Abstract
Background and Aims
Pollinator-limited seed-set in some terrestrial orchids is compensated for by the presence of long-lived flowers. This study tests the hypothesis that pollen from these insect-pollinated orchids should be desiccation tolerant and relatively long lived using four closely related UK terrestrial species; Anacamptis morio, Dactylorhiza fuchsii, D. maculata and Orchis mascula.
Methods
Pollen from the four species was harvested from inflorescences and germinated in vitro, both immediately and also after drying to simulate interflower transit. Their tolerance to desiccation and short-term survival was additionally assessed after 3 d equilibration at a range of relative humidities (RHs), and related to constructed sorption isotherms (RH vs. moisture content, MC). Ageing of D. fuchsii pollen was further tested over 2 months against temperature and RH, and the resultant survival curves were subjected to probit analysis, and the distribution of pollen death in time (σ) was determined. The viability and siring ability, following artificial pollinations, were determined in D. fuchsii pollen following storage for 6 years at –20 °C.
Key Results
The pollen from all four species exhibited systematic increases in germinability and desiccation tolerance as anthesis approached, and pollen from open flowers generally retained high germinability. Short-term storage revealed sensitivity to low RH, whilst optimum survival occurred at comparable RHs in all species. Similarly, estimated pollen life spans (σ) at differing temperatures were longest under the dry conditions. Despite a reduction in germination and seeds per capsule, long-term storage of D. fuchsii pollen did not impact on subsequent seed germination in vitro.
Conclusions
Substantial pollen desiccation tolerance and life span of the four entomophilous orchids reflects a resilient survival strategy in response to unpredictable pollinator visitation, and presents an alternative approach to germplasm conservation.
Keywords: Anacamptis morio, Orchis mascula, Dactylorhiza maculata, Dactylorhiza fuchsii, orchid pollination, pollen germination, storage, longevity, desiccation tolerance, seed-set, entomophilous pollen
INTRODUCTION
Reproductive success in higher plants is measured by the exchange of germplasm in the form of pollen from donor to recipient flowers, and the subsequent seed-set following fertilization. A major factor in the efficiency of this process is the large abundance of pollen produced in many angiosperm species, where a broad range of pollen:ovule ratios, from 418:1 (Camissonia ovata) to 17 661:1 (Larrea divaricata), have been reported (Cruden and Miller-Ward, 1981). In temperate orchids, however, ratios are in the region of 10:1 (Goodyera repens) to 24:1 (Platanhtera chlorantha) and such low ratios are typical of entomophilous European species. Thus when compared with anemophilous species in general, seed production is potentially pollinator limited; to increase the likelihood of pollination, orchids tend to produce long-lived flowers (Neiland and Wilcock, 1995). However, in many species, including those in the tribe Orchideae, the flowers are food-deceptive; no food reward is offered to the pollinator, so reducing the chances of a pollinator revisiting those flowers (Bellusci et al., 2010). Consequently, to increase the probability of pollination, evolutionary selection would favour long-lived pollen in combination with long-lived flowers.
In orchids, pollen is packed into discrete waxy masses (massulae) held together in pollinia, creating a highly efficient delivery system containing, for example, >400 000 pollen grains (Wolter and Schill, 1986). The pollinia are attached by a stalk (caudicle) to a sticky organ (the viscidium) to form a pollinarium, which adheres to the body of a visiting pollinator (Pacini and Franchii, 1998; Pridgeon et al., 1999). In the Orchideae, the pollen is present in tetrads (Endress, 1996; Pacini and Hesse, 2002), and these are contained within two pollinia in each flower (Pridgeon et al., 2001). The presence of pollinia ensures minimal pollen wastage during transit, and a high probability of deposition on a conspecific stigma. Pollinia tend not to be readily released from the pollinator, and can be retained for up to several hours, so reducing the chance of self-pollination in geitonogamous species (Johnson and Edwards, 2000).
Pollinia have been shown to vary in volume and moisture content (MC) during dispersal, but to remain ‘partially hydrated’ after anthesis (Borba and Semir, 1999; Franchi et al., 2002). A relatively high water content may be a consequence of their structure, with a relatively small surface area to volume ratio and further protection from the presence of an anther wall, or as a consequence of being sheltered within the flower prior to removal by the pollinator (Pandolfi and Pacini, 1995). That these factors reduce the likelihood of dehydration affected by atmospheric temperature and humidity may reflect two important aspects of their physiology: a hydrated state could facilitate more rapid germination, but also indicates a reduced ability to tolerate (desiccation avoidance) drying (Franchi et al., 2002; Hoekstra, 2002). However, detailed analyses of orchid pollen dehydration tolerance are limited, but suggest that germination is reduced at low MC (Pritchard and Prendergast, 1989).
The modulating roles of MC and temperature on seed longevity are known for some orchid species (for a review, see Pritchard and Dickie, 2003), including Dactylorhiza fuchsii (Pritchard et al., 1999). Generally, systematic information of this type is lacking for pollen and may be similarly species specific. For Typha latifolia, another tetrad pollen species, modelling of longevity (survival) curves using equations developed for seed longevity studies have described similar negative logarithmic relationships between longevity and increasing MC (Buitink et al., 1998). Corresponding information for orchids would better describe the physiology of its pollen behaviour in the natural environment (Neiland and Wilcock, 1995) and inform strategies for pollen conservation (Towill, 1985).
The ability of pollen to survive for extended periods on the open flower, and during transit on a pollinator, raises the possibility of its ex situ storage to facilitate both asynchronous pollination and germplasm conservation. Extended storage of orchid pollinia was first reported by Ito (1965), where successful pollen germination was observed in Dendrobium ‘Lady Hamilton’, D. nobile and Calanthe furcata following storage for up to 957 d at –79 °C. Pollinia were variously desiccated and treated with cryoprotectants, but the results were not quantified. More detailed studies of Anacamptis pyramidalis and D. fuchsii showed that air-dried pollinia (approx. 15 % MC) tolerated 12 months storage at both –20 and –196 °C (Pritchard and Prendergast, 1989), whereas pollinia of Dendrobium ‘Sena Red’ and ‘Mini WRL’ desiccated over silica gel for 24 h (approx. 5 % MC) maintained high levels of germination following storage at –196 °C for 48 h (Vendrame et al., 2008). Conversely storage over silica gel or calcium chloride for up to 12 months reduced viability in four Dendrobium species and in Oncidium stipitatum (Meeyot and Kamemoto, 1969).
Whilst an in vitro germination assay is favoured for its speed and may accurately reflect pollen viability, the most definitive test of pollen quality is in vivo, i.e. measuring pollen tube growth in situ (Pandolfi and Pacini, 1995) or, more commonly, the seed-set that occurs (Towill, 1985). Although pollen germination in vitro and in vivo are similar, e.g. in white spruce (Dawkins and Owens, 1993), there can be a lack of correlation between germination in vitro and seed-siring/fruit-setting ability, e.g. in Erythronium gradiflorum (Thomson et al., 1994). However, successful seed-set and seed germination have been observed in Dendrobium cultivars following pollen storage at –196 °C for 48 h (Vendrame et al., 2008).
This study reports the use of pollen from four entomophilous non-rewarding UK native species with relatively long-lived flowers, to determine; (1) the relationship between pollen maturity and its ability to tolerate changes in water status; (2) the ability of mature pollen to respond to changes in temperature and relative humidity (RH) as might be experienced during transit on a pollinator; and (3) the potential to use mature pollen for long-term germplasm storage.
MATERIALS AND METHODS
Plant material
Four species of terrestrial orchids native to the UK were investigated: Anacamptis morio (L.) R.M.Bateman, Pridgeon & M.W.Chase, D. fuchsii (Druce) Soó, D. maculata (L.) Soó and Orchis mascula (L.) L. The flowering period, and the numbers of inflorescences appearing on a flower varied among the species, with A. morio both having the shortest flowering period and generally bearing the least number of flowers. The differences in habitats of each species sampled varied and may have influenced physiological responses; D. fuchsii grew in open shade, O. mascula in woodland, A. morio in open grassland and D. maculata in partially shaded conditions. The variation in numbers of flowers produced, and further details of their general habitats, ecology and taxonomy are described by Lang (2004) and Pridgeon et al. (2001). Between April and June, a small number of inflorescences were removed from plants growing in low-density populations in the grounds of the Royal Botanic Gardens, Kew (Wakehurst Place, UK) and under licence from other natural sites in Sussex (UK), and used immediately for study or placed briefly (hours) in water prior to use. There was considerable variation in the number of flowers per inflorescence both within and between species, and the two pollinia present in each flower were taken from along individual flower spikes rather than from replicate plants, to prevent the variation this would inevitably capture from confounding positional differences within the population of plants. The two pollinia sampled from each species were attached to a single viscidium and, although the number of composite massulae was not recorded, they conform to observations made by Darwin (1862) and Pridgeon et al. (2001). To ensure that differences in pollen developmental age were adequately represented, between seven and 14 flower positions were sampled in order along each inflorescence, encompassing both open and unopened flowers (Fig. 1).
Fig. 1.
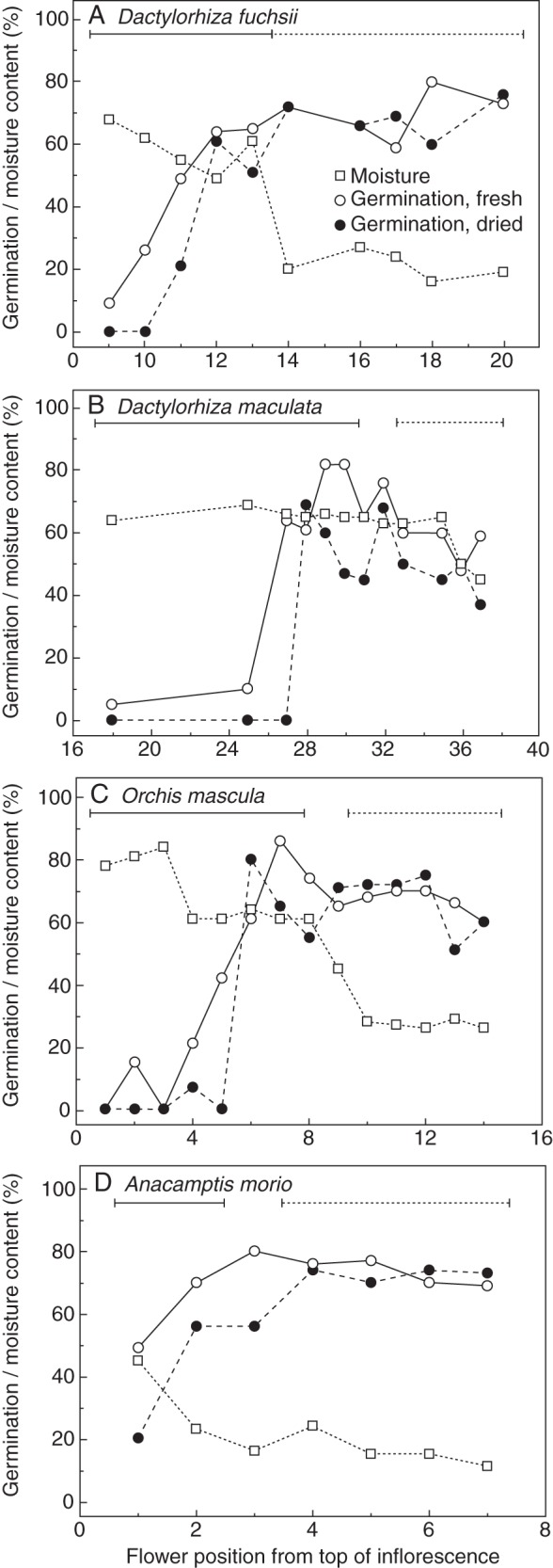
Development of pollen quality traits of moisture content and germination of fresh and dried pollen (as indicated in the key) in four orchids, Dactylorhiza fuchsii (A), Dactylorhiza maculata (B), Orchis mascula (C) and Anacamptis morio (D). Horizontal solid and dashed lines signify pollinia extracted from closed and open flowers, respectively, while the gap represents partially open flowers.
Pollen development
To describe pollen physiology at different levels of maturity from each species, pollinia were routinely removed from each flower using fine pointed microforceps, and a record was taken of whether the flower was closed or open. No colour differences were observed between pollinia from within individual flowers, and as such they were assumed to be of the same level of maturity. The top half of one pollinium was sown immediately to assess their viability by their ability to germinate in vitro. These were sown on an agar-gelled (1 % w/v) medium consisting of 1 % (w/v) sucrose and 0·1 % (w/v) boric acid, corrected to pH 5·6, and held at 26 °C in the dark (Pritchard and Prendergast, 1989). Fifty tetrads (200 pollen grains) per pollinium were scored for germination after 48 h on the media by placing pollinia in a drop of deionized water, and teasing out the pollen grains. An individual grain was deemed to have germinated only when the pollen tube was longer than the grain diameter. The top half of the other pollinium was used in a desiccation tolerance test by equilibrium for 3 d at 21 °C and 33 % RH above a saturated salt solution of calcium chloride (CaCl2) in a sealed glass jar. Thereafter, pollen was germinated as described above. The remaining two lower half-pollinia were combined, minus their caudicles and viscidia, for determination of the MC (fresh weight basis) by oven drying at 103 °C for 17 h (Pritchard and Prendergast, 1989). Only single values were determined for the pair of half-pollinia as attempting to replicate the precise position on multiple flower spikes was liable to introduce confounding variation.
Pollen hydration and dehydration
To test the effects of short-term exposure to changes in ambient RH upon pollen physiology, pollinia of the four species were isolated from up to nine recently opened flowers in similar positions along separate additional inflorescences of plants not used in the developmental study. After the removal of pollinia from each flower, they were equilibrated for 3 d at 21 °C over silica gel and a range of saturated salt solutions providing RHs of 5 % (freshly regenerated silica gel), 23 % (potassium acetate, CH3COOK), 33 % (CaCl2), 44 % (potassium carbonate, K2CO3), 53 % (magnesium nitrate, Mg(NO3)2), 64 % (sodium nitrite, NaNO2), 75 % (sodium chloride, NaCl), 84 % (potassium bromide, KBr) and 90 % (zinc sulphate, ZnSO4) (see Sun, 2002). Upon removal, the top half of each pollinium was used to assess viability, while the remaining two lower half-pollinia from each flower were used for an MC determination.
To establish further the relationship between temperature and RH upon changes in pollen survival (longevity), additional pollinia of D. fuchsii were removed from recently opened flowers on other inflorescences and stored over four saturated salt solutions producing RHs of 23, 44, 64 and 79 % (ammonium chloride, NH4Cl) at two temperatures (6 and 16 °C, the latter to match median summer temperatures in southern England). Samples of two pollinia were taken from each treatment periodically over a 76 d period at 6 °C and 62 d at 16 °C, and germinated as previously described. For each pollinium, 50 tetrads were assessed for germination, giving a total of 400 pollen grains for each storage period.
Long-term pollen storage
To assess long-term storage potential of D. fuchsia pollen, about 100 pollinia were removed from recently opened flowers and equilibrated at 33 % RH over saturated CaCl2 overnight at 16 °C, and transferred to 2 cm3 capacity polypropylene ‘cryovials’. The vials were then placed directly into a freezer (–20 °C) and left for 6 years. Vials were later thawed at ambient temperature on the laboratory bench. Both the stored and fresh pollen, collected 6 years later from the same population of D. fuchsii, were used to pollinate recently opened flowers adjacent to one another on ten individual inflorescences from the same population at Wakehurst Place. To reduce the risk of using single, non-viable pollinia in the pollinations, five half-pollinia were used per stigma, the remaining five halves from both fresh and stored pollinia being sown for in vitro germination. Fifty tetrads comprising 200 pollen grains were assessed. Seed capsules were removed 11 weeks later when they were turning brown, and the seeds were extracted by hand and equilibrated overnight in aluminium vials at 33 % RH and 16 °C. The seeds from each capsule were kept separate and weighed on a seven-place balance to give a total seed yield per capsule. Two aliquots of seeds per capsule were used for individual seed weight determination, using the same balance and by counting the exact number of seeds for each determination (n = 57–302 seeds) under a binocular microscope. Mean seed weights were then used to estimate the number of seeds extracted from each capsule.
For germination, seeds were surface sterilized for 20 min in 10 % commercial bleach (approx. 0·5 % sodium hypochlorite) at room temperature and then sown in duplicate on both Norstog (1973) and Knudson C (Knudson, 1946) medium in Petri dishes held in the dark at 21 °C (Pritchard et al., 1999) and assessed after 2 months. The percentage of full seeds per capsule was estimated when scoring the germination tests, which involved a mean of 175 and 138 full seeds per sowing for seeds gathered from capsules produced from fresh and stored pollen, respectively.
Statistical analysis
Pollen viability was measured using either 50 or 100 tetrads comprising 200 or 400 pollen grains, and differences between fresh and stored pollen were determined by a two-way binomial test. Prior to analysis, percentage germination data were normalized by arcsine transformation, but expressed as non-transformed data. One-way analysis of variance (ANOVA) was used to determine differences between fresh and stored pollen treatments for D. fuchsii seed fecundity and quality, and for asymbiotic germination (Table 2), whereas linear regression analysis was used to assess any effect of resource allocation between the number of flowers per inflorescence and the number of full seeds set. Probit analysis of pollen viability following storage at 6 and 16 °C under modified RHs was used to fit curves to probability plots and estimate values of initial viability (Ki), and the standardization of the distribution of pollen death in time (σ) was plotted for both temperatures using values derived from Table 1. All analyses were carried out using GenStat Version 12 (VSN International, 2011) software.
Table 2.
Effects of long-term pollen storage upon viability and seed quantity and germination in Dactylorhiza fuchsii
| Parameter | Fresh pollen | Stored pollen | P-value |
|---|---|---|---|
| Pollen germination (%)* | 80·4 ± 2·80 | 64·0 ± 3·39 | <0·001 |
| Total seed weight per capsule (mg) | 3·50 ± 0·56 | 1·62 ± 0·27 | 0·008 |
| Mean seed weight (μg) | 1·9 ± 0·15 | 1·67 ± 0·18 | 0·266 |
| Number of seeds per capsule | 1943 ± 399 | 997 ± 143 | 0·040 |
| Full seeds (%)† | 89·09 ± 2·00 | 81·30 ± 2·35 | 0·005 |
| Germination on Knudson C medium (%)† | 45·10 ± 4·58 | 40·05 ± 4·84 | 0·665 |
| Germination on Norstog medium (%)† | 71·71 ± 4·00‡ | 65·68 ± 4·42 | 0·548 |
Values are shown ± standard error (s.e.).
*Binominal analysis of fresh and stored pollen.
†Data were arcsine-transformed before analysis; non-transformed means are shown.
‡In vitro germination tests on seeds from one capsule were lost due to contamination (i.e. n = 8).
Table 1.
Probit regression analysis of the loss of viability in Dactylorhiza fuchsii pollen following ageing held at 6 and 16 °C, and equilibrated to 23, 44, 64 and 79 % relative humidities
| Temperature (°C) | Relative humidity (%) | Ki probits (%) | Slope (probit loss d–1) ± s.e. | Time to lose 1 probit (σ, d) |
|---|---|---|---|---|
| 6 | 23 | 5·032 (51·3) | –0·0105 ± 0·0009 | 94·8 |
| 6 | 44 | 5·125 (55·0) | –0·0285 ± 0·0013 | 35·1 |
| 6 | 64 | 5·163 (56·5) | –0·0414 ± 0·0018 | 24·2 |
| 6 | 79 | 5·711 (76·1) | –0·1290 ± 0·0045 | 7·8 |
| 16 | 23 | 4·764 (40·7) | –0·0135 ± 0·0012 | 74·3 |
| 16 | 44 | 4·897 (45·9) | –0·0374 ± 0·0016 | 28·8 |
| 16 | 64 | 5·406 (65·8) | –0·1774 ± 0·0070 | 5·6 |
| 16 | 79 | 5·448 (67·3) | –0·1999 ± 0·0083 | 5·0 |
RESULTS
Development of pollen quality
Samples were taken from along the inflorescences of each species to encompass open, partially open and closed flowers, and hence different degrees of pollen maturity. Flower position on the inflorescences affected the pollinia MC, and the germination of both fresh and dried pollen (Fig. 1). In all four species, MC decreased basipetally, although the extent of this decrease varied between species. In D. fuchsii (Fig. 1A) and O. mascula (Fig. 1C), this decrease clearly coincided with anthesis, dropping from approx. 60 % to approx, 20 % MC. Dactylorhiza maculata MC (Fig. 1B) remained similar in both open and closed flowers, and only dropped from 60 to 40 % in the oldest flowers, whereas A. morio (Fig. 1D) produced drier pollen, with similar MC in open flowers.
Flower position also influenced pollen germination before and after artificial drying at 33 % RH (Fig. 1). In the three species for which at least five unopened flowers could be sampled, the initial pollen germination capacity increased from 0–10 % levels to 60–80 % at the point of anthesis (Fig. 1A–C). Germination also increased to 80 % at the beginning of anthesis in A. morio, although germination was already higher in the two unopened flowers than in the other species (Fig. 1D). In contrast, there was little consistent position-dependent variability in germination levels for pollen extracted from open flowers in D. fuchsii (Fig. 1A), O. mascula (Fig. 1C) and A. morio (Fig. 1D). For D. maculata, however, viability fell by about 20 % from its maximum in pollen progressively towards the basal positions on the inflorescence (Fig. 1B). Pollen removed from unopened flowers was particularly sensitive to desiccation stress, and germination was generally lower than in fresh pollen in all species. However, this sensitivity was not observed in pollen in open flowers of D. fuchsii, O. mascula and A. morio (Fig. 1A, C and D), whereas in D. maculata (Fig. 1B) the more mature pollen remained partially sensitive to this low RH (33 %), with germination reduced by up to 20 % after drying.
Pollen hydration status
The isotherms produced for all species showed a similar reverse sigmoidal response, such that an RH of 90 % produced MCs of 22–29 % (Fig. 2), which was similar to MCs of the open flowers of all except D. maculata (Fig. 1). However, this equated with sub-optimal germination in all species. Moisture contents varied from 4–7 % to 22–29 % over the 5–90 % RH range, with a great similarity between patterns. For example, in all four species, the equilibrium MC was approx. 11 % at 50 % RH (Fig. 2). The effect of equilibrium to various RHs over 3 d at 21 °C, and hence MC, upon pollen germination varied between species. For D. maculata, O. mascula and A. morio, optimal survival occurred close to 30 % RH, corresponding to approx. 10 % MC in these three species. Germination in D. fuchsii pollen was depressed at this RH, and optimal at approx. 75 % RH, where pollen MC was somewhat higher (15 %). Orchis mascula pollen was observed to be the least sensitive to changes in RH (i.e. variation in germination was the most limited). In contrast, the loss of viability with increasing RH above the optimal level for germination was increasingly abrupt through A. morio (Fig. 2D), D. maculata (Fig. 2B) and D. fuchsii (Fig. 2A). Pollen of these three species was also very sensitive to the lowest RH (5 %) generated by silica gel.
Fig. 2.
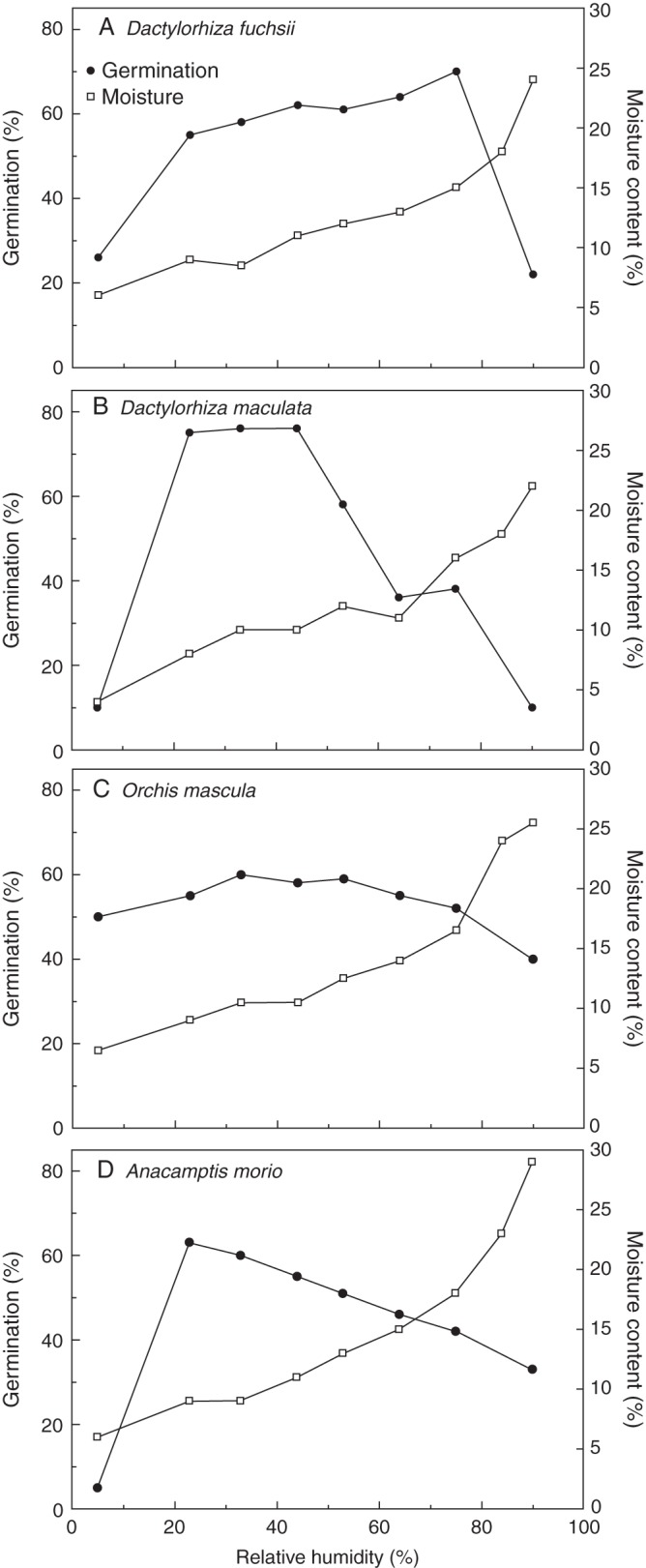
Germination and isotherms (moisture content) of Dactylorhiza fuchsii (A), Dactylorhiza maculata (B), Orchis mascula (C) and Anacamptis morio (D) following 3 d exposure to different levels of relative humidity.
Pollen longevity
Plotted as probability response curves, D. fuchsia pollen viability was progressively lost over time in storage at either 6 or 16 °C (Fig. 3). Linear regression analysis for pollen responses after storage over different RH solutions showed that the rate of loss of viability was increased as the RH, and thus the MC, increased. The slopes for the rate of loss of viability were greater at 16 °C than at 6 °C (Fig. 3), and the survival times (σ) correspondingly longer at 6 °C. The slopes of these respective regression lines were used to calculate the longevity values shown in Table 1. The time taken for viability to change by one probit, i.e. a standard deviation of the frequency of pollen death in time, decreased with increasing RH, ranging from approx. 95 to 8 d at 6 °C, and from approx. 74 to 5 d at 16 °C. An estimation of the value of Ki could be made from these slopes, and showed a degree of variation over the RH and temperature ranges used. When converted to percentage viability values, these were most similar for the three lowest RH regimes stored at 6 °C, and were more variable at 16 °C.
Fig. 3.
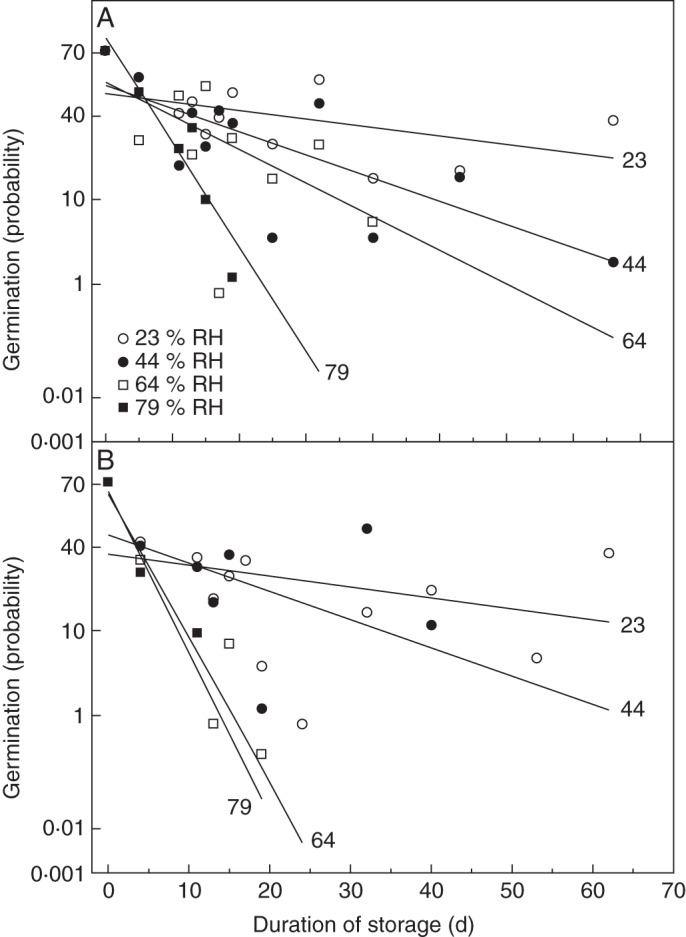
Germination probability of Dactylorhiza fuchsii pollen following storage in equilibrium relative humidities of 23, 44, 64 and 79 % (as indicated in the key) at both (A) 6 °C and (B) 16 C. The slopes of the fitted linear regression lines are described in Table 1.
A negative logarithmic relationship was observed between longevity and storage RH for pollen held at both 6 and 16 °C, where this increased with decreasing RH, and was consistently higher at the lower temperature over the entire range. At both temperatures there was a >10-fold increase in longevity between storage in the highest and lowest (79 and 23 %) RH environments (Fig. 4).
Fig. 4.
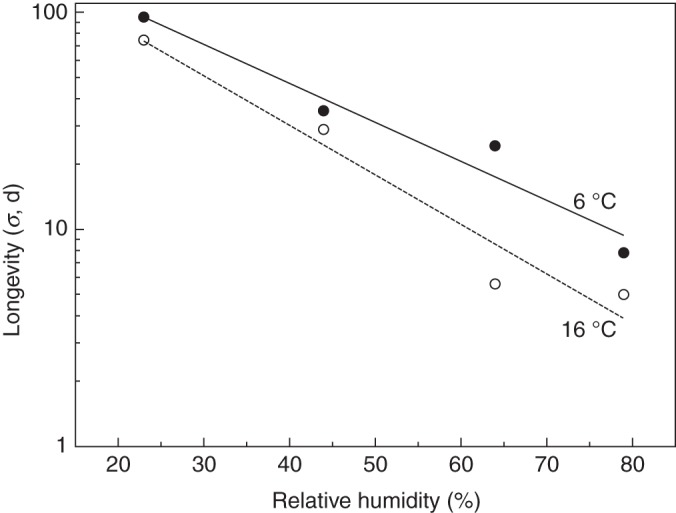
Negative logarithmic relationship between longevity (days) and equilibrium relative humidity (RH) for Dactylorhiza fuchsii pollen stored at 6 and 16 °C. Lines are fitted following probit analysis of data presented in Fig. 3. At 6 °C, log σ = 2·392–0·018 RH (r2 = 0·951); and at 16 °C, log σ = 2·390–0·023 RH (r2 = 0·948).
Long-term pollen storage and seed set
Of the ten inflorescences used for the D. fuchsii pollination experiments, one was damaged so only nine survived for analysis. The total number of flowers present (35–79) and capsules set (8–24) per inflorescence varied. After pollination, this equated to an average of 30 ± 9 (s.d.) % capsules set per inflorescence. All remaining 18 assisted pollinations using the fresh and stored pollen resulted in capsule- and seed-set across the remaining nine inflorescences (i.e. 90 %).
Pollen stored for 6 years at –20 °C showed a lower level of germination (64 %) than fresh pollen (80 %). This reduced viability was also expressed in seed yield, being significantly higher from fresh pollen measured as either total seed weight (P = 0·008) or the presence of full seeds (P = 0·005; as opposed to empty testa). The mean number of seeds produced when pollinating with fresh pollen was also higher; P = 0·04 (Table 2). Linear regression analysis relating the proportion of full seeds to the number of flowers present on an inflorescence was not significant (P = 0·228 and r2 = 0·18 for fresh pollen, and P = 0·476 and r2 = 0·07 for stored pollen). However, the mean seed weights from either source of pollination did not differ, nor did their germination on either Knudson C or Norstog medium, although the latter medium allowed a higher level of germination overall (P < 0·001).
DISCUSSION
Development of pollen quality
The sequential harvesting of pollen down the inflorescence of each orchid species has been used as a surrogate for ‘developmental’ age, showing that as the flowers approach anthesis, the pollen develops the ability to germinate, rapidly followed by the onset of tolerance to artificial drying (Fig. 1). Improving germination and tolerance to desiccation in orchid pollen leading up to anthesis are similar to what was observed previously in both Papaver pollen (Hoekstra and van Roekel, 1988) and developing seeds (Hay and Smith, 2003). The near coincidence of maximum germinability with anthesis in all four species investigated indicated that the pollen was available to pollinators for transferral when at its highest viability. Persistence of high viability for an extended time post-anthesis, as observed in the more mature lower inflorescences, therefore maximizes the likelihood of successful fertilization in these non-rewarding flowers. For three of the species, it appears that pollen further down the inflorescence are either at, or slightly below the maximum quality observed, suggesting that some ageing may have taken place in situ (Hoekstra, 2002), despite protection of the pollen within the flower (Pandolfi and Pacini, 1995). The exception to this trend was for D. fuchsii pollen (Fig. 1A). This may suggest greater inherent pollen longevity related to its habitat in this species compared with the others tested, but direct comparisons are confounded by at least two factors. First, flower opening rates may have been different across the species, thereby reducing post-anthesis ageing time in situ. Secondly, the environmental conditions prevailing post-anthesis may also have a potential impact on pollen quality; these were not measured and will have differed as flowering and collection times varied from April to June. In comparison, Neiland and Wilcock (1995) observed that the stigma of A. morio remained receptive to pollen for at least 20 d post-anthesis, although fruit set was optimal (100 %) when pollination occurred after 4 d. Also, D. maculata pollen was still viable after 41 d.
The MC of pollinia within opened flowers remained high, and varied between 45 and 65 % for D. maculata and between 11 and 45 % for the other three species. In contrast, the freshly collected pollinia of Dendrobium hybrids were reported to have an MC of only 8 % (Vendrame et al., 2008), and an MC of 5–12 % was reported in fresh Juglans regia pollen (Luza and Polito, 1987). However, germination in Juglans fell away rapidly at lower MCs, while in Dendrobium germination remained above 60 %, even when the MC was reduced to 5 %. This is likely to reflect resistance to rapid (hours) equilibration to natural conditions, necessary in an entomophilous orchid species displaying an extended flowering period. Indeed this demonstrates that there is a considerable difference in tolerance to low MC between diverse species of orchid. The study by Bellusci et al. (2010) showed that this could be related to pollination strategy, where the pollinia of food-deceptive (non-rewarding) species were longer lived than those of species exhibiting sexual- and shelter-deceptive strategies.
Reduced life span in developing pollen could result from limited desiccation tolerance, as shown by Curcurbita pepo: approx. 10 % viability after 24 h (Pacini et al., 1997). However, pollen of all four species had, by anthesis, acquired the ability to survive enforced drying to 33 % RH with little detriment to viability (Fig. 1); equivalent to approx. 10 % MC based on their isotherms (Fig. 2), and slightly below that of their fresh counterparts. Such a level of air-drying might easily occur during transit by pollinators between these relatively open-habitat species. Thus, it appears that at anthesis orchid pollen is at its highest physiological competence for both germinability and desiccation tolerance. Similar developmental changes in physiological traits were described in Papaver dubium, where pollen became progressively more germinable and desiccation tolerant in the 3 d prior to anthesis (Hoekstra and van Roekel, 1988). In Pennisetum typhoides and Zea mays (Hoekstra et al., 1989), increased desiccation tolerance was correlated with pollen sucrose content, and it is suggested that this sugar acts as a membrane stabilizer at low MCs (Hoekstra, 2002). A similar, protective mechanism may be operative in developing orchid pollen, but remains to be confirmed.
The term ‘partially hydrated’ has been used to describe pollen with an MC >30 % at anthesis, which could support rapid germination (Franchi et al., 2002). However, the arrangement of orchid pollen into tightly packed tetrads in massulae within a pollinium (Pandolfi and Pacini, 1995) may account for some high MCs observed compared with those in naked pollen. Also, pollen MC at anthesis can vary across the Orchidaceae (Franchi et al., 2002), and the evidence that high levels of germination occur in pollen of A. morio at 20 % MC, or Dendrobium ‘Sena Red’ and ‘Mini WRL’ at 5–8 % MC (Vendrame et al., 2008), would more probably suggest an ability to rehydrate readily, and no necessity to retain a high MC. Aspects of orchid reproductive ecophysiology (e.g. time of anthesis, local environmental conditions, etc.) are known to vary (Lang, 2004), making direct comparisons of in situ pollen longevity between species complex (Pacini et al., 1997).
Short-term ex situ storage and desiccation tolerance in pollen
Sustained viability after drying to a relatively low RH (approx. 30 %; Fig. 1) indicates that the mature pollen of all four species have considerable desiccation tolerance, unlike the pollen of some species in the Araceae, Cucurbitaceae, Gramineae and Zingiberaceae families (see Hoekstra, 2002). An ability to tolerate low MCs may be attributed to the presence of a small vacuole (0·1–0·5 μm; Pacini and Hesse, 2002) which is often associated with the ability of various plant cells to tolerate freeze-induced, and other forms of dehydration (Pritchard et al., 1986; Black and Pritchard, 2002). Complete tolerance of desiccation did not, however, extend to the ultra-dry conditions imposed by the use of silica gel (RH approx. 5 %), which reduced MCs to 4–6·5 % (Fig. 2). Similarly, J. regia pollen was sensitive to extreme drying and, from about 5 % to 3 % MC, viability was reduced from 100 % to 0 % (Luza and Polito, 1987). Orchis mascula was least affected by ultra-drying, and supports the previous observation of Pritchard and Prendergast (1989) for this species. These authors also showed that some post-drying germination potential could be recovered by rehydration of the pollen of D. fuchsii and D. musculata prior to sowing, which would occur naturally when the pollinium is deposited on the stigma. Rehydration has also been used to improve germination in aged Pistachia vera pollen by exposure to a high-humidity atmosphere. This increased the duration for recoverable pollen from 5 to 25 d (Polito and Luza, 1988). However, there is a risk of rapid ageing when pollen is held at higher RHs and MCs (Fig. 2). A similar relationship between high MC and reduced viability was seen in J. regia pollen (Luza and Polito, 1987). Our results indicate greater pollen ageing the higher the equilibrating RH, and the onset of sensitivity to extreme drying at the lowest RH. The risk to longevity of these two physiological stresses has been observed in numerous other species, both for pollen and for seeds (see Dickie and Pritchard, 2002).
The relationship between viability, exposure to different storage RHs and temperature (Fig. 3) is analogous to the findings for T. latifolia pollen, where viability was progressively lost in higher RH environments, and was exacerbated by higher storage temperatures (Buitink et al., 1998). In comparison, longevity for Typha pollen stored at 66 % RH and temperatures of 5 and 15 °C was >50 and >25 d, respectively, whereas for D. fuchsii, at 6 and 16 °C, this was >80 and <30 d, respectively. Under lower RH conditions, longevity of Typha pollen would exceed 300 d at both temperatures. A similar relationship showing loss of viability with increasing storage RH was also seen in the ageing of D. fuchsii, D. anosmum and E. gonychila seeds (Pritchard et al., 1999). The different RH environments affect the MC of the pollen, and both Typha (Hong et al., 1999) and D. fuchsii pollen longevity is negatively affected by increasing MC. This predictable impact of elevated MC on life span has been described for the seeds of many species, e.g. lettuce (Roberts and Ellis, 1989), and in other single-celled propagules, such as spores of the fungus Metarhizium flavoviride (Hong et al., 1997, 1998). In D. fuchsii pollen, this relationship was only explored over RHs down to 23 %, so the breakdown in the relationship to longevity at low RH (<10 %) seen in Typha (Hong et al., 1999) was not observed.
Long-term pollen storage and seed-set
Pollen of D. fuchsii stored for 6 years at –20 °C and at an MC of approx. 8·5 % retained a relatively high level of germination, and the ability to sire functional seeds. The precise conditions favourable for optimal retention of viability over the longer term are likely to vary with species, and with the temperature or MCs under which they are stored. Using storage of pollen in a hydrated state on agar at 2 °C, Pritchard and Prendergast (1989) showed a species-dependent rate of loss of pollen viability in a range of terrestrial and epiphytic orchids, whereas viability was maintained in dried pollen (MC <8 %) of D. fuchsii and A. pyrimidalis over a 12 month period of storage at either –20 or –196 °C. Failure to recover viability after storage may also be related to the rate of rehydration in the viability test. Gradual rehydration under conditions of high humidity is known to increase the level of germination in the pollen of Pistachio (Polito and Luza, 1988) and A. pyramidalis (Pritchard and Prendergast, 1989).
Different levels of pollen germination may be achieved post-storage, but the principal criterion for success is the level of seed-set achieved using stored pollen for fertilization. Stanley and Linskens (1974) raised the point that pollen may germinate in vitro, but be dysfunctional in completing the stages of zygote formation. In this study, the 90 % successful fruit-set in D. fuchsii when using either fresh or stored pollen would fulfil this requirement, and sits well with the findings of Burd (1994), who showed that in artificial and natural outcrossing of some 18 orchid species, fruit-set was 24 and 73 % (derived data), respectively. Seed yields per capsule of D. fuchsii may have been reduced following storage, but the subsequent germination of seed was not significantly affected (Table 2). Highly germinable seed was also produced from Dendrobium hybrid pollen following storage at –196 °C, although the level of seed-set was not reported (Vendrame et al., 2008). However, pollination of Cypripedium reginae with 8-day-old pollen produced lighter fruits with fewer seeds, although the percentage of embryonated seeds was not affected (Proctor, 1998). In the cacti Hylocereus polyrhizus and H. undatus, storage of pollen at –196, –70 and –18 °C for 9 months before being used for fertilization had little effect upon subsequent fruit weight, seed number per fruit and seed germinability (Metz et al., 2000), whereas storage at 4 °C reduced fruit weight and seed yield. However, as with D. fuchsii, the level of germination of these seeds was the same as for seed derived from fresh pollen.
Seed production in entomophilous species is potentially pollinator limited, especially when extreme pollinator specificities occur, such as in sexual mimicry, although the relationship between pollination rate and population size is reported to be highly variable (Phillips et al., 2014). However, the packaging of orchid pollen into pollinia makes for a highly efficient delivery system; pollinia may contain >400 000 grains (Wolter and Schill, 1986), and low pollen to ovule ratios of 10–24:1 are a feature of European orchids (Neiland and Wilcock, 1995). This means that even if massulae become detached on different stigmas, high levels of fertilization are likely to occur. Thus, it seems unlikely that the generally lower number of seeds per capsule observed here is a function of poor delivery of pollen to the ovule, rather a feature of reduced vigour of the pollen that was deposited.
We can conclude that the pollen of these four species are adapted to maintaining their viability under conditions of reduced RH and for extended periods of time, which would facilitate their survival both in the flower and during transfer by insect pollinators to other flowers. The ability to survive under reduced MC and lower temperature for extended periods of time suggests that they can be conserved ex situ, and that they have a similar storage physiology to orthodox seeds. The ability of the species tested to retain a seed-siring capability following 6 years of storage without significant loss of quality would support this argument.
ACKNOWLEDGEMENTS
Ryan Davies, Keith Manger, Andrew Poynter and Grace Prendergast are thanked for their technical assistance. Financial support from the Millennium Commission, the Wellcome Trust and Orange Plc is gratefully acknowledged. The Royal Botanic Gardens, Kew is supported by a grant-in-aid from DEFRA
LITERATURE CITED
- Bellusci F, Musacchio A, Stabile R, Pellegrino G. Differences in pollen viability in relation to different deceptive pollination strategies in Mediterranean orchids. Annals of Botany. 2010;106:769–774. doi: 10.1093/aob/mcq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford, UK: CABI Publishing; 2002. [Google Scholar]
- Borba EL, Semir J. Temporal variation in pollinarium size after its removal in species of Bulbophyllum: a different mechanism presenting self-pollination in Orchidaceae. Plant Systematics and Evolution. 1999;217:197–204. [Google Scholar]
- Buitink J, Walters C, Hoekstra FA, Cranbe J. Storage behaviour of Typha latifolia pollen at low water contents: interpretation on the basis of water activity and glass concepts. Physiologia Plantarum. 1998;103:145–153. [Google Scholar]
- Burd M. Bateman's Principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review. 1994;60:83–139. [Google Scholar]
- Cruden RB, Miller-Ward S. Pollen–ovule ratio, pollen size, and the ratio of stigmatic area to the pollen-bearing area of the pollinator: an hypothesis. Evolution. 1981;35:964–974. doi: 10.1111/j.1558-5646.1981.tb04962.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the various contrivances by which British and foreign orchids are fertilised by insects, and on the good effects of intercrossing. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Dawkins MD, Owens JN. In vitro and in vivo pollen hydration, germination, and pollen-tube growth in white spruce, Picea glauca (Moench) Voss. International Journal of Plant Sciences. 1993;154:506–521. [Google Scholar]
- Dickie JB, Pritchard HW. Systematic and evolutionary aspects of desiccation tolerance in seeds. In: Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford, UK: CABI Publishing; 2002. pp. 239–259. [Google Scholar]
- Endress PK. Diversity and evolutionary trends in angiosperm anthers. In: D'Arcy WG, Keating RC, editors. The anther: form, function and phylogeny. Cambridge: Cambridge University Pres; 1996. pp. 92–110. [Google Scholar]
- Franchi GG, Nepi M, Dafni A, Pacini E. Partially hydrated pollen: taxonomic distribution, ecological and evolutionary significance. Plant Systematics and Evolution. 2002;234:211–227. [Google Scholar]
- Hay F, Smith RD. Seed maturity: knowing when to collect seeds from wild plant species. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. Kew, UK: Royal Botanic Gardens; 2003. pp. 97–133. [Google Scholar]
- Hoekstra F. Pollen and spores: desiccation tolerance in pollen and the spores of lower plants and fungi. In: Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford, UK: CABI Publishing; 2002. pp. 185–205. [Google Scholar]
- Hoekstra F, van Roekel T. Desiccation tolerance of Papaver dubium L. pollen during its development in the anther. Plant Physiology. 1988;88:626–632. doi: 10.1104/pp.88.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra F, Crowe LM, Crowe JH. Differential desiccation sensitivity of corn and Pennisetum pollen linked to their sucrose contents. Plant, Cell and Environment. 1989;12:83–91. [Google Scholar]
- Hong TD, Ellis RH, Moore D. Development of a model to predict the effect of temperature and moisture on fungal spore longevity. Annals of Botany. 1997;79:121–128. [Google Scholar]
- Hong TD, Jenkins NE, Ellis RH, Moore D. Limits to the negative logarithmic relationship between moisture content and longevity in conidia of Metarhizium flavoviride. Annals of Botany. 1998;81:625–630. [Google Scholar]
- Hong TD, Ellis RH, Buitink J, Walters C, Hoekstra FA, Crane J. A model of the effect of temperature and moisture on pollen longevity in air-dry storage environments. Annals of Botany. 1999;83:167–173. [Google Scholar]
- Ito I. Ultra-low temperature storage of pollinia and seeds of orchids. Japanese Orchid Society Bulletin. 1965;11:4–15. [Google Scholar]
- Johnson SD, Edwards TJ. The structure and function of orchid pollinaria. Plant Systematics and Evolution. 2000;222:243–269. [Google Scholar]
- Knudson L. A new nutrient solution for orchid seed germination. American Orchid Society Bulletin. 1946;15:214–217. [Google Scholar]
- Lang D. Britain's orchids: a guide to the identification and ecology of the wild orchids of Great Britain and Ireland. Old Basing, UK: Wild Guides Ltd; 2004. [Google Scholar]
- Luza JG, Polito BS. Effects of desiccation and controlled rehydration on germination in vitro of pollen of walnut (Juglans spp.) Plant, Cell and Environment. 1987;10:487–492. [Google Scholar]
- Meeyot W, Kamemoto H. Studies on storage of orchid pollen. American Orchid Society Bulletin. 1969;38:388–393. [Google Scholar]
- Metz C, Nerd A, Mizrahi Y. Viability of pollen of two fruit crop cacti of the genus Hylocereus is affected by temperature and duration of storage. HortScience. 2000;35:22–24. [Google Scholar]
- Neiland MRM, Wilcock CC. Maximisation of reproductive success by European Orchidaceae under conditions of infrequent pollination. Protoplasma. 1995;187:39–48. [Google Scholar]
- Norstog K. New synthetic medium for the culture of premature barley embryos. Vitro. 1973;8:307–308. doi: 10.1007/BF02615911. [DOI] [PubMed] [Google Scholar]
- Pacini E, Franchi GG. Pollen dispersal units, gynoecium and pollination. In: Owens SJ, Rudall PJ, editors. Reproductive biology. Kew, UK: Royal Botanic Gardens; 1998. pp. 183–195. [Google Scholar]
- Pacini E, Hesse M. Types of pollen dispersal units in orchids, and their consequences for germination and fertilization. Annals of Botany. 2002;89:653–664. doi: 10.1093/aob/mcf138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini E, Franchi GG, Lisci M, Nepi M. Pollen viability related to type of pollination in six angiosperm species. Annals of Botany. 1997;80:83–87. [Google Scholar]
- Pandolfi T, Pacini E. The pollinium of Loroglossum hircinum (Orchidaceae) between pollination and the pollen tube emission. Plant Systematics and Evolution. 1995;196:141–151. [Google Scholar]
- Phillips RD, Peakall R, Hutchinson MF, et al. Specialized ecological interactions and plant species rarity: the role of pollinators and mycorrhizal fungi across multiple spatial scales. Biological Conservation. 2014;169:285–295. [Google Scholar]
- Polito VS, Luza JG. Longevity of pistachio pollen determined by in vitro germination. Journal of the American Society for Horticultural Science. 1988;113:214–217. [Google Scholar]
- Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera Orchidacearum. Volume 1: general introduction, Apostasioideae, Cypripedioideae. Kew, UK: Royal Botanic Gardens; 1999. [Google Scholar]
- Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera Orchidacearum. Volume 2: Orchidoideae (Part one) Kew, UK: Royal Botanic Gardens; 2001. [Google Scholar]
- Pritchard HW, Dickie JB. Predicting seed longevity: the use and abuse of seed viability equations. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. Kew, UK: Royal Botanic Gardens; 2003. pp. 653–722. [Google Scholar]
- Pritchard HW, Prendergast FG. Factors influencing the germination and storage characteristics of orchid pollen. In: Pritchard HW, editor. Modern methods in orchid conservation: the role of physiology, ecology and management. Cambridge: Cambridge University Press; 1989. pp. 1–16. [Google Scholar]
- Pritchard HW, Grout BWW, Short KC. Osmotic stress as a pregrowth procedure for cryopreservation. 1. Growth and ultrastructure of sycamore and soybean cell suspensions. Annals of Botany. 1986;57:41–48. [Google Scholar]
- Pritchard HW, Poynter AC, Seaton PT. Interspecific variation in orchid seed longevity in relation to ultra-drying and cryopreservation. Lindleyana. 1999;14:92–101. [Google Scholar]
- Proctor HC. Effect of pollen age on fruit set, fruit weight, and seed set in three orchid species. Canandian Journal of Botany. 1998;76:420–427. [Google Scholar]
- Roberts EH, Ellis RH. Water and seed survival. Annals of Botany. 1989;63:39–52. [Google Scholar]
- Stanley RG, Linskens HF. New York: Springer-Verlag; 1974. Pollen: biology, biochemistry, management. [Google Scholar]
- Sun W. Methods for the study of water relations under desiccation stress. In: Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford, UK: CABI Publishing; 2002. pp. 47–91. [Google Scholar]
- Thompson JD, Rigney LP, Karoly KM, Thomson BA. Pollen viability, vigour, and competitive ability in Erythronium grandiflorum (Liliaceae) American Journal of Botany. 1994;81:1257–1266. [Google Scholar]
- Towill LE. Low temperature and freeze-vacuum-drying preservation of pollen. In: Kartha KK, editor. Cryopreservation of plant cells and organs. Boca Raton, FL: CRC Press; 1985. pp. 171–198. [Google Scholar]
- Vendrame WA, Carvalho VS, Dias JMM, Maguire I. Pollination of Dendrobium hybrids using cryopreserved pollen. HortScience. 2008;43:264–267. [Google Scholar]
- VSN International. GenStat for Windows. 12th edn. Hemel Hempstead, UK: VSN International; 2011. [Google Scholar]
- Wolter M, Schill R. Ontogenie von pollen, massulae und pollinien bei den orchideen. Tropische und Subtropische Pflanzenwelt. 1986;56:1–93. [Google Scholar]


