | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.02, 0.94] |
| 2 Citalopram versus heterocyclics | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.65, 0.29] |
| 2.1 versus Maprotiline | 1 | 73 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.42, 0.50] |
| 2.2 versus Mianserin | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.96, 0.09] |
| 3 Citalopram versus other SSRIs | 16 | 3610 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.11, 0.10] |
| 3.1 versus Escitalopram | 7 | 1872 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [0.05, 0.27] |
| 3.2 versus Fluoxetine | 3 | 672 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.46, 0.11] |
| 3.3 versus Fluvoxamine | 1 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.50, 0.12] |
| 3.4 versus Paroxetine | 1 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.39, 0.16] |
| 3.5 versus Sertraline | 4 | 703 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.31, 0.04] |
| 4 Citalopram versus SNRIs | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 versus Venlafaxine XR | 1 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐2.93, 1.93] |
| 5 Citalopram versus MAOIs or newer ADs | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 versus Moclobemide | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐4.6 [‐8.28, ‐0.92] |
| 6 Citalopram versus other conventional ADs | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 versus Mirtazapine | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.26, 0.22] |
| 6.2 versus Reboxetine | 2 | 866 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.33, 0.04] |
Comparison 9. Standardised mean difference (16‐24 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 versus Imipramine | 1 | 168 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐1.02, 2.82] |
| 2 Citalopram versus SNRIs | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 versus Venlafaxine XR | 1 | 148 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.61, 2.61] |
| 3 Citalopram versus other conventional ADs | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 versus Reboxetine | 1 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.62, 0.02] |
9.2. Analysis.
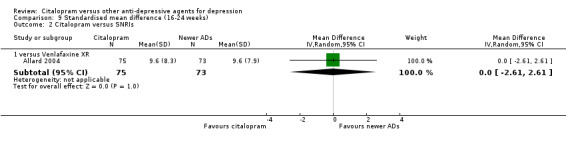
Comparison 9 Standardised mean difference (16‐24 weeks), Outcome 2 Citalopram versus SNRIs.
Comparison 10. Failure to complete (any cause).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 8 | 1209 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.61, 1.07] |
| 1.1 versus Amitriptyline | 4 | 535 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.04] |
| 1.2 versus Clomipramine | 1 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.61, 4.36] |
| 1.3 versus Imipramine | 2 | 517 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.55, 1.41] |
| 1.4 versus Nortriptyline | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.19, 2.08] |
| 2 Citalopram versus heterocyclics | 3 | 492 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.46, 1.22] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.35, 1.96] |
| 2.2 versus Mianserin | 2 | 396 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.29] |
| 3 Citalopram versus other SSRIs | 18 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 8 | 2206 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.64, 1.31] |
| 3.2 Versus Fluoxetine | 4 | 799 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.81, 1.67] |
| 3.3 Versus Fluvoxamine | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.33] |
| 3.4 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.62, 1.63] |
| 3.5 Versus Sertraline | 5 | 911 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 4 Citalopram versus other conventional ADs | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Reboxetine | 4 | 1095 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.42, 1.21] |
| 4.2 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 1.01] |
| 5 Citalopram versus non‐conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Hypericum (St. John's wort) | 1 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 3.01 [0.93, 9.72] |
Comparison 11. Failure to complete (side effects).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 8 | 1216 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.38, 0.78] |
| 1.1 versus Amitriptyline | 3 | 484 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.87] |
| 1.2 versus Clomipramine | 1 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.97] |
| 1.3 versus Imipramine | 2 | 517 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.19] |
| 1.4 versus Nortriptyline | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.34] |
| 2 Citalopram versus heterocyclics | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.21, 1.18] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 4.10] |
| 2.2 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.32] |
| 3 Citalopram versus other SSRIs | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 7 | 1989 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.65, 1.82] |
| 3.2 Versus Fluoxetine | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.80, 2.67] |
| 3.3 Versus Fluvoxamine | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.28, 1.11] |
| 3.4 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.36, 2.09] |
| 3.5 Versus Sertraline | 4 | 860 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.43, 1.09] |
| 4 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 2.02] |
| 5 Citalopram versus other conventional ADs | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.21, 3.12] |
| 5.2 versus Reboxetine | 3 | 494 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.13, 1.27] |
Comparison 12. Failure to complete (inefficacy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 9 | 1267 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.84, 2.57] |
| 1.1 versus Amitriptyline | 4 | 535 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.09] |
| 1.2 versus Clomipramine | 1 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 2.48 [0.72, 8.59] |
| 1.3 versus Imipramine | 2 | 517 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.24, 11.24] |
| 1.4 versus Nortriptyline | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [0.76, 8.53] |
| 2 Citalopram versus heterocyclics | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.24, 1.69] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.17, 2.42] |
| 2.2 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.15, 2.68] |
| 3 Citalopram versus other SSRIs | 14 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 8 | 2206 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.38, 1.66] |
| 3.2 Versus Fluoxetine | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.64, 2.08] |
| 3.3 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.13, 2.42] |
| 3.4 Versus Sertraline | 3 | 760 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.34, 1.60] |
| 4 Citalopram versus other conventional ADs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.28] |
| 4.2 versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.17, 2.57] |
Comparison 13. SE ‐ Subjects with at least one TEAEs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 7 | 1088 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.41] |
| 1.1 versus Amitriptyline | 4 | 528 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.28, 0.65] |
| 1.2 versus Imipramine | 2 | 517 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [1.14, 2.89] |
| 1.3 versus Nortriptyline | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.20, 4.39] |
| 2 Citalopram versus heterocyclics | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.37] |
| 2.1 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.37] |
| 3 Citalopram versus other SSRIs | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 7 | 1979 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.97, 1.47] |
| 3.2 Versus Fluoxetine | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.81, 1.47] |
| 3.3 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.83, 2.18] |
| 3.4 Versus Sertraline | 5 | 902 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.39, 1.16] |
| 4 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.24, 0.88] |
| 5 Citalopram versus MAOIs or newer ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Moclobemide | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.20, 2.35] |
| 6 Citalopram versus other conventional ADs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.73, 2.04] |
| 6.2 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.42, 0.97] |
| 7 Citalopram versus non‐conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 versus Hypericum (St. John's wort) | 1 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [1.01, 2.83] |
Comparison 14. SE ‐ Abdominal pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
| 1.2 Versus Fluoxetine | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.55, 4.53] |
14.1. Analysis.
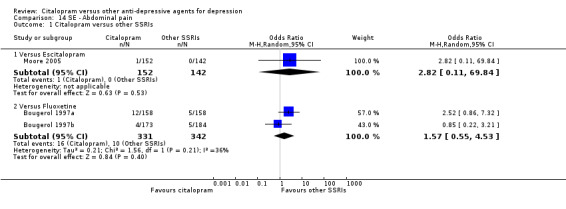
Comparison 14 SE ‐ Abdominal pain, Outcome 1 Citalopram versus other SSRIs.
Comparison 15. SE ‐ Accidental injury.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.21] |
15.1. Analysis.
Comparison 15 SE ‐ Accidental injury, Outcome 1 Citalopram versus other SSRIs.
Comparison 16. SE ‐ Aggressive behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.65] |
16.1. Analysis.
Comparison 16 SE ‐ Aggressive behaviour, Outcome 1 Citalopram versus other SSRIs.
Comparison 17. SE ‐ Anorexia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 2 | 448 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.06, 7.29] |
17.1. Analysis.
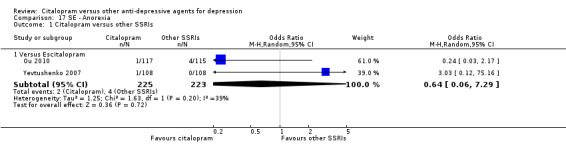
Comparison 17 SE ‐ Anorexia, Outcome 1 Citalopram versus other SSRIs.
Comparison 18. SE ‐ Anxiety/agitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 1.4 [0.35, 5.54] |
| 1.1 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 1.4 [0.35, 5.54] |
| 2 Citalopram versus heterocyclics | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.16, 5.16] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 4.10] |
| 2.2 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.58, 3.81] |
| 3 Citalopram versus other SSRIs | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 2 | 437 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.07, 9.51] |
| 3.2 Versus Fluoxetine | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.50, 2.16] |
| 3.3 Versus Sertraline | 1 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 2.96 [0.30, 29.12] |
Comparison 19. SE ‐ Appetite increased.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.46] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.46] |
| 2 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.03, 0.72] |
19.1. Analysis.
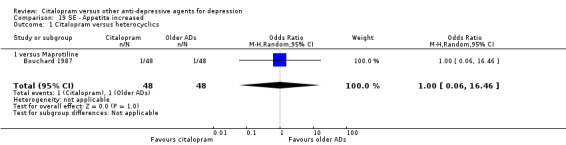
Comparison 19 SE ‐ Appetite increased, Outcome 1 Citalopram versus heterocyclics.
Comparison 20. SE ‐ Asthenia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Amitriptyline | 1 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.11, 2.35] |
| 1.2 Versus Imipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.6 [0.09, 4.01] |
| 2 Citalopram versus other SSRIs | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.13, 6.72] |
| 2.2 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.96, 3.00] |
| 2.3 Versus Sertraline | 2 | 442 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.11, 1.37] |
20.1. Analysis.
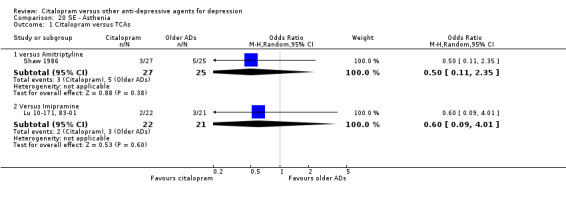
Comparison 20 SE ‐ Asthenia, Outcome 1 Citalopram versus TCAs.
20.2. Analysis.
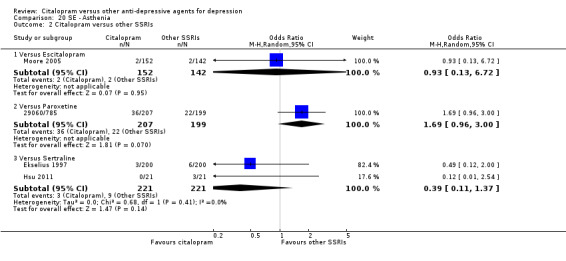
Comparison 20 SE ‐ Asthenia, Outcome 2 Citalopram versus other SSRIs.
Comparison 21. SE ‐ Back pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.22, 1.75] |
| 1.1 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.22, 1.75] |
| 2 Citalopram versus other SSRIs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 2 | 605 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.34, 5.51] |
| 2.2 Versus Fluoxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 12.04 [0.66, 219.46] |
21.1. Analysis.
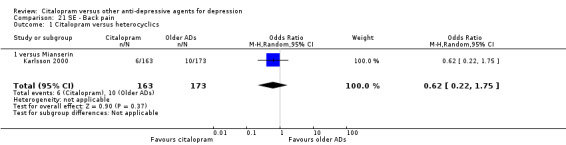
Comparison 21 SE ‐ Back pain, Outcome 1 Citalopram versus heterocyclics.
21.2. Analysis.
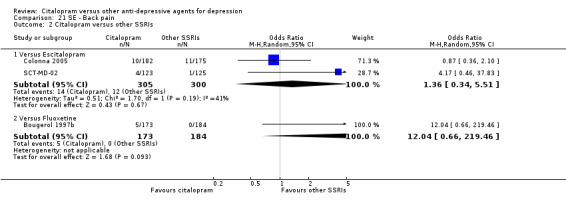
Comparison 21 SE ‐ Back pain, Outcome 2 Citalopram versus other SSRIs.
Comparison 22. SE ‐ Brest surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
22.1. Analysis.
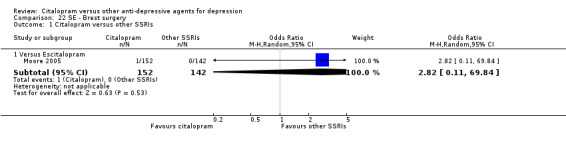
Comparison 22 SE ‐ Brest surgery, Outcome 1 Citalopram versus other SSRIs.
Comparison 23. SE ‐ Bronchitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.02] |
| 1.2 Versus Fluoxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.23, 2.42] |
23.1. Analysis.
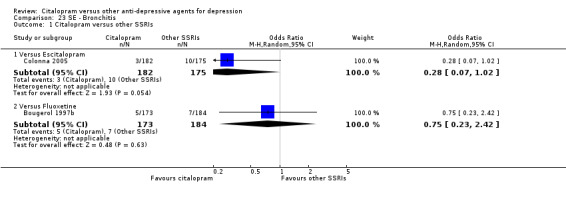
Comparison 23 SE ‐ Bronchitis, Outcome 1 Citalopram versus other SSRIs.
Comparison 24. SE ‐ Chest pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
24.1. Analysis.
Comparison 24 SE ‐ Chest pain, Outcome 1 Citalopram versus other SSRIs.
Comparison 25. SE ‐ Chicken pox.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.65] |
25.1. Analysis.
Comparison 25 SE ‐ Chicken pox, Outcome 1 Citalopram versus other SSRIs.
Comparison 26. SE ‐ Common cold.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 4.11] |
26.1. Analysis.
Comparison 26 SE ‐ Common cold, Outcome 1 Citalopram versus SNRIs.
Comparison 27. SE ‐ Concentration decrease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 2 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.04, 5.53] |
| 3 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 4.13] |
27.1. Analysis.
Comparison 27 SE ‐ Concentration decrease, Outcome 1 Citalopram versus heterocyclics.
27.2. Analysis.
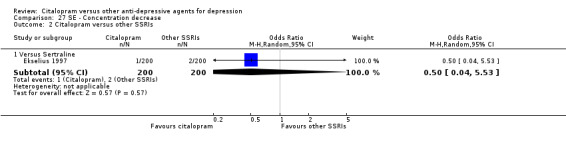
Comparison 27 SE ‐ Concentration decrease, Outcome 2 Citalopram versus other SSRIs.
27.3. Analysis.
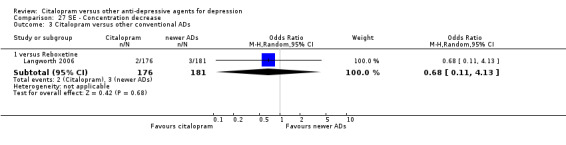
Comparison 27 SE ‐ Concentration decrease, Outcome 3 Citalopram versus other conventional ADs.
Comparison 28. SE ‐ Confusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Amitriptyline | 1 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.06, 1.83] |
| 1.2 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 3.36 [0.13, 88.39] |
| 2 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.69] |
28.1. Analysis.
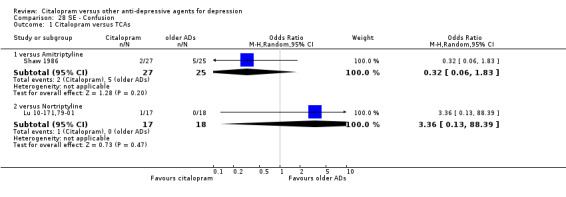
Comparison 28 SE ‐ Confusion, Outcome 1 Citalopram versus TCAs.
28.2. Analysis.
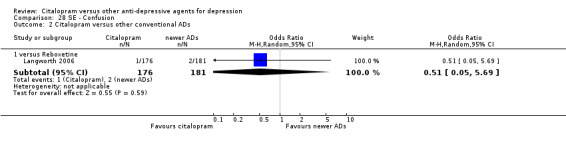
Comparison 28 SE ‐ Confusion, Outcome 2 Citalopram versus other conventional ADs.
Comparison 29. SE ‐ Conjunctivitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 2.77 [0.11, 71.35] |
| 1.1 versus Amitriptyline | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 2.77 [0.11, 71.35] |
29.1. Analysis.
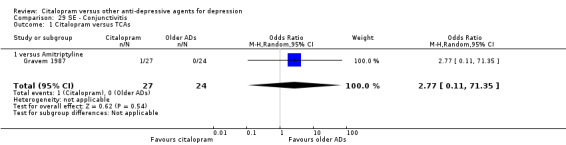
Comparison 29 SE ‐ Conjunctivitis, Outcome 1 Citalopram versus TCAs.
Comparison 30. SE ‐ Constipation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 6 | 1018 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.55] |
| 1.1 versus Amitriptyline | 3 | 468 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.23, 0.90] |
| 1.2 versus Imipramine | 2 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.18, 0.53] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.09, 2.09] |
| 2 Citalopram versus heterocyclics | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.24, 2.00] |
| 2.1 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.24, 2.00] |
| 3 Citalopram versus other SSRIs | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
| 3.2 Versus Fluoxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.07, 1.74] |
| 3.3 Versus Sertraline | 2 | 442 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.88] |
| 4 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [0.50, 14.07] |
| 5 Citalopram versus other conventional ADs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 0.90] |
30.4. Analysis.
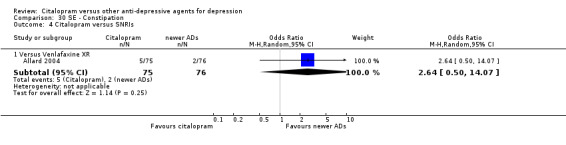
Comparison 30 SE ‐ Constipation, Outcome 4 Citalopram versus SNRIs.
Comparison 31. SE ‐ Craving for sweets.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 5.22 [0.24, 111.55] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 5.22 [0.24, 111.55] |
31.1. Analysis.
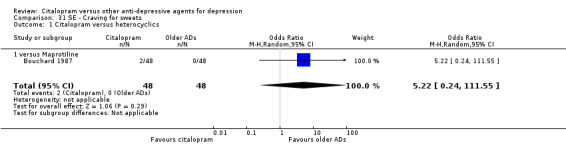
Comparison 31 SE ‐ Craving for sweets, Outcome 1 Citalopram versus heterocyclics.
Comparison 32. SE ‐ Decreased weight.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Fluoxetine | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.25, 1.50] |
| 1.2 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 2.23 [0.98, 5.05] |
| 2 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 1.00] |
32.1. Analysis.
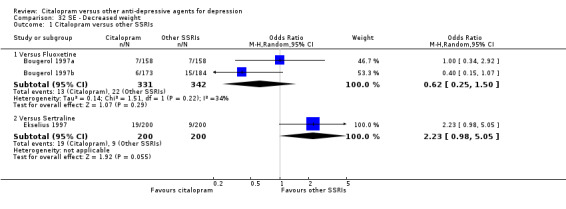
Comparison 32 SE ‐ Decreased weight, Outcome 1 Citalopram versus other SSRIs.
32.2. Analysis.
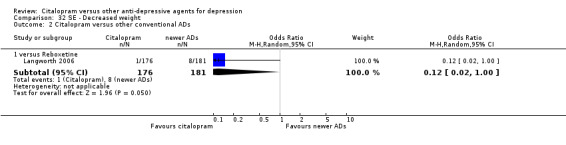
Comparison 32 SE ‐ Decreased weight, Outcome 2 Citalopram versus other conventional ADs.
Comparison 33. SE ‐ Dermatological problems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.05, 14.96] |
| 1.1 versus Amitriptyline | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.05, 14.96] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.46] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.46] |
| 3 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 1 | 219 | Odds Ratio (M‐H, Random, 95% CI) | 2.0 [0.18, 22.38] |
| 3.2 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.36, 4.02] |
| 4 Citalopram versus non‐conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Hypericum (St. John's wort) | 1 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.43, 5.72] |
33.1. Analysis.
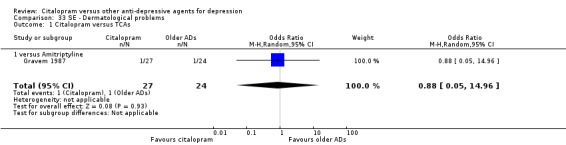
Comparison 33 SE ‐ Dermatological problems, Outcome 1 Citalopram versus TCAs.
33.2. Analysis.
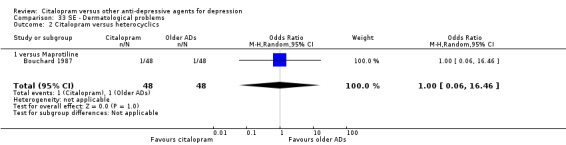
Comparison 33 SE ‐ Dermatological problems, Outcome 2 Citalopram versus heterocyclics.
33.3. Analysis.
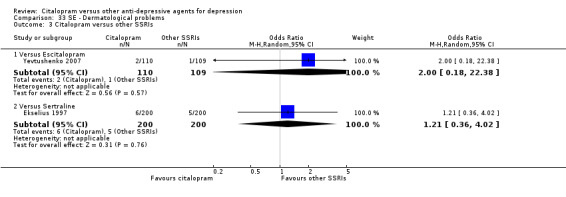
Comparison 33 SE ‐ Dermatological problems, Outcome 3 Citalopram versus other SSRIs.
33.4. Analysis.
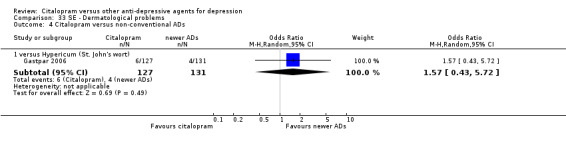
Comparison 33 SE ‐ Dermatological problems, Outcome 4 Citalopram versus non‐conventional ADs.
Comparison 34. SE ‐ Diarrhoea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 2 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.26, 6.16] |
| 1.1 versus Amitriptyline | 1 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [0.16, 22.58] |
| 1.2 versus Imipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.12, 7.44] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 7.40] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 7.40] |
| 3 Citalopram versus other SSRIs | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 4 | 1247 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.78, 1.92] |
| 3.2 Versus Fluoxetine | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 2.11 [0.34, 13.22] |
| 3.3 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.41, 1.32] |
| 3.4 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.29, 1.37] |
| 4 Citalopram versus other conventional ADs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [0.63, 7.24] |
| 4.2 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.17] |
34.2. Analysis.
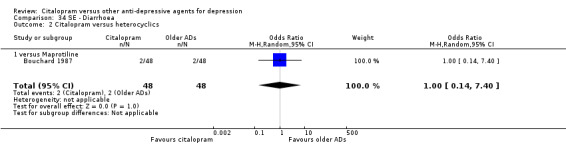
Comparison 34 SE ‐ Diarrhoea, Outcome 2 Citalopram versus heterocyclics.
Comparison 35. SE ‐ Dizziness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 5 | 546 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.27, 1.27] |
| 1.1 versus Amitriptyline | 3 | 468 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.15, 1.44] |
| 1.2 versus Imipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.10, 1.22] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.44, 7.48] |
| 2 Citalopram versus heterocyclics | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.22, 2.68] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.43, 5.00] |
| 2.2 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.33] |
| 3 Citalopram versus other SSRIs | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 5 | 1136 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.43, 1.81] |
| 3.2 Versus Sertraline | 2 | 545 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.41, 1.39] |
| 4 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.16, 3.47] |
| 5 Citalopram versus other conventional ADs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.18, 1.35] |
| 5.2 versus Reboxetine | 1 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.31, 1.81] |
35.1. Analysis.
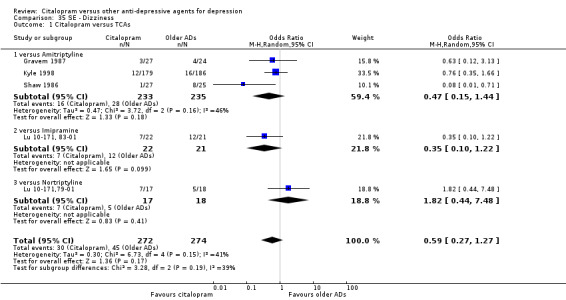
Comparison 35 SE ‐ Dizziness, Outcome 1 Citalopram versus TCAs.
35.2. Analysis.
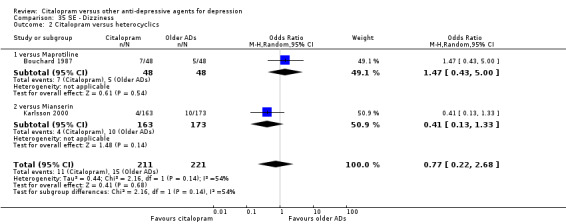
Comparison 35 SE ‐ Dizziness, Outcome 2 Citalopram versus heterocyclics.
35.3. Analysis.
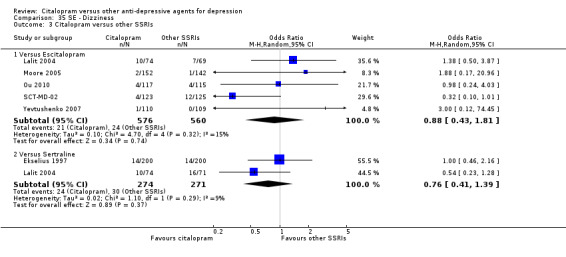
Comparison 35 SE ‐ Dizziness, Outcome 3 Citalopram versus other SSRIs.
35.4. Analysis.
Comparison 35 SE ‐ Dizziness, Outcome 4 Citalopram versus SNRIs.
35.5. Analysis.
Comparison 35 SE ‐ Dizziness, Outcome 5 Citalopram versus other conventional ADs.
Comparison 36. SE ‐ Dry mouth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 7 | 1078 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.18, 0.35] |
| 1.1 versus Amitriptyline | 4 | 528 | Odds Ratio (M‐H, Random, 95% CI) | 0.17 [0.10, 0.28] |
| 1.2 versus Imipramine | 2 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.21, 0.50] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.11, 1.70] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.30, 1.79] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.30, 1.79] |
| 3 Citalopram versus other SSRIs | 10 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 5 | 1457 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.60, 1.62] |
| 3.2 Versus Fluoxetine | 2 | 416 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.02, 11.57] |
| 3.3 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.60, 1.79] |
| 3.4 Versus Sertraline | 2 | 442 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.35, 1.20] |
| 4 Citalopram versus SNRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Venlafaxine XR | 1 | 151 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.42, 3.18] |
| 5 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.25, 1.10] |
Comparison 37. SE ‐ Dyspepsia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [0.18, 23.32] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [0.18, 23.32] |
| 2 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 219 | Odds Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 74.45] |
37.1. Analysis.
Comparison 37 SE ‐ Dyspepsia, Outcome 1 Citalopram versus heterocyclics.
37.2. Analysis.
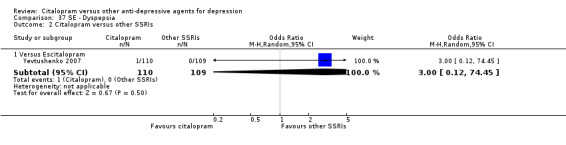
Comparison 37 SE ‐ Dyspepsia, Outcome 2 Citalopram versus other SSRIs.
Comparison 38. SE ‐ Dyspnea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.22] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.22] |
38.1. Analysis.
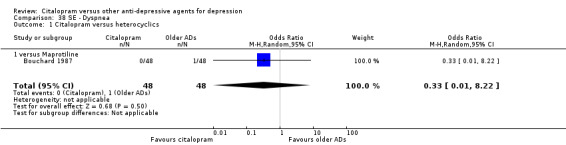
Comparison 38 SE ‐ Dyspnea, Outcome 1 Citalopram versus heterocyclics.
Comparison 39. SE ‐ Emotional indifference.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 2.01 [0.18, 22.35] |
| 2 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.28] |
39.1. Analysis.
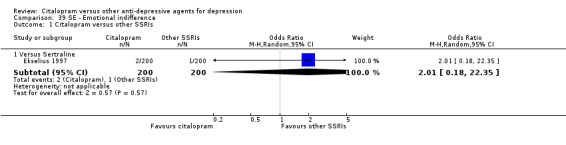
Comparison 39 SE ‐ Emotional indifference, Outcome 1 Citalopram versus other SSRIs.
39.2. Analysis.
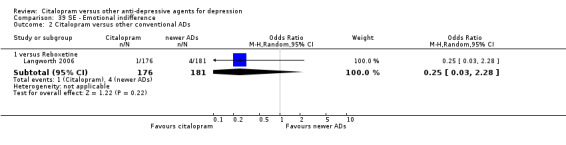
Comparison 39 SE ‐ Emotional indifference, Outcome 2 Citalopram versus other conventional ADs.
Comparison 40. SE ‐ Enuresis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.65] |
40.1. Analysis.
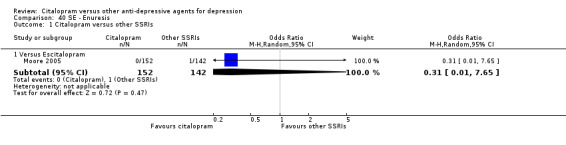
Comparison 40 SE ‐ Enuresis, Outcome 1 Citalopram versus other SSRIs.
Comparison 41. SE ‐ Exacerbation of depressive disorder.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
41.1. Analysis.
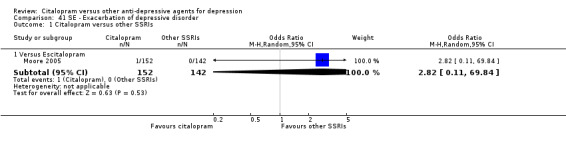
Comparison 41 SE ‐ Exacerbation of depressive disorder, Outcome 1 Citalopram versus other SSRIs.
Comparison 42. SE ‐ Fatigue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.53] |
| 1.1 versus Amitriptyline | 1 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.53] |
| 2 Citalopram versus heterocyclics | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.76] |
| 2.1 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.76] |
| 3 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 2 | 467 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.84] |
| 4 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.54, 2.25] |
42.1. Analysis.
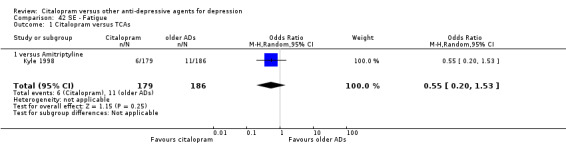
Comparison 42 SE ‐ Fatigue, Outcome 1 Citalopram versus TCAs.
42.4. Analysis.
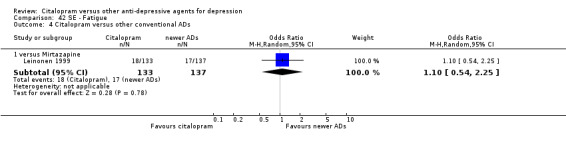
Comparison 42 SE ‐ Fatigue, Outcome 4 Citalopram versus other conventional ADs.
Comparison 43. SE ‐ Feeling of numbness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 5.22 [0.24, 111.55] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 5.22 [0.24, 111.55] |
43.1. Analysis.
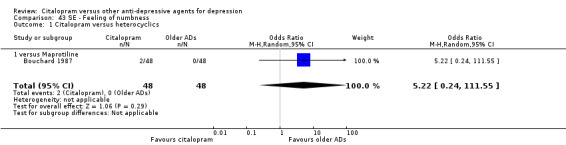
Comparison 43 SE ‐ Feeling of numbness, Outcome 1 Citalopram versus heterocyclics.
Comparison 44. SE ‐ Forgetfulness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 1.78 [0.51, 6.17] |
44.1. Analysis.
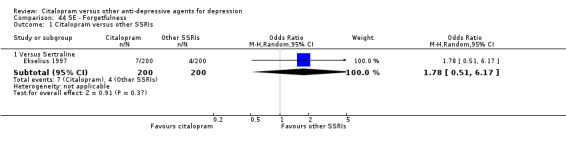
Comparison 44 SE ‐ Forgetfulness, Outcome 1 Citalopram versus other SSRIs.
Comparison 45. SE ‐ Gastrointestinal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 3 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.28, 2.15] |
| 1.1 versus Amitriptyline | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.10, 2.07] |
| 1.2 versus Imipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 1.2 [0.30, 4.74] |
| 2 Citalopram versus other SSRIs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 2 | 375 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.54, 2.40] |
| 2.2 Versus Sertraline | 2 | 545 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.30, 1.30] |
| 3 Citalopram versus MAOIs or newer ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Moclobemide | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.28, 4.47] |
| 4 Citalopram versus non‐conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Hypericum (St. John's wort) | 1 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [1.12, 5.18] |
45.1. Analysis.
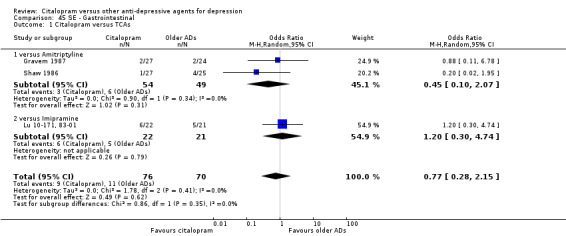
Comparison 45 SE ‐ Gastrointestinal, Outcome 1 Citalopram versus TCAs.
45.2. Analysis.
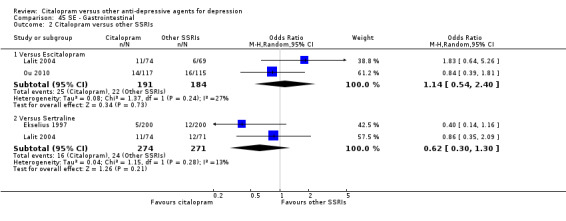
Comparison 45 SE ‐ Gastrointestinal, Outcome 2 Citalopram versus other SSRIs.
45.3. Analysis.
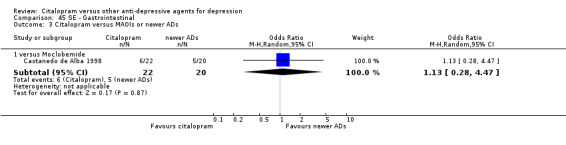
Comparison 45 SE ‐ Gastrointestinal, Outcome 3 Citalopram versus MAOIs or newer ADs.
Comparison 46. SE ‐ Headache.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 6 | 606 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.78, 2.42] |
| 1.1 versus Amitriptyline | 4 | 528 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.65, 2.42] |
| 1.2 versus Imipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 3.56 [0.63, 20.15] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.25, 4.70] |
| 2 Citalopram versus heterocyclics | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.62, 2.60] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [0.50, 9.12] |
| 2.2 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.46, 2.45] |
| 3 Citalopram versus other SSRIs | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 5 | 1261 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.64, 1.81] |
| 3.2 Versus Fluoxetine | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.51, 1.60] |
| 3.3 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.79, 1.96] |
| 3.4 Versus Sertraline | 3 | 587 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.33, 0.91] |
| 4 Citalopram versus IMAOs or newer ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Moclobemide | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 3.64] |
| 5 Citalopram versusother conventional ADs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.75, 3.37] |
| 5.2 versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.25, 1.00] |
46.1. Analysis.
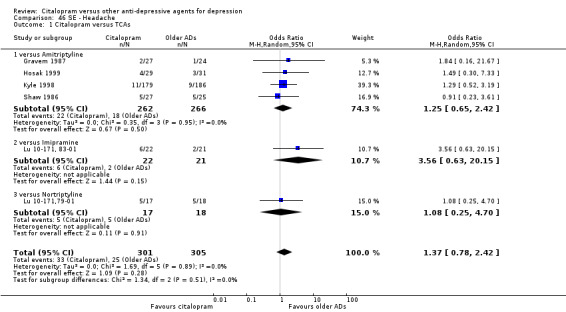
Comparison 46 SE ‐ Headache, Outcome 1 Citalopram versus TCAs.
46.2. Analysis.
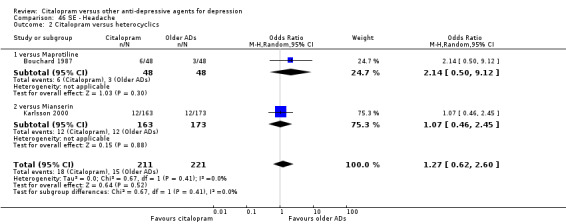
Comparison 46 SE ‐ Headache, Outcome 2 Citalopram versus heterocyclics.
46.4. Analysis.
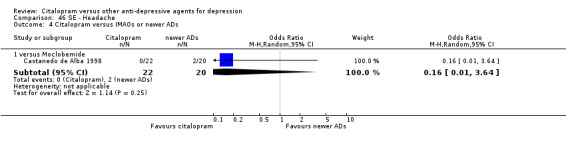
Comparison 46 SE ‐ Headache, Outcome 4 Citalopram versus IMAOs or newer ADs.
46.5. Analysis.
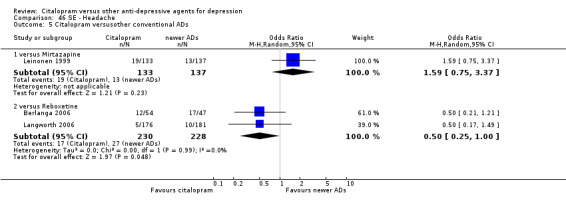
Comparison 46 SE ‐ Headache, Outcome 5 Citalopram versusother conventional ADs.
Comparison 47. SE ‐ Hot flush.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.65] |
47.1. Analysis.
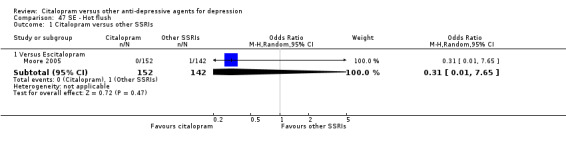
Comparison 47 SE ‐ Hot flush, Outcome 1 Citalopram versus other SSRIs.
Comparison 48. SE ‐ Hypertonia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.46] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.46] |
48.1. Analysis.
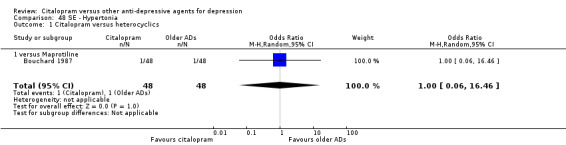
Comparison 48 SE ‐ Hypertonia, Outcome 1 Citalopram versus heterocyclics.
Comparison 49. SE ‐ Hypotension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Imipramine | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.19, 0.75] |
| 2 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.65] |
Comparison 50. SE ‐ Increased dream activity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.5 [0.17, 1.49] |
50.1. Analysis.
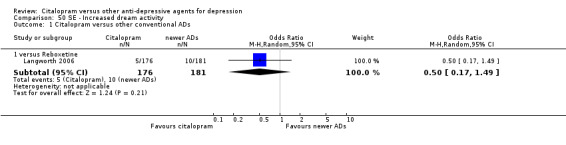
Comparison 50 SE ‐ Increased dream activity, Outcome 1 Citalopram versus other conventional ADs.
Comparison 51. SE ‐ Increased salivation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus older ADs | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 2 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 3.02 [0.12, 74.46] |
| 3 Citalopram versus newer ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.27] |
51.1. Analysis.
Comparison 51 SE ‐ Increased salivation, Outcome 1 Citalopram versus older ADs.
51.2. Analysis.
Comparison 51 SE ‐ Increased salivation, Outcome 2 Citalopram versus other SSRIs.
51.3. Analysis.
Comparison 51 SE ‐ Increased salivation, Outcome 3 Citalopram versus newer ADs.
Comparison 52. SE ‐ Infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus non‐conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Hypericum (St. John's wort) | 1 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.43, 1.72] |
52.1. Analysis.
Comparison 52 SE ‐ Infection, Outcome 1 Citalopram versus non‐conventional ADs.
Comparison 53. SE ‐ Influenza‐like symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Fluoxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.07, 1.74] |
| 2 Citalopram versus other conventional ADs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.11, 1.69] |
| 2.2 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.44, 3.09] |
53.1. Analysis.
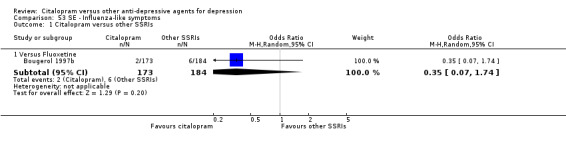
Comparison 53 SE ‐ Influenza‐like symptoms, Outcome 1 Citalopram versus other SSRIs.
53.2. Analysis.
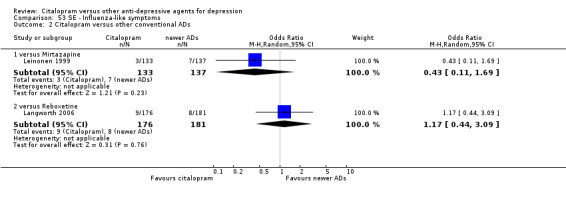
Comparison 53 SE ‐ Influenza‐like symptoms, Outcome 2 Citalopram versus other conventional ADs.
Comparison 54. SE ‐ Insomnia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 2 | 532 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.58, 4.69] |
| 1.1 versus Amitriptyline | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 3.78 [0.70, 20.53] |
| 1.2 versus Imipramine | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.52, 2.59] |
| 2 Citalopram versus heterocyclics | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 2.94 [1.20, 7.25] |
| 2.1 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 2.94 [1.20, 7.25] |
| 3 Citalopram versus other SSRIs | 12 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 6 | 1613 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.60, 1.30] |
| 3.2 Versus Fluoxetine | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.60, 2.23] |
| 3.3 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.53, 1.59] |
| 3.4 Versus Sertraline | 3 | 587 | Odds Ratio (M‐H, Random, 95% CI) | 1.54 [0.82, 2.91] |
| 4 Citalopram versus MAOIs or newer ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Moclobemide | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 7.51] |
| 5 Citalopram versus other conventional ADs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.05, 1.99] |
Comparison 55. SE ‐ Irritability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.26, 1.09] |
| 1.1 versus Imipramine | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.26, 1.09] |
55.1. Analysis.
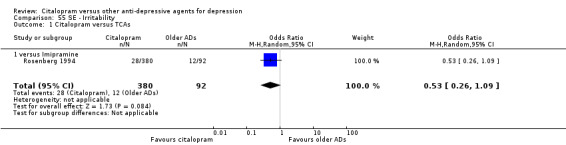
Comparison 55 SE ‐ Irritability, Outcome 1 Citalopram versus TCAs.
Comparison 56. SE ‐ Loss of hair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 2.77 [0.11, 71.35] |
| 1.1 versus Amitriptyline | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 2.77 [0.11, 71.35] |
56.1. Analysis.
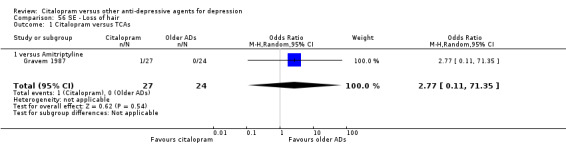
Comparison 56 SE ‐ Loss of hair, Outcome 1 Citalopram versus TCAs.
Comparison 57. SE ‐ Memory impairment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 4.73 [0.23, 99.47] |
| 2 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 4.13] |
57.1. Analysis.
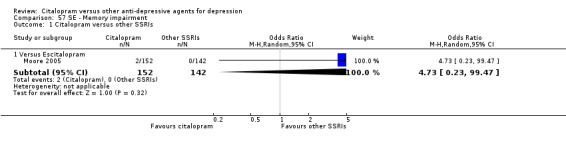
Comparison 57 SE ‐ Memory impairment, Outcome 1 Citalopram versus other SSRIs.
57.2. Analysis.
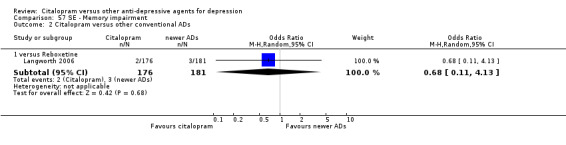
Comparison 57 SE ‐ Memory impairment, Outcome 2 Citalopram versus other conventional ADs.
Comparison 58. SE ‐ Meteorism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 7.33] |
| 1.1 versus Amitriptyline | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 7.33] |
58.1. Analysis.
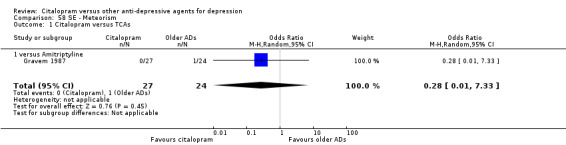
Comparison 58 SE ‐ Meteorism, Outcome 1 Citalopram versus TCAs.
Comparison 59. SE ‐ Musculoskeletal and connective tissue disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus non‐conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Hypericum (St. John's wort) | 1 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.25, 2.87] |
59.1. Analysis.
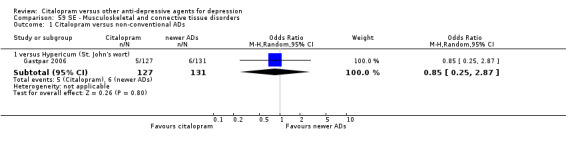
Comparison 59 SE ‐ Musculoskeletal and connective tissue disorders, Outcome 1 Citalopram versus non‐conventional ADs.
Comparison 60. SE ‐ Nasal congestion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.46] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.46] |
60.1. Analysis.
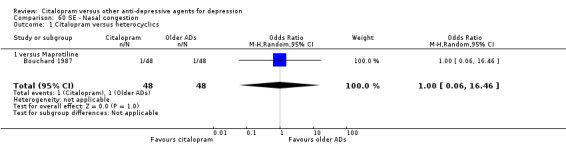
Comparison 60 SE ‐ Nasal congestion, Outcome 1 Citalopram versus heterocyclics.
Comparison 61. SE ‐ Nausea/vomiting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 6 | 1027 | Odds Ratio (M‐H, Random, 95% CI) | 1.78 [0.96, 3.30] |
| 1.1 versus Amitriptyline | 3 | 477 | Odds Ratio (M‐H, Random, 95% CI) | 2.44 [1.27, 4.66] |
| 1.2 versus Imipramine | 2 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.55, 1.73] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 7.11 [1.23, 40.98] |
| 2 Citalopram versus heterocyclics | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.60, 13.23] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 12.26 [0.66, 228.27] |
| 2.2 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.89, 3.97] |
| 3 Citalopram versus other SSRIs | 12 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 7 | 2055 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.49, 1.74] |
| 3.2 Versus Fluoxetine | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.91, 2.35] |
| 3.3 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.67, 1.95] |
| 3.4 Versus Sertraline | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.14, 3.64] |
| 4 Citalopram versus other conventional ADs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 2.24 [1.12, 4.49] |
| 4.2 versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 3.46 [0.40, 29.92] |
Comparison 62. SE ‐ Nervousness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Fluoxetine | 1 | 316 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.36, 4.04] |
62.1. Analysis.
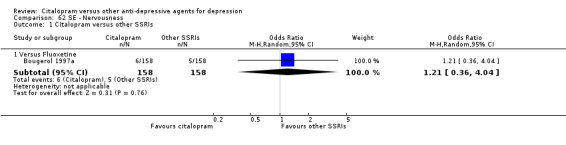
Comparison 62 SE ‐ Nervousness, Outcome 1 Citalopram versus other SSRIs.
Comparison 63. SE ‐ Orthostatic symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.19, 5.22] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.19, 5.22] |
| 2 Citalopram versus newer ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.13, 1.47] |
63.1. Analysis.
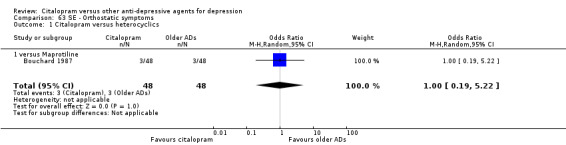
Comparison 63 SE ‐ Orthostatic symptoms, Outcome 1 Citalopram versus heterocyclics.
63.2. Analysis.
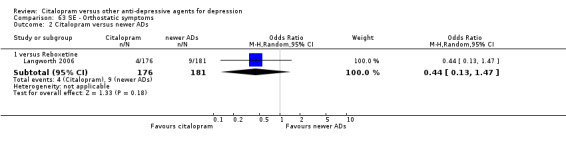
Comparison 63 SE ‐ Orthostatic symptoms, Outcome 2 Citalopram versus newer ADs.
Comparison 64. SE ‐ Pain (general).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.24, 2.00] |
| 1.1 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.24, 2.00] |
64.1. Analysis.
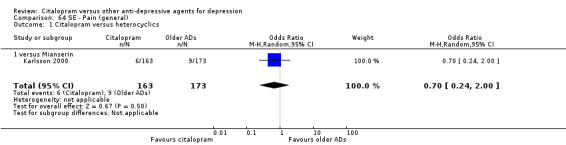
Comparison 64 SE ‐ Pain (general), Outcome 1 Citalopram versus heterocyclics.
Comparison 65. SE ‐ Palpitations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 3 | 138 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.21, 1.41] |
| 1.1 versus Amitriptyline | 2 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.24] |
| 1.2 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.22, 5.22] |
| 2 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.65] |
| 2.2 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.46, 3.96] |
65.1. Analysis.
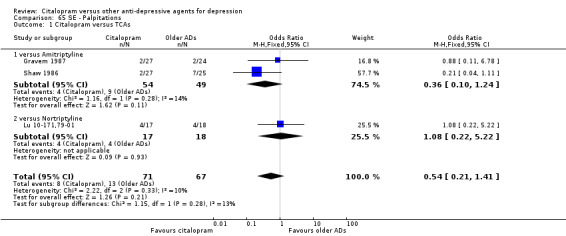
Comparison 65 SE ‐ Palpitations, Outcome 1 Citalopram versus TCAs.
65.2. Analysis.
Comparison 65 SE ‐ Palpitations, Outcome 2 Citalopram versus other SSRIs.
Comparison 66. SE ‐ Panic attack.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
66.1. Analysis.
Comparison 66 SE ‐ Panic attack, Outcome 1 Citalopram versus other SSRIs.
Comparison 67. SE ‐ Paraesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.29, 3.62] |
67.1. Analysis.
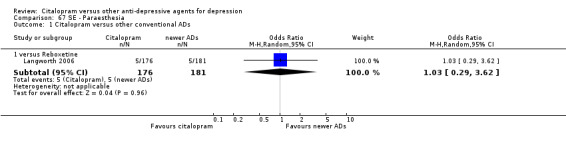
Comparison 67 SE ‐ Paraesthesia, Outcome 1 Citalopram versus other conventional ADs.
Comparison 68. SE ‐ Pharyngitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.65] |
68.1. Analysis.
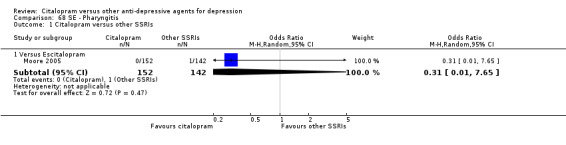
Comparison 68 SE ‐ Pharyngitis, Outcome 1 Citalopram versus other SSRIs.
Comparison 69. SE ‐ Pruritus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.41, 10.53] |
| 2 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
| 2.2 Versus Fluoxetine | 1 | 316 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.07, 2.05] |
69.1. Analysis.
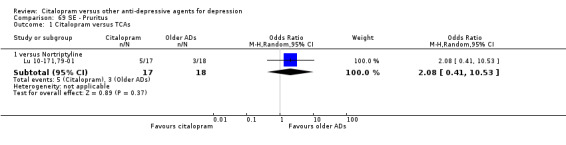
Comparison 69 SE ‐ Pruritus, Outcome 1 Citalopram versus TCAs.
69.2. Analysis.
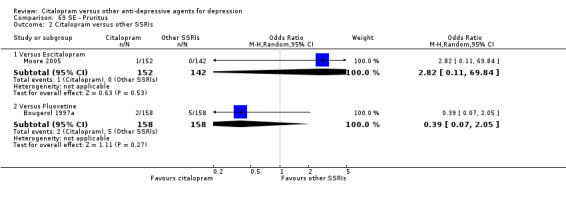
Comparison 69 SE ‐ Pruritus, Outcome 2 Citalopram versus other SSRIs.
Comparison 70. SE ‐ Rash.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Amitriptyline | 1 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.05, 15.59] |
| 2 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Escitalopram | 1 | 219 | Odds Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 74.45] |
| 3 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.09, 2.81] |
70.1. Analysis.
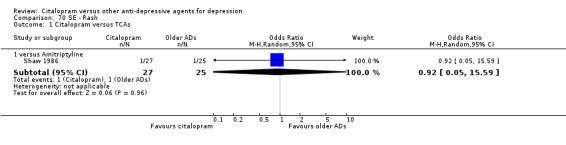
Comparison 70 SE ‐ Rash, Outcome 1 Citalopram versus TCAs.
70.2. Analysis.
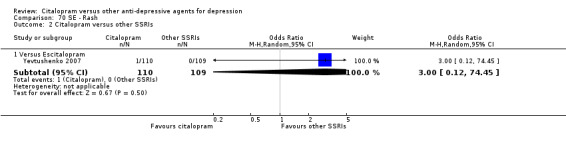
Comparison 70 SE ‐ Rash, Outcome 2 Citalopram versus other SSRIs.
70.3. Analysis.
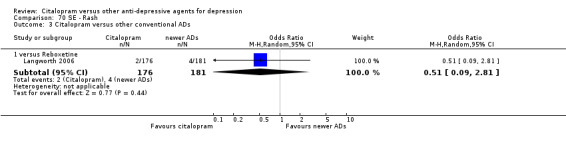
Comparison 70 SE ‐ Rash, Outcome 3 Citalopram versus other conventional ADs.
Comparison 71. SE ‐ Reduced salivation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.67] |
Comparison 72. SE ‐ Sedation/drowsiness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 2 | 112 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.70] |
| 1.1 versus Amitriptyline | 2 | 112 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.70] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.20, 1.90] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.20, 1.90] |
| 3 Citalopram versus other SSRIs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.65] |
| 3.2 Versus Fluoxetine | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.14, 7.90] |
| 3.3 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.36, 2.25] |
| 4 Citalopram versus other conventional ADs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.29, 1.88] |
| 4.2 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.34, 2.41] |
Comparison 73. SE ‐ Rhinitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 3 | 922 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.87] |
73.1. Analysis.
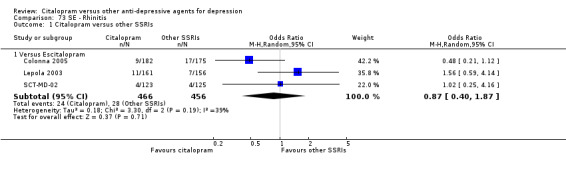
Comparison 73 SE ‐ Rhinitis, Outcome 1 Citalopram versus other SSRIs.
Comparison 74. SE ‐ Restlessness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 3 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.24, 1.99] |
| 1.1 versus Amitriptyline | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.18, 2.82] |
| 1.2 versus Imipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.13, 3.44] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.63] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.63] |
74.1. Analysis.
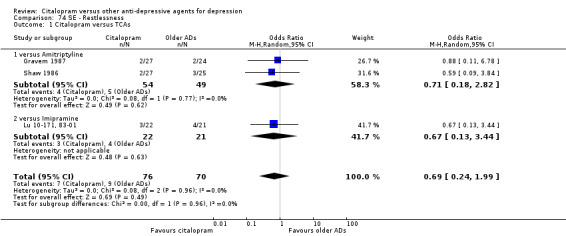
Comparison 74 SE ‐ Restlessness, Outcome 1 Citalopram versus TCAs.
74.2. Analysis.
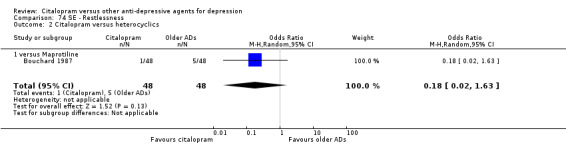
Comparison 74 SE ‐ Restlessness, Outcome 2 Citalopram versus heterocyclics.
Comparison 75. SE ‐ Sexual problems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anorgasmia | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 1.96 [0.97, 3.97] |
| 2 Erectile dysfunction | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Escitalopram | 1 | 317 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 4.02] |
| 2.2 versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 2.38 [0.61, 9.34] |
| 3 Increased sexual desire | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Escitalopram | 1 | 248 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.43, 3.10] |
| 3.2 versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [0.82, 5.26] |
| 4 Loss of sexual interest | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Moclobemide | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 7.51] |
| 4.2 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.41, 7.37] |
| 4.3 versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.66] |
| 5 Orgastic dysfunction | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 3.74 [1.56, 8.95] |
| 6 Other sexual problems | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 versus Escitalopram | 4 | 1015 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.43] |
| 6.2 versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.50, 2.62] |
| 6.3 versus Reboxetine | 1 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 8.65 [1.86, 40.22] |
| 6.4 versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.68, 4.12] |
75.1. Analysis.
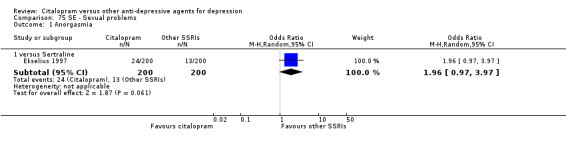
Comparison 75 SE ‐ Sexual problems, Outcome 1 Anorgasmia.
75.2. Analysis.
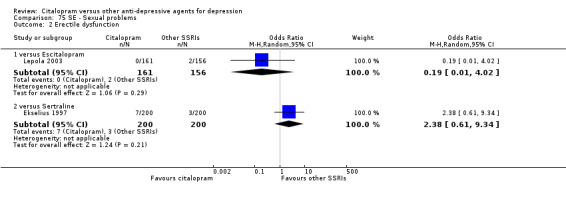
Comparison 75 SE ‐ Sexual problems, Outcome 2 Erectile dysfunction.
75.3. Analysis.
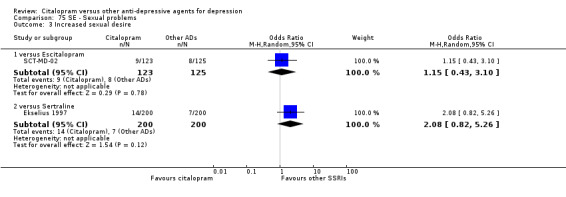
Comparison 75 SE ‐ Sexual problems, Outcome 3 Increased sexual desire.
75.4. Analysis.
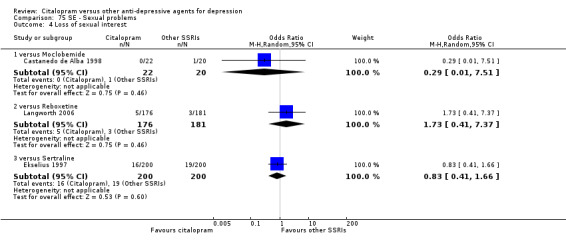
Comparison 75 SE ‐ Sexual problems, Outcome 4 Loss of sexual interest.
Comparison 76. SE ‐ Sleepiness/somnolence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 5 | 966 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.33, 0.74] |
| 1.1 versus Amitriptyline | 2 | 416 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.85] |
| 1.2 versus Imipramine | 2 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.83] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.9 [0.24, 3.41] |
| 2 Citalopram versus heterocyclics | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 0.94] |
| 2.1 versus Mianserin | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 0.94] |
| 3 Citalopram versus other SSRIs | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 3 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.34, 1.64] |
| 3.2 Versus Fluoxetine | 1 | 316 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.44, 4.57] |
| 3.3 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.92, 2.90] |
| 3.4 Versus Sertraline | 2 | 442 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.31, 1.51] |
| 4 Citalopram versus MAOIs or newer ADs | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 2.86 [0.11, 74.31] |
| 4.1 versus Moclobemide | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 2.86 [0.11, 74.31] |
| 5 Citalopram versus other conventional ADs | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 2.46 [0.63, 9.66] |
| 5.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 2.46 [0.63, 9.66] |
Comparison 77. SE ‐ Sweating.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 5 | 653 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.31, 0.77] |
| 1.1 versus Amitriptyline | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.49] |
| 1.2 versus Imipramine | 2 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.30, 0.83] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.14, 2.21] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.2 [0.62, 7.87] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.2 [0.62, 7.87] |
| 3 Citalopram versus other SSRIs | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 3 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.39, 1.78] |
| 3.2 Versus Fluoxetine | 1 | 316 | Odds Ratio (M‐H, Random, 95% CI) | 3.08 [0.61, 15.49] |
| 3.3 Versus Sertraline | 2 | 442 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.76, 2.27] |
| 4 Citalopram versus other conventional ADs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 7.91 [2.29, 27.29] |
| 4.2 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.16, 0.90] |
77.2. Analysis.
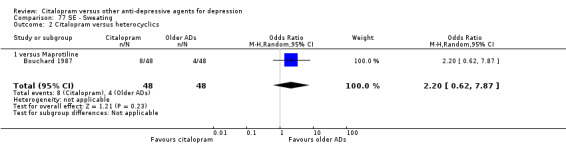
Comparison 77 SE ‐ Sweating, Outcome 2 Citalopram versus heterocyclics.
77.3. Analysis.
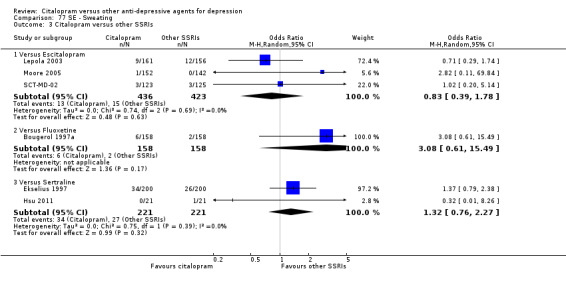
Comparison 77 SE ‐ Sweating, Outcome 3 Citalopram versus other SSRIs.
Comparison 78. SE ‐ Syncope.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 7.33] |
| 1.1 versus Amitriptyline | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 7.33] |
| 2 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Paroxetine | 1 | 406 | Odds Ratio (M‐H, Random, 95% CI) | 2.90 [0.12, 71.57] |
78.1. Analysis.
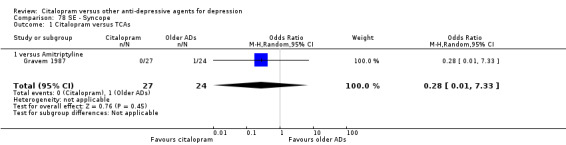
Comparison 78 SE ‐ Syncope, Outcome 1 Citalopram versus TCAs.
78.2. Analysis.
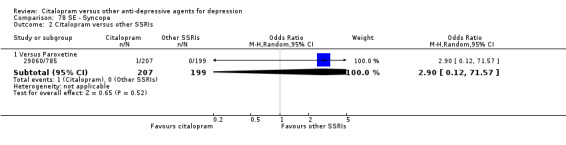
Comparison 78 SE ‐ Syncope, Outcome 2 Citalopram versus other SSRIs.
Comparison 79. SE ‐ Tachycardia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 2 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.13, 0.99] |
| 1.1 versus Imipramine | 2 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.13, 0.99] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.13, 2.55] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.13, 2.55] |
| 3 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Escitalopram | 1 | 248 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.33] |
| 4 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.17] |
79.2. Analysis.
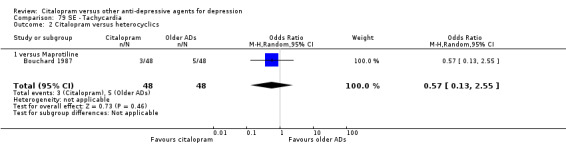
Comparison 79 SE ‐ Tachycardia, Outcome 2 Citalopram versus heterocyclics.
79.3. Analysis.
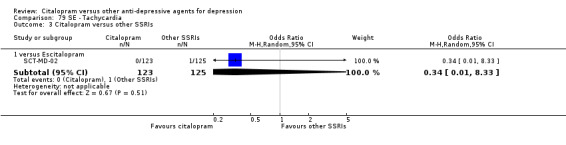
Comparison 79 SE ‐ Tachycardia, Outcome 3 Citalopram versus other SSRIs.
79.4. Analysis.
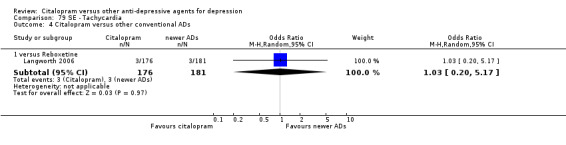
Comparison 79 SE ‐ Tachycardia, Outcome 4 Citalopram versus other conventional ADs.
Comparison 80. SE ‐ Taste abnormalities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 7.33] |
| 1.1 versus Amitriptyline | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 7.33] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
80.1. Analysis.
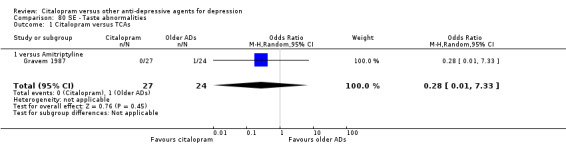
Comparison 80 SE ‐ Taste abnormalities, Outcome 1 Citalopram versus TCAs.
80.2. Analysis.
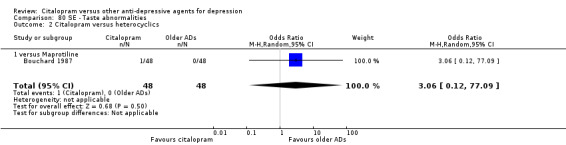
Comparison 80 SE ‐ Taste abnormalities, Outcome 2 Citalopram versus heterocyclics.
Comparison 81. SE ‐ Tension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Fluoxetine | 1 | 316 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.32, 3.17] |
| 1.2 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.39, 3.55] |
| 2 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.30, 6.26] |
81.1. Analysis.
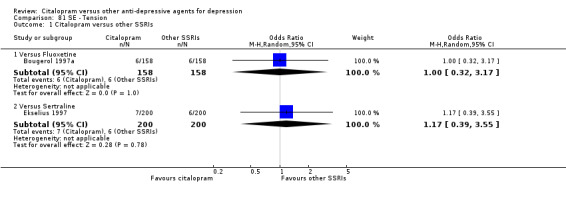
Comparison 81 SE ‐ Tension, Outcome 1 Citalopram versus other SSRIs.
81.2. Analysis.
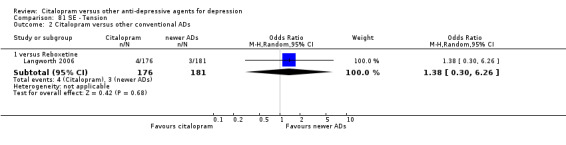
Comparison 81 SE ‐ Tension, Outcome 2 Citalopram versus other conventional ADs.
Comparison 82. SE ‐ Tremor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 5 | 653 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.28, 0.76] |
| 1.1 versus Amitriptyline | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.15, 1.75] |
| 1.2 versus Imipramine | 2 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.25, 0.80] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.11, 2.10] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.18, 1.93] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.18, 1.93] |
| 3 Citalopram versus other SSRIs | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.89] |
| 3.2 Versus Sertraline | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.54] |
| 4 Citalopram versus MAOIs or newer ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Moclobemide | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.9 [0.16, 22.72] |
| 5 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 2.11] |
82.2. Analysis.
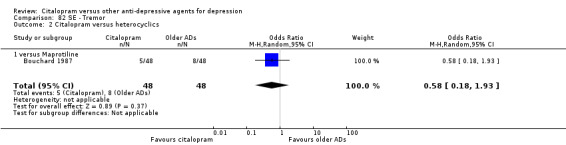
Comparison 82 SE ‐ Tremor, Outcome 2 Citalopram versus heterocyclics.
82.3. Analysis.
Comparison 82 SE ‐ Tremor, Outcome 3 Citalopram versus other SSRIs.
82.4. Analysis.
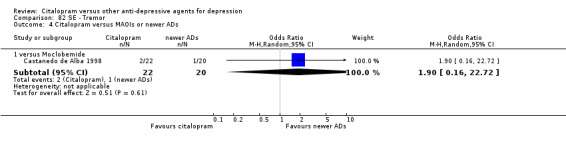
Comparison 82 SE ‐ Tremor, Outcome 4 Citalopram versus MAOIs or newer ADs.
82.5. Analysis.
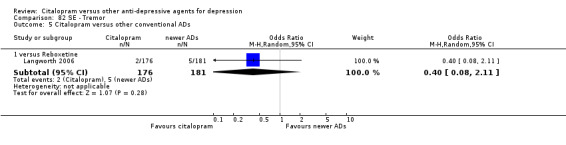
Comparison 82 SE ‐ Tremor, Outcome 5 Citalopram versus other conventional ADs.
Comparison 83. SE ‐ Urination problems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 3 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.12] |
| 1.1 versus Amitriptyline | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.49] |
| 1.2 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.34] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 4.10] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 4.10] |
| 3 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.42, 5.45] |
| 4 Citalopram versus other conventional ADs | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 5.61] |
| 4.1 versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 5.61] |
Comparison 84. SE ‐ Upper respiratory tract infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 1 | 248 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.26, 1.66] |
| 2 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.54, 5.23] |
84.1. Analysis.
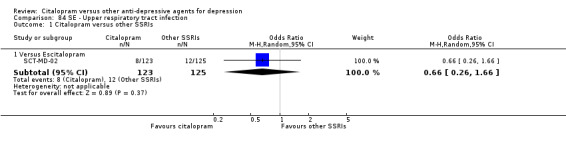
Comparison 84 SE ‐ Upper respiratory tract infection, Outcome 1 Citalopram versus other SSRIs.
84.2. Analysis.
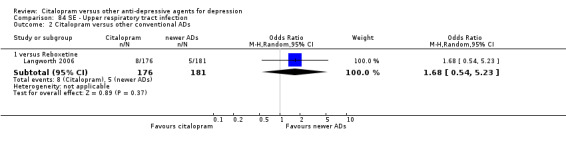
Comparison 84 SE ‐ Upper respiratory tract infection, Outcome 2 Citalopram versus other conventional ADs.
Comparison 85. SE ‐ Vertigo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Fluoxetine | 1 | 316 | Odds Ratio (M‐H, Random, 95% CI) | 2.40 [0.61, 9.43] |
| 2 Citalopram versus other conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.50, 3.38] |
| 3 Citalopram versus non‐conventional ADs | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 versus Hypericum (St. John's wort) | 1 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 6.12 [1.33, 28.17] |
85.1. Analysis.
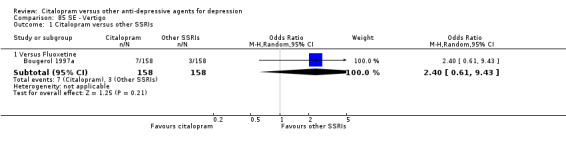
Comparison 85 SE ‐ Vertigo, Outcome 1 Citalopram versus other SSRIs.
85.2. Analysis.
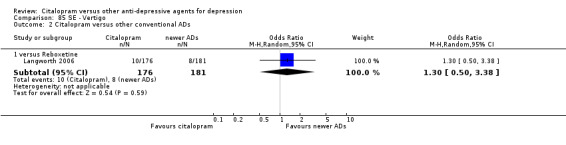
Comparison 85 SE ‐ Vertigo, Outcome 2 Citalopram versus other conventional ADs.
Comparison 86. SE ‐ Visual problems (accommodation disorders, blurred vision, detached retina, mydriasis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus TCAs | 4 | 181 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.69] |
| 1.1 versus Amitriptyline | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 0.82] |
| 1.2 versus Imipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.84] |
| 1.3 versus Nortriptyline | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.16, 2.68] |
| 2 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 2.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 3 Citalopram versus other SSRIs | 2 | 694 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.24, 1.63] |
| 3.1 Versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
| 3.2 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.19, 1.47] |
| 4 Citalopram versus other conventional ADs | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.28] |
| 4.1 versus Reboxetine | 1 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.28] |
86.2. Analysis.
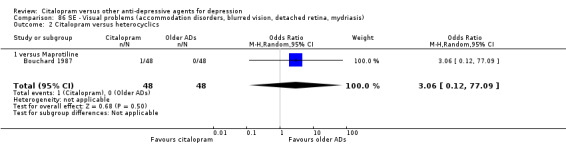
Comparison 86 SE ‐ Visual problems (accommodation disorders, blurred vision, detached retina, mydriasis), Outcome 2 Citalopram versus heterocyclics.
86.3. Analysis.
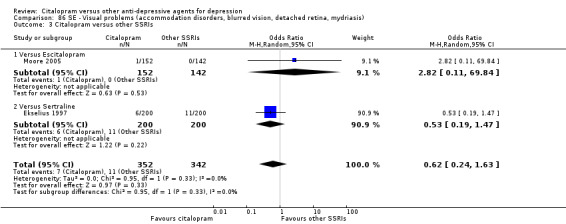
Comparison 86 SE ‐ Visual problems (accommodation disorders, blurred vision, detached retina, mydriasis), Outcome 3 Citalopram versus other SSRIs.
86.4. Analysis.
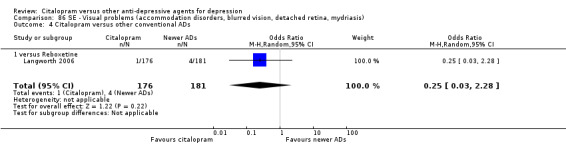
Comparison 86 SE ‐ Visual problems (accommodation disorders, blurred vision, detached retina, mydriasis), Outcome 4 Citalopram versus other conventional ADs.
Comparison 87. SE ‐ Weight gain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus other SSRIs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Escitalopram | 2 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.55, 2.64] |
| 1.2 Versus Sertraline | 1 | 400 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.48, 1.49] |
| 2 Citalopram versus other conventional ADs | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Mirtazapine | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.10, 0.67] |
| 2.2 versus Reboxetine | 2 | 458 | Odds Ratio (M‐H, Random, 95% CI) | 2.37 [0.61, 9.19] |
87.1. Analysis.
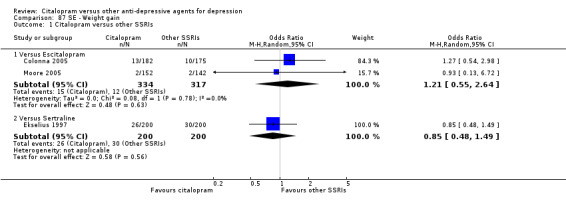
Comparison 87 SE ‐ Weight gain, Outcome 1 Citalopram versus other SSRIs.
Comparison 88. SE ‐ Yawning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Citalopram versus heterocyclics | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 1.1 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
88.1. Analysis.
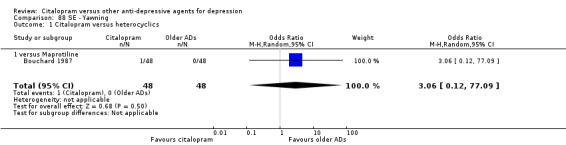
Comparison 88 SE ‐ Yawning, Outcome 1 Citalopram versus heterocyclics.
Comparison 89. Deaths, suicide and suicidality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DSH | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 versus Amitriptyline | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 2.77 [0.11, 71.35] |
| 1.2 versus Escitalopram | 1 | 248 | Odds Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.21] |
| 1.3 versus Fluoxetine | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.33, 3.23] |
| 1.4 versus Fluvoxamine | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.18] |
| 1.5 versus Imipramine | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.14, 10.51] |
| 2 Suicide ‐ Tendency/Ideation | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 versus Escitalopram | 1 | 248 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.33] |
| 3 Suicide ‐ completed | 4 | 1079 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.29, 6.42] |
| 3.1 versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
| 3.2 versus Fluvoxamine | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 75.85] |
| 3.3 versus Imipramine | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 3.88] |
| 3.4 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
| 4 Deaths (any cause) | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 versus Escitalopram | 1 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.11, 69.84] |
| 4.2 versus Fluvoxamine | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 75.85] |
| 4.3 versus Imipramine | 1 | 472 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.06, 25.67] |
| 4.4 versus Maprotiline | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 3.06 [0.12, 77.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
29060/785.
| Methods | Six‐week, double‐blind, placebo‐controlled, multicentre, parallel group, randomised study. | |
| Participants | Patients with major depressive disorder (DSM‐IV criteria), with a Montgomery and Asberg Depression Rating Scale (MADRS) score of at least 17 (both at the screening and baseline visits). Exclusion criteria: patient who have taken other psychotropic drugs, had a history of schizophrenia or schizoaffective disorder, had current (or within 6 months prior to screening) Axis I anxiety disorder or Axis I affective disorder other than major depressive disorder. Patient who, in the investigator's judgement, posed a current homicidal or suicidal risk. Women who had a positive pregnancy test or who were lactating, women of child‐bearing potential who were not practicing a clinically accepted method of contraception. Patient with a serious medical disorder or condition that, in the investigator's opinion, precluded the administration of paroxetine controlled release (CR) or citalopram. Patient undergoing any form of psychotherapy. Age range: 18‐65 years. |
|
| Interventions | Citalopram 20 mg/day: 107 participants Citalopram 40 mg/day: 100 participants Paroxetine CR 12.5 mg/day: 96 participants Paroxetine CR 25 mg/day: 103 participants Placebo: 105 participants |
|
| Outcomes | Primary outcome: proportion of MADRS responders at the week 6 (last observation carried forward at endpoint). Response was defined as reduction of 50% or more in the MADRS total score, relative to the baseline total score. Secondary outcomes: mean change from baseline in the MADRS total score; proportion of subjects with a positive response (score of 1 or 2) on the global improvement rating of the Clinical Global Impression (CGI); mean change from baseline in Hamilton Anxiety Rating Scale (HAM‐A) total score; mean change from baseline in CGI severity of illness rating; mean change from baseline in Hospital Anxiety and Depression Scale (HAD) total score; mean change from baseline in HAD, Anxiety and Depression sub‐scales and mean change from baseline in Sheehan Disability Scale (SDS) total score. Safety was assessed via adverse event monitoring, vital signs, laboratory evaluation, serum pregnancy test, ECGs, physical exam and weight. |
|
| Notes | This study was funded by GSK (paroxetine manufacturer). One death for suicide in the placebo group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized (1:1:1:1:1) to either paroxetine CR 12.5 mg, paroxetine CR 25 mg, citalopram 20 mg, citalopram 40 mg, or placebo". |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "paroxetine CR and citalopram were provided as over‐encapsulated tablets (...) placebo capsules were identical in appearance to the active study medication capsules". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "all subjects who were randomized to double‐blind medication and had at least one valid post baseline efficacy assessment comprised the Intention‐to‐treat (ITT) efficacy population. The Last Observation Carried Forward (LOCF) data at week 6 were the primary dataset of interest". |
| Selective reporting (reporting bias) | High risk | Remission rate are missing. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Allard 2004.
| Methods | Twenty‐two‐week, double‐blind, randomised, parallel group study. | |
| Participants | Outpatients meeting DSM‐IV criteria for major depression, having a minimum score of 20 on Montgomery and Asberg Depression Rating Scale (MADRS) and a ≤ 20% change in MADRS score between pre‐study and baseline visits, which were one‐week apart. Age‐range: 64‐89 years |
|
| Interventions | Venlafaxine: 76 participants. Citalopram: 75 participants Venlafaxine dose range: 75‐150 mg/day Citalopram dose range: 20‐40 mg/day Zopiclone (≤ 7.5 mg/day) or zolpidem (≤ 5 mg/day) for insomnia and medications for treatment of somatic disorders were allowed. |
|
| Outcomes | Primary outcome: change in MADRS score from baseline to week 8. Secondary outcomes: Clinical Global Impression (CGI), subscale Severity of Illness and Global Improvement. Geriatric Depression Scale (GDS‐20). |
|
| Notes | This study was funded by Wyeth (venlafaxine manufacturer). One death in the citalopram group (unknown cause of death). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the study was designed as a randomized". Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "both venlafaxine and citalopram were administered orally in identically appearing capsules to maintain the double‐blind integrity of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Even though an Intention‐to‐treat (ITT) approach was used, no reliable information was provided in the paper to check the consistency between methods and results (for instance, see figures in Table 1 of the published paper). Quote: "Analyses of the efficacy variables were performed on an ITT patient population, defined as all randomised patients who had received at least one dose of study medication and with at least one efficacy evaluation while on treatment [...] In case of missing values at 8 or 22 weeks, the last prior on‐therapy value was carried forward (LOCF). Analyses of safety were performed on all patients who had received at least one dose of study medication." |
| Selective reporting (reporting bias) | High risk | No clear data about dropout rate in each group. Quote: "There were 33 withdrawals, nine of which due to adverse events (...). 118 patients completed the 6‐month study...". |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Andersen 1986.
| Methods | Five‐week controlled, double‐blind, multicentre study | |
| Participants | In‐patients having a total score of ≥ 18 on the Hamilton Depression Rating Scale (HDRS) or a score of ≥ 9 on the Hamilton Depression Sub‐scale (HDSS). Exclusion criteria: patients with age below 19 or above 65 years, schizophrenia, paranoid psychoses, oligophrenia, organic brain syndrome, chronic drug or alcohol abuse or serious somatic disease, such as myocardial infarction within the last 6 months, acute glaucoma, severe liver disease, hypertension, endocrine disorder, etc...Patients treated with MAO inhibitors or tricyclic antidepressants within the last 3 weeks were also excluded. Other reasons for exclusion were pregnancy, current depressive episode of more than 12 months duration, and severe retardation or suicidal behaviour (requiring ECT) |
|
| Interventions | Citalopram: 57 participants Clomipramine: 57 participants Citalopram: 40 mg/day Clomipramine: 150 mg/day Additional medication was restricted to oxazepam or nitrazepam as sedative/hypnotic. Other sedatives or neuroleptics were not allowed. Some patients received occasional doses of acetylsalicylic acid. |
|
| Outcomes | Primary outcome: change in HDRS and HDSS that is assumed to represent core symptoms in depressed patients. | |
| Notes | Data on rating scale score at baseline are missing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients meeting the inclusion criteria were stratified according to diagnostic rating (endogenous versus non‐ endogenous) and department before being randomly allocated in a double blind fashion to treatment with either citalopram or clomipramine for five weeks". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information provided is "double blind", without clear description of method. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | High risk | Data on rating scale score at baseline are missing. Information about side‐effects are missing. |
| Other bias | Unclear risk | This study was not sponsored by pharmaceutical industry. |
Berlanga 2006.
| Methods | Eight‐week double‐blind clinical trial. | |
| Participants | Patients between 18 and 40 years, meeting the DSM‐IV criteria for Major Depressive Disorder after two independent clinical interviews, and scoring at least 18 in the 21‐item Hamilton Depression Rating Scale (HDRS). Patients were excluded if psychotic symptoms were present or a history of past manic, hypomanic or mixed episodes was confirmed. Also participants with uncontrolled medical illnesses, evidence of drug abuse or severe personality disorders were not included. In the case of women individuals with irregular menstrual cycles, pregnancy, breastfeeding, current hormonal treatments and biological or surgical menopause were also excluded. |
|
| Interventions | Citalopram: 54 participants Reboxetine: 47 participants Citalopram dose range: 20‐40 mg/day (mean dose: 25.8 SD 3.7). Reboxetine dose range: 4‐8 mg/day (mean dose: 5.8 SD 1.5) |
|
| Outcomes | Change in HDRS scores from baseline to endpoint. | |
| Notes | This study was funded by Lundbeck (citalopram manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned to an 8‐week double blind comparative trial with reboxetine or citalopram". Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "drugs were administered orally at bedtime using identical capsules containing 4 mg of reboxetine or 20 mg of citalopram as starting doses". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "comparison were done only with patients having at least five evaluations (basal and four weeks of treatment). In patients who had a minimum of five evaluations but did not complete the 8‐week of follow‐up, Last Observation Carried Forward (LOCF) procedure was used. |
| Selective reporting (reporting bias) | High risk | Continous data about the two groups are missing. The paper reported only data for men or for women. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Bouchard 1987.
| Methods | Six‐week multicentre, controlled, double‐blind trial. | |
| Participants | Patients who suffered from a depression which required drug treatment and which was of a severity corresponding to a total score of at least 15 on the Montgomery and Asberg Depression Rating Scale (MADRS) after a wash‐out period of 3‐7 days. The depression was classified as endogenous, doubtfully endogenous or non‐endogenous, using the Newcastle rating scale and the DSM‐III, as belonging to one of the following groups: major depressive episode with melancholia, major depressive episode without melancholia, atypical depression. Exclusion criteria: pregnancy or absence of use of an effective contraceptive methods, severe somatic disease (particularly severe cardiac, renal or hepatic disease), organic brain syndrome, schizophrenia or paranoid psychosis, epilepsy, abuse of alcohol or narcotics, treatment with MAO‐inhibitors within the last 3 weeks preceding entry into the trial, previous unsuccessful treatment with one of the test drugs, patient's refusal to participate in the trial. |
|
| Interventions | Citalopram: 48 participants Maprotiline: 48 participants Citalopram dose range: 40‐60 mg/day Maprotiline dose range: 75‐150 mg/day Among psychotropic drugs, only benzodiazepines were allowed. |
|
| Outcomes | Primary outcome: mean score on MADRS or Clinical Global Impression (CGI). | |
| Notes | This study was funded Lundbeck (citalopram manufacturer). One death for suicide in the citalopram group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were allocated at random in blocks of four to double‐blind treatment with either citalopram or maprotiline once daily for a period of 6 weeks". Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No data provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Using the double‐ dummy half patients received active citalopram tablets and placebo maprotiline tablets and the other half was given placebo citalopram tablets and active maprotiline tablets". Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No clear data provided |
| Selective reporting (reporting bias) | High risk | CGI‐S score at baseline are missing. Remission rate are reported only at endpoint (week 1‐4 are missing). |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Bougerol 1997a.
| Methods | Eight‐week double‐blind, multicentre study in a psychiatrist setting. | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for a major depressive disorder or bipolar disorder. The severity of depression should be 25 or more on the Montgomery and Asberg Depression Rating Scale (MADRS). Age range: 18‐65 years old. Exclusion criteria: pregnancy, lactation, failure to use a safetable contraceptive method, alcohol or drug abuse within the last year, patients with severe somatic, neurologic or psychiatric disease, treatment with MAOI within 2 weeks prior to entry the trial, hypersensitivity to study drugs, suicide risk. | |
| Interventions | Fluoxetine: 158 participants. Citalopram: 158 participants. Fluoxetine dose: 20 mg. Citalopram dose range: 20‐40 mg/day. Concomitant psychotropic medication was prohibited, but use of benzodiazepines for insomnia was allowed. | |
| Outcomes | Primary outcome: MADRS. Secondary outcomes: Hamilton Depression Rating Scale (HDRS‐17), Clinical Global Impression (CGI). | |
| Notes | Three attempted suicides in citalopram group, and three attempted suicides in fluoxetine group. This study was funded Lundbeck (citalopram manufacturer). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear data provided |
| Allocation concealment (selection bias) | Unclear risk | No data provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "due to the different appearance of the two drugs the "double‐dummy" principle was used to blind the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "patients populations were defined as the Intention‐to‐treat (ITT) group and the Efficacy (EFF) group. The ITT population comprised all randomized patients. The EFF population consisted of all patients who fulfilled the entry criteria and had completed at least 14 days double‐blind treatment. All efficacy analyses were made on the basis of the EFF population". |
| Selective reporting (reporting bias) | High risk | Some endpoint scores and baseline scores are missing. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Bougerol 1997b.
| Methods | Eight‐week, double‐blind, multicentre, parallel group study in general practice. | |
| Participants | Outpatients (primary care) fulfilling DSM‐III‐R criteria for a major depressive disorder. The severity of depression should be 22 or more on the Montgomery and Asberg Depression Rating Scale (MADRS).
Age range: 18‐70 years. Exclusion criteria: pregnancy, lactation, failure to use a safetable contraceptive method, alcohol or drug abuse within the last year, patients with severe somatic, neurologic or psychiatric disease, treatment with MAOI within two weeks prior to entry the trial, hypersensitivity to study drugs, suicide risk. |
|
| Interventions | Fluoxetine: 184 participants. Citalopram: 173 participants. Fluoxetine dose: 20 mg. Citalopram dose: 20 mg/day. Concomitant psychotropic medication was prohibited, but use of benzodiazepines for insomnia. | |
| Outcomes | Primary outcome: MADRS. Secondary outcomes: Hamilton Rating Scale for Depression (HDRS‐17), Clinical Global Impression (CGI). | |
| Notes | This study was funded Lundbeck (citalopram manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized to double blind treatment". Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No data provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "due to the different appearance of the two drugs the "double‐dummy" principle was used to blind the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: " patients populations were defined as the Intention‐to‐treat (ITT) group and the Efficacy (EFF) group. The ITT population comprised all randomized patients. The EFF population consisted of all patients who fulfilled the entry criteria and had completed at least 14 days double‐blind treatment. All efficacy analyses were made on the basis of the EFF population". |
| Selective reporting (reporting bias) | Unclear risk | Some endpoint scores and baseline scores are missing. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Burke 2002.
| Methods | Eight‐week, double‐blind, randomised, parallel group, multicentre study. | |
| Participants | Outpatients meeting DSM‐IV criteria for Major Depressive Disorder, having a minimum score of 22 on Montgomery‐Asberg Depression Rating Scale (MADRS) and a minimum score of 2 on Item 1 of Hamilton Depression Rating Scale (HDRS). Age range: 18‐65 years. Exclusion criteria: any DSM‐IV Axis I disorder other than major depression, any personality disorder, a history of substance abuse, a suicide attempt within the past year or evidence of active suicidal ideation (as indicated by a score of at least 5 on item 10 of the MADRS), pregnancy, lactation, women of childbearing potential if they didn't agree to use a medically acceptable method of contraception, concomitant psychotropic medication. | |
| Interventions | Escitalopram: 252 participants. Citalopram: 127 participants. Escitalopram dose range: 10‐20 mg/day. Citalopram dose: 40 mg/day. Zolpidem for insomnia was allowed (no more than three times per week). | |
| Outcomes | Primary Outcome: Change from baseline to week 8 in MADRS, HDRS‐24, HDRS Depressed Mood Item, Clinical Global Impression‐Improvement (CGI‐I), Clinical Global Impression‐Severity (CGI‐S). Secondary Outcomes: change in Hamilton Rating Scale for Anxiety (HAM‐A), Center for Epidemiological Studies‐Depression Scale (CES‐D), Quality of Life Questionnaire (QOL) and a 16‐item instrument derived from the QOL enjoyment and satisfaction questionnaire from baseline to endpoint. | |
| Notes | This study was funded by escitalopram manufacturer. One suicide attempted in escitalopram 20 mg group. One intentional overdose in placebo group. One non‐intentional overdose in citalopram 40 mg group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients meeting eligibility criteria at a screening visit entered a 1‐week, single blind, placebo lead‐in period, returning for a baseline visit at the end of the lead‐in period. Patients completing the placebo lead‐in, who continued to meet all entry criteria, were then randomly assigned to receive 8 weeks of double blind treatment". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "in order to maintain the blind, all double blind study medication was administered as one capsule per day, regardless of dose of treatment group. No further adjustment of dosage was permitted". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy was assessed in the Intention‐to‐treat (ITT) population which included all patients who had received at least 1 dose of double blind study medication and had at least 1 post‐baseline MADRS assessment". |
| Selective reporting (reporting bias) | Low risk | Remission rate are missing. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Castanedo de Alba 1998.
| Methods | Six‐week, open‐label, controlled study. | |
| Participants | Forty‐two patients of both sexes ranging in age from 18 to 65 years were included in this trial if they fulfilled the criteria of major depressive disorder according to the DSM‐III‐R and had a minimum score of 17 on Hamilton Depression Rating Scale 17 Item (HDRS‐17). All patients gave their written informed consent. The exclusion criteria were: patients with severe depression and suicidal tendencies, patients with psychotic symptoms, pregnant women, alcoholic or drug abuse patients, patients with epilepsy, with schizophrenia or other form of psychosis, patients with hepatic and/or renal disease, cardiac patients, patients with acquired immunodeficiency syndrome, with hepatitis or diabetes, patients who are been treated with other antidepressants within two weeks before the study, and patients with known hypersensitivity or resistance to citalopram or moclobemide. |
|
| Interventions | Citalopram: 22 participants. Moclobemide: 20 participants. Citalopram dose range: 20‐60 mg/day (mean dose: 28.0 mg) Moclobemide dose range: 300‐600 mg/day (mean dose: 545 mg) |
|
| Outcomes | Primary Outcome: Change from baseline to week 6 in HDRS‐17. | |
| Notes | This study was not sponsored by pharmaceutical industry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to two groups..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "this was an open label study..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "data from patients who withdrew from the study were not taken into account for the final analysis and were considered only for the statistical analysis of adverse reactions". |
| Selective reporting (reporting bias) | Unclear risk | Response rate and remission rate are missing. |
| Other bias | Unclear risk | This study was not sponsored by pharmaceutical industry. |
Colonna 2005.
| Methods | Twenty‐four‐week, double‐blind, randomised, parallel group, multicentre study. | |
| Participants | Outpatients meeting DSM‐IV criteria for Major Depressive Disorder, having a total score between 22 and 40 on Montgomery‐Asberg Depression Rating Scale (MADRS). Age range: 18‐65 years. Exclusion criteria: other serious illnesses on the basis of medical history and the screening results of a physical examination, electrocardiogram (ECG) and clinical laboratory tests, pregnancy, breast‐feeding, non adequate contraception at time of screening, mania or any bipolar disorder, schizophrenia or any psychotic disorder, obsessive‐compulsive disorder, eating disorders, mental retardation or any pervasive developmental or cognitive disorder, MADRS score >= 5 on item 10, concomitant treatment with antipsychotics, antidepressants, hypnotics, anxiolytics, antiepileptics, barbiturates, chloral hydrate, 5‐HT receptor agonists, electroconvulsive treatment, behaviour therapy or psychotherapy, use of any investigational drug within the past 30 days, history of schizophrenia, psychotic disorder or drug abuse, history of severe drug allergy or hypersensitivity (including to citalopram), a lack of response to more than one antidepressant treatment (including citalopram) during the present depressive episode. | |
| Interventions | Escitalopram: 175 participants. Citalopram: 182 participants. Escitalopram dose: 10 mg/day. Citalopram dose: 20 mg/day. Benzodiazepines in low doses for insomnia were allowed. | |
| Outcomes | Primary Outcome: Change from baseline in the mean of the MADRS during the 24 weeks. Secondary Outcomes: MADRS single items, Clinical Global Impression ‐ Improvement (CGI‐I), Clinical Global Impression ‐ Severity (CGI‐S). | |
| Notes | This study was funded by Lundbeck. Three suicide attempted in citalopram group; three suicide attempted in escitalopram group. Remission: a score equal or less than 12 on MADRS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "There was an initial 1‐week single‐blind, placebo period, followed by randomization of patients in a 1:1 ratio to treatment (...). Patients were assigned to escitalopram or citalopram treatment according to a computer‐generated randomization list drawn‐up by Lundbeck". |
| Allocation concealment (selection bias) | Low risk | Quote: "The details of the randomization series were unknown to any of the investigators and were contained in a set of sealed opaque envelopes. At each study centre, sequentially enrolled patients were assigned the lowest randomization number available". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "all study personnel and participants were blinded (...), the study products were tablets of identical appearance, taste and smell". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention‐to‐treat (ITT) population included all randomised patients who took at least one dose of double‐blind study product and who had at least one valid post‐baseline MADRS assessment." |
| Selective reporting (reporting bias) | High risk | Missing standard deviations. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
de Wilde 1985.
| Methods | Six‐week controlled, double‐blind, randomised trial. | |
| Participants | In‐patients suffering from endogenous depression or chronic dysthymic disorder (Spitzer's research Diagnostic Criteria), with a total score of at least 25 on the 10‐item Comprehensive Psychopathological Rating Scale (CPRS) sub‐scale for depression. Age range: 18‐70 years Exclusion criteria: pregnancy/lactation, serious somatic disease (particularly of the heart, liver or kidneys), organic brain syndrome, need for ECT, abuse of alcohol or drugs, and treatment with MAO inhibitors within the previous 3 weeks. |
|
| Interventions | Citalopram: 30 participants Mianserin: 30 participants Citalopram dose range: 40‐80 mg/die Mianserin dose range:60‐120 mg/die Additional medication with benzodiazepine as sedatives/hypnotics was permitted. |
|
| Outcomes | Primary outcome: mean change at endpoint on the 10‐item CPRS sub‐scale for depression and on Clinical Global Impression ‐ Severity (CGI‐S). | |
| Notes | This study was funded by Lundbeck (citalopram manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " Patients were randomly allocated". Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind treatment with either citalopram or mianserin, administered as identically looking capsules". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Observed‐case (completers) analysis only |
| Selective reporting (reporting bias) | High risk | No reliable data about response rate. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Ekselius 1997.
| Methods | Twenty four‐week, double‐blind, randomised multicentre study. | |
| Participants | General Practice patients fulfilling DSM‐III‐R criteria for major depression with a minimum baseline score of 21 on Montgomery‐Asberg Depression Rating Scale (MADRS). Age range: 18‐70 years old. Exclusion criteria: pregnancy, lactating, inadequate method of contraception, severe depression of psychotic dimension, history of serious suicide attempt or suicide risk, therapy refractory depression, previous treatment with sertraline or citalopram without significant effect, bipolar disorder, previous or present history of alcohol or drug abuse, history of epilepsy, known intolerance or allergic reactions to SSRIs, therapy with lithium within the preceding month, currently receiving and unable to discontinue any other psychotropic medication, except for a hypnotic for insomnia or a daytime anxiolytic, currently receiving treatment with cimetidine, warfarin or tryptophan, significant hepatic or renal disease, previous participation in the study. Patients who had been receiving antidepressants drugs required to have a washout period of at least 3 weeks. | |
| Interventions | Sertraline: 200 participants. Citalopram: 200 participants. Sertraline dose: 50‐150 mg/day. Citalopram dose: 20‐60 mg/day. Permitted Nitrazepam 2.5‐10 mg/day, flunitrazepam 0.5‐2 mg/day and oxazepam 15‐25 mg/day. | |
| Outcomes | Primary Outcome: change in MADRS, Clinical Global Impression‐Severity (CGI‐S) and Clinical Global Impression‐Improvement (CGI‐I). | |
| Notes | This study was funded by Pfizer (sertraline manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized". Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐dummy" but we have no other information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing standard deviations on MADRS data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
Gastpar 2006.
| Methods | Six‐week, double‐blind, multicentre, placebo‐controlled, randomised study. | |
| Participants | Outpatients with a moderate depressive episode having depression with a score of 20‐24 on the first 17 items of the 21‐item Hamilton Depression Rating Scale (HDRS‐21) and diagnosis of moderate depression (first manifestation or recurrent depressive disorder) defined by ICD‐10 criteria or according to DSM‐IV criteria for major depressive episode and recurrent major depression; females taking adequate contraceptive or without child‐bearing potential. Exclusion criteria: diagnosis of resistance to depression treatment, known schizophrenia, psychosis or dementia, depressive mood due to a serious general disease, known hypersensitivity to study medication, known photosensitivity, specific psychotherapy during the last two months or treatment with psychoactive drugs (antidepressants, neuroleptic drugs, anxiolytic drugs, etc...) during the last 3 weeks (6 weeks for fluoxetine) prior to study enrolment, and determined suicidal tendency by scores of > 2 in item 3 of HDRS‐21 scale or known attempted suicide. |
|
| Interventions | Citalopram: 127 participants. Hypericum extract STW3‐VI: 131 participants. Placebo: 130 participants. Citalopram: 20 mg/day Hypericum extract STW3‐VI: 900 mg/day |
|
| Outcomes | Primary outcome: endpoint total score on HDRS. Secondary outcomes: endpoint total score on the Von Zerssen's Adjective Mood Scale (BfS) and Clinical Global Impression (CGI) scale. |
|
| Notes | This study was funded by STW3‐VI manufacturer (EPA EuroPharma). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a randomization schedule using the randomization program IDV‐Rancode 3.6, patients were chronologically randomized by the investigators to treatment groups by assigning them the lowest yet unassigned treatment number available at the trial centre". |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "the double‐dummy technique was used to guarantee complete blinding for both investigator and patient at any time in the trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "the tests for superiority (STW3‐VI over placebo) were carried out on the Intention‐to‐treat (ITT) population, the test for non‐inferiority (of STW3‐VI to citalopram) on the Per Protocol (PP) population." |
| Selective reporting (reporting bias) | Unclear risk | No clear data about dropout rate in each group. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Gravem 1987.
| Methods | Six‐week, double‐blind, multicentre trial | |
| Participants | In‐ and out‐patients who were referred to hospital for a depression requiring drug treatment. The patient's depression was classified as endogenous or non‐endogenous by means of the Newcastle Depression Scale I. Age range: 18‐70 years Exclusion criteria: serious physical disease, pregnancy, previous resistance to therapy with amitriptyline or citalopram in doses considered to be adequate. |
|
| Interventions | Citalopram: 27 participants Amitriptyline: 24 participants Citalopram dose range: 20‐60 mg/day Amitriptyline dose range: 75‐225 mg/day Additionally treatment was not allowed apart from low doses of diazepam or nitrazepam for severe anxiety or insomnia, if necessary. |
|
| Outcomes | Primary outcome: endpoint total score on the 10‐item Comprehensive Psychopathological Rating Scale (CPRS) sub‐scale for depression. | |
| Notes | This study was funded by Lundbeck (citalopram manufacturer). No signed informed consent was required, neither from the patient nor from his relatives. The clinician informed the patient of the object of the study and that he/she was quite free to participate. At that time there were no ethical committees in Norway to evaluate the design of study (Health Autorities approved the study). One suicide attempted in citalopram group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No reliable information provided (no data about sequence generation). |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "tablets of identical appearance were prepared". Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about incomplete data in each group. |
| Selective reporting (reporting bias) | High risk | No MADRS scores were reported (neither at baseline nor at endpoint). Response rate and remission rate are missing. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Hosak 1999.
| Methods | Four‐week, randomised and open study. | |
| Participants | Hospitalised patients. Diagnoses for inclusion (according to the ICD‐10 criteria) were: bipolar affective disorder, most recent episode depressed (8 participants); major depressive episode, single (44 participants), major depressive episode, recurrent (38 participants). Average age: 44.5 years (SD14.3). |
|
| Interventions | Citalopram: 29 participants. Amitriptyline: 31 participants. Fluoxetine: 30 participants. Citalopram dose range: 20‐60 mg/day Amitriptyline dose range: 150‐300 mg/day Fluoxetine: 20‐60 mg/day |
|
| Outcomes | Primary Outcome: mean change on Hamilton Depression Rating Scale 21‐item (HDRS‐21). | |
| Notes | Study report published only in Czech. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the subjects were randomized to the study antidepressant using computer randomization program (Excel) at the beginning of the initial hospitalization at the Dpt. of Psychiatry in Hradec Kralovc." |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information reported. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Other bias | Unclear risk | No information reported. |
Hsu 2011.
| Methods | Six‐week, randomised, double‐blind study. | |
| Participants | Outpatients aged between 20 and 65 years, who met the DSM‐IV criteria for MDD, experiencing a drug naive first depressive episode, exhibited a total score on the Montgomery‐Asberg Depression Rating Scale (MADRS) (MADRS of > or = 25 at screening, and displayed a < or = 20% decrease in MADRS score between screening and baseline visits). Patients were excluded from the trial if they had a history of severe allergy or major medical illness. Were also excluded patients who displayed acutely suicidal tendencies, or had a history of drug or alcohol dependence or abuse. In addition, patients were excluded if they had previously received treatment of any antidepressant or had taken monoamine oxidase inhibitors. Women who were pregnant, lactating, and women with childbearing potential who were not using a medically acceptable form of contraception were also excluded. |
|
| Interventions | Citalopram: 25 participants. Sertraline: 26 participants. Citalopram dose: 20 mg/day. Setraline dose: 50 mg/day. |
|
| Outcomes | Primary outcomes: MADRS total score, response and remission rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned". Likely done |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The primary efficacy end points were the mean difference in MADRS total score at baseline and weeks 1, 3, and 6. Other efficacy end points included the percentage of patients with MADRS remission (MADRS total scores e10) and response (Q50% reduction from randomization in MADRS total score) at treatment week 6. Tolerability was assessed as the percentage of patients who developed specific adverse events during the treatment period." |
| Selective reporting (reporting bias) | High risk | MADRS scores were reported only in figures. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Karlsson 2000.
| Methods | Twelve‐week, randomised, multicentre, double‐blind study. | |
| Participants | In‐ or out‐patients being treated in psychiatric hospitals, psychiatric specialist or general practices, or geriatrics units. Patients were to have a diagnosis of major depression (DSM‐III‐R criteria), a Montgomery and Asberg Rating Scale for Depression (MADRS) total score of ≥ 20 and a Mini Mental State Examination (MMSE) total score of at least 16. For patients with a MMSE score of 16‐24, the DSM‐III‐R diagnosis forms for dementia were completed. Exclusion criteria: patients having schizophrenia or related psychotic disorder, neurological disease other than vascular or primary degenerative dementia, focal cortical deficit or chronic drug or alcohol abuse. Patients with severe somatic disorders, such as cardiac, renal, hepatic or endocrinological disorders or blood laboratory abnormalities, which, in the opinion of investigator, interfered with participation in the study. Patients were not to have received other antidepressants during previous 4‐7 days; irreversible MAO‐inhibitors (A or B), lithium or carbamazepine during the previous 2 weeks, or fluoxetine during the previous 5 weeks. Patients were also excluded if they had received electroconvulsive therapy within the previous 8 weeks, oral or parenteral neuroleptics during the previous week, depot neuroleptics during the previous 4 weeks, an investigational drug during the previous 3 months, or were known to be intolerant to or have had a non‐response to the study drugs. Patients at risk for suicide in the investigator's opinion and patients treated with oral anticoagulants. Age: 65 years or older. |
|
| Interventions | Citalopram: 163 participants. Mianserin: 173 participants. Citalopram dose range: 20‐40 mg/day Mianserin dose range: 30‐60 mg/day |
|
| Outcomes | Primary outcome: mean change at endpoint on MADRS. Secondary outcomes: mean change in Clinical Global Impression‐Severity (CGI‐S) of Illness and Clinical Global Impression‐ Improvement (CGI‐I) scales, Gottfries‐Brane‐Steen (GBS) sub‐scale 3 ("emotional functions") and sub‐scale 4 ("symptoms common in dementia disorders") and MMSE. In addition, a modified Well‐Being Questionnaire was completed at baseline and week 12. |
|
| Notes | This study was funded by Lundbeck (citalopram manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear information about sequence generation. Quote: "Patients were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "to ensure blinding, the citalopram and mianserin tablets were identical in appearance and were taken once daily, preferably in the evening". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "two different populations were analysed: for primary efficacy analysis the efficacy population was chosen. (...) For secondary analysis, the Intention‐to‐treat (ITT) population was chosen. Primary and secondary efficacy variables were evaluated in both of these populations". |
| Selective reporting (reporting bias) | High risk | MADRS score and remission rate are missing. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Khanzode 2003.
| Methods | Twelve‐week, prospective, open‐label, randomised study | |
| Participants | Patients with major depression according to the DSM‐IV criteria. Exclusion criteria: patients having a score less than 14 were excluded from the study, patients with other axis I and axis II diagnoses besides major depression. Medical illnesses including endocrine, metabolic or autoimmune disorders known to affect free radical status |
|
| Interventions | Citalopram: 33 participants. Fluoxetine: 34 participants. Citalopram dose: 20 mg/day. Fluoxetine dose: 20 mg/die. |
|
| Outcomes | Primary outcome: MDA and SOD concentration levels. Secondary outcomes: change in Hamilton Depression Rating Scale (HDRS) score from baseline to week 12. |
|
| Notes | Indian study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients who were suitable for drug treatment were allocated randomly". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Kyle 1998.
| Methods | Eight‐week, double‐blind, parallel group, multicentre study. | |
| Participants | Patients over 65 years of age diagnosed with major depression as defined by DSM‐III‐R criteria, with a Mini Mental State Examination (MMSE) score ≥ 24 and a score ≥ 22 on the Montgomery and Asberg Rating Scale for Depression (MADRS) at both the screening (day 7) and baseline visits (day 0). Exclusion criteria: patients with renal or hepatic disorders, cardiovascular disorders, prostatism or urinary retention, glaucoma, epilepsy, organic mental disease, marked mental retardation, other psychiatric disorders, alcohol or drug dependence, uncontrolled diabetes or other endocrine disease, or uncontrolled hypertension, or if they required treatment with guanethidine or bethanidine. Patients receiving treatment with a psychotropic medication, those considered at suicide risk, with a recent depressive episode lasting less than 2 weeks, those with a known resistance to treatment with an SSRI or TCA, those who had taken MAO inhibitors in the last 2 weeks, and those who had taken fluoxetine in the last 5 weeks. Age range: 65‐90 in citalopram group. 65‐89 in amitriptyline group. |
|
| Interventions | Citalopram: 179 participants. Amitriptyline: 186 participants. Citalopram dose range: 20‐40 mg/day Amitriptyline dose range: 50‐100 mg/day |
|
| Outcomes | Primary outcome: mean change on MADRS score from baseline to endpoint of study. | |
| Notes | This study was funded by Lundbeck (citalopram manufacturer). Information about suicide attempts are not clear. Quote: "suicide attempts were observed exclusively in the amitriptyline group". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients (...) were randomly assigned to receive citalopram or amitriptyline". |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind, "double‐dummy". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "all analyses were performed on data from all randomized patients who had at least one post‐baseline measurement (ITT population). Patients who remained in therapy for at least 4 weeks with an average compliance of at least 50% constituted the efficacy (EFF) population". |
| Selective reporting (reporting bias) | High risk | Hamilton Depression Rating Scale (HDRS) and Clinical Global Impression (CGI) scores are missing. MADRS score at baseline is missing. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Lalit 2004.
| Methods | Four‐week controlled, randomised, double‐blind study. | |
| Participants | Outpatients, 18 to 65 years of age, with ICD‐10 diagnosis of Major Depressive Episode and a minimum score of 18 on the Hamilton Rating Scale for Depression. Patients were excluded if they had recent ongoing significant non‐psychiatric medical disorder, a history of substance abuse, chronic suicidal ideation and behaviour, participated in any drug trial within 4 weeks, schizoaffective or bipolar disorder, seizure disorder, anorexia nervosa, hepatic and renal system dysfunction, therapy with lithium within the preceding month, treatment with cimetidine, warfarin or MAO inhibitors, hypersensitivity to citalopram, escitalopram and sertraline and non responders to citalopram and sertraline. Women of childbearing age not using contraceptives, pregnant women, lactating mothers, women desiring to have children were also excluded. |
|
| Interventions | Citalopram: 74 participants. Escitalopram: 69 participants. Sertraline: 71 participants. Citalopram dose: 20‐40 mg/day. Escitalopram dose: 10‐20 mg/day. Sertraline dose: 100‐150 mg/day. |
|
| Outcomes | Primary outcomes: change in Hamilton Rating Scale for Depression, Clinical Global Impression scores, response rate, remission rate. | |
| Notes | This study was sponsored by Torrent pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized". Likely done |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No clear information provided. Probably done Quote: "...double–blind, single dummy, titratable dose, parallel group, multi‐centric study". And "...In order to maintain the blind, all double blind study medication was administered in alu ‐ alu (aluminum – aluminum) strips." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about secondary outcome were reported. Quote: "Primary Efficacy Measures: 1) Change in HAM‐D total score (The sum of all 17 items); 2) CGI –S score and CGI –I score; 3) Response rate: HAM‐D score decrease by 50% from baseline; 4) Remission rate: HAM‐D score below 8. |
| Selective reporting (reporting bias) | High risk | Primary outcomes data such as HDRS total scores and CGI total scores were reported only in figures. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Langworth 2006.
| Methods | Twenty‐four‐week, double‐blind, parallel group, randomised, multicentre study | |
| Participants | Outpatients or day hospital clinic patients having a total score of 22 or more on the 21‐item Hamilton Depression Rating Scale (HDRS‐21) at screening and baseline, with major depressive disorder without psychotic features, diagnosed using DSM‐IV criteria. Age range: 16‐71 years. Exclusion criteria: medical complication or physical finding that could interfere with study activities or drug absorption, distribution, metabolism or excretion, a history of electroconvulsive therapy within the previous 6 months, hypersensitivity or a lack of response to a previous course of reboxetine or citalopram, or a positive serum pregnancy test or breast‐feeding. |
|
| Interventions | Citalopram: 176 participants. Reboxetine: 181 participants. Citalopram: 20‐40 mg/day Reboxetine: 8‐10 mg/day Sedatives/hypnotics taken on an as‐needed basis for sleep were allowed. Other psychotropic medications were not allowed. |
|
| Outcomes | Primary outcome: endpoint score on the HDRS‐21. Secondary outcomes: change from baseline in total score on Montgomery‐Asberg Depression Rating Scale (MADRS), Clinical Global Impression (CGI), Social Adaptation Self‐evaluation Scale (SASS) and Sexual Function Scale (SF), response rate (reduction of at least 50% in HDRS total score from baseline), remission rate (HDRS total score of 10 or less at each post‐baseline visit), time to response and time to remission. |
|
| Notes | This study was founded by Pfizer (reboxetine manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess whether adequate sequence generation was made. Quote "patients were randomized to receive 24 weeks of treatment with reboxetine or citalopram". |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "two types of analyses were performed for the primary variable (HDRS total score), namely Last Observation Carried Forward (LOCF) and Observed Cases (OC). (...) when data were analysing, it was however concluded that the LOCF analysis was less valid because there was a huge amount of missing data. Another reason for nor using the LOCF was that the treatment effect was increasing over time, which would have been ignored in an LOCF analysis. The OC was therefore finally considered as the most valid analysis for the primary efficacy variable". |
| Selective reporting (reporting bias) | High risk | No clear data about dropout rate in each group. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Leinonen 1999.
| Methods | Eight‐week, double‐blind, multicentre, randomised study. | |
| Participants | Patients fulfilling the DSM‐IV criteria for a major depressive episode according to the DSM‐IV check‐list with a total score of ≥ 22 on the Montgomery and Asberg Rating Scale for Depression (MADRS). Exclusion criteria: patients with a history or presence of bipolar disorder, depressive disorder (not otherwise specified), schizophrenia, adjustment disorder, schizotypal or borderline personality disorder, organic mental disorder, anxiety disorders preceding depression, or presence of eating disorders (anorexia or bulimia nervosa), post‐partum depression, epilepsy or a history of seizure disorder or treatment with anticonvulsant medication for epilepsy or seizures, alcohol or substance abuse during the lat 12 months, with actual risk of committing suicide defined as MADRS score 5 or 6 or assessed by investigators as being at high risk of committing suicide. Patients with a previous history or actual presence of any meaningful renal, hepatic, respiratory, cardiovascular or cerebrovascular disease or other serious, progressive physical disease, or with any clinically meaningful abnormal finding uncovered during the physical examination and/or clinically significant abnormal laboratory results at screen and still present at baseline. Non‐responders to antidepressant treatment. Patients participating in any other clinical trials or treated before the start of active treatment with MAO inhibitors (2 weeks), fluoxetine (4 weeks), citalopram (current episode), electroconvulsive therapy (3 months), benzodiazepines (2 weeks), other psychotropic drugs (1 week). Women pregnant or lactating, or women who intended to become pregnant during the course of the study were not eligible for participation. |
|
| Interventions | Citalopram: 133 participants. Mirtazapine: 137 participants. Citalopram dose range: 20‐60 mg/day (mean: 36,6 sd: 9,7) Mirtazapine dose range: 15‐60 mg/day (mean: 35,0 sd: 6,9) |
|
| Outcomes | Outcomes: mean change on MADRS, Hamilton Anxiety Scale (HAM‐A), Clinica Global impression (CGI), Leeds Sleep Evaluation Questionnaire (LSEQ) and Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ) score. | |
| Notes | This study was founded by Mirtazapine manufacturer (Organon). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were allocated to treatment with either mirtazapine or citalopram, according to the centrally prepared randomization list". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "active medication was prepared as indistinguishable looking tablets and packaging was performed using a double‐dummy technique". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "efficacy analyses were based on Intention‐to‐treat (ITT) patient sample, thus including all randomized subject who received at least one dose of study medication and had at least one post‐baseline efficacy assessment on MADRS, using the Last Observation Carried forward (LOCF) method. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Lepola 2003.
| Methods | Eight‐week, double‐blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM‐IV criteria for Major Depressive Disorder and having a total score on Montgomery‐Asberg Depression Rating Scale (MADRS) between 22 and 40. Age range: 18‐65 years. Exclusion criteria: mania or any bipolar disorder, schizophrenia or any psychotic disorder, obsessive‐compulsive disorder, eating disorder, mental retardation, any pervasive developmental disorder or cognitive disorder (according to DSM‐IV criteria), MADRS score >= 5 on item 10, treatment with antipsychotics, antidepressants, hypnotics, anxiolytics, barbiturates, chloral hydrate or other 5‐hydroxytryptamine receptor agonists, electroconvulsive treatment, treatment with behaviour therapy or psychotherapy. | |
| Interventions | Escitalopram: 156 participants. Citalopram: 161 participants. Escitalopram dose range: 10‐20 mg/day. Citalopram dose range: 20‐40 mg/day. Benzodiazepines for insomnia were allowed. |
|
| Outcomes | Primary outcome: Change from baseline to week 8 in MADRS. Secondary Outcomes: Clinical Global Impression‐Improvement (CGI‐I), Clinical Global Impression‐Severity (CGI‐S), MADRS Individuals Items (apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, suicidal thoughts). | |
| Notes | This study was funded by escitalopram manufacturer. One fetal death in female patient treated with citalopram; one unintended pregnancy in female patient treated with escitalopram. Remission: a score equal or less than 12 on MADRS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: " there was an initial single blind placebo period, followed by randomization of eligible patients in a 1:1:1 ratio of escitalopram, citalopram and placebo treatment". The following weeks are in double‐blind conditions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention‐to‐treat (ITT) population included all randomized patients who took at least one dose of double‐blind study product and who had at least one valid post‐baseline MADRS assessment." |
| Selective reporting (reporting bias) | High risk | Many rating scales listed in Methods, but only a few reported. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Lewis 2011.
| Methods | Twelve‐week, randomised controlled trial. | |
| Participants | Patients with depression, recruited in primary care, aged 18‐74 years who had already agreed with their general practitioner that antidepressant should be prescribed. Patients who had taken antidepressant medication within the 2‐weeks prior to the baseline assessment and those who could not complete self‐administered scales were excluded. General practitioner also excluded those with medical contraindications, psychosis, bipolar affective disorder, major substance or alcohol misuse and others whose participation was deemed inappropriate. |
|
| Interventions | Citalopram: 298 participants. Reboxetine: 303 participants. Citalopram dose: 20 mg/day. Reboxetine dose: 8 mg/day. |
|
| Outcomes | Primary outcome: total Beck depression Inventory Score (BDI) at 6‐weeks. Secondary outcomes: remission rates (defined as BDI score < 10) at 6‐weeks, Short Form Health Survey mental and physical sub‐scale scores and Hospital Anxiety and Depression Scale total scores. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted using a computer‐generated code, administered centrally and communicated by telephone and thereby concealed in advance from the researcher. Allocation was stratified by severity of symptoms and by centre, using variable block sized to maximise concealment". |
| Allocation concealment (selection bias) | Low risk | Quote: "(Randomization was) concealed in advance from the researcher. Allocation was stratified by severity of symptoms and by centre, using variable block sized to maximise concealment". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No data reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate and unbalance between treatment groups (about 20% of lost to follow up in the citalopram group and about 30% in the reboxetine group) |
| Selective reporting (reporting bias) | Low risk | Primary outcome data were reported. |
| Other bias | Low risk | Sponsorship bias can be ruled out. |
Lu 10‐171, 83‐01.
| Methods | Six‐week, randomised, double‐blind study. | |
| Participants | In‐and outpatients of either sex, 18‐65 years old, who had given their informed consent to participate in the study, and who were suffering from a major depressive episode (DSM‐III classification) of a severity corresponding to a total score of at least 18 points on the HDRS‐17 items. Exclusion criteria: concurrent somatic disease (particularly severe liver, heart or kidney disease); pregnancy or absence of use of an effective contraceptive method; a history of epilepsy, glaucoma, urinary retention, alcoholism, pyloric stenosis or symptomatic prostatic hypertrophy, marked mental subnormality, need of ECT or administration of ECT during the previous month, treatment with TCA in adequate dosage (100 mg/day of amitriptyline or equivalent) during the last month or with a MAO‐I during the last 2 weeks prior to entry into the study. |
|
| Interventions | Citalopram: 23 participants. Citalopram dose range: 20‐60 mg/day. Imipramine: 22 participants. Imipramine dose range: 50‐150 mg/day. |
|
| Outcomes | Outcomes: Change from baseline to week 6 in HDRS‐17 items, Leeds self rating scale. | |
| Notes | This study was funded by Lundbeck. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the randomization was made in block of 4 according to a code prepared by the Biostatistical Department of Lundbeck". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Lu 10‐171,79‐01.
| Methods | Eight‐week, double‐blind, randomised study. | |
| Participants | Hospitalised depressed patients who needed antidepressant medication. Age range: 18‐65 years. Exclusion criteria: patients with severe somatic disorders (particularly in heart, liver and kidney), pregnant patients and patients who did not wish to participate after having been informed of the trial. |
|
| Interventions | Citalopram: 21 participants. Citalopram dose range: 40‐60 mg/day. Nortriptyline: 22 participants. Nortriptyline dose range: 50‐150 mg/day. |
|
| Outcomes | Outcomes: change in the severity of depression assessed using the HDRS and CGI scores. | |
| Notes | This study was funded by Lundbeck. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients fulfilling the inclusion criteria were allocated randomly". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "in order to ensure blindness, the nortriptyline tablets were supplemented with placebo tablets up to a total of 4 tablets. The initial dose of nortriptyline was estimated for all patients, and in accordance with the randomization list the drugs were packed by the hospital laboratory in doses boxes containing citalopram only or nortriptyline plus any necessary placebo tablets". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported. |
| Selective reporting (reporting bias) | Unclear risk | No information reported. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Matreja 2007.
| Methods | Six‐week, open‐label, prospective, randomised study. | |
| Participants | Patients suffering from Major Depressive Disorder as per DSM‐IV criteria were enrolled in the study, with Hamilton Depression Rating Scale (HDRS) score >18. Age range: 18‐75 years. |
|
| Interventions | Citalopram: 50 participants. Sertraline: 50 participants. Citalopram dose range: 20‐60 mg/day (mean dose: 33 sd: 13). Sertraline dose range: 50‐150 mg/day (mean dose 96 sd: 35). |
|
| Outcomes | Outcomes: change in the severity of depression assessed using the HDRS, Montgomery and Asberg Rating Scale for Depression (MADRS) and Amritstar Depressive Inventory (ADI) scores. | |
| Notes | No information provided about study sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a total of 100 patients were randomized into two groups as per random number table". Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the primary statistical analysis was Intention‐to‐treat (ITT) for all safety and efficacy variables with the Last Observation Carried Forward (LOCF) for those patients who had at least 2 weeks data". |
| Selective reporting (reporting bias) | High risk | Also data about individual side‐effects are missing. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Moeller 2003.
| Methods | Four‐week, prospective, randomised study. | |
| Participants | In‐patients fulfilling DSM‐IV criteria for unipolar depression. Exclusion criteria: patients who were not physically healthy, needed further medication, had a history of endocrine disorders, were pregnant or were suffering from alcohol or drug abuse. Age range: 19‐67 in citalopram group; 16‐64 in reboxetine group. |
|
| Interventions | Citalopram: 19 participants. Reboxetine: 17 participants. Citalopram fixed dose: 40 mg/day. Reboxetine fixed dose: 8 mg/day. Only diazepam and zaleplon were allowed as additional medications. |
|
| Outcomes | Primary outcome: basal prolactin levels from baseline to endpoint. Secondary outcomes: mean change on Hamilton Depression Rating Scale (HDRS) and Montgomery and Asberg Rating Scale for Depression (MADRS) scores from baseline to endpoint. |
|
| Notes | Three days before tests started, patients were treated exclusively with diazepam (for agitation) and zaleplon (for insomnia) in order to wash out previous antidepressant medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were assigned randomly". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "patients were not blinded about medication". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Moore 2005.
| Methods | Eight‐week, double‐blind, prospective, multicentre, randomised study. | |
| Participants | Outpatients meeting DSM‐IV criteria for Major Depressive Disorder (MDD) and having a Montgomery‐Asberg Depression Rating Scale (MADRS) total score at baseline of at least 30. Age range: 18‐65 years. Exclusion criteria: patients meeting criteria for primary diagnoses of any axis I disorder other than MDD, or those with a history of mania, bipolar disorder, schizophrenia or other psychotic disorder, obsessive‐ compulsive disorder, cognitive disorder including mental retardation or personality disorder. Patients who met DSM criteria for substance abuse or dependence within the past 12 months, or who used a depot antipsychotic within 6 months before study inclusion, or any antipsychotic, anxiolytic or anticonvulsant medication within 2 weeks before the first administration of study medication. |
|
| Interventions | Escitalopram: 138 participants. Citalopram: 142 participants. Escitalopram fixed dose: 20 mg/day. Citalopram fixed dose: 40 mg/day. |
|
| Outcomes | Primary outcome: mean change from baseline to endpoint on the MADRS. Change from baseline to last assessment score in the Clinical Global Impression‐Severity Scale (CGI‐S). |
|
| Notes | This study was funding by H. Lundbeck A/S. One suicide completed in citalopram group after 12 days of treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients meeting eligibility criteria were randomly assigned (...) with equal block randomization at baseline". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind" but author not give other information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy analysis on Intention‐to‐treat (ITT) population (all patients who took at least one dose of study medication and who had at least one valid post‐baseline MADRS assessment). |
| Selective reporting (reporting bias) | High risk | Many rating scales listed in Methods, but only a few reported. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Navarro 2001.
| Methods | Twelve‐week, randomised, single‐blind study. | |
| Participants | In‐ and out‐patients with unipolar major depression fulfilling the DSM‐IV criteria for a current major depressive episode, with or without endogenous or psychotic features. Only elderly patients with late‐onset depression were included (depression late‐onset had to have begun after the age of 50). Age: 60 years or over. Exclusion criteria: patients with significant abnormal biological findings on electrocardiographic or laboratory examination, those with focal neurological findings or systemic neurological disorder (e.g. seizure disorders, stroke, Parkinson's disease) and those with uncontrolled medical illness at the time of recruitment. Patients with a manic or hypomanic episode, any history of psychosis, current substance dependence and electroconvulsive therapy within 6 months of recruitment. |
|
| Interventions | Citalopram: 29 participants. Nortriptyline: 29 participants. Citalopram dose range: 30‐40 mg/day (mean dose: 33.45; SD 4.84) Nortriptyline dose range: 50‐100 mg/day (mean dose: 61.11; SD 17.45) Lorazepam up to 4 mg/day was allowed for management of anxiety or insomnia. |
|
| Outcomes | Primary outcome: mean change in Hamilton Depression Rating Scale (HDRS) score from baseline to endpoint. | |
| Notes | Six patients with psychotic symptoms (two in nortriptyline group and four in citalopram group) received haloperidol up to 4 mg/day during the first 4 weeks. Eligible patients underwent a 2‐week washout period. Rapid wash‐out responders (HDRS decreased by 25% or more) were excluded from the study. This study was partially supported by a research grant from the Investicacions Biomediques August Pi i Sunyer Institut (IDIBAPS) to Victor Navarro and by FIS grant 99/0171. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomly divided into two subgroups". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "single‐blind", but author not give other information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "statistical analyses of safety data were conducted on all patients randomized to treatment who took at last one dose of study medication. Efficacy analyses included all modified intent to treat patients: that is all patients randomized to treatment who took their assigned medication for 4 weeks or more". |
| Selective reporting (reporting bias) | Unclear risk | No reliable data provided. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Ou 2010.
| Methods | Six‐week, randomised, parallel group, controlled study. | |
| Participants | In‐ and out‐patients were recruited if they met the following criteria: age 18‐65 years, diagnosis of Major depressive Disorder (MDD) as defined as Axis I of the DSM‐IV, total score of the Hamilton Depression Rating Scale 17 Item (HDRS‐17) > or = 17, in the opinion of the treating psychiatrist, potential benefit from treatment with one or the other study drugs. Exclusion criteria: patients were excluded if they met DSM‐IV Axis I criteria for mania or any bipolar disorder, schizophrenia or any psychotic disorder or displayed any psychotic features, obsessive‐compulsive disorder, mental retardation or any pervasive developmental disorder, eating disorder (anorexia nervosa or bulimia nervosa), dementia, or alcohol or drug abuse within the previous 12 months. further exclusion criteria were a history of severe drug allergy or hypersensitivity, other serious illness or sequela of serious illness, citalopram or escitalopram treatment within 60 days prior to inclusion, and/or inability to comply with the protocol in the investigator's opinion. Patients were also excluded if they serious tended to suicide. Patients who had joined any other clinical trial or who received oral antipsychotic drugs, monoamine oxidase inhibitors, or electroconvulsive therapy within 4 weeks prior to initiation of the study were also excluded. Women who were pregnant or breast feeding were also excluded. |
|
| Interventions | Citalopram: 120 participants. Escitalopram: 120 participants. Citalopram dose range: 20 mg/day. Escitalopram dose range: 10 mg/day. |
|
| Outcomes | Primary outcome: change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint. Secondary outcome: patients who responded to treatment, patients who remitted. |
|
| Notes | Eligible patients underwent a 1‐ to 7‐day washout period. This study was funded by the National institutes of Pharmaceutical Research and Development Co., Ltd., and all drugs were provided by the company. The sponsor’s only role was in the design and monitoring. The company had no further role in data collection, analysis, and interpretation or writing of this paper, or in the decision to submit the paper for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized (without restriction or stratification) through a computer‐generated table to one of the two treatments in blocks of four to ensure approximately equal numbers in the two treatment groups". |
| Allocation concealment (selection bias) | Low risk | Quote: "to ensure concealment of the randomization, which was conducted independently of the investigators by a research pharmacist at a separate facility, medication was provided in coded packages". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "medication was provided in coded packages containing the drugs, which were identical in appearance, taste and odor". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy analysis was conducted in the Intention‐to‐treat population, which included all patients who received at least one dose of medication and had data available from at least one valid post‐baseline efficacy assessment". |
| Selective reporting (reporting bias) | Unclear risk | No reliable data provided. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Rosenberg 1994.
| Methods | Twenty‐two‐week, multicentre study. The primary treatment period was 6 weeks. However, patients who in the opinion of the investigator would benefit from further treatment could continue treatment under double‐blind conditions for a further 16 weeks, i.e. for a total of 22 weeks. | |
| Participants | Depressed patients of either sex, who were assessed as being in need of antidepressant treatment and who had a total score of 14 or more on the Hamilton Depression Rating Scale (HDRS) Age range: 18‐65 years. Exclusion criteria: pregnancy, failure to use an acceptable contraceptive method, known alcohol or drug abuse within the past year, psychosis, serious somatic disease, treatment with MAO inhibitors within the last 2 weeks or with other antidepressants within the last week before inclusion and hypersensitivity to test drugs. Patients who required psychiatric in‐patient treatment were also excluded. |
|
| Interventions | Citalopram 10‐30 mg/day: 187 participants. Citalopram 20‐60 mg/day: 193 participants. Imipramine 50‐150 mg/day: 92 participants. Benzodiazepines or sedatives antihistamines could be prescribed for sleep disturbance, but other psychotropic drugs were not allowed. |
|
| Outcomes | Primary outcome: mean change in HDRS score from baseline to endpoint. Secondary outcomes: mean change in Clinical Global Impression (CGI) and Visual Analogue Scale (VAS) score, HDRS factors as depression, sleep disturbances, anxiety/somatization, retardation. |
|
| Notes | This study was funded by Lundbeck (citalopram manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated to one or two dose levels of citalopram or imipramine treatment. In each block of five patients one patient received imipramine and two pairs of patients each received one of the two citalopram dose". Randomization ratio 1:2:2. |
| Allocation concealment (selection bias) | Unclear risk | No data provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "This study was a double blind comparison (...) tablets of identical appearance were used". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients receiving at least one tablet constituted the Intention‐to‐treat (ITT) population. Patients who met the inclusion and exclusion criteria, had a compliance of 50% or higher and who completed at least 14 days of treatment constituted the Efficacy Population (EFF). |
| Selective reporting (reporting bias) | Unclear risk | No clear information reported. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
SCT‐MD‐02.
| Methods | Eight‐week, double‐blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM‐IV criteria for Major Depressive Disorder (MDD) and having a minimum score of 22 on Montgomery‐Asberg Depression Rating Scale (MADRS) and a minimum score of 2 on Item 1 of Hamilton Depression Rating Scale (HDRS). Age range: 18‐80 years. | |
| Interventions | Escitalopram: 129 participants. Citalopram: 128 participants. Escitalopram dose range: 10‐20 mg/day. Citalopram dose range: 20‐40 mg/day. | |
| Outcomes | Primary Outcome: Change from baseline to week 8 in MADRS. Secondary outcomes: HDRS, HDRS Depressed Mood Item, Clinical Global Impression‐Improvement (CGI‐I), Clinical Global Impression‐Severity (CGI‐S). | |
| Notes | This study was funded by escitalopram manufacturer. Only unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized". Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No clear information reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: Intention‐to‐treat (ITT) analysis ("all patients with at least one post‐baseline assessment of MADRS"). |
| Selective reporting (reporting bias) | Unclear risk | No clear information reported. |
| Other bias | Unclear risk | No reliable information reported. |
Shaw 1986.
| Methods | Six‐week, double‐blind, randomised study. | |
| Participants | In‐ and out‐patients who met the DSM‐III criteria for Major Depressive illness, scored 18 or more on the 17‐item Hamilton Depression Rating Scale Scale (HDRS). All participants entered into the trial within 36 hours of admission (48 hours at week end). Age range: 18‐70 years. |
|
| Interventions | Citalopram: 29 participants. Amitriptyline: 30 participants. Citalopram dose range: 30‐60 mg/day. Amitriptyline dose range: 112.5‐225 mg/day. |
|
| Outcomes | Outcomes: mean change on HDRS and Montgomery‐Asberg Depression Rating Scale (MADRS), Newcastle Scale, Leeds Self‐rating Depression Scale. | |
| Notes | The study was funded by Lundbeck (citalopram manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the trial was randomized in blocks of four". |
| Allocation concealment (selection bias) | Unclear risk | No reliable information reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reliable information reported. |
| Selective reporting (reporting bias) | Unclear risk | No reliable information reported. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Stahl 2000.
| Methods | Twenty‐four‐week, eight centres, double‐blind randomised trial. | |
| Participants | Patients who satisfied DSM‐IV criteria for Major Depressive Disorder (MDD) with a minimum 2 months duration of illness, with a score of at least 22 on the Hamilton Depression Rating Scale (HDRS) , a minimum score of 2 on Depressed Mood Item and a minimum score of 8 on Raskin Depression Scale together with a lower score on the Covi Anxiety Scale.
Age range: 18‐60 years old. Exclusion criteria: pregnancy, inadequate contraception, another DSM‐IV Axis I diagnosis, use of other psychotropic medication, increased risk of suicide, treatment resistance, history of sertraline intolerance or SSRI hypersensitivity reactions, history of alcohol or substance abuse. |
|
| Interventions | Sertraline: 108 participants. Citalopram: 107 participants. Placebo: 108 participants. Sertraline dose range: 50‐150 mg/day. Citalopram dose range: 20‐60 mg/day. Chloral Hydrate was permitted. | |
| Outcomes | 21‐HDRS, MADRS, Clinical Global Impression‐Severity (CGI‐S) andClinical Global Impression‐Improvement (CGI‐I), Hamilton Anxiety Scale (HAM‐A), Symptom Checklist‐56 (SCL‐56), Quality of Life Enjoyment and Satisfaction Questionnaire (Q‐LES‐Q). | |
| Notes | This study was funded by Lundbeck (citalopram manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "randomized". Probably done, as a similar trial by these investigators included the same phrase and used a proper method of allocation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote "double‐blind" but authors did not give other information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data and standard deviations. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Timmerman 1993.
| Methods | Six‐week, double‐blind, randomised, parallel group, multicentre study. | |
| Participants | Cooperative out‐patients of either sex with a reasonable knowledge of the Dutch language, who met the DSM‐III‐R criteria for "Major Depression, single episode", "Major Depression, recurrent", "Bipolar Disorder, depressed", with a score of a least 16 on the 17 items Hamilton Depression Rating Scale (HDRS). Age range: 18‐70 years. Exclusion criteria: patients who had been treated with MAO inhibitors or fluoxetine within the last 3 weeks or with other psychotropic drugs within the last week, with the exception of benzodiazepines. Patients with another primary psychiatric diagnosis than the above mentioned, or with a history of epilepsy, alcohol and/or drug abuse, pregnant or lactating women and women with childbearing potential failing to use standard birth control methods as well as patients with renal, hepatic, cardiovascular, neurological or somatic disorders, and/or significant abnormal laboratory findings. |
|
| Interventions | Citalopram: 108 participants. Fluvoxamine: 109 participants. Citalopram dose range: 20‐40 mg/day Fluvoxamine dose range: 100‐200 mg/day |
|
| Outcomes | Primary outcome: mean change on HDRS score from baseline to endpoint. Secondary outcomes: change in Clinical Global Impression‐Severity (CGI‐S) score, in the Zung Self‐rating Scale for depression score. |
|
| Notes | The study was funded by Lundbeck (citalopram manufacturer). One suicide completed in citalopram group, one fatal myocardial infarction in citalopram group, two suicide attempted in fluvoxamine group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | No reliable information reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "patients were randomly assigned to double‐blind treatment". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the Intention‐to‐treat (ITT) population included all patients who had been allocated a randomization number to entry of double‐blind treatment". |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
Yevtushenko 2007.
| Methods | Six‐week, prospective, randomised, double‐blind, active‐controlled trial was conducted at eight psychiatric outpatient clinics across the Federation of Russia. | |
| Participants | Outpatients, aged 25 (this minimum age limit was a requirement of one of the ethics committees) to 45 years, with a diagnosis of Major Depressive Disorder (MDD), as defined in the DSM‐IV criteria and a total score more than or equal to 25 on Montgomery‐Asberg Depression Rating Scale (MADRS). Patients were not eligible if they met DSM‐IV criteria for mania or any bipolar disorder, schizophrenia, or any psychotic disorder, or displayed any psychotic features, obsessive‐compulsive disorder, mental retardation or any pervasive developmental disorder, eating disorder (anorexia nervosa or bulimia nervosa), dementia, or alcohol or drug abuse within the previous12 months, a history of severe drug allergy or hypersensitivity, other serious illness or sequela of serious illness, citalopram or escitalopram treatment within 60 days prior to inclusion. Patients were also excluded if they had received an oral antipsychotic drug or monoamine oxidase inhibitor within 2 weeks prior to inclusion; a depot antipsychotic preparation within 6 months prior to inclusion; an SSRI (except fluoxetine), a serotonin‐noradrenaline reuptake inhibitor, or a TCA within 1 week prior to inclusion; or fluoxetine within 5 weeks before inclusion; an antiparkinsonian compound, barbiturate, chloral hydrate, lithium, anticonvulsant, or hypnotic and anxiolytic (except for benzodiazepines used for insomnia at a stable dose for the previous 6 months or used episodically at a lower recommended dose). Women who were pregnant or breast feeding were also excluded from the study. | |
| Interventions | Using equal (˜110 patients per group) block randomisation, patients were assigned to receive a once‐daily fixed dose of escitalopram 10 mg (109 participants), citalopram10 mg (111 participants), or citalopram 20 mg (110 participants) for 6 weeks. | |
| Outcomes | The primary efficacy measure was the change in the MADRS total score from baseline to end of study. Secondary efficacy measures were changes from baseline in MADRS total score in a subgroup of severely depressed patients (MADRS total score more than or equal to 35), MADRS core depression subscale score (in the overall population and severely depressed subgroup), Clinical Global impression‐ Severity (CGI‐S), and Clinical Global impression‐ Improvement (CGI‐I). In addition, the proportions of patients classified a priori as responders (decrease in MADRS total score by at least 50% of the baseline value) or remitters (primary definition, MADRS total score less than or equal to 12; secondary definition, less than or equal to 10) were analysed. | |
| Notes | The present study was part of the S‐citalopram development program for approval in some European Countries through a bridging procedure using results from studies of the racemate, citalopram. Care and medication were free of charge for the patients enrolled in the trial. This study was specifically designed a priori as a superiority study. The sample size was calculated using Singer’s method. The largest between‐group difference was estimated at 5 points, with an SD of 12. Given this assumption, and with an a level of 5% (2‐tailed) and a b level of 20%, it was calculated that 100 patients per arm would be needed to achieve sufficient power. Assuming a 10% withdrawal rate, 10 additional patients per arm were included in the design to ensure sufficient power, giving 110 patients per arm (330 patients in total). This research was sponsored by OOO ARBACOM (Moscow, Federation of Russia) (it's unclear the relationship with the escitalopram manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...Eight equal block randomizations were generated, 1 per center." Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "To maintain blinding, all study medication was provided in capsules (tablets were encapsulated in a lactose powder) that were identical in appearance, taste, and odor.Investigators and patients were blinded to treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Last Observation Carried Forward (LOCF) method of replacing missing values. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Sponsorship bias cannot be ruled out. |
MAO: Monoamine oxidase MAOIs: Monoamine oxidase inhibitors MDA: malondialdehyde SOD: superoxide dismutase SSRIs: Selective serotonin re‐uptake inhibitors TCA: tricyclic antidepressant
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adli 2008 | Wrong design (non‐randomised). |
| Altamura 2008 | Wrong intervention. |
| Altamura 2008b | Wrong intervention. |
| Amiaz 2008 | Wrong diagnosis. |
| Amsterdam 2006 | Wrong design (non‐randomised). |
| Amsterdam 2008 | Medical/psychiatric comorbidity. |
| Anderer 2002 | Wrong comparison. |
| Andersen 1993 | Post‐stroke depression. |
| Angermann 2007 | Depressed heart failure patients. |
| Anon 1995 | Not compared with other antidepressive agents. |
| Anonymous 2011 | Wrong design (not randomised). |
| Azorin 2004 | This study pooled from three different clinical trials. |
| Baldwin 2006 | Duplicate publication. |
| Barone 2011 | Medical/psychiatric comorbidity. |
| Bauer 2010 | Wrong comparison. |
| Baumann 1998a | Not compared with other antidepressive agents. |
| Baumann 1998b | Not compared with other antidepressive agents. |
| Benkelfat 1987 | Only two patients randomised to citalopram. |
| Berney 2008 | Not compared with other antidepressive agents. |
| Bersani 1997 | Not compared with other antidepressive agents. |
| Beving 1985 | Non‐randomised study. |
| Bhagwagar 2004 | Patients with a previous history of depression. |
| Bigos 2008 | Wrong population. |
| Bijl 2004 | Wrong comparison (Escitalopram for depression). |
| Bjerkenstedt 1985 | Not compared with other antidepressive agents. |
| Blier 2006 | Patients randomised to various treatment strategies, including augmentation strategies and psychotherapy (STAR*D study). |
| Bouchard 1997 | Wrong formulation of intervention (e.v.). |
| Boulenger 2010 | Wrong comparison. |
| Bowden 1998 | Wrong diagnosis (bipolar patients were included). |
| Brown 2004 | Not compared with other antidepressive agents. |
| Bryan 2007 | Association of diabetes mellitus with response to depression treatment. |
| Bun'kova KM | Wrong comparison. |
| Carman 2002 | Patients with major depression or bipolar disorder. |
| Chakravarti 2002 | Wrong design. |
| Chan 2009 | Medical comorbidity. |
| Chen 2005 | Post‐stroke depression. |
| Conte 1997 | Not compared with other antidepressive agents. |
| Cooper‐Kazaz 2011 | Double publication of Stahl 2000. |
| Court 2010 | Wrong diagnosis. |
| Culang 2009 | Wrong comparison. |
| Daly 2011 | Wrong design (not randomised). |
| Davis 2006 | Patients randomizsd to various treatment strategies, including augmentation strategies and psychotherapy (STAR*D study). |
| Davis 2010 | Wrong comparison. |
| Davis 2010b | Wrong design (non‐randomised). |
| Deakin 2002 | Not compared with other antidepressive agents. |
| DeBattista 2011 | Wrong comparison. |
| Dell'Agnello 2001 | Medical/psychiatric comorbidity. |
| Dell'Osso 2008 | Wrong comparison. |
| Deng 2006 | Wrong comparison (citalopram combined with quetiapine). |
| Denko 2007 | Patients randomized to various treatment strategies, including augmentation strategies and psychotherapy (STAR*D study). |
| Devos 2008 | Medical/psychiatric comorbidity. |
| Di Simplicio 2010 | Wrong design (non‐randomised). |
| Diniz 2010 | Wrong diagnosis. |
| Doggrell 2006 | Wrong diagnosis (resistant depression). |
| Domelas 2007 | Patients with coronary artery disease. |
| Doree 2007 | Quetiapine augmentation for treatment resistant depression. |
| Dougherty 2009 | Wrong diagnosis. |
| Dozois 2009 | Wrong comparison. |
| Dunbar 2010 | Wrong comparison. |
| Eriksson 1996 | Wrong diagnosis. |
| Eyding 2010 | systematic review and meta‐analysis (citalopram studies already included in the present review). |
| Fava 2006 | Patients randomised to various treatment strategies, including augmentation strategies and psychotherapy (STAR*D study). |
| Feighner 1997 | Not compared with other antidepressive agents. |
| Feighner 1997b | Wrong comparison. |
| Feighner 1999 | Not compared with other antidepressive agents. |
| Fernandez 2005 | Double reference. |
| Fernandez 2009 | Medical/psychiatric comorbidity. |
| Flicker 1998 | Patients with or without dementia. |
| Ford 2010 | Wrong comparison. |
| Fraguas 2009 | Medical/psychiatric comorbidity. |
| Frank 2004 | Wrong design. |
| Garriock 2010 | Wrong design (non‐randomised). |
| Gilbert 2008 | Wrong design (non‐randomised). |
| Gilmer 2008 | Wrong design (non‐randomised). |
| Glod 2004 | Patients are adolescents. |
| Goder 2011 | Wrong design (not randomised). |
| Gommol 2010 | Wrong comparison. |
| Gonsai 2000 | Wrong population. |
| Gorman 2002a | Wrong design. |
| Gorwood 2007 | Escitalopram for preventing relapse. |
| Guelfi 1998 | Not compared with other antidepressive agents. |
| Hannestad 2011 | Wrong comparison. |
| Harrington 2002 | Not compared with other antidepressive agents. |
| Hegerl 2005 | Non randomised design. |
| Hellerstein 2010 | Wrong comparison. |
| Hemels 2004 | Economic evaluation. |
| Herrera‐Guzman 2009 | Wrong comparison. |
| Hflich 2011 | Wrong design. |
| Hindmarch 2000 | Discontinuation treatment. |
| Hochstrasser 2001 | Maintenance therapy. |
| Holtzheimer 2008 | Wrong comparison. |
| Howland 2011 | Wrong design. |
| Huezo‐Diaz 2009 | Wrong comparison. |
| Johnson 2002 | Wrong design. |
| Judge 2000 | Non randomised design. |
| Kapitany 1999 | Not compared with other antidepressive agents. |
| Kasckow 2010 | Wrong diagnosis. |
| Kasper 2009 | Wrong comparison. |
| Ketter 2006 | Wrong diagnosis. |
| Khazaie 2006 | Not randomised design. |
| Khazaie 2011 | Medical comorbidity. |
| Kiosses 2010 | Wrong intervention. |
| Klysner 2000 | Not compared with other antidepressive agents. |
| Kornstein 2006 | Escitalopram for relapse prevention. |
| Kovacs 1998 | Not compared with other antidepressive agents. |
| Kraus 2008 | Medical/psychiatric comorbidity. |
| Kroenke 2009 | Medical/psychiatric comorbidity. |
| Kuhn 2003 | Medical comorbidity. |
| Kupfer 2000 | Double reference. |
| Lakey 2008 | Non major depression. |
| Lam 2008 | Wrong comparison. |
| Lavretsky 2010 | Wrong comparison. |
| Leuchter 2009 | Wrong comparison. |
| Li WQ 2006 | Vascular depression. |
| Lindsley 2010 | Wrong comparison. |
| Linnet 1996 | Wrong design. |
| Liu 2006b | Medical/psychiatric comorbidity. |
| Liu 2006c | Medical/psychiatric comorbidity. |
| Llacer 2007 | Depressed patients with anxiety and insomnia. |
| Lydiatt 2006 | Wrong population. |
| Maas 2010 | Wrong comparison. |
| Maksinczyk 1997 | Bipolar depression. |
| Malik 2002 | Treatment for depression as risk factor for ischemic heart disease. |
| Mannu 2009 | Wrong comparison. |
| Martinez 2012 | Wrong design. |
| Martini 2007 | Not compared with other antidepressive agents. |
| Martiny 2004 | Not compared with other antidepressive agents. |
| Martire 2008 | Wrong population. |
| McCabe 2010 | Wrong population (healthy people). |
| Mcgrath 2008 | Wrong design (non‐randomised). |
| Mendels 1990 | Not compared with other antidepressive agents. |
| Meyer 2001 | Not randomised design. |
| Miao 2004 | Post‐stroke depression. |
| Minelli 2010 | Wrong comparison. |
| Miskowiak 2009 | Wrong comparison. |
| Moltzen 2005 | Wrong comparison. |
| Morasco 2010 | Medical/psychiatric comorbidity. |
| Moretti 2002 | Depression and Alzheimer’s disease. |
| Muhonen 2008 | Medical/psychiatric comorbidity. |
| NCT00048815 | Wrong diagnosis. |
| Nierenberg 2004 | Minor depression. |
| Nowak 2003 | Zinc supplementation on antidepressant therapy. |
| Nunez 1999 | Not compared with other antidepressive agents. |
| Nurnberg 2008 | Wrong comparison. |
| Nyth 1990 | Not compared with other antidepressive agents. |
| Oberpichler‐Schwenk 2000 | Wrong design. |
| Pae 2011 | Wrong comparison. |
| Palmer 2002 | Not compared with other antidepressive agents. |
| Papakostas 2000 | Not compared with other antidepressive agents. |
| Parvin 2011 | Wrong intervention. |
| Perlis 2009 | Wrong comparison. |
| Petersen 1998 | Double reference. |
| Pogosova 2004 | Not compared with other antidepressants. |
| Portella 2010 | Wrong comparison. |
| Prasko 2003 | Cognitive behavioural therapy (short or long term) combined with pharmacotherapy. |
| Quante 2010 | Wrong comparison. |
| Raisi 2007 | Combination of citalopram and nortriptyline. |
| Rampello 2004 | Wrong population. |
| Rampello 2004a | Citalopram alone or in combination with amitriptyline; patients with different diagnosis in comorbidity. |
| Rampello 2004b | Post‐stroke depression. |
| Rampello 2006 | Treatment for panic attack. |
| Rapaport 2010 | Wrong comparison. |
| Rapaport 2011 | Wrong diagnosis. |
| Rapoport 2010 | Wrong comparison. |
| Raskin 2011 | Wrong population. |
| Rasmussen 1992 | Not compared with other antidepressive agents. |
| Riva 2006 | Evaluation of integrated pharmacologic and psychotherapeutic treatment. |
| Robinson 2008 | Post‐stroke depression. |
| Robinson 2009 | Wrong population. |
| Rocca 2005 | Non major depression. |
| Roose 2004 | Not compared with other antidepressants. |
| Rosenthal 2002 | Wrong comparison. |
| Rush 2008 | Wrong comparison. |
| Salloway 2002 | Not compared with other antidepressants. |
| Schaefer 2008 | Medical/psychiatric comorbidity. |
| Schfer 2010 | Medical/psychiatric comorbidity. |
| Schmitt 2006 | Escitalopram as continuation treatment of intravenous citalopram. |
| Segal 2010 | Wrong population. |
| Serfaty 2010 | Wrong comparison. |
| Sharp 2010 | Wrong comparison. |
| Smith 2011 | Wrong intervention. |
| Sneed 2007 | Wrong comparison. |
| Soares 2006 | Peri and post menopausal women. |
| Soares 2010 | Wrong comparison. |
| Souery 2010 | Wrong population. |
| Stein 2001 | Wrong diagnosis. |
| Stein 2005 | Psychotherapy plus pharmacotherapy for drug users. |
| Sun 2004 | Refractory depression. |
| Swartz 2008 | Wrong comparison. |
| Talati 2007 | Patients randomised to various treatment strategies, including augmentation strategies and psychotherapy (STAR*D study). |
| Targacept 2008 | Wrong design and wrong intervention. |
| Thase 2010 | Pooled‐analysis (citalopram studies already included in the present review) |
| Thase 2011 | Wrong design. |
| Thorell 1999 | Seasonal affective disorder. |
| Uher 2010 | Wrong comparison. |
| Van Bemmel 1993 | Not compared with other antidepressants. |
| Voirol 1999 | Wrong design (non‐randomised). |
| Wade 2000 | Not compared with other antidepressants. |
| Wade 2006 | Wrong intervention. |
| Wagner 2002 | Patients are children and adolescents. |
| Wang 2005 | Diagnosis is “depression induced by Alzheimer”. |
| Warden 2009 | Wrong intervention. |
| Wermuth 1998 | Parkinson’s disease; not compared with other antidepressants. |
| Wise 2011 | Wrong population. |
| Wisniewski 2009 | Wrong intervention. |
| Wu 2008 | Wrong design (non‐randomised). |
| Yang 2005 | Refractory depression. |
| Yang 2010 | Wrong intervention. |
| Zhao 2005 | Post stroke depression. |
| Zimbroff 2004 | Citalopram for non responders depressive‐patients. |
| Zisook 2007 | Patients with schizophrenia. |
| Zisook 2010 | Wrong diagnosis. |
| Zou 2005 | Citalopram combined with psychological morning exercise. |
Characteristics of studies awaiting assessment [ordered by study ID]
Ahlfors 1988.
| Methods | Four‐week, randomised, double‐blind, multicentre study |
| Participants | Patients with depression, aged from 18 to 70 years and referred to a psychiatric hospital for a depression requiring treatment. |
| Interventions | Citalopram: 37 participants. Mianserin: 34 participants. Citalopram dose‐range: 20‐60 mg/day. Mianserin dose‐range: 60‐90 mg/day. |
| Outcomes | Change in Montgomery and Asberg Depression Rating Scale (MADRS) from baseline to endpoint. |
| Notes | Two patients in Mianserin group died (the reason could not be ascribed to the test treatment). |
Akimova 2010.
| Methods | Double‐blind, randomised, longitudinal PET study using radioligand [11C]DASB |
| Participants | 18 patients |
| Interventions | 10 mg/d escitalopram or 20 mg/d citalopram, i.e. equal doses of the enantiomer S‐Citalopram |
| Outcomes | Serotonin transporter availability in the unmedicated state and SERT occupancy after a single‐dose and later after the first 3 weeks of treatment with SSRIs. The Hamilton Depression Rating scale (HAM‐D, 17 items) was assessed at the screening visit and before each PET scan. |
| Notes | Radioligand [11C]DASB is a new, highly selective PET radiotracer that shows a high affinity for serotonin transporter |
Akimova 2011.
| Methods | Double‐blind, longitudinal study. |
| Participants | Patients with MDD. |
| Interventions | Citalopram: 20mg/day. Escitalpram: 10mg/day. |
| Outcomes | Alterations in different brain regions assessed with PET scans using the radioligand [11C]DASB. |
| Notes |
Aydemir 2011.
| Methods | In the treatment of major depressive disorder, in addition to the remission of symptoms, improvement in functionality and subjective quality of life of the patients is desirable. In this study, we aimed to evaluate and compare the changes in quality of life measures in citalopram‐ versus escitalopram‐treated major depressive disorder patients, and to compare the scores of the patients who achieved remission at the end of treatment with standard scores of the Turkish population. |
| Participants | 74 outpatients with major depressive disorder |
| Interventions | Citalopram was started at a dose of 20 mg/day, and escitalopram was started at a dose of 10 mg/day. At the end of the 6th week, the mean dose for the citalopram treated patients was 24.6 mg/day and for the escitalopram treated patients it was 11.8 mg/day. |
| Outcomes | Treatment response was accepted as a 50% decrease in the index assessment and remission was accepted as HAM‐D<=7 |
| Notes |
Du 2004.
| Methods | Six‐week, (likely) randomised study. |
| Participants | Patients with depression according to CCM‐II criteria. |
| Interventions | Citalopram: 32 participants. Amitriptyline: 32 participants. Citalopram dose‐range: 20‐40 mg/day. Amitriptyline dose‐range: 75‐250 mg/day. |
| Outcomes | Change in Hamilton Depression rating scale (HDRS) from baseline to endpoint, number of patients who responded to treatment, number of patients who remitted. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Fu 2006.
| Methods | Six‐week, randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 34 participants. Amitriptyline: 34 participants. Citalopram dose‐range: 20‐60 mg/day (mean dose: 28.82 SD: 10.67). Amitriptyline dose‐range: 50‐175 mg/day (mean dose: 113.24 SD: 29.02). |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) from baseline to endpoint, number of patients who responded to treatment, number of patients who remitted. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Galecki 2004.
| Methods | Six‐ week study. |
| Participants | 89 elderly patients with a serious depressive episode were involved in the study. |
| Interventions | Citalopram: 44 participants. Venlafaxine: 45 participants. |
| Outcomes | The clinical state of patients was assessed by Hamilton Depression rating Scale (HDRS), a geriatric depressive scale (GDS) and a clinical general impression scale (CGI). Cognitive functions were examined by Mini‐Mental scale. |
| Notes | Waiting for translation from Polish to English (only abstract available in English). |
Gao 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Amitriptyline: 30 participants. Citalopram dose‐rage: 20‐50 mg/day. Amitriptyline dose‐range: 100‐200 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Gong 2005.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 49 participants. Mirtazapine: 49 participants. Citalopram dose‐range: 20‐40 mg/day (mean dose: 29.4 SD: 5.2). Mirtazapine dose‐range: 30‐45 mg/day (mean dose: 37.2 SD: 5.7). |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Huang 2004.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 29 participants. Fluoxetine: 28 participants. Citalopram dose‐range: 20‐40 mg/day. Fluoxetine dose‐range: 20‐40 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17‐ Item (HDRS‐17) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Huang 2006.
| Methods | Six‐week, randomised study. |
| Participants | In and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Fluoxetine: 30 participants. Citalopram dose‐range: 10‐40 mg/day (mean dose: 34 SD: 6.7). Fluoxetine dose‐range: 10‐40 mg/day (mean dose: 33 SD: 6.5). |
| Outcomes | Change in Hamilton Depression Rating Scale 17‐ Item (HDRS‐17) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Huang b 2006.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | Out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 26 participants. Fluoxetine: 25 participants. Citalopram dose‐range: 20‐60 mg/day. Fluoxetine dose‐range: 20‐60 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Juckel 2007.
| Methods | Randomized prospective study |
| Participants | 35 unmedicated in‐patients with a DSM‐IV or ICD‐10 diagnosis of major depressive disorder |
| Interventions | Citalopram versus reboxetine (dose range not specified) |
| Outcomes | Change on Hamilton Rating Scale for Depression |
| Notes | Waiting for supplemental data about efficacy and tolerability from authors |
Li 2004.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Amitriptyline: 30 participants. Citalopram dose‐rage: 20‐40 mg/day (mean dose: 26 SD: 7.42). Amitriptyline dose‐range: 25‐150 mg/day (mean dose116 SD: 24). |
| Outcomes | Change in Hamilton Depression Rating Scale 21 item (HDRS‐21) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Li 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | Out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 25 participants. Venlafaxine: 25 participants. Citalopram dose‐range: 20‐40 mg/day. Venlafaxine dose‐range: 25‐250 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) from baseline to endpoint, number of patients who responded to treatment, number of patients who remitted. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Li 2006.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 28 participants. Escitalopram: 28 participants. Citalopram dose‐range: 20‐40 mg/day. Escitalopram dose‐range: 10‐20 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Li DS 2006.
| Methods | Six‐week, (likely) randomised study. |
| Participants | Out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 41 participants. Paroxetinee: 41 participants. Citalopram dose‐rage: 20‐40 mg/day. Paroxetinee dose‐range: 20‐40 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) from baseline to endpoint, number of patients who responded to treatment, number of patients who remitted. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Li X 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Amitriptyline: 30 participants. Citalopram dose‐rage: 20‐40 mg/day. Amitriptyline dose‐range: 50‐200 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 24 Item (HDRS‐24) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Li Z 2004.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 23 participants. Amitriptyline: 23 participants. Citalopram dose‐rage: 20‐40 mg/day. Amitriptyline dose‐range: 150‐300 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) from baseline to endpoint. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Liang 2005.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Fluoxetine: 30 participants. Citalopram dose range: 10‐60 mg/day. Fluoxetine: dose range: 10‐40 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 24 Item (HDRS‐24) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Liang 2006.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 53 participants. Mianserin: 53 participants. Citalopram dose range: 10‐40 mg/day (mean dose: 27.5 SD: 10.8). Mianserin: dose range: 15‐60 mg/day (mean dose: 40.3 SD: 12.2). |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Lin 2001.
| Methods | Six‐week, (likely) randomised study. |
| Participants | Out‐patients with depression according to CCMD‐II‐R criteria. |
| Interventions | Citalopram: 89 participants. Amitriptyline: 89 participants. Citalopram dose‐rage: 20‐40 mg/day (mean dose: 22 SD: 6). Amitriptyline dose‐range: 50‐150 mg/day (mean dose: 100 SD: 10). |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) from baseline to endpoint. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Liu 2006.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | Out‐patients with depression according to CCMD‐II‐R criteria. |
| Interventions | Citalopram: 50 participants. Amitriptyline: 50 participants. Citalopram dose‐rage: 20‐40 mg/day. Amitriptyline dose‐range: 100‐200 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 24 Item (HDRS‐24) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Liu 2006d.
| Methods | Randomized study (likely) |
| Participants | Patients with senile depression |
| Interventions | Citalopram versus unclear comparator |
| Outcomes | Unclear |
| Notes | Waiting for abstract and full text to check for eligibility |
Lu 2008.
| Methods | Control study. |
| Participants | Patients with depressive disorder. |
| Interventions | Citalopram Doxepin |
| Outcomes | Unclear (full text to retrieve). |
| Notes |
Ma 2004.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐ III criteria. |
| Interventions | Citalopram: 30 participants. Amitriptyline: 30 participants. Citalopram dose‐rage: 20 mg/day. Amitriptyline dose‐range: 25‐175 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Moeller 1986.
| Methods | Four‐week, double‐blind study. |
| Participants | Female in‐patients with a Major Depressive Disorder (MDD) according to the DSM‐III criteria, and with a pretreatment score of at least 18 on the Hamilton Depression Rating Scale‐17 Item (HDRS‐17). Age range: 18‐65 years. Exclusion criteria: patients who did not give their informed consent, pregnant patients, patients with serious concomitant disease (heart, liver, kidney), patients with an organic cerebral syndrome, schizophrenics or patients with a paranoid psychosis, alcoholics or patients addicted to narcotics, patients with epilepsy, and patients having received MAO‐inhibitors within the last 3 weeks. |
| Interventions | Citalopram: 14 participants. Maprotiline: 13 participants. Citalopram dose range: 40‐60 mg/day. Maprotiline dose range: 75‐150 mg/day. |
| Outcomes | Primary outcome: plasma ratios of tryptophan (Trp) and Tyriosine (Tyr) to other large neutral amino acids. |
| Notes | This study was funded by Lundbeck (citalopram manufacturer). One patient in maprotiline group committed suicide. |
NCT00269334.
| Methods | Randomised, open‐label study. |
| Participants | Self‐identified as of Taiwanese ethnic background, and report that both of their parents and all four of their grandparents are members of the same ethnic group; non‐responders: have a 21‐item HDRS score of > 17; partial responders: have a 21‐item HDRS score between 8 and 15; responders: have a 21‐item HDRS score of < 7. Only the non‐responder group will be included in Study II. male or female, who, if of child‐bearing potential, agrees to use effective contraception including the regular use of contraceptive pills, intra‐uterine devises or abstinence; age > 18; capable of giving informed consent. |
| Interventions | Citalopram Paroxetine |
| Outcomes | Structured Clinical Interview for DSM‐IV Disorders (SCID) at week baseline. Hamilton Depression Rating Scale (HDRS) at week 1,2,4,6,8. Beck Depression Inventory (BDI) at week 1,2,4,6,8. Clinical Global Impression Scale (CGI) at week 1,2,4,6,8. Patient's Global Improvement Scale (PGI) at week 1,2,4,6,8. Treatment Emergent Symptoms Scale (TESS) at week 1,2,4,6,8. Arizona Sexual Experience Scale (ASEX) at week 1,2,4,6,8. |
| Notes |
NCT00993876.
| Methods | Randomised, open‐label trial. |
| Participants | Patients with MDD according to DSM‐IV criteria. |
| Interventions | Citalopram: 20‐30 mg/day. Reboxetine: 4‐8 mg/day. |
| Outcomes | Cognitive performance with respect to cognitive flexibility, memory and attention. |
| Notes |
Norra 2011.
| Methods | Randomised study. |
| Participants | Unmedicated patients with major depression and a healthy control group. |
| Interventions | Citalopram Reboxetine |
| Outcomes | Comparison of Auditory Mismatch negativity (MMN) between unmedicated patients with major depression and a healthy control group, longitudinal examination of the patient group to investigate differential monoaminergic treatment effects of antidepressants on MMN. |
| Notes |
Pan 2005.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | In‐patients with depression according to CCMD‐ III criteria. |
| Interventions | Citalopram: 30 participants. Paroxetine: 30 participants. Venlafaxine: 30 participants. Citalopram dose range: 20‐60 mg/day. Paroxetine dose range: 20‐50 mg/day. Venlafaxine dose range: 75‐375 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Qiao 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐ III criteria. |
| Interventions | Citalopram: 34 participants. Paroxetinee: 34 participants. Citalopram dose‐rage: 30‐60 mg/day. Paroxetine dose‐range: 20‐60 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) from baseline to endpoint, number of patients who responded to treatment, number of patients who remitted. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Qiu 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | Out‐patients with depression according to CCMD‐ III criteria. |
| Interventions | Citalopram: 28 participants. Amitriptyline: 28 participants. Citalopram dose‐rage: 20‐40 mg/day. Amitriptyline dose‐range: 75‐250 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Ren 2006.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | In‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 54 participants. Sertraline: 48 participants. Citalopram dose range: 20‐60 mg/day. Sertraline dose range: 50‐150 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 24 Item (HDRS‐24) score from baseline to endpoint, number of patients who responded to treatment, number of patients who remitted. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Rutherford 2010.
| Methods | Preliminary results will be presented from a clinical trial and integrated functional Magnetic Resonance Imaging (fMRI) study randomising adult outpatients with MDD to 8 weeks of treatment in high vs. low expectancy conditions. Expectancy is measured using items 2 and 4 of the CES, which measure the subject’s expected likelihood and magnitude of improvement, respectively. Subjects are treated for 8 weeks with the study medication and are classified as responders (50% decrease from baseline HRSD) or remitters (HRSD < 7). |
| Participants | Included patients are men and women aged 18 to 65 years with unipolar MDD (DSM‐IV) and 24‐item HRSD score = 16. |
| Interventions | Patients are randomised to (1) Placebo‐controlled Track (random assignment to escitalopram or placebo), or (2) Comparator Track (random assignment to escitalopram or citalopram) and are informed of their Track assignment but are blinded to their specific treatment assignment. |
| Outcomes | Well‐validated fMRI paradigms are used to investigate the activity of neural circuits underlying subjects’ response to emotional stimuli, reward processing, and memory retrieval. |
| Notes |
Shi 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Maprotiline: 30 participants. Citalopram dose range: 20 mg/day. Maprotiline dose range: 100‐200 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Song 2004.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 43 participants. Fluoxetine: 46 participants. Citalopram dose range: 20 mg/day. Fluoxetine dose range: 20 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Tan 2004.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 25 participants. Amitriptyline: 26 participants. Citalopram dose range: 20‐40 mg/day. Amitriptyline dose range: 100‐200 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Tang 2005.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | In‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 40 participants. Amitriptyline: 40 participants. Citalopram dose range: 20‐60 mg/day (mean dose: 37.2 SD:17.4). Amitriptyline dose range: 150‐250 mg/day (mean dose: 191.3 SD: 37.8). |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Tao 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 33 participants. Paroxetine: 30 participants. Citalopram dose range: 20‐40 mg/day. Paroxetine dose range: 20‐40 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Thomas 2008.
| Methods | Twelve‐week, multi‐centred randomised controlled trial. |
| Participants | Patients with depression according to ICD‐10 criteria, recruited in primary care setting. |
| Interventions | Citalopram dose range: 20 mg/day. Reboxetine dose range: 8 mg/day. |
| Outcomes | Change in Beck Depression Inventory (BDI) from baseline to week 6. |
| Notes | Only study protocol available. |
Wan 2006.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 35 participants. Amitriptyline: 34 participants. Citalopram dose range: 20 mg/day. Amitriptyline dose range: 150mg/day . |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Wang 2003.
| Methods | Six‐week, (likely) randomised study. |
| Participants | Patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 39 participants. Imipramine: 39 participants. Citalopram dose range: 20‐40 mg/day. Imipramine dose range: 100‐200 mg/day . |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Wang 2004.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 42 participants. Amitriptyline: 42 participants. Citalopram dose range: 20‐40 mg/day (mean dose: 28.6 SD: 5.2). Amitriptyline dose range: 100‐300 mg/day(mean dose: 220 SD: 48). |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Wang 2006.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 25 participants. Mirtazapine: 25 participants. Citalopram dose range: 20‐40 mg/day. mirtazapine dose range: 15‐30 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Xu 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | Out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Amitriptyline: 30 participants. Citalopram dose range: 20 ‐ ? mg/day (the upper dose limit is unclear ‐ mean dose: 25.5 SD: 15.5). Amitriptyline dose range: 50 ‐ ? mg/day (the upper dose limit is unclear ‐ mean dose: 117.4 SD: 35.1). |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment, number of patients who remitted. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Yu 2006.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 29 participants. Venlafaxine: 29 participants. Citalopram dose range: 10‐40 mg/day (mean dose: 15 SD: 7.1). Venlafaxine dose range: 50‐200 mg/day (mean dose: 165 SD: 17). |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Zhang 2005.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to DSM‐IV criteria. |
| Interventions | Citalopram: 32 participants. Venlafaxine: 34 participants. Citalopram dose range: 10‐40 mg/day. Venlafaxine dose range: 50‐225 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Zhang 2006.
| Methods | Six‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Maprotyline: 30 participants. Citalopram dose range: 10‐40 mg/day (mean dose: 21.90 SD:6.93). Maprotyline dose range: 25‐200 mg/day (mean dose: 141.52 SD: 30.4). |
| Outcomes | Change in Hamilton Depression Rating Scale 17 Item (HDRS‐17) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Zhao 2006.
| Methods | Eight‐week, (likely) randomised study. |
| Participants | In‐ and out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 30 participants. Fluoxetine: 30 participants. Citalopram dose range: 20‐60 mg/day. Fluoxetine dose range: 20‐60 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale 24 Item (HDRS‐24) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
Zhou 2005.
| Methods | Seven‐week, (likely) randomised study. |
| Participants | Out‐patients with depression according to CCMD‐III criteria. |
| Interventions | Citalopram: 29 participants. Venlafaxine: 28 participants. Citalopram dose range: 20‐40 mg/day. Venlafaxine dose range: 50‐300 mg/day. |
| Outcomes | Change in Hamilton Depression Rating Scale (HDRS) score from baseline to endpoint, number of patients who responded to treatment. |
| Notes | Waiting for translation from Chinese to English (only abstract available in English). |
MAOIs: Monoamine oxidase inhibitors PET: Positron emission tomography SSRIs: Selective serotonin re‐uptake inhibitors
Characteristics of ongoing studies [ordered by study ID]
NCT01407094.
| Trial name or title | Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care for Depression (NCT01407094). |
| Methods | Randomised, double‐blind study. |
| Participants | Adults, age 18‐65. Outpatients with a current diagnosis of nonpsychotic recurrent MDD per the SCID‐I. QIDS‐SR score of ≥ 14 at Screening Visit and Randomization (Baseline) Visit. No failed antidepressant trials of adequate dose and duration. Agrees to, and is eligible for, all biomarkers procedures (EEG/psychological testing, MRI, and blood draws). |
| Interventions | Citalopram
Bupropion XL Placebo |
| Outcomes | Primary Outcome Measure: HDRS score. |
| Starting date | July 2011. |
| Contact information | David W Morris, Ph.D. 214‐648‐0162 davidw.morris@utsouthwestern.edu Ben T Kurian, M.D. 214‐648‐0158 benji.kurian@utsouthwestern.edu |
| Notes |
NCT01473381.
| Trial name or title | Safety and Efficacy of Vilazodone in Major Depressive Disorder (NCT01473381). |
| Methods | Randomised, double‐blind study. |
| Participants | Patients aged 18‐70 years, with MDD (according to DSM‐IV criteria). The patient's current major depressive episode must be at least 8 weeks and no longer than 12 months in duration. |
| Interventions | Vilazodone Citalopram Placebo |
| Outcomes | Primary Outcome Measure: MADRS score at 10 Weeks. |
| Starting date | November 2011. |
| Contact information | Sandra Beaird, PhD 1‐800‐678‐1605 ext 66297, info@forestpharm.com |
| Notes |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders
MADRS: Montgomery and Asberg Depression Rating Scale MMD: major depressive disorder MRI: magnetic resonance imaging QIDS‐SR: Quick Inventory of Depressive Symptomatology Self‐Report
Differences between protocol and review
In the analyses, the cut‐off point for remission was set at 12 or less on the MADRS (instead of 10), because all studies included in the present review used this cut‐off point for defining remission.
Contributions of authors
AC, CB, TAF and RC conceived and designed the meta‐analysis. AC, MP, CT, AS, GI and SD identified and acquired reports of trials, and extracted data. AC, MP, CT and AS contacted authors of trials and pharmaceutical industries for additional information. AC, CB and MP analysed and interpreted the data. TAF provided statistical advice and input. RC, NW, CT, AS, GI and SD contributed to the interpretation of the data. AC and MP drafted the manuscript. CB, MP, CT, AS, GI, TAF, RC and SD critically reviewed the manuscript.
Sources of support
Internal sources
Department of Public Health and Community Medicine, Section of Psychiatry and Clinical PsychologyUniversity of Verona, Italy.
External sources
No sources of support supplied
Declarations of interest
AC, MP, CB, CT, AS, RC, SD: none declared TAF has received research funds and speaking fees from Asahi Kasei, Astellas, Dai‐Nippon, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Kyowa Hakko, Meiji, Organon, Pfizer, Tsumura, Yoshitomi and Zelia. The Japanese Ministry of Education, Science, and Technology and the Japanese Ministry of Health Labor and Welfare have also funded his research. GI has received speaking fees from Abbott, Angelini, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Lundbeck, Pfizer, Wyeth. NW has received speaking fees from GlaxoSmithKline, but his speech did not deal with pharmacological agents but with methodology of evidence‐based medicine.
New
References
References to studies included in this review
29060/785 {unpublished data only}
- Jefferson J, Griest J. A double‐blind comparison of citalopram and paroxetine in the treatment of patients with depression and anxiety. 39th Annual Meeting of the American College of Neuropsychopharmacology; Dec 10‐14; San Juan; Puerto Rico. 2000.
- Unpublished study. A double‐blind, placebo controlled, fixed‐dosage study comparing the efficacy and tolerability of paroxetine CR and citalopram to placebo in the treatment of Major Depressive Disorder with anxiety. www.gsk‐clinicalstudyregister.com.
Allard 2004 {published data only (unpublished sought but not used)}
- Allard P, Gram L, Timdahl K, Behnke K, Hanson M, Sogaard J. Efficacy and tolerability of venlafaxine in geriatric outpatients with major depression: a double‐blind, randomised 6‐month comparative trial with citalopram. International Journal of Geriatric Psychiatry 2004;19(12):1123‐30. [DOI] [PubMed] [Google Scholar]
Andersen 1986 {published data only}
- Andersen J, Bech P, Benjaminsen S, Bjerre M, Bojhlm S, Christensen P, et al. Citalopram: clinical effect profile in comparison with clomipramine. A controlled multicentre study. Psychopharmacology 1986;90:131‐8. [DOI] [PubMed] [Google Scholar]
- Bech P, Allerup P, Gram LF, Kragh‐Sorensen P, Rafaelsen OJ, Reisby N, et al. The Diagnostic Melancholia Scale (DMS): dimensions of endogenous and reactive depression with relationship to the newcastle scales. Journal of Affective Disorders 1988;14(2):161‐70. [DOI] [PubMed] [Google Scholar]
- Christensen P, Thomsen HY, Pedersen OL, Gram LF, Kragh‐Sorensen P. Orthostatic side effects of clomipramine and citalopram during treatment for depression. Psychopharmacology 1985;86(4):383‐5. [DOI] [PubMed] [Google Scholar]
- Fuglum E, Rosenberg C, Damsbo N, Stage K, Lauritzen L, Bech P. Screening and treating depressed patients A comparison of two controlled citalopram trials across treatment settings: hospitalized patients vs patients treated by their family doctors. Acta Psychiatrica Scandinavica 1996;94(1):18‐25. [DOI] [PubMed] [Google Scholar]
Berlanga 2006 {published and unpublished data}
- Berlanga C, Flores M. Are gender differences in antidepressant response specific to serotoninergic agents? A comparative study of citalopram vs reboxetine. International Journal of Neuropsychopharmacology 2004;7(Suppl 2):S167‐8. [Google Scholar]
- Berlanga C, Flores‐Ramos M. Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. Journal of Affective Disorders 2006;95:119‐23. [DOI] [PubMed] [Google Scholar]
- Eyding D, Lelgemann M, Grouven U, Harter M, Kromp M, Kaiser T. Reboxetine for acute treatment of major depression: systematic review and meta‐analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 2010;341:4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouchard 1987 {published data only}
- Bouchard JM, Delaunay J, Delisle JP, Grasset N, Mermberg PF, Molczadzki M, et al. Citalopram versus maprotiline: a controlled, clinical multicentre trial in depressed patients. Acta Psychiatrica Scandinavica 1987;76(5):583‐92. [DOI] [PubMed] [Google Scholar]
Bougerol 1997a {published data only}
- Bougerol T, Scotto JC, Patris M, Strub N, Lemming O, Hopfner Petersen HE. Citalopram and fluoxetine in major depression: Comparison of two clinical trials in a psychiatrist setting and in general practice (first trial). Clinical Drug Investigation 1997;14(2):77‐89. [Google Scholar]
Bougerol 1997b {published data only}
- Bougerol T, Scotto J‐C, Patris M, Strub N, Lemming O, Hopfner Petersen HE. Citalopram and fluoxetine in major depression: Comparison of two clinical trials in a psychiatrist setting and in general practice (second trial). Clinical Drug Investigation 1997;14(2):77‐89. [Google Scholar]
- Patris M, Bouchard JM, Bougerol T, Charbonnier JF, Chevalier JF, Clerc G, et al. Citalopram versus fluoxetine: a double‐blind, controlled, multicentre, phase III trial in patients with unipolar major depression treated in general practice.. International Clinical Psychopharmacology 1996;11(2):129‐36. [PubMed] [Google Scholar]
Burke 2002 {published and unpublished data}
- Burke WJ, Gergel I, Bose A. Fixed‐dose trial of the single isomer SSRI escitalopram in depressed outpatients. Journal of Clinical Psychiatry 2002;63(4):331‐6. [DOI] [PubMed] [Google Scholar]
Castanedo de Alba 1998 {published data only}
- Castanedo de Alba L, Meixueiro‐Montes De Oca R. An open‐label, controlled study of citalopram versus moclobemide in patients with major depression. Current Therapeutic Research 1998;59(2):107‐15. [Google Scholar]
Colonna 2005 {published and unpublished data}
- Colonna L, Andersen HF, Reines EH. A randomized, double‐blind, 24‐week study of escitalopram (10 mg/day) versus citalopram (20 mg/day) in primary care patients with major depressive disorder. Current Medical Research and Opinion 2005;21(10):1659‐68. [DOI] [PubMed] [Google Scholar]
de Wilde 1985 {published data only}
- Wilde J, Mertens C, Fredricson‐Overo K, Petersen HE. Citalopram versus mianserin. A controlled, double‐blind trial in depressed patients. Acta Psychiatrica Scandinavica 1985;72(1):89‐96. [DOI] [PubMed] [Google Scholar]
Ekselius 1997 {published data only}
- Ekselius L, Knorring L, Eberhard G. A double‐blind multicenter trial comparing sertraline and citalopram in patients with major depression treated in general‐practice. International Clinical Psychopharmacology 1997;12(6):323‐31. [DOI] [PubMed] [Google Scholar]
Gastpar 2006 {published data only}
- Gastpar M, Bassler D, Zeller K. Comparative, placebo‐controlled study of the hypericum extract STW 3‐VI with Citalopram in patients with moderate depression. Medizinische Klinik 2005;100:117. [Google Scholar]
- Gastpar M, Singer A, Zeller K. Comparative efficacy and safety of a once‐daily dosage of hypericum extract STW3‐VI and citalopram in patients with moderate depression: A double‐blind, randomised, multicentre, placebo‐controlled study. Pharmacopsychiatry 2006;39(2):66‐75. [DOI] [PubMed] [Google Scholar]
- Singer A, Schmidt M, Hauke W, Stade K. Duration of response after treatment of mild to moderate depression with Hypericum extract STW 3‐VI, citalopram and placebo: A reanalysis of data from a controlled clinical trial. Phytomedicine 2011;18(8‐9):739‐42. [DOI] [PubMed] [Google Scholar]
Gravem 1987 {published data only}
- Gravem A, Amthor KF, Astrup C, Elgen K, Gjessing LR, Gunby B er al. A double‐blind comparison of citalopram (Lu 10‐171) and amitriptyline in depressed patients. Acta Psychiatrica Scandinavica 1987;75(5):478‐86. [DOI] [PubMed] [Google Scholar]
Hosak 1999 {published data only}
- Hosak L, Tuma I. Comparative study of three antidepressants: Preliminary results. Homeostasis in Health and Disease 1996;37(3):138‐9. [Google Scholar]
- Hosak L, Tuma I, Hanus H. [A comparative study of three antidepressants with a different mechanism of action in hospitalized patients]. Ceska a Slovenska Psychiatrie 1999;95(3):146‐56. [Google Scholar]
- Hosak L, Tuma I, Hanus H, Straka L. Costs and outcomes of use of amitriptyline, citalopram and fluoxetine in major depression: exploratory study. Acta Medica (Hradec Kralove) 2000;43(4):133‐7. [PubMed] [Google Scholar]
Hsu 2011 {published data only}
- Hsu J‐W, Su T‐P, Huang C‐Y, Chen Y‐S, Chou Y‐H. Faster onset of antidepressant effects of citalopram compared with sertraline in drug‐naive first‐episode major depressive disorder in a Chinese population: A 6‐week double‐blind, randomized comparative study. Journal of Clinical Psychopharmacology 2011;31(5):577‐81. [DOI] [PubMed] [Google Scholar]
Karlsson 2000 {published data only}
- Karlsson I, Godderis J, Augusto De Mendonca Lima C, Nygaard H, Simanyi M, Taal M, et al. A randomised, double‐blind comparison of the efficacy and safety of citalopram compared to mianserin in elderly, depressed patients with or without mild to moderate dementia. International Journal of Geriatric Psychiatry 2000;15:295‐305. [DOI] [PubMed] [Google Scholar]
Khanzode 2003 {published data only}
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin‐re‐uptake inhibitors. Redox Report 2003;8(6):365‐70. [DOI] [PubMed] [Google Scholar]
Kyle 1998 {published data only}
- Kyle CJ, Petersen HE, Overo KF. Comparison of the tolerability and efficacy of citalopram and amitriptyline in elderly depressed patients treated in general practice. Depression and Anxiety 1998;8(4):147‐53. [PubMed] [Google Scholar]
Lalit 2004 {published data only}
- Lalit V, Appaya PM, Hegde RP, Mital AK, Mittal S, Nagpal R, et al. Escitalopram Versus Citalopram And Sertraline : A Double‐Blind Controlled, Multi‐Centric Trial In Indian Patients With Unipolar Major Depression. Indian Journal of Psychiatry 2004;46(4):333‐41. [PMC free article] [PubMed] [Google Scholar]
Langworth 2006 {published data only}
- Eyding D, Lelgemann M, Grouven U, Harter M, Kromp M, Kaiser T. Reboxetine for acute treatment of major depression: systematic review and meta‐analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 2010;341:4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langworth S, Bodlund O, Agren H. Efficacy and tolerability of reboxetine compared with citalopram: A double‐blind study in patients with major depressive disorder. Journal of Clinical Psychopharmacology 2006;26(2):121‐7. [DOI] [PubMed] [Google Scholar]
Leinonen 1999 {published data only}
- Agren H, Leinonen E, Skarstein J, Behke K, Helsdingen JT. Efficacy and tolerability of mirtazapine vs citalopram: a double‐blind, randomized study in patients with major depressive disorder. European Neuropsychopharmacology 1999;9(Suppl 5):228. [DOI] [PubMed] [Google Scholar]
- Agren H, Skarstein J, Behke K, Schutte A‐J, Leinonen E. Efficacy and tolerability of mirtazapine versus citalopram in major depression: a double‐blind, randomized study [Abstract No. NR476]. 152nd Annual Meeting of the American Psychiatric Association. 1999 May 15‐20, Washington DC. 1999.
- Leinonen E, Skarstein J, Behnke K, Agren H, Helsdingen JT. Efficacy and tolerability of mirtazapine versus citalopram: a double‐blind, randomized study in patients with major depressive disorder. International Clinical Psychopharmacology 1999;14(6):329‐37. [DOI] [PubMed] [Google Scholar]
- Leinonen E, Skarstein J, Behnke K, Agren H, Schutte AJ. Long‐term mirtazapine versus citalopram in major depression [Abstract No.476]. 153rd Annual Meeting of the American Psychiatric Association, 2000 May 13‐18, Chicago. 2000.
- Leinonen E, Skarstein J, Behnke K, Angren H, Schutte AJ. Mirtazapine has similar long‐term efficacy and tolerability to citalopram and faster onset of action in the treatment of major depression. European Neuropsychopharmacology 2000;10(Suppl 3):265. [Google Scholar]
- Schutte AJ, Leinonen E, Skarstein J, Behnke K, Angren H. Mirtazapine has similar long‐term efficacy and tolerability to citalopram and faster onset of action in the treatment of major depression. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):193. [Google Scholar]
Lepola 2003 {published and unpublished data}
- Lepola U M, Loft H, Reines E H. Escitalopram (10‐20 mg/day) is effective and well tolerated in a placebo‐controlled study in depression in primary care. International Clinical Psychopharmacology 2003;18(4):211‐7. [DOI] [PubMed] [Google Scholar]
- Lepola U, Wade A, Andersen HF. Do equivalent doses of escitalopram and citalopram have similar efficacy? A pooled analysis of two positive placebo‐controlled studies in major depressive disorder. International Clinical Psychopharmacology 2004;19(3):149‐55. [DOI] [PubMed] [Google Scholar]
- Lepola UM, Loft H, Reines EH. Escitalopram: efficacious and well tolerated in depression management in primary care [Abstract No.NR431]. 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans LA. 2001.
- Reines EH, Loft H, Lepola U. Escitalopram is efficacious and well tolerated in the treatment of depression in primary care. European Neuropsychopharmacology 2002;12(Suppl 3):S254. [Google Scholar]
Lewis 2011 {published and unpublished data}
- Lewis G, Mulligan J, Wiles N, Cowen P, Craddock N, Ikeda M, et al. Polymorphism of the 5‐HT transporter and response to antidepressants: randomised controlled trial.. British Journal of Psychiatry 2011;198:464‐71. [DOI] [PubMed] [Google Scholar]
- Thomas L, Mulligan J, Mason V, Tallon D, Wiles N, Cowen P, et al. GENetic and clinical predictors of treatment response in depression: the GenPod randomised trial protocol. Trials 2008;22(9):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 10‐171,79‐01 {unpublished data only}
- Lundbeck. A controlled clinical study on citalopram and nortriptyline in patients with depressive illness. H. Lundbeck A/S 1984;Report No. 1/831 1984.
Lu 10‐171, 83‐01 {unpublished data only}
- www.lundbeck.com. A controlled clinical comparison on citalopram and imipramine in depressed patients. H. Lundbeck A/S 1987;Report No. 42/831 1987.
Matreja 2007 {published data only (unpublished sought but not used)}
- Matreja PS, Badyal DK, Khosla P, Deswal RS. Effectiveness and acceptability of sertraline and citalopram in major depressive disorder: pragmatic randomized open‐label comparison. Human Psychopharmacology 2007;22(7):477‐82. [DOI] [PubMed] [Google Scholar]
Moeller 2003 {published data only}
- Moeller O, Hetzel G, Rothermundt M, Michael N, Nyhuis PW, Suslow T, et al. Oral citalopram and reboxetine challenge tests before and after selective antidepressant treatment. Journal of Psychiatric Research 2003;37(3):261‐2. [DOI] [PubMed] [Google Scholar]
Moore 2005 {published data only}
- Fantino B, Moore N. The self‐reported Montgomery‐Asberg depression rating scale is a useful evaluative tool in major depressive disorder. BMC Psychiatry 2009;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N, Verdoux H, Fantino B. Prospective, multicentre, randomized, double‐blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. International Clinical Psychopharmacology 2005;20(20):131‐7. [DOI] [PubMed] [Google Scholar]
Navarro 2001 {published data only}
- Navarro V, Gasto C, Torres X, Marcos T, Pintor L. Citalopram versus nortriptyline in late‐life depression: a 12‐week randomized single‐blind study. Acta Psychiatrica Scandinavica 2001;103(6):435‐40. [DOI] [PubMed] [Google Scholar]
Ou 2010 {published data only}
- Ou JJ, Xun GL, Wu RR, Li LH, Fang MS, Zhang HG, et al. Efficacy and safety of escitalopram versus citalopram in major depressive disorder: a 6‐week, multicenter, randomized, double‐blind, flexible‐dose study. Psychopharmacology (Berl) 2011;213:639‐46. [DOI] [PubMed] [Google Scholar]
Rosenberg 1994 {published data only}
- Fuglum E, Rosenberg C, Damsbo N, Stage K, Lauritzen L, Bech P. Screening and treating depressed patients A comparison of two controlled citalopram trials across treatment settings: hospitalized patients vs patients treated by their family doctors. Acta Psychiatrica Scandinavica 1996;94(1):18‐25. [DOI] [PubMed] [Google Scholar]
- Rosenberg C, Damsbo N, Fuglum E, Jacobsen LV, Horsgard S. Citalopram and imipramine in the treatment of depressive patients in general‐practice. A Nordic multicentre clinical study. International Clinical Psychopharmacology 1994;9(Suppl 1):41‐8. [DOI] [PubMed] [Google Scholar]
SCT‐MD‐02 {unpublished data only}
- Forest Laboratories. Flexible‐dose comparison of the safety and efficacy of Lu 26‐054 (escitalopram), citalopram, and placebo in the treatment of major depressive disorder. www.forestclinicaltrials.com.
Shaw 1986 {published and unpublished data}
- Shaw DM, Thomas DR, Briscoe MH, Watkins SE, Crimmins R, Harris B, et al. A comparison of the antidepressant action of citalopram and amitriptyline.. British Journal of Psychiatry 1986;149:515‐7. [DOI] [PubMed] [Google Scholar]
Stahl 2000 {published data only}
- Cooper‐Kazaz R, Rigbi A, Lerer B. Targeting remission by 8 weeks: When should supplementation be considered in patients with major depression treated with a specific serotonin reuptake inhibitor?. Comprehensive Psychiatry 2011;52(1):9‐16. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Double‐blind comparison of citalopram, sertraline and placebo. 152nd Annual Meeting of the American Psychiatric Association. 1999 May 15‐20, Washington DC. 1999.
- Stahl SM. Placebo‐controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biological Psychiatry 2000;48:894‐901. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Wilcox C, Overo K. Citalopram vs. sertraline vs. placebo in the treatment of major depression. European Neuropsychopharmacology 1999;9(Suppl 5):232. [Google Scholar]
Timmerman 1993 {published data only}
- Haffmans PM, Timmerman L, Hoogduin CA. Efficacy and tolerability of citalopram in comparison with fluvoxamine in depressed outpatients: a double‐blind, multicentre study The lucifer group. International Clinical Psychopharmacology 1996;11(3):157‐64. [DOI] [PubMed] [Google Scholar]
- Timmerman L, Haffmans PMJ, Hoogduin CA. Citalopram in major depression: a comparative study with Fluvoxamine, Preliminary results. Past, Present and Future of Psychiatry 1993;2:982‐6. [Google Scholar]
Yevtushenko 2007 {published data only}
- Yevtushenko VY, Belous AI, Yevtushenko YG, Gusinin SE, Buzik OJ, Agibalova TV. Efficacy and tolerability of escitalopram versus citalopram in major depressive disorder: a 6‐week, multicenter, prospective, randomized, double‐blind, active‐controlled study in adult outpatients. Clinical Therapeutics 2007;29(11):2319‐32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adli 2008 {published data only}
- Adli M, Wiethoff K, Baethge C, Pfennig A, Stamm T, Bauer M. Olanzapine in the treatment of depression with psychotic features: A prospective open‐label study. Journal of Psychiatry in Clinical Practice 2008;12:202‐9. [DOI] [PubMed] [Google Scholar]
Altamura 2008 {published data only}
- Altamura AC, Dell'Osso B, Buoli M, Bosi M, Mundo E. Short‐term intravenous citalopram augmentation in partial/nonresponders with major depression: a randomized placebo‐controlled study. International Clinical Psychopharmacology 2008;23:198‐202. [DOI] [PubMed] [Google Scholar]
Altamura 2008b {published data only}
- Altamura AC, Dell'Osso B, Buoli M, Zanoni S, Mundo E. Intravenous augmentative citalopram versus clomipramine in partial/nonresponder depressed patients: a short‐term, low dose, randomized, placebo‐controlled study. Journal of Clinical Psychopharmacology 2008;28:406‐10. [DOI] [PubMed] [Google Scholar]
Amiaz 2008 {published data only}
- Amiaz R, Fostick L, Gershon A, Zohar J. Naltrexone augmentation in OCD: A double‐blind placebo‐controlled cross‐over study. European Neuropsychopharmacology 2008;18(6):455‐61. [DOI] [PubMed] [Google Scholar]
Amsterdam 2006 {published data only}
- Amsterdam JD, Shults J, Rutherford N, Schwartz S. Safety and efficacy of s‐citalopram in patients with co‐morbid major depression and diabetes mellitus. Neuropsychobiology 2006;54:208‐14. [DOI] [PubMed] [Google Scholar]
Amsterdam 2008 {published data only}
- Amsterdam JD, Shults J, Rutherford N. Open‐label study of s‐citalopram therapy of chronic fatigue syndrome and co‐morbid major depressive disorder. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2008;32:100‐6. [DOI] [PubMed] [Google Scholar]
Anderer 2002 {published data only}
- Anderer P, Saletu B, Semlitsch HV, Pascual‐Marqui RD. Structural and energetic processes related to P300: LORETA findings in depression and effects of antidepressant drugs. Methods and Findings in Experimental and Clinical Pharmacology 2002;24(suppl D):85‐91. [PubMed] [Google Scholar]
Andersen 1993 {published data only}
- Andersen G, Vestergaard K, Lauritzen L. Post‐stroke depression treated with Citalopram ‐ A selective serotonin reuptake inhibitor. Canadian Journal of Neurological Sciences 1993;20:S115. [Google Scholar]
Angermann 2007 {published data only}
- Angermann CE, Gelbrich G, Stork S, Fallgatter A, Deckert J, Faller H, et al. Rationale and design of a randomised, controlled, multicenter trial investigating the effects of selective serotonin re‐uptake inhibition on morbidity, mortality and mood in depressed heart failure patients (MOOD‐HF). European Journal of Heart Failure 2007;9(12):1212‐22. [DOI] [PubMed] [Google Scholar]
Anon 1995 {unpublished data only}
- Unknown. Citalopram a double‐blind parallel group comparison with placebo in depressed outpatients. Unpublished study supplied by Lundbeck 1995.
Anonymous 2011 {published data only}
- Anonymous. Therapy of moderately severe depressions in daily practice: first patient care research study reinforces clinical data. MMW Fortschritte der Medizin 2011;153(41):38‐9. [PubMed] [Google Scholar]
Azorin 2004 {published data only}
- Azorin JM, Llorca PM, Despiegel N, Verpillat P. Escitalopram is more effective than citalopram for the treatment of severe major depressive disorder. Encephale 2004;30(2):158‐66. [DOI] [PubMed] [Google Scholar]
Baldwin 2006 {published data only}
- Baldwin D, Bridgman K, Buis C. Resolution of sexual dysfunction during double‐blind treatment of major depression with reboxetine or paroxetine. Journal of Psychopharmacology 2006;20(1):91‐6. [DOI] [PubMed] [Google Scholar]
Barone 2011 {published data only}
- Barone P. Treatment of depressive symptoms in Parkinson's disease. European Journal of Neurology 2011;18:11‐5. [DOI] [PubMed] [Google Scholar]
Bauer 2010 {published data only}
- Bauer M, El‐Khalili N, Datto C, Szamosi J, Eriksson H. A pooled analysis of two randomised, placebo‐controlled studies of extended release quetiapine fumarate adjunctive to antidepressant therapy in patients with major depressive disorder. Journal of Affective Disorders 2010;127:19‐30. [DOI] [PubMed] [Google Scholar]
Baumann 1998a {unpublished data only}
- Baumann P, Guelfi JD, Nil R, Strub N, Loft H. The benefits of intravenous versus oral citalopram in severely depressed patients: results of two double‐blind studies. 11th European College of Neuropsychopharmacology Congress, Paris. 1998.
Baumann 1998b {published and unpublished data}
- Baumann P, Nil R, Bertschy G, Jecker A, Brandli H, Morand J, et al. A double‐blind double‐dummy study of citalopram comparing infusion versus oral administration. Journal of Affective Disorders 1998;49:203‐10. [DOI] [PubMed] [Google Scholar]
Benkelfat 1987 {published data only}
- Benkelfat C, Poirier MF, Leouffre P, Gay C, Loo H. Dexamethasone suppression test and the response to antidepressant depending on their central monoaminergic action in major depression. Canadian Journal of Psychiatry 1987;32:175‐8. [DOI] [PubMed] [Google Scholar]
Berney 2008 {published data only}
- Berney A, Nishikawa M, Benkelfat C, Debonnel G, Gobbi G, Diksic M. An index of 5‐HT synthesis changes during early antidepressant treatment: alpha‐[(11)C]methyl‐l‐tryptophan PET study. Neurochemistry International 2008;52(4‐5):701‐8. [DOI] [PubMed] [Google Scholar]
Bersani 1997 {published data only}
- Bersani G, Saito A, Pallanti S, Sasso E, Tosca P. Clinical variables and response to citalopram in Major Depression: An open multicentric study. Rivista Di Psichiatria 1997;32(6):260‐7. [Google Scholar]
Beving 1985 {published data only}
- Beving H, Bjerkenstedt L, Malmgren R, Olsson P, Unge G. The effects of citalopram (Lu 10‐171) on the serotonin (5‐HT) uptake kinetics in platelets from endogenously depressed patients. Journal of Neural Transmission 1985;61(1‐2):95‐104. [DOI] [PubMed] [Google Scholar]
Bhagwagar 2004 {published data only}
- Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. American Journal of Psychiatry 2004;161(1):166‐8. [DOI] [PubMed] [Google Scholar]
Bigos 2008 {published data only}
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5‐HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology 2008;33:3221‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bijl 2004 {published data only}
- Bijl D. Escitalopram (Cipralex and Lexapro) for the treatment of depression. Geneesmiddelenbulletin 2004;38(12):89‐90. [Google Scholar]
Bjerkenstedt 1985 {published data only}
- Bjerkenstedt L, Edman G, Flyckt L, Hagenfeldt L, Sedvall G, Wiesel FA. Clinical and biochemical effects of citalopram, a selective 5‐HT reuptake inhibitor‐ a dose‐response study in depressed patients. Psychopharmacology 1985;87(3):253‐9. [DOI] [PubMed] [Google Scholar]
Blier 2006 {published data only}
- Blier P. The sequenced treatment alternatives to relieve depression (STAR*D): A first look at the initial pharmacotherapy results. Clinical Neuropsychiatry 2006;3(4):265‐7. [Google Scholar]
Bouchard 1997 {published data only}
- Bouchard JM, Strub N, Nil R. Citalopram and viloxazine in the treatment of depression by means of slow drop infusion. A double‐blind comparative trial. Journal of Affective Disorders 1997;46:51‐8. [DOI] [PubMed] [Google Scholar]
Boulenger 2010 {published data only}
- Boulenger JP, Hermes A, Huusom AK, Weiller E. Baseline anxiety effect on outcome of SSRI treatment in patients with severe depression: escitalopram vs paroxetine. Current Medical Research and Opinion 2010;26:605‐14. [DOI] [PubMed] [Google Scholar]
Bowden 1998 {published data only}
- Bowden CL. Citalopram versus imipramine in the treatment of inpatient depression ‐ results from a double‐blind, placebo‐controlled trial. 151st Annual Meeting of the American Psychiatric Association. Toronto, Ontario, Canada. 30th May 4th June 1998.
Brown 2004 {published data only}
- Brown ES, Khan DA, Vigil L, Rush AA. A randomized, double‐blind, placebo‐controlled trial of citalopram in outpatient adults with asthma and major depressive disorder. Neuropsychopharmacology 2004;29:S92. [Google Scholar]
Bryan 2007 {published data only}
- Bryan C. The association of diabetes mellitus with response to depression treatment. Dissertation Abstracts International 2007;68(3‐B):1588. [Google Scholar]
Bun'kova KM {published data only}
- Bun'kova KM. Efficacy and tolerability of clomipramine, pirlindole and escitalopram in the treatment of neurotic level depression. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2008;108:29‐32. [PubMed] [Google Scholar]
Carman 2002 {unpublished data only}
- Carman JS. Double‐blind parallel comparison of citalopram, imipramine and placebo in patients with major depression or bipolar disorder, depressed. Unpublished 2002.
Chakravarti 2002 {unpublished data only}
- Chakravarti SK. Double‐blind comparison of citalopram versus amitriptyline in the treatment of depressive illness in Great Britain. Unpublished/ LUNDBECK 2002.
Chan 2009 {published data only}
- Chan F, Lanctacutet KL, Herrmann N, Kiss A, McCullagh S, Feinstein A, et al. A randomized controlled trial of citalopram in major depression following mild traumatic brain injury. World Psychiatry 2009;8:184. [Google Scholar]
Chen 2005 {published data only}
- Chen KN, Chen SL, Luo F, Tan YY. Changes of neurotransmitter in patients with post‐stroke depression observed with encephalofluctuography technology. Chinese Journal of Clinical Rehabilitation 2005;9(16):118‐9. [Google Scholar]
Conte 1997 {unpublished data only}
- Conte G, Cauli G, Sanna C, Scarone S. Citalopram use and treatment outcome in an unselected Italian sample of major depressives. 10th European College of Neuropsychopharmacology Congress. Vienna, Austria. 13th to 17th September 1997. 1997.
Cooper‐Kazaz 2011 {published data only}
- Cooper‐Kazaz R, Rigbi A, Lerer B. Targeting remission by 8 weeks: When should supplementation be considered in patients with major depression treated with a specific serotonin reuptake inhibitor?. Comprehensive Psychiatry 2011;52:9‐16. [DOI] [PubMed] [Google Scholar]
Court 2010 {published data only}
- Court A, Mulder C, Kerr M, Yuen HP, Boasman M, Goldstone S. Investigating the effectiveness, safety and tolerability of quetiapine in the treatment of anorexia nervosa in young people: A pilot study. Journal of Psychiatric Research 2010;44:1027‐34. [DOI] [PubMed] [Google Scholar]
Culang 2009 {published data only}
- Culang ME, Sneed JR, Keilp JG, Rutherford BR, Pelton GH, Devanand DP. Change in cognitive functioning following acute antidepressant treatment in late‐life depression. American Journal of Geriatric Psychiatry 2009;17:881‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daly 2011 {published data only}
- Daly EJ, Trivedi MH, Fava M, Shelton R, Wisniewski SR, Morris DW, et al. The relationship between adverse events during selective serotonin reuptake inhibitor treatment for major depressive disorder and non remission in the suicide assessment methodology study. Journal of Clinical Psychopharmacology 2011;31(1):31‐8. [DOI] [PubMed] [Google Scholar]
Davis 2006 {published data only}
- Davis LL, Frazier E, Husain MM, Warden D, Trivedi M, Fava M, Cassano P, McGrath PJ, Balasubramani GK, Wisniewski SR, Rush AJ. Substance use disorder comorbidity in major depressive disorder: a confirmatory analysis of the STAR*D cohort. American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2006;15(4):278‐285. [DOI] [PubMed] [Google Scholar]
Davis 2010 {published data only}
- Davis A, Gilhooley M, Agius M. Using non‐steroidal anti‐inflammatory drugs in the treatment of depression. Psychiatria Danubina 2010;22:S49‐S52. [PubMed] [Google Scholar]
Davis 2010b {published data only}
- Davis LL, Wisniewski SR, Howland RH, Trivedi MH, Husain MM, Fava M. Does comorbid substance use disorder impair recovery from major depression with SSRI treatment? An analysis of the STAR*D level one treatment outcomes. Drug Alcohol Dependence 2010;107:161‐70. [DOI] [PubMed] [Google Scholar]
Deakin 2002 {unpublished data only}
- Deakin JFW. A double‐blind, controlled phase III of citalopram versus placebo in the treatment of depressive illness. Unpublished 2002.
DeBattista 2011 {published data only}
- DeBattista C, Kinrys G, Hoffman D, Goldstein C, Zajecka J, Kocsis J. The use of referenced‐EEG (rEEG) in assisting medication selection for the treatment of depression. Journal of Psychiatric Research 2011;45:64‐75. [DOI] [PubMed] [Google Scholar]
Dell'Agnello 2001 {published data only}
- Dell'Agnello G, Ceravolo R, Nuti A, Bellini G, Piccinni A, D'Avino C. SSRIs do not worsen Parkinson's disease: evidence from an open‐label, prospective study. Clinical Neuropharmacology 2001;24:221‐7. [DOI] [PubMed] [Google Scholar]
Dell'Osso 2008 {published data only}
- Dell'Osso B, Hadley S, Allen A, Baker B, Chaplin WF, Hollander E. Escitalopram in the treatment of impulsive‐compulsive internet usage disorder: an open‐label trial followed by a double‐blind discontinuation phase. The Journal of Clinical Psychiatry 2008;69:452‐6. [DOI] [PubMed] [Google Scholar]
Deng 2006 {published data only}
- Deng W, Xu C, Ma T. A control study of citalopram combined with quetiapine in the treatment of female depression. Journal of Clinical Psychosomatic Diseases 2006;12(4):274‐9. [Google Scholar]
Denko 2007 {published data only}
- Denko TC, Friedman ES. Augmentation strategies in STAR*D: A review. Primary Psychiatry 2007;14(1):46‐50. [Google Scholar]
Devos 2008 {published data only}
- Devos D, Dujardin K, Poirot I, Moreau C, Cottencin O, Thomas P. Comparison of desipramine and citalopram treatments for depression in Parkinson's disease: a double‐blind, randomized, placebo‐controlled study. Movement Disorders 2008;23:850‐7. [DOI] [PubMed] [Google Scholar]
Diniz 2010 {published data only}
- Diniz JB, Pereira CAB, Miguel EC, Shavitt RG. Clomipramine and quetiapine augmentation for obsessive compulsive disorder compared to sustained fluoxetine treatment. European Neuropsychopharmacology 2010;20:S547‐S548. [Google Scholar]
Di Simplicio 2010 {published data only}
- Simplicio M, Norbury R, Harmer CJ. Antidepressant treatment modulates neural responses to self‐referential words in subjects with high neuroticism. European Neuropsychopharmacology 2010;20:S172. [Google Scholar]
Doggrell 2006 {published data only}
- Doggrell SA. After the failure of citalopram for depression, what next?. Expert Opinion on Pharmacotherapy 2006;7(11):1515‐8. [DOI] [PubMed] [Google Scholar]
Domelas 2007 {published data only}
- Dornelas EA, Burg MM. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease. JAMA 2007;297(17):1879‐80. [DOI] [PubMed] [Google Scholar]
Doree 2007 {published data only}
- Doree J‐P, Rosiers J, Lew V, Gendron A, Elie R, Stip E, et al. Quetiapine augmentation of treatment‐resistant depression: A comparison with lithium. Current Medical Research and Opinion 2007;23(2):333‐41. [DOI] [PubMed] [Google Scholar]
Dougherty 2009 {published data only}
- Dougherty DD, Jameson M, Deckersbach T, Loh R, Thompson‐Hollands J, Jenike M. Open‐label study of high (30 mg) and moderate (20 mg) dose escitalopram for the treatment of obsessive‐compulsive disorder. International Clinical Psychopharmacology 2009;24:306‐11. [DOI] [PubMed] [Google Scholar]
Dozois 2009 {published data only}
- Dozois DJA, Bieling PJ, Patelis‐Siotis I, Hoar L, Chudzik S, McCabe K. Changes in self‐schema structure in cognitive therapy for major depressive disorder: a randomized clinical trial. Journal of Consulting and Clinical Psychology 2009;77:1078‐88. [DOI] [PubMed] [Google Scholar]
Dunbar 2010 {published data only}
- Dunbar G, Hosford D. The potential of the nicotinic channel blocker tc‐5214 as augmentation treatment in patients with major depression. European Neuropsychopharmacology 2010;20:S334. [Google Scholar]
Eriksson 1996 {published data only}
- Eriksson E, Andersch B, Bergman L, Bing O, Kakaoulidis P, Sundblad C. On the Possible Role of Testosterone in Serotonin‐Related Psychiatric Disorders in Women. XXth Collegium Internationale Neuro‐psychopharmacologicum. Melbourne, Australia. 23rd‐27th June, 1996.
Eyding 2010 {published data only}
- Eyding D, Lelgemann M, Grouven U, Härter M, Kromp M, Kaiser T, et al. Reboxetine for acute treatment of major depression: systematic review and meta‐analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 2010;341:c4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fava 2006 {published data only}
- Fava M, Rush AJ, Wisniewski SR, Nierenberg AA, Alpert JE, McGrath PJ, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. American Journal of Psychiatry 2006;163(7):1161‐72. [DOI] [PubMed] [Google Scholar]
Feighner 1997 {unpublished data only}
- Feighner JP, Fieve RR, Carman JS, Cunningham LA, Schwartz G. A fixed‐dose comparison of citalopram versus placebo. 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego, CA. 1997.
Feighner 1997b {published data only}
- Feighner JP, Fieve RR, Carman JS, Cunningham LA, Schwartz G. A Fixed‐Dose Comparison of Citalopram Versus Placebo. 150th Annual Meeting of the American Psychiatric Association. San Diego, California, USA 17‐22 May, 1997.
Feighner 1999 {published data only}
- Feighner JP, Overo K. Multicenter, placebo‐controlled, fixed‐dose study of citalopram in moderate‐to‐severe depression. Journal of Clinical Psychiatry 1999;60(12):824‐30. [DOI] [PubMed] [Google Scholar]
Fernandez 2005 {published data only}
- Fernandez JL, Montgomery S, Francois C. Evaluation of the cost effectiveness of escitalopram versus venlafaxine XR in major depressive disorder. Pharmacoeconomics 2005;23(2):155‐67. [DOI] [PubMed] [Google Scholar]
Fernandez 2009 {published data only}
- Fernandez M. Comparison of desipramine and citalopram treatments for depression in Parkinson's disease: A double‐blind, randomized, placebo‐controlled study. Revista de Neurologia 2009;48:S23. [DOI] [PubMed] [Google Scholar]
Flicker 1998 {unpublished data only}
- Flicker C, Gottfries CG. Citalopram treatment of depression in elderly patients with or without dementia: results of a placebo‐controlled study. 11th Annual Meeting of the American Association for Geriatric Psychiatry. San Diego, California, USA. 8th 11th March. 1998.
Ford 2010 {published data only}
- Ford AH, Flicker L, McCaul K, Bockxmeer F, Hegarty S, Hirani V. The B‐VITAGE trial: A randomized trial of homocysteine lowering treatment of depression in later life. Trials 2010;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraguas 2009 {published data only}
- Fraguas R, Silva Telles RM, Alves TCTF, Andrei AM, Rays J, Iosifescu DV. A double‐blind, placebo‐controlled treatment trial of citalopram for major depressive disorder in older patients with heart failure: The relevance of the placebo effect and psychological symptoms. Contemporary Clinical Trials 2009;30:205‐11. [DOI] [PubMed] [Google Scholar]
Frank 2004 {published data only}
- Frank MG, Hendricks SE, Burke WJ, Johnson DR. Clinical response augments NK cell activity independent of treatment modality: A randomized double‐blind placebo controlled antidepressant trial.. Psychological Medicine 2004;34(3):491‐8. [DOI] [PubMed] [Google Scholar]
Garriock 2010 {published data only}
- Garriock HA, Tanowitz M, Kraft JB, Dang VC, Peters EJ, Jenkins GD. Association of mu‐opioid receptor variants and response to citalopram treatment in major depressive disorder. American Journal of Psychiatry 2010;167:565‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2008 {published data only}
- Gilbert G. Adults with both anxiety and depression respond poorly to treatment. Journal of the National Medical Association 2008;100:870‐1. [Google Scholar]
Gilmer 2008 {published data only}
- Gilmer WS, Gollan JK, Wisniewski SR, Howland RH, Trivedi MH, Miyahara S. Does the duration of index episode affect the treatment outcome of major depressive disorder? A STAR*D report. Journal of Clinical Psychiatry 2008;69:1246‐56. [DOI] [PubMed] [Google Scholar]
Glod 2004 {unpublished data only}
- Glod CA, Lynch A, Berkowitz C, Hennen J, Baldessarini RJ. Bupropion versus citalopram versus placebo in adolescents with major depression. 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York, NY , NR484. 2004. 2004.
Goder 2011 {published data only}
- Goder R, Seeck‐Hirschner M, Stingele K, Huchzermeier C, Kropp C, Palaschewski M, et al. Sleep and cognition at baseline and the effects of REM sleep diminution after 1week of antidepressive treatment in patients with depression. Journal of Sleep Research 2011;20(4):544‐51. [DOI] [PubMed] [Google Scholar]
Gommol 2010 {published data only}
- Gommoll C, Greenberg WM, Forero G, Wang Q. Comparison of safety, efficacy, and tolerability of modified and immediate release escitalopram and placebo in adults with major depressive disorder. 163rd Annual Meeting of the American Psychiatric Association; New Orleans. May 22‐26 2010.
Gonsai 2000 {published data only}
- Gonsai KR, Meandzija B, George TP, Chawaraski M, Falcioni J, Schottenfeld RS. Comparison of nefazodone vs. citalopram for depression in methadone maintained subjects. NIDA Research Monograph 2000:264‐9.
Gorman 2002a {published data only}
- Gorman J, Korotzer A, Jin J. Escitalopram in the treatment of severe depression. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):147. [Google Scholar]
Gorwood 2007 {published data only}
- Gorwood P, Weiller E, Lemming O, Katona C. Escitalopram prevents relapse in older patients with major depressive disorder. American Journal of Geriatric Psychiatry 2007;15(7):581‐93. [DOI] [PubMed] [Google Scholar]
Guelfi 1998 {published data only}
- Guelfi JD, Strub N, Loft H. Efficacy of intravenous citalopram compared with oral citalopram for severe depression. Safety and efficacy data from a double‐blind, double‐dummy trial. Journal of Affective Disorders 2000;58(3):201‐9. [DOI] [PubMed] [Google Scholar]
Hannestad 2011 {published data only}
- Hannestad J, DellaGioia N, Ortiz N, Pittman B, Bhagwagar Z. Citalopram reduces endotoxin‐induced fatigue. Brain, Behavior, and Immunity 2011;25:256‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrington 2002 {unpublished data only}
- Harrington RN. A controlled clinical comparison of citalopram and placebo in depressed patients. Unpublished 2002.
Hegerl 2005 {published data only}
- Hegerl U, Mergl R, Henkel V, Pogarell O, Muller‐Siecheneder F, Frodl T, et al. Differential effects of reboxetine and citalopram on hand‐motor function in patients suffering from major depression. Psychopharmacology 2006;178(1):58‐66. [DOI] [PubMed] [Google Scholar]
Hellerstein 2010 {published data only}
- Hellerstein DJ, Batchelder ST, Hyler S, Arnaout B, Toba C, Benga I. Escitalopram versus placebo in the treatment of dysthymic disorder. International Clinical Psychopharmacology 2010;25:143‐8. [DOI] [PubMed] [Google Scholar]
Hemels 2004 {published data only}
- Hemels MEH, Kasper S, Walter E, Einarson TR. Cost‐effectiveness of escitalopram versus citalopram in the treatment of severe depression. Annals of Pharmacotherapy 2004;38(6):954‐60. [DOI] [PubMed] [Google Scholar]
Herrera‐Guzman 2009 {published data only}
- Herrera‐Guzman I, Gudayol‐Ferre E, Herrera‐Guzman D, Guardia‐Olmos J, Hinojosa‐Calvo E, Herrera‐Abarca JE. Effects of selective serotonin reuptake and dual serotonergic‐noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. Journal of Psychiatric Research 2009;43:855‐63. [DOI] [PubMed] [Google Scholar]
Hflich 2011 {unpublished data only}
- Hflich AS, Philippe C, Savli M, Baldinger P, Kranz GS, Mller S, et al. Prediction of steady‐state occupancy of the serotonin transporter based on single‐dose occupancy: A [11C]DASB pet study. European Psychiatry,19th European Congress of Psychiatry. 2011.
Hindmarch 2000 {published data only}
- Hindmarch I, Kimber S, Cockle SM. Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. International Clinical Psychopharmacology 2000;15(6):305‐18. [DOI] [PubMed] [Google Scholar]
Hochstrasser 2001 {published data only}
- Hochstrasser B, Isaksen PM, Koponen H, Lauritzen L, Mahnert FA, Rouillon F, et al. Prophylactic effect of citalopram in unipolar, recurrent depression: placebo‐controlled study of maintenance therapy. British Journal of Psychiatry 2001;178:304‐10. [DOI] [PubMed] [Google Scholar]
Holtzheimer 2008 {published data only}
- Holtzheimer PE III, Meeks TW, Kelley ME, Mufti M, Young R, McWhorter K. A double blind, placebo‐controlled pilot study of galantamine augmentation of antidepressant treatment in older adults with major depression. International Journal of Geriatric Psychiatry 2008;23:625‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howland 2011 {unpublished data only}
- Howland RH, Wisniewski S, Balasubramani GK, Fava M, Trivedi M, Rush AJ. Thyroid disease, major depression, and treatment outcome in the COMED trial. Biological Psychiatry, 66th Annual Meeting of the Society of Biological Psychiatry San Francisco, CA. 2011.
Huezo‐Diaz 2009 {published data only}
- Huezo‐Diaz P, Uher R, Smith R, Rietschel M, Henigsberg N, Marusic A. Moderation of antidepressant response by the serotonin transporter gene. British Journal of Psychiatry 2009;195:30‐8. [DOI] [PubMed] [Google Scholar]
Johnson 2002 {unpublished data only}
- Johnson DA. A controlled, clinical comparison of citalopram and imipramine in depressed patients. Unpublished 2002.
Judge 2000 {published data only}
- Judge R, Parry M, Quail D, Campbell A, Koke S. Clinical consequences of non‐compliance with antidepressant therapy: A comparison of fluoxetine and citalopram. International Journal of Neuropsychopharmacology 2000;3(1):211. [Google Scholar]
Kapitany 1999 {published data only}
- Kapitany T, Schindl M, Schindler SD, Hesselmann B, Fureder T, Barnas C, et al. The citalopram challenge test in patients with major depression and in healthy controls. Psychiatry Research 1999;88:75‐88. [DOI] [PubMed] [Google Scholar]
Kasckow 2010 {published data only}
- Kasckow J, Fellows I, Golshan S, Solorzano E, Meeks T, Zisook S. Treatment of subsyndromal depressive symptoms in middle‐age and older patients with schizophrenia: Effect of age on response. American Journal of Geriatric Psychiatry 2010;18:853‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasper 2009 {published data only}
- Kasper S, Baldwin DS, Larsson Lonn S, Boulenger JP. Superiority of escitalopram to paroxetine in the treatment of depression. European Neuropsychopharmacology 2009;19:229‐37. [DOI] [PubMed] [Google Scholar]
Ketter 2006 {published data only}
- Ketter TA, Greist JH, Graham JA, Roberts JN, Thompson TR, Nanry KP. The effect of dermatologic precautions on the incidence of rash with addition of lamotrigine in the treatment of bipolar I disorder: A randomized trial. Journal of Clinical Psychiatry 2006;67:400‐6. [DOI] [PubMed] [Google Scholar]
Khazaie 2006 {unpublished data only}
- Khazaie H, Moradi M, Chehri A. Antidepressant induced sexual dysfunction during treatment with trazodon, florentine, citalopram and moclobemide. World Psychiatric Association, International Congress 2006; July 12 ‐ 16 2006; Istanbul, Turkey, 327. 2006.
Khazaie 2011 {published data only}
- Khazaie H, Rahimi M, Tatari F, Rezaei M, Najafi F, Tahmasian M. Treatment of depression in type 2 diabetes with Fluoxetine or Citalopram?. Neurosciences 2011;16(1):42‐5. [PubMed] [Google Scholar]
Kiosses 2010 {published data only}
- Kiosses DN, Arean PA, Teri L, Alexopoulos GS. Home‐delivered problem adaptation therapy (PATH) for depressed, cognitively impaired, disabled elders: A preliminary study. American Journal of Geriatric Psychiatry 2010;18:988‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klysner 2000 {published data only}
- Klysner R, Pleidrup E, Hansen HL, Bent‐Hansen J, Loldrup PD, Lunde M, et al. The effectiveness of citalopram in the prevention of depression recurrence in elderly patients. International Journal of Neuropsychopharmacology 2000;3(1):211. [Google Scholar]
Kornstein 2006 {published data only}
- Kornstein SG, Bose A, Li D, Saikali KG, Gandhi C. Escitalopram maintenance treatment for prevention of recurrent depression: a randomized, placebo‐controlled trial. Journal of Clinical Psychiatry 2006;67(11):1767‐75. [DOI] [PubMed] [Google Scholar]
Kovacs 1998 {unpublished data only}
- Kovacs G, Kelemen. Citalopram infusion therapy of uni‐ and bipolar depression. 9th Congress of the Association of European Psychiatrists. Copenhagen, Denmark. 20 24th September 1998. 1998.
Kraus 2008 {published data only}
- Kraus MR, Schäfer A, Schöttker K, Keicher C, Weissbrich B, Hofbauer I. Therapy of interferon‐induced depression in chronic hepatitis C with citalopram: a randomised, double‐blind, placebo‐controlled study. Gut 2008;57:531‐6. [DOI] [PubMed] [Google Scholar]
Kroenke 2009 {published data only}
- Kroenke K, Theobald D, Norton K, Sanders R, Schlundt S, McCalley S. The Indiana Cancer Pain and Depression (INCPAD) trial. Design of a telecare management intervention for cancer‐related symptoms and baseline characteristics of study participants. General Hospital Psychiatry 2009;31:240‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuhn 2003 {published data only}
- Kühn KU, Quednow BB, Thiel M, Falkai P, Maier W, Elger CE. Antidepressive treatment in patients with temporal lobe epilepsy and major depression: a prospective study with three different antidepressants. Epilepsy & Behavior 2003;4(6):674‐9. [DOI] [PubMed] [Google Scholar]
Kupfer 2000 {unpublished data only}
- Kupfer DJ, Chengappa KN, Gelenberg AJ, Hirschfeld RM, Kocsis JH, Sachs GS. Citalopram treatment of bipolar depression. 39th Annual Meeting of the American Collegeof Neuropsychopharmacology. 2000; Dec 10‐14; San Juan; Puerto Rico. 2000.
Lakey 2008 {published data only}
- Lakey SL, Gray SL, Ciechanowsk P, Schwartz S, LoGerfo J. Antidepressant use in nonmajor depression: Secondary analysis of a program to encourage active, rewarding lives for seniors (PEARLS), a randomized controlled trial in older adults from 2000 to 2003. American Journal Geriatric Pharmacotherapy 2008;6(1):12‐20. [DOI] [PubMed] [Google Scholar]
Lam 2008 {published data only}
- Lam RW, Andersen HF, Wade AG. Escitalopram and duloxetine in the treatment of major depressive disorder: a pooled analysis of two trials. International Clinical Psychopharmacology 2008;23:181‐7. [DOI] [PubMed] [Google Scholar]
Lavretsky 2010 {published data only}
- Lavretsky H, Siddarth P, Irwin MR. Improving depression and enhancing resilience in family dementia caregivers: a pilot randomized placebo‐controlled trial of escitalopram. American Journal of Geriatric Psychiatry 2010;18:154‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leuchter 2009 {published data only}
- Leuchter AF, Cook IA, Marangell LB, Gilmer WS, Burgoyne KS, Howland RH. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in Major Depressive Disorder: results of the BRITE‐MD study. Psychiatry Research 2009;169:124‐31. [DOI] [PubMed] [Google Scholar]
Lindsley 2010 {published data only}
- Lindsley CW. (S)‐(+)‐mecamylamine (TC‐5214): A neuronal nicotinic receptor modulator enters phase III trials as an adjunct treatment for major depressive disorder (MDD). ACS Chemical Neuroscience 2010;1:530‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linnet 1996 {published data only}
- Linnet K, Olesen OV. Citalopram and desmethylcitalopram for psychiatric patients. Ugeskrift for Laeger 1996;158(35):4920‐3. [PubMed] [Google Scholar]
Liu 2006b {published data only}
- Liu Y, Xu R. Effect of citalopram treatment on post‐stroke depression and neurological functional rehabilitation. Chinese Journal of Rehabilitation 2006;21:174‐5. [Google Scholar]
Liu 2006c {published data only}
- Liu G, Liu R, Wang Y, He G. Clinical control study of citalopram and amitriptyline in the treatment of post‐stroke depression. Journal of Clinical Psychological Medicine 2006;16:153‐4. [Google Scholar]
Li WQ 2006 {published data only}
- Li WQ, Chen ZH, Li DX. Efficacy of nimodipine combined with citalopram in treatment of 68 patients with vascular depression. Chinese Journal of New Drugs and Clinical Remedies 2006;25(7):504‐7. [Google Scholar]
Llacer 2007 {published data only}
- Llacer JMB, Gandia I, Espla R, Matarredona Catala J, Martinez EP. Trazadone associated with SSRIs and SNRIs in depressed patients with anxiety and insomnia. Psiquiatria Biologica 2007;14(6):204‐10. [Google Scholar]
Lydiatt 2006 {published data only}
- Lydiatt WM, Denman D, Burke WJ. Randomized placebo‐controlled trial of citalopram demonstrating depression prevention during treatment for HN cancer. Archives of Otolaryngology ‐ Head and Neck Surgery 2006;132:845. [DOI] [PubMed] [Google Scholar]
Maas 2010 {published data only}
- Maas DW, Westendorp RGJ, Willems JM, Craen AJM, Mast RC. TNF‐alpha antagonist infliximab in the treatment of depression in older adults: Results of a prematurely ended, randomized, placebo‐controlled trial. Journal of Clinical Psychopharmacology 2010;30(3):343‐5. [DOI] [PubMed] [Google Scholar]
Maksinczyk 1997 {published data only}
- Maksinczyk. Double‐blind, multicentre, phase III study to compare the efficacy, safety and tolerability of citalopram and amitriptyline in elderly depressed patients in general practice. National Research Register 1997.
Malik 2002 {unpublished data only}
- Malik NA. Comparison of treatment to influence depression as a risk factor for Ischemic Heart Disease with new generation antidepressants. XII World Congress of Psychiatry, Aug 24‐9, 2002, Yokohama, Japan. 2002.
Mannu 2009 {published data only}
- Mannu P, Rinaldi S, Fontani V, Castagna A, Margotti ML. Radio electric treatment vs. Es‐Citalopram in the treatment of panic disorders associated with major depression: an open‐label, naturalistic study. Acupuncture & Electro‐Therapeutics Research 2009;34:135‐49. [DOI] [PubMed] [Google Scholar]
Martinez 2012 {published data only}
- Martinez JM, Katon W, Greist JH, Kroenke K, Thase ME, Meyers AL, et al. A pragmatic 12‐week, randomized trial of duloxetine versus generic selective serotonin‐reuptake inhibitors in the treatment of adult outpatients in a moderate‐to‐severe depressive episode. International Clinical Psychopharmacology 2012;27(1):17‐26. [DOI] [PubMed] [Google Scholar]
Martini 2007 {published data only}
- Martini B. Antidepressants versus psychotherapy: Successful treatment in patients with coronary heart disease. Deutsche Apotheker Zeitung 2007;147(19):50‐2. [Google Scholar]
Martiny 2004 {published data only}
- Martiny K, Lunde M, Simonsen C, Clemmensen L, Poulsen DL, Solstad K, et al. Relapse prevention by citalopram in SAD patients responding to 1 week of light therapy. A placebo‐controlled study. Acta Psychiatrica Scandinavica 2004;109(3):230‐4. [DOI] [PubMed] [Google Scholar]
Martire 2008 {published data only}
- Martire LM, Schulz R, Reynolds CF, Morse JQ, Butters MA, Hinrichsen GA. Impact of close family members on older adults' early response to depression treatment. Psychology & Aging 2008;23:447‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCabe 2010 {published data only}
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological Psychiatry 2010;67(5):439‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mcgrath 2008 {published data only}
- McGrath PJ, Khan AY, Trivedi MH, Stewart JW, Morris DW, Wisniewski SR. Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: a STAR*D report. Journal of Clinical Psychiatry 2008;69:1847‐55. [DOI] [PubMed] [Google Scholar]
Mendels 1990 {unpublished data only}
- Mendels J, Fabre L, Kiev A. A double‐blind parallel comparison of citalopram and placebo in out‐patients with major depression or bipolar disorder, depressed. 143rd Annual Meeting of the American Psychiatric Association; 1996 May 12‐17; New York, NY. 1990. 1990.
Meyer 2001 {published data only}
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a DASB PET imaging study. American Journal of Psychiatry 2001;58(12):1839‐49. [DOI] [PubMed] [Google Scholar]
Miao 2004 {published data only}
- Miao S‐Y, Shi Y‐J. Related factors of post‐stroke depression and therapeutical effect of citalopram. Chinese Journal of Clinical Rehabilitation 2004;8(19):3718‐9. [Google Scholar]
Minelli 2010 {published data only}
- Minelli A, Bortolomasi M, Scassellati C, Salvoro B, Avesani M, Manganotti P. Effects of intravenous antidepressant drugs on the excitability of human motor cortex: a study with paired magnetic stimulation on depressed patients. Brain Stimulation 2010;3:15‐21. [DOI] [PubMed] [Google Scholar]
Miskowiak 2009 {published data only}
- Miskowiak KW, Favaron E, Hafizi S, Inkster B, Goodwin GM, Cowen PJ. Effects of erythropoietin on emotional processing biases in patients with major depression: An exploratory fMRI study. Psychopharmacology 2009;207:133‐42. [DOI] [PubMed] [Google Scholar]
Moltzen 2005 {published data only}
- Moltzen LS, Ninan PT, Ventura D, Wang J. Escitalopram in the treatment of severe depression. XIII World Congress of Psychiatry, Cairo, Egypt. 10‐15th September 2005.
Morasco 2010 {published data only}
- Morasco BJ, Loftis JM, Indest DW, Ruimy S, Davison JW, Felker B. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: A double‐blind, placebo‐controlled trial. Psychosomatics 2010;51:401‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moretti 2002 {published data only}
- Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Depression and alzheimer's disease: symptom or comorbidity?. American Journal of Alzheimer's Disease and Other Dementias 2002;17(6):338‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muhonen 2008 {published data only}
- Muhonen LH, Lahti J, Sinclair D, Lönnqvist J, Alho H. Treatment of alcohol dependence in patients with co‐morbid major depressive disorder‐‐predictors for the outcomes with memantine and escitalopram medication. Substance abuse treatment, prevention, and policy 2008;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhonen LH, Lonnqvist J, Lahti J, Alho H. Age at onset of first depressive episode as a predictor for escitalopram treatment of major depression comorbid with alcohol dependence. Psychiatry Research 2009;167:115‐22. [DOI] [PubMed] [Google Scholar]
- Muhonen LH, Lonnqvist J, Juva K, Alho H. Double‐blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. Journal of Clinical Psychiatry 2008;69(3):392‐9. [DOI] [PubMed] [Google Scholar]
NCT00048815 {unpublished data only}
- NCT00048815. Drug Therapy to Treat Minor Depression. www.clinicaltrials.gov.
Nierenberg 2004 {published and unpublished data}
- Nierenberg AA, Henderson J, Rapaport MH, Burns A. Pharmacotherapy for minor depression. ClinicalTrials.gov 2004.
Nowak 2003 {published data only}
- Nowak G, Siwek M, Dudek D, Zieba A, Pilc A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: A preliminary placebo‐controlled study. Polish Journal of Pharmacology 2003;55(6):1143‐7. [PubMed] [Google Scholar]
Nunez 1999 {unpublished data only}
- Nunez R, Doran WE, Freund B, Kumar A, Goodnick PJ. Citalopram in depression: response and serotonin. 152nd Annual Meeting of the American Psychiatric Association, 1999 May 15‐20, Washington, DC1999 . 1999.
Nurnberg 2008 {published data only}
- Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant‐associated sexual dysfunction: A randomized controlled trial. JAMA 2008;300:395‐404. [DOI] [PubMed] [Google Scholar]
Nyth 1990 {published data only}
- Nyth AL, Gottfries CG. The clinical efficacy of citalopram in treatment of emotional disturbances in dementia disorders. A Nordic multicentre study. British Journal of Psychiatry 1990;157:894‐901. [DOI] [PubMed] [Google Scholar]
Oberpichler‐Schwenk 2000 {published data only}
- Oberpichler‐Schwenk HO. Safe treatment of depression with mirtazapine. Psychopharmakotherapie 2000;7(5):1‐4. [Google Scholar]
Pae 2011 {published data only}
- Pae CU, Forbes A, Patkar AA. Aripiprazole as adjunctive therapy for patients with major depressive disorder: Overview and implications of clinical trial data. CNS Drugs 2011;25:109‐27. [DOI] [PubMed] [Google Scholar]
Palmer 2002 {published data only}
- Palmer SM, Clary GL, Babyak MA, Wilkerson N, Silvertooth E, Hellegars C, et al. A preliminary randomised double‐blind placebo controlled study of the effects of citalopram (Celexa) on depression, anxiety and quality of life in patients with severe COPD. Chest 2002;122(4):144. [Google Scholar]
Papakostas 2000 {published data only}
- Papakostas YG, Markianos M, Zervas IM, Theodoropoulou M, Vaidakis N, Daras M. Administration of citalopram before ECT: Seizure duration and hormone responses. Journal of ECT 2000;164(4):356‐60. [DOI] [PubMed] [Google Scholar]
Parvin 2011 {unpublished data only}
- Parvin N, Farzaneh S, Nikfarjam M, Shahinfard N, Asarzadegan N. The effect of lavandula angustifolia in the treatment of depression. European Psychiatry, 19th European Congress of Psychiatry, Vienna. 2011.
Perlis 2009 {published data only}
- Perlis RH, Fijal B, Adams DH, Sutton VK, Trivedi MH, Houston JP. Variation in catechol‐O‐methyltransferase is associated with duloxetine response in a clinical trial for major depressive disorder. Biological Psychiatry 2009;65:785‐91. [DOI] [PubMed] [Google Scholar]
Petersen 1998 {published data only}
- Petersen HE, Patris M, Mackle M. Double‐blind comparison of citalopram and fluoxetine ‐ treatment of depression with and without benzodiazepines. 151st Annual Meeting of the American Psychiatric Association. Toronto, Ontario, Canada. 30th May 4th June 1998. 1998.
Pogosova 2004 {published data only}
- Pogosova GV, Gudkova OA, Iufereva IuM, Tikhomirova EA. Clinical efficacy of citalopram in patients with hypertension and concomitant depression. Kardiologiia 2004;44(10):49‐53. [PubMed] [Google Scholar]
Portella 2010 {published data only}
- Portella MJ, Diego‐Adeliño J, Ballesteros J, Puigdemont D, Oller S, Santos B. Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect? A randomized clinical trial and a meta‐analysis of pindolol in nonresistant depression. Journal of Clinical Psychiatry 2010 [ePub ahead of print]. [DOI] [PubMed]
Prasko 2003 {published data only}
- Prasko J, Johanovska E, Klar I, Pec O, Ondrackova I, Sipek J, et al. Cognitive‐behavioral Therapy and Pharmacotherapy in the Treatment of Patients Suffering with Unipolar Recurrent Depression. Ceska a Slovenska Psychiatrie 2003;99(2):103‐5. [Google Scholar]
Quante 2010 {published data only}
- Quante A, Zeugmann S, Luborzewski A, Schommer N, Langosch J, Born C. Aripiprazole as adjunct to a mood stabilizer and citalopram in bipolar depression: A randomized placebo‐controlled pilot study. Human Psychopharmacology 2010;25:126‐32. [DOI] [PubMed] [Google Scholar]
Raisi 2007 {published data only}
- Raisi F, Habibi N, Nasehi AA, Akhondzadeh S. Combination of citalopram and nortriptyline in the treatment of severe major depression: A double‐blind, placebo‐controlled trial. Therapy 2007;4(2):187‐92. [Google Scholar]
Rampello 2004 {published data only}
- Rampello L, Alvano A. Prediction of the response to citalopram and reboxetine in post‐stroke depressed patients: The reasons of the enrollment of patients without cognitive impairment. Psychopharmacology 2004;175:264. [DOI] [PubMed] [Google Scholar]
Rampello 2004a {published data only}
- Rampello L, Alvano A, Chiechio S, Malaguarnera M, Raffaele R, Vecchio I, et al. Evaluation of the prophylactic efficacy of amitriptyline and citalopram, alone or in combination, in patients with comorbidity of depression, migraine, and tension‐type headache. Neuropsychobiology 2004;50(4):322‐8. [DOI] [PubMed] [Google Scholar]
Rampello 2004b {published data only}
- Rampello L, Chiechio S, Nicoletti G, Alvano A, Vecchio I, Raffaele R, et al. Prediction of the response to citalopram and reboxetine in post‐stroke depressed patients. Psychopharmacology 2004;173(1‐2):73‐8. [DOI] [PubMed] [Google Scholar]
Rampello 2006 {published data only}
- Rampello L, Alvano A, Raffaele R, Malaguarnera M, Vecchio I. New possibilities of treatment for panic attacks in elderly patients: escitalopram versus citalopram. Journal of Clinical Psychopharmacology 2006;26(1):67‐70. [DOI] [PubMed] [Google Scholar]
Rapaport 2010 {published data only}
- Rapoport MJ, Mitchell RA, McCullagh S, Herrmann N, Chan F, Kiss A, et al. A randomized controlled trial of antidepressant continuation for major depression following traumatic brain injury. Journal of Clinical Psychiatry 2010;71(9):1125‐30. [DOI] [PubMed] [Google Scholar]
Rapaport 2011 {published data only}
- Rapaport MH, Nierenberg AA, Howland R, Dording C, Schettler PJ, Mischoulon D. The treatment of minor depression with St. John's Wort or citalopram: Failure to show benefit over placebo. Journal of Psychiatric Research 2011;45(7):931‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rapoport 2010 {published data only}
- Rapoport MJ, Mitchell RA, McCullagh S, Herrmann N, Chan F, Kiss A. A randomized controlled trial of antidepressant continuation for major depression following traumatic brain injury. Journal of Clinical Psychiatry 2010;71:1125‐30. [DOI] [PubMed] [Google Scholar]
Raskin 2011 {unpublished data only}
- Raskin J, Granger RE, Hussain N, Zhao GW, Marangell LB. Apathy in SSRI‐treated patients with depression: Outcomes after switch to duloxetine or escitalopram. European Neuropsychopharmacology, 24th Congress of the European College of Neuropsychopharmacology, Paris France. 2011.
Rasmussen 1992 {published data only}
- Rasmussen S, Bech P. Citalopram in the treatment of depression in specialist practice. Morning versus evening administration. Clinical Neuropharmacology 1992;15(1):529. [Google Scholar]
Riva 2006 {published data only}
- Riva M, Lurati C, Durbano F, Regispani F, Mencacci C. Treatment and treatments: Evaluation of therapeutic efficacy of integrated pharmacologic and psychotherapeutic treatment in outpatients with major depression. Italian Journal of Psychopathology 2006;12(3):323‐31. [Google Scholar]
Robinson 2008 {published data only}
- Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, et al. Escitalopram and problem‐solving therapy for prevention of poststroke depression: a randomized controlled trial. Journal of American Medical Association 2008;299(20):2391‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2009 {published data only}
- Robinson RG, Tenev V, Jorge RE. Citalopram for continuation therapy after repetitive transcranial magnetic stimulation in vascular depression. American Journal of Geriatric Psychiatry 2009;17:682‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rocca 2005 {published data only}
- Rocca P, Calvarese P, Faggiano F, Marchiaro L, Mathis F, Rivoira E, et al. Citalopram versus sertraline in late‐life nonmajor clinically significant depression: a 1‐year follow‐up clinical trial. Journal of Clinical Psychiatry 2005;66(3):360‐9. [DOI] [PubMed] [Google Scholar]
Roose 2004 {published data only}
- Roose SP, Sackeim HA, Krishnan KR, Pollock BG, Alexopoulos G, Lavretsky H, et al. Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo‐controlled trial. American Journal of Psychiatry 2004;161(11):2050‐9. [DOI] [PubMed] [Google Scholar]
Rosenthal 2002 {published data only}
- Rosenthal M, Zornberg G, Li D. Efficacy and tolerability of escitalopram in patients intolerant of other SSRIs. International Journal of Neuropsychopharmacology 2002;5(1):147. [Google Scholar]
Rush 2008 {published data only}
- Rush AJ, Wisniewski SR, Warden D, Luther JF, Davis LL, Fava M. Selecting among second‐step antidepressant medication monotherapies: predictive value of clinical, demographic, or first‐step treatment features. Archives of General Psychiatry 2008;65:870‐80. [DOI] [PubMed] [Google Scholar]
Salloway 2002 {published data only}
- Salloway S, Correia S, Boyle P, Malloy P, Schneider L, Lavretsky H, et al. Mri subcortical hyperintensities in old and very old depressed outpatients: the important role of age in late‐life depression. Journal of the Neurological Sciences 2002;203‐204:227‐33. [DOI] [PubMed] [Google Scholar]
Schaefer 2008 {published data only}
- Schaefer M. Es‐Citalopram for the prevention of PEG‐IFN‐alpha and ribavirin associated depression in HCV‐infected patients without psychiatric risk factors. Hepatology 2008;48:432A‐433A. [Google Scholar]
Schfer 2010 {published data only}
- Schfer A, Scheurlen M, Seufert J, Keicher C, Weissbrich B, Rieger P. Platelet serotonin (5‐HT) levels in interferon‐treated patients with hepatitis C and its possible association with interferon‐induced depression. Journal of Hepatology 2010;52:10‐5. [DOI] [PubMed] [Google Scholar]
Schmitt 2006 {published data only}
- Schmitt L, Tonnoir B, Arbus C. Safety and efficacy of oral escitalopram as continuation treatment of intravenous citalopram in patients with major depressive disorder. Neuropsychobiology 2006;54(4):201‐7. [DOI] [PubMed] [Google Scholar]
Segal 2010 {published data only}
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness‐based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression vs sequential pharmacotherapy and mindfulness‐based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Archives of General Psychiatry 2010;67:1256‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Serfaty 2010 {published data only}
- Serfaty MA, Osborne D, Buszewicz MJ, Blizard R, Raven PW. A randomized double‐blind placebo‐controlled trial of treatment as usual plus exogenous slow‐release melatonin (6 mg) or placebo for sleep disturbance and depressed mood. International Clinical Psychopharmacology 2010;25:132‐42. [DOI] [PubMed] [Google Scholar]
Sharp 2010 {published data only}
- Sharp DJ, Chew‐Graham CA, Tylee A, Lewis G, Howard L, Anderson I. A pragmatic randomised controlled trial to compare antidepressants with a community‐based psychosocial intervention for the treatment of women with postnatal depression: The RESPOND trial. Health Technology Assessment 2010;14:1‐181. [DOI] [PubMed] [Google Scholar]
Smith 2011 {published data only}
- Smith GS, Workman CI, Kramer E, Hermann CR, Ginsberg R, Ma Y. The relationship between the acute cerebral metabolic response to citalopram and chronic citalopram treatment outcome. The American Journal of Geriatric Psychiatry 2011;19:53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sneed 2007 {published data only}
- Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late‐life depression. American Journal of Geriatric Psychiatry 2010;18:128‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. American Journal of Geriatric Psychiatry 2007;15(7):553‐563. [DOI] [PubMed] [Google Scholar]
Soares 2006 {published data only}
- Soares CN, Arsenio H, Joffe H, Bankier B, Cassano P, Petrillo LF, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri‐ and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006;13(5):780‐6. [DOI] [PubMed] [Google Scholar]
Soares 2010 {published data only}
- Soares CN, Thase ME, Clayton A, Guico Pabia CJ, Focht K, Jiang Q. Desvenlafaxine and escitalopram for the treatment of postmenopausal women with major depressive disorder. Menopause 2010;17:700‐11. [DOI] [PubMed] [Google Scholar]
Souery 2010 {published data only}
- Souery D, Serretti A, Calati R, Oswald P, Massat I, Konstantinidis A, et al. Citalopram versus desipramine in treatment resistant depression: Effect of continuation or switching strategies. A randomized open study. World Journal of Biological Psychiatry 2011;12(5):364‐75. [DOI] [PubMed] [Google Scholar]
- Souery D, Serretti A, Montgomery S, Kasper S, Zohar J, Mendlewicz J. Advances on the treatment of resistant depression. International Journal of Psychiatry in Clinical Practice 2010;14:13. [Google Scholar]
Stein 2001 {published data only}
- Stein DJ, Montgomery SA, Kasper S, Tanhoj P. Predictors of response to pharmacotherapy with citalopram in obsessive‐compulsive disorder. World Journal of Biological Psychiatry 2001;2(Suppl 1):345‐9. [DOI] [PubMed] [Google Scholar]
Stein 2005 {published data only}
- Stein MD, Solomon DA, Anderson BJ, Herman DS, Anthony JL, Brown RA, et al. Persistence of antidepressant treatment effects in a pharmacotherapy plus psychotherapy trial for active injection drug users.. American Journal on Addictions 2005;14(4):346‐57. [DOI] [PubMed] [Google Scholar]
Sun 2004 {published data only}
- Sun Q, Zen D, Luo S. Comparative study of citalopram combined with buspirone for treatment of refractory depression. Journal of Clinical Psychological Medicine 2004;14(4):221‐2. [Google Scholar]
Swartz 2008 {published data only}
- Swartz HA, Frank E, Zuckoff A, Cyranowski JM, Houck PR, Cheng Y. Brief interpersonal psychotherapy for depressed mothers whose children are receiving psychiatric treatment. American Journal of Psychiatry 2008;165:1155‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talati 2007 {published data only}
- Talati A, Wickramaratne PJ, Pilowsky DJ, Alpert JE, Cerda G, Garber J, et al. Remission of maternal depression and child symptoms among single mothers. A STAR*D‐child report. Social Psychiatry and Psychiatric Epidemiology 2007;42(12):962‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Targacept 2008 {unpublished data only}
- Targacept Inc. A Multi‐Center, Double Blind, Randomized, Placebo‐Controlled, Parallel Group, Flexible Dose Titration, Add‐On Study of TC‐5214 in the Treatment of MDD With Subjects Who Are Partial Responders or Non‐Responders to Citalopram Therapy. www.clinicaltrials.gov 2008.
Thase 2010 {published data only}
- Thase ME, Nierenberg AA, Vrijland P, Oers HJ, Schutte AJ, Simmons JH. Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta‐analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. International Clinical Psychopharmacology 2010;25:189‐98. [DOI] [PubMed] [Google Scholar]
Thase 2011 {published data only}
- Thase ME, Ninan PT, Musgnung JJ, Trivedi MH. Remission with venlafaxine extended release or selective serotonin reuptake inhibitors in depressed patients: A randomized, open‐label study. Primary Care Companion to the Journal of Clinical Psychiatry 2011;13(1):0m00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorell 1999 {published data only}
- Thorell LH, Kjellman B, Arned M, Lindwall‐Sundel K, Walinder J, Wetterberg L. Light treatment of seasonal affective disorder in combination with citalopram or placebo with 1‐year follow‐up. International Clinical Psychopharmacology 1999;14(2):7‐11. [PubMed] [Google Scholar]
Uher 2010 {published data only}
- Uher R, Perroud N, Ng MY, Hauser J, Henigsberg N, Maier W. Genome‐wide pharmacogenetics of antidepressant response in the GENDEP project. American Journal of Psychiatry 2010;167:555‐64. [DOI] [PubMed] [Google Scholar]
Van Bemmel 1993 {published data only}
- Bemmel AL, Hoofdakker RH, Beersma DG, Bouhuys AL. Changes in sleep polygraphic variables and clinical state in depressed patients during treatment with citalopram. Psychopharmacology 1993;113(2):225‐30. [DOI] [PubMed] [Google Scholar]
Voirol 1999 {published data only}
- Voirol P, Rubin C, Bryois C, Kosel M, Buclin T, Baumann P. Pharmacokinetic consequences of a citalopram treatment discontinuation. Therapeutic drug monitoring 1999;21:263‐6. [DOI] [PubMed] [Google Scholar]
Wade 2000 {published data only}
- Wade AG, Hochstrasser B. Prevention of depression recurrence with citalopram: Results from a double‐blind, placebo‐controlled trial. International Journal of Neuropsychopharmacology 2000;3(1):209. [Google Scholar]
Wade 2006 {published data only}
- Wade A, Despiegel N, Reines EH. Escitalopram in the long‐term treatment of major depressive disorder. Annals of Clinical Psychiatry 2006;18(2):83‐9. [DOI] [PubMed] [Google Scholar]
Wagner 2002 {published data only}
- Wagner KD, Robb AS, Findling R, Tiseo P. Citalopram is effective in the treatment of major depressive disorder in children and adolescents: results of a placebo‐controlled trial. International Journal of Neuropsychopharmacology 2002;5(1):161. [Google Scholar]
Wang 2005 {published data only}
- Wang X, Hu X, Li H. Comparative study between citalopram and amitriptyline in treatment of depression induced by Alzheimer Disease. Journal of Clinical Psychological Medicine 2005;15(2):84‐5. [Google Scholar]
Warden 2009 {published data only}
- Warden D, Rush AJ, Wisniewski SR, Lesser IM, Kornstein SG, Balasubramani GK. What predicts attrition in second step medication treatments for depression?: a STAR*D Report. International Journal of Neuropsychopharmacology 2009;12:459‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wermuth 1998 {published data only}
- Wermuth L, Sorensen PS, Timm S, Christensen B, Utzon NP, Boas J, et al. Depression in idiopathic Parkinson's disease treated with citalopram. A placebo‐controlled trial. Nordic Journal of Psychiatry ‐ Nordisk Psykiatrisk Tidsskrift 1998;52(2):163‐9. [Google Scholar]
Wise 2011 {published data only}
- Wise TN. Prophylactic citalopram treatment in hepatitis C patients on antiviral therapy: Will it limit drug‐induced depression and enhance adherence?. Current Psychiatry Reports 2011;13:1‐2. [DOI] [PubMed] [Google Scholar]
Wisniewski 2009 {published data only}
- Wisniewski SR, Rush AJ, Nierenberg AA, Gaynes BN, Warden D, Luthe, JF. Can phase III trial results of antidepressant medications be generalized to clinical practice? A STAR*D report. American Journal of Psychiatry 2009;166:599‐607. [DOI] [PubMed] [Google Scholar]
Wu 2008 {published data only}
- Wu E, Greenberg PE, Yang E, Yu A, Erder MH. Comparison of escitalopram versus citalopram for the treatment of major depressive disorder in a geriatric population. Current Medical Research & Opinion 2008;24:2587‐95. [DOI] [PubMed] [Google Scholar]
Yang 2005 {published data only}
- Yang C, Wen Q, Wang X, Liu X. Comparative study of citalopram combined with amitriptyline for treatment of refractory depression. International Medicine and Health Guidance News 2005;11(4):69‐70. [Google Scholar]
Yang 2010 {published data only}
- Yang LPH, Scott LJ. Escitalopram: In the treatment of major depressive disorder in adolescent patients. Pediatric Drugs 2010;12:155‐62. [DOI] [PubMed] [Google Scholar]
Zhao 2005 {published data only}
- Zhao FT, Xu SM, Zhang QH, Wang XL, Liu HH. Citalopiam versus venlafaxine for the improvement of post‐stroke depression. Chinese Journal of Clinical Rehabilitation 2005;9(12):12‐3. [Google Scholar]
Zimbroff 2004 {unpublished data only}
- Zimbroff DL, Bose A, Li D. Escitalopram treatment of SSRI nonresponders can lead to remission in patients who fail initial SSRI therapy. 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York, NY , NR758. 2004. 2004.
Zisook 2007 {published data only}
- Zisook S, Montross L, Kasckow J, Mohamed S, Palmer BW, Patterson TL, et al. Subsyndromal depressive symptoms in middle‐aged and older persons with schizophrenia. American Journal of Geriatric Psychiatry 2007;15(12):1005‐14. [DOI] [PubMed] [Google Scholar]
Zisook 2010 {published data only}
- Zisook S, Kasckow JW, Lanouette NM. Augmentation with citalopram for suicidal ideation in middle‐aged and older outpatients with schizophrenia and schizoaffective disorder who have subthreshold depressive symptoms: a randomized controlled trial. Journal of Clinical Psychiatry 2010;71:915‐22. [DOI] [PubMed] [Google Scholar]
Zou 2005 {published data only}
- Zou XB, Lin ZX, Lin JD, Lu D, Chen GM. Interventional efficacy of citalopram combined with shining and psychological morning exercise in the attack of depression in elderly people. Chinese Journal of Clinical Rehabilitation 2005;9(12):24‐5. [Google Scholar]
References to studies awaiting assessment
Ahlfors 1988 {published data only}
- Ahlfors UG, Elovaara S, Harma P, Suoniemi I, Heikkila L, Nummi K, et al. Clinical multicentre study of citalopram compared double‐blindly with mianserin in depressed patients in Finland. Nord Psykiatr Tidsskr 1988;42:201‐10. [Google Scholar]
Akimova 2010 {published data only}
- Akimova E, Lanzenberger R, Savli M, Hausler D, Wadsak W, Spindelegger C. The serotonin transporter (SERT) occupancy in median raphe nucleus quantified with PET predicts treatment response to SSRIs in major depressive disorder. European Neuropsychopharmacology 2010;20:S389. [Google Scholar]
Akimova 2011 {unpublished data only}
- Akimova E. Area‐specific occupancy of the serotonin transporter by escitalopram and citalopram in major depressive disorder. European Psychiatry. 2011; Vol. abstracts from the 19th European Congress of Psychiatry, EPA 2011 Mar 12‐15; Vienna, Austria.
Aydemir 2011 {published data only}
- Aydemir O, Ergun H, Kesebir S, Soygur H, Tulunay C. Effect of citalopram versus escitalopram on quality of life in the treatment of the acute phase of major depressive disorder: A comparative, open‐label study. Klinik Psikofarmakoloji Bulteni 2011;21(3):210‐8. [Google Scholar]
Du 2004 {published data only}
- Du C, Che L. Efficacy analysis of citalopram and amitriptyline in the treatment of depression. Chinese Journal of health Psychology 2004;12(6):424‐6. [Google Scholar]
Fu 2006 {published data only}
- Fu R, Zhu C. Compariaon of citalopram and amitriptyline in treatment of depression. Medical Journal of the Chinese People's Armed Police Forces 2006;17(4):250‐1. [Google Scholar]
Galecki 2004 {published data only}
- Galecki P, Florkowski A, Pietras T, Kolodziejska I, Nowakowski T, Pawelczyk A er al. Efficiency and safety of citalopram and venlafaxine in treatment of depressive disorders in elderly patients.. Polski Merkuriusz Lekarski 2004;17(102):621‐4. [PubMed] [Google Scholar]
Gao 2005 {published data only}
- Gao B, Yue S, Liu J. Study on citalopram in the treatment of aged depression. Practical Geriatrics 2005;19(1):42‐3. [Google Scholar]
Gong 2005 {published data only}
- Gong C, Xu H, Xiang D, Zhou X. Comparative study on depression treated by citalopram or mirtazapine. Journal of Clinical Psychological Medicine 2005;15(3):154‐5. [Google Scholar]
Huang 2004 {published data only}
- Huang P, Li Z, Wang C. A clinical controlled study of citalopram and fluoxetine in the treatment of depression. Journal of Clinical Psychological Medicine 2004;14(6):364‐5. [Google Scholar]
Huang 2006 {published data only}
- Huang K. Contrast study of citalopram and fluoxetine in treatment of depression. Modern Medicine and Health 2006;20(10):1456‐1457. [Google Scholar]
Huang b 2006 {published data only}
- Huang J, Sun Z. A control study of citalopram and fluoxetine in first‐episode senile depression. Journal of Clinical Psychosomatic Diseases 2006;12(3):175‐6. [Google Scholar]
Juckel 2007 {published data only}
- Juckel G, Pogarell O, Augustin H, Mulert C, Muller‐Siecheneder F, Frodl T. Differential prediction of first clinical response to serotonergic and noradrenergic antidepressants using the loudness dependence of auditory evoked potentials in patients with major depressive disorder. Journal of Clinical Psychiatry 2007;68:1206‐12. [DOI] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li XH, Zhang HW, Pang YD. A comparative evaluation on the effect of citalopram and amitriptyline in treating senile depression. Chinese Journal of Clinical Rehabilitation 2004;8(27):5762‐3. [Google Scholar]
Li 2005 {published data only}
- Li X, Sun L, Li Z. A comparative study of the efficacy of citalopram in the treatment of depression. Sichuan Mental Health 2005;18(4):214‐6. [Google Scholar]
Li 2006 {published data only}
- Li J, Shen WW, Liu Y, Xu L, Liu SM, Kuang WH. The effectiveness and safety of escitalopram in the treatment of major depression: A randomized double‐blind active‐drug controlled trial. Chinese Journal of Evidence‐Based Medicine 2006;6(8):552‐6. [Google Scholar]
Liang 2005 {published data only}
- Liang C, Liu L, Zhang X. A comparative study of citalopram and fluoxetine in the treatment of depression. Shandong Archives of Psychiatry 2005;18(2):82‐3. [Google Scholar]
Liang 2006 {published data only}
- Liang K, Fan X. Comparative study of citalopram and mianserin in the treatment of senile depression. Journal of Clinical Psychological Medicine 2006;16(1):32‐3. [Google Scholar]
Li DS 2006 {published data only}
- Li DS, Wang C, Yu J. A comparative study of citalopram and paroxetine in the treatment of depression. Medical Journal of Chinese People's Health 2006;18(6):460‐1. [Google Scholar]
Lin 2001 {published data only}
- Lin J, HUuang Y, Chen G, Lin Z, Zhu G. Comparison of citalopram and amitriptyline in treatment of depression. Chinese Journal of New Drugs and Clinical Remedies 2001;20(5):351‐4. [Google Scholar]
Liu 2006 {published data only}
- Liu J, Gan L, Feng D. A control study on citalopram and amitriptyline in the treatment of aged patients with depression. China Journal of Health Psychology 2006;14(4):450‐1. [Google Scholar]
Liu 2006d {published data only}
- Liu H, Du H, Liu Y, Li H. Study of citalopram in treatment of patients with senile depression. Journal of Clinical Psychological Medicine 2006;16:88‐9. [Google Scholar]
Li X 2005 {published data only}
- Li D, Yu J, Pang Y. Comparative study of citalopram and venlafaxine in the treatment of depression. Journal of Clinical Psychological Medicine 2005;15(3):158‐9. [Google Scholar]
Li Z 2004 {published data only}
- Li Z, Zeng Z, Liu Q. Clinical controlled study of citalopram versus amitriptyline in treatment of depressive disorder. Sichuan Mental Health 2004;17(2):76‐8. [Google Scholar]
Lu 2008 {published data only}
- Lu Q, Li Z, Zeng Z. Controlled study of citalopram versus amitriptyline in treatment of depressive disorder. Journal of Clinical Psychological Medicine 2008;18:69‐70. [Google Scholar]
Ma 2004 {published data only}
- Ma Z, Zheng Z, Zhuang X. A comparative study of citalopram and amitriptyline in the treatment of depression. Shandong Archives of Psychiatry 2004;17(2):79‐81. [Google Scholar]
Moeller 1986 {published data only}
- Moeller SE, Beurs P, Timmerman L, Tan BK, Leijnse‐Ybema HJ, Cohen Stuart MH, et al. Plasma tryptophan and tyrosine ratios to competing amino acids in relation to antidepressant response to citalopram and maprotiline. A preliminary study. Psychopharmacology 1986;5:96‐100. [DOI] [PubMed] [Google Scholar]
NCT00269334 {published data only}
- Chen W, Deng C, Liu J‐Y, Hsiao N, Wen J‐K, Wu C‐K. Phase 4 Study of Clinical Pharmacogenomics of Antidepressant Response. ClinicalTrials.gov [www.clinicaltrials.gov] 2005:http://clinicaltrials.gov/ct2/show/ NCT00269334 (accessed 11 Jun 2012). [NCT00269334]
NCT00993876 {published data only}
- Kock, J M. Cognitive Flexibility and Its Correlation to Sleep and Neuroplasticity In The Course Of Depression During Different Treatments. ClinicalTrials.gov 2009:http://clinicaltrials.gov/ct2/show/ NCT00993876?term= NCT00993876&rank=1. (accessed 11 Jun 2012). [NCT00993876]
Norra 2011 {published data only}
- Norra C, Peddersen A, Juckel G, Waniek S. Mismatch negativity in depressed patients under selective noradrenergic and serotonergic antidepressants. European Neuropsychopharmacology 2011;21(Suppl 3):251‐2. [Google Scholar]
Pan 2005 {published data only}
- Pan K, Liu X, Yang J, Zhu L, Wang X, Wang X, et al. Cost‐effectiveness analysis of depression treatment with paroxetine, venlafaxine and citalopram. Chinese Journal of Clinical rehabilitation 2005;9(28):16‐7. [Google Scholar]
Qiao 2005 {published data only}
- Qiao J, Yu J, Hao Z. Comparative study of citalopram and paroxetine in treatment of depression. Journal of Clinical Psychological Medicine 2005;15(5):281‐2. [Google Scholar]
Qiu 2005 {published data only}
- Qiu C, Xiao B, Shi Z, Xie W, Zhang F, Qiu K. The effect observation of citalopram in the treatment of patients with depression. Journal of Shantou University Medical College 2005;18(4):216‐7. [Google Scholar]
Ren 2006 {published data only}
- Ren Y. Controlled study of citalopram and sertraline the treatment of depression. Medical Journal of Chinese People's Health 2006;18(7):580‐1. [Google Scholar]
Rutherford 2010 {published data only}
- Rutherford BR, Roose SP, Sneed J, Devanand D. Expectancy effects and treatment of depression: cognitive and neural mechanisms. 163rd Annual Meeting of the American Psychiatric Association; New Orleans. May 22‐26 2010.
Shi 2005 {published data only}
- Shi Y, Liu H, Ding D. Comparative study of citalopram and maprotiline in treatment of depression. Journal of Clinical Psychological Medicine 2005;15(6):356‐7. [Google Scholar]
Song 2004 {published data only}
- Song H, Guo B, Chen Z. Comparisons of citalopram and fluoxetine in first‐episode depression. Journal of Clinical Psychosomatic Disease 2004;10(3):180‐1. [Google Scholar]
Tan 2004 {published data only}
- Tan X, Li H, Du Z. A double blind study of citalopram and amitriptyline in the treatment of aged depression. Shandong Archives of Psychiatry 2004;17(4):202‐3. [Google Scholar]
Tang 2005 {published data only}
- Tang W, Huang W. Efficacy of citalopram and amitriptyline in the treatment of patients with depression. Shangai Archives of Psychiatry 2005;17(2):87‐8. [Google Scholar]
Tao 2005 {published data only}
- Tao W, LV C, Li W. A comparative study between citalopram and paroxetine in the treatment of depression. Medical Journal of Chinese People's Health 2005;17(9):491‐2. [Google Scholar]
Thomas 2008 {published data only}
- Thomas L, Mulligan J, Mason V, Tallon D, Wiles N, Cowen P, et al. GENetic and clinical Predictors Of treatment response in Depression: the GenPod randomised trial protocol. Trials 2008;9(29):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2006 {published data only}
- Wan Y, Li M, Han Y. A control study of citalopram in the treatment of senile depression. Journal of Clinical Psychosomatic Diseases 2006;12(4):243‐4. [Google Scholar]
Wang 2003 {published data only}
- Wang X. A double‐blind control study on the effect of citalopram and imipramine on depression. Modern Forums in Basic 2003;7(12):1111‐2. [Google Scholar]
Wang 2004 {published data only}
- Wang J, Liu X, Yang G. A study of citalopram in the treatment of depression. Journal of Clinical Psychological Medicine 2004;14(1):16‐7. [Google Scholar]
Wang 2006 {published data only}
- Wang J, Yang C, Wang Y. Comparative study of citalopram vs mirtazapine in treatment of senile depressive patients. China Journal of Health Psychology 2006;14(5):552‐3. [Google Scholar]
Xu 2005 {published data only}
- Xu M, Ji L. A comparative study of citalopram and amitriptyline in the treatment of out‐patients with depression. Chinese Mental Health Journal 2005;19(5):353‐4. [Google Scholar]
Yu 2006 {published data only}
- Yu J, Wang P, Zhang G, Hao Z. Controlled study of citalopram and venlafaxine in treatment of patients with senile depression. Journal of Clinical Psychological Medicine 2006;16(2):92‐3. [Google Scholar]
Zhang 2005 {published data only}
- Zhang H, Cheng J, Wang G. Comparison of citalopram and venlafaxine for depressive disorder. Chinese journal of Clinical Rehabilitation 2005;9(36):4‐6. [Google Scholar]
Zhang 2006 {published data only}
- Zhang F, Mao Q. A study of citalopram in treatment of old age depression. Chinese Journal of Clinical Rehabilitation 2006;14(4):434‐5. [Google Scholar]
Zhao 2006 {published data only}
- Zhao H, Guan T. A control study in the treatment of depressive disorder with citalopram and fluoxetine. Evaluation and Analysis of Drug‐Use in Hospitals of China 2006;6(2):106‐8. [Google Scholar]
Zhou 2005 {published data only}
- Zhou X, Liu Z, Zhu H, Ding Y, Zhang A, Tao Y, et al. Treatment of depression with citalopram and venlafaxine: a clinical comparative study. Medical Journal of National Defending Forces in North China 2005;17(2):90‐1. [Google Scholar]
References to ongoing studies
NCT01407094 {unpublished data only}
- Trivedi M H, McGrath P J, Weissman M, Parsey R, Fava M. Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care (EMBARC) for Depression. ClinicalTrials.gov 2011; Vol. NCT01407094:http://clinicaltrials.gov/ct2/show/ NCT01407094?term= NCT01407094&rank=1. (accessed 11 Jun 2012).
NCT01473381 {unpublished data only}
- Forest Laboratories. A Double‐Blind, Placebo‐ and Active‐Controlled, Fixed‐Dose Study of Vilazodone in Patients With Major Depressive Disorder. ClinicalTrials.gov 2011:http://clinicaltrials.gov/ct2/show/ NCT01473381?term= NCT01473381&rank=1. (accessed 11 Jun 2012). [Forest VLZ‐MD‐01]
Additional references
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. JAMA 2003;290(7):921‐8. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. .. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). American Journal of Psychiatry 2000;157(4 Suppl):1‐45. [PubMed] [Google Scholar]
APA 2006
- American Psychiatric Association. American Psychiatric Association Practice Guidelines for the Treatment of Psychiatric Disorders: Compendium 2006. American Psychiatric Association, 2006. [Google Scholar]
Barbui 2004
- Barbui C, Cipriani A, Brambilla P, Hotopf M. "Wish bias" in antidepressant drug trials?. Journal of Clinical Psychopharmacology 2004;24(2):126‐30. [DOI] [PubMed] [Google Scholar]
Barbui 2011
- Barbui C, Cipriani A. What are evidence‐based treatment recommendations?. Epidemiology and Psychiatric Sciences 2011;20(1):29‐31. [DOI] [PubMed] [Google Scholar]
Barker 1995
- Barker EL, Blakely RD. Norepinephrine and serotonin transporters: molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ editor(s). Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, 1995:321–34. [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. [DOI] [PubMed] [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170(4):477‐80. [PMC free article] [PubMed] [Google Scholar]
Bollini 1999
- Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta‐analysis of dose‐effect relationships in randomised clinical trials. British Journal of Psychiatry 1999;174:297‐303. [DOI] [PubMed] [Google Scholar]
Buchkowsky 2004
- Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Annals of Pharmacotherapy 2004;38(4):579‐85. [DOI] [PubMed] [Google Scholar]
Cipriani 2005a
- Cipriani A, Brambilla P, Furukawa T, Geddes J, Gregis M, Hotopf M, et al. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2005b
- Cipriani A, Barbui C, Geddes JR. Suicide, depression, and antidepressants. BMJ 2005;330(7488):373‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2006
- Cipriani A, Barbui C, Brambilla P, Furukawa TA, Hotopf M, Geddes JR. Are all antidepressants really the same? The case of fluoxetine: a systematic review. Journal of Clinical Psychiatry 2006;67(6):850‐64. [DOI] [PubMed] [Google Scholar]
Cipriani 2007
- Cipriani A, Geddes JR, Barbui C. Venlafaxine for major depression. BMJ 2007;334(7587):215‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2009a
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments metaanalysis. Lancet 2009;373:746–58. [DOI] [PubMed] [Google Scholar]
Cipriani 2009b
- Cipriani A, Ferla T, Furukawa TA, Signoretti A, Nakagawa A, Churchill R, et al. Sertraline versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006117] [DOI] [PubMed] [Google Scholar]
Cipriani 2009c
- Cipriani A, Santilli C, Furukawa TA, Signoretti A, Nakagawa A, McGuire H, et al. Escitalopram versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006532] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2009d
- Cipriani A, Purgato M, Barbui C. Why internal and external validity of experimental studies are relevant for clinical practice?. Epidemiology and Psychiatric Sciences 2009;18(2):101‐3. [PubMed] [Google Scholar]
Ciuna 2004
- Ciuna A, Andretta M, Corbari L, Levi D, Mirandola M, Sorio A, et al. Are we going to increase the use of antidepressants up to that of benzodiazepines?. European Journal of Clinical Pharmacology 2004;60(9):629‐34. [DOI] [PubMed] [Google Scholar]
Depression Guideline Panel 1993
- Depression Guideline Panel. Depression in primary care: Vol 2. Treatment of major depression, Clinical Practice Guideline, Number 5, AHCPR Publication No. 93‐0551. Rockville, MD: U. S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, 1993. [Google Scholar]
Dozois 2004
- Dozois D J A, Dobson K S. Depression. In: Antony M M, Barlow D H editor(s). Handbook of Assessment and Treatment Planning for Psychological Disorders. New York: Guilford Press, 2004:259‐99. [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] [DOI] [PubMed] [Google Scholar]
Feighner 1972
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26:57‐63. [DOI] [PubMed] [Google Scholar]
Furukawa 2002a
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. [DOI] [PubMed] [Google Scholar]
Furukawa 2002b
- Furukawa TA, McGuire H, Barbui C. Meta‐analysis of effects and side effects of low dosage tricyclic antidepressants in depression: systematic review. BMJ 2002;325(7371):991‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20(1):49‐52. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa TA, Akechi T, Azuma H, Okuyama T, Higuchi T. Evidence‐based guidelines for interpretation of the Hamilton Rating Scale for Depression. Journal of Clinical Psychopharmacology 2007;27:531‐4. [DOI] [PubMed] [Google Scholar]
Gartlehner 2010
- Gartlehner G, Chapman A, Strobelberger M, Thaler K. Differences in efficacy and safety of pharmaceutical treatments between men and women: an umbrella review. PLoS One 2010;5(7):e11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gartlehner 2011
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, Noord M, et al. Comparative benefits and harms of second‐generation antidepressants for treating major depressive disorder: an updated meta‐analysis. Annals of Internal Medicine 2011;155(11):772‐85. [DOI] [PubMed] [Google Scholar]
Geddes 2004
- Geddes JR, Cipriani A. Selective serotonin reuptake inhibitors. BMJ 2004;329(7470):809‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gourion 2008
- Gourion D. Antidepressants and their onset of action: a major clinical, methodological and pronostical issue. Encéphale 2008;34(1):73–81. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions ‐ ECDEU Asessment Manual Psychopharmacology (DHEW Publ No ADM 76‐338). Revised. Rockville MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH, 1976. [Google Scholar]
Hall 1995
- Hall RC. Global Assessment of Functioning ‐ a modified scale. Psychosomatics 1995;36:267‐75. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].. The Cochrane Collaboration, 2011.. Available from www.cochrane‐handbook.org., 2011. [Google Scholar]
Imperadore 2007
- Imperadore G, Cipriani A, Signoretti A, Furukawa TA, Watanabe N, Churchill R et al. Meta‐Analysis of New Generation Antidepressants (MANGA) Study Group. Citalopram versus other anti‐depressive agents for depression. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006534] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 2005
- Ioannidis JP. Why most published research findings are false. PLoS Medicine 2005;2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2003
- Khan A, Khan SR, Walens G, Kolts R, Giller EL. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacology 2003;28(3):552‐7. [DOI] [PubMed] [Google Scholar]
Leonard 2010
- Leonard B, Taylor D. Escitalopram ‐ translating molecular properties into clinical benefit: reviewing the evidence in major depression. The Journal of Psychopharmacology 2010;24(8):1143‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326(7400):1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2008
- Linde K, Berner MM, Kriston L. St John's wort for major depression. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000448.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Montgomery 2004
- Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F, et al. An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Controlled Clinical Trials 2004;25(6):598‐612. [DOI] [PubMed] [Google Scholar]
Mottram 2006
- Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003491.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Müller 2003
- Müller M J, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery‐Asberg depression rating scale (MADRS). Journal of Affective Disorders 2003;77(3):255‐60. [DOI] [PubMed] [Google Scholar]
Nakagawa 2009
- Nakagawa A, Watanabe N, Omori IM, Barbui C, Cipriani A, McGuire H, et al. Milnacipran versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006529] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care ‐ NICE guidance. London: National Institute for Clinical Excellence, 2010. [Google Scholar]
Omori 2010
- Omori IM, Watanabe N, Nakagawa A, Cipriani A, Barbui C, McGuire H, et al. Fluvoxamine versus other anti‐depressive agents for depression. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD006114] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [DOI] [PubMed] [Google Scholar]
Perlis 2005
- Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, Nierenberg AA. Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. American Journal of Psychiatry 2005;162(10):1957‐60. [DOI] [PubMed] [Google Scholar]
Procyshyn 2004
- Procyshyn RM, Chau A, Fortin P, Jenkins W. Prevalence and outcomes of pharmaceutical industry‐sponsored clinical trials involving clozapine, risperidone, or olanzapine. Canadian Journal of Psychiatry ‐ Revue Canadienne De Psychiatrie 2004;49(9):601‐6. [DOI] [PubMed] [Google Scholar]
Rothwell 2005
- Rothwell PM. External validity of randomised controlled trials: to whom do the results apply?. Lancet 2005;365:82–93. [DOI] [PubMed] [Google Scholar]
Sanchez 2004
- Sánchez C, Bøgesø KP, Ebert B, Reines EH, Braestrup C. Escitalopram versus citalopram: the surprising role of the R‐enantiomer. Psychopharmacology (Berl) 2004;174(2):163‐76. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Spitzer 1972
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives General Psychiatry 1978;35(6):773‐82. [DOI] [PubMed] [Google Scholar]
Stahl 1994
- Stahl SM. Is serotonin receptor desensitization linked to the mechanism of action of antidepressant drugs?. Psychopharmacology Bulletin 1994;30:39–43. [PubMed] [Google Scholar]
Stahl 1998
- Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors: Serotonin receptors and pathways mediate therapeutic effects and side effects. Journal of Affective Disorders 1998;51(3):215‐35. [DOI] [PubMed] [Google Scholar]
Sterne 2000
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119‐29. [PUBMED: 11106885] [DOI] [PubMed] [Google Scholar]
Suh 1997
- Suh T, Gallo J J. Symptom profiles of depression among general medical service users compared with specialty mental health service users. Psychological Medicine 1997;27(5):1051‐63. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor M J, Freemantle N, Geddes J R, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta‐analysis. Archives of General Psychiatry 2006;63(11):1217‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trespidi 2011
- Trespidi C, Barbui C, Cipriani A. Why it is important to include unpublished data in systematic reviews. Epidemiology and Psychiatric Sciences 2011;20(2):133‐5. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short form health survey (SF‐36). Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
Ware 1998
- Ware JE, Kosinski M, Keller SD. SF‐12: How to Score the SF‐12.Physical and Mental Health Summary Scales. Lincoln RI: QualityMetric Inc, 1998. [Google Scholar]
WHO 1992
- World Health Organization. The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD‐10). Geneva: World Health Organization, 1992. [Google Scholar]
WHO 2006
- World Health Organization. WHO Collaborative Centre for Drug Statistics Methodology. http://www.whocc.no/atcddd/.
WHO 2009
- WHO Collaborative Centre for Drug Statistics Methodology. ATC/DDD Index 2009. http://www.whocc.no/atcddd/ 2009.
WHOQOL Group 1998
- WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Social Science and Medicine 1998;46(12):1569‐85. [DOI] [PubMed] [Google Scholar]
Wing 1998
- Wing JK, Beevor AS, Curtis RH, Park SBG, Burns A. Health of the nation outcome scales (HoNOS): Research and development. British Journal of Psychiatry 1998;172:11‐8. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


