Abstract
Covalent attachment of a phenolic antioxidant analogue of α-tocopherol to graphite-coated magnetic cobalt nanoparticles (CoNPs) provided a novel magnetically responsive antioxidant capable of preventing the autoxidation of organic materials and showing a reduced toxicity toward human cells.
Keywords: antioxidants, cobalt, cytotoxicity, nanoparticles, tocopherol
Antioxidants are actively investigated not only for technological purposes (i.e., for the stabilization of plastic, oils, and food)[1a–c] but also because they can modulate the redox balance inside the cells, and can thus influence some important biological processes, such as oxidative damage and cell death in a wide range of pathologies.[2a,b] Engineered nanoparticles (NPs) have recently emerged as an innovative and little explored method to obtain novel antioxidants with enhanced characteristics. For instance, bio-degradable NPs have been used to improve the bioavailability of natural antioxidants (such as curcumin),[3a–c] and a covalent link between SiO2–NPs and gallic acid was proposed for reducing its leaching and volatility.[4] Cerium oxide NPs (nanoceria) have been shown to be powerful antioxidants in biological systems acting as superoxide dismutase mimics.[5] Additionally, the generation of free radicals and the induction of oxidative stress have been invoked to rationalize the toxicity of many types of NPs in biological systems.[6a–f] This is a particularly serious limitation to medical applications of NPs, such as targeted drug delivery or contrast agents, that require to avoid the use of intrinsically cytotoxic materials.[7a–b] Linking antioxidants to NPs may therefore represent a new strategy to reduce the toxicity of NPs.
Herein, we report the synthesis, the study of the chain-breaking antioxidant activity and the evaluation of the toxicity in human cells of graphite-coated cobalt magnetic NPs (CoNPs)[8] covalently linked to a phenolic vitamin E analogue antioxidant. These CoNPs have been recently proposed for many applications including catalysis,[9] water purification,[10] and in vivo blood detoxification,[11] and represent a promising scaffold to obtain novel “magnetic antioxidants”. Our results enlighten an unexpected role of NPs in increasing the radical scavenging, and suggest a possible role of the pendant vitamin E analogue in the reduction of the cytotoxicity of CoNPs.
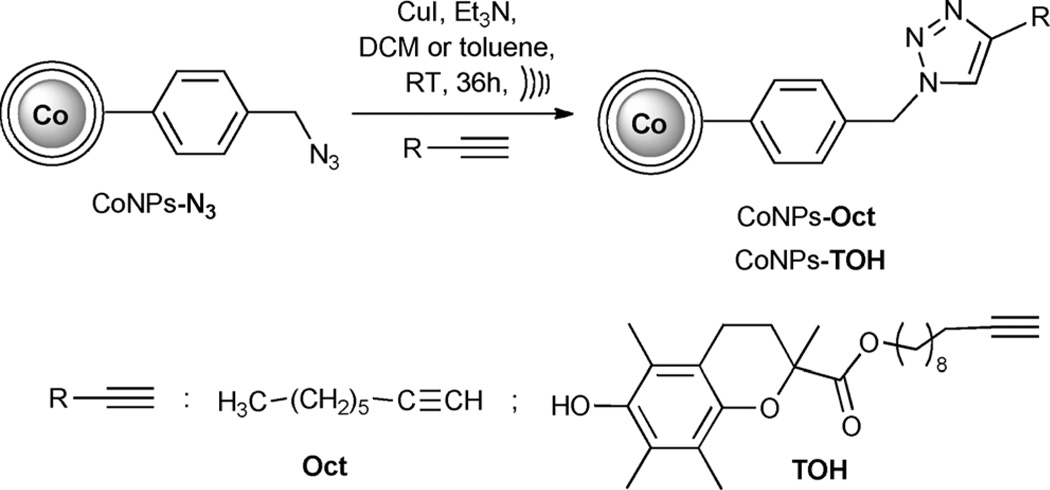
Magnetic CoNPs functionalized with azido moieties (CoNPs–N3), were purchased from TurboBeads®, Switzerland. The nanoparticles have a metallic cobalt core, with a diameter of about 30 nm, coated by approximately three layers of graphitic carbon that render them air stable.[8] In CoNPs–N3, the concentration of azido functional groups is about 0.1 mmolg−1.[9] From the density of the CoNPs and their mean radius,[8] we could calculate that about 6000 -N3 groups are attached to each CoNP. Preliminary experiments showed that these CoNPs (either with or without the azido functionalization) were inert toward the reaction with tert-butylhydroperoxide, a model for the hydroperoxides often present in organic materials (see the Supporting Information). The graphite layers efficiently isolate the metallic core, avoiding the pro-oxidant activity arising from homolytic decomposition of hydroperoxides, commonly observed for magnetic NPs.[12] For use in our studies the CoNPs–N3 were covalently linked to selected alkynes using an azide–alkyne cycloaddition catalyzed by CuI (CuAAC) under reaction conditions previously reported for similar multi functionalized systems (Scheme 1).[9]
Scheme 1.
Synthesis of functionalized CoNPs–Oct and CoNPs–-TOH. Only one pendant is shown.
Two particles were generated for our study. A control particle (CoNPs–Oct) was obtained through reaction of CoNPs–N3 with 1-octyne (Oct). The terminal acetylenic antioxidant portion (TOH) was obtained by condensing undec-10-yn-1-ol with Trolox, a phenolic antioxidant analogue of α-tocopherol (the main and more active component of vitamin E).[1] The resulting alkyne TOH was then reacted with CoNPs–N3 in toluene under ultrasound irradiation (that was a safe operation for TOH pendants, see the Supporting Information for details) as depicted in Scheme 1. The conjugated CoNPs–Oct and CoNPs–TOH were isolated by magnetic separation and repeatedly washed with fresh solvent and a NH3/EtOH solution to completely remove residual reagents and/or catalysts possibly dispersed in the NPs surface coating.[13] The functionalization of CoNPs–N3 could be easily detected by FT-IR by monitoring the disappearance of the N3 stretching peak at 2090 cm−1.[9] The concentration of the TOH moieties in CoNPs–TOH, considering the weight increase after functionalization, is 0.096 mmolg−1 (details of the preparation of the coupling reagent, of the coupling procedure as well as the purification of conjugated CoNPs are reported in the Supporting Information). Dried CoNPs–TOH could be stored in a closed vessel at room temperature in the dark for several months without any loss of activity.
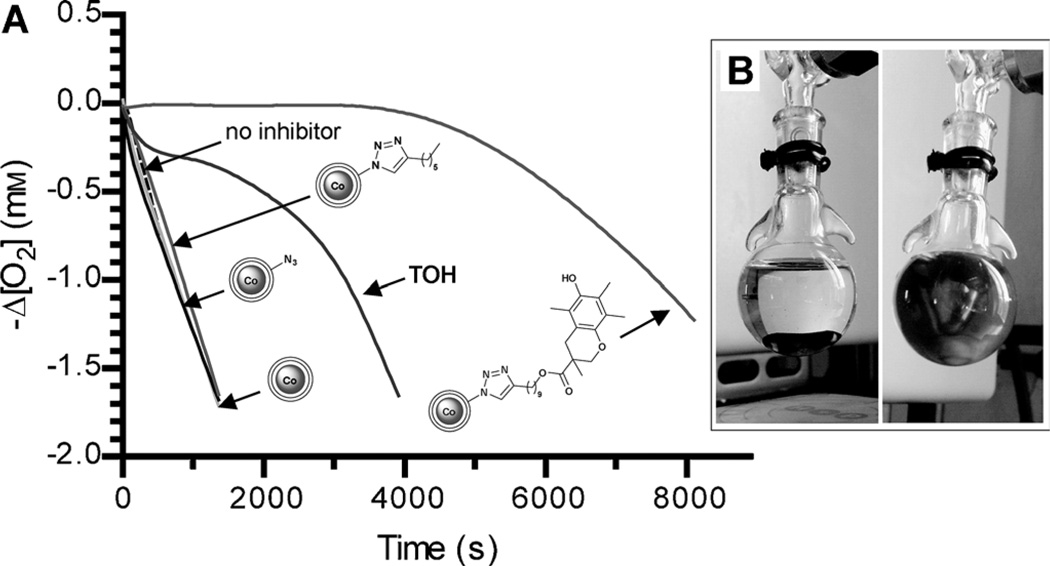
As a first test of the radical-trapping ability of CoNPs–TOH, we studied the reaction with the stable purple colored dpphC (diphenylpycrylhydrazyl) radical.[4] Although the reactivity with dpph˙ does not necessarily guarantee antioxidant activity,[14] this method is indeed valuable for screening purposes and, as reported in the Supporting Information, it showed clear evidence of the ability of CoNPs–TOH to quench dpph˙ radicals. Thus we moved on determining the antioxidant activity by measuring the reaction of functionalized CoNPs with alkylperoxyl radicals (ROO˙), which are the radicals responsible for the chain propagation in the autoxidation of organic compounds.[1a,c] The activity of the particles was determined through inhibition of the autoxidation of styrene.[15a–c] The reaction was initiated by azobis(isobutyronitrile) (AIBN) at 30°C in PhCN and was followed by measuring the oxygen consumption with an automatic gas uptake recording apparatus (see Figure 1).
Figure 1.
A) Oxygen consumption during the autoxidation of styrene (4.3 m) in PhCN initiated by AIBN (25 mm) at 30°C without inhibitor, 3.8 µm of TOH or with 0.15 mg mL−1 of CoNPs, CoNPs–N3, CoNPs–Oct and CoNPs–TOH (corresponding to a concentration of TOH groups of 14.4 µm) ; Ri = 3.6 × 10−9 ms−1. B) Photograph of the reaction vessels containing the NPs in the absence (left) and in the presence (right) of magnetic stirring.
The rotation of a magnetic stir bar was sufficient to keep CoNPs suspended in solution, as shown in Figure 1 B. From the O2 consumption rate, measured in the presence of the antioxidants, the rate constants for the reaction with ROO˙ radicals (kROO) reported in Table 1 could be measured (see the Supporting Information for details).[15a–c] The length of the inhibited period (τ) provided the number of radicals trapped by each TOH (n) by the equation n = Ri τ/[TOH], where Ri is the rate of radical production by AIBN. In the case of CoNPs–TOH, the molar concentration of TOH moieties could be obtained from the amount (w/v) of nanoparticles in the sample and the loading of TOH moieties on the nanoparticles. In these experiments, CoNPs, CoNPs–N3, and CoNPs–Oct did not show any antioxidant effect, whereas TOH and CoNPs–TOH inhibited styrene autoxidation, due to the highly reactive α-tocopherol-like moiety.[15a,b] CoNPs–TOH showed an inhibition period followed by a weaker antioxidant effect (see Figure 1) and had a n value smaller than TOH, presumably because in the heterogeneous system not all pendant antioxidants have a similar exposure to the solution.
Table 1.
Rate constants for the reaction with peroxyl radicals (kROO) in PhCN, at 30 °C, and number of radicals trapped by each phenolic moiety (n) determined from styrene autoxidation studies.
| kROO [104 m−1 s−1] | n | |
|---|---|---|
| CoNPs | <0.1 | – |
| CoNPs–N3 | <0.1 | – |
| CoNPs–Oct | <0.1 | – |
| CoNPs–TOH | 560±150[a] | 1.1±0.1[a] |
| TOH | 64±10 | 2.2±0.2 |
Data referred to the first inhibition period, see text.
Remarkably, CoNPs–TOH showed a much larger kROO than TOH itself (see Table 1), suggesting that CoNPs play a unique role in promoting the reaction with alkylperoxyl radicals. Interestingly, a larger reactivity of antioxidants linked to NPs, with respect to those free in solution, has been previously noticed also in the case of gold nanoparticles functionalized with Trolox[16a] or salvianic acid.[16b] It has been speculated that these effects may be due to preconcentration of the radicals near the reactive OH moieties,[16a] or to π–π stacking between the phenolic aromatic rings.[16a,b] In our system however, we cannot exclude the possibility that the increased reactivity of antioxidants linked to NPs is due to the catalytic effect of the basic triazole group in proximity to the TOH moiety,[17a,b] or to a synergistic effect of the graphite surface that, like fullerenes, nanotubes or graphene,[18a–c] could act as a “sponge” of free radicals which would be subsequently quenched by the nearby pendant antioxidants.[19]
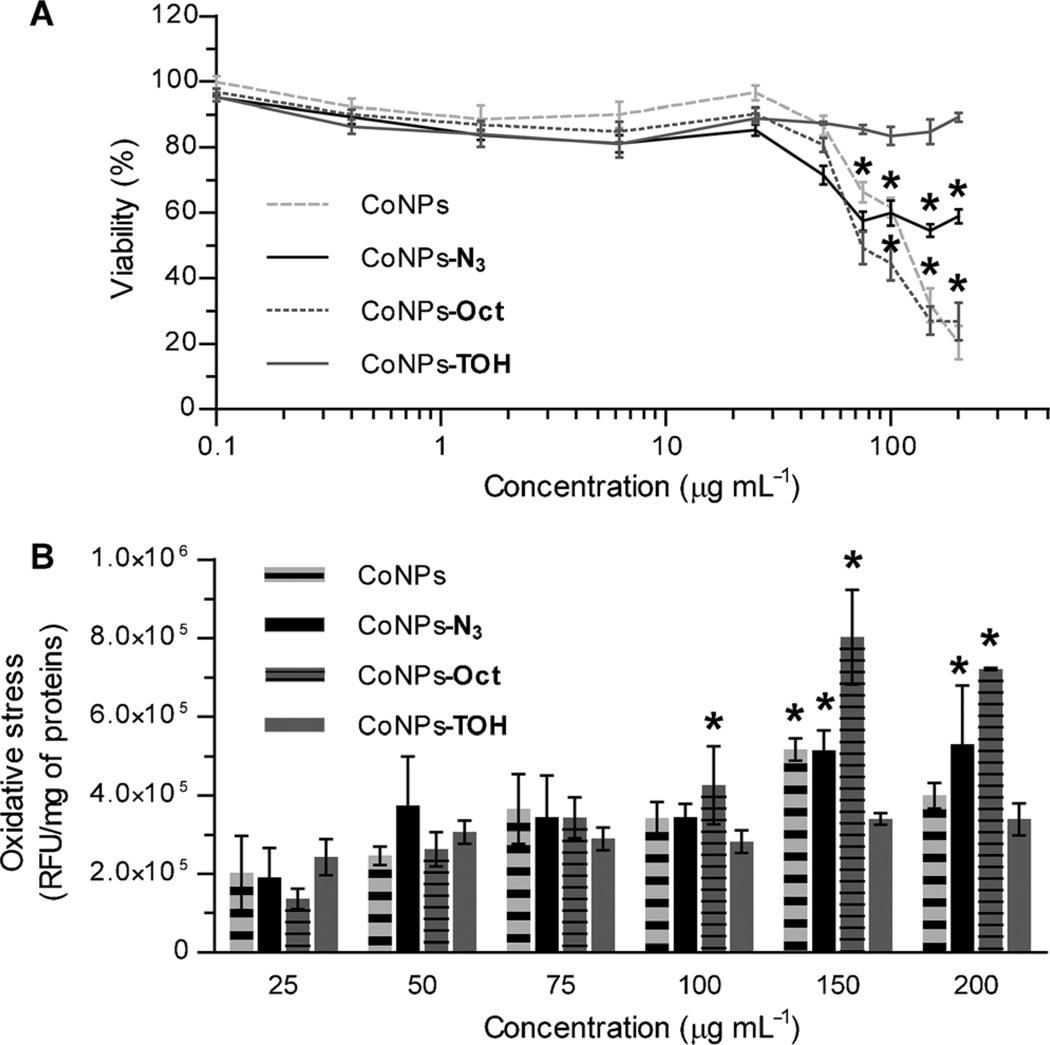
A preliminary indication of the effect of surface-bound antioxidants on the biological effects of selected CoNPs at high exposure concentrations was determined by cytotoxicity and oxidative stress induction studies on human cancer cell lines (HeLa, from uterine cervical cancer, and MG63, from bone osteosarcoma). The cytotoxicity of CoNPs was evaluated by measuring the ability of the cells to convert non-fluorescent resazurin to red-fluorescent resurofin (AlamarBlue test), while the oxidative stress levels were assessed by measuring the intracellular oxidation of the fluorogenic 2′,7′-dichlorofluoroscein diacetate (DCFH-DA) to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) upon reaction with reactive oxygen species (ROS).[20] Figure 2A shows that the viability of HeLa cells, measured after 24 h of incubation with suspensions of CoNPs, drastically decreased by increasing the concentration of NPs beyond 75 µg mL−1. Significantly, CoNPs–TOH showed almost no toxicity (p <0.05 vs. all), while either CoNPs and CoNPs–Oct showed the higher cytotoxicity. Several mechanisms have been proposed, but still debated, to possibly explain the reasons behind these adverse cytotoxic effects,[6a–f] certainly the particles hydrophobicity and the induction of oxidative stress must be taken into account.[21] Indeed, the intracellular oxidative stress levels in HeLa cells steadily increased with the NPs concentration, particularly in the case of highly cytotoxic CoNPs–Oct (Figure 2 B), while CoNPs–TOH displayed the smallest oxidative stress among the graphite CoNPs tested. When the same measures were repeated on MG63 cells, we observed a very small induction of radical stress and mild toxicity for all the NPs tested (data shown in the Supporting Information). Taken together, these results are in good agreement with literature data[6c–f, 22] and indicate that the CoNPs induced production of ROS may be responsible for their adverse toxic effects, although with a large variability among different cell lines, and suggest that a pending antioxidant is able to reduce both oxidative stress and cytotoxicity.
Figure 2.
A) Viability and B) intracellular oxidative stress in HeLa cells after 24 h of incubation with increasing amount of NPs. Results are expressed as mean ± SD. * p <0.05 vs. CoNPs–TOH for each given concentration).
In conclusion, magnetic CoNPs–TOH synthesized in the present work showed an outstanding antioxidant activity, having a reactivity toward peroxyl radicals nine times larger than that of TOH free in solution. Additionally, CoNPs–TOH have the smallest cytotoxicity and oxidative stress induction among all the CoNPs tested independently by their functionalization. Nevertheless, the effects of altered surface and potentially altered uptake and intracellular behavior require further (separate) investigations. Our results suggest that the attachment of antioxidant moieties on the surface of NPs represents a promising operation to obtain novel effective antioxidants and an expedient to reduce the cellular toxicity of nanodevices.
Acknowledgements
We acknowledge PRIN 2010PFLRJR (PROxi) and NIH EB014277 grants.
References
- 1.a) Burton GW, Ingold KU. Acc. Chem. Res. 1986;19:194–201. [Google Scholar]; b) Boragno L, Stagnaro P, Losio S, Sacchi MC, Menichetti S, Viglianisi C, Piergiovanni L, Limbo S. J. Appl. Polym. Sci. 2012;124:3912–3920. [Google Scholar]; c) Pratt DA, DiLabio GA, Brigati G, Pedulli GF, Valgimigli L. J. Am. Chem. Soc. 2001;123:4625–4626. doi: 10.1021/ja005679l. [DOI] [PubMed] [Google Scholar]
- 2.a) Murphy MP. Free Radical Biol. Med. 2014;66:20–23. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]; b) Sheu S-S, Nauduri D, Anders MW. Biochim. Biophys. Acta Mol. Basis Dis. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.a) Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y, Wang H, Zhou Q, Yu S. J. Agric. Food Chem. 2011;59:9280–9289. doi: 10.1021/jf202135j. [DOI] [PubMed] [Google Scholar]; b) Astete CE, Dolliver D, Whaley M, Khachatryan L, Sabliov CM. ACS nano. 2011;5:9313–9325. doi: 10.1021/nn102845t. [DOI] [PubMed] [Google Scholar]; c) Hu B, Ting Y, Yang X, Tang W, Zeng X, Huang Q. Chem. Commun. 2012;48:2421–2423. doi: 10.1039/c2cc17295j. [DOI] [PubMed] [Google Scholar]
- 4.Deligiannakis Y, Sotiriou GA, Pratsinis SE. ACS Appl. Mater. Interfaces. 2012;4:6609–6617. doi: 10.1021/am301751s. [DOI] [PubMed] [Google Scholar]
- 5.Karakoti AS, Singh S, Kumar A, Malinska M, Kuchibhatla SVNT, Wozniak K, Self WT, Seal S. J. Am. Chem. Soc. 2009;131:14144–14145. doi: 10.1021/ja9051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Nel A, Xia T, Madler L, Li N. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]; b) Liu G, Gao J, Ai H, Chen X. Small. 2013;9:1533–1545. doi: 10.1002/smll.201201531. [DOI] [PubMed] [Google Scholar]; c) Guildford AL, Poletti T, Osbourne LH, Di Cerbo A, Gatti AM, Santin M. J. R. Soc. Interface. 2009;6:1213–1221. doi: 10.1098/rsif.2009.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Papis E, Rossi F, Raspanti M, Dalle-Donne I, Colombo G, Milzani A, Bernardini G, Gornati R. Toxicol. Lett. 2009;189:253–259. doi: 10.1016/j.toxlet.2009.06.851. [DOI] [PubMed] [Google Scholar]; e) Sekhon BS, Kamboj SR. Nanomed. Nanotechnol. 2010;6:612–618. doi: 10.1016/j.nano.2010.04.003. [DOI] [PubMed] [Google Scholar]; f) Jiang H, Liu F, Yang H, Li Y. Biol. Trace Elem. Res. 2012;146:23–29. doi: 10.1007/s12011-011-9221-8. [DOI] [PubMed] [Google Scholar]
- 7.a) Kim CK, Ghosh P, Pagliuca C, Zhu Z-J, Menichetti S, Rotello VM. J. Am. Chem. Soc. 2009;131:1360–1361. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim ST, Saha K, Kim C, Rotello VM. Acc. Chem. Res. 2013;46:681–691. doi: 10.1021/ar3000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grass RN, Athanassiou EA, Stark WJ. Angew. Chem. 2007;119:4996–4999. doi: 10.1002/anie.200700613. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:4909–4912. doi: 10.1002/anie.200700613. [DOI] [PubMed] [Google Scholar]
- 9.Schätz A, Grass RN, Stark WJ, Reiser O. Chem. Eur. J. 2008;14:8262–8266. doi: 10.1002/chem.200801001. [DOI] [PubMed] [Google Scholar]
- 10.Koehler FM, Rossier M, Waelle M, Athanassiou EK, Limbach LK, Grass RN, Gunther D, Stark WJ. Chem. Commun. 2009;4862:4864. doi: 10.1039/b909447d. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann IK, Schlegel A, Graf R, Schumacher CM, Senn N, Hasler M, Gschwind S, Hirt A-M, Gunther D, Clavien P-A, Stark WJ, Beck-Schimmer B. Nanoscale. 2013;5:8718–8723. doi: 10.1039/c3nr02468g. [DOI] [PubMed] [Google Scholar]
- 12.Voinov MA, Pagan JOS, Morrison E, Smirnova TI, Smirnov AI. J. Am. Chem. Soc. 2011;133:35–41. doi: 10.1021/ja104683w. [DOI] [PubMed] [Google Scholar]
- 13.Ornelas C, Aranzaes JR, Cloutet E, Alves S, Astruc D. Angew. Chem. 2007;119:890–895. doi: 10.1002/anie.200602858. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:872–877. doi: 10.1002/anie.200602858. [DOI] [PubMed] [Google Scholar]
- 14.Amorati R, Foti MC, Valgimigli L. J. Agric. Food Chem. 2013;61:10835–10847. doi: 10.1021/jf403496k. [DOI] [PubMed] [Google Scholar]
- 15.a) Burton GW, Ingold KU. J. Am. Chem. Soc. 1981;103:6472–6477. [Google Scholar]; b) Menichetti S, Amorati R, Bartolozzi MG, Pedulli GF, Salvini A, Viglianisi C. Eur. J. Org. Chem. 2010;2218:2225. [Google Scholar]; c) Amorati R, Menichetti S, Mileo E, Pedulli GF, Viglianisi C. Chem. Eur. J. 2009;15:4402–4410. doi: 10.1002/chem.200802454. [DOI] [PubMed] [Google Scholar]
- 16.a) Nie Z, Liu KJ, Zhong C-J, Wang L-F, Yang Y, Tian Q, Liu Y. Free Radical Biol. Med. 2007;43:1243–1254. doi: 10.1016/j.freeradbiomed.2007.06.011. [DOI] [PubMed] [Google Scholar]; b) Du L, Suo S, Wang G, Jia H, Liu KJ, Zhao B, Liu Y. Chem. Eur. J. 2013;19:1281–1287. doi: 10.1002/chem.201203506. [DOI] [PubMed] [Google Scholar]
- 17.a) Litwinienko G, Ingold KU. Acc. Chem. Res. 2007;40:222–230. doi: 10.1021/ar0682029. [DOI] [PubMed] [Google Scholar]; b) Valgimigli L, Amorati R, Petrucci S, Pedulli GF, Hu D, Hanthorn JJ, Pratt DA. Angew. Chem. 2009;121:8498–8501. doi: 10.1002/anie.200903360. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:8348–8351. doi: 10.1002/anie.200903360. [DOI] [PubMed] [Google Scholar]
- 18.a) McEwen CN, McKay RG, Larsen BS. J. Am. Chem. Soc. 1992;114:4412–4414. [Google Scholar]; b) Galano A. Nanoscale. 2010;2:373–380. doi: 10.1039/b9nr00364a. [DOI] [PubMed] [Google Scholar]; c) Zhang L, Zhou L, Yang M, Liu Z, Xie Q, Peng H, Liu Z. Small. 2013;9:1134–1143. doi: 10.1002/smll.201203152. [DOI] [PubMed] [Google Scholar]
- 19.Although on the basis of the literature data (see Ref. [18]) the graphite surface is expected to react with peroxyl radicals, in our conditions this does not translate into antioxidant activity, similarly, for instance, to C60 which under air is devoid of any antioxidant activity: Enes RF, Farinha ASF, Tome AC, Cavaleiro JAS, Amorati R, Petrucci S, Pedulli GF. Tetrahedron. 2009;65:253–262.
- 20.Pezzoli D, Zanda M, Chiesa R, Candiani G. J. Controlled Release. 2013;165:44–53. doi: 10.1016/j.jconrel.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Chompoosor A, Saha K, Ghosh PS, Macarthy DJ, Miranda OR, Zhu ZJ, Arcaro KF, Rotello VM. Small. 2010;6:2246–2249. doi: 10.1002/smll.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alarifi S, Ali D, Y AOS, Ahmed M, Siddiqui MA, Al-Khedhairy AA. Int. J. Nanomed. 2013;8:189–199. doi: 10.2147/IJN.S37924. [DOI] [PMC free article] [PubMed] [Google Scholar]