Abstract
Background and Aims
Although it is well known that fire acts as a selective pressure shaping plant phenotypes, there are no quantitative estimates of the heritability of any trait related to plant persistence under recurrent fires, such as serotiny. In this study, the heritability of serotiny in Pinus halepensis is calculated, and an evaluation is made as to whether fire has left a selection signature on the level of serotiny among populations by comparing the genetic divergence of serotiny with the expected divergence of neutral molecular markers (QST–FST comparison).
Methods
A common garden of P. halepensis was used, located in inland Spain and composed of 145 open-pollinated families from 29 provenances covering the entire natural range of P. halepensis in the Iberian Peninsula and Balearic Islands. Narrow-sense heritability (h2) and quantitative genetic differentiation among populations for serotiny (QST) were estimated by means of an ‘animal model’ fitted by Bayesian inference. In order to determine whether genetic differentiation for serotiny is the result of differential natural selection, QST estimates for serotiny were compared with FST estimates obtained from allozyme data. Finally, a test was made of whether levels of serotiny in the different provenances were related to different fire regimes, using summer rainfall as a proxy for fire regime in each provenance.
Key Results
Serotiny showed a significant narrow-sense heritability (h2) of 0·20 (credible interval 0·09–0·40). Quantitative genetic differentiation among provenances for serotiny (QST = 0·44) was significantly higher than expected under a neutral process (FST = 0·12), suggesting adaptive differentiation. A significant negative relationship was found between the serotiny level of trees in the common garden and summer rainfall of their provenance sites.
Conclusions
Serotiny is a heritable trait in P. halepensis, and selection acts on it, giving rise to contrasting serotiny levels among populations depending on the fire regime, and supporting the role of fire in generating genetic divergence for adaptive traits.
Keywords: Heritability, heritable plant traits, fire ecology, serotiny, QST, FST, selection, Pinus halepensis, Aleppo pine
INTRODUCTION
Wildfires have occurred in many ecosystems for millennia (Pausas and Keeley, 2009), and species from fire-prone ecosystems possess traits conferring fitness benefits under recurrent fires (Keeley et al., 2011). These traits vary among populations subjected to different fire regimes (Pausas et al., 2012; Hernández-Serrano et al., 2013). To unravel whether this divergence is the product of a selective process, it is necessary to demonstrate that traits conferring fitness are heritable. Narrow-sense heritability (h2) and quantitative genetic differentiation of phenotypes among populations (QST; Spitze, 1993) are key parameters to describe the genetic architecture of quantitative traits (Falconer and Mackay, 1996). The divergence of a given trait among populations can be interpreted as the result of diversifying selection when it is higher than the differentiation expected under a neutral process (Leinonen et al., 2008). In other cases, lower differentiation than expected may indicate uniform selection or trait canalization (Lamy et al., 2012). The neutral expectation for trait divergence is usually provided by another statistic known as neutral genetic differentiation (typically FST; Wright, 1951) which is calculated with data from putatively neutral molecular markers; it quantifies neutral forces such as gene flow or genetic drift. Thus, QST–FST comparisons provide information about whether the divergence/convergence of traits among populations can be caused by selective processes or not. Despite the role of fire acting as a selective pressure shaping plant phenotypes has been repeatedly evoked (e.g. Keeley et al., 2011, 2012; Pausas et al., 2012), little is known about the genetic basis of fire traits (Budde et al., 2014; Moreira et al., 2014). In fact, there are neither quantitative estimations of heritability nor information about the quantitative genetic differentiation of any trait related to the regeneration under recurrent fires. This lack of information on the genetic basis of trait variability is limiting our understanding of fire as an evolutionary process (Pausas and Schwilk, 2012).
A trait clearly associated with fire is serotiny, which is the prolonged storage of mature seeds in a canopy seedbank. This seedbank is released in response to an environmental trigger, mainly the heat from a fire (Lamont et al., 1991). Serotiny provides fitness benefits in fire-prone environments because it induces massive seed dispersal in a favourable post-fire seed bed (Keeley et al., 2011, 2012). There are many studies showing that different fire regimes produce variability in the degree of serotiny, with higher serotiny under recurrent crown fires (see the meta-analysis in Hernández-Serrano et al., 2013). In addition, phylogenetic analyses have reconstructed serotiny as an ancestral character linked to an ancient fire regime (He et al., 2011, 2012). Altogether these findings support a selective role of fire on serotiny levels. Moreover, previous studies have supported that serotiny is under genetic control (Rudolph et al., 1959; Lotan, 1967; Teich, 1970), and recent works using association genetics and molecular techniques have provided evidence on the polygenic genetic architecture of serotiny (Parchman et al., 2012; Budde et al., 2014). However, although these results point towards serotiny as a heritable trait which has been selected by fire, there is no specific quantitative genetic study estimating its heritability or quantitative genetic differentiation.
Here, we estimate narrow-sense heritability and genetic differentiation of serotiny in Pinus halepensis, a pine living in fire-prone ecosystems of the Mediterranean Basin (Ne'eman and Trabaud, 2000), using a provenance–progeny common-garden trial. Common-garden trials of forest trees are a useful tool to determine the evolutionary potential of plant species because the uniformity of environmental conditions allows a focus on genetically based differences of the evaluated traits. Specifically, provenance–progeny trials allow quantification of the genetic variation of phenotypic traits both between and within populations. Previous research on P. halepensis has shown that the serotiny level is related to recent disturbances (Goubitz et al., 2004), and that populations under high crown-fire recurrence have a higher proportion of serotinous cones than populations where fires are rare (Hernández-Serrano et al., 2013). To test whether fire has left a selective signature on the level of serotiny among populations, we compared quantitative (QST) and neutral (FST) estimators of genetic differentiation. A QST < FST or QST > FST means that there is globally stabilizing or divergent selection, respectively. Conversely, trait divergence may be the result of a neutral process if QST = FST (Leinonen et al., 2013). We hypothesize that fire drives a divergent selection (QST > FST) towards high levels of serotiny in populations subjected to high recurrent fires.
MATERIALS AND METHODS
Study site and sampling procedure
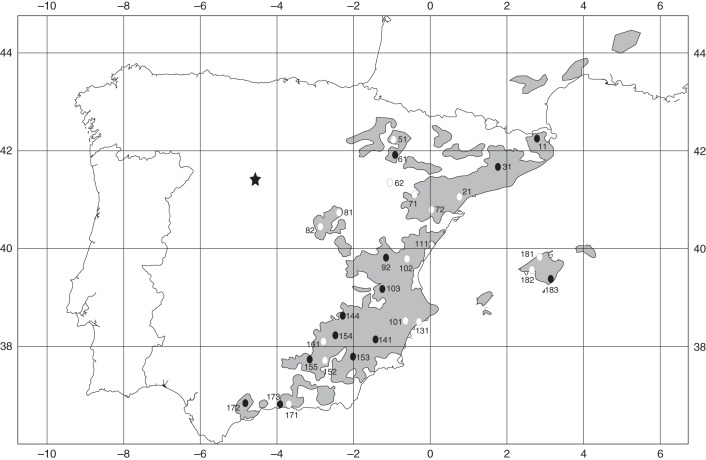
This study was carried out in a Pinus halepensis provenance–progeny common garden, located in inland Spain (Megeces, Valladolid, 4°33′30′′W, 41°25′18′′N; Fig. 1). The trial was established in 1995 with 1-year-old seedlings at a spacing of 2·5 × 2 m in a randomized complete block design with seven blocks. It is composed of 145 open-pollinated families (expected half-sib family structure, see below) of 29 provenances covering the entire natural range of P. halepensis in the Iberian Peninsula and Balearic Islands (Fig. 1). When establishing the trial, the mothers of the progenies were not selected for any trait and therefore this experiment is suitable for the study of evolutionary processes without any genetic bias. The trial site is located at 779 m elevation on a dry shallow calcareous soil with <15 % slope. The average temperature is approx. 12 °C and the annual rainfall reaches 413 mm (66 mm of rainfall during the summer). Even though the trial is located outside the natural distribution of the species (Fig. 1), the existence of extensive P. halepensis plantations surrounding it (for erosion control) shows that the species can thrive in the area.
Fig. 1.
Location of the Pinus halepensis provenance–progeny trial (star) and the provenances of which it is composed (circles). Filled circles indicate the provenances used for QST–FST comparison. The grey areas represent the natural distribution of the species.
In October 2011, we sampled 29 provenances each of which was composed of five families and each family comprising 4–14 trees (see Supplementary Data Table S1), yielding a total of 1375 trees of 17 years old. Of these trees, 1227 (89 %) had reached reproductive maturity (having from 1 to 151 cones) and thus these were the trees considered in all subsequent analyses. All open and closed cones borne by each tree were counted, except the last two cohorts (easily distinguishable by their green and reddish-brown colour), which were discarded because it is not possible to know whether they would later become serotinous or not. Thus, the serotiny level for each tree was estimated as the proportion of closed cones (after full maturation) with respect to the total fully ripe (open and closed) cones.
For serotiny to be a good indicator of the potential capacity to regenerate after fire it should reflect the amount of seeds in the canopy seed bank. This would not be the case if there was a negative relationship at the provenance level between serotiny and the number or quality of seeds in the serotinous cones. To evaluate this trade-off, we compared serotinous cones of the three least and the three most serotinous provenances. For this purpose, we collected six serotinous cones in each of five individuals (one per family; total of 30 cones per provenance), and counted and weighed the seeds in each cone.
Data analyses
To ensure that serotiny variation among populations was not biased by the environmental conditions of the trial site, we compared the serotiny levels with the environmental distances between the trial site and the conditions of origin of each provenance (for a similar procedure, see Rutter and Fenster, 2007; Climent et al., 2008). Climatic data were obtained from the model by Gonzalo et al. (2010) for all provenances except for the three Balearic islands for which data were not available (see Supplementary Data Table S1). We first calculated the Gower's distance between climatic variables of each provenance and those of the trial site, and then we correlated the serotiny level of each provenance with its environmental (Gower) distance. This correlation was not significant (Pearson r = 0·24, P = 0·24), indicating that the particular environment of the trial site did not differentially affect the level of serotiny of the evaluated provenances.
Narrow-sense heritability (h2) and genetic differentiation among populations (QST) for serotiny of the 29 provenances were derived from an ‘animal model’ fitted by Bayesian inference with the version 2·17 of package MCMCglmm (Hadfield, 2010) for R. Because serotiny was defined as the proportion of closed cones in relation to total cones at tree level, we assumed a logit link function and binomial error distribution. The incorporation of the pedigree in an animal model makes it unnecessary to nest individuals within family. With this model, we estimated variance components, namely additive genetic variance (σ2a), among-population variance (σ2p) and error variance (σ2ϵ). Heritability was then calculated as:
with σ2l being the implicit variance due to the link function, π2/3 in the case of a logit link. QST was calculated as
The pedigree was constructed with known mothers (trees from which the seeds were collected) and unknown fathers and assuming that both mothers and fathers were unrelated. This assumption was possible because seeds had been sampled in trees (mothers) separated by at least 30 m, and previous studies on species of the genus Pinus (e.g. De Lucas et al., 2009) and our preliminary data on P. halepensis suggest that the spatial genetic structure is only significant at short distances (<30 m). Regarding father trees, the probability of two individuals sharing the same parent is very low in anemophyllous forest trees such as P. halepensis. In any case, to evaluate the sensitivity of this assumption, we recomputed heritability and the QST estimate simulating a scenario with approx. 18 % of the individuals being full-sibs, which is an extreme value compared with the observations for closely related pine species (Gaspar et al., 2009). Specifically, we estimated family variance with a Bayesian mixed model including family as a random factor. Then, we inferred additive genetic variance for a scenario where, on average, 1·5 trees per family (18 %) were full-sibs and the rest half-sibs (82 %), corresponding to a relationship coefficient of 1/3·6 (because full-sibs share half of their genes and half-sibs share a quarter). Thus, additive genetic variance was calculated as 3·6 times the family variance. In the ‘animal model’, we used parameter expanded priors for the variance components, being the normally distributed working parameter prior, with a mean of 0 and a variance of 1000. The location effect prior was inverse-Wishart distributed, forming a scaled non-central F distribution, with a degree of belief parameter and a limit variance of 1. Models were run for 6 050 000 iterations, discarding the first 50 000 and sampling every 6000, yielding a population of 1000 estimates, and ensuring a low autocorrelation (<0·10). To assess model convergence, the model was run twice with overdispersed starting values. Because the Gelman–Rubin statistic was very close to 1 (i.e. suggesting chain convergence), we only report the results [h2, QST; and 95 % credible intervals (CIs)] of the first model fit.
To determine whether genetic differentiation for serotiny is the result of differential natural selection, we compared QST estimates for serotiny with FST estimates based on the 13 provenances that were studied by Agúndez et al. (1999) using the allozyme data (five loci, average of 50 individuals per population). FST distribution was derived from 1000 bootstraps over allozyme loci. QST distribution was obtained directly from Bayesian MCMC models either fitted to the full phenotypic dataset (29 provenances) or restricted to the 13 above-mentioned populations. The QST–FST comparison was carried out comparing the 95 % CIs (Sahli et al., 2008) as well as comparing the QST and FST distributions following the procedure outlined in Whitlock (2008). For this latter method, we used each of the 1000 FST bootstraps to calculate the predicted χ2 FST distribution using the Lewontin–Krakauer approach. Then, for each FST distribution, we recorded the tail probability of a QST value drawn at random from the QST distribution (see above), and tail probabilities were averaged over the 1000 replicates to obtain the test of neutrality for serotiny (see further details in Whitlock 2008, p. 1894).
Given that in the study area summer rainfall is negatively related to annual area burned (Pausas, 2004; Pausas and Paula, 2012), we used summer rainfall as a proxy of fire regime in each provenance. Serotiny in the provenance–progeny trial was related to summer rainfall of the populations by fitting a generalized linear model with binomial distribution of errors using the lme4 package for R (Bates et al., 2011). In this model, the serotiny level was the dependent variable, summer rainfall was a fixed factor and family nested within provenance was considered as a random factor. To test whether this relationship is not confounded by geographic distance, we carried out a partial Mantel test between dissimilarity in serotiny and dissimilarity in summer rainfall (among provenances), controlling for the effect of geographic distance, using the vegan package for R (Oksanen et al., 2013).
To test whether values of serotiny may be balanced by the quantity or quality of the seeds, we fitted the number of seeds per cone and the weight of the seeds against serotiny in two different linear mixed models where the proportion of serotiny was a fixed factor and individual nested within provenance a random factor. These models were fitted using the nlme package (Pinheiro et al., 2012).
RESULTS
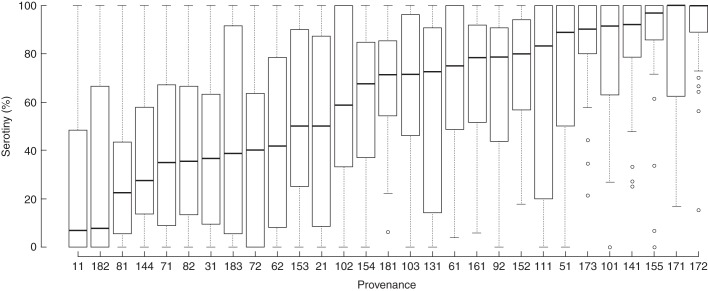
The serotiny level of 1227 individuals ranged from 0 to 100 % and showed a mean value of 60·37 % (s.d. = 35·60). At provenance level, serotiny ranged from 24·59 to 90·69 %, with high within-provenance variability and a mean value of 59·48 % (s.d. = 18·75; Fig. 2; see Supplementary Data Table S1).
Fig. 2.
Proportion of serotinous cones on trees for each of the studied Pinus halepensis provenances. Boxplots indicate the median (horizontal line), the first and third quartiles (box), the range that excludes outliers (whiskers) and the outliers (circles).
Serotiny showed a significant narrow-sense heritability (h2) of 0·20 (CI 0·09–0·40) and a significant genetic differentiation (QST) of 0·32 (CI 0·17–0·60). Neutral genetic differentiation (FST) calculated using allozymes for 13 populations of the provenance–progeny trial was 0·12 (CI 0·09–0·15) (as recomputed from Agúndez et al., 1999). The QST for these 13 populations was 0·44 (CI 0·15–0·76), which is not significantly different from that obtained with all the 29 provenances. Genetic differentiation for serotiny (QST) was significantly higher than expected under a neutral process (FST) when comparing either the CIs (which do not really overlap) or the distribution of values (P = 0·02; Whitlock's procedure). If we calculate heritability and QST simulating a new scenario with a very high proportion of full-sibs (18 %), we obtain a heritability of 0·17 (CI 0·07–0·31) and genetic differentiation among populations (QST) for serotiny of 0·40 (CI 0·21–0·63); these values are not different from our initial results.
The serotiny level of trees in the common garden was significantly related to the summer rainfall (a proxy of fire regime) of their provenance sites (Fig. 3). The negative relationship between both variables (estimate ± s.e. = –0·016 ± 0·003, P < 0·001) indicated a higher levels of serotiny in those provenances with lower summer rainfall, i.e. with higher expected activity of crown fires. Removing the most extreme value in Fig. 3 (with the highest summer rainfall), the relationship remains very similar (estimate ± s.e. = –0·019 ± 0·005, P < 0·001). The partial Mantel test between serotiny and summer rainfall dissimilarity matrices, while controlling for the effect of geographic distance, was significant (Pearson's r = 0·33, P = 0·001), indicating that the relationship between serotiny and summer rainfall is not confounded by geographic distance.
Fig. 3.
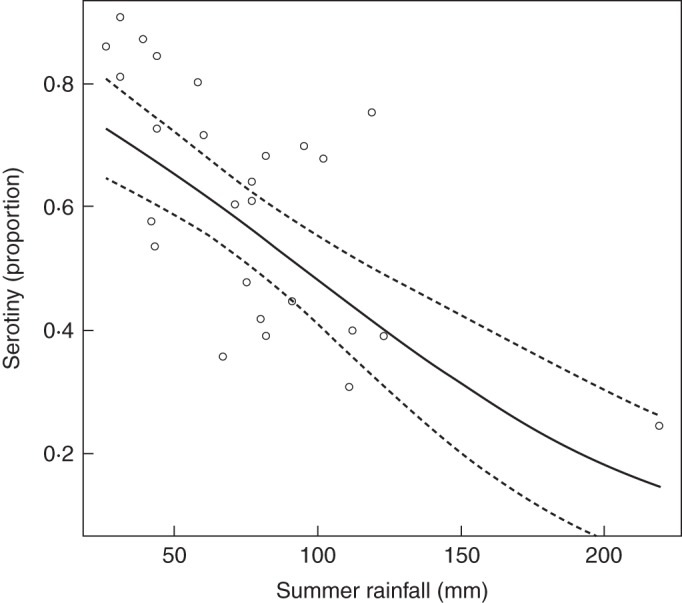
Proportion of serotiny in relation to summer rainfall (a proxy of fire activity) for the 26 provenances. Lines refer to the fit of the fixed effects and the associated 95 % credible interval.
The two groups of different serotiny levels (the three least and the three most serotinous provenances) showed no significant differences either in total seed weight per cone (P-value = 0·50; overall mean = 1·53 ± 0·68 g) or in number of seeds per cone (P-value = 0·58; overall mean = 75·16 ± 23·06). This suggests that the higher proportion of serotinous cones in populations subjected to high recurrent fires was not counterbalanced by a lower number or weight of seeds per cone.
DISCUSSION
Although previous studies have explored the genetic basis of serotiny in different pine species, our study in a P. halepensis provenance–progeny trial is the first providing a quantitative estimation of heritability and genetic differentiation for this trait. Rudolph (1959) identified the frequencies of Pinus banksiana trees bearing serotinous and non-serotinous cones in a progeny test to describe the genetic control of serotiny, and Teich (1970) described it in natural populations of lodgepole pine (Pinus contorta). Both studies suggested that serotiny was a heritable trait probably governed by two alleles of a single gene. However, recent genome-wide association genetic studies that link serotiny with underlying molecular variation have highlighted that this trait has a more complex genetic architecture than previously thought, with many unlinked loci contributing to phenotypic variation of serotiny (P. contorta, Parchman et al., 2012; P. pinaster, Budde et al., 2014), thus showing the need for a quantitative genetics approach.
The relationship between serotiny and fire regime has been widely explored, especially in pines (see review by Hernández-Serrano et al., 2013). Despite the fact that dry winds might facilitate cone opening and seed dispersal (Nathan et al., 1999), the driver that produces large gaps ensuring reproduction and an increase in population size is linked to fire (Pausas et al., 2004; Keeley et al., 2011). In P. halepensis, despite the existence of high within-population variability of serotiny levels, populations under recurrent fire regimes are more serotinous than those subjected to infrequent fires (Hernández-Serrano et al., 2013). Similarly to observations in wild populations, individuals in the common garden showed variable serotiny levels both within and among provenances (Fig. 2). Genetic differentiation estimates showed that variability of serotiny among provenances was substantial (QST = 0·32), in comparison with other studies with pine species where 32 of 39 QST estimates for different traits were <0·32 (range: 0·006–0·97; see details in Supplementary Data Table S2). However, genetic differentiation among provenances for serotiny was still lower than the variation among families within provenances. The presence of additive genetic variation within populations (h2 = 0·20; CI 0·09–0·40) enables short-term evolutionary change responding to natural selection. Moreover, the lower neutral (FST) than quantitative (QST) genetic differentiation found among provenances suggests that there is a spatially divergent selection on serotiny, giving rise to provenances with contrasting levels of serotiny. Significantly, we have been able to link these divergent serotinous strategies shown in the common-garden trial with the fire regime in the provenance sites (Fig. 3). In addition, the comparison of the QST–FST difference of this study with that of 53 studies performed in different plant species [51 studies compiled by De Kort et al. (2013) and two additional studies with pine species that provide QST and FST values (Mahalovich and Hipkins, 2011; Lamy et al., 2012)] shows that our QST–FST difference is relatively high (81 % of the QST–FST differences presented by these studies were below our value).
Several explanations may help to understand why despite divergent selection among provenances, there is high variability in serotiny within provenances (Fig. 2). For instance, gene flow might preclude the fixation of the trait even in the presence of strong selection (Pannell and Fields, 2014). Although average seed dispersal is relatively low in P. halepensis (<20 m; Nathan et al., 2000), long-distance seed dispersal events (>500 m) have also been reported (Steinitz et al., 2011), and gene flow by pollen can reach hundreds of kilometres in conifers (Kremer et al., 2012). In addition, selection only favours the evolution of invariant phenotypes if individuals experience the same conditions throughout their life span (Bradshaw, 1965; Givnish, 2002). Therefore, temporal and spatial variation in fire recurrence could also explain the variation of serotiny at the population level, allowing the emergence of some offspring in fire-free periods or fire-free locations through gap dynamics unrelated to fire. In any case, our data show that within-population variability in serotiny levels is not masking the differences between populations under different fire regimes.
There are limitations regarding QST–FST comparisons based on common gardens. There is little information on maternal effects in conifers (Zas et al., 2013), but it is widely known that maternal effects generally appear at early ontogenetic stages, affecting early life traits such as seed mass, germination rate and seedling growth (Castro et al., 2008; Donohue, 2009; De Kort et al., 2013). Accordingly, maternal effects should not greatly affect serotiny, because it is expressed when individuals are mature (in our case study, measurement took place in 17-year-old trees). Marker type may overestimate FST values if markers are non-neutral, or underestimate it when highly polymorphic markers are used. The fact that some allozymes could be non-neutral (with a low mutation rate) would suggest a possible overestimation of the FST value. Given that in our case the QST estimate is higher than FST, our estimation of the significant effect of natural selection acting on serotiny would be conservative. Moreover, non-additive genetic interactions, such as functional epistasis, may affect QST–FST estimates because serotiny is controlled by several genes (Parchman et al., 2012; Budde et al., 2014). Antagonistic effects between loci in epistatic interactions can modify the QST–FST difference by decreasing QST values; therefore, our comparison would be conservative in this case too. Finally, a significant negative relationship was found between the serotiny level of trees in the common garden and summer rainfall (a proxy of fire regime) of their provenance sites, supporting spatially divergent selection on serotiny.
In conclusion, serotiny is a heritable trait in P. halepensis and selection acts on it, giving rise to contrasting serotiny levels among populations depending on the fire regime. In provenances where crown fires are rare and recruitment is independent of fire, trees have lower serotiny than in provenances growing under high crown fire recurrence. Nevertheless, high levels of within-population genetic variation for this trait can foster evolutionary responses to changes in fire regime. This study provides the first quantitative estimate of inheritance of a trait related to plant persistence under recurrent fires, confirming the role of fire in generating phenotypic divergence and, ultimately, driving local adaptation.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank David Lafuente and Eduardo Ballesteros for fieldwork and laboratory support. Data used in this research are part of the Spanish Network of Genetic Trials (GENFORED). We thank all persons and institutions linked to the establishment and measurement of the field trials and to the maintenance of the network. This work was supported by the Spanish Ministry of Economy and Competitiveness through the following projects: VAMPIRO (CGL2008–05289-C02–01/02), SOBACO (CGL2011–29585-C02–01/02), TREVOL (CGL2012–39938-C02–01) and MITIGENFOR (RTA2011–00016).
LITERATURE CITED
- Agúndez D, Degen B, Von Wuehlisch G, Alía R. Multilocus analysis of Pinus halepensis Mill. from Spain: genetic diversity and clinal variation. Silvae Genetica. 1999;48:173–178. [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: linear mixed-effects models using S4 classes. 2011. R package version 0·999375–42. http://CRAN.R-project.org/package=lme4 . [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Budde KB, Heuertz M, Hernández-Serrano A, et al. In situ genetic association for serotiny, a fire-related trait, in Mediterranean maritime pine (Pinus pinaster Aiton) New Phytologist. 2014;201:230–241. doi: 10.1111/nph.12483. [DOI] [PubMed] [Google Scholar]
- Castro J, Reich PB, Sánchez-Miranda A, Guerrero JD. Evidence that the negative relationship between seed mass and relative growth rate is not physiological but linked to species identity: a within-family analysis of Scots pine. Tree Physiology. 2008;28:1077–1082. doi: 10.1093/treephys/28.7.1077. [DOI] [PubMed] [Google Scholar]
- Climent J, Prada MA, Calama R, Chambel MR, Sanchez de Ron D, Alía R. To grow or to seed: ecotypic variation in reproductive allocation and cone production by young female Aleppo pine (Pinus halepensis, Pinaceae) American Journal of Botany. 2008;95:1–10. doi: 10.3732/ajb.2007354. [DOI] [PubMed] [Google Scholar]
- De Kort H, Vandepitte K, Honnay O. A meta-analysis of the effects of plant traits and geographical scale on the magnitude of adaptive differentiation as measured by the difference between QST and FST. Evolutionary Ecology. 2013;27:1081–1097. [Google Scholar]
- De Lucas A, González-Martínez SC, Vendramin GG, Hidalgo E, Heuertz M. Spatial genetic structure in continuous and fragmented populations of Pinus pinaster Aiton. Molecular Ecology. 2009;18:4564–4576. doi: 10.1111/j.1365-294X.2009.04372.x. [DOI] [PubMed] [Google Scholar]
- Donohue K. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1059–1074. doi: 10.1098/rstb.2008.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th edn. London: Longman; 1996. [Google Scholar]
- Gaspar MJ, De Lucas A, Alía R, et al. Use of molecular markers for estimating breeding parameters: a case study in a Pinus pinaster Ait. progeny trial. Tree Genetics and Genomes. 2009;5:609–616. [Google Scholar]
- Givnish TJ. Ecological constraints on the evolution of plasticity in plants. Evolutionary Ecology. 2002;16:213–242. [Google Scholar]
- Gonzalo J. Diagnosis fitoclimática de la España peninsular. Hacia un modelo de clasificación funcional de la vegetación y de los ecosistemas peninsulares españoles. Madrid: Organismo Autónomo Parques Nacionales; 2010. [Google Scholar]
- Goubitz S, Nathan R, Roitemberg R, Shmida A, Ne'eman G. Canopy seed bank structure in relation to: fire, tree size and density. Plant Ecology. 2004;173:191–201. [Google Scholar]
- Hadfield JD. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software. 2010;33:1–22. [Google Scholar]
- He T, Lamont BB, Downes KS. Banksia born to burn. New Phytologist. 2011;191:184–196. doi: 10.1111/j.1469-8137.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- He T, Pausas JG, Belcher CM, Schwilk DW, Lamont BB. Fire-adapted traits of Pinus arose in the fiery Cretaceous. New Phytologist. 2012;194:751–759. doi: 10.1111/j.1469-8137.2012.04079.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Serrano A, Verdú M, González-Martínez SC, Pausas JG. Fire structures pine serotiny at different scales. American Journal of Botany. 2013;100:2349–2356. doi: 10.3732/ajb.1300182. [DOI] [PubMed] [Google Scholar]
- Keeley JE, Pausas JG, Rundel PW, Bond WJ, Bradstock RA. Fire as an evolutionary pressure shaping plant traits. Trends in Plant Science. 2011;16:406–411. doi: 10.1016/j.tplants.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Keeley JE, Bond WJ, Bradstock RA, Pausas JG, Rundel PW. Fire in Mediterranean ecosystems: ecology, evolution and management. Cambridge: Cambridge University Press; 2012. [Google Scholar]
- Kremer A, Ronce O, Robledo-Arnuncio JJ, et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecology Letters. 2012;15:378–392. doi: 10.1111/j.1461-0248.2012.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont BB, Le Maitre DC, Cowling RM, Enright NJ. Canopy seed storage in woody plants. Botanical Review. 1991;57:277–317. [Google Scholar]
- Lamy JB, Lagane F, Plomion C, Cochard H, Delzon S. Micro-evolutionary patterns of juvenile wood density in a pine species. Plant Ecology. 2012;213:1781–1792. [Google Scholar]
- Leinonen T, O'Hara RB, Cano JM, Merilä J. Comparative studies of quantitative trait and neutral marker divergence: a meta-analysis. Journal of Evolutionary Biology. 2008;21:1–17. doi: 10.1111/j.1420-9101.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- Leinonen T, McCairns RJS, O'Hara RB, Merilä J. QST–FST comparisons: evolutionary and ecological insights from genomic heterogeneity. Nature Reviews Genetics. 2013;14:179–190. doi: 10.1038/nrg3395. [DOI] [PubMed] [Google Scholar]
- Lotan JE. Cone serotiny of lodgepole pine near West Yellowstone, Montana. Forest Science. 1967;13:55–59. [Google Scholar]
- Mahalovich MF, Hipkins VD. Molecular genetic variation in whitebark pine (Pinus albicaulis Engelm.) in the Inland West. In: Keane RE, Tomback DF, Murray MP, Smith CM, editors. The future of high-elevation, five-needle white pines in Western North America: Proceedings of the High Five Symposium. CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station; 2011. pp. 28–30. June 2010; Missoula, MT. Proceedings RMRS-P-63. Fort Collins. [Google Scholar]
- Moreira B, Castellanos MC, Pausas JG. Genetic component of flammability variation in a Mediterranean shrub. Molecular Ecology. 2014;23:1213–1223. doi: 10.1111/mec.12665. [DOI] [PubMed] [Google Scholar]
- Nathan R, Safriel UN, Noy-Meir I, Schiller G. Seed release without fire in Pinus halepensis, a Mediterranean serotinous wind-dispersed tree. Journal of Ecology. 1999;87:659–669. [Google Scholar]
- Nathan R, Safriel UN, Noy-Meir I, Schiller G. Spatiotemporal variation in seed dispersal and recruitment near and far from Pinus halepensis trees. Ecology. 2000;81:2156–2169. [Google Scholar]
- Ne'eman G, Trabaud L. Ecology, biogeography and management of Pinus halepensis and Pinus brutia forest ecosystems in the Mediterranean Basin. Leiden, The Netherlands: Backhuys Publishers; 2000. [Google Scholar]
- Oksanen J, Kindt R, Legendre P, et al. vegan: community ecology package. R package version 2.0–10. 2013. http://CRAN.R-project.org/package=vegan . [Google Scholar]
- Pannell JR, Fields PD. Evolution in subdivided plant populations: concepts, recent advances and future directions. New Phytologist. 2014;201:417–432. doi: 10.1111/nph.12495. [DOI] [PubMed] [Google Scholar]
- Parchman TL, Gompert Z, Mudge J, Schilkey FD, Benkman CW, Buerkle C. Genome-wide association genetics of an adaptive trait in lodgepole pine. Molecular Ecology. 2012;21:2991–3005. doi: 10.1111/j.1365-294X.2012.05513.x. [DOI] [PubMed] [Google Scholar]
- Pausas JG. Changes in fire and climate in the eastern Iberian Peninsula (Mediterranean basin) Climatic Change. 2004;63:337–350. [Google Scholar]
- Pausas JG, Keeley JE. A burning story: the role of fire in the history of life. BioScience. 2009;59:593–601. [Google Scholar]
- Pausas JG, Paula S. Fuel shapes the fire–climate relationship: evidence from Mediterranean ecosystems. Global Ecology and Biogeography. 2012;21:1074–1082. [Google Scholar]
- Pausas JG, Schwilk DW. Fire and plant evolution. New Phytologist. 2012;193:301–303. doi: 10.1111/j.1469-8137.2011.04010.x. [DOI] [PubMed] [Google Scholar]
- Pausas JG, Alessio G, Moreira B, Corcobado G. Fires enhance flammability in Ulex parviflorus. New Phytologist. 2012;193:18–23. doi: 10.1111/j.1469-8137.2011.03945.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D R Development Core Team. nlme: linear and nonlinear mixed effects models. 2012. R package version 3.1–105. [Google Scholar]
- Rudolph TD, Schoenike RE, Schantz-Hanse T. Results of one-parent progeny tests relating to the inheritance of open and closed cones in jack pine. Minnesota Forestry Notes 78. 1959 School of Forestry, University of Minnesota. [Google Scholar]
- Rutter MT, Fenster CB. Testing for adaptation to climate in Arabidopsis thaliana: a calibrated common garden approach. Annals of Botany. 2007;99:529–536. doi: 10.1093/aob/mcl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli HF, Conner JK, Shaw FH, Howe S, Lale A. Adaptive differentiation of quantitative traits in the globally distributed weed, wild radish (Raphanus raphanistrum) Genetics. 2008;180:945–955. doi: 10.1534/genetics.107.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitze K. Population structure in Daphnia obtusa: quantitative genetic and allozymic variation. Genetics. 1993;135:367–374. doi: 10.1093/genetics/135.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz O, Troupin D, Vendramin GG, Nathan R. Genetic evidence for a Janzen–Connell recruitment pattern in reproductive offspring of Pinus halepensis trees. Molecular Ecology. 2011;20:4152–4164. doi: 10.1111/j.1365-294X.2011.05203.x. [DOI] [PubMed] [Google Scholar]
- Teich AH. Cone serotiny and inbreeding in natural populations in Pinus banksiana and Pinus contorta. Canadian Journal of Botany. 1970;48:1805–1809. [Google Scholar]
- Whitlock MC. Evolutionary inference from Qst. Molecular Ecology. 2008;17:1885–1896. doi: 10.1111/j.1365-294X.2008.03712.x. [DOI] [PubMed] [Google Scholar]
- Wright S. The genetical structure of populations. Annals of Eugenics. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Zas R, Cendán C, Sampedro L. Mediation of seed provisioning in the transmission of environmental maternal effects in Maritime pine (Pinus pinaster Aiton) Heredity. 2013;111:248–255. doi: 10.1038/hdy.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.