Abstract
Background/Aims
Hyperglycemia is associated with decreased 2-18[F]fluoro-2-deoxy-D-glucose (FDG) uptake by tumors assessed by positron emission tomography (PET). In this retrospective study we investigated a comparison of standardized uptake values (SUVs) in patients with primary colorectal cancers who either had diabetes mellitus (DM) or were otherwise healthy.
Methods
The medical records of 397 patients who were diagnosed with colorectal cancer and underwent PET-CT between January 2006 and December 2012 were analyzed. Eighty patients with DM and 317 patients without DM were included. Clinical characteristics were reviewed and maximal standardized uptake values (SUVmax) were calculated in the primary colorectal lesions.
Results
There was no significant difference between tumor SUVmax in DM patients (10.60±5.78) and those without DM (10.92±5.44). In addition, no significant difference was detected between tumor SUVmax in DM patients with glycated hemoglobin (HbA1c) levels <8% (10.34±5.17) and those with HbA1c levels ≥8% (10.61±7.27). The maximum size of the primary colorectal tumor was associated with SUVmax in a linear regression analysis.
Conclusion
The results of this study showed that DM did not influence FDG uptake values in colorectal cancer patients regardless of glucose levels.
Keywords: Diabetes mellitus, Positron emission tomography, Colorectal neoplasms
INTRODUCTION
The incidence of colorectal cancer, the fourth and second leading causes of death in men (15.4%) and women (12.1%), respectively,1 has been gradually increasing in Korea since 2000. Additionally, the prevalence of diabetes mellitus (DM) has increased rapidly in the last three decades in Korea and is forecasted to increase from 8.6% in 2007 to 10.8% in 2015.2 As the prevalence of DM has increased, the risk of colon cancer, pancreatic cancer, bladder cancer, breast cancer, and others has been increasing as well.3,4 Contrast-enhanced abdomino-pelvic CT and PET-CT are used in preoperative local staging evaluations and in diagnosing metastasis. A PET-CT scan is performed after injecting a non-metabolizable glucose sugar analogue, such as 18[F]-Fluorodeoxyglucose (FDG),1 that remains in cells as an intermediate product. The degree of 18[F]-FDG accumulation in cells with active metabolism is referred to as the standardized uptake value.5 Although FDG uptake in the bowel is often influenced by the amount of intracellular mucus, cell size, peristaltic movement, white blood cell count, and others,6 maximal standardized uptake values (SUVmax) are elevated by an increase in the FDG accumulation in cancer cells with active metabolism. When blood glucose levels are high, elevated glucose levels can prevent FDG uptake in malignant tumor cells due to a competitive reaction. This may lead to decreased SUVmax values, as FDG uptake is relatively lowered in malignant neoplasms.7,8 However, previous studies on the association between blood glucose levels and SUVmax in PET-CT have mainly been focused on patients without DM. This study aimed to investigate the effect of DM in patients with colorectal cancer on SUVmax, as well as the difference in SUVmax based on the degree of blood glucose control.
METHODS
1. Subjects
This study included 397 patients diagnosed with colorectal cancer through histopathological examination in the SMG-SNU Boramae Medical Center from January 2006 to December 2012 who also underwent FDG PET-CT scans. Data were collected from patients through reviewing clinical and radiologic records and pathologic examination and included age, sex, BMI, presence of DM, blood glucose levels, glycated hemoglobin (HbA1c) levels, postoperative lesion sizes, histological findings and stages, and others. We excluded patients with severe infections and inflammatory diseases that could affect test results, those with tumors in other organs, and those with unknown SUVmax values due to lack of FDG PET-CT scan data. In addition, we also excluded DM patients without confirmed blood glucose examinations and those without HbA1c levels collected in the two months prior to the PET scan. Colorectal cancer was diagnosed based on pathologic results and Tumour, Node, Metastases staging was based on the results of contrast-enhanced abdomino-pelvic CT, PET scan, colonoscopy, and pathologic results. This study received approval from the Institutional Review Board of the SMG-SNU Boramae Medical Center.
2. PET-CT Scan
Patients underwent FDG PET-CT scan after 8-hours of fasting. Blood glucose levels were measured prior to scanning, and patients without and with DM having levels <120 mg/dL and <180 mg/dL, respectively, received PET-CT. Patients drank sufficient water before the exam and then received 5.18 MBq of 18[F]-FDG per kg of body weight intravenously. PET scan images were obtained after 60 min using a GEMINI TF-64 scanner (Phillips Medical System, Cleveland, OH, USA). CT scanning was conducted from the basal skull to the femoral region, and attenuation correction was performed in CT images. After CT scanning, PET scans were conducted for 2.5 min per frame according to standard protocols. Sixty minutes have passed after intravenous injection of FDG, the image taking was performed. If the focal FDG uptake was seen in the captured image, and then further delayed images were taken after 4 hours later. Two nuclear medicine specialists evaluated uptake regions and SUVmax by interpreting PET scan images. SUVmax, the maximum amount of FDG accumulation in tumors depending on the dose of 18[F]-FDG injected with FDG-PET activity, was calculated using the percentage of the average rate of whole body uptake and the uptake rate for lesions.
3. Pathological Findings
Surgical resection was performed in 397 patients diagnosed with colorectal cancer and confirmed with endoscopic biopsy. Tumor size and the degree of infiltration were measured through pathologic examination after resection. The diameter of lesions was measured as lesion size after formalin fixation. The location of lesions was categorized into the proximal portion from the cecum to the transverse colon, the distal portion from the splenic flexure to the sigmoid colon, and the rectum area below the sigmoid colon. Anastomosis was defined in patients with a history of intestinal surgery. Lesions were classified into polypoid, ulceroinfiltrative, and ulcerofungating based on gross type, and neoplasms were generally categorized into well differentiated, moderately differentiated, and poorly differentiated carcinoma. In addition, lymph node metastasis, perineural infiltration, lymphatic invasion, and vascular invasion were examined in surgically resected tissues.
4. FDG-PET Findings according to the Level of Blood Glucose Control
We examined differences in patients with DM and colorectal cancer to identify the effects of HbA1c levels on SUVmax by dividing them into two groups, well-controlled and poorly controlled. By reviewing HbA1c levels measured in the two months prior to the PET scan, SUVmax values could be compared between patients with DM and HbA1c levels <8% and those with HbA1c levels ≥8%.
5. Statistical Analysis
Statistical analyses were performed using the SPSS version 20.0 for Windows (SPSS Inc., Chicago, IL, USA). P-values of less than 0.05 were considered statistically significant. T-tests and one-way ANOVAs were used to analyze continuous variables, and X2-tests or Fisher's exact tests were performed to analyze categorical variables. Linear regression was conducted to examine the association between SUVmax and colorectal cancer-related factors. Multiple linear regression was performed on factors demonstrating P-values of less than 0.25 in a simple linear regression analysis.
RESULTS
1. Characteristics of Patients by the Presence of DM
Among 397 patients, 268 (67.5%) were men and 129 (32.5%) were women. Of these patients with colorectal cancer, 80 (20.2%) were patients with DM and 317 (79.8%) did not have DM. Of the 80 patients with DM, 57 (71.3%) were undergoing therapy with oral hypoglycemic agents (OHA), 10 (12.5%) were receiving insulin, 3 (3.8%) were undergoing combined insulin-OHA therapy, and 10 (12.5%) were applying lifestyle modification therapies without medication. The mean HbA1c level in patients with DM was 8.06±2.15% and the mean duration of DM was 8.25±6.81 years.
The mean age of patients with DM was 66.88±9.79 years, significantly older than that of patients without DM (64.24±11.65 years; P=0.040), however no difference was found in the BMIs of patients in the two groups. Blood glucose levels were significantly different between patients with and without DM (154.71±69.38 mg/dL vs. 103.17±18.50 mg/dL; P<0.001).
According to the results of the pathologic examinations, adenocarcinoma was the most common type of colorectal cancer in both groups (DM vs. Non-DM: 97.5% vs. 93.4%). Regarding the location, colorectal cancers occurred most commonly in the rectum in both groups (DM vs. Non-DM: 41.2% vs. 37.5%). Analysis of gross type indicated that ulceroinfiltrative and ulcerofungating lesions accounted for 45.0% and 43.8%, respectively, of all patients with DM, and 46.4% and 41.3%, respectively, of all patients without DM, showing no difference between the groups. According to the American Joint Committee on Cancer staging system, stages III and IV colorectal cancer accounted for 32.5% and 33.8%, respectively, of all patients with DM, and 33.1% and 26.2%, respectively, of all patients without DM, showing no difference between the groups (Table 1).
Table 1.
Baseline Characteristics of Patients with and without Diabetes Mellitus
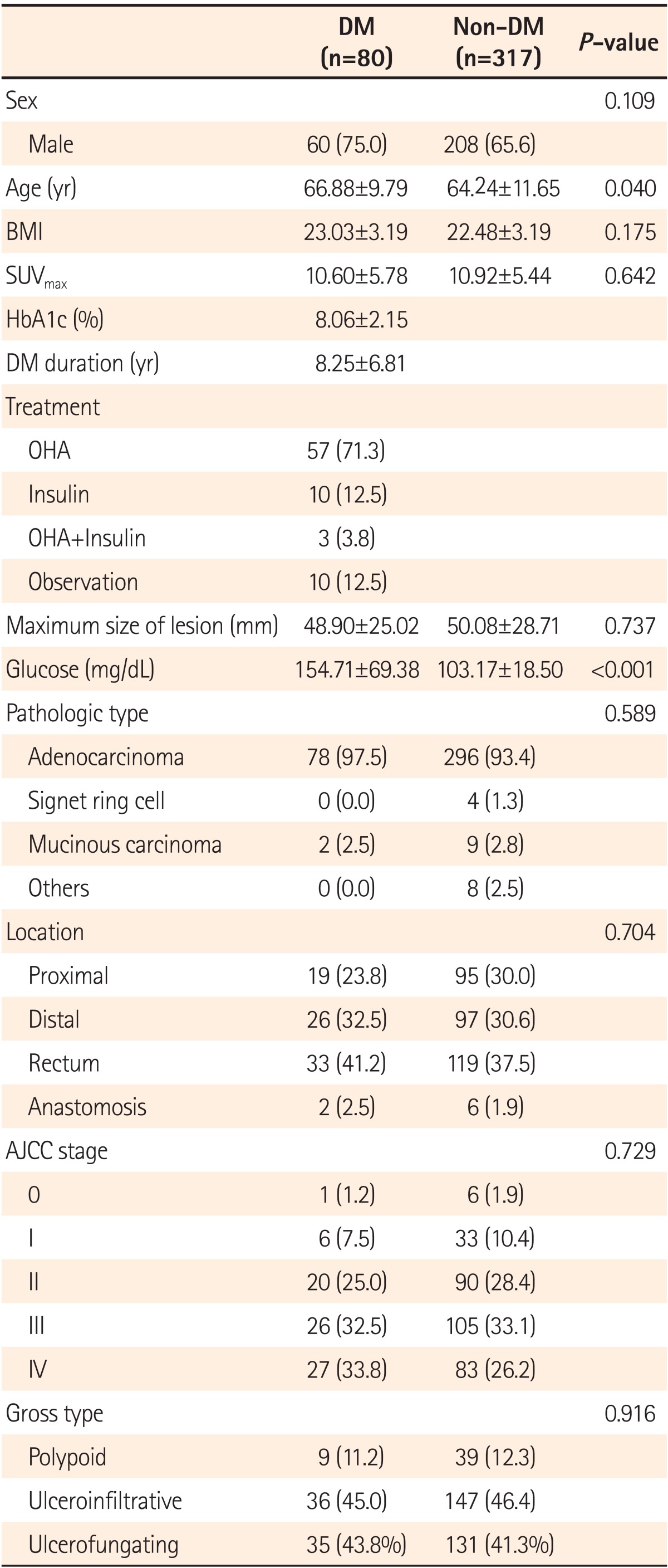
Values are presented as mean±SD or n (%).
SUVmax, maximum standardized uptake values; HbA1c, glycated hemoglobin; DM, diabetes mellitus; OHA, oral hypoglycemic agent; AAJC, American Joint Committee on Cancer.
2. Characteristics of FDG Uptake in the Presence of DM
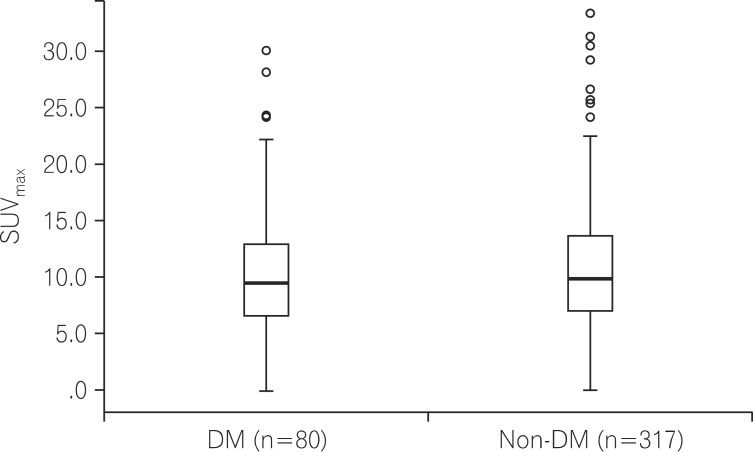
When the SUVmax values of colorectal cancer were compared according to the presence of DM, the SUVmax was 10.60±5.78 in patients with DM and 10.92±5.44 in patients without DM, exhibiting no difference between the groups (P=0.642; Fig. 1).
Fig. 1.
Comparison of maximal standardized uptake values (SUVmax) in colorectal cancer patients with or without diabetes mellitus (DM) (P=0.642).
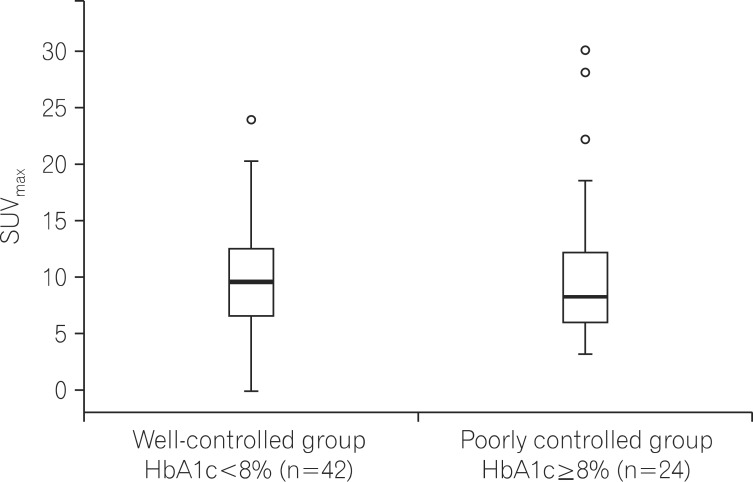
Among the 80 total patients with DM, differences in SUVmax were examined according to glucose levels in 66 patients who had available HbA1c data. Patients with DM were classified into a well-controlled group (n=42) with HbA1c levels <8% and a poorly controlled group (n=24) with HbA1c levels ≥8%. SUVmax values were 10.34±5.17 in the well-controlled group and 10.61±7.27 in the poorly controlled group, showing no significant difference between the groups (P=0.860; Fig. 2). Moreover, no differences were found in age, sex, BMI, lesion size, or the duration of DM between two groups (Table 2).
Fig. 2.
Comparison of maximal standardized uptake values (SUVmax) in colorectal cancer patients with diabetes mellitus (DM) according to control of glucose (P=0.860). HbA1c, glycated hemoglobin.
Table 2.
Characteristics according to Level of Glucose Control in Patients with Diabetes Mellitus
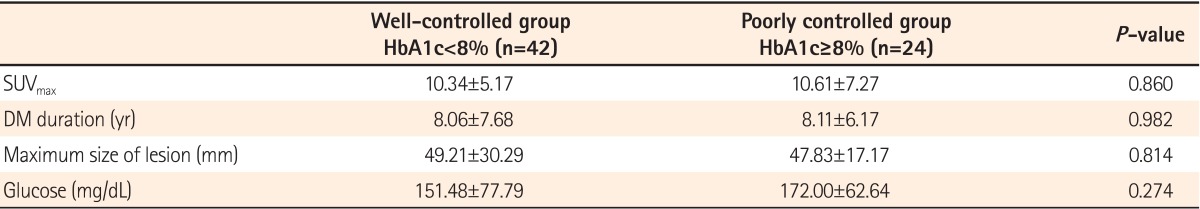
Values are presented as mean±SD.
HbA1c, glycated hemoglobin; SUVmax, maximum standardized uptake values; DM, diabetes mellitus.
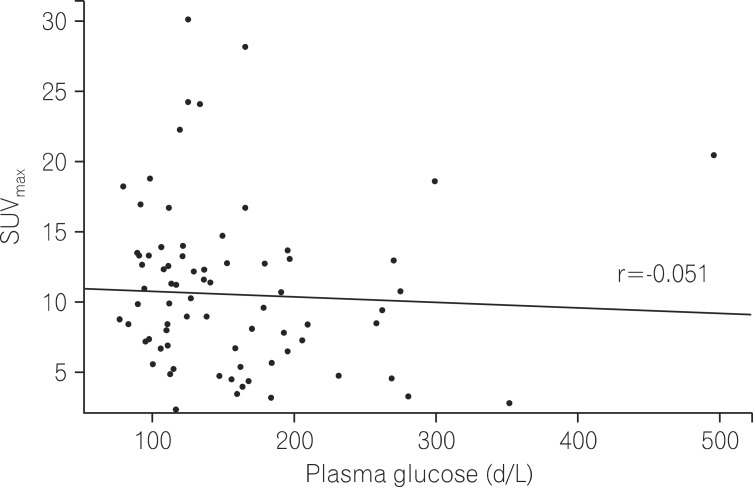
To identify a correlation between SUVmax and blood glucose levels, scattergrams of blood glucose levels and SUVmax were compared. As a result, no correlation was found between blood glucose levels and SUVmax (correlation coefficient -0.051 [P=0.657]; Fig. 3).
Fig. 3.
Distribution between maximal standardized uptake values (SUVmax) and glucose in diabetes mellitus (DM) patients. The correlation coefficient(r) was -0.051.
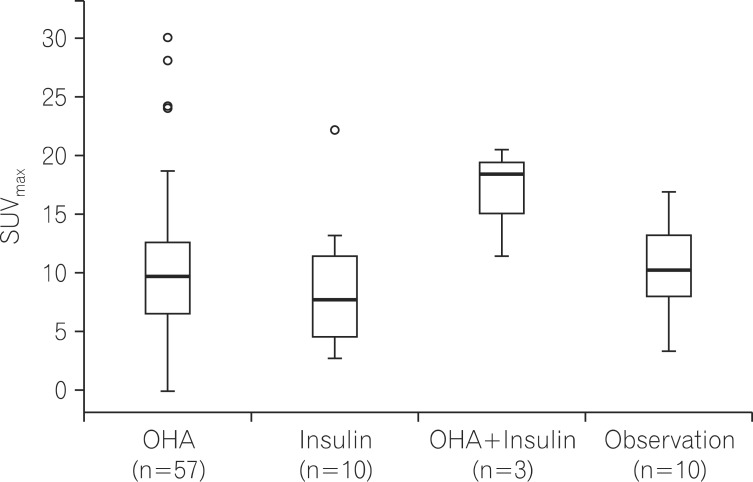
When SUVmax values were compared according to treatment methods used by patients with DM, a trend towards higher SUVmax values was observed in the group using combined insulin-OHA therapy as compared to the values from groups of patients using only an OHA, only insulin, or only being observed. However, no significant difference was detected between these groups (P=0.239; Fig. 4).
Fig. 4.
Difference of maximal standardized uptake values (SUVmax) in diabetes mellitus (DM) patients (n=80) according to treatment (P=0.239). OHA, oral hypoglycemic agent.
3. Factors Affecting FDG Uptake
In the analysis of factors influencing SUVmax in patients with DM, the maximum size of the colorectal lesion was identified as the only factor that affected SUVmax. There was no correlation between SUVmax values and BMI, the duration of DM, blood glucose levels, HbA1c levels, gross types, or Tumour, Node, Metastases staging (Table 3).
Table 3.
Predictive Factors Associated with Maximum Standardized Uptake Values
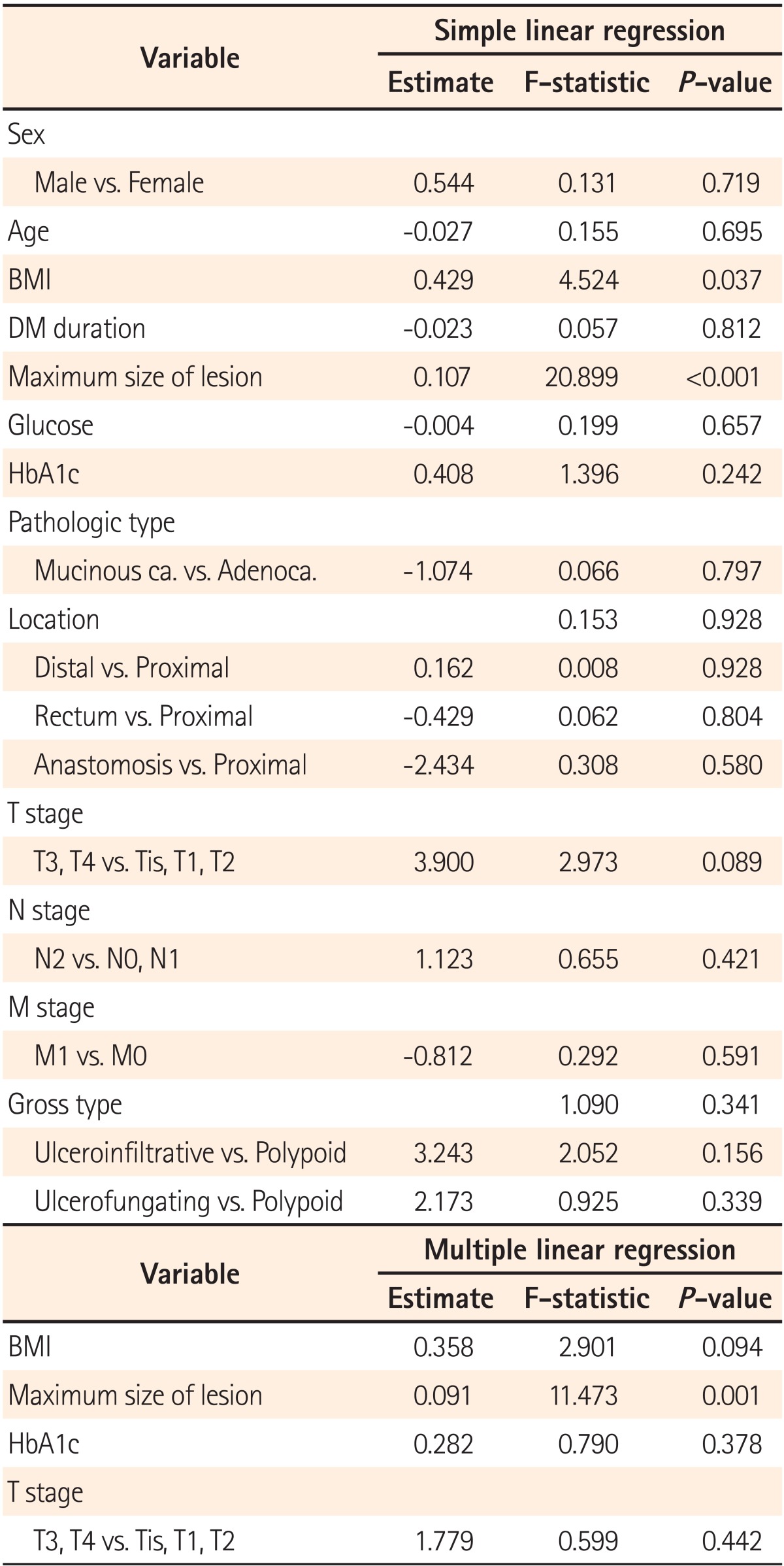
DM, diabetes mellitus; HbA1c, glycated hemoglobin; Mucinous ca., mucinous carcinoma; Adenoca., adenocarcinoma.
DISCUSSION
FDG uptake is elevated in malignant tumor tissues, as these tissues use energy mainly from glucose metabolic processes for survival. When blood glucose levels are high, elevated glucose levels can prevent FDG uptake in malignant tumor tissues due to a competitive reaction. This process is known to lower both the resolution of PET-CT scans and diagnostic sensitivity.7 Only a few studies have investigated changes in FDG uptake in tumors of patients with DM and chronically elevated glucose levels.9 The current study is the first to compare the differences in tumor SUVmax values during PET-CT according to the presence of DM in patients with colorectal cancer. No significant difference was found in SUVmax values between patients with and without DM. Only tumor size was identified as a factor that could influence SUVmax.
These results were comparable to those of a previous study that compared SUVmax values between patients with and without DM with esophageal cancer. Haley et al.10 compared pre-treatment SUVmax values in 18 esophageal cancer patients with DM and 45 esophageal cancer patients without DM. There was no significant difference between tumor SUVmax in patients with and without DM (10.1±5.9 vs. 8.7±5.6 [P=0.44]). When Gorenberge et al.11 compared SUVmax between 40 lung cancer patients with DM and 145 lung cancer patients without DM, no significant difference in SUVmax was shown between patients with and without DM (5.86±3.97 vs. 6.47±5.48). Collectively, these results indicate that DM has an insignificant effect on the SUVmax values of PET-CT scans.
In the current study we used HbA1c levels to compare SUVmax according to the degree of blood glucose controlled in patients with DM. When patients with well-controlled glucose levels (HbA1c<8%) and poorly controlled glucose levels (HbA1c≥8%) were compared, no significant difference was found in SUVmax between the groups. Gorenberge et al.11 reported no difference in SUVmax in patients with DM when comparing groups with different fasting blood sugar levels (≥126 mg/dL vs. <126 mg/dL). Moreover, a study of 219 subjects with cervical cancer compared 16 patients with DM and hyperglycemia, 12 patients with DM and normal glucose levels, and 191 patients without DM. SUVmax values were 12.83±11.47, 13.86±7.90, and 13.34±7.78 in each group, respectively, showing no difference.12 These results imply that chronic hyperglycemia also has an insignificant influence on the SUVmax values of PET-CT scans.
There are several reasons why the presence of DM in patients with colorectal cancer would not result in differences in tumor SUVmax values, one being elevated tumor FDG uptake.
First, as part of the mechanism of increased glucose metabolism in cancer cells, a number of glucose transporter (GLUT1-GLUT7) genes are involved in the uptake of glucose to the plasma membrane. Of these GLUTs, an increase in the expression of GLUT1 and GLUT3 within tumors has been previously reported.13,14 Because increased expression of GLUTs in tumors is sufficient to offset tumor uptake of FDG caused by increased glucose levels through competition, no differences were found in SUVmax values according to DM and blood glucose regulation.
Second, invasive cancer associated with ulceration is characterized by molecular biological instability and a large number of inflammatory cells.15 Most of the subjects in our study had ulcerative-type lesions of colorectal cancer (88.8% in patients with DM, 87.7% in patients without DM). The lack of differences in SUVmax between these groups may be related to increased FDG uptake attributable to the large number of inflammatory cells that had a greater effect than the competitive inhibition of FDG uptake within tumors with elevated glucose levels.
The results of previous studies have suggested that OHAs can influence intestinal absorption of FDG in patients with DM. In particular, metformin has been shown to stimulate adenosine monophosphate-activated protein kinase in cells and to increase FDG uptake by mobilizing GLUT4 from the microsomal membrane to the plasma membrane.16,17 Gontier et al.18 performed PET scanning in 32 patients with DM receiving OHA therapy with metformin and insulin, 23 undergoing OHA therapy without metformin and insulin, and 95 patients without DM. As a result, a significant increase was observed in intestinal FDG uptake in patients undergoing OHA therapy with metformin and insulin as compared to the other groups. Moreover, a comparative study on 77 patients with DM and 77 healthy individuals reported that a significant increase in intestinal FDG uptake was observed in patients with DM receiving metformin.19 In the current study, no differences in SUVmax were found among patients receiving OHA (71.2%), insulin (12.5%), combined insulin-OHA therapy (3.8%), or follow-up (12.5%). However, different OHAs including metformin, sulfonylureas, meglitinides, and alpha-glucosidase were administered to patients, and only three patients underwent combined therapy with insulin. For these reasons, large-scale studies are needed to further investigate the effects of OHAs on SUVmax.
The current study displayed a few limitations. First, blood glucose levels and PET scan periods may have varied in subjects due to the retrospective nature of the study. However, the impact of different blood collection times is thought to be minimal in the comparison of SUVmax by DM, as PET-CT scans were performed in patients during their hospital stay and at a glucose level <180 mg/dL. Second, there was no difference in SUVmax according to the types of OHAs used by patients in this study. However, the scope of this study was limited to analyze only the results based on the presence of DM. Third, a PET scan is usually done about an hour after the injection of 18[F]-FDG. The difference in FDG uptake may have been partially caused by inconsistent periods between PET-CT scans. This may not have been problematic, however, considering the outcomes of recent studies that have demonstrated that the SUV does not reach maximum until 130-500 min after injection of 18[F]-FDG in animal tests20 and that 18[F]-FDG uptake is known to persist for 2-3 hours.21,22
In conclusion, there was no difference in SUVmax values dependent on the presence of DM when performing 18[F]-FDG PET-CT scans in patients with colorectal cancer. Additionally, no association was found between glucose levels and SUVmax. Therefore, the results of the current study demonstrated that DM did not influence FDG uptake values in colorectal cancer patients.
Footnotes
Financial support: None.
Conflict of interest: None.
References
- 1.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SK, Park MK, Suk JH, et al. Cause-of-death trends for diabetes mellitus over 10 years. Korean Diabetes J. 2009;33:65–72. [Google Scholar]
- 3.Yi BD, Bae YP, Kim BG, et al. The association between type 2 diabetes mellitus and colorectal cancer. Endocrinol Metab. 2011;26:126–132. [Google Scholar]
- 4.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 5.Endo K, Oriuchi N, Higuchi T, et al. PET and PET/CT using 18F-FDG in the diagnosis and management of cancer patients. Int J Clin Oncol. 2006;11:286–296. doi: 10.1007/s10147-006-0595-0. [DOI] [PubMed] [Google Scholar]
- 6.Lee JC, Hartnett GF, Hughes BG, Ravi Kumar AS. The segmental distribution and clinical significance of colorectal fluorodeoxyglucose uptake incidentally detected on PET-CT. Nucl Med Commun. 2009;30:333–337. doi: 10.1097/MNM.0b013e32832999fa. [DOI] [PubMed] [Google Scholar]
- 7.Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. J Nucl Med. 1993;34:1–6. [PubMed] [Google Scholar]
- 8.Diederichs CG, Staib L, Glatting G, Beger HG, Reske SN. FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med. 1998;39:1030–1033. [PubMed] [Google Scholar]
- 9.Langen KJ, Braun U, Rota Kops E, et al. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med. 1993;34:355–359. [PubMed] [Google Scholar]
- 10.Haley M, Konski A, Li T, et al. Influence of diabetes on the interpretation of PET scans in patients with esophageal cancer. Gastrointest Cancer Res. 2009;3:149–152. [PMC free article] [PubMed] [Google Scholar]
- 11.Gorenberg M, Hallett WA, O'Doherty MJ. Does diabetes affect [18F]FDG standardised uptake values in lung cancer? Eur J Nucl Med Mol Imaging. 2002;29:1324–1327. doi: 10.1007/s00259-002-0887-1. [DOI] [PubMed] [Google Scholar]
- 12.Chang YC, Yen TC, Ng KK, et al. Does diabetes mellitus influence the efficacy of FDG-PET in the diagnosis of cervical cancer? Eur J Nucl Med Mol Imaging. 2005;32:647–652. doi: 10.1007/s00259-004-1744-1. [DOI] [PubMed] [Google Scholar]
- 13.Brown RS, Leung JY, Kison PV, Zasadny KR, Flint A, Wahl RL. Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer. J Nucl Med. 1999;40:556–565. [PubMed] [Google Scholar]
- 14.Marom EM, Aloia TA, Moore MB, et al. Correlation of FDG-PET imaging with Glut-1 and Glut-3 expression in early-stage non-small cell lung cancer. Lung Cancer. 2001;33:99–107. doi: 10.1016/s0169-5002(00)00250-6. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, Lee SY, Lim HK, et al. Relationship between positron emission tomography uptake and macroscopic findings of colorectal cancer. Intest Res. 2012;10:168–175. [Google Scholar]
- 16.Zou MH, Kirkpatrick SS, Davis BJ, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 17.Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gontier E, Fourme E, Wartski M, et al. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging. 2008;35:95–99. doi: 10.1007/s00259-007-0563-6. [DOI] [PubMed] [Google Scholar]
- 19.Bybel B, Greenberg ID, Paterson J, Ducharme J, Leslie WD. Increased F-18 FDG intestinal uptake in diabetic patients on metformin: a matched case-control analysis. Clin Nucl Med. 2011;36:452–456. doi: 10.1097/RLU.0b013e318217399e. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang H, Pourdehnad M, Lambright ES, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42:1412–1417. [PubMed] [Google Scholar]
- 21.Lodge MA, Lucas JD, Marsden PK, Cronin BF, O'Doherty MJ, Smith MA. A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med. 1999;26:22–30. doi: 10.1007/s002590050355. [DOI] [PubMed] [Google Scholar]
- 22.Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med. 2002;43:871–875. [PubMed] [Google Scholar]