Summary
Purpose
To investigate the toxicity profile, activity, pharmacokinetics, and pharmacodynamics of pemetrexed in leukemia.
Patients and Methods
Patients with refractory or relapsed acute leukemia were eligible. A phase I 3+3 design was implemented. Pemetrexed was infused intravenously (IV) over 25 min with vitamin supplementation. Courses were repeated every 3 to 4 weeks according to toxicity and efficacy. The starting dose of 900 mg/m2 was escalated by approximately 33% until the dose-limiting toxicity (DLT) was determined.
Results
Twenty patients with acute myeloid (AML) or lymphocytic (ALL) leukemia received therapy. The main non-hematologic adverse event was liver dysfunction at several dose levels, including 2 DLTs at 3,600 mg/m2. One patient with ALL (3,600 mg/m2 dose level) achieved a partial response. Pemetrexed pharmacokinetics were linear with escalated dosing. Elevated plasma deoxyuridine was observed in a subset of patients following pemetrexed infusion, but was not correlated with dose levels. Changes in the nucleotide pools of circulating mononuclear cells were observed, but were variable.
Conclusions
The recommended phase II dose of pemetrexed for future leukemia studies is 2,700 mg/m2 IV over 25 min every 3 to 4 weeks with vitamin supplementation. Deoxyuridine levels did not increase with increasing pemetrexed dose, suggesting pemetrexed inhibition of thymidylate synthase (TS) may be saturated by the 900 mg/m2 dose level. However, no firm conclusion can be made regarding TS saturation in tumor cells. While tolerable, pemetrexed monotherapy had limited activity in this highly refractory population. Exploration of pemetrexed in combination with other active agents in leukemia is a reasonable future endeavor.
Keywords: Pemetrexed, Leukemia, Phase 1
Introduction
The American Cancer Society estimated that approximately 18,700 new cases of acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML) were expected to occur in 2008, with 10,300 associated deaths [1]. Although treatments for some acute leukemias have improved significantly over the years, most afflicted adults eventually die from disease progression; the 5-year survival for patients with AML is 20% to 30%, whereas the 5-year survival for patients with ALL is 30% to 45%. Because many older patients with leukemia are unable to tolerate intensive chemotherapy regimens, there is a need for more efficacious agents and regimens with improved tolerability [2].
Pemetrexed is a multitargeted antifolate with known activity in a number of tumor types [3]. It exerts its cytotoxic effect predominantly through inhibition of thymidylate synthase (TS), which is a folate-dependent enzyme that catalyzes the transformation of deoxyuridine monophosphate (dUMP) to thymidine monophosphate (TMP). Inhibition of TS disrupts DNA synthesis via a reduction in intracellular thymidine levels [4, 5].
Pemetrexed (Alimta®) in combination with cisplatin is approved by the Food and Drug Administration (FDA) for the treatment of patients with malignant pleural mesothelioma whose disease is unresectable or who are otherwise not candidates for curative surgery, and for the initial treatment of patients with advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) [6]. As a single agent, pemetrexed is approved for the treatment of patients with locally advanced or metastatic non-squamous NSCLC after prior chemotherapy and for maintenance treatment of patients with locally advanced or metastatic non-squamous NSCLC whose disease has not progressed after 4 cycles of platinum-based, first-line chemotherapy. The approved dose schedule is 500 mg/m2 intravenous (IV) administration every 3 weeks. Early studies with pemetrexed were performed without vitamin B12 and folic acid supplementation [7]. However, it was subsequently shown that patients with elevated homocysteine, a marker for folic acid deficiency, were more likely to experience pemetrexed-associated toxicities [8]. More recent clinical trials involving pemetrexed were amended to include vitamin B12 and folic acid supplementation [7] with subsequent reduction in the incidence of severe toxicities [6].
In vitro cytotoxic activity against a leukemia cell line and myelosuppression as a dose-limiting toxicity (DLT) in patients with solid tumors [9] provided the rationale for testing pemetrexed in patients with leukemia. Because pemetrexed has been safely evaluated at a dose of 900 mg/m2 in patients with solid tumors [10, 11], higher doses were explored in this clinical trial involving patients with relapsed acute leukemia. The primary objective of this phase I study was to define the DLT and maximum tolerated dose (MTD) of pemetrexed (900–3,600 mg/m2) with vitamins and folic acid in patients with relapsed leukemia when administered as a single dose every 3 to 4 weeks. Secondary objectives were to conduct a pharmacokinetic (PK) assessment of pemetrexed in the study population and to study the pharmacodynamics (PD) of pemetrexed in plasma and peripheral blood mononuclear cells. A preliminary efficacy analysis was also performed.
Materials and methods
Study group
Patients who were at least 15 years of age, with a diagnosis of relapsed or refractory acute leukemia, were eligible for this study. The first patient was enrolled on 25 March 2005 and the last patient visit was on 10 October 2006. This study was conducted at one study center (MD Anderson Cancer Center) in the United States. The protocol was approved by the institutional review board prior to enrolling any patient, and all patients provided written informed consent according to regulatory guidelines prior to under-going any study procedure or receiving any study therapy. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices. Other eligibility criteria included: Eastern Cooperative Oncology Group performance score 0 to 2; serum bilirubin <1.5 mg/dL; aspartate transaminase (AST) and alanine transaminase (ALT) levels <3–5 times upper limit of normal (ULN); creatinine <1.5 mg/dl or calculated creatinine clearance (CrCl) ≥45 mL/min based on the standard Cockcroft and Gault formula; estimated life expectancy ≥16 weeks; use of adequate contraception; and negative pregnancy test in women of childbearing potential.
Exclusion criteria were: treatment within the last 30 days with an investigational anti-leukemia drug; cytotoxic chemotherapy (other than imatinib, corticosteroids, or hydroxyurea) or radiotherapy within the last 7 days; known central nervous system (CNS) disease; history of allergic reactions attributed to compounds of similar chemical or biologic composition to pemetrexed; uncontrolled intercurrent illness including active infection, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or psychiatric illness/social disorders that would limit compliance with study requirements; clinically relevant third-space fluid collections that could not be controlled by drainage or other procedures prior to study entry; and second primary malignancy (except in situ carcinoma of the cervix or adequately treated non-melanomatous carcinoma of the skin or other malignancy treated ≥5 years previously with no evidence of recurrence; prior low grade [Gleason score ≤6] localized prostate cancer was allowed). Additional exclusion criteria were inability or unwillingness to take folic acid, vitamin B12 supplementation, or dexamethasone and inability to interrupt aspirin or other nonsteroidal anti-inflammatory agents for a 5-day period (8-day period for long-acting agents such as piroxicam.
Therapy
Pemetrexed was administered IV over 25 min every 3 to 4 weeks. In addition, all patients received daily folic acid (350 to 600µg) and vitamin B12 (1,000µg) administered ≥12 h prior to pemetrexed. The starting dose of pemetrexed was 900 mg/m2. Dose escalations were to 1,200, 1,500, 2,000, 2,700, and 3,600 mg/m2 (Table 1) using a classical 3+3 design. At each dose level, 3 patients were enrolled. If one DLT was observed, up to 3 additional patients were enrolled at the same dose level. If no DLT was observed, enrollment began at the next higher dose level. All patients within a dose level were observed for a period of 3 weeks following drug administration before any patient was enrolled at the next dose level. The MTD was defined as the dose below that at which at least 2 patients experienced a DLT.
Table 1.
Patient characteristics and details of dose-limiting toxicities
| Dose level Pem mg/m2 |
Age (years), Gender |
Disease type |
No. of salvage regimens |
Induction therapy |
Cytogenetic abnormalities |
DLT details |
|---|---|---|---|---|---|---|
| 1 | 46, F | AML | 2 | IA | Diploid | |
| 900 | 71, M | AML | 5 | IA | Pseudodiploid | |
| 75, M | AML | 2 | vincristine | Monosomy 7 | ||
| 2 | 47, F | AML | 2 | IA | Monosomy 7 | 1 Gr 3 liver dysfunctionb |
| 1200 | 31, M | AML | 2 | 3+7 | Tetraploid | |
| 59, M | AML | 1 | TAD | Pseudodiploid | 1 Gr 3 AST/ALTa+Gr 3 bilirubinc | |
| 3 | 18, M | ALL | 2 | IA | Multiple | 1 Gr 3 ALTa |
| 1500 | 61, M | ALL | 2 | HCVAD | Diploid | |
| 68, M | AML | 2 | 3+7 | Multiple | 1 Gr 4 bilirubina | |
| 4 | 36, M | AML | 4 | 3+7 | t(8;21) | |
| 2000 | 62, M | AML | 4 | IA | Multiple | |
| 50, F | ALL | 2 | HCVAD | Pseudodiploid | ||
| 5 | 38, F | ALL | 1 | HCVAD | Multiple | |
| 2700 | 61, F | ALL | 2 | HCVAD | Diploid | |
| 50, M | AML | 3 | DCTR | Diploid | ||
| 6e | 36, F | ALL | 5 | HCVAD | Multiple | |
| 3600 | 69, M | ALL | 4 | HCVAD | Diploid | |
| 35, F | AML | 3 | IA | Pseudodiploid | 1 Gr 3 ASTb | |
| 54, F | AML | 2 | IA | Pseudodiploid | 1 Gr 3 diarrheab | |
| 35, F | AML | 3 | 3+7 | Monosomy 7 | 1 Gr 4 bilirubind |
ALL acute lymphocytic leukemia, ALT alanine transaminase, AML acute myeloid leukemia, AST aspartate transaminase, DCTR daunorubicin, cytarabine, thioguanine, DLT dose-limiting toxicities, F female, Gr Grade, HCVAD hyperCVAD regimen, IA Idarubicin+cytarabine, M male, No. number, Pem pemetrexed, TAD thioguanine, cytarabine, daunorubicin, 3+7 daunorubicin+cytarabine
Not treatment-related
Possibly treatment-related
Unrelated to treatment
Definitely treatment-related
Patient achieved a partial response
A DLT was defined as a clinically significant adverse event (AE) or abnormal laboratory value assessed as unrelated to disease progression, intercurrent illness, or concomitant medication(s) occurring in the first cycle of treatment. A DLT must have met the following criteria: 1) Common Terminology Criteria for Adverse Events (CTCAE) Grade 4 AST/serum glutamic oxaloacetic transaminase (SGOT) or ALT/serum glutamic pyruvic transaminase (SGPT) of any duration; or 2) all other clinically significant non-hematologic National Cancer Institute Common Toxicity Criteria version 3.0 (NCI-CTC v3.0) AEs that were CTCAE Grade 3 or 4. Due to the nature of this disease, hematologic AEs were not considered DLTs.
There was no dose reduction for hematological toxicities. Patients were supported with blood products or hematopoietic growth factors as per institutional guidelines for neutropenic fever or documented infections. Retreatment with pemetrexed, if judged beneficial, was allowed if >5% blasts were present in the bone marrow or the peripheral blood on Days 14–21 following pemetrexed treatment. Consolidation therapy was allowed at the discretion of the treating physician if ≤5% blasts were present in the bone marrow after induction therapy. This was given within 1–2 weeks after hematologic recovery, defined as absolute neutrophil count (ANC) > 109/L and platelet count >100×109/L, but within 42 days after the last dose of pemetrexed. Additional doses could be given to patients who were not candidates for bone marrow or peripheral blood stem cell transplantation or who were experiencing hematologic improvement with therapy. The use of hydroxyurea to control peripheral blasts, up to a maximum of 5 g daily for 5 days, was allowed during the first cycle of therapy with pemetrexed.
Methods
Plasma sampling and processing
For PK assays, blood was drawn immediately prior to the pemetrexed infusion, at the end of the infusion, and at 1, 2, 4, 6, 24, 48, and 168 h after the infusion. Samples were analyzed for pemetrexed at Taylor Technology, Inc. (Princeton, NJ) using a validated liquid chromatography/electrospray ionization-tandem mass spectrometry (LC/MS/MS) method that generated a linear response over the concentration ranges of 10 to 2,000 ng/mL and 1,000 to 200,000 ng/mL [12].
Plasma deoxyuridine samples were obtained immediately prior to pemetrexed administration and at 2, 6, 24, 48, 72, and 168 h after the infusion. Plasma deoxyuridine was determined at Taylor Technology Inc. (Princeton, NJ) using a LC/MS/MS analytic method validated over the concentration range of 5 to 500 ng/mL.
Pharmacokinetic analyses
Pharmacokinetic analyses were done using a population PK approach in the nonlinear mixed-effect modeling program, NONMEM (version V) with PREDPP (version V), using first-order conditional estimation (FOCE) with interaction [13]. A 3-compartment model with constant rate input (IV infusion) and parameterized in terms of clearance (CL), central volume of distribution (V1), intercompartmental clearances (Q2 and Q3), and peripheral volumes of distribution (V2 and V3) was used to describe the PK of pemetrexed. Previously established covariates (creatinine clearance estimated by the Cockcroft-Gault method [CrCLCG, std] relative to pemetrexed clearance and body surface area [BSA] relative to pemetrexed volume of distribution) were incorporated into the model. Dose proportionality of pemetrexed exposure over the dose range of 900 to 3,600 mg/m2 was assessed using a linear regression model in the statistical software, S-Plus.
Cellular pharmacology
Mononuclear cells that were collected concurrent with PK samples, were isolated using Ficoll-Hypaque density-gradient step-gradient centrifugation procedures as described [14]. An aliquot of the washed cells was extracted and treated with rat plasma hydrolase to degrade polyglutamates to parent pemetrexed. Total pemetrexed was solvent-extracted and levels determined at a central laboratory (Taylor Technology, Princeton, NJ) as described above for plasma samples. Inhibition of DNA synthesis was assessed by determining [3H]deoxycytidine (1 µCi/ml) uptake by mononuclear cells as described [15]. The results were expressed as a percentage of control value from cells obtained before therapy. For determination of cellular nucleoside triphosphate pools (NTPs), another aliquot of the cells was extracted with 60% methanol and extracted NTPs were assayed as described [15, 16], whereas extracted deoxy-NTPs were assayed using the DNA polymerase assay as described elsewhere [17].
Response and toxicity criteria
Complete remission (CR) was defined as normalization of the blood and bone marrow with ≤5% blasts, normocellular or hypercellular bone marrow, a granulocyte count ≥109/L, and a platelet count ≥100×109/L lasting for ≥4 weeks. Patients who met these criteria but still had 6% to 25% marrow blasts were considered to have a partial remission (PR). Hematologic improvement (HI) was defined as for CR, but with platelet counts remaining <100×109/L. Other responses were considered as treatment failures. Toxicity was graded using the NCI-CTCAE v3.0 criteria. All patients receiving at least one pemetrexed dose were considered evaluable for toxicity. Prolonged myelosuppression, as defined by the leukemia-specific NCI criteria (marrow cellularity <5% without evidence of leukemia on Day 42 or later [6 weeks] from start of therapy), was considered in defining the MTD and DLT.
Results
Study population
Twenty-two patients were enrolled into the study and 20 received therapy (Table 1). The median age was 50 years (range: 18 to 75 years). Nine patients were female. The diagnosis was AML in 13 patients and ALL in 7. The median number of prior salvage regimens was 2 (range: 1 to 5). Fifteen patients had chromosomal abnormalities. All patients were refractory to their immediate prior therapy.
Maximum tolerated dose and safety
At the specified dose escalations, transient Grade 1 and 2 liver function abnormalities that were not clinically relevant were observed. Other clinically significant AEs are shown in Table 2. Five patients were treated at Dose Level 6 (3,600 mg/m2) (Table 1). Of these, one patient experienced a Grade 3 AST, another patient experienced a DLT of Grade 3 diarrhea that were both judged to be possibly treatment-related, and a third patient experienced a DLT (Grade 4 bilirubin) that was considered definitely treatment-related. Therefore, the MTD and dose recommended for phase II studies in leukemia was 2,700 mg/m2. No patient received more than one dose of pemetrexed.
Table 2.
Adverse events (NCI-CTCAE version 3.0)
| AE, n(%) | Grade | Pemetrexed dose, mg/m2 |
|||||
|---|---|---|---|---|---|---|---|
| 900 | 1,200 | 1,500 | 2,000 | 2,700 | 3,600 | ||
| Febrile neutropenia | 3 | 1 (33.3) | 1 (33.3) | 3 (100) | 1 (33.3) | 1 (33.3) | 1 (20.0) |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Leukopenia | 3 | 1 (33.3) | 0 | 0 | 0 | 0 | 1 (20.0) |
| 4 | 0 | 0 | 0 | 0 | 0 | 1 (20.0) | |
| Neutropenia | 3 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 0 | 0 | 2 (40.0) |
| 4 | 2 (66.7) | 0 | 0 | 0 | 0 | 1 (20.0) | |
| Thrombocytopenia | 3 | 1 (33.3) | 2 (66.7) | 0 | 0 | 2 (66.7) | 0 |
| 4 | 0 | 1 (33.3) | 1 (33.3) | 2 (66.7) | 0 | 4 (80.0) | |
| Anemia | 3 | 0 | 0 (0) | 0 | 0 | 0 | 0 |
| 4 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | |
| ALT, SGPT | 3 | 0 | 1 (33.3) | 1 (33.3) | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AST, SGOT | 3 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (20.0) |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Bilirubin | 3 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (20.0) | |
| Diarrhea | 3 | 0 | 0 | 0 | 0 | 0 | 1 (20.0) |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
AE adverse event, ALT alanine aminotransferase, AST aspartate aminotransferase, n number of events, NCI-CTCAE National Cancer Institute Common Toxicity Criteria Adverse Events Version 3.0, SGOT serum glutamic oxaloacetic transaminase, SGPT serum glutamic pyruvic transaminase
As expected, the predominant Grade 3 and 4 toxicities were hematologic (Table 2). Grade 3 and 4 liver abnormalities affected up to 33% of patients at some dose levels. With the possible exception of Grade 3 diarrhea, there did not appear to be a relationship between pemetrexed dose and frequency of AEs at pemetrexed doses ranging from 900–3,600 mg/m2. No other clinically relevant drug-related toxicities were observed.
There were three discontinuations from this study due to an adverse event or death. One patient discontinued due to progression of an intercurrent illness (frontal subdural hematoma); the relationship of the event to study drug treatment was uncertain. There were two deaths on study. One death was attributed to disease progression, and the second death involved a patient with a history of hypertension, who was neutropenic and thrombocytopenic at the time of study treatment. This patient subsequently developed acute renal failure, pulmonary edema secondary to congestive heart failure, and hepatic toxicity while receiving antibiotics and antifungals for febrile neutropenia. This patient then developed progressive disease after study treatment and died.
Pemetrexed pharmacokinetics (PK)
Samples from 12 patients (85 observations) were available and were included in the analysis.
Exposure to pemetrexed appeared to increase in a linear fashion over the pemetrexed dose range of 900 to 3,600 mg/m2. The PK of pemetrexed 900–3,600 mg/m2 were consistent with those for a reference population administered pemetrexed 600–1,400 mg/m2 [7]. Table 3 shows calculated maximum observed drug concentration (Cmax), area under the concentration-time curve (AUC), total body clearance of drug (CL), and volume of distribution at steady-state (Vss) for each dose of pemetrexed.
Table 3.
Geometric mean (CV%)a pemetrexed pharmacokinetic parameters by dose group
| Dose (mg/m2) | n | Cmax (µg/mL) | AUC (µg×h/mL) | CL (mL/min) | Vss (L) |
|---|---|---|---|---|---|
| 900 | 3 | 160 (5.68) | 460 (19.7) | 64.6 (17.9) | 18.7 (6.34) |
| 1,500 | 2 | 269, 239 | 820, 779 | 66.1, 59.0 | 19.2, 16.8 |
| 2,000 | 2 | 369, 403 | 780, 887 | 88.4, 88.7 | 17.5, 20.2 |
| 2,700 | 3 | 349 (28.4) | 1020 (14.5) | 80.0 (5.98) | 16.0 (5.86) |
| 3,600 | 2 | 388b, 573 | 2580b, 1670 | 35.4b, 69.9 | 16.0b, 18.3 |
AUC area under the concentration-time curve from time zero to infinity, BSA body surface area, CL pemetrexed systemic clearance, Cmax maximum plasma concentration, n number of patients at each dose level, PK pharmacokinetic, Vss volume of distribution at steady-state
Geometric mean (CV%) is reported for dose groups with 3 or more observations
Parameter estimates for Patient 16 are predictions based on BSA (1.49 m2) and CrCLCG,std (54.8 mL/min) and the population PK model
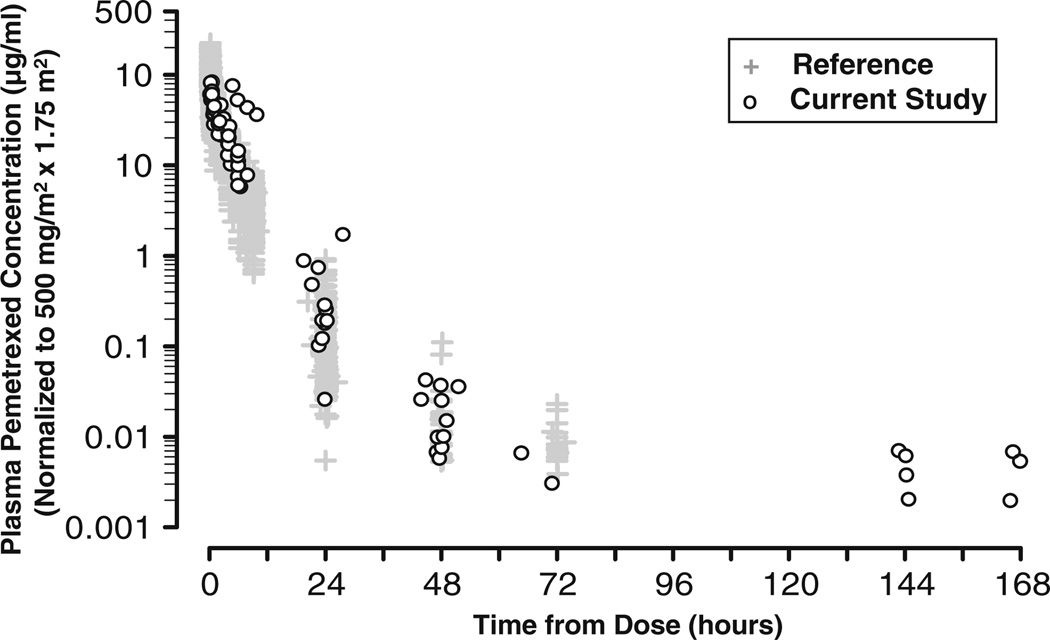
The individual concentration-time profiles, which were normalized to a pemetrexed dose of 500 mg/m2 for a BSA of 1.75 m2, are presented in Fig. 1. The exposure to pemetrexed appeared to increase linearly with increasing dose. One patient (Patient 16) exhibited slightly elevated dose-normalized concentrations relative to other concentrations obtained at times prior to 48 h (data not shown), and was excluded from the primary analysis of PK. The final population PK model was used to calculate individual PK parameters for Patient 16 based on the BSA (1.49 m2) and CrCLCG, std (54.8 mL/min). The concentration-time profiles for all patients followed a similar pattern of multi-exponential decay.
Fig. 1.
Dose-normalized pemetrexed plasma concentration versus time from dose after administration of pemetrexed 900, 1,500, 2,000, 2,700, or 3,600 mg/m2. Twelve patients were included in the PK analysis. Pemetrexed PK evaluations used a 3-compartment model in non-linear mixed effects modeling (NONMEM). Estimates of pemetrexed systemic clearance (CL) and volume of distribution at steadystate (Vss) based on this model for a “typical patient” (mean CrCLCG, std [114 mL/min] and mean BSA [1.92 m2]) are 72 mL/min and 17 L, respectively)
Pharmacodynamics
The primary effect of pemetrexed is inhibition of TS, which causes fluctuations in the nucleotide pools and subsequent changes in DNA synthesis. In addition, inhibition of TS results in the intracellular accumulation of deoxyuridine monophosphate. Subsequent hydrolysis of the phosphate moiety causes deoxyuridine efflux into the circulation. Therefore, plasma deoxyuridine has been regarded as a PD marker for systemic TS inhibition [18]. It was therefore of interest, to examine the effect of pemetrexed dosing on the above parameters in mononuclear cells.
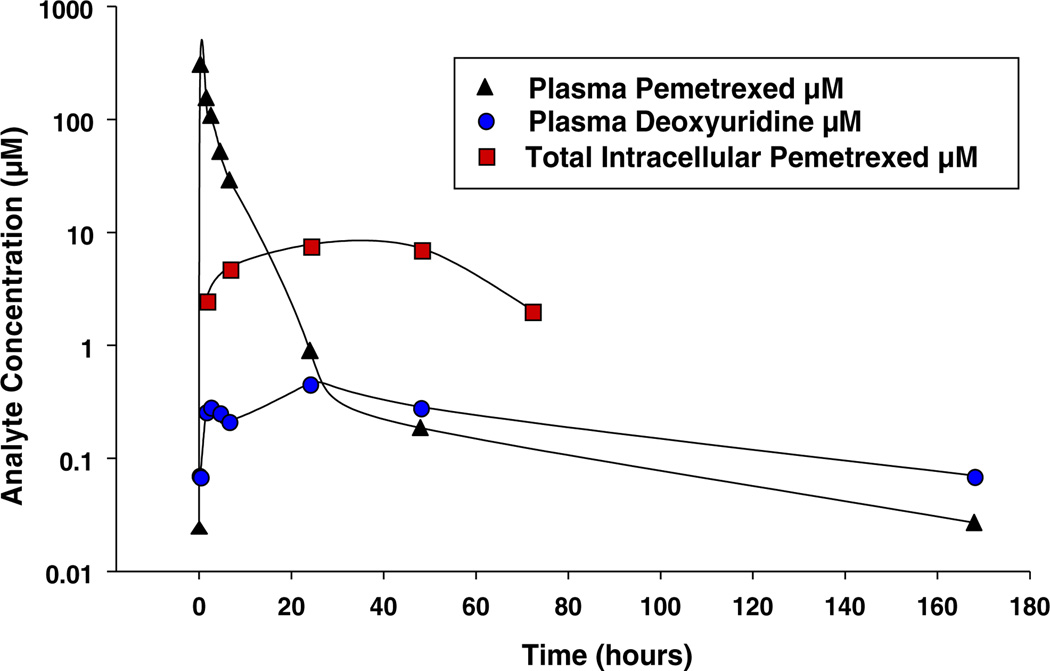
Pharmacodynamic measurements were available for only a subset of patients who were able to provide sufficient and/or suitable samples. Only one patient (patient 100, treated at the 900 mg/m2 dose level) provided sufficient material for both PK and PD measurements. In this patient, plasma pemetrexed peaked rapidly (Fig. 2). The clearance of plasma pemetrexed was followed by accumulation of intracellular pemetrexed, which persisted for approximately 48 h. Additionally, plasma deoxyuridine levels increased transiently, with its elimination lagging slightly behind the clearance of plasma pemetrexed. Patient 100 demonstrated variation in nucleotide pools over this time frame (Fig. 3b).
Fig. 2.
Correlation of pharmacokinetics/pharmacodynamics in Patient 100 (900 mg/m2). One patient was treated with a single dose of pemetrexed and blood was drawn at the indicated time points. Plasma pemetrexed, plasma deoxyuridine, and total intracellular pemetrexed (mononuclear cells) were determined as described in Methods. Shown are analyte concentration (plasma pemetrexed, intracellular pemetrexed, and plasma deoxyuridine) versus time following pemetrexed infusion
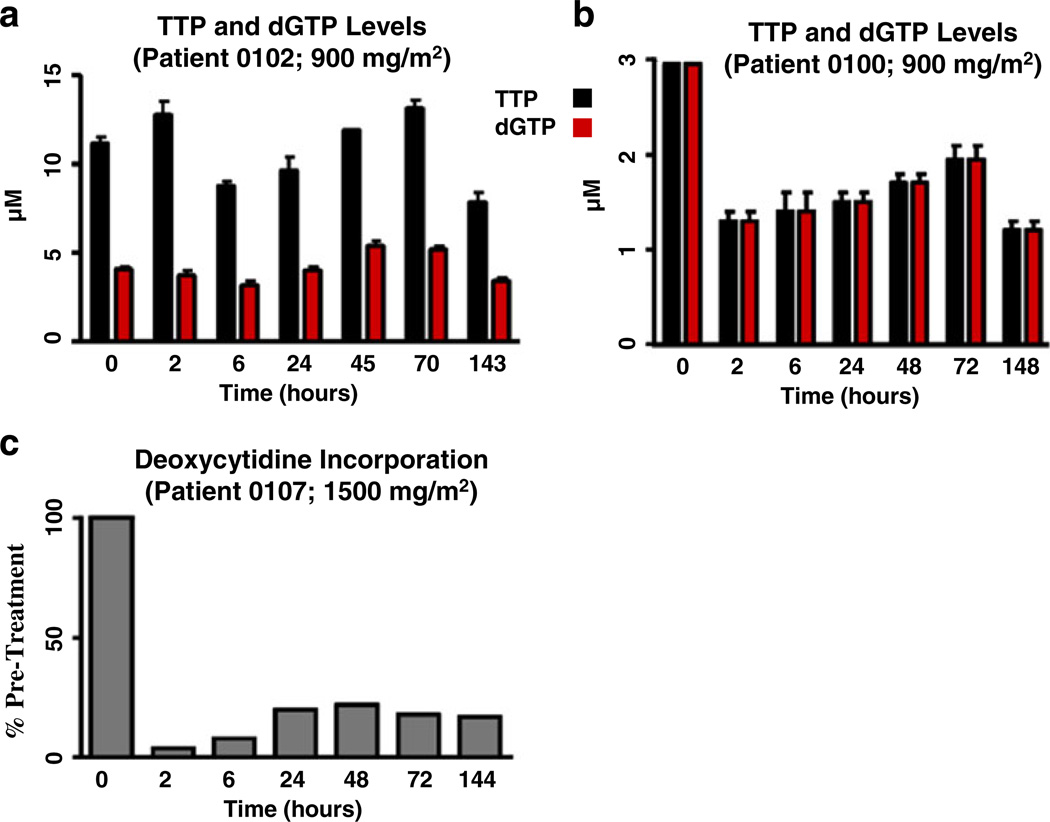
Fig. 3.
Examples of cellular pharmacodynamics data. Three patients were treated with a single dose of pemetrexed (Panels a–b, 900 mg/m2 or Panel c, 1,500 mg/m2) and blood was collected at the indicated time points. Panels a–b, Mononuclear cells were isolated and intracellular TTP and dGTP were extracted and measured as described in Methods. Panel c, Mononuclear cells were isolated and incorporation of radiolabelled deoxycytidine into mononuclear cell DNA was determined as described in Methods. Panels a–b, Shown are TTP and dGTP concentrations (uM) versus time following pemetrexed infusion; Panel c, Shown is the change of the amount of 3H-deoxycytidine taken up by mononuclear cells following pemetrexed administration expressed as percent of pretreatment value
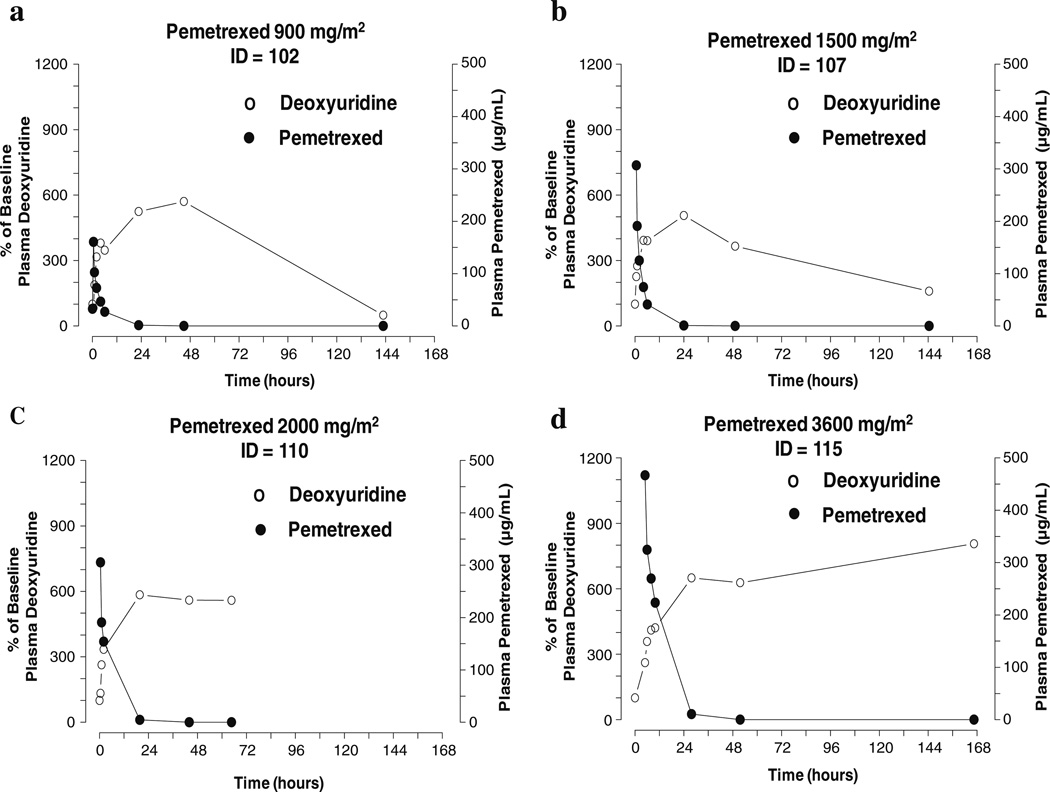
Changes in thymidine triphosphate (TTP) and deoxyguanosine triphosphate (dGTP) pools were variable among patients (Fig. 3a and b). Because of the limited PD data, it was not possible to make definitive correlations between pemetrexed plasma levels and subsequent PD effects on mononuclear cells. The effect of pemetrexed on DNA synthesis was evaluated in one patient treated at the 1,500 mg/m2 dose level. In this patient, inhibition of deoxycytidine incorporation into cellular DNA was observed (Fig. 3c). In five patients receiving pemetrexed doses ranging from 900–3,600 mg/m2, the administration of a single dose of pemetrexed was associated with an elevation of plasma deoxyuridine peaking between 24 and 48 h (Figs. 2 and 4). Peak (not shown) and relative deoxyuridine levels were similar among patients regardless of pemetrexed dose, and the peak occurred within 24 to 48 h (Fig. 4).
Fig. 4.
Pemetrexed pharmacodynamics analysis (representative samples). Four patients were treated with a single dose of pemetrexed (Panel a, 900 mg/m2; Panel b, 1,500 mg/m2; Panel c, 2,000 mg/m2; Panel d, 3,600 mg/m2) and blood was drawn at the indicated time points. Plasma pemetrexed and plasma deoxyuridine concentrations were determined as described in Methods. Shown is percent of baseline plasma deoxyuridine (left Y-axis) and plasma pemetrexed (right Y-axis) versus time following pemetrexed infusion
Response
Among the 20 patients who were evaluable for response, there was one PR in a patient with ALL receiving pemetrexed 3,600 mg/m2. This patient achieved a reduction of marrow blasts from 25% to 2% but without recovery of platelets. No other significant reductions of marrow blasts were noted. Three patients had >90% reduction of peripheral blasts.
Discussion
This phase I study of pemetrexed in relapsed/refractory leukemia established a dose of 2,700 mg/m2 IV over 1 h every 3 weeks with folic acid and vitamin B12 as the MTD and recommended phase II dose. At dose level 6 (3,600 mg/m2), two patients experienced liver dysfunction, which manifested as hyperbilirubinemia and elevated transaminases. Also at dose level 6, a patient who was receiving multiple antimicrobial agents experienced Grade 3 diarrhea, which was considered possibly related to study treatment. There were no DLTs at 2,000 mg/m2 and 2,700 mg/m2, but one patient experienced Grade 3 liver dysfunction that was possibly treatment-related at dose level 2 (1,200 mg/m2). Otherwise, as expected, myelosuppression was a common Grade 3 or 4 toxicity. Thus, pemetrexed was well tolerated at its MTD in this patient population.
The MTD in patients with relapsed/refractory leukemia is 5 times higher than the approved dose of pemetrexed 500 mg/m2 every 3 weeks in solid tumors. In 2007, Takimoto et al reported that the MTD of pemetrexed 600–1,400 mg/m2 with vitamin supplementation in adult patients (age range: 18–80 years) with locally advanced or metastatic solid tumors was 800 mg/m2 in heavily pretreated patients and 1,050 mg/m2 in lightly pretreated patients [7]. In Takimoto’s study, DLTs were mostly hematologic with only 3.6% to 5% of heavily pretreated patients experiencing Grade 3 hyperbilirubinemia and 0% to 4.4% of lightly pretreated patients experiencing Grade 3 hyperbilirubinemia; likewise, 2.9% to 8.7% of lightly pretreated patients experienced Grade 3 elevated transaminases and 0% of heavily pretreated patients experienced elevated transaminases. In children aged 1–21 years with refractory solid tumors, the MTD of pemetrexed 400–2,480 mg/m2 with vitamin supplementation was 1,910 mg/m2, and the primary DLTs were neutropenia and rash [19]. In this trial, only one patient experienced Grade 3 elevation of ALT/AST at 1,470 mg/m2 pemetrexed; the remainder of liver abnormalities were Grade 1 or Grade 2. Thus, the MTD of 2,700 mg/m2 reported here in patients with leukemia is the highest tolerable dose of pemetrexed reported to date. With consideration of the disease state differences in hematologic toxicities, these patients with leukemia were able to tolerate higher doses of pemetrexed than those with solid tumors.
The PK behavior of pemetrexed revealed that exposure increased linearly over a dose range of 900 to 3,600 mg/m2. Pharmacodynamic measurements confirmed the inhibition of TS in this study involving patients with leukemia. However, such systemic TS inhibition did not always translate into cellular PD response (see below). The data described herein (Figs. 2 and 4) for patients with leukemia show plasma deoxyuridine peaked at 24–48 h following a single dose of pemetrexed 900–3,600 mg/m2, and gradually returned to baseline over a period of several days for some patients and longer for others. This suggests effective and persistent systemic inhibition of TS for at least 48 h following an infusion of pemetrexed. However, this marker is not specific for tumor cells, and may not reflect intratumoral dose-response. A complete set of studies could not be obtained for all patients. However, at the 900 mg/m2 dose level, studies in patient 100 showed an interesting PK-PD correlation. For this patient, plasma pemetrexed clearance was followed first by transient intracellular pemetrexed accumulation, which persisted for about 48 h, then subsequent modulation in the deoxyuridine profile (Fig. 2) coupled with reduction of TTP and dGTP pools (Fig. 3), which is in accord with preclinical data [20]. Furthermore, the incorporation of deoxycytidine into mononuclear cell DNA was inhibited in another patient studied at the 1,500 mg/m2 dose level (Fig. 3).
The peak, and relative elevation of deoxyuridine expressed as a percentage of baseline, was not correlated to pemetrexed dose, suggesting that TS inhibition may be saturated perhaps even at the lowest pemetrexed dose employed in this study. This may be relevant to the experience in solid tumor trials, where no consistent efficacy advantage has been observed with pemetrexed doses higher than 500 mg/m2 [11, 21]. It would be of value to know whether the CNS or other sanctuary sites require higher pemetrexed dosing to replicate these findings in those tissues.
Although the PK and PD data from this study suggest that pemetrexed at doses of 900–3,600 mg/m2 inhibits TS in patients with acute leukemia, pemetrexed demonstrated limited activity in this cohort of heavily pretreated patients. In fact, the observed cellular PD responses in two patients (Patient 100 and Patient 107) did not translate into any observable clinical response. It is possible that prolonged inhibition of TS beyond several days is required to induce death of the blasts. Alternatively, it is likely that leukemia cells from these patients contain a combination of genetic and biochemical abnormalities that enable the blast cells to circumvent the toxic effects of pemetrexed, such that single agent pemetrexed, despite inhibition of several components of the folic acid pathway, had limited clinical activity in these heavily pretreated patients. Based on the known biology of tumor cells, especially those originating from patients with relapsed/refractory disease, it is likely that multiple cellular pathways need to be targeted to maximize tumor cell death with concomitant clinical efficacy. Variability in nucleotide pools observed in circulating blasts suggests that among patients with leukemia, some have different nucleotide or folic acid metabolic patterns, and therefore varying susceptibility to antimetabolite agents. Thus, in patients with refractory leukemia, the development of pemetrexed in combination with other complementary agents is a reasonable future endeavor.
Acknowledgments
This study was funded by a grant from Lilly USA, LLC, Indianapolis, IN, USA. The authors wish to acknowledge the patients and study personnel who participated in this clinical trial. The authors would like to acknowledge the expert assistance of Guochang George Zhu, MD (Eli Lilly and Company), who facilitated many aspects of protocol implementation and support. The authors wish to thank Lori Kornberg, PhD (i3 Statprobe, funded by Lilly USA, LLC) for assistance in the technical writing of this manuscript.
Contributor Information
Isam Abdel-Karim, San Antonio Cancer Center, San Antonio, TX, USA.
William K. Plunkett, Jr, Department of Leukemia, M.D. Anderson Cancer Center, The University of Texas, Box 428, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Susan O’Brien, Department of Leukemia, M.D. Anderson Cancer Center, The University of Texas, Box 428, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Francis Giles, San Antonio Cancer Center, San Antonio, TX, USA.
Deborah Thomas, Department of Leukemia, M.D. Anderson Cancer Center, The University of Texas, Box 428, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Stefan Faderl, Department of Leukemia, M.D. Anderson Cancer Center, The University of Texas, Box 428, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Farhad Ravandi, Department of Leukemia, M.D. Anderson Cancer Center, The University of Texas, Box 428, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Mary Beth Rios, Department of Leukemia, M.D. Anderson Cancer Center, The University of Texas, Box 428, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Min Du, Department of Leukemia, M.D. Anderson Cancer Center, The University of Texas, Box 428, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Karen B. Schneck, Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA
Victor J. Chen, Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA
Boris K. Lin, Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA
Steven J. Nicol, Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA
Hagop M. Kantarjian, Email: hkantarj@mdanderson.org, Department of Leukemia, M.D. Anderson Cancer Center, The University of Texas, Box 428, 1515 Holcombe Blvd, Houston, TX 77030, USA.
References
- 1.American Cancer Society. Cancer Facts and Figures 2008. [Accessed 1 Sep 2009];American Cancer Society. 2008 Available at: http://www.cancer.org.
- 2.Kantarjian HM. Therapy for elderly patients with acute myeloid leukemia: a problem in search of solutions. Cancer. 2007;109:1007–1010. doi: 10.1002/cncr.22502. [DOI] [PubMed] [Google Scholar]
- 3.Adjei AA. Pemetrexed: a multitargeted antifolate agent with promising activity in solid tumors. Ann Oncol. 2000;11:1335–1341. doi: 10.1023/a:1008379101017. [DOI] [PubMed] [Google Scholar]
- 4.Grem J. Fluorinated pyrimidines. In: Chabner BA, Collins JM, editors. Cancer chemotherapy: principles and practice. Philadelphia: Lippincott; 1990. pp. 180–224. [Google Scholar]
- 5.Schilsky RL. Antimetobolites. In: Perry MC, editor. The chemotherapy source book. Baltimore: Williams and Wilkins; 1992. pp. 301–315. [Google Scholar]
- 6.Alimta Prescribing Information. Indianapolis, IN: Eli Lilly and Company; 2009. [Accessed 1 Sep 2009]. Available at: http://pi.lilly.com/us/alimta-pi.pdf. [Google Scholar]
- 7.Takimoto CH, Hammond-Thelin LA, Latz JE, Forero L, et al. Phase I and pharmacokinetic study of pemetrexed with high-dose folic acid supplementation or multivitamin supplementation in patients with locally advanced or metastatic cancer. Clin Cancer Res. 2007;13:2675–2683. doi: 10.1158/1078-0432.CCR-06-2393. [DOI] [PubMed] [Google Scholar]
- 8.Niyikiza C, Baker SD, Seitz DE, Walling JM, et al. Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther. 2002;1:545–552. [PubMed] [Google Scholar]
- 9.Hanauske AR, Chen V, Paoletti P, Niyikiza C. Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist. 2001;6:363–373. doi: 10.1634/theoncologist.6-4-363. [DOI] [PubMed] [Google Scholar]
- 10.Miller DS, Blessing JA, Krasner CN, Mannel RJ, et al. A phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. J Clin Oncol. 2009;27:2686–2691. doi: 10.1200/JCO.2008.19.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen MH, Zatloukal P, Sorenson S, Novello S, et al. A randomized phase III trial comparing standard and high-dose pemetrexed as second-line treatment in patients with locally advanced or metastatic non-small-cell lung cancer. Ann Oncol. 2008;19:939–945. doi: 10.1093/annonc/mdm592. [DOI] [PubMed] [Google Scholar]
- 12.Latz JE, Chaudhary A, Ghosh A, Johnson RD. Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol. 2006;57:401–411. doi: 10.1007/s00280-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 13.Beal SL, Sheiner LB. NONMEM User's Guide. NONMEM Project Group. San Francisco, CA: University of California at San Francisco; 1992. [Google Scholar]
- 14.Plunkett W, Hug V, Keating MJ, Chubb S. Quantitation of 1-beta-D-arabinofuranosylcytosine 5'-triphosphate in the leukemic cells from bone marrow and peripheral blood of patients receiving 1-beta-D-arabinofuranosylcytosine therapy. Cancer Res. 1980;40:588–591. [PubMed] [Google Scholar]
- 15.Gandhi V, Plunkett W, Du M, Ayres M, Estey EH. Prolonged infusion of gemcitabine: clinical and pharmacodynamic studies during a phase I trial in relapsed acute myelogenous leukemia. J Clin Oncol. 2002;20:665–673. doi: 10.1200/JCO.2002.20.3.665. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez CO, Jr, Plunkett W, Paff MT, Du M, et al. High-performance liquid chromatography method for the determination and quantitation of arabinosylguanine triphosphate and fludarabine triphosphate in human cells. J Chromatogr B Biomed Sci Appl. 2000;745:421–430. doi: 10.1016/s0378-4347(00)00303-0. [DOI] [PubMed] [Google Scholar]
- 17.Sherman PA, Fyfe JA. Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers. Anal Biochem. 1989;180:222–226. doi: 10.1016/0003-2697(89)90420-x. [DOI] [PubMed] [Google Scholar]
- 18.Li KM, Rivory LP, Clarke SJ. Pemetrexed pharmacokinetics and pharmacodynamics in a phase I/II study of doublet chemotherapy with vinorelbine: implications for further optimisation of pemetrexed schedules. Br J Cancer. 2007;97:1071–1076. doi: 10.1038/sj.bjc.6603995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malempati S, Nicholson HS, Reid JM, Blaney SM, et al. Phase I trial and pharmacokinetic study of pemetrexed in children with refractory solid tumors: the Children's Oncology Group. J Clin Oncol. 2007;25:1505–1511. doi: 10.1200/JCO.2006.09.1694. [DOI] [PubMed] [Google Scholar]
- 20.Chen VJ, Bewley JR, Andis SL, et al. Preclinical cellular pharmacology of LY231514 (MTA): a comparison with methotrexate, LY309887 and raltitrexed for their effects on intracellular folate and nucleoside triphosphate pools in CCRF-CEM cells. Br J Cancer. 1998;78(Suppl 3):27–34. doi: 10.1038/bjc.1998.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohe Y, Ichinose Y, Nakagawa K, Tamura T, et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin Cancer Res. 2008;14:4206–4212. doi: 10.1158/1078-0432.CCR-07-5143. [DOI] [PubMed] [Google Scholar]