Abstract
Background and Aims
Vulnerability of the leaf hydraulic pathway to water-stress-induced dysfunction is a key component of drought tolerance in plants and may be important in defining species' climatic range. However, the generality of the association between leaf hydraulic vulnerability and climate across species and sites remains to be tested.
Methods
Leaf hydraulic vulnerability to drought (P50leaf, the water potential inducing 50 % loss in hydraulic function) was measured in a diverse group of 92 woody, mostly evergreen angiosperms from sites across a wide range of habitats. These new data together with some previously published were tested against key climate indices related to water availability. Differences in within-site variability in P50leaf between sites were also examined.
Key Results
Values of hydraulic vulnerability to drought in leaves decreased strongly (i.e. became more negative) with decreasing annual rainfall and increasing aridity across sites. The standard deviation in P50leaf values recorded within each site was positively correlated with increasing aridity. P50leaf was also a good indicator of the climatic envelope across each species' distributional range as well as their dry-end distributional limits within Australia, although this relationship was not consistently detectable within sites.
Conclusions
The findings indicate that species sorting processes have influenced distributional patterns of P50leaf across the rainfall spectrum, but alternative strategies for dealing with water deficit exist within sites. The strong link to aridity suggests leaf hydraulic vulnerability may influence plant distributions under future climates.
Keywords: Leaf hydraulic vulnerability, interspecific variation, drought, rainfall, aridity, climate change, species distribution
INTRODUCTION
Plant functional traits that vary systematically across environmental gradients are considered to be adaptive because they enhance species performance and survival under particular environmental conditions (Ackerly, 2003). In cases where they have a known mechanistic relationship with specific environmental stress, such as drought, these traits offer insight into how specific changes in the environment might affect vegetation structure and function (McGill et al., 2006).
Under drought stress, the water transport pathway from the roots to the sites of evaporation in the leaves is vulnerable to dysfunction. The ability of plants to resist such drought-induced hydraulic stress varies widely across species. Drought stress vulnerability has been shown to correlate with site water availability (Pockman and Sperry, 2000; Choat et al., 2007; Nardini et al., 2012), with mean annual rainfall (Maherali et al., 2004), and with the dry-end rainfall boundaries of species distributions (Blackman et al., 2012; Brodribb and Hill, 1999). These correlations imply that resistance to hydraulic dysfunction is adaptive, meaning both that it can confer benefits to plants from environments that experience strong water deficit, and also that it incurs costs so that strong resistance to hydraulic dysfunction should not be found where it is not needed.
Most studies of interspecific variation in the ability of plants to resist drought-induced hydraulic dysfunction have examined plant stems. However, more recent research has begun to reveal the functional significance of this ability in leaves. Leaves represent a disproportionately large bottleneck in the whole-plant hydraulic continuum (>30 % of whole-plant hydraulic resistance), and thus may limit maximum rates of photosynthetic gas exchange (Sack and Holbrook, 2006). As in stems, the vulnerability of the leaf hydraulic pathway is generally measured as P50, or the water potential value inducing 50 % loss of maximum hydraulic conductance (Brodribb and Holbrook, 2003). Such losses in leaf hydraulic conductance have been linked to cavitation and the formation of embolisms in the water conducting xylem (Johnson et al., 2012); however, conduit collapse (Cochard et al., 2004; Brodribb and Holbrook, 2005) and extravascular processes such as turgor loss (Brodribb and Holbrook, 2004b; Knipfer and Steudle, 2008) and leaf shrinkage (Scoffoni et al., 2014) may also be important in driving changes in leaf hydraulic function.
Compared with stems, leaves are typically more vulnerable to hydraulic dysfunction (Brodribb et al., 2003; Choat et al., 2005; Hao et al., 2008). Hydraulic conductance in leaves (Kleaf) is also typically more dynamic, with many species losing and recovering more than 50 % of Kleaf diurnally (Johnson et al., 2009) as a function of changes in evaporative demand (Brodribb and Holbrook, 2004a) and water availability (Scoffoni et al., 2011). In some species, the onset of this reduction in Kleaf has been shown to coincide with reduced stomatal conductance and photosynthesis (Brodribb and Holbrook, 2003; Blackman et al., 2009), which suggests leaf hydraulic vulnerability may play an important role in defining the short-term response of plants to water stress. Leaf hydraulic vulnerability has also been linked to species absolute drought tolerance in both conifers (Brodribb and Cochard, 2009) and angiosperms (Blackman et al., 2009).
Recent studies of interspecific variation in P50leaf suggest leaf hydraulic vulnerability influences species distributions across water availability gradients at local scales (Nardini et al., 2012) and at the very dry-end of their geographical ranges (Blackman et al., 2012). These studies have indicated an adaptive link between P50leaf and site water availability using small groups of angiosperms within a relatively narrow band of climate. Here we seek to test this relationship across a much wider spread of climate zones and species.
Based on a functional link between leaf hydraulic vulnerability and drought resistance, across species we expected P50leaf would (1) become more negative (stronger resistance to hydraulic dysfunction) with both decreasing site rainfall and increasing site aridity, and (2) influence species climatic range. Given that within-site variation in stem hydraulic vulnerability to drought can be high, particularly in arid (Jacobsen et al., 2007b; Pratt et al., 2012) and seasonally dry tropical environments (Markesteijn et al., 2011; Pineda-Garcia et al., 2013), we also assessed and compared the level of within-site variability in P50leaf across sites that might indicate divergences in drought strategy among co-occurring species.
MATERIALS AND METHODS
Species and sites
We made new measurements of P50leaf in species from five sites across eastern Australia. Four of these sites (Kur-ing-gai, Yengo, Round Hill and Fowler's Gap) were associated with a strong east–west aridity gradient in New South Wales, while a fifth site (Princess Hills) was located in seasonally dry eucalyptus woodland in tropical Queensland. Together with species previously studied from Tasmania (Mount Field) and central Peru (Cordillera Yanachaga) (Blackman et al., 2010, 2012), these sites spanned a variety of habitats, ranging from sparse arid scrubland to ever-wet montane cloud forest. Across sites, rainfall varied widely, ranging from 236 to 3170 mm per year, while aridity index (rainfall/pan evaporation) varied 17-fold (Table 1). With the exception of monsoonal Princess Hills, the Australian sites are characterized by relatively even monthly rainfall throughout the year (Supplementary Data Fig. S1). The cloud forest site in Peru has a moderate dry season during the austral winter (Supporting Information Fig. S1), but persistent canopy cloud cover approx. 75 % of the year (Catchpole, 2012) probably maintains ever-wet soil conditions.
Table 1.
Details and climate characteristics of each sample site in the study; climate characteristics include mean annual precipitation (MAP), minimum and maximum average monthly temperature, and aridity index (AI)
| Site | Habitat | Coordinates | Elevation (m) | MAP (mm) | Min. temp. (°C) | Max. temp. (°C) | AI |
|---|---|---|---|---|---|---|---|
| Yanachaga | Montane cloud forest | 10·516°S, 75·35°W | 2800 | 3170 | 3·4 | 18·1 | 2·524 |
| Mount Field | Sub-alpine rain forest | 42·679°S, 46·623°E | 900 | 1530 | –1·1 | 15·8 | 2·096 |
| Ku-ring-gai | Coastal woodland/scrub | 33·679°S, 151·147°E | 201 | 1210 | 5·3 | 26·3 | 1·038 |
| Princess Hills | Seasonally dry woodland | 18·295°S, 145·492°E | 677 | 1139 | 10·1 | 30·6 | 0·717 |
| Yengo | Sclerophyllous woodland | 32·778°S, 150·922°E | 310 | 779 | 2·8 | 28·6 | 0·598 |
| Round Hill | Semi-arid shrubland | 32·976°S, 146·156°E | 180 | 383 | 3·6 | 33·2 | 0·287 |
| Fowler's Gap | Sparse arid scrub | 31·073°S, 141·678°E | 182 | 236 | 4·3 | 34·0 | 0·146 |
A total of 92 woody angiosperm species were used in the analysis, representing 32 families (Supplementary Data Table S1). These species were largely evergreen, but varied widely in aspects of leaf form and function; leaf size varied by nearly four orders of magnitude, ranging from 0·012 to 104·5 cm2, while leaf dry mass per area (LMA) ranged from 43 g m–2 in the succulent leaves of Atriplex angulata to 772 g m–2 in the extremely scleromorphic needles of Hakea lissosperma (Table S1). The sample also included a drought deciduous species Planchonia careya, the winter deciduous species Nothofagus gunnii and three leaf succulent species from Amaranthaceae.
Leaf hydraulic vulnerability (P50leaf)
For each mainland Australian site leaf hydraulic vulnerability was measured in 11–16 of the most common co-occurring woody species. Leaf hydraulic vulnerability data for the Tasmanian and Peruvian species were sourced from previous work (Blackman et al., 2010, 2012). Vulnerability curves were generated for each species by measuring Kleaf at a range of water potentials (Ψleaf). Kleaf was measured using a modified rehydration technique whereby leaves were allowed to rehydrate while connected to a flow meter (Brodribb and Cochard, 2009). Branches 1–2 m in length were cut from the upper canopies of three individuals of each species and immediately placed inside plastic bags for transport back to the laboratory. Most sampling was done early in the morning when plants were well hydrated. Cut branches were laid on the bench top and allowed to dehydrate slowly in approx. 1-MPa steps. At each 1-MPa step, the branch was carefully re-bagged to arrest water loss and ensure water potential equilibrium. After equilibration, two adjacent leaves or small shoot tips with fully expanded leaves were cut from each branch for determination of Ψleaf (pressure chamber Model 1000, PMS Instruments, Corvallis, OR, USA). An adjacent sample shoot was then cut under water to prevent vessel cavitation and immediately connected via silicon tubing to a beaker of ultra-pure milli-Q water placed on a laboratory balance (Sartorius CP225D, Göttingen, Germany). A pressure release valve ensured zero pressure in the system upon connection of the shoot to the tubing. Water was then drawn into the rehydrating leaves out of the balance, which logged the change in mass every 2 s. The maximum rate of water flow was calculated from the first 3–4 data points at the very beginning of the rehydration (exponential decay) curve. This rate was then normalized by leaf area, pressure gradient (i.e. leaf water potential) and water viscosity. For each species, normalized flow rates (Kleaf) were maximal in shoots near full hydration (between –1 and –2 MPa) and declined as shoots became progressively drier. Loss in Kleaf was plotted against initial leaf (xylem) water potential and fitted with an exponential sigmoidal equation (Pammenter and Vander Willigen, 1998). We assumed losses in hydraulic conductance in our sample group were primarily due to cavitation and the formation of embolisms in the water conducting xylem; however, we acknowledge that processes such as turgor loss and leaf shrinkage may also act to drive changes in Kleaf (Scoffoni et al., 2014), especially in the small number of non-sclerophyllous species from moist sites. Embolism resistance was expressed as the water potential at which 50 % of the initial conductance was lost (P50leaf; see Supplementary Data Fig. S2 for species vulnerability curves).
Climate data
To examine how P50leaf related to site-specific climate indices of water availability we collected data on mean annual precipitation (MAP) and aridity index [AI, the ratio of MAP to potential evapotranspiration (Thornthwaite, 1948)]. For each Australian site, long-term rainfall averages were sourced via the Australian Bureau of Meteorology from adjacent or nearby (<10 km) meteorological stations. For the montane cloud forest site in Peru, rainfall data were based on 7 years of within-site measurements (2003–2009; D. Catchpole, University of Tasmania, unpubl. res.). Potential evapotranspiration data were downloaded from the CGIAR-CSI geospatial database (http://www.cgiar-csi.org/data) and were modelled after Hargreaves and Allen (2003).
To test whether leaf hydraulic vulnerability relates to species climatic range we downloaded MAP and AI data for all observation points across each species distribution within Australia (Atlas of Living Australia website, http://www.ala.org.au). Peruvian species were excluded from this analysis because sufficient location data do not exist for them. Species climatic range was then characterized by mean annual rainfall, minimum rainfall (calculated as the 5th percentile of mean annual rainfall) and maximum aridity (calculated as the 95th percentile of AI). Note that this analysis assumes our P50leaf measurements are representative for each species across their respective distributions and do not take into account possible intraspecific variation (Matzner et al., 2001).
Statistical analysis
Ordinary least squares regression was used to model cross-species relationships between P50leaf and climate variables. P50leaf data were significantly right-skewed and were log-scaled prior to analysis. Relationships between the within-site variation in P50leaf (standard deviation around site means) and site climate were also fitted with ordinary least squares regression models using untransformed data. Differences in P50leaf between sites were compared using ANOVA.
RESULTS
Leaf hydraulic vulnerability to drought (P50leaf) ranged from –1·03 MPa recorded in the most vulnerable species in montane cloud forest (Yanachaga) to –8·35 MPa for the least vulnerable species in semi-arid shrubland (Round Hill; Supplementary Data Table S1). Significant differences in species P50leaf were detected between sites (ANOVA, P < 0·0001), with site means (±s.d.) ranging from –1·38 ± 0·25 MPa among species in montane cloud forest (Yanachaga) to –5·69 ± 1·60 MPa among species in semi-arid shrubland (Round Hill; Table 2). Across species, P50leaf became significantly more negative with decreasing site rainfall (r2 = 0·68, P < 0·0001; Fig. 1A) and with increasing site aridity index (r2 = 0·72, P < 0·0001; Fig. 1B). We thought it important to investigate aridity index as well as precipitation because aridity index also considers evaporation and therefore should more accurately represent water availability across the wide range in latitude examined here. Also, evaporation might shape plant adaptations somewhat independently of precipitation (Eamus, 2003). However, aridity index was only a marginally better predictor of P50leaf, relative to precipitation, across the sites and species studied here.
Table 2.
The mean, standard deviation (s.d.) and coefficient of variation (CV) for P50leaf values within each sample site; significant differences (P < 0·05) in P50leaf between site means are denoted with different lower case subscripts (Bonferroni mean separation)
| Site | n | Mean P50leaf (MPa) | s.d. | CV |
|---|---|---|---|---|
| Yanachaga | 14 | –1·38a | 0·25 | 0·18 |
| Mount Field | 15 | –1·76a | 0·46 | 0·26 |
| Kur-ing-gai | 14 | –2·83b | 0·53 | 0·19 |
| Princess Hills | 16 | –3·25b | 0·60 | 0·18 |
| Yengo | 11 | –3·40b | 0·83 | 0·24 |
| Round Hill | 11 | –5·69c | 1·60 | 0·28 |
| Fowler's Gap | 11 | –4·70c | 1·12 | 0·24 |
Fig. 1.
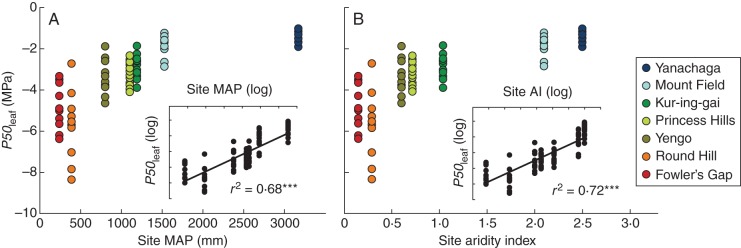
The relationship between P50leaf and site-specific climate parameters related to water availability: (A) mean annual precipitation (MAP) and (B) aridity index (AI). Levels of significance for regressions through log-transformed data are indicated (see insets; ***P < 0·0001). The sites from where species were sampled are colour-coded as indicated in the key in (B).
Within-site variability in P50leaf, expressed using the non-transformed standard deviation from the mean (s.d.), increased significantly with decreasing site rainfall and increasing site aridity (r2 = 0·63, P < 0·05; r2 = 0·64, P < 0·05, respectively). However, when expressed as a coefficient of variation within sites (Table 2), within-site variability in P50leaf showed no significant relationship to rainfall or aridity (r2 = 0·34, n.s.; r2 = 0·12, n.s., respectively).
P50leaf among the Australian species was significantly correlated with mean annual rainfall (r2 = 0·55, P < 0·0001), the 5th percentile of mean annual rainfall (r2 = 0·53, P < 0·0001) and the 5th percentile of aridity index (r2 = 0·61, P < 0·0001) across each species distribution (Fig. 2A, B, C, respectively). That is, species with more negative P50leaf tended to have distributions characterized by lower mean annual rainfall, and which also extended into regions with lower rainfall and higher aridity. However, with the exception of species from Mount Field in Tasmania, these relationships were not significant when analysed across species within each site separately (absence of correlation within sites in Fig. 2, Supporting Information Table S2).
Fig. 2.
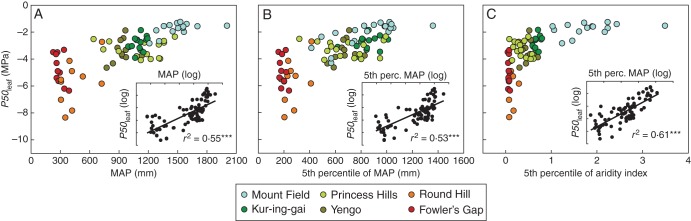
The relationship between leaf hydraulic vulnerability to drought-induced dysfunction (P50leaf) and climate parameters related to water availability across species distributions: (A) mean annual precipitation; (B) the 5th percentile of mean annual precipitation; (C) the 5th percentile of aridity index. Significant relationships using log-transformed data (***P < 0·0001) are fitted with least-squares regressions. The sites from where species were sampled are colour-coded as indicated in the key in (C).
DISCUSSION
This study examined how variation across species in the vulnerability of leaves to drought-induced hydraulic dysfunction relates to key aspects of climate using a broad group of woody angiosperms from sites that varied widely in water availability. Leaf hydraulic vulnerability decreased significantly with both decreasing site rainfall and increasing site aridity (Fig. 1A, B, respectively). P50leaf was also related to species climatic envelope within Australia, in terms of both the average rainfall across each species biogeographical range and the very dry-end limits of their distributions (Fig. 2). Given the overall relationship between leaf hydraulic vulnerability and the low-rainfall boundary of species distributions, it might have been expected that the variation in hydraulic vulnerability between species within each site would also be correlated, with those species whose distribution extended further towards low rainfall also having more negative leaf P50. However, this pattern was statistically significant only for species from the Mt Field site.
Our results substantially broaden the findings from smaller sets of species and relatively narrow aridity ranges that have been studied in the past. Our results largely support these previous studies, linking variation in leaf hydraulic vulnerability to species distribution patterns across gradients of mean annual precipitation (Nardini et al., 2012), as well as the dry-end limits of species distributional ranges (Blackman et al., 2012). Indeed, the generality of the association between leaf vulnerability and climate is made clear considering P50leaf was assessed across such a large and diverse group of species with a climate-coverage that captured a substantial portion of the global water availability spectrum in terms of both rainfall and aridity. Aridity index as an indicator of site water availability did not, for these data, provide any stronger insight into the patterns of variation in P50leaf across sites and species distributions than did rainfall (MAP).
It is clear from our results that leaf hydraulic vulnerability is strongly tuned to the apparent level of water stress experienced by plants in the field. We note that xylem water potentials regularly fall below levels associated with P50leaf (Brodribb and Holbrook, 2004a; Johnson et al., 2009). Such hydraulic dysfunction is recoverable once water tension is relieved (e.g. during the night under well-watered conditions), or else requires metabolically active refilling of embolized conduits in cases where leaf water potential remains substantially below zero. While there is clear evidence of embolism reversal under tension in many plants (Brodersen and McElrone, 2013), including those under moderate drought (Trifilo et al., 2014), it remains unclear whether this process is viable in plants that experience severe and prolonged drought where dry soils and strong water tension prevents embolism repair (Hacke and Sperry, 2003). In the present study, the seasonal midday plant water potential (measured at most of the Australian sites at the height of a multi-year drought) was significantly correlated with leaf hydraulic vulnerability, and was more strongly negative than P50leaf in only a small number of species (data not shown). This suggests species in our sample group generally avoid substantial losses in Kleaf and that P50leaf represents a robust indicator of plant drought resistance across the rainfall spectrum. Indeed, our sample group largely comprised evergreen species that retain their leaves over long periods of water deficit, maintain viable hydraulic pathways year round and ideally respond promptly to rainfall events. Thus, low leaf hydraulic vulnerability among species in the more arid sites that are associated with unpredictable rainfall and large water deficits probably reflects all these needs. Conversely, a high level of vulnerability among species from ever-wet montane cloud forest reflects the almost complete absence of water stress in these environments.
The variation among P50leaf values within sites increased significantly with increasing site aridity. This increase was in the spread of absolute P50leaf values (s.d.), but not in the coefficient of variation, i.e. the percentage variation around the site mean. Within-site variation in hydraulic vulnerability in arid environments has been attributed to differences in plant water-use and life-history strategies, often involving co-variation in plant hydraulic properties such as rooting depth, drought deciduousness and water storage capacity (Jacobsen et al., 2007b; Pratt et al., 2012); however, we do not know whether it is the spread of absolute P50leaf or rather the percentage variation around the mean that should be regarded as the better indicator of variation in strategies for water use. But in either case, our results contrast with reports of converging water-use strategies found among arid plant communities in Western Australia (Mitchell et al., 2008). Wider hydraulic variation among co-occurring species from our most arid sites probably reflects greater heterogeneity in the soil water profile or differing life-history strategies among the species themselves. Co-variation between P50leaf and traits such as rooting depth and/or water storage capacity may even shape the drought tolerance vs. avoidance strategies of particular species in these environments. However, there was inconsistency in these trait trade-offs across species within individual sites (data not shown), which suggests that drought avoidance versus resistance strategies in plants may be influenced by a range of traits that do not necessarily co-vary (Jacobsen et al., 2007a; Miranda et al., 2010).
Overall, our findings considerably extend the evidence that resistance to hydraulic dysfunction in leaves, especially among evergreen species, is functionally tuned to site rainfall and aridity and is a key component of drought resistance in arid environments. Furthermore, leaf hydraulic vulnerability may be important in shaping the climatic envelope across species distributions as well as defining their distributional limits in terms of minimum water availability. Because these relationships are based on a fundamental understanding of the drought tolerance limits in plants (Blackman et al., 2009; Brodribb and Cochard, 2009; Urli et al., 2013), greater knowledge of the hydraulic vulnerability of leaves may prove to be a very useful functional trait in predicting the consequences of changing climate for species distributions.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This research was funded by an Australian Research Council Laureate Fellowship grant awarded to M.W. We thank Damien Catchpole for his contribution of climate data for the Peruvian site. We also thank Keith Leggett and staff for their support of our research at Fowler's Gap.
LITERATURE CITED
- Ackerly DD. Community assembly, niche conservatism, and adaptive evolution in changing environments. International Journal of Plant Sciences. 2003;164:S165–S184. [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species. Plant, Cell and Environment. 2009;32:1584–1595. doi: 10.1111/j.1365-3040.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Brodribb T, Jordan GJ. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytologist. 2010;188:1113–1123. doi: 10.1111/j.1469-8137.2010.03439.x. [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulic vulnerability influences species’ bioclimatic limits in a diverse group of woody angiosperms. Oecologia. 2012;168:1–10. doi: 10.1007/s00442-011-2064-3. [DOI] [PubMed] [Google Scholar]
- Brodersen CR, McElrone AJ. Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Frontiers in Plant Science. 2013;4:108. doi: 10.3389/fpls.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Cochard H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T, Hill RS. The importance of xylem constraints in the distribution of conifer species. New Phytologist. 1999;143:365–372. [Google Scholar]
- Brodribb TJ, Holbrook NM. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology. 2003;132:2166–2173. doi: 10.1104/pp.103.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant Cell and Environment. 2004a;27:820–827. [Google Scholar]
- Brodribb TJ, Holbrook NM. Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytologist. 2004b;162:663–670. doi: 10.1111/j.1469-8137.2004.01060.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiology. 2005;137:1139–1146. doi: 10.1104/pp.104.058156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM, Edwards EJ, Gutierrez MV. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell and Environment. 2003;26:443–450. [Google Scholar]
- Catchpole D. Orographic gradients in climate and forest cover at the Cordillera Yanachaga, Peru. Australia: University of Tasmania; 2012. PhD thesis. [Google Scholar]
- Choat B, Ball MC, Luly JG, Holtum JAM. Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees – Structure and Function. 2005;19:305–311. [Google Scholar]
- Choat B, Sack L, Holbrook NM. Diversity of hydraulic traits in nine Cordia species growing in tropical forests with contrasting precipitation. New Phytologist. 2007;175:686–698. doi: 10.1111/j.1469-8137.2007.02137.x. [DOI] [PubMed] [Google Scholar]
- Cochard H, Froux F, Mayr FFS, Coutand C. Xylem wall collapse in water-stressed pine needles. Plant Physiology. 2004;134:401–408. doi: 10.1104/pp.103.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamus D. How does ecosystem water balance affect net primary productivity of woody ecosystems? Functional Plant Biology. 2003;30:187–205. doi: 10.1071/FP02084. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS. Limits to xylem refilling under negative pressure in Laurus nobilis and Acer negundo. Plant Cell and Environment. 2003;26:303–311. [Google Scholar]
- Hao GY, Hoffmann WA, Scholz FG, et al. Stem and leaf hydraulics of congeneric tree species from adjacent tropical savanna and forest ecosystems. Oecologia. 2008;155:405–415. doi: 10.1007/s00442-007-0918-5. [DOI] [PubMed] [Google Scholar]
- Hargreaves G, Allen R. History and evaluation of Hargreaves evapotranspiration equation. Journal of Irrigation and Drainage Engineering. 2003;129:53–63. [Google Scholar]
- Jacobsen AL, Pratt RB, Davis SD, Ewers FW. Cavitation resistance and seasonal hydraulics differ among three arid Californian plant communities. Plant Cell and Environment. 2007a;30:1599–1609. doi: 10.1111/j.1365-3040.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen AL, Pratt RB, Ewers FW, Davis SD. Cavitation resistance among 26 chaparral species of southern California. Ecological Monographs. 2007b;77:99–115. [Google Scholar]
- Johnson DM, Woodruff DR, McCulloh KA, Meinzer FC. Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiology. 2009;29:879–887. doi: 10.1093/treephys/tpp031. [DOI] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC. Evidence for xylem embolism as a primary factor in dehydration-induced declines in leaf hydraulic conductance. Plant Cell and Environment. 2012;35:760–769. doi: 10.1111/j.1365-3040.2011.02449.x. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Steudle E. Root hydraulic conductivity measured by pressure clamp is substantially affected by internal unstirred layers. Journal of Experimental Botany. 2008;59:2071–2084. doi: 10.1093/jxb/ern064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- Markesteijn L, Poorter L, Paz H, Sack L, Bongers F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant, Cell and Environment. 2011;34:137–148. doi: 10.1111/j.1365-3040.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- Matzner SL, Rice KJ, Richards JH. Intra-specific variation in xylem cavitation in interior live oak (Quercus wislizenii A. DC.) Journal of Experimental Botany. 2001;52:783–789. doi: 10.1093/jexbot/52.357.783. [DOI] [PubMed] [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Miranda JD, Padilla FM, Martinez-Vilalta J, Pugnaire FI. Woody species of a semi-arid community are only moderately resistant to cavitation. Functional Plant Biology. 2010;37:828–839. [Google Scholar]
- Mitchell P, Veneklaas E, Lambers H, Burgess S. Using multiple trait associations to define hydraulic functional types in plant communities of south-western Australia. Oecologia. 2008;158:385–397. doi: 10.1007/s00442-008-1152-5. [DOI] [PubMed] [Google Scholar]
- Nardini A, Peda G, La Rocca N. Trade-offs between leaf hydraulic capacity and drought vulnerability: morpho-anatomical bases, carbon costs and ecological consequences. New Phytologist. 2012;196:788–798. doi: 10.1111/j.1469-8137.2012.04294.x. [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Vander Willigen C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology. 1998;18:589–593. doi: 10.1093/treephys/18.8-9.589. [DOI] [PubMed] [Google Scholar]
- Pineda-Garcia F, Paz H, Meinzer FC. Drought resistance in early and late secondary successional species from a tropical dry forest: the interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell and Environment. 2013;36:405–418. doi: 10.1111/j.1365-3040.2012.02582.x. [DOI] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS. Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. American Journal of Botany. 2000;87:1287–1299. [PubMed] [Google Scholar]
- Pratt RB, Jacobsen AL, Jacobs SM, Esler KJ. Xlem transport safety and efficiency differ among Fynbos shrub life history types and between two sites differing in mean rainfall. International Journal of Plant Sciences. 2012;173:474–483. [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, McKown AD, Rawls M, Sack L. Dynamics of leaf hydraulic conductance with water status: quantification and analysis of species differences under steady state. Journal of Experimental Botany. 2011;63:643–658. doi: 10.1093/jxb/err270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoffoni C, Vuong C, Diep S, Cochard H, Sack L. Leaf shrinkage with dehydration: coordination with hydraulic vulnerability and drought tolerance. Plant Physiology. 2014;164:1772–1788. doi: 10.1104/pp.113.221424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornthwaite CW. An approach toward a rational classification of climate. Geographical Review. 1948;38:55–94. [Google Scholar]
- Trifilo P, Barbera PM, Raimondo F, Nardini A, Lo Gullo MA. Coping with drought-induced xylem cavitation: coordination of embolism repair and ionic effects in three Mediterranean evergreens. Tree Physiology. 2014;34:109–122. doi: 10.1093/treephys/tpt119. [DOI] [PubMed] [Google Scholar]
- Urli M, Porte AJ, Cochard H, Guengant Y, Burlett R, Delzon S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology. 2013;33:672–683. doi: 10.1093/treephys/tpt030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


