Abstract
Background and Aims
A major challenge in plant ecophysiology is understanding the effects of multiple sub-optimal environmental conditions on plant performance. In most Mediterranean areas soil salinity builds up during the summer because of low availability of soil water coupled with hot temperatures. Although sunlight and soil salinity may strongly interact in determining a plant's performance, this has received relatively little attention.
Methods
Two-year-old seedlings of Fraxinus ornus were grown outdoors in pots during a Mediterranean summer in either 45 % (shaded plants) or 100 % (sun plants) sunlight irradiance and were supplied with either deionized water or deionized water plus 75 mm NaCl. Morpho-anatomical traits, water and ionic relations, gas exchange and photosystem II performance, concentrations of individual carotenoids, activity of antioxidant enzymes, concentrations of ascorbic acid and individual polyphenols were measured in leaves. Leaf oxidative stress and damage were estimated by in vivo analysis of stable free radicals and ultrastructural analyses.
Key Results
Leaf concentrations of potentially toxic ions did not markedly differ in shaded or sun plants in response to salinity. Leaves of sun plants displayed superior water use efficiency compared with leaves of shaded plants, irrespective of salinity treatment, and had both better stomatal control and higher CO2 carboxylation efficiency than leaves of shaded plants. In the salt-treated groups, the adverse effects of excess midday irradiance were greater in shade than in sun plants. The activity of enzymes responsible for detoxifying hydrogen peroxide decreased in shaded plants and increased in sun plants as a result of salinity stress. In contrast, the activity of guaiacol peroxidase and the concentration of phenylpropanoids increased steeply in response to salinity in shaded plants but were unaffected in sun plants.
Conclusions
It is concluded that salinity may constrain the performance of plants growing under partial shading more severely than that of plants growing under full sun during summer. The results suggest co-ordination within the antioxidant defence network aimed at detoxifying salt-induced generation of reactive oxygen species.
Keywords: Antioxidant enzymes, electron spin resonance (ESR), Fraxinus ornus, gas exchange performance, ionic relations, water relations, leaf morpho-anatomy, oxidative damage, photosynthesis, shade versus sun, salinity, transmission electron microscopy (TEM)
INTRODUCTION
Root-zone salinity has long been hypothesized to deleteriously affect a plant's performance more in full sunlight than under partial shading (Munns and James, 2003; Tattini et al., 2006; Munns and Tester, 2008). This is because ‘sun’ plants may have greater uptake and translocation rates of potentially toxic ions than their shaded counterparts (Munns and James, 2003). The matter is, however, more complex, and involves not only light-induced effects on ionic and water relations (García-Sánchez et al., 2006; Melgar et al., 2009; Cimato et al., 2010), but also effects on the suite of morpho-anatomical and biochemical features of the leaf/plant that are inextricably linked to both uptake and transport of water and salt (Tattini et al., 2006; López-Hoffman et al., 2007).
First, the accumulation of potentially toxic ions in sensitive shoot organs largely depends on the irradiance at which shaded plants grow. In leaves of olive trees grown in full sunlight the leaf concentrations of Na+ and Cl– were 73 % greater than those in leaves growing in 15 % sunlight, and were only in part countered by 20 % greater K+ concentration (Melgar et al., 2009). On the other hand, García-Sánchez et al. (2006) showed that leaf Na+ concentration was higher in Citrus plants growing under moderate shade (50 % of full sunlight) compared with in full sun. These contrasting results may depend simply on stomatal conductance and transpiration rates of plants growing under deep or moderate shade (Barradas et al., 2005; Nicolás et al., 2008). Furthermore, interaction effects of sunlight irradiance and root-zone salinity on physiological and biochemical traits depend upon a species-specific strategy to control the entry and allocation of potentially toxic ions to sensitive shoot organs and to manage properly the flux of sunlight irradiance reaching the leaf (broadly ‘salt excluders’ versus ‘salt includers’ and ‘sun tolerant’ versus ‘sun sensitive’; Tattini et al., 2006; Melgar et al., 2009; Fusaro et al., 2014).
Second, leaves growing in full sun usually display a suite of morpho-anatomical traits that may confer great capacity to counter salt-induced water/osmotic stress (Evans, 1999; Poorter et al., 2009). There is clear evidence that drought stress may be more deleterious for leaves adapted to shade than to full sun (Valladares and Pearcy, 2002; Fini et al., 2012). Salt-induced osmotic imbalance and leaf dehydration may more severely constrain plants growing at limited sunlight availability (García-Sánchez et al., 2006; Cimato et al., 2010), as sun plants may limit cuticular water loss more effectively and have superior control of stomatal aperture compared with shaded plants (Rhizopoulou et al., 1991; Ponton et al., 2002). This in turn greatly affects photosynthetic performance, particularly when the usage of radiant energy for CO2 assimilation decreases as a consequence of stomatal and mesophyll limitations (for reviews see Flexas et al., 2004; Chaves et al., 2009). This is of great significance for plants inhabiting the Mediterranean regions, as excess soil salinity builds up concomitantly with hot temperatures, reduced soil water availability and high sunlight irradiance during the summer months (Tattini et al., 2006; Fusaro et al., 2014). In other words, Mediterranean plants in their natural habitats are challenged by severe excess light stress and hence by massive generation of reactive oxygen species (ROS) when suffering from an excess of soil salinity.
Finally, leaves of plants growing under partial shade or full sunlight also differ considerably in their capacity to avoid and counter stress-induced generation of ROS. In leaves acclimated to high sunlight, carotenoids, particularly violaxanthin-cycle pigments, may effectively dissipate excess radiant energy (Müller et al., 2001; Demmig-Adams and Adams, 2006) while directly protecting chloroplast membranes against oxidative damage (e.g. by chemically quenching singlet oxygen; Ramel et al., 2012). Furthermore, the activity of antioxidant enzymes and the concentrations of low molecular-weight antioxidants (such as ascorbate and phenylpropanoids) are usually greater in well-watered plants growing in full sun than in those growing in shade (Agati and Tattini, 2010; Agati et al., 2012). Exploring the extent to which salt-induced limitation of photosynthesis actually translates into excess light stress under natural conditions is a central issue for the ecology of Mediterranean plants exposed to a wide array of abiotic stresses, including root-zone salinity. Indeed, stress-induced excess excitation energy received by the chloroplast (Zhu et al., 2009; Fini et al., 2012) may have great impact on the plant's suite of primary and secondary antioxidant defences. There is evidence that excess excitation energy depresses the activity of enzymes devoted to H2O2 removal, particularly ascorbate peroxidase (APX) (Mullineaux and Karpinski, 2002; Hatier and Gould, 2008; Fini et al., 2011). Recently, phenylpropanoids in conjunction with vacuolar peroxidases (POXs) have been suggested to complement the depletion of primary antioxidant defences to scavenge excess H2O2 generated in the chloroplast and the peroxisome under severely excessive excitation energy (Ferreres et al., 2011; Fini et al., 2011; Agati et al., 2012).
Here we investigated Fraxinus ornus (manna ash), a deciduous member of the Oleaceae family. This species is largely used for restoring vegetation in urban and rural areas in the Mediterranean basin. We are unaware of experiments exploring the strategies adopted by this species to cope with excess soil salinity. Woody deciduous species usually display greater fluxes of Na+ and Cl– than woody evergreens, even at moderate soil salinities (Munns and Tester, 2008; Fusaro et al., 2014), likely as a consequence of their relative inability to sustain both drastic reductions in transpiration rates and severe dehydration (Tattini et al., 2006; Munns and Tester, 2008; Fusaro et al., 2014). The extent to which this affects gas exchange performance and hence biochemical adjustments in response to root-zone salinity during summer is central for understanding the ecophysiology of the Mediterranean flora.
We hypothesized that in F. ornus (1) transpiration rates, and hence the rates of Na+ and Cl– transport to the leaf, may not differ greatly between plants growing under moderate shade and those growing fully exposed to sunlight; but (2) sun plants are likely to display a suite of morpho-anatomical features conferring greater ability to counter salt-induced water stress than their shaded counterparts. To test these hypotheses, we grew plants at 45 or 100 % full solar irradiance and supplied them with deionized water (control, C) or this water with the addition of 75 mm NaCl (salt-treated, ST) during the Mediterranean summer. Measurements were performed of morpho-anatomy, water and ionic relations, gas exchange and photosystem (PS) II performance, the concentration of individual carotenoids, the activity of antioxidant enzymes and the concentrations of ascorbic acid and individual phenylpropanoids (mostly coumarins and quercetin 3-O-glycosides; Fini et al., 2012). Leaf oxidative damage was investigated by in vivo analysis of stable free radical content using electron spin resonance (ESR) spectrometry)and ultrastructural analysis using electron transmission microscopy (TEM).
MATERIALS AND METHODS
Plant material and growth conditions
Two-year-old Fraxinus ornus seedlings (whole-plant dry weight 16·7 ± 1·4 g, mean ± s.d., n = 10) were grown outdoors in 5·0-L pots in a peat/pumice substrate (50:50, v:v) fertilized with 2 kg m−3 of a controlled release fertilizer (Osmocote Exact, 15:9:12, 5–6-month formulation, Everris International, Geldermalsen, Netherlands). Plants grew under 60 % sunlight irradiance (∼1100 μmol m–2 s–1 at midday, corresponding to the light saturation point in healthy F. ornus leaves; see Table 3) over a 4-week period in Florence (43°46′ N, 11°15′ E) from 1 to 28 July. Plants were then transferred to screen houses (3 × 2 × 2 m, length × width × height) constructed with a roof and walls using plastic foils with specific transmittances, to receive 45 or 100 % solar irradiance over a 4-week experimental period. Plants grew under a 100-μm ethylene tetrafluoroethylene fluoropolymer transparent film (Nowoflon® ET-6235, Nowofol® Kunststoffprodukte, Siegsdorf, Germany) with the addition or not of a proper black polyethylene net. Photosynthetic photon flux density (PPFD) at midday inside the screen houses was measured with a calibrated Li-190 quantum sensor (Li-Cor, Lincoln, NE, USA), and was on average 1917 and 885 μmol m–2 s–1 at the sun and shaded sites, respectively, on a clear day. Final light treatments were achieved by the end of an 8-d period during which plants were exposed to final light treatments for 2, 4, 8 and 12 h, every 2 d, when the experiment started. Temperature maxima/minima were measured daily with Tinytag Ultra2 data loggers (Gemini Dataloggers, UK) and averaged 31·4/18·7 °C and 32·6/17·5 °C at the shade and sun sites, respectively. Plants were supplied at 2-d intervals with deionized water (treatment C) or this water with the addition of 75 mm NaCl (treatment ST) until complete leaching of the substrate.
Table 3.
Gas exchange and PSII photochemistry in control (C) and salt-treated (ST) F. ornus grown at 45 (shade) or 100 % (sun) solar irradiance
| Shade |
Sun |
NIVlight | NIVsalt | |||
|---|---|---|---|---|---|---|
| C | ST | C | ST | |||
| A (μmol m–2 s–1) | 13·4 ± 1·2 | 4·7 ± 0·8 | 14·1 ± 1·4 | 7·1 ± 0·7 | +0·08 | −0·40 |
| gs (mmol m–2 s–1) | 245 ± 21 | 74 ± 12 | 267 ± 29 | 112 ± 10 | +0·08 | −0·47 |
| gm (mmol m–2 s–1) | 138 ± 19 | 69 ± 10 | 129 ± 10 | 65 ± 9 | −0·04 | −0·33 |
| Vc,max (μmol m–2 s–1) | 75 ± 8 | 19 ± 4 | 106 ± 9 | 33 ± 5 | +0·19 | −0·56 |
| Jmax (μmol m–2 s–1) | 120 ± 11 | 33 ± 5 | 96 ± 7 | 46 ± 6 | −0·04 | −0·46 |
| Ci (μmol mol–1) | 217 ± 21 | 276 ± 16 | 210 ± 11 | 239 ± 14 | −0·05 | +0·09 |
| Cc (μmol mol–1) | 141 ± 13 | 248 ± 22 | 128 ± 8 | 198 ± 14 | −0·09 | +0·25 |
| PPFDsat (μmol quanta m–2 s–1) | 965 ± 80 | 356 ± 58 | 1056 ± 93 | 871 ± 85 | +0·19 | −0·24 |
| Fv/Fm | 0·78 ± 0·01 | 0·77 ± 0·01 | 0·77 ± 0·02 | 0·76 ± 0·01 | −0·01 | −0·01 |
| ΦPSII | 0·56 ± 0·02 | 0·36 ± 0·04 | 0·49 ± 0·03 | 0·38 ± 0·03 | −0·02 | −0·17 |
| NPQ | 0·95 ± 0·10 | 1·91 ± 0·09 | 1·30 ± 0·12 | 1·97 ± 0·13 | +0·07 | +0·29 |
| 1 – qP | 0·14 ± 0·03 | 0·29 ± 0·03 | 0·18 ± 0·02 | 0·23 ± 0·02 | −0·02 | +0·24 |
Data are mean ± s.d. (n = 6).
Leaves sampled for measurements were fully developed (5–6 weeks old).
Measurements were conducted under laboratory conditions.
A, net CO2 assimilation rate; gs, stomatal conductance; gm, mesophyll conductance to CO2; Vc,max, apparent rate of maximum carboxylation by Rubisco; Ci, internal CO2 concentration; Cc, chloroplast CO2 concentration; Fv/Fm, maximum efficiency of PSII photochemistry; ΦPSII, actual efficiency of PSII photochemistry; 1 – qP, reduction state of primary acceptors.
Plant growth, leaf morphology and anatomy and water and ionic relations
Plant growth was estimated in terms of relative shoot dry weight increments, i.e. relative growth rate of the shoot (RGRshoot), using the equation:
| (1) |
where W is shoot dry weight, t is time and subscripts 0 and 1 denote initial and final harvest. Leaf lamina size and leaf mass per area (LMA) were determined as reported previously (Tattini et al., 2000). Whole-leaf thickness and the thicknesses of adaxial and abaxial cuticular layers were estimated in 1-μm transverse sections that were fixed and embedded following standard methodology (Tattini et al., 2005), using an Axiophot microscope (Carl Zeiss, Jena, Germany) equipped with a high-resolution TK 870E video camera (JVC, Yokoama, Japan). Cuticular waxes were quantified on six leaves immersed in chloroform (2 × 100 mL) for 30 s, following the gravimetric protocol of Hauke and Schreiber (1998).
Predawn water (ψw) and osmotic (ψπ) potentials were measured for fully developed leaves (35 d old) using a standard method (Tattini et al., 2002). Leaf osmotic potential was measured on expressed sap of frozen and thawed leaves using a boiling-point Vapro 5520 osmometer (Wescor, South Logan, UT, USA). Leaf osmotic potential at full turgor (ψπFT) was then calculated as:
| (2) |
where RWC is relative water content and AWF is apoplastic water fraction. RWC was measured as previously reported (Tattini et al., 2002) and AWF was estimated from the analysis of pressure/volume isotherms at 6 %. The contribution of dehydration (D) to osmotic adjustment was calculated as:
| (3) |
where Δψ is the difference in leaf osmotic potential between salt-treated and control plants. The osmotic contributions of Na+, Cl– and K+ to ψπFT were calculated by the Van't Hoff equation:
| (4) |
where ψπ,s is the contribution (–MPa) of individual solutes (s) to ψπFT, RDW is relative dry weight at saturation (kg m−3), C is the molar concentration of solutes (mol kg−1 DW) and 0·002479 m3 MPa−1 mol−1 is the RT value at 25 °C.
Cation analysis was performed with a PerkinElmer 1100 emission-absorption spectrophotometer (PerkinElmer, Norwalk, CT, USA) and chloride was quantified with a Quanta 4000E Ion Capillary Electrophoresis Unit (Waters, Milford, MA, USA), based on the protocols previously reported by Tattini and Gucci (1999). Soluble carbohydrates were identified and quantified by high-performance liquid chromatography with refractive index detection (HPLC-RI) as reported previously (Tattini et al., 1996).
Leaf gas exchange, mesophyll conductance and PSII performance
Net photosynthesis (A), stomatal conductance (gs), transpiration rate (E) and intercellular CO2 concentration (Ci) were measured on the medial leaflet of fully expanded leaves, under laboratory conditions (leaf temperature 28 °C), using a portable infrared gas analyser (Ciras-2; PP-systems, Amesbury, MA, USA) operating at 380 μL L–1 ambient CO2. Leaves were enclosed in a 2·5 cm2 Parkinson cuvette and provided with saturating light irradiance using actinic light (CFM; PP-systems). The CFM also provides measurement of modulated chlorophyll fluorescence, allowing simultaneous measurements of gas exchange and fluorescence parameters. Mesophyll conductance to CO2 diffusion (gm) was estimated using the variable J method (Harley et al., 1992):
| (5) |
where A is CO2 assimilation rate, Γ* is the chloroplastic CO2 compensation point, Jf is the rate of linear electron transport estimated from chlorophyll fluorescence and (A + Rday) is the gross photosynthetic rate (including non-photorespiratory respiration). Γ* was calculated using the Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase)-specific factor estimated for deciduous species (Galmés et al., 2005) and Jf was calculated as described by Genty et al. (1989):
| (6) |
where ΦPSII is the quantum yield of PSII in the light and α is leaf absorptance.
Leaf absorptance was measured with a spectroradiometer equipped with an integrating sphere (model 1800-12S; LI-Cor, Lincoln, NE, USA); the factor 0·5 was chosen assuming an equal distribution of absorbed photons between PSI and PSII (Loreto et al., 1992). Before measurements, we verified that Jf measurements corresponded to the quantification of electron transport rate estimated by gas exchange in non-photorespiratory conditions (i.e. lowering O2 concentration to 1 %; Laisk and Loreto, 1996).
The CO2 concentration in the chloroplasts (Cc) was then calculated as:
| (7) |
Responses of A to changes in intercellular CO2 concentration (A/Ci curves) were analysed by decreasing stepwise external CO2 concentration (Ca) from 380 to 30 μL L–1, then restoring Ca to 380 μL L–1 and finally increasing Ca stepwise to 1800 μL L–1. The apparent maximum rate of carboxylation by Rubisco (referred to as carboxylation efficiency throughout this paper) was calculated as described by Long and Bernacchi (2003). In addition, the activity of Rubisco in vitro was measured following the protocol of Ouerghi et al. (2000).
A quantitative analysis of stomatal (SL), mesophyll conductance (ML) and biochemical (BL) limitations on A was carried out as described by Grassi and Magnani (2005). Responses of A to changes in photosynthetic photon flux density (A/PPFD curves) were measured in the range 0–1800 μmol quanta m–2 s–1. Daily changes in A and stomatal conductance (gs) were also measured in situ, from 06:00 to 21:00 h. In situ measurements were conducted at 380 μL L–1 CO2, ambient temperature and natural irradiance, as described by Valladares and Pearcy (2002). Net daily carbon gain (CO2,daily) was then calculated by the integration procedures reported by Tattini et al. (2004).
A modulated chlorophyll a fluorescence analysis was conducted on dark-adapted (over a 40-min period) leaves using a PAM-2000 fluorometer (Walz, Effeltrich, Germany) connected to a Walz 2030-B leaf-clip holder through a Walz 2010-F trifurcated fibre optic, under laboratory conditions. The maximum efficiency of PSII photochemistry was calculated as Fv/Fm = (Fm − F0)/Fm, where Fv is the variable fluorescence and Fm is the maximum fluorescence of dark-adapted (over a 40-min period) leaves. The minimal fluorescence, F0, was measured using a modulated light pulse <1 μmol m−2 s−1, to avoid appreciable variable fluorescence. Values of Fm and Fm′ were determined at 20 kHz using a 0·8-s saturating light pulse of white light at 8000 μmol m−2 s−1. We then estimated ΦPSII and non-photochemical quenching [NPQ = (Fm/Fm′) – 1] as described by Genty et al. (1989). The excitation pressure on PSII, (1 − qP), qP being the coefficient of photochemical quenching, was calculated as described by Schreiber et al. (1995).
Analysis of photosynthetic pigments and phenylpropanoids
Individual carotenoids in leaves sampled at predawn and midday were identified and quantified as reported by Beckett et al. (2012). Leaves were immediately frozen in liquid nitrogen and kept at −80 °C until analysis. Fresh leaf material (120–150 mg) was extracted with 2 × 4 ml acetone (containing 0·5 g l–1 CaCO3) and 15 μL aliquots were injected into a PerkinElmer Flexar chromatograph equipped with a quaternary 200Q/410 pump and LC 200 diode array detector (all from PerkinElmer, Bradford, CT, USA). Photosynthetic pigments were separated using a 250 × 4·6 mm Waters Spherisorb ODS1 (5 μm) column operating at 30 °C, eluted with a linear gradient solvent system, at a flow rate of 1·2 mL min–1, consisting of CH3CN/MeOH/H2O (8·4/0·8/0·7, A) and MeOH/ethyl acetate (6·8/3·2, B) during an 18-min run: 0–12 min from 100 to 0 % A;12–18 min at 0 % A. Carotenoids were tentatively identified using visible spectral characteristics and retention times. Violaxanthin and antheraxanthin were quantified using the calibration curve of an authentic lutein standard (Extrasynthese, Lyon-Nord, Genay, France); for zeaxanthin we used the calibration curve of an authentic zeaxanthin standard (Extrasynthese). Chlorophylls a and b were quantified using calibration curves of chlorophyll authentic standards (Sigma-Aldrich, Milan, Italy).
Phenylpropanoids, mostly coumarins and flavonols, were extracted, identified and quantified following the protocol of Fini et al. (2012). Freeze-dried leaf tissue (30–40 mg) was extracted with 3 × 5 mL of 75 % ethanol (adjusted to pH 3·0 with formic acid), and the supernatant was partitioned with 3 × 8 mL of n-hexane. The ethanol fraction was reduced to dryness under vacuum at room temperature with a Büchi P12 Multivapor unit equipped with a Büchi V-855 vacuum controller (Büchi, Flawil, Switzerland). The residue was dissolved in 1 mL of MeOH/H2O (9:1, v:v) and 10 μL aliquots were injected into the Perkin-Elmer LC equipment described above. Phenylpropanoids were separated using a 250 mm × 4·6 mm Hypersil BDS C18 column (5 μm) operating at 30 °C and eluted with a linear gradient solvent system, at a flow rate of 1·0 mL min−1, consisting of CH3CN (A) and H2O (pH 3·2 by H3PO4, B), during a 60-min run; 0–15 min from 5 to 15 % A; 15–35 min from 15 to 30 % A; 35–40 min from 30 to 40 % A: 40–50 min from 40 to 60 % A; 50–55 min from 60 to 90 % A; 55–60 min from 90 to 100 % A. Individual coumarins and flavonoids were identified using retention times and UV spectral characteristics of authentic standards (all from Extrasynthese) and by MS data. HPLC–mass spectrometry (MS) analysis was performed with an Agilent LC1200 chromatograph coupled with an Agilent 6410 triple quadrupole MS detector equipped with an electrospray ion source (all from Agilent Technologies, Santa Clara, CA, USA). Coumarins were quantified at 350 nm for esculetin and at 334 nm for both esculin (esculetin 6-O-glucoside) and chicorin (esculetin glycosides were pooled and are referred as to esculin throughout this article, as the chicorin concentration never exceeded 10 % of the esculin concentration) using calibration curves for authentic standards. Quercetin derivatives (quercetin 3-O-glucoside, quercetin 3-O-galactoside and quercetin 3-O-rutinoside) were quantified at 350 nm using calibration curves for the individual compounds.
Antioxidant enzymes, oxidative stress and oxidative damage
Antioxidant enzyme activities and ascorbate concentration were measured in fresh leaf material as described by Guidi et al. (2008). Superoxide dismutase (SOD, EC 1.15.1.1) activity was measured photometrically at 560 nm, based on the inhibition by SOD of nitroblue tetrazolium reduction. One unit of SOD was defined as the amount needed to bring about 50 % inhibition of the nitroblue tetrazolium reduction state. Catalase (CAT, EC 1.11.1.6) activity was measured photometrically at 270 nm by determining the rate of conversion of H2O2 to O2. Total APX (EC 1.1.11.1) activity was measured as the decrease in absorbance at 290 nm resulting from ascorbate oxidation. Polyphenol oxidase (PPO, EC 1.14.18.1) activity was determined according to Espín et al. (1997) with 3,4-dihydroxyphenylpropionic acid as substrate, measuring the formation of o-quinones. Guaiacol POX (EC 1.11.1.7) was assayed as described by Bestwick et al. (1998), using 10 mm guaiacol (in 50 mm potassium phosphate buffer) as substrate and, following the addition of 0·25 % H2O2, measuring the concentration of oxidized guaiacol. The concentration of total ascorbate (ASA) was determined spectrophotometrically as described by Guidi et al. (2008).
Oxidative stress was estimated by determining the content of stable free radicals using ESR spectroscopy. Intact leaves were placed in a wide-bore (8·0 mm i.d.) quartz tube, and analyses were performed in continuous wave (CW) X-band using a Brüker Elexsys E500 series spectrometer equipped with a Brüker ER4122 SHQE cavity at 100 K (using an ER411VT Brüker variable temperature unit) as reported by Melgar et al. (2009). Spectra were recorded under the following experimental conditions: modulation frequency 100 kHz, microwave power 20 mW, modulation amplitude 0·3 mT and frequency 9·39 GHz. The signal area was calculated using a double integration of the experimental spectrum. Our interest was in a signal centred at g ∼ 2·003 (peak-to-peak line width 1 mT), detectable on the fourth peak of the Mn(II) sextet component, using a scan width of 7 mT.
Cell ultrastructure and H2O2 accumulation were examined using TEM. Accumulation of H2O2 was determined cytochemically by detection of cerium perhydroxide formation upon the reaction of cerium chloride (CeCl3) with endogenous H2O2, following the protocols of Guidi et al. (2010). Leaf pieces (area ∼0·15 mm2) were vacuum-infiltrated in 5 mm CeCl3 in 50 mm 3-(N-morpholino)-propanesulphonic acid (pH 7·2). Samples treated with CeCl3 were then fixed in 10 % glutaraldehyde, 10 % acrolein and 6 % paraformaldehyde in 0·2 m phosphate buffer (pH 7·2) for 1 h, and washed twice with the same buffer before post-fixing with 2 % osmium tetroxide in phosphate buffer (pH 7·2). Specimens were dehydrated in a graded ethanol series (25, 50, 75, 90 and 100 %), and gradually embedded in Epon–Araldite. Ultrathin sections (∼80–90 nm) were obtained with an LKB IV ultramicrotome (LKB Produkter, New York, USA), mounted on Formvar-coated copper grids, stained with uranyl acetate and lead citrate and examined using a Philips CM12 transmission electron microscope (Philips, Eindhoven, Netherlands) operating at 80 kV.
Statistics
The experimental set up was a split-plot design with eight replicates in which light intensity was the main plot and salinity the subplot. Measurements were made on fully developed leaves aged 5–6 weeks. Data collected under laboratory conditions, with the exception of ψπ,s (the contribution of individual solutes to osmotic potential at full turgor), were analysed using two-way ANOVA (light and salinity as fixed factors and their interaction factor). Daily trends in A and gs in situ were analysed using repeated measures ANOVA. Statistical analysis was performed using the SPSS statistical package (IBM, New Orchard Road, Armonk, NY, USA). The extent to which a plant trait X varied in response to sunlight irradiance (light) or root-zone salinity (salt) was quantified in terms of the normalized index of variation (NIV) as follows (Tattini et al., 2006):
| (8) |
| (9) |
RESULTS
Morpho-anatomy and water and ionic relations
Salinity stress did not markedly affect the whole set of leaf morpho-anatomical features examined in our study, which instead were greatly altered by sunlight irradiance (Table 1 and Supplementary Data Table S1). There were major light-induced alterations in cuticle thickness and cuticular wax content (average +58 %), but whole-leaf thickness, LMA and the proportion of palisade parenchyma to spongy parenchyma tissue were also significantly greater in leaves of sun plants than on those of shaded plants (average +34 %). Interestingly, leaf lamina size, which was on average 40 % greater in shaded than in full sun plants, decreased because of salinity stress only in leaves of shaded plants. The RGRshoot, which was slightly higher in control plants growing in full sun than in partial shade) declined more in shaded (–58 %) than in sun (–44 %) plants because of root-zone salinity (Table 1).
Table 1.
Shoot dry weight increments (RGRshoot) and leaf morpho-anatomical features in control (C) and salt-treated (ST) F. ornus grown at 45 (shade) or 100 % (sun) solar irradiance
| Trait | Shade |
Sun |
NIVlight | NIVsalt | ||
|---|---|---|---|---|---|---|
| C | ST | C | ST | |||
| RGRshoot (d–1 × 103) | 23·2 ± 2·8 | 9·8 ± 2·2 | 29·1 ± 3·6 | 15·6 ± 3·2 | +0·15 | −0·35 |
| Leaf area (cm2) | 15·4 ± 1·8 | 11·5 ± 1·3 | 10·3 ± 1·1 | 8·9 ± 1·0 | −0·17 | −0·11 |
| Leaf mass per area (g DW m–2) | 68·2 ± 4·6 | 70·5 ± 5·4 | 98·4 ± 7·3 | 95·3 ± 5·0 | +0·17 | +0·00 |
| Cuticle thickness (μm) | 3·2 ± 0·3 | 3·1 ± 0·2 | 4·7 ± 0·5 | 4·8 ± 0·3 | +0·21 | +0·01 |
| Cuticular wax (μg cm–2) | 65·7 ± 7·7 | 74·2 ± 8·1 | 109·1 ± 8·8 | 116·0 ± 9·9 | +0·29 | +0·05 |
| Leaf thickness (μm) | 187 ± 21 | 194 ± 19 | 244 ± 19 | 252 ± 17 | +0·13 | +0·01 |
| Palisade to spongy ratio | 0·42 ± 0·4 | 0·40 ± 0·3 | 0·53 ± 0·3 | 0·54 ± 0·4 | +0·13 | −0·00 |
Data are mean ± s.d. (n = 6).
Leaves sampled for measurement were fully developed (5–6 weeks old).
Sunlight irradiance (NIV = 0·08) had a relatively minor effect compared with root-zone salinity (NIV = 0·31) on water and ionic relations (Table 2 and Supplementary Data Table S1). Nonetheless, osmotic potential decreased more in leaves of sun plants than in shaded plant leaves in response to salinity. Furthermore, dehydration contributed more in shaded (D = −0·27 MPa, 26 % of Δψπ) than in sun plants (–0·25 MPa, 16 % of Δψπ) to the salt-induced decline in ψπ. The greater capacity for active osmotic adjustment in response to salinity stress displayed by leaves of sun plants than those of shaded plants (Table 2) was mostly due to higher concentrations of Na+ (122 mm in shaded versus 167 mm in sun plants), Cl− (142 mm in shaded versus 195 mm in sun plants) and K+ (240 mm in shaded versus 293 mm in sun plants). On the contrary, soluble carbohydrates (which contributed more to ψπFT in sun than in shaded plants in the control) made a greater contribution to salt-induced osmotic adjustment in shaded than in sun plants (Table 2). This resulted from a much greater (+61 %) salt-induced increase in the molar concentration of mannitol in shaded than in sun plants.
Table 2.
Water and ionic relations in control (C) salt-treated (ST) F. ornus grown at 45 (shade) or 100 % (sun) solar irradiance
| Shade |
Sun |
NIVlight | NIVsalt | |||
|---|---|---|---|---|---|---|
| C | ST | C | ST | |||
| RWC (%) | 93·4 ± 2·8 | 86·0 ± 2·0 | 92·5 ± 1·8 | 89·1 ± 1·5 | +0·00 | −0·03 |
| ψw (–MPa) | 0·44 ± 0·3 | 0·83 ± 0·5 | 0·48 ± 0·5 | 0·86 ± 0·4 | +0·03 | +0·29 |
| ψπ (–MPa) | 1·88 ± 0·09 | 2·95 ± 0·15 | 1·94 ± 0·17 | 3·51 ± 0·22 | +0·06 | +0·32 |
| ψπFT (–MPa) | 1·75 ± 0·13 | 2·51 ± 0·16 | 1·78 ± 0·15 | 3·10 ± 0·18 | +0·07 | +0·30 |
| ψπ,FT,Na+ (–MPa) | 0·05 ± 0·01 | 0·25 ± 0·03 | 0·07 ± 0·01 | 0·34 ± 0·03 | +0·15 | +0·66 |
| ψπFT,Cl– (–MPa) | 0·06 ± 0·01 | 0·29 ± 0·04 | 0·09 ± 0·02 | 0·40 ± 0·02 | +0·17 | +0·64 |
| ψπFT,K+ (–MPa) | 0·35 ± 0·03 | 0·49 ± 0·05 | 0·38 ± 0·02 | 0·60 ± 0·04 | +0·08 | +0·22 |
| ψπFT,mannitol (–MPa) | 0·21 ± 0·02 | 0·42 ± 0·01 | 0·23 ± 0·02 | 0·36 ± 0·03 | −0·03 | +0·28 |
| ψπFT,sugars (–MPa) | 0·55 ± 0·03 | 0·50 ± 0·04 | 0·66 ± 0·04 | 0·65 ± 0·02 | +0·11 | −0·03 |
Data are mean ± s.d. (n = 6).
Leaves sampled for measurement were fully developed (5–6 weeks old).
Sugars include sucrose, glucose, galactose and fructose.
ψw, water potential; ψπ osmotic potential; ψπFT, osmotic potential at full turgor; ψπFT,s, contribution of solutes to ψπFT.
Gas exchange and PSII photochemistry
Salinity stress markedly (NIV = 0·33) affected leaf gas exchange performance under laboratory conditions, whereas the overall effect of sunlight irradiance (NIV = 0·10) was of relatively minor significance (Table 3 and Supplementary Data Table S1). Sunlight irradiance did not significantly alter net photosynthesis and stomatal conductance measured at saturating light under laboratory conditions in control plants, whereas salt-induced declines in gas exchange were more pronounced in shaded (on average –67·5 %) than in full sun plants (average –55 %). Sun plants had much greater carboxylation efficiency (Vc,max) than leaves of shaded plants (+47 %), as estimated by A/Ci curves, particularly when exposed to salinity stress (+68 %). The apparent maximum electron transport rate contributing to Rubisco regeneration (Jmax) was higher (+25 %) in control plants growing in the shade than in full sun plants, but salt-induced decreases in Jmax were again greater in leaves of shaded plants (–72 %) than in leaves of sun plants (–52 %) (Table 3). The photosynthetically active radiation at which photosynthesis saturated (PPFDsat), which did not differ significantly between leaves of sun and shaded control plants, decreased unexpectedly much more in shaded (–61 %) than in full sun plants (–17 %) in response to salinity stress. At midday PPFD exceeded PPFDsat by 255 % in shaded plants and by 210 % in sun plants (Table 3).
Mesophyll conductance to CO2 did not vary significantly with sunlight, but decreased in response to salinity in both sun and shaded plants (Table 3). In leaves of sun plants and especially in shaded plants, root-zone salinity increased the intercellular CO2 concentration compared with control leaves (Table 3), indicating that, though stomatal limitations increased because of root-zone salinity, non-stomatal limitations played the major role in constraining A (Fig. 1). Diffusive limitations (SL + ML) accounted for 40 and 50 % of the total limitation on A in shaded and sun salt-treated plants, respectively (Fig. 1). Thus, biochemical limitations (BL) constrained A more in shaded than in sun plants when suffering from salinity stress. Consistently, though salinity depressed gm, the CO2 concentration in the chloroplast (Cc) increased greatly in response to salinity, particularly in shaded plants (Table 3).
Fig. 1.
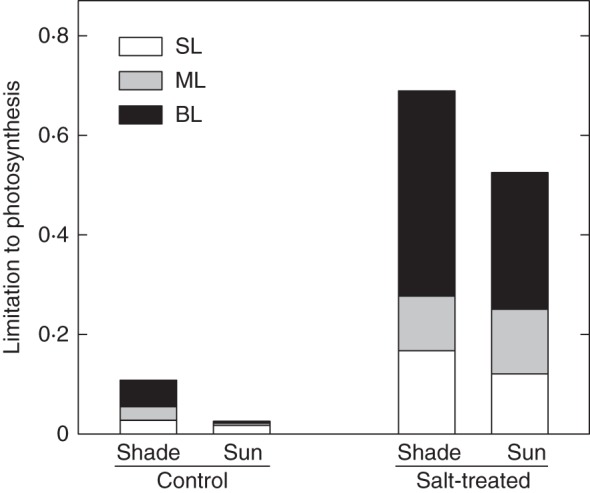
Stomatal (SL), mesophyll (ML) and biochemical (BL) limitations on photosynthesis in leaves (5–6 weeks old) of F. ornus grown at 45 (shade) or 100 % sunlight irradiance (sun) and supplied with 0 (control) or 75 mm NaCl (salt-treated). Data are means for four replicate leaves.
Shaded and sun plants displayed more evident differences in gas exchange performance under field conditions irrespective of salt treatment, as revealed by the daily courses of net photosynthesis and stomatal conductance estimated in situ (Fig. 2). Briefly, in control plants in full sun, stomatal conductance (Fig. 2B) decreased more than in corresponding shaded plants during the central hours of the day, without a concomitant depression in net CO2 assimilation rate (Fig. 2A). In salt-treated leaves, photosynthesis was much higher in the sun than in the shade (+33 %), though sun plants had slightly higher stomatal conductance (+9 %) compared with shaded plants. Overall, on a daily basis, instantaneous water use efficiency (WUE, inset in Fig. 2A) was greater in sun (+16 %) than in shaded plants, especially during the hottest hours. This occurred despite sun plants having to cope with a higher vapour pressure deficit (VPD; +23 %) than shaded plants (inset in Fig. 2B).
Fig. 2.
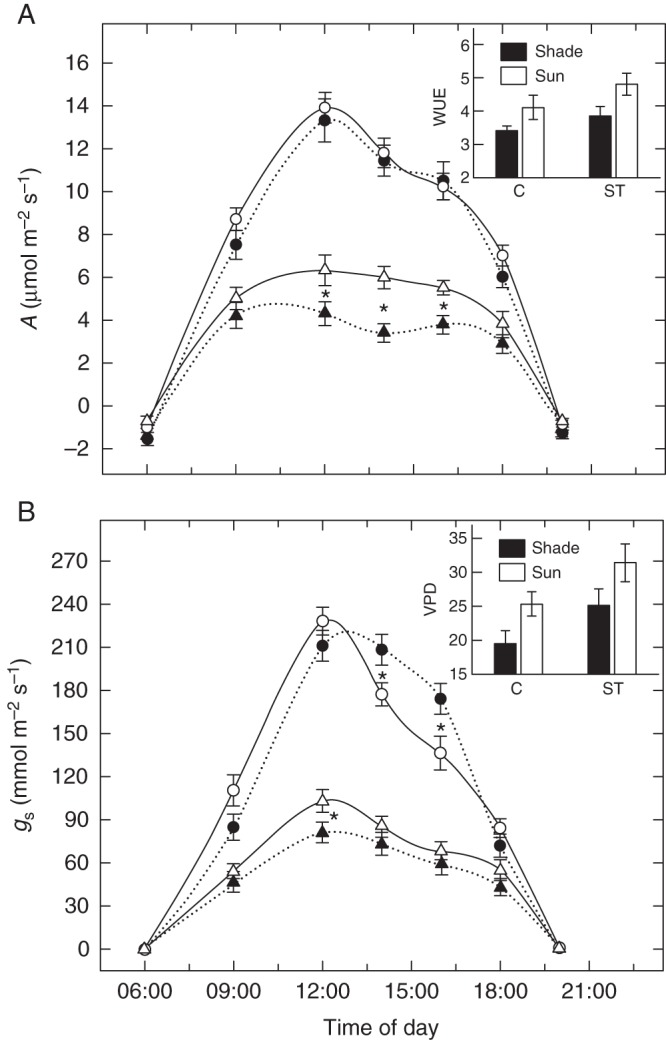
(A) Daily changes in net photosynthesis (A) and (B) stomatal conductance (gs) in leaves of F. ornus grown at 45 (black symbols) or 100 % sunlight irradiance (open symbols) and supplied with 0 (circles) or 75 mm NaCl (triangles). Insets in (A) and (B) denote water use efficiency (A/E) and VPD, respectively, for control (C) and salt treatment (ST). Measurements were conducted after 2 (4-week-old leaves) and 4 (6-week-old leaves) weeks of treatment on four replicate plants, and data were pooled prior to statistical analysis. Data are means ± s.d. (n = 8). *P < 0·05 (LSD test).
PSII photochemistry in the dark-adapted state, i.e. Fv/Fm, did not vary in response to either sunlight or salt treatment (Table 3 and Supplementary Data Table S1). This is consistent with light- and salt-induced declines in PSII quantum yield (ΦPSII) and increases in NPQ, the extent of these changes being greater in leaves of shaded plants (on average 44 %) than in those of sun (28 %) plants. The reduction state of primary acceptors, 1 – qP, here taken as a measure of excess excitation pressure on PSII (Maxwell and Johnson, 2000), increased much more in the shade (+107 %) than in full sun (+28 %) in response to salinity stress. Overall, leaves of salt-treated plants had greater excess excitation energy when grown under partial shading than in full sun (Table 3).
Photosynthetic pigments
Consistent with gas exchange and leaf photochemistry, the concentrations and composition of photosynthetic pigments varied more in shaded than in sun plants in response to salinity (Table 4 and Supplementary Data Table S1). First, total chlorophyll (Chltot) concentration was higher in shaded than in sun plants under control conditions, but salt-induced declines in Chltot were detected in shaded plants only. Second, sun plants had a higher pool of violaxanthin-cycle pigments relative to Chltot compared with shaded plants, irrespective of salt treatment (149 versus 119 mmol mol–1 Chltot). The de-epoxidation state (DES) of violaxanthin cycle pigments was also greater (+47 %) in sun than in shaded plants under control conditions. However, salt-induced increases in DES were much higher in shaded (+195 %) than in sun plants (+68 %). This did not result from higher concentrations of antheraxanthin and zeaxanthin in shaded than in sun plants, but depended on a salt-induced decline in violaxanthin concentration, which was indeed much greater in shaded (–33 %) than in sun plants (–13 %). On the contrary, the concentration of β-carotene decreased and remained constant in sun and shaded plant leaves, respectively, in response to salinity stress (Table 4).
Table 4.
Concentration of total chlorophyll (μmol g–1 DW), concentrations of individual carotenoids relative to total chlorophyll concentration (mmol mol–1) and de-epoxidation state (DES) of violaxanthin cycle pigments in leaves of control (C) and salt-treated (ST) F. ornus grown at 45 (shade) or 100 % (sun) solar irradiance
| Pigment | Shade |
Sun |
NIVlight | NIVsalt | ||
|---|---|---|---|---|---|---|
| C | ST | C | ST | |||
| Total chlorophyll | 6·8 ± 0·5 | 4·9 ± 0·4 | 5·6 ± 0·3 | 5·0 ± 0·4 | –0·05 | –0·11 |
| β-carotene | 111 ± 14 | 114 ± 12 | 117 ± 9 | 83 ± 10 | −0·06 | −0·07 |
| Lutein | 130 ± 14 | 145 ± 8 | 122 ± 10 | 138 ± 12 | −0·03 | +0·06 |
| Violaxanthin (V) | 86 ± 7 | 58 ± 8 | 100 ± 6 | 87 ± 5 | +0·09 | −0·10 |
| Antheraxanthin (A) | 11 ± 2 | 35 ± 4 | 16 ± 2 | 36 ± 3 | +0·10 | +0·47 |
| Zeaxanthin (Z) | 9 ± 1 | 40 ± 3 | 20 ± 3 | 40 ± 4 | +0·10 | +0·47 |
| DES = (Z + A)/ (V + A + Z) | 0·19 ± 0·02 | 0·56 ± 0·04 | 0·28 ± 0·02 | 0·47 ± 0·03 | +0·00 | +0·37 |
Data are mean ± s.d. (n = 4).
Leaves (5–6 weeks old) were collected at 09:00 and 13:00 h and pooled before analysis.
Antioxidant defences and oxidative damage
Sun and shade plants mostly differed in the pool of antioxidant defences (NIVlight = 0·19) compared with all other traits examined in our study, particularly when supplied with good-quality water (Table 5 and Supplementary Data Table S1). These differences mostly concerned SOD, CAT and APX, as well as total ASA and quercetin 3-O-glycosides, the activities and the concentrations of which were significantly higher (on average +76 %) in leaves of sun plants than in those of shaded plants. Enzymes and metabolites aimed at removing ROS varied either little in the sun (25 %) or markedly in the shade (118 %) in response to salinity. Notably, CAT and particularly APX decreased markedly in the shade or increased in the sun because of salinity stress. Such decreases were paralleled by steep increases in the activities of POX and PPO in leaves of shaded plants (Table 5). Salinity stress decreased the concentration of total ASA in the shade but did not affect it in the sun. This was due to salt-induced reduction of the concentration of oxidized ascorbate (DHASA) in leaves of shaded plants, thus increasing the ASA/DHASA ratio from 0·80 (0·88 in sun) in the control to 2·32 (0·61 in sun) in leaves of salt-treated plants. The phenylpropanoid concentration and composition did not vary with salinity in sun plants, whereas major phenylpropanoids increased in concentration with salinity in leaves of shaded plants.
Table 5.
Activity of antioxidant enzymes (normalized to mg of protein) and concentrations of ascorbate and phenylpropanoids in leaves of well-watered and salt-treated F. ornus grown at 45 (shade) or 100 % (sun) solar irradiance
| Parameter | Shade |
Sun |
NIVlight | NIVsalt | ||
|---|---|---|---|---|---|---|
| C | ST | C | ST | |||
| SOD (U) | 0·26 ± 0·04 | 0·30 ± 0·04 | 0·57 ± 0·03 | 0·62 ± 0·06 | +0·35 | +0·04 |
| CAT (mmol H2O2 min–1) | 10·4 ± 1·5 | 7·8 ± 0·9 | 15·3 ± 2·30 | 18·6 ± 1·24 | +0·30 | +0·00 |
| APX (mol ASA min–1) | 0·17 ± 0·02 | 0·06 ± 0·02 | 0·15 ± 0·03 | 0·25 ± 0·01 | +0·27 | −0·01 |
| POX (μmol oxidized guaiacol) | 0·24 ± 0·04 | 0·53 ± 0·06 | 0·38 ± 0·02 | 0·34 ± 0·04 | −0·03 | +0·16 |
| PPO (μmol quinones) | 0·15 ± 0·03 | 0·63 ± 0·06 | 0·14 ± 0·02 | 0·36 ± 0·04 | −0·22 | +0·55 |
| ASA (μg g–1 DW) | 48·0 ± 5·6 | 56·5 ± 4·4 | 74·7 ± 6·0 | 60·5 ± 5·8 | +0·05 | −0·02 |
| DHASA (μg g–1 DW) | 60·2 ± 3·6 | 24·3 ± 5·1 | 84·1 ± 4·3 | 98·4 ± 8·0 | +0·38 | −0·08 |
| Esculetin (μmol g–1 DW) | 45·1 ± 7·1 | 68·1 ± 5·5 | 45·9 ± 5·7 | 43·4 ± 4·1 | −0·12 | +0·12 |
| ESC (μmol g–1 DW) | 54·1 ± 3·6 | 40·6 ± 5·0 | 58·2 ± 3·7 | 60·5 ± 7·2 | +0·11 | −0·04 |
| QUE (μmol g–1 DW) | 4·3 ± 0·8 | 12·9 ± 1·8 | 8·9 ± 0·7 | 8·7 ± 1·1 | +0·01 | +0·24 |
Data are mean ± s.d. (n = 4).
Leaves sampled for measurement were fully developed (5–6 weeks old), collected at midday.
ESC, esculin (esculetin 6-O + esculetin 7-O-glucoside); QUE, quercetin 3-O-glycosides.
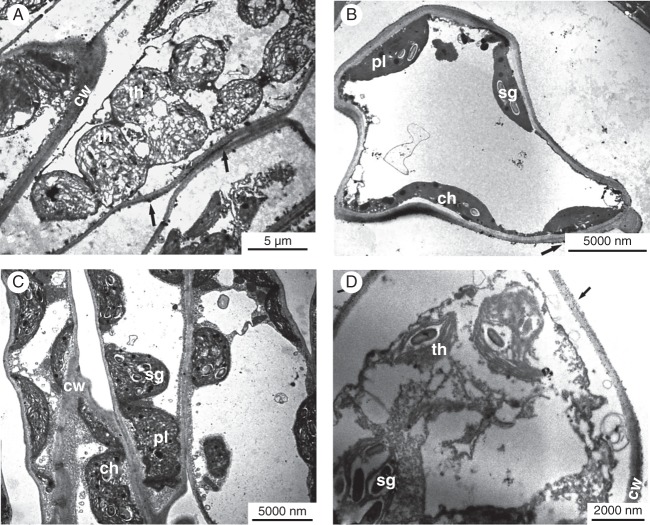
Overall, salt-treated F. ornus suffered from oxidative damage both in the shade and in full sun, as revealed by the TEM images in Fig. 3. In particular, damage was evident in palisade parenchyma cells in sun plants: aggregated chloroplasts occurred in the centre of the cell, were round in shape and displayed a disorganized lamellar system (Fig. 4A). No signs of damage were apparent in spongy cells (Fig. 4B). Palisade parenchyma cells suffered less from salinity stress in leaves of shaded plants: chloroplasts had swollen thylakoid membranes and an increased number of plastoglobules (Fig. 4C). By contrast, spongy cells in leaves of shaded plants showed clear signs of degeneration, such as disruption of both the chloroplast envelope and the tonoplast membrane (Fig. 4D). Actually, our ESR analysis performed on intact leaves showed marked differences in the content of a stable free radical, centred on the fourth peak of the Mn(II) sextet, between salt-treated shaded and sun plants (Fig. 5). The area of this signal, with g = 2·0034 (consistent with the presence of semiquinone radicals) was indeed 88 % higher in leaves of salt-treated plants grown in the shade than in those of salt-treated sun plants.
Fig. 3.
TEM of palisade (A, C) and spongy (B, D) mesophyll cells in salt-treated F. ornus leaves (5–6 weeks old) grown at 45 (C, D) or 100 % sunlight irradiance (A, B). Arrows denote H2O2 accumulation. Palisade parenchyma cells in sun plants (A) have round-shaped chloroplasts with disorganized lamellar system. Disruption of both the chloroplast envelope and the tonoplast membrane is evident in spongy cells of leaves of shaded plants (D). ch, chloroplast; cw, cell wall; th, thylakoids; pl, plastoglobules; sg, starch grains.
Fig. 4.
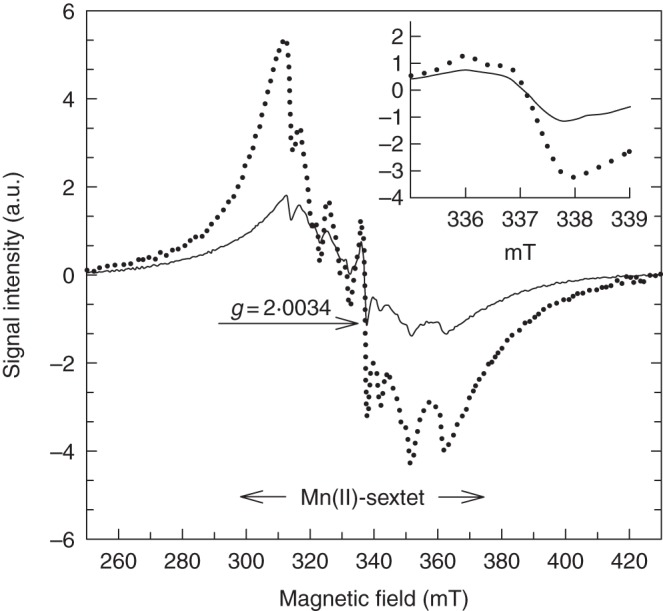
ESR analysis of intact leaves (5–6 weeks old) of salt-treated F. ornus grown at 45 (dotted line) or 100 % sunlight irradiance (solid line). The signal centred on the fourth peak of the Mn(II) sextet, with g = 2·0034 (inset shows an enlarged view) is attributable to semiquinone radicals. a.u., arbitrary units.
DISCUSSION
Our study draws a comprehensive picture of the effects of sunlight irradiance on the performance of F. ornus, a salt-sensitive species, under mild root-zone salinity. The results highlight that light-induced changes in morpho-anatomy, in addition to relevant biochemical characters, have great impact on the use of assimilated carbon in plants challenged by root zone salinity during summer, and profoundly affect the biochemical machinery aimed at countering salt-induced oxidative stress.
First, we suggest that the suite of morpho-anatomical features were responsible for greater net photosynthesis in leaves of sun plants than in those of shaded plants during the hottest hours of the day, irrespective of salinity treatment (Evans, 1999; Poorter et al., 2009). Light-induced increases in cuticle thickness and cuticular wax content may effectively limit cuticular transpiration, which is of particular significance in thin leaves, such as those of F. ornus (leaves acclimated for 5 weeks to full sunlight were 250 μm thick), particularly when stomatal conductance is depressed by salt-induced osmotic unbalance (Boyer et al., 1997; Ristic and Jenks, 2002). Light-induced reduction in leaf lamina size also confers on sun plants a great capacity for dissipating heat by conduction and convection (Nobel, 2005), a key feature for plants challenged by hot temperatures during summer. We calculate, using the method described by Nobel (2005), that sun plants dissipated 37 % more heat by conduction and convection during the warmest hours of the day (air temperature was on average 32·5 °C between 11:00 and 15:00 h). This may also help explain why stomatal conductance was higher in shaded than in sun plants during the hottest hours of the day. Sun plants regulated transpiration through stomata more effectively than shaded plants; stomata are indeed very sensitive to VPD in the sun (Nicolás et al., 2008), whereas daily changes in light irradiance, rather than VPD, have been reported as the main factor controlling stomatal opening in leaves of shaded plants (Nicolás et al., 2008). In our study, VPD was 14 % higher on a daily basis, and 33 % higher at midday, in full sun than in partial shading. Thus, enhanced stomatal control of water loss and heat dissipation by conduction/convection allowed control leaves in the sun to maintain transpiration rates similar to those in leaves of shaded plants during the warmest hours of the day. This is remarkable, as sun plants had to cope with both higher temperature (air temperature was ∼1 °C higher in the sun than in the shade) and higher VPD than shaded plants. Salt-induced decrease in soil water potential was instead the main driver of stomatal responsiveness, as stomatal conductance did not differ markedly between leaves of sun and shaded plants supplied with root-zone NaCl, in agreement with the results of Perez-Martin et al. (2009).
Despite greater stomatal control, stomata did not limit net photosynthesis more in the sun than in the shade under either the control condition or with root-zone salinity. Salt-induced decreases in mesophyll conductance were indeed similar in leaves of sun and shaded plants (Loreto et al., 2003). Therefore, diffusive limitations (SL + ML) were not the main determinants of the salt-induced declines in A observed in our study. In contrast, biochemical limitations on photosynthesis were markedly higher in shaded than in sun plants under control conditions, and these differences became even larger during salinity. This conforms to the values of both carboxylation efficiency (Vc,max) and mesophyll conductance (gm) estimated in our study as well as in a number of previous experiments (Flexas et al., 2004; Galmés et al., 2013). Lower constitutive (i.e. in control leaves) coupled with steeper salt-induced reductions in Vc,max may have been responsible for the larger increases in Cc observed in shaded than in sun plants in response to salinity (Galmés et al., 2011). An increase in Cc mostly occurs under severe stress conditions, as a result of biochemical limitations largely prevailing over the diffusive ones (Flexas et al., 2004; Galmés et al., 2011), as was also the case in our study in leaves of shaded salt-treated plants. Therefore, greater photosynthetic rates – not lower transpiration rates – were responsible for higher WUE in leaves of sun plants than in those of shaded plants during the hottest hours of the day (Medrano et al., 2012), particularly in salt-treated leaves. As mentioned above, morpho-anatomical features indeed have a crucial role in leaf water balance under field conditions, a role that is not revealed in experiments conducted under laboratory (or controlled) conditions (Mielke and Schaffer, 2010; Gardiner and Krauss, 2011). WUE was indeed similar in leaves of sun and shaded plants measured under laboratory conditions at saturating light as well as in leaves sampled in situ at 09:00 and 18:00 h.
The relatively small differences in the accumulation of potentially toxic ions and K+ detected in our experiment relate to similar daily gs (and instantaneous transpiration rate, not reported; García-Sánchez et al., 2006) displayed by salt-treated plants growing under contrasting sunlight. Our study therefore suggests that the effect of sunlight irradiance on ionic transport to actively growing shoot organs may have relatively minor significance under field conditions (Reynolds, 2000; López-Hoffman et al., 2007), particularly when soil salinity builds up in concomitance with hot temperatures. In turn, this leads us to conclude that in our study the performance of salt-treated shaded and sun plants depended mostly on the osmotic component of excess soil salinity rather than on the ionic component. In support of this, the K+/Na+ ratio was apparently high in salt-treated leaves (Greenway and Munns, 1980), ranging from 1·96 in shaded to 1·76 in sun plants. In contrast, the steeper salt-induced increase in mannitol concentration observed in shaded than in sun plants reveals that the leaves of shaded plants were less effective in active osmotic adjustment: dehydration indeed made a much greater contribution in shaded than in sun plants to salt-induced osmotic changes (Gucci et al., 1997; Tattini et al., 2002). Nonetheless, it is worth noting that sun plants made considerable use of ‘cheap’ osmolytes, i.e. Na+, Cl– and K+, for osmotic adjustment (Greenway and Munns, 1980; Gucci et al., 1997) compared with shaded plants. This likely allowed sun plants to devote more fresh carbon to growth than to biosynthesis of compatible solutes (Cimato et al., 2010) – a very costly process (Chen et al., 2007) – compared with shade plants. This conforms to greater salt-induced decreases in RGRshoot observed in shaded than in sun plants in our study. Furthermore, in salt-treated sun plants photosynthetic performance might have benefited from higher concentrations of K+ compared with their shaded counterparts (Anschütz et al., 2014; Shabala and Potosin, 2014). On the contrary, the steeper salt-induced increase in mannitol concentration possibly feedback-inhibited photosynthesis more in shaded than in sun plants (Paul and Pellny, 2003). However, the massive use of Na+ and Cl– for osmotic adjustment may raise concern about the performance of ‘glycophytes’ on a long-term basis (Gucci et al., 1997; Tattini et al., 2002). This issue merits further investigation (e.g. by performing field experiment), as F. ornus and other deciduous tree species are transiently challenged by excess soil salinity in Mediterranean areas.
By the end of the experiment, salt-treated leaves in the shade were subjected to a more severe excess of light during the central hours of the day compared with sun plants. Saturation of photosynthesis occurred at 33·2 and 42·2 % of the maximum sunlight irradiance at which shaded and sun plants grew, respectively. In the leaves of salt-treated shaded plants, A and CO2,daily accounted for 63 and 38 %, respectively, of the corresponding values in sun plants. This is also consistent with the salt-induced increase in the reduction state of primary acceptors, 1 – qP, which was markedly higher in shaded than in sun plants. Consequently, the salt-induced activation of de-epoxidation processes within the violaxanthin cycle (VAZ) pool to sustain thermal dissipation of excess energy through NPQ was greater in the shade than in the sun. A correlation between DES and NPQ has been reported in many instances (Müller, 2001; Demmig-Adams and Adams, 2006). However, in our study, DES, but not NPQ, was significantly higher in shaded than in sun plants suffering from salinity stress. We note first that salt-treated leaves in the shade underwent greater decreases (–33 %) in violaxanthin compared with sun plants (–13 %). In sun plants, therefore, the salt-induced increase in zeaxanthin may have been in part derived from β-carotene, the concentration of which decreased (–29 %) in response to salinity (Davison et al., 2002; Du et al., 2010). Second, zeaxanthin-dependent NPQ occurs at relatively low concentrations of VAZ relative to Chltot (usually <50 mmol mol–1 Chltot; Havaux and Niyogy, 1999; Niinemets et al., 2003), and in our study the concentration of the VAZ pool greatly exceeded 100 mmol mol–1 Chltot. This supports a role of zeaxanthin, located in other parts of the thylakoid membranes, which is not directly involved in NPQ, possibly aimed at directly preserving the chloroplast membrane from photo-oxidation (Havaux and Niyogy, 1999; Beckett et al., 2012). Third, we conducted NPQ measurements under laboratory conditions, whereas we analysed carotenoids in leaves sampled in the field. Components of the VAZ cycle have key roles in preserving chloroplast membranes from heat (Havaux, 1998; Fernández-Marin et al., 2011), and this may have been of particular value in leaves of salt-treated shaded plants, which underwent steep declines in A, particularly during the central hours of the day. In other words, leaves have to sustain greater NPQs in the field during the hottest hours of the day, which is not revealed by NPQ measurements under laboratory conditions. Our data do not support the notion that slow-relaxing components that are independent of xanthophylls play a prominent role in NPQ. Slow-relaxing components of NPQ become relevant when leaves are severely photo-inhibited (Müller et al., 2001), which was not the case in our study.
The activity of antioxidant enzymes has been reported to be related to stress tolerance in some instances, though it is still uncertain whether a constitutive or stress-induced increase in antioxidant enzyme activity confers greater tolerance to abiotic stresses (for reviews see Smirnoff, 1998; Gill and Tuteja, 2010). The results of our study therefore support the idea that sun plants were more tolerant of root-zone salinity than shaded plants (Mittal et al., 2012; Turkan et al., 2013). Indeed, enzymes aimed at H2O2 removal were more active in control and particularly in salt-treated leaves growing in the sun than in those in partial shade, as recently observed in tomato fruits (Zushi et al., 2014). We hypothesize that greater reduction in radiation use efficiency coupled with hot temperatures may have detrimentally affected the activities of enzymes aimed at detoxifying ROS in leaves of shaded plants under salinity stress (Peltzer and Polle, 2001; Hatier and Gould, 2008; Fini et al., 2011, 2012). A salt-induced decrease in total ASA concentration in leaves of shaded plants, mostly due to reduction in DHASA concentration, is indeed consistent with the very low APX activity in leaves of salt-treated shaded plants. Interestingly, following salt-induced reduction of CAT and APX activities, the activity of guaiacol POX increased in leaves of shaded plants (Fini et al., 2012; Maia et al., 2013). This increase occurred in parallel with marked increases in the concentrations of effective antioxidant phenylpropanoids (e.g. the ratio of esculetin to esculin increased by as much as 102 % in leaves of shaded plants in response to salinity). It is therefore possible that POX and antioxidant phenylpropanoids will have complemented the action of primary antioxidant defences in countering salt-induced ROS generation in the shade (Ferreres et al., 2011; Agati et al., 2012).
Both shaded and sun plants underwent salt-induced damage at the sub-cellular level. Interestingly, in leaves of shaded plants damage mostly occurred in spongy mesophyll cells, which might indicate that direct flux of solar energy was not the main driver of oxidative stress, which was likely the case in sun plants. It is apparent that, in sun plants, palisade cells preserved the underlying tissues from severe photo-oxidative stress, whereas in leaves of shaded plants the radiant energy that reached the spongy cells was likely much more that usable for photosynthesis, due to the very low ability to carboxylate CO2. The signal centred on the fourth line of the Mn(H2O)62+ sextet at g = 2·0034 is consistent with the formation of semiquinone radicals (Hatano et al., 1997; Gallez et al., 2000). Semiquinones had possibly originated in the incomplete recycling of phenylpropanoids following their oxidation mediated by vacuolar POX (Hernández et al., 2009; Agati and Tattini, 2010). However, it is likely that polyphenols flowed out from the vacuole of severely damaged cells and were then oxidized by PPO, the activity of which rapidly increased because of salinity stress, particularly in leaves of shaded plants.
CONCLUSIONS
Fraxinus ornus, a glycophyte distributed mostly in the southern Mediterranean basin, may transiently face excess soil salinity during the summer months. Here we show that root-zone salinity may be more deleterious for plants growing in partial shade than for those growing in full sunlight, though the accumulation of potentially toxic ions is greater in sun than in shaded plants. This is because the suite of morpho-anatomical traits coupled with an inherently greater efficiency to carboxylate CO2 allows greater photosynthetic rates in leaves of sun plants than in those of shaded plants, particularly when CO2 diffusion in the leaf declines considerably because of salinity. Furthermore, potentially toxic ions and K+ may contribute to active osmotic adjustment to a greater extent in leaves of sun plants than in those of shaded plants (which mostly use compatible solutes for osmotic adjustment), thus diverting less carbon and energy from growth while exerting a positive effect on photosynthetic performance. Following more severe reductions in photosynthesis, leaves of shaded plants are more likely to unexpectedly experience excess light stress compared with leaves growing in full sunlight. Drastic salt-induced reductions in radiation use efficiency in leaves of shaded plants may therefore result in severe depletion of primary antioxidant defences and photo-oxidative damage. We therefore conclude that, under mild root-zone salinity [relatively low soil salt concentration on a short-term (weeks) basis], deciduous tree species, such as F. ornus, are more resistant when inhabiting sunny than shaded habitats.
SUPPLEMENTARY DATA
ABBREVIATIONS
1 – qP, reduction state of primary acceptors; A, net CO2 assimilation rate (net photosyntesis); APX, ascorbate peroxidase; ASA, ascorbate; BL, biochemical limitations on net CO2 assimilation rate; C, control (group); C, molar concentration of solutes (mol kg−1 dry weight); Ca, external CO2 concentration; CAT, catalase; Cc, CO2 concentration in chloroplasts; Chltot, total chlorophyll; Ci, intercellular CO2 concentration; Ci, internal CO2 concentration; CO2,daily, net daily carbon gain; D, dehydration; DES, de-epoxidation state; DHASA, oxidized ascorbate; DW, dry weight; E, transpiration rate; F0, minimal fluorescence; Fm, maximum fluorescence of dark-adapted leaves (over a 40-min period); Fv, variable fluorescence of dark-adapted leaves (over a 40-min period); Fv/Fm, maximum efficiency of PSII photochemistry; gm, mesophyll conductance to CO2 diffusion; gs, stomatal conductance; Jf, rate of linear electron transport estimated from chlorophyll fluorescence; Jmax, maximum electron transport rate contributing to Rubisco regeneration; LMA, leaf mass per area; ML, mesophyll conductance limitations on net CO2 assimilation rate; MS, mass spectrometry; NIV, normalized index of variation; NPQ, non-photochemical quenching; POX, peroxidase; PPFD, photosynthetic photon flux density; PPFDsat photosynthetic active radiation at which saturation of photosynthesis occurs; PPO, polyphenol oxidase; POX, peroxidase; PS photosystem; qP, coefficient of photochemical quenching; RDW, relative dry weight; RGRshoot, relative growth rate of the shoot; Rubisco, activity of ribulose-1,5-bis-phosphate carboxylase/oxygenase; ROS, reactive oxygen species; RWC, relative water content; SL, stomatal limitations on net CO2 assimilation rate; SOD, superoxide dismutase; ST, salt-treated (group); TEM, transmission electron microscopy; VAZ, violaxanthin cycle; Vc,max, apparent rate of maximum carboxylation by Rubisco; Vc,max, carboxylation efficiency; VPD, vapour pressure deficit; W, shoot dry weight; WUE, water use efficiency; ΦPSII, quantum yield of photosystem II in the light; ψπ, osmotic potential; ψπFT, osmotic potential at full turgor; ψπFT,s, contribution of solutes to ψπFT; ψπ,s, contribution (–MPa) of individual solutes (s) to ψπFT; ψw, water potential; ψπ, osmotic potential; ψπFT, osmotic potential at full turgor; ФPSII, actual efficiency of PSII photochemistry; Δψ, difference in leaf osmotic potential between salt-treated and control plants;
Supplementary Material
ACKNOWLEDGEMENTS
Work in the authors' laboratory was funded partly by the PRIN Project TreeCity (MIUR, Rome, Italy) and Uniser Consortium Pistoia.
LITERATURE CITED
- Agati G, Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytologist. 2010;186:786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Science. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Anschütz U, Becker D, Shabala S. Going beyond nutrition: regulation of potassium homeostasis as a common denominator of plant adaptive responses to environment. Journal of Plant Physiology. 2014;171:670–787. doi: 10.1016/j.jplph.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Barradas VL, Nicolàs E, Torrecillas A, Alarcón JJ. Transpiration and canopy conductance in young apricot (Prunus armeniaca L.) trees subjected to different PAR levels and water stress. Agricultural Water Management. 2005;77:323–333. [Google Scholar]
- Beckett M, Loreto F, Velikova V, et al. Photosynthetic limitations and volatile and non-volatile isoprenoids in the poikilochlorophyllous resurrection plant Xerophyta humilis during dehydration and rehydration. Plant, Cell & Environment. 2012;35:2061–2074. doi: 10.1111/j.1365-3040.2012.02536.x. [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown JR, Mansfield JW. Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a non-host hypersensitive reaction in lettuce. Plant Physiology. 1998;118:1067–1078. doi: 10.1104/pp.118.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS, Wong SC, Farquhar GD. CO2 and water vapour exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiology. 1997;114:185–191. doi: 10.1104/pp.114.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis during drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. Journal of Experimental Botany. 2007;58:4245–4255. doi: 10.1093/jxb/erm284. [DOI] [PubMed] [Google Scholar]
- Cimato A, Castelli S, Tattini M, Traversi ML. An ecophysiological analysis of salinity tolerance in olive. Environmental and Experimental Botany. 2010;68:214–221. [Google Scholar]
- Davison PA, Hunter CN, Horton P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature. 2002;418:203–206. doi: 10.1038/nature00861. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytologist. 2006;172:11–21. doi: 10.1111/j.1469-8137.2006.01835.x. [DOI] [PubMed] [Google Scholar]
- Du H, Wang N, Cui F, Li X, Xiao J, Xiong L. Characterization of the β-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiology. 2010;154:1304–1318. doi: 10.1104/pp.110.163741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espín JC, Morales M, García-Ruiz PA, Tudela J, García-Cánovas F. Improvement of a continuous spectrophotometric method for determining the monophenolase and diphenolase activities of mushroom polyphenol oxidase. Journal of Agricultural and Food Chemistry. 1997;45:1084–1090. [Google Scholar]
- Evans JR. Leaf anatomy enables more equal access to light and CO2 between chloroplasts. New Phytologist. 1999;143:93–104. [Google Scholar]
- Fernández-Marín B, Míguez F, Becerril JM, García-Plazaola JI. Activation of violaxanthin cycle is a common response to different abiotic stresses: a case study in Pelvetia canaliculata. BMC Plant Biology. 2011;11:181. doi: 10.1186/1471-2229-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreres F, Figuereido R, Bettencourt S, et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidases: an H2O2 affair? Journal of Experimental Botany. 2011;62:2841–2854. doi: 10.1093/jxb/erq458. [DOI] [PubMed] [Google Scholar]
- Fini A, Brunetti C, Di Ferdinando M, Ferrini F, Tattini M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling and Behavior. 2011;6:709–711. doi: 10.4161/psb.6.5.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini A, Guidi L, Ferrini F, et al. Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: an excess light stress affair? Journal of Plant Physiology. 2012;169:929–939. doi: 10.1016/j.jplph.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology. 2004;6:269–279. doi: 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- Fusaro L, Mereu S, Brunetti C, et al. Photosynthetic performance and biochemical adjustments in two co-occurring Mediterranean evergreens, Quercus ilex and Arbutus unedo, differing in salt-exclusion ability. Functional Plant Biology. 2014;41:391–400. doi: 10.1071/FP13241. [DOI] [PubMed] [Google Scholar]
- Gallez B, Baudelet C, Debuyst R. Free radicals in licorice-flavored sweets can be detected noninvasively using low frequency electron paramagnetic resonance after oral administration to mice. Journal of Nutrition. 2000;830:1831–1833. doi: 10.1093/jn/130.7.1831. [DOI] [PubMed] [Google Scholar]
- Galmés J, Flexas J, Keys AJ, et al. Rubisco specificity factor tends to be larger in plant species from dryer habitats and in species with persistent leaves. Plant, Cell & Environment. 2005;28:571–579. [Google Scholar]
- Galmés J, Ribas-Carbó M, Medrano H, Flexas J. Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. Journal of Experimental Botany. 2011;62:653–665. doi: 10.1093/jxb/erq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Aranjuelo I, Medrano H, Flexas J. Variation in Rubisco content and activity under variable climatic factors. Photosynthesis Research. 2013;117:73–90. doi: 10.1007/s11120-013-9861-y. [DOI] [PubMed] [Google Scholar]
- García-Sánchez F, Syvertsen JP, Martínez V, Melgar JC. Salinity tolerance of ‘Valencia’ orange trees on rootstocks with contrasting salt tolerance is not improved by moderate shade. Journal Experimental Botany. 2006;57:3697–3706. doi: 10.1093/jxb/erl121. [DOI] [PubMed] [Google Scholar]
- Gardiner ES, Krauss KW. Photosynthetic light response of flooded cherrybark oak (Quercus pagoda) seedlings grown in two light regimes. Tree Physiology. 2001;21:1103–1111. doi: 10.1093/treephys/21.15.1103. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Grassi G, Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell & Environment. 2005;28:834–849. [Google Scholar]
- Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annual Review of Plant Physiology. 1980;31:149–190. [Google Scholar]
- Guidi L, Degl'Innocenti E, Remorini D, Massai R, Tattini M. Interactions of water stress and sunlight irradiance on the physiology and biochemistry of Ligustrum vulgare. Tree Physiology. 2008;28:873–883. doi: 10.1093/treephys/28.6.873. [DOI] [PubMed] [Google Scholar]
- Guidi L, Degl'Innocenti E, Giordano C, Biricolti S, Tattini M. Ozone tolerance in Phaseolus vulgaris depends on more than one mechanism. Environmental Pollution. 2010;158:3164–3171. doi: 10.1016/j.envpol.2010.06.037. [DOI] [PubMed] [Google Scholar]
- Gucci R, Lombardini L, Tattini M. Analysis of leaf water relations in leaves of two olive (Olea europaea L.) cultivars differing in tolerance to salinity. Tree Physiology. 1997;17:13–21. doi: 10.1093/treephys/17.1.13. [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology. 1992;98:1429–1436. doi: 10.1104/pp.98.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano T, Takagi M, Ito H, Yoshida T. Phenolic constituents of liquorice. VII. A new chalcone with a potent radical scavenging activity and accompanying phenolics from liquorice. Chemical Pharmaceutical Bulletin. 1997;45:1485–1492. [Google Scholar]
- Hatier JHB, Gould KS. Foliar anthocyanins as modulators of stress signals. Journal of Theoretical Biology. 2008;253:625–627. doi: 10.1016/j.jtbi.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Hauke V, Schreiber L. Ontogenetic and seasonal development of wax composition and cuticular transpiration of ivy (Hedera helix L.) sun and shade leaves. Planta. 1998;207:67–75. [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends in Plant Sciences. 1998;3:147–151. [Google Scholar]
- Havaux M, Niyogy KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism; Proceedings of the National Academy of Sciences of the USA; 1999. pp. 8762–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández I, Alegre L, van Breusegem F, Munné-Bosch S. How relevant are flavonoids as antioxidants in plants? Trends in Plant Science. 2009;14:125–132. doi: 10.1016/j.tplants.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Laisk A, Loreto F. Determining photosynthetic parameters from leaf CO2 exchange and chlorophyll fluorescence. Plant Physiology. 1996;110:903–912. doi: 10.1104/pp.110.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S, Bernacchi C. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany. 2003;392:2393–2401. doi: 10.1093/jxb/erg262. [DOI] [PubMed] [Google Scholar]
- López-Hoffman L, Anten NPR, Martinez-Ramos M, Ackerly DD. Salinity and light interactively affect neotropical mangrove seedlings at the leaf and whole plant levels: Oecologia. 2007;150:545–556. doi: 10.1007/s00442-006-0563-4. [DOI] [PubMed] [Google Scholar]
- Loreto F, Harley PC, Di Marco G, Sharkey TD. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology. 1992;98:1437–1443. doi: 10.1104/pp.98.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Centritto M, Chartzoulakis K. Photosynthetic limitations in olive cultivars with different sensitivity to salt stress. Plant, Cell & Environment. 2003;26:595–601. [Google Scholar]
- Maia JM, Voigt EL, Ferreira-Silva SL, Fontenele A., de V, Macedo CEC, Silveira JAG. Differences in cowpea root growth triggered by salinity and dehydration are associated with oxidative modulation involving types I and III peroxidases and apoplastic ascorbate. Journal of Plant Growth Regulation. 2013;32:376–387. [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Medrano H, Pou A, Tomás M, et al. Average daily light interception determines leaf water use efficiency among different canopy locations in grapevine. Agricultural Water Management. 2012;114:4–10. [Google Scholar]
- Melgar JC, Guidi L, Remorini D, et al. Antioxidant defences and oxidative damage in salt-treated olive plants under contrasting sunlight irradiance. Tree Physiology. 2009;29:1187–1198. doi: 10.1093/treephys/tpp047. [DOI] [PubMed] [Google Scholar]
- Mielke SM, Schaffer B. Photosynthetic and growth responses of Eugenia uniflora L. seedlings to soil flooding and light intensity. Environmental and Experimental Botany. 2010;68:113–121. [Google Scholar]
- Mittal S, Kumari N, Sharma V. Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiology and Biochemistry. 2012;54:17–26. doi: 10.1016/j.plaphy.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Müller P, Li X-P, Niyogi KK. Non-photochemical quenching. A response to excess light energy. Plant Physiology. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux P, Karpinski S. Signal transduction in response to excess light: getting out of the chloroplast. Current Opinion in Plant Biology. 2002;5:43–48. doi: 10.1016/s1369-5266(01)00226-6. [DOI] [PubMed] [Google Scholar]
- Munns R, James RA. Screening methods for salinity tolerance: a case of study with tetraploid wheat. Plant and Soil. 2003;253:201–218. [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nicolás E, Barradas VL, Ortuño MF, Navarro A, Torrecillas A, Alarcón JJ. Environmental and stomatal control of transpiration and decoupling coefficient in young lemon trees under shading net. Environmental and Experimental Botany. 2008;63:200–206. [Google Scholar]
- Niinemets Ü, Kollist H, García-Plazaola JI, Hernández A, Becerril JM. Do the capacity and kinetics for modification of xanthophylls cycle pool size depend on growth irradiance in temperate trees? Plant, Cell & Environment. 2003;26:1787–1801. [Google Scholar]
- Nobel PS. Physicochemical and environmental plant physiology. 3rd edn. Burlington, MA: Academic Press; 2005. [Google Scholar]
- Ouerghi Z, Cornic G, Roudani M, Ayadi A, Brulfert J. Effect of NaCl on photosynthesis of two wheat species (T. durum and T. aestivum) differing in their sensitivity to salt stress . Journal of Plant Physiology. 2000;156:335–340. [Google Scholar]
- Paul MJ, Pellny TK. Carbon metabolite feedback regulation of leaf photosynthesis and development. Journal of Experimental Botany. 2003;54:539–547. doi: 10.1093/jxb/erg052. [DOI] [PubMed] [Google Scholar]
- Peltzer D, Polle A. Diurnal fluctuations of antioxidative systems in leaves of field-grown beech trees (Fagus sylvatica): responses to light and temperature. Physiologia Plantarum. 2001;111:158–164. [Google Scholar]
- Perez-Martin A, Flexas J, Ribas-Carbó M, et al. Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea. Journal of Experimental Botany. 2009;60:2391–2405. doi: 10.1093/jxb/erp145. [DOI] [PubMed] [Google Scholar]
- Ponton S, Dupouey J-L, Bréda N, Dreyer E. Comparison of water-use efficiency of seedlings from two sympatric oak species: genotype × environment interactions. Tree Physiology. 2002;22:413–422. doi: 10.1093/treephys/22.6.413. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Causes and consequences of variations in leaf mass per area (LMA): a meta-analysis. New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Ramel F, Birtic S, Cuiné S, Triantaphylidès C, Ravanat J-L, Havaux M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiology. 2012;158:1267–1278. doi: 10.1104/pp.111.182394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CS. Gloomy predictions – modelling photoautotrophy in low-light environments. New Phytologist. 2000;146:182–184. doi: 10.1046/j.1469-8137.2000.00650.x. [DOI] [PubMed] [Google Scholar]
- Rhizopoulou S, Meletiou-Christou MS, Diamantoglou S. Water relations for sun and shade leaves of four Mediterranean evergreen sclerophylls. Journal of Experimental Botany. 1991;42:627–635. [Google Scholar]
- Ristic Z, Jenks MA. Leaf cuticle and water loss in maize lines differing in dehydration avoidance. Journal of Plant Physiology. 2002;159:645–651. [Google Scholar]
- Shabala S, Potosin I. Regulation of potassium transport in hostile conditions: implications for abiotic and biotic stress tolerance. Physiologia Plantarum. 2014 doi: 10.1111/ppl.12165. doi:10.1111/ppl.12165. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Bilger W, Neubaer C. Chlorophyll fluorescence as a non-intruder indicator of rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM, editors. Ecophysiology of photosynthesis. Berlin: Springer; 1995. pp. 49–70. [Google Scholar]
- Smirnoff N. Plant resistance to environmental stresses. Current Opinion in Biotechnology. 1998;9:214–219. doi: 10.1016/s0958-1669(98)80118-3. [DOI] [PubMed] [Google Scholar]
- Tattini M, Gucci R. Ionic relations of Phyllirea latifolia L. plants during NaCl stress and relief from stress. Canadian Journal of Botany. 1999;77:969–975. [Google Scholar]
- Tattini M, Gucci R, Romani A, Baldi A, Everard JD. Changes in non-structural carbohydrates in olive (Olea europaea) leaves during root zone salinity stress. Physiologia Plantarum. 1996;98:117–124. [Google Scholar]
- Tattini M, Gravano E, Pinelli P, Mulinacci N, Romani A. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytologist. 2000;148:69–77. doi: 10.1046/j.1469-8137.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- Tattini M, Montagni G, Traversi ML. Gas exchange, water relations and osmotic adjustment in Phyllirea latifolia grown at various salinity concentrations. Tree Physiology. 2002;22:403–412. doi: 10.1093/treephys/22.6.403. [DOI] [PubMed] [Google Scholar]
- Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytologist. 2004;163:547–561. doi: 10.1111/j.1469-8137.2004.01126.x. [DOI] [PubMed] [Google Scholar]
- Tattini M, Guidi L, Morassi-Bonzi L, et al. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to high solar radiation. New Phytologist. 2005;167:457–470. doi: 10.1111/j.1469-8137.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Tattini M, Remorini D, Pinelli P, et al. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytologist. 2006;170:779–794. doi: 10.1111/j.1469-8137.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Turkan I, Demiral T, Sekmen AH. The regulation of antioxidant enzymes in two Plantago species differing in salinity tolerance under combination of waterlogging and salinity. Functional Plant Biology. 2013;40:484–493. doi: 10.1071/FP12147. [DOI] [PubMed] [Google Scholar]
- Valladares F, Pearcy RW. Drought can be more critical in the shade than in the sun: a field study of carbon gain and photo-inhibition in a Californian shrub during a dry El Niño year. Plant, Cell & Environment. 2002;25:749–759. [Google Scholar]
- Zhu JJ, Zhang JL, Liu HC, Cao KF. Photosynthesis, non-photochemical pathways and activities of antioxidant enzymes in a resilient evergreen oak under different climatic conditions from a valley-savanna in southwest China. Physiologia Plantarum. 2009;135:62–72. doi: 10.1111/j.1399-3054.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- Zushi K, Ono M, Matsuzoe N. Light intensity modulates antioxidant systems in salt-stressed tomato (Solanum lycopersicum L. cv. Micro-Tom) fruits. Scientia Horticulturae. 2014;165:384–391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.