Abstract
Background and aims
Seed dormancy enhances fitness by preventing seeds from germinating when the probability of seedling survival and recruitment is low. The onset of physical dormancy is sensitive to humidity during ripening; however, the implications of this mechanism for seed bank dynamics have not been quantified. This study proposes a model that describes how humidity-regulated dormancy onset may control the accumulation of a dormant seed bank, and seed experiments are conducted to calibrate the model for an Australian Fabaceae, Acacia saligna. The model is used to investigate the impact of climate on seed dormancy and to forecast the ecological implications of human-induced climate change.
Methods
The relationship between relative humidity and dormancy onset was quantified under laboratory conditions by exposing freshly matured non-dormant seeds to constant humidity levels for fixed durations. The model was field-calibrated by measuring the response of seeds exposed to naturally fluctuating humidity. The model was applied to 3-hourly records of humidity spanning the period 1972–2007 in order to estimate both temporal variability in dormancy and spatial variability attributable to climatic differences among populations. Climate change models were used to project future changes in dormancy onset.
Key Results
A sigmoidal relationship exists between dormancy and humidity under both laboratory and field conditions. Seeds ripened under field conditions became dormant following very short exposure to low humidity (<20 %). Prolonged exposure at higher humidity did not increase dormancy significantly. It is predicted that populations growing in a temperate climate produce 33–55 % fewer dormant seeds than those in a Mediterranean climate; however, dormancy in temperate populations is predicted to increase as a result of climate change.
Conclusions
Humidity-regulated dormancy onset may explain observed variation in physical dormancy. The model offers a systematic approach to modelling this variation in population studies. Forecast changes in climate have the potential to alter the seed bank dynamics of species with physical dormancy regulated by this mechanism, with implications for their capacity to delay germination and exploit windows for recruitment.
Keywords: Acacia saligna, Fabaceae, wattle, humidity, after-ripening, physical dormancy, fire, invasive species, climate change, seed bank
INTRODUCTION
Seed dormancy enhances seedling survival by delaying germination to avoid competition from established plants, unfavourable weather conditions or the presence of parasites or herbivores (Silvertown and Lovett Doust, 1993; Fenner and Thompson, 2005). Dormancy is critical to population persistence, particularly if the standing population is periodically eliminated, e.g. by drought, frost or fire. In these circumstances, the size of the seed bank is an important factor determining the size of the replacement cohort. However, the onset of dormancy is sensitive to environmental factors (Roach and Wulff, 1987; Andersson and Milberg, 1998) and may potentially be affected by human-induced climate change (Walck et al., 2011; Ooi, 2012).
Physical seed dormancy (sensu Baskin and Baskin, 1998) is widely represented in species of the family Fabaceae (Bell et al., 1982, 1993; Auld and O'Connell, 1991; Auld, 1996; Herranz et al., 1998; Santana et al., 2010; Ooi et al., 2012). Dormancy onset occurs when the epidermal cells of the seed become impermeable by dehydration after the seed reaches maturity. Moisture loss is regulated by the hilum acting as a hygroscopically activated valve that opens in response to low humidity (Hyde, 1954; Baskin et al., 2000). A relationship between relative humidity (RH), seed moisture content and dormancy has been described in a number of species with physical dormancy in the Fabaceae and it is likely the mechanism is common to all or most species (Quinlivan, 1971; Pukittayacamee and Hellum, 1987). Variation in dormancy among populations of the same species, between seed crops produced in different years, between individuals in the same population and between seeds produced in different parts of the same plant has been attributed to macro- or microclimatic effects on humidity-regulated dormancy onset (D'hondt et al., 2010; Ooi et al., 2012). However, the ecological implications of this phenomenon and the potential impact of climate change are largely unexplored (D'hondt et al., 2010).
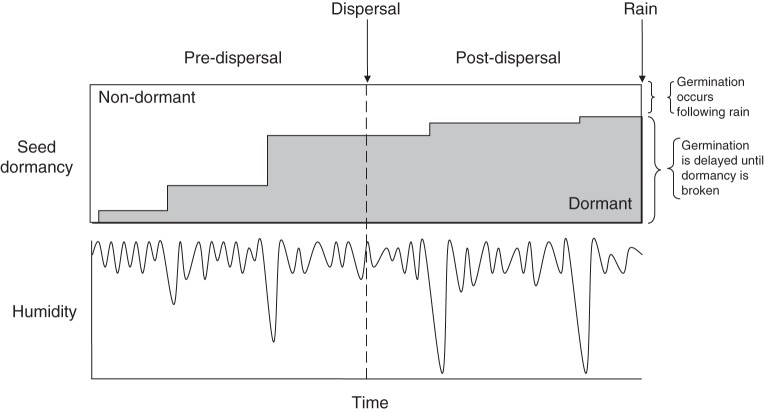
We propose a model (Fig. 1) that describes how humidity-regulated dormancy onset may influence the accumulation of a dormant seed bank in species of Fabaceae. The model postulates that: (1) seeds are non-dormant at maturity; (2) dormancy onset occurs in a variable proportion of seeds determined by fluctuations in humidity during daily and short-term weather cycles (Goss and Madliger, 2007); (3) dormant seeds accumulate in a seed bank as a protection against the loss of the standing population; and (4) non-dormant seeds germinate when soil moisture is sufficient and thus do not enter the seed bank. Two periods are identified during which dormancy onset may occur (Fig. 1). Following maturation and prior to primary dispersal, seeds are held in the canopy as the pods open. Germination cannot occur during this time and dormancy onset is highly responsive to fluctuations in ambient humidity. Following primary dispersal, seeds are buffered against fluctuations in atmospheric humidity (Baskin et al., 1993) within a diffusion gradient from the soil pores (where the air is highly buffered at >60 % RH; Goss and Madliger, 2007), through the humus and litter zones to the atmosphere. The post-dispersal potential for dormancy onset is therefore lowest for seeds incorporated into the soil and highest for seeds exposed on the soil surface.
Fig. 1.
Conceptual model of seed bank input as a function of humidity-regulated dormancy onset under variable weather conditions. We hypothesize that there is a higher potential for seeds to respond to humidity fluctuations prior to dispersal when the seeds are held in the tree canopy compared with post-dispersal, when seeds fall to the soil surface, after which they may be removed to ants' nests or otherwise buried in soil or leaf litter after some period of time. Germination of non-dormant seed follows rainfall and dormant seeds accumulate in a seed bank.
In this paper we describe evidence in support of this model and illustrate its ecological significance in the case of naturalized populations of Acacia saligna occurring in the Sydney region of south-east Australia. First, we quantify the relationship between humidity and dormancy under experimental conditions in the field and laboratory. Second, we use weather records and global climate change models to estimate past and future trends in dormancy percentage for populations growing under the climate conditions that prevail in both its native distribution in south-west Australia and where it has become naturalized in south-east Australia. Since the degree to which seeds are buffered from atmospheric conditions in surface leaf litter is unknown, we measured humidity fluctuations within the litter layer in the field and simulated dormancy onset scenarios representing the extreme cases: (1) dormancy onset is curtailed at the time of primary dispersal; and (2) dormancy onset continues unimpeded by soil moisture buffering until rainfall sufficient for germination occurs (nominally the end of January in Sydney). Finally, we invoke our model to explore the ecological implications for A. saligna in the range of habitats to which it has been introduced, and for the changes in climate that are projected to occur by 2050.
METHODS
Study area and species
Acacia saligna is a small tree endemic to the Swan Coastal Plain in the Mediterranean climate region of south-west Australia and widely naturalized in Mediterranean-type ecosystems throughout the world. It is physiologically and demographically suited to fire-prone, oligotrophic environments experiencing a Mediterranean or temperate climate and a fire-return interval in the range 5–30 years. Acacia saligna is highly invasive in South African fynbos, where populations develop large dormant seed banks which result in prolific post-fire seedling establishment and are difficult to eradicate (Cohen et al., 2008; Strydom et al., 2012). South African populations exhibit dormancy percentages comparable to those of native populations (typically >90 %; Jones, 1963; Larson, 1964; Aveyard, 1968; Shaybany and Rouhari, 1976; Jeffrey et al., 1988; Holmes, 1989; Bell et al., 1993); however, an absence of pathogens and seed predators accounts for a higher recruitment potential in South African populations (Maslin, 1974; New, 1984; Richardson et al., 1992; French and Major, 2001; Bar et al., 2004; Yelenik et al., 2004; Manor et al., 2008; Richardson et al., 2011).
Acacia saligna was planted extensively along major roads and during the restoration of coastal sand mines in the latter half of the twentieth century and has become widely naturalized in south-east Australia (Armitage, 1977). In the Sydney region it occurs as scattered populations along the edge of roads and tracks on the coast and adjacent plateaus (Beadle et al., 1982). Flowering occurs from August to September and the fruits ripen and open in early to late December, after which most seeds are held on the plant for ∼2 weeks before dispersing passively to the ground below.
We harvested seeds from populations located within 40 km north or south of the central business district of Sydney on the east coast of Australia (Table 1). Sydney experiences a maritime temperate climate (Peel et al., 2007) characterized by warm, humid summers and mild winters with significant rainfall in all seasons. Average annual rainfall recorded at the Sydney Observatory, located in the city centre (33·86°S 151·21°E), is 1213 mm (1858–2011), with monthly totals ∼25 % above average from February to June and ∼25 % below average from August to December. January is the warmest month and July the coolest, with average maxima and minima ranging from 18·7 to 25·9 °C and 8 to 16·3 °C, respectively.
Table 1.
Seed viability (V, %) and dormancy (D, %) estimated by germination trials (mean ± s.e.m.). Dates indicate when the seeds were harvested in the field
| Site | Location | Distance to sea (km) | Test | 2 December 1991, RH 22 % | 26 December 1992, RH 32 % | 13 December 1993, RH 8 % |
|---|---|---|---|---|---|---|
| MVSt | 33°42′27″ S, 151°10′51″ E | 15 | V | 98 ± 2A | 93 ± 4Aa | 99 ± 1Aa |
| D | 26 ± 2A | 2 ± 2Ba | 91 ± 3Ca | |||
| MVNt | 33°42′28″ S, 151°12′00″ E | 15 | V | – | 80 ± 5b | 100 ± 0a |
| D | 0 ± 0a | 98 ± 1b | ||||
| Harb | 33°45′46″ S, 151°16′46″ E | 1·5 | V | 92 ± 2 | 91 ± 1a | 76 ± 5b |
| D | 43 ± 6 | 68 ± 5b | 77 ± 8c | |||
| WeHd | 33°35′34″ S, 151°16′44″ E | 2 | V | – | 98 ± 1a | 100 ± 0a |
| D | 5 ± 2a | 81 ± 2c | ||||
| WP1 | 33°46′4″ S, 151°14′4″ E | 6 | V | – | 98 ± 3a | 99 ± 1a |
| D | 48 ± 8c | 100 ± 0b | ||||
| WP4 | 33°44′43″ S, 151°14′7″ E | 6 | V | – | – | 98 ± 1a |
| D | 85 ± 0c |
Identical letters indicate statistical invariance among years (upper case; site MVSt only) or among sites (lower case; 1992/93 only).
Seed viability and dormancy
Ripe fruits were harvested as late as possible, at the time when passive dispersal from the pods was commencing, from samples of 20 fruiting individuals from multiple populations and fruiting seasons (seed harvesting dates and locations are contained in Table 1). Seeds were removed from the fruits by hand, mixed thoroughly and those consumed by weevil larvae (Melanterius sp.) were discarded. Four replicates of 30 seeds were placed on moistened filter paper in Petri dishes and watered with distilled water as required. The number of germinated (radicle emergence) or dead (soft when pressed) seeds was recorded daily for 6 weeks. Ungerminated seeds were scarified with sandpaper to break physical dormancy then placed back on moist filter paper to germinate. The viable portion of the seed lot was calculated as the total number of germinated seeds as a proportion of the total number of seeds tested. The non-dormant portion of the seed lot was calculated as the number of seeds germinated before scarification as a proportion of the total number of viable seeds. Variation in seed dormancy and viability was tested by one-factor analysis of variance (ANOVA) across years of sampling (site MVSt) and among sites within the year 1993. Cochran's test was used to check the assumption of homogeneity of variances and data were arcsine-transformed where necessary. Comparisons between treatment levels were made using Student–Newman–Keuls tests (Underwood, 1981). The relationship between dormancy and ambient conditions prior to dispersal was explored using 3-hourly records of RH measured at Sydney Observatory. A period of 2 weeks leading up to the time of harvesting was nominally designated the pre-dispersal period.
Effect of RH on seed dormancy: laboratory experiment
Seeds were harvested at the time of first opening at site MVSt (Table 1) and transferred immediately to a water vapour-saturated storage container in order to minimize their dehydration potential. Dormancy and viability were tested as described above, then two samples of 50 seeds were transferred to each of 21 airtight containers in which RH was regulated at different levels by a saturated solution of one of six different salts or precipitated silicic acid (silica gel) (NaCl 75 %, NH4NO3 62 %, MgNO3 53 %, K2CO3, 43 %, MgCl2 33 %, CH3COOK 22 %, silica gel <1 %).
Temperature during storage was regulated by laboratory air-conditioning and was in the range 20–25 °C. One sample of seeds was removed after 24 h and the second was left for a further 49 d. Factorial combinations of storage duration and RH were replicated three times. A logistic model with a binomial error structure was used to model the non-dormant proportion of the seed against the RH in storage following the procedure of Crawley (1993). Williams' procedure was used to correct for over-dispersion. Plots of standardized residuals against the fitted values and the normal order statistics were examined to check the validity of the error specification and model structure.
Effect of RH on seed dormancy: field experiment
We tested the sensitivity of dormancy onset to different levels of RH varying under field conditions as a function of daily cycles (morning–afternoon–evening) and the origin of dominant air masses (e.g. maritime versus continental). Typical daily cycles during December and January reflect the predominant influence of maritime air masses. RH is highest overnight (>80 %) and decreases from dawn to a minimum in early to mid-afternoon before rising again with the advent of sea breezes. Humidity may drop suddenly with the arrival of hot, dry continental air and can remain low even during the evening. Humidity is maintained at 100 % during precipitation.
We measured the sensitivity of seeds to ambient fluctuations of humidity over single and multiple daily cycles. By measuring the response to a single daily cycle we aimed to quantify the minimum fraction of dormant seed produced as a function of the minimum humidity recorded during a cycle. By exposing seeds to multiple cycles we aimed to test for cumulative effects of exposure over multiple days. A relatively small proportion of our samples were exposed to low humidity conditions (because they were rare) and the maximum exposure time was limited to 8 d to ensure the treatment was terminated before a dry day occurred, which would swamp the signal of the earlier days.
Seeds were harvested at site MVSt in December 2006, tested for dormancy and viability and stored in water-saturated vapour as described above. Batches of 30 seeds were removed from storage at intervals during the period 18 December 2006 to 24 January 2007 (the period of natural seed fall in the wild) and exposed to ambient conditions in Petri dishes in the field for periods ranging from 24 h to 8 d. RH was recorded at 15-min intervals during this period using a Tinytag logger coupled with an internal capacitive sensor (accuracy 3·0 %; http://www.geminidataloggers.com/). Exposure commenced at 0700 h, when humidity was close to the night-time maximum, and was terminated at the same time following the requisite number of days, when the dormancy test was commenced by saturating the filter paper. The relative contributions to dormancy of minimum RH and duration of exposure were tested by fitting a logistic model with a binomial error structure and performing stepwise deletion from the maximal model (Crawley, 1993).
Humidity fluctuations within surface leaf litter
RH was recorded at a depth of 15 mm below the surface of the leaf litter (∼5 mm above the soil surface) adjacent to site MVSt at hourly intervals from 24 December 2013 to 10 February 2014. Three Thermodata (http://thermodata.com.au/) ThermoLogger (Model TL-TH) devices were deployed at locations ∼5 m apart, with a single device suspended 1 m above ground in a central gap between shrubs. The relationship between daily ambient humidity minima and the respective minima recorded in the litter was tested using log-linear regression.
Proportion of dormant seed under historical and future weather patterns
We analysed daily weather records from Sydney and Perth spanning the period 1972–2007 and calculated the minimum humidity attained during the period relevant to dormancy onset. We assumed pre-dispersal dormancy onset occurred during a 14-d window commencing at any time during December and considered two post-dispersal scenarios. First, we assumed that humidity at and below the soil surface is buffered by soil moisture, preventing dormancy onset following primary dispersal. In the second scenario we assumed soil moisture buffering is negligible; thus dormancy onset could continue post-dispersal up to a nominal endpoint at the end of January (by which time an average of 100 mm of rainfall has occurred). We calculated the dormant fraction (1972–2007) in each hypothetical seed crop with the pre-dispersal dormancy onset period commencing progressively later from 1 December to 31 December. We calculated the dormancy from the minimum humidity attained in the relevant period using the equation fitted to the data collected in our field experiment (see Results). Potential changes in the minimum humidity level under scenarios of future climate change were examined by repeating the analyses using daily observations simulated for a period centred on 2050 using the Australian Commonwealth Scientific and Industrial Research Organisation (CSIRO) global climate models Mark 2 and Mark 3 (Hennessey et al., 2005). Simulations considered only extreme scenarios (low emission/low climate sensitivity and high emission/high climate sensitivity).
RESULTS
Seed viability and dormancy
Seed viability was almost uniformly high, with only MVNt (1992) and Harb (1993) recording slightly lower levels (Table 1). Dormancy was highly variable among years (ranging from 0 to 100 %) and also varied significantly among sites within the 1992 season (0–68 %) and, to a lesser extent, the 1993 season (77–100 %). The fraction of dormant seed was highest in 1993, when dry conditions were prevalent, and lowest in 1992, when humidity remained high during the pre-dispersal period. Indicative RH minima recorded at Sydney Airport during the pre-dispersal period explained ∼89 % of variation in dormancy via a log-log response model (Table 2).
Table 2.
Analysis of deviance for models fitting the fraction of dormant seed to levels of RH and duration of exposure. Change in deviance was calculated by deletion from the maximal model and assessed for significance by comparison with values in χ2 tables (Crawley, 1993)
| Sample details and humidity conditions | Model | Fitted terms | Change in deviance (d.f.) | Scaled deviance (d.f.) |
|---|---|---|---|---|
| Seed ripened under field conditions in the years 1991–93 and 2006 (see Table 1) | log-log | Minimum RH Sydney Airport | 688·9 (1) | 93·05 (41) |
| Seeds harvested from MVSt and exposed for 24 h under laboratory-regulated humidity conditions | log-log | Humidity | 315·4 (1) | 48·37 (15) |
| Seeds harvested from MVSt and exposed for 50 d under laboratory-regulated humidity conditions | logit | Humidity | 256·7 (1) | 28·31 (18) |
| Seeds harvested from MVSt and exposed to field conditions for variable duration | log-log | RH minimum during exposure measured on site | 289·9 (1) | 105·05 (41) |
| Duration (<60 %) | 0·04 (1)* | 103·05 (40) | ||
| Duration (<40 %) | 3·5 (1)* | 99·59 (40) | ||
| Duration (<30 %) | 13·9 (1) | 89·18 (40) | ||
| Duration (<20 %) | 14·35 (1) | 88·74 (40) |
*Non-significant change in deviance; all other terms were significant at P < 0·001.
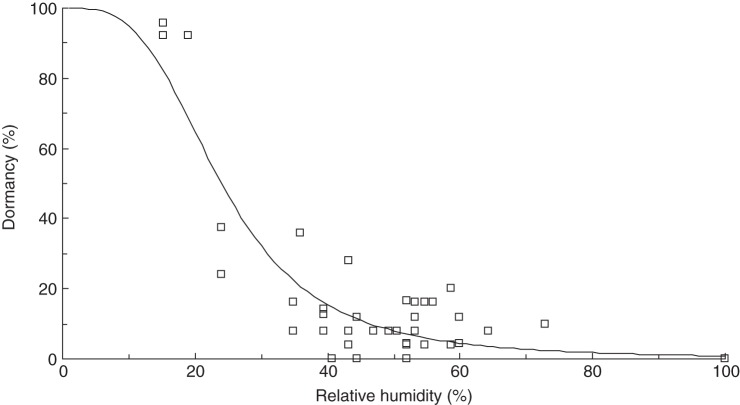
Dormancy was negatively related to RH during storage under constant conditions (Table 2, Fig. 2). Storage for 50 d at humidity levels ranging from 33 to 62 % resulted in higher levels of dormancy than the corresponding treatments stored for 24 h. Seeds stored at 22 % RH were almost uniformly dormant irrespective of storage duration. More than 80 % of seeds were dormant following 50 d of storage at 53 % RH but a humidity of ≤43 % was required to achieve >80 % dormancy in seeds stored for 24 h.
Fig. 2.
Fraction of dormant seed (y) measured in germination trials as a function of the minimum RH (x) maintained during storage over 24 h (y = 1/(1 + 100/(e(13·62–3·406(ln(x)))))) or 50 d (y = 100/(1 + 1/(e(7·184–0·1051x)))), as indicated in the key.
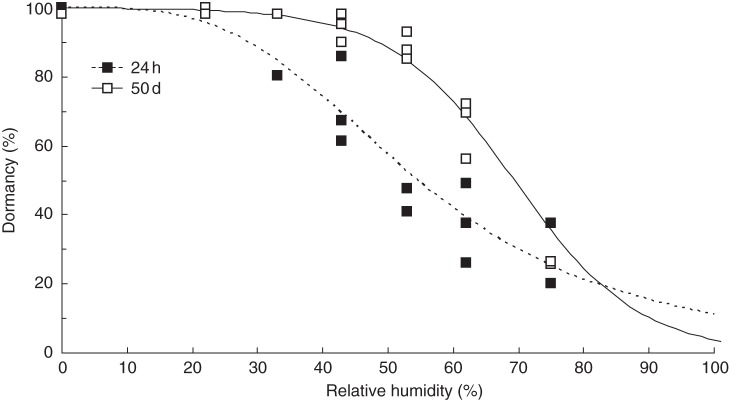
Non-dormant seeds under field conditions were highly sensitive to conditions of low humidity (<20 %) and attained levels of dormancy >92 % following 15 min to 2 h of exposure (Fig. 3, Table 3). Overall, 73 % of variation in the dormant fraction was explained by the minimum RH experienced during exposure (Table 2), but only 4 % could be attributed to the duration of exposure to <30 % humidity and dormancy was apparently insensitive to longer exposure at higher humidity (Tables 2 and 3).
Fig. 3.
Fraction of dormant seed (y) measured in germination trials as a function of the minimum RH (x) recorded under field conditions: the fitted line is y = 1/(1 + 1/(e(10·59–3·332(ln(x))))).
Table 3.
Sensitivity of dormancy onset to variation in RH under field conditions. Each row represents samples exposed during the specified dates; columns 2–5 are the duration of exposure (h) below the RH threshold specified, column 6 is the minimum humidity experienced during exposure and column 7 is the level of dormancy measured following exposure. The control sample was maintained at 75 % RH throughout the experiment and tested upon conclusion
| Date | Time <20 % | Time <30 % | Time <40 % | Time <60 % | Minimum RH (%) | Dormancy (%) |
|---|---|---|---|---|---|---|
| 18 Dec. 2006 | 0 | 0 | 2 | 8·75 | 34·9 | 8 |
| 19 Dec. 2006 | 0 | 0 | 0 | 1 | 53·3 | 12 |
| 20 Dec. 2006 | 0 | 0 | 0 | 0 | 100 | 0 |
| 21 Dec. 2006 | 0 | 0 | 0 | 0·25 | 58·7 | 20 |
| 22 Dec. 2006 | 0 | 0 | 0 | 0 | 64·2 | 8 |
| 18–23 Dec. 2006 | 0 | 0 | 2 | 10 | 34·9 | 16 |
| 23 Dec. 2006 | 0 | 0 | 0 | 0·25 | 56 | 16 |
| 27 Dec. 2006 | 0 | 0 | 0 | 9·75 | 44·4 | 0 |
| 26–27 Dec. 2006 | 0 | 3 | 9·25 | 23·75 | 24 | 24 |
| 28 Dec. 2006 | 0 | 0 | 0 | 8·75 | 43·2 | 4 |
| 26–28 Dec. 2006 | 0 | 3 | 9·25 | 32·5 | 24 | 38 |
| 30 Dec. 2006 | 0 | 0 | 0 | 3·5 | 52 | 4 |
| 31 Dec. 2006 | 0 | 0 | 0 | 0 | 72·9 | 10 |
| 30 Dec. 2006 to 1 Jan. 2007 | 0 | 0 | 0 | 3·5 | 52 | 0 |
| 01 Jan. 2007 | 0 | 0 | 0 | 0 | 60 | 4 |
| 30 Dec. 2006 to 2 Jan. 2007 | 0 | 0 | 0 | 3·5 | 52 | 17 |
| 02 Jan. 2007 | 0 | 0 | 0 | 0 | 43·2 | 28 |
| 30 Dec. 2006 to 3 Jan. 2007 | 0 | 0 | 0 | 3·5 | 52 | 4 |
| 1–3 Jan. 2007 | 0 | 0 | 0 | 0 | 60 | 12 |
| 3 Jan. 2007 | 0 | 0 | 0 | 6 | 53·3 | 8 |
| 4 Jan. 2007 | 0 | 0 | 0·25 | 2·75 | 54·7 | 4 |
| 3–5 Jan. 2007 | 0 | 0 | 0·25 | 8·75 | 43·2 | 8 |
| 5 Jan. 2007 | 0 | 0 | 0 | 8·75 | 39·5 | 8 |
| 30 Dec. 2006 to 6 Jan. 2007 | 0 | 0 | 0·25 | 21 | 39·5 | 14 |
| 2–6 Jan. 2007 | 0 | 0 | 0·25 | 17·5 | 39·5 | 13 |
| 6 Jan. 2007 | 0 | 0 | 0 | 10 | 49·3 | 8 |
| 5–7 Jan. 2007 | 0 | 0 | 0 | 18·75 | 39·5 | 0 |
| 9 Jan. 2007 | 0 | 0 | 0 | 11·25 | 43·2 | 8 |
| 10 Jan 2007 | 0 | 0 | 0 | 8·25 | 49·3 | 8 |
| 11 Jan. 2007 | 0 | 0 | 0 | 1·5 | 58·7 | 4 |
| 14 Jan. 2007 | 0 | 0 | 0 | 7 | 49·3 | 8 |
| 12–14 Jan 2007 | 0 | 0 | 0 | 0·25 | 44·4 | 12 |
| 15 Jan. 2007 | 0 | 0 | 0·25 | 9·75 | 50·6 | 8 |
| 9–17 Jan. 2007 | 0 | 0 | 0·25 | 56·75 | 36 | 36 |
| 18 Jan. 2007 | 0 | 0 | 0 | 1·75 | 53·3 | 16 |
| 19 Jan. 2007 | 0 | 0 | 0 | 2·25 | 54·7 | 16 |
| 20 Jan. 2007 | 0 | 0 | 0 | 13·75 | 46·9 | 8 |
| 21 Jan. 2007 | 2 | 5·25 | 10 | 21·5 | 15·2 | 92 |
| 21 Jan. 2007 | 2 | 5·25 | 10 | 21·5 | 15·2 | 96 |
| 17–22 Jan. 2007 | 2 | 5·25 | 10 | 39·25 | 15·2 | 96 |
| 22 Jan. 2007 | 0·25 | 1 | 5·25 | 9 | 19·1 | 92 |
| 23 Jan. 2007 | 0 | 0 | 0 | 2·5 | 39·5 | 8 |
| Control | 0 | 0 | 0 | 0 | 100 | 0 |
| Total | 2·5 | 10·75 | 37·75 | 223·25 | 15·2 | 100 |
Temporal patterns in dormancy
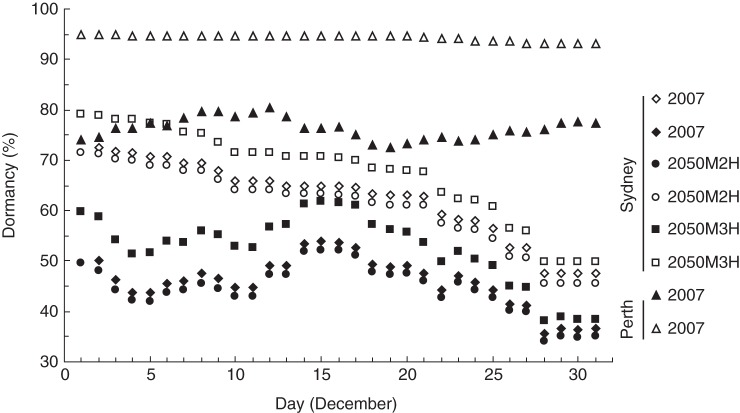
Between 1972 and 2007, conditions conducive to pre-dispersal dormancy onset in Sydney (<20 % RH) occurred erratically in the first half of December, were most frequent in the third week of December and declined in frequency thereafter (Fig. 4). The frequency of years in which low humidity was encountered post-dispersal declined steadily as a function of the commencement date. Although conditions in Perth were also erratic in the first half of December, conditions conducive to pre-dispersal dormancy onset occurred more frequently than in Sydney and frequency rose steadily from a mid-December minimum. Post-dispersal exposure to low humidity was assured within the historical range of seed maturation times.
Fig. 4.
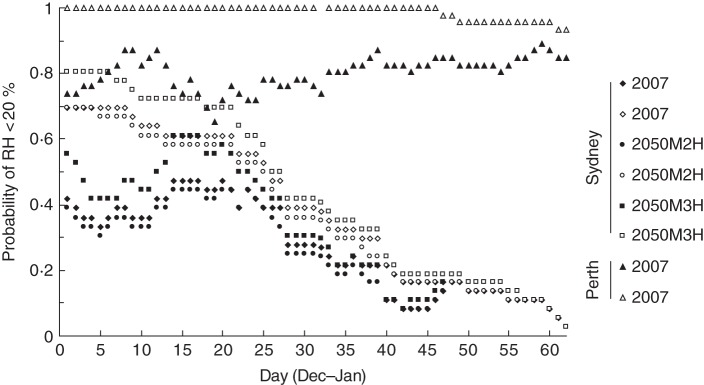
Frequency of years when RH falls below 20 % during a nominal 2-week ripening period; the x-axis represents the date ripening commences. Diamonds represent frequency of years calculated from weather records spanning the period 1972–2007 at Sydney Airport. Circles and squares are estimated frequencies in the future (nominally 2050) based on, respectively, the CSIRO Mark 2 (M2H) and Mark 3 (M3H) climate models, assuming a scenario of high carbon emissions (Hennessey et al., 2005). Triangles represent the corresponding frequencies for Perth (south-west Australia) for the period 1974–2007. Closed symbols represent statistics for a nominal 2-week pre-dispersal period. Open symbols represent statistics for the pre-dispersal period through to the end of January.
Estimates for the average percentage of dormant seeds at the point of primary dispersal for the period 1972–2007 varied from 34 to 54 % (Sydney) and from 72 to 80 % (Perth) as a function of commencement date, and either declined (Sydney) or increased (Perth) steadily from the third week of December (Fig. 5). Post-dispersal conditions could have increased dormancy by up to 66 % (Sydney) or 33 % (Perth) depending on the commencement date (and assuming no rainfall).
Fig. 5.
Estimated seed bank dormancy as a function of the date ripening commences averaged over the years 1972–2007. Dormancy was estimated for each year in the 34-year sequence from the minimum humidity occurring in a nominal 2-week ripening period using the equation in Fig. 3. Diamonds represent averages calculated from weather records at Sydney Airport. Circles and squares are the estimated averages in the future (nominally 2050) based on, respectively, the CSIRO Mark 2 and Mark 3 climate models, assuming a high carbon emissions scenario (Hennessey et al., 2005). Triangles represent the corresponding averages for Perth (south-west Australia) for the period 1972–2007. Closed symbols represent statistics for a nominal 2-week pre-dispersal period. Open symbols represent statistics for the pre-dispersal period through to the end of January.
Changes in the frequency of occurrence of low humidity conditions under climate change varied depending on the climate change model and emissions scenario chosen. These ranged from a slight decrease in the frequency of days where humidity falls below 20 % (Mark 2 model, high emissions scenario) to an increase in the frequency of low-humidity days, resulting in an increase in dormancy (Mark 3 model, high emissions scenario) depending on the commencement date (Fig. 4). Under a high emissions scenario, the Mark 3 model predicts that the 30-year average dormant percentage would increase by ∼20 % if dormancy onset was restricted to the pre-dispersal period, or up to 10 % if dormancy onset continued post-dispersal until sufficient rainfall occurs (Fig. 5).
Humidity fluctuations within surface leaf litter
RH within the leaf litter fluctuated in response to fluctuations in ambient humidity but was buffered to a variable degree depending on location (Fig. 6). Some 48 % of variation in litter humidity was accounted for by a log-linear relationship with ambient humidity. On average, this relationship predicts humidity in the litter to be higher than ambient humidity by 21 percentage points when ambient humidity falls to the critical threshold of 20 %, and by 6 percentage points when ambient humidity falls to 10 % (Fig. 6). The trajectory of change during the driest day recorded suggests that humidity within the litter bed lags behind ambient humidity as a function of moisture content (Fig. 7). Humidity levels in the litter can apparently fall as low as the ambient level, presumably in certain positions conducive to the litter drying out (Figs 6 and 7).
Fig. 6.
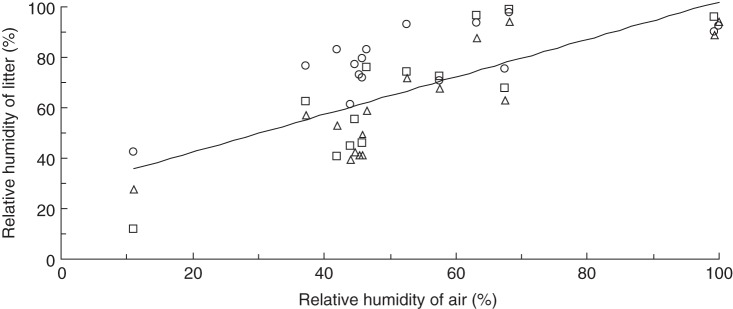
Daily minimum RH recorded in the litter (y) plotted against the corresponding minimum ambient humidity (x) during the period 24 December 2013 to 10 February 2014. Symbols represent three locations ∼5 m apart (y = 35.8ln(x) – 66·1; r2 = 0·48).
Fig. 7.
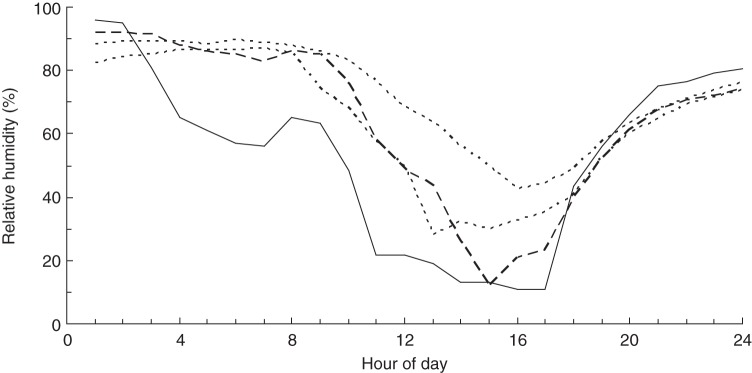
Patterns of humidity recorded at the same litter locations as in Fig. 6 (broken lines) and the atmosphere (solid line) on the driest day during the period 24 December 2013 to 10 February 2014.
DISCUSSION
Humidity-regulated dormancy onset in Acacia saligna
Naturalized populations of A. saligna in the Sydney region exhibit patterns in seed dormancy that are broadly consistent with our proposed model of humidity-regulated dormancy onset (Fig. 1). Seeds were non-dormant at the end of maturation (when the pods start to cure and dehisce) but attained variable levels of dormancy at the point of dispersal (0–100 %), consistent with the conditions (humid or dry) predominating during the pre-dispersal period (Table 1). Although we demonstrated the sensitivity of this mechanism to both the level of humidity and the duration of exposure under laboratory conditions, the nature of (and response to) humidity fluctuations under field conditions suggests that dormancy onset is primarily regulated by the minimum humidity to which seeds are exposed. Thus, our representation of dormancy increasing in steps through time (as opposed to gradually) is plausible (Fig. 1). These results are consistent with a plastic response to humidity conditions and thus we conclude that a hygroscopic mechanism governs dormancy onset in A. saligna, as described in other species of Fabaceae (Hyde, 1954; Hagon and Ballard, 1970; Quinlivan, 1971; Pukittayacamee and Hellum, 1988).
Examples of variation in dormancy attributed to macro- or microclimatic effects on humidity-regulated dormancy onset are uncommon. D'hondt et al. (2010) identified patterns in the level of dormancy in Trifolium repens associated with climatic and habitat factors operating at a range of scales. Wet conditions recurring on a 6-yearly climate cycle were associated with low dormancy at the population level, while variation among individuals or inflorescences was related to the degree of exposure in the landscape. D'hondt et al. (2010) speculated that the population dynamics of T. repens could vary substantially along altitude or latitude gradients as a consequence of this mechanism. Similarly, Jones and Bunch (1987) found that dormancy of the annual seed lot varied between 6 and 94 % over a 12-year period for seeds of the physically dormant Macroptilium atropurpureum, and was highest when dry periods preceded seed collection. Humidity and rainfall accounted for 77 % of the variance in dormancy levels between years.
Further investigations may reveal patterns in A. saligna dormancy relating to climatic gradients, as postulated by D'hondt et al. (2010). For example, conditions of very low humidity in the Sydney region are generated by large continental air masses driven by strong westerly winds, which may be partially mitigated by moist air adjacent to the coast. Accordingly, there is some evidence of lower dormancy percentages in our 1993 samples from populations located near the coast (Table 1: Harb, WeHd). Conversely, the same pattern could be attributed to the influence of humid afternoon sea-breezes; these affect near-coastal areas in the early afternoon but their influence is delayed and diminished with increasing distance from the coast, thus prolonging conditions suitable for dormancy onset in inland relative to coastal populations.
Differences in seed handling and storage prior to testing may be responsible for some variability in dormancy among the populations we sampled. The primary anomalies in our germination data concern the higher percentages of dormancy recorded in Harb and WP1 relative to other populations in 1992. We speculate that these results relate to a period of days during which seed from those populations was stored in an air-conditioned office prior to testing (humidity 50–60 %). This highlights the importance of standardized seed-handling procedures such as we employed in the latter part of our study.
Impact on the accumulation of dormant seed banks
The impact of humidity-regulated dormancy onset on the rate of seed bank accumulation depends on the post-dispersal fate of seeds. There is experimental evidence suggesting that the most likely outcome for A. saligna is that seeds are encountered by ants, moved distances in the order of metres and incorporated in subterranean nests, although removal rates are potentially highly variable as a function of the proximity and activity of species attracted to Acacia seeds (M. Tozer, unpublished data). Once incorporated into the soil, there appears to be negligible potential for further dormancy onset even when the soil appears dry. Goss and Madliger (2007) demonstrated that humidity in soil pores was strongly buffered against atmospheric fluctuations and remained >60 % at depths greater than 3 cm in deep, well-drained Haplic Acrisol (sand, 49 %; silt, 4 %; clay, 47 %) even while vegetation wilted (buffering was weaker at 1·5 cm depth, but RH remained >35 %).
Seeds not encountered by ants are most likely to remain close to the parent plant and to be incorporated passively into the litter and soil. Our measurements suggest that seeds incorporated into litter are moderately buffered from fluctuations in atmospheric humidity while seeds exposed above soil or litter are subject to atmospheric conditions. In the specific case of our naturalized populations, the post-dispersal phase corresponds to a declining probability of conditions of low humidity and a period of generally reliable rainfall. Thus, under an aseasonal or weakly summer dominant rainfall pattern (such as experienced in Sydney), potential post-dispersal increases in dormancy (in the order of 30–50 %) are unlikely to be realized, with both the likelihood and magnitude of increased dormancy declining with progressively later ripening (Fig. 5). Furthermore, there is experimental evidence showing that seeds that enter the seed bank in a non-dormant state germinate (and thus are lost to the seed bank) within 3 months of burial (M. Tozer, unpublished data). In contrast, populations occurring in areas experiencing a Mediterranean-type climate may have a higher potential for post-dispersal dormancy onset due to the prolonged dry summer (Fig. 5), but presumably only if the seeds remain above the soil surface.
Ecological implications of climate change
Projected changes in the climate caused by atmospheric CO2 enrichment (Clarke et al., 2011) have implications for dormancy in A. saligna and thus its potential to develop dormant seed banks in humid regions like Sydney. The magnitude and direction of changes in dormancy resulting from changes in average humidity vary depending on the emissions scenario and climate model chosen, although the extremes in the range of outcomes occur under the highest emission scenario considered (Hennessey et al., 2005). Under the Mark 2 model a slight decrease in the 30-year average percentage of dormant seed is predicted, whereas under the Mark 3 model we predict dormancy to increase by 15–18 % depending on commencement of ripening. More substantial changes in dormancy are likely to result from changes in climate stochasticity, which are widely predicted but not included in either Mark 2 or Mark 3 models. There is general agreement that modest decreases in average humidity will be partly driven by increases in the frequency of heat waves (hot dry conditions) (Tryhorn and Risbey, 2006; Lucas et al., 2007; Alexander and Arblaster, 2009; Clarke et al., 2011). This would result in an increase in the output of dormant seed and, by implication, increase the capacity of A. saligna to develop seed banks and exploit intermittent disturbance events such as fire.
There is further potential for an increase in dormancy if changes in phenology result in ripening occurring at a more favourable time for the production of dormant seeds. There is widespread evidence of a trend towards earlier ripening in a range of species (Fitter and Fitter, 2002) and conditions of low humidity are more likely to occur in Sydney during spring relative to summer; however, the potential for interactions between changes in climate and changes in phenology make predicting dormancy response difficult.
Ecological implications: invasive potential of A. saligna
The implications of humidity-regulated dormancy onset specific to populations of A. saligna naturalized in the Sydney region derive from a combination of two main factors. First, invasion theory emphasizes the role of critical biotic and abiotic constraints to reproduction and maturation in determining the success of species introduced to novel environments (Noble, 1989). For example, the invasive success of A. saligna in South Africa has been largely attributed to the reproductive advantage it derives from an absence of natural seed predators (Richardson et al., 1992, 2011). Second, dormancy implicitly controls the potential for disturbance-coupled recruitment (Silvertown and Lovett Doust, 1993). Thus, failure to produce dormant seeds at the level achieved in native populations of a species corresponds to reproductive disadvantage upon translocation to a novel environment with similar opportunity and/or necessity for disturbance-coupled recruitment. Conversely, a lack of dormancy confers an advantage in colonizing habitats where space-creating disturbance events occur more frequently, such as where humans maintain road verges.
The highly variable dormancy exhibited by Sydney populations implies a smaller proportion of the seed output accumulates as a dormant seed bank than is in the case where dormancy is consistently higher. Based on historical weather records (1972–2007), the average input of dormant seed may be as low as 33–54 % depending on when ripening commences.
The production of non-dormant seed equates to potential reproductive losses in the range 40–60 % relative to populations with a nominal 90 % output of dormant seed. Such losses are similar in magnitude to average losses of 13–37 % attributable to pre-dispersal seed predation in indigenous populations of a range of Acacia species (Auld, 1983, 1986, 1991; New, 1984; Auld and O'Connell, 1989).
Invasive success is frequently linked to enhanced reproductive output in the absence of seed predators (Milton and Hall, 1981; Weiss and Milton, 1984; Gill and Neser, 1984; Neser and Kluge, 1986; Richardson et al., 1987, Pieterse and Cairns, 1988; Honig et al., 1992). By implication, reproductive losses due to variable dormancy are likely to be sufficient to offset any enhancement of reproductive output due to predator release experienced in populations naturalized in the Sydney area. Furthermore, generalist predators of the genus Melanterius were recorded at six out of seven sites surveyed from 1992 to 1993 and destroyed 0·2–27 % of seed crops prior to dispersal (M. Tozer, unpublished data). Thus we conclude that the humidity-regulated dormancy mechanism confers a disadvantage on A. saligna with respect to re-establishment following the elimination of the standing population, as occurs periodically as a result of fire in this type of vegetation. Conversely, the production of non-dormant seed is likely to enhance the spread and growth of populations in areas of human-disturbance, such as roadsides.
Climatic differences between west and east Australia are a plausible explanation for the high variability in dormancy percentages we observed in Sydney populations compared with the consistently high dormancy reported for (native) Western Australian and (naturalized) South African populations (typically >90 %; Jones, 1963; Larson, 1964; Aveyard, 1968; Shaybany and Rouhari, 1976; Jeffrey et al., 1988; Holmes, 1989; Bell et al., 1993). Based on the dormancy attained under field conditions in our experiment and the historical weather data available for Perth, we predict that our seed lots would have attained at least 74–83 % dormancy in Perth (depending on the commencement of ripening), since our model underestimates dormancy attained at very low humidity (Fig. 3). Populations of A. saligna occurring in south-western Australia and South Africa's Cape Province experience a Mediterranean climate under which the pattern of average humidity is essentially the reverse of that experienced in Sydney (Deacon et al., 1992). Conditions of low humidity are much more likely to occur under such a climate (Fig. 4) and thus are more conducive to the production of dormant seed (Fig. 5).
Further investigations are required to investigate the possibility that genetic factors associated with founder populations impact on dormancy onset in A. saligna (Millar and Byrne, 2012; Harris et al., 2012). Both a plastic dormancy response and genetic bottlenecks (if they affect dormancy) have implications for determining the invasive potential of A. saligna and other hard-seeded species in fire-prone oligotrophic shrub lands. We predict that variable dormancy as a plastic response to variable humidity will be an impediment to the invasion of regions experiencing a humid–temperate climate. Although, by our arguments, there should be selective pressure against genotypes unable to attain dormancy under humid conditions, there exist two mechanisms which may act to slow any potential evolutionary response. First, infrequent low-humidity events will ensure that low-sensitivity genotypes will be well represented in the dormant seed bank. Second, the genetic pool of populations establishing in native bushland is likely to be continually diluted with that of populations growing along roadsides, for which a lack of dormancy is not an impediment.
Conclusions
Our results suggest a more general hypothesis concerning humidity-regulated dormancy for hard-seeded Fabaceae translocated from Mediterranean-type to humid-temperate climate zones. Assuming dormancy functions to prevent germination in unfavourable conditions and seeds vary in their sensitivity to humidity (to become dormant), then selective pressure against genotypes with low sensitivity will be higher in areas where high humidity prevails compared with areas where dry conditions prevail. As a consequence, we predict species native to Mediterranean climates are likely to exhibit a wider range in the threshold humidity to which seeds are sensitive relative to species from humid–temperate climes, and thus lower levels of dormancy when seeds are produced under higher levels of humidity than occur in their native range.
Finally, our results highlight the importance of considering polymorphic seed banks or plasticity in dormancy onset in the study of dormancy in hard-seeded species in fire-prone ecosystems. We argue that there is a tendency for demographers to consider dormancy as a fixed trait and thus overlook a potentially significant variable influencing the size of the dormant seed bank. Factors relating to seed handling, the timing of ripening and harvesting and weather conditions in the year of sampling have the potential to influence the dormancy of seed and thus must be systematically accounted for if models of seed bank dynamics are to be realistic.
ACKNOWLEDGEMENTS
M.O. is supported by an Australian Research Council Linkage Project fellowship (LP110100527). We thank Tony Auld and Andrew Denham for their constructive comments.
LITERATURE CITED
- Alexander LV, Arblaster JM. Assessing trends in observed and modelled climate extremes over Australia in relation to future projections. International Journal of Climatology. 2009;29:417–435. [Google Scholar]
- Andersson L, Milberg P. Variation in seed dormancy among mother plants, populations and years of seed collection. Seed Science Research. 1998;8:29–38. [Google Scholar]
- Armitage I. Acacias of New South Wales. Sydney NSW: Society For Growing Australian Plants; 1977. [Google Scholar]
- Auld TD. Seed predation in native legumes of south-eastern Australia. Australian Journal of Ecology. 1983;8:367–376. [Google Scholar]
- Auld TD. Population dynamics of the shrub Acacia suaveolens (Sm.) Willd.: dispersal and the dynamics of the soil seed bank. Australian Journal of Ecology. 1986;11:235–254. [Google Scholar]
- Auld TD. Patterns of predispersal seed predators in the Fabaceae of the Sydney region, south-eastern Australia. Australian Journal of Zoology. 1991;39:519–528. [Google Scholar]
- Auld TD. Ecology of the Fabaceae in the Sydney region: fire, ants and the soil seed bank. Cunninghamia. 1996;4:531–551. [Google Scholar]
- Auld TD., O'Connell MA. Changes in predispersal seed predation levels after fire for two Australian legumes, Acacia elongata and Sphaerolobium vimineum. Oikos. 1989;54:55–59. [Google Scholar]
- Auld TD, O'Connell MA. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Australian Journal of Ecology. 1991;16:53–70. [Google Scholar]
- Aveyard JM. The effect of seven pre-sowing seed treatments on total germination and germination rate of six Acacia species. Journal of the Soil Conservation Service of New South Wales. 1968;24:43–54. [Google Scholar]
- Bar P, Cohen O, Shoshany M. The invasion rate of the alien species Acacia saligna within the coastal sand dune habitats of Israel. Israeli Journal of Plant Science. 2004;52:115–124. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin CC, Chesson PL, Baskin JM. Annual seed dormancy cycles in two desert winter annuals. Journal of Ecology. 1993;81:551–556. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Beadle NCW, Evans OD, Carolin RC. Flora of the Sydney region. Chatswood, NSW: AH and AW Reed; 1982. [Google Scholar]
- Bell DT, Hopkins AJM, Pate JS. Fire in the Kwongan. In: Pate JS, Beard JS, editors. Kwongan – plant life of the sandplain. Nedlands, WA: University of Western Australia Press; 1982. pp. 178–204. [Google Scholar]
- Bell DT, Plummer JA, Taylor SK. Seed germination ecology in south-western Western Australia. Botanical Review. 1993;59:24–73. [Google Scholar]
- Clarke HG, Smith PL, Pitman AJ. Regional signatures of future fire weather over eastern Australia from global climate models. International Journal of Wildland Fire. 2011;20:550–562. [Google Scholar]
- Cohen O, Riov J, Katan J, Gamliel A, Bar P. Reducing persistent seed banks of invasive plants by soil solarization – the case of Acacia saligna. Weed Science. 2008;56:860–865. [Google Scholar]
- Crawley MJ. GLIM for ecologists. Oxford, UK: Blackwell Scientific Publications; 1993. [Google Scholar]
- Deacon HJ, Jury MR, Ellis F. Selective regime and time. In: Cowling RM, editor. The ecology of fynbos. Nutrients, fire and diversity. Cape Town: Oxford University Press; 1992. pp. 6–22. [Google Scholar]
- D'hondt B, Brys R, Hoffmann M. The incidence, field performance and heritability of non-dormant seeds in white clover (Trifolium repens L.) Seed Science Research. 2010;20:169–177. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- French K, Major RE. Effect of an exotic Acacia (Fabaceae) on ant assemblages in South African fynbos. Austral Ecology. 2001;26:303–310. [Google Scholar]
- Gill AM, Neser S. Acacia cyclops and Hakea sericea at home and abroad. In: Bell D, editor. Proceedings of the 4th International Conference on Mediterranean Ecosystems. Nedlands, WA: University of Western Australia; 1984. pp. 57–58. [Google Scholar]
- Goss KU, Madliger M. Estimation of water transport based on in situ measurements of relative humidity and temperature in a dry Tanzanian soil. Water Resources Research. 2007;43:W05433. [Google Scholar]
- Hagon MW, Ballard LAT. Reversibility of strophiolar permeability to water in seeds of subterranean clover (Trifolium subterraneum L.) Australian Journal of Biological Sciences. 1970;23:519–528. [Google Scholar]
- Herranz JM, Ferrandis P, Martínez-Sánchez JJ. Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecology. 1998;136:95–103. [Google Scholar]
- Harris CJ, Dormontt EE, Le Roux JJ, Lowe A, Leishman MR. No consistent association between changes in genetic diversity and adaptive responses of Australian acacias in novel ranges. Evolutionary Ecology. 2012;26:1345–1360. [Google Scholar]
- Hennessey K, Lucas C, Nicholls N, Bathols J, Suppiah R, Rickets J. Climate change impacts on fire-weather in south-east Australia. Canberra, ACT: CSIRO; 2005. [Google Scholar]
- Holmes PM. Decay rates in buried alien Acacia seed populations of different density. South African Journal of Botany. 1989;55:299–303. [Google Scholar]
- Honig MA, Cowling RM, Richardson DM. The invasive potential of Australian banksias in South-African fynbos – a comparison of the reproductive potential of Banksia ericifolia and Leucadendron laureolum. Australian Journal of Ecology. 1992;17:305–314. [Google Scholar]
- Hyde EOC. The function of the hilum in some Papilionaceae in relation to the ripening of the seed and the permeability of the testa. Annals of Botany. 1954;18:241–56. [Google Scholar]
- Jeffrey DJ, Holmes PM, Rebelo AG. Effects of dry heat on seed germination in selected indigenous and alien legume species in South Africa. South African Journal of Botany. 1988;54:28–34. [Google Scholar]
- Jones RM. Preliminary studies of the germination of seed of Acacia cyclops and Acacia cyanophylla. South African Journal of Science. 1963;59:296–298. [Google Scholar]
- Jones RM, Bunch GA. The effect of stocking rate on the population dynamics of siratro in siratro (Macroptilium atropurpureum)–setaria (Setaria sphacelata) pastures in south-east Queensland. II. Seed set, soil seed reserves, seedling recruitment and seedling survival. Australian Journal of Agricultural Research. 1987;39:221–234. [Google Scholar]
- Larson E. Germination response of Acacia seeds to boiling. Australian Forest Research. 1964;1:51–53. [Google Scholar]
- Lucas C, Hennessy K, Mills G, Bathols J. Bushfire weather in southeast Australia: recent trends and projected climate change impacts. Melbourne: CSIRO; 2007. [Google Scholar]
- Manor R, Cohen O, Saltz D. Community homogenization and the invasiveness of commensal species in Mediterranean afforested landscapes. Biological Invasions. 2008;10:507–515. [Google Scholar]
- Maslin BR. Studies in the genus Acacia-3: the taxonomy of A. saligna (Labill.) H. Wendl. Nuytsia. 1974;1:332–340. [Google Scholar]
- Millar MA, Byrne M. Biogeographic origins and reproductive mode of naturalised populations of Acacia saligna. Australian Journal of Botany. 2012;60:383–395. [Google Scholar]
- Milton SJ, Hall AV. Reproductive biology of Australian Acacias in the South-Western Cape Province, South Africa. Transactions of the Royal Society of South Africa. 1981;44:465–487. [Google Scholar]
- Neser S., Kluge RL. The importance of seed-attacking agents in the biological control of invasive alien plants. In: Macdonald IAW, Kruger FJ, Ferrar AA., editors. The ecology and management of biological invasions in southern Africa. Cape Town: Oxford University Press; 1986. pp. 285–293. [Google Scholar]
- New TR. Colonisation of seedling Acacias by arthropods in southern Victoria. Australian Entomological Magazine. 1984;10:13–18. [Google Scholar]
- Noble IR. Attributes of invaders and the invading process: terrestrial and vascular plants. In: Drake JA, Mooney HA, di Castri F, et al., editors. Biological invasions – a global perspective. New York: John Wiley and Sons; 1989. pp. 301–310. [Google Scholar]
- Ooi MKJ. Seed bank persistence and climate change. Seed Science Research. 2012;22:S53–S60. [Google Scholar]
- Ooi MKJ, Auld TD, Denham AD. Projected soil temperature increase and seed dormancy response along an altitudinal gradient: implications for seed bank persistence under climate change. Plant and Soil. 2012;353:289–303. [Google Scholar]
- Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences Discussions. 2007;4:439–473. doi:10.5194/hessd-4-439-2007. [Google Scholar]
- Pieterse PJ, Cairns ALP. Factors affecting the reproductive success of Acacia longifolia (Andr.) Willd. in the Banhoek Valley, South-Western Cape, Republic of South Africa. South African Journal of Botany. 1988;54:461–464. [Google Scholar]
- Pukittayacamee P, Hellum AK. Seed germination in Acacia auriculiformis: developmental aspects. Canadian Journal of Botany. 1988;66:388–93. [Google Scholar]
- Quinlivan BJ. Seed coat impermeability in legumes. Journal of the Australian Institute of Agricultural Science. 1971;37:283–295. [Google Scholar]
- Richardson DM, Van Wilgen BW, Mitchell DT. Aspects of the reproductive ecology of four Australian Hakea species (Proteaceae) in South Africa. Oecologia. 1987;71:345–354. doi: 10.1007/BF00378706. [DOI] [PubMed] [Google Scholar]
- Richardson DM, Macdonald IAW, Holmes PM, Cowling RM. Plant and animal invasions. In: Cowling RM, editor. The ecology of fynbos. Nutrients, fire and diversity. Cape Town: Oxford University Press; 1992. pp. 271–308. [Google Scholar]
- Richardson DM, Carruthers J, Hui C, et al. Human-mediated introductions of Australian acacias – a global experiment in biogeography. Diversity and Distributions. 2011;17:771–787. [Google Scholar]
- Roach DA, Wulff RD. Maternal effects in plants. Annual Review of Ecology and Systematics. 1987;18:209–235. [Google Scholar]
- Santana VM, Bradstock RA, Ooi MKJ, Denham AJ, Auld TD, Baeza MJ. Effects of soil temperature regimes after fire on seed dormancy and germination in six Australian Fabaceae species. Australian Journal of Botany. 2010;58:539–545. [Google Scholar]
- Shaybany B, Rouhani I. Effect of pre-sowing treatments and temperatures on seed germination of Acacia cyanophylla Lindl. HortScience. 1976;11:381–383. [Google Scholar]
- Silvertown JW, Lovett Doust J. Introduction to plant population biology. 3rd edn. Oxford: Blackwell Scientific Publications; 1993. [Google Scholar]
- Strydom M, Esler KJ, Wood AR. Acacia saligna seed banks: sampling methods and dynamics, Western Cape, South Africa. South African Journal of Botany. 2012;79:140–147. [Google Scholar]
- Tryhorn L, Risbey J. On the distribution of heat waves over the Australian region. Australian Meteorological Magazine. 2006;55:169–182. [Google Scholar]
- Underwood AJ. Techniques of analysis of variance in experimental marine biology and ecology. Oceanography and Marine Biology: Annual Review. 1981;19:513–605. [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. Climate change and plant regeneration. Global Change Biology. 2011;17:2145–2161. [Google Scholar]
- Weiss PJ, Milton SJ. Chrysanthemoides monilifera and Acacia longifolia in Australia and South Africa. In: Bell D, editor. Proceedings of the 4th International Conference on Mediterranean Ecosystems. Nedlands, WA: University of Western Australia; 1984. pp. 159–160. [Google Scholar]
- Yelenik SG, Stock WD, Richardson DM. Ecosystem level impacts of invasive Acacia saligna in the South African fynbos. Restoration Ecology. 2004;12:44–51. [Google Scholar]