Abstract
Objectives
Scleroderma (SSc)-associated pulmonary arterial hypertension (PAH) is a major cause of mortality in SSc patients and represents an important diagnostic and therapeutic target. Our aims were to evaluate the relationship between echocardiogram-derived right heart hemodynamics and “gold standard” right heart catheterization (RHC) measurements in a scleroderma population, and investigate whether this relationship is modified by a subset of pulmonary hypertension.
Methods
We performed right heart catheterization and echocardiography on the same day, with pulmonary function testing in 21 consecutive subjects (age 57 ± 10, 81% female) with scleroderma and pre-capillary pulmonary hypertension.
Results
RHC measures including PA systolic and mean pressure, and pulmonary vascular resistance (PVR) correlated strongly with echo-derived data. RHC-derived pulmonary vascular resistance was negatively associated with RV systolic performance as measured by tricuspid annular plane systolic excursion (TAPSE, rho −0.70, p < 0.001), tissue Doppler tricuspid s’ velocity (rho −0.68, p 0.002), and RV fractional area change (rho −0.78, p < 0.001). Correlations with TAPSE and s’ velocity were strengthened when FVC%/DLCO% ≥ 1.6 used to identify pure PAH phenotypes in SSc. Bland-Altman analyses demonstrated strong agreement between RHC and echo-derived hemodynamic measures.
Conclusions
Our findings suggest that echocardiography may play a clinical role in identifying pulmonary hypertension and RV dysfunction non-invasively, particularly in a subset of SSc patients stratified by pulmonary function testing. This method may establish specific disease phenotypes with differential cardiovascular impact and prove useful as a marker of disease progression/risk stratification in SSC patients that warrants further investigation in larger cohorts.
Keywords: pulmonary hypertension, scleroderma, right heart
Systemic sclerosis (SSc), also known as scleroderma, is a systemic inflammatory connective tissue disorder associated with autoimmune activation and collagen overproduction that progressively leads to fibrosis of skin, joints, and internal organs with a predilection for pulmonary, gastrointestinal, and renal involvement (1). Specifically, scleroderma-associated pulmonary hypertension (SSc-PAH) is a leading cause of mortality in this population and represents an important therapeutic target in clinical studies (2, 3). Pre-capillary pulmonary hypertension in scleroderma is characterized as either isolated pulmonary arterial hypertension (PAH) or secondary to interstitial lung disease (PH-ILD).
Right heart catheterization (RHC) is the “gold standard” for the assessment of cardiopulmonary hemodynamics, however its invasive nature limits use in large population studies or longitudinal clinical trials that require repeat procedures (4, 5). Echocardiography provides a non-invasive tool for not only evaluating right heart structure such as chamber size and function, but also comprehensive assessment of hemodynamics (6). As such, transthoracic Doppler echocardiography is currently the recommended initial screening modality for the evaluation of PAH (7). While the clinical role of echocardiography in SSc-PAH continues to be an area of significant interest, few studies have directly simultaneously compared echocardiography to right heart catheterization in this population (5, 8, 9).
The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Expert Consensus Document on Pulmonary Hypertension identifies pulmonary vascular resistance (PVR) as a key measure integral to the evaluation and diagnosis of pre-capillary PAH (7). Both right heart hemodynamics and right ventricular (RV) function predict all-cause mortality in pulmonary hypertension (5, 10, 11). Non-invasive assessment of intracardiac pressures in SSc-PAH patients, particularly in relation to right heart functional measures including RV tissue Doppler, fractional area change, and tricuspid annular plane systolic excursion (TAPSE) have not been well investigated. Thus, the aims of this study were to 1) evaluate the relationship of echo-derived right heart hemodynamics to invasive RHC measurements in a scleroderma population, and 2) investigate whether the association is modified by etiology of pulmonary hypertension (with or without significant ILD).
METHODS
Study population
The early, simple and reliable DETECTion of PAH in SSc (DETECT) Study was a prospective, observational, cross-sectional cohort study in scleroderma patients to evaluate screening tests and the incidence of pulmonary hypertension. The study involved 62 centers in 18 countries with longitudinal data collection over three years. This study included 21 patients enrolled at the Boston University Medical Center. The Institutional Review Board at the Boston University Medical Center approved the research protocol and written informed consent was obtained from all participants prior to enrollment.
Inclusion criteria included age ≥18 years old with a definite diagnosis of systemic sclerosis by American College of Rheumatology (ACR) criteria including all patients with any other connective tissue diseases who in parallel also meet the ACR criteria for SSc, disease duration of greater than 3 years dated from onset of first non-Raynaud feature, and diffusing capacity of the lung for carbon monoxide (DLC0%) < 60% of predicted. Excluded from enrollment were subjects with a prior history of known PH with mean pulmonary arterial pressure (mPAP) ≥ 25 mm Hg at rest or ≥ 30 mm Hg during exercise independent of pulmonary capillary wedge pressure (PCWP) prior to enrollment, RHC within past 12 months, use of therapy considered definite PAH/PH treatment for any indication within 6 weeks of enrollment and/or for a total of greater than 6 weeks during the previous 12 months, forced vital capacity (FVC) < 40%, estimated glomerular filtration rate (eGFR) < 40 mL/min/1.73 m2, known prior PWCP > 15 mmHg, previous evidence or diagnosis of clinically relevant left heart disease (including left ventricular ejection fraction < 50%, significant diastolic dysfunction, significant valvulopathy, known significant coronary disease, uncontrolled blood pressure, hypertrophic cardiomyopathy, decompensated congestive heart failure, congenital heart disease, prior cardiac surgery, and pregnancy).
Echocardiography
Two-dimensional transthoracic echocardiograms were performed using commercially available ultrasound machines (IE33, Phillips Medical Systems, Andover, Massachusetts, USA). All echocardiograms were performed in our Intersocietal Accreditation Commission in Echocardiography Laboratory in accordance with the American Society of Echocardiography (ASE) guidelines (12), and by the same sonographer to minimize technical variability. No patients in this study were noted to have atrial fibrillation/arrhythmias. Left atrial (LA) diameter was determined from parasternal long-axis view and right atrial (RA) area and right ventricle (RV) was measured utilizing the four-chamber apical view. LA volume was quantified using the area-length method with measurements taken in the apical 4-chamber and 2-chamber views at ventricular end systole. Left ventricular EF was measured by modified Simpson's method. RV fractional area change (FAC) was determined using measurements of end diastolic and end systolic areas and applying the following formula: FAC % = 100 x enddiastolic area – end-systolic area/end-diastolic area (6). Doppler measurements included continuous wave Doppler through the tricuspid valve, utilizing the highest velocity obtained from multiple views to determine the peak tricuspid regurgitant velocity (TRV) (m/s). The modified Bernoulli equation was used for calculation of the right ventricular systolic pressure, which in the absence of RV outflow tract obstruction, determined pulmonary arterial systolic pressure (PASP). RA pressure (RAP) was determined by inferior vena cava size and collapsibility and assigned a value of 3, 8, or 15 mmHg according to ASE guidelines (6). Pulsed-wave Doppler to assess peak E (early diastolic) and A (late diastolic) velocities were obtained using standard methodology as previously described (13). Tissue Doppler early (e’) and late (a’) diastolic velocities and systolic velocity (s’) were obtained on both mitral and tricuspid annular planes, respectively. Tissue Doppler measures the intrinsic myocardial velocities in a longitudinal fashion, thus limitations can occur due to beam angle when it is not parallel to myocardial motion. The septal annulus was utilized for mitral measurements and tricuspid measurements were obtained at the junction of the right ventricular free wall and anterior leaflet. Calculation of the RV myocardial performance index (MPI), a global measurement of both systolic and diastolic function of the RV, was performed using tissue Doppler and defined as the ratio of tricuspid valve closure-opening time - ejection time/ejection time (6). TAPSE was obtained by placing a M-mode cursor across the tricuspid annulus in a four-chamber apical view and capturing the maximum annular movement during peak systole. The right ventricular outflow tract time-velocity integral (TVIRVOT) (cm) was obtained by placing a 1-2-mm pulsed wave Doppler sample volume in the proximal right ventricular outflow tract just below the pulmonary valve in the parasternal short-axis view. PVRECHO was calculated by previously validated method using the formula: PVRECHO = 0.1618 + 10.006 x TRV/ TVIRVOT (14).
Cardiac catheterization
Right heart catheterization was performed using a standard protocol and no sedation was administered for the procedure. Except for one participant, all subjects underwent RHC and echocardiography on the same day within one hour. All catheterization procedures were performed by the same operator who was blinded to real-time echocardiographic data. Cardiac output was measured using the Fick method. The following cardiopulmonary measurements were recorded: RAP, right ventricular pressure (RVP), PASP, mean pulmonary artery pressure (MPAP), pulmonary artery diastolic pressure (PADP) and pulmonary capillary wedge pressure (PCWP). Pulmonary vascular resistance by catheterization (PVRcath) was calculated by the following equation: PVRcath = MPAP – PCWP/cardiac output (15).
Pulmonary function evaluation
All patients underwent standard pulmonary function testing (PFT) as entry into the study requiring total lung capacity (TLC) ≥ 40%. Measurements (reported as % predicted) used for the analyses included: forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), TLC, and diffusion capacity of carbon monoxide (DLCO).
Imaging
In addition, all patients underwent a high-resolution CT scans for ILD evaluation. Patients were classified as having ILD based on criteria used in the main DETECT study which was defined as PCWP ≤ 15 mmHg and FVC< 60%, or FVC 60-70% plus HRCT data not available or determined as “moderate – severe” as determined by radiology staff blinded to any echo or invasive hemodynamic data (16).
Statistical analyses
Descriptive statistics are presented as mean (± standard deviation) for continuous variables and percentages for categorical variables. Relationships between echocardiographic and RHC variables were assessed by Spearman rank correlations and linear regression. Agreement between echocardiographic and RHC parameters were analyzed using Bland-Altman plots. Analyses were further stratified by PFT cut-point ratios of FVC%/DLCO% ≥ 1.6 or < 1.6, previously reported to discriminate patients with PAH versus PH-ILD (17) and risk stratify in scleroderma (18, 19). FVC%/ DLCO % stratification was evaluated in all correlations, however only statistically significant analyses were displayed. A two-sided p < 0.05 was accepted as statistically significant. Analyses and graphics were performed with SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline characteristics
As displayed in Table 1, subjects were age 57 ± 10 years and predominantly white females, consistent with the expected demographics of the population afflicted with scleroderma. All participants were non-diabetic with normal renal function. Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were both slightly decreased with normal ratio (75.1 ± 19.5 and 75.4 ± 18.9, respectively). Total lung capacity was decreased and the diffusion capacity (DLCO) was severely reduced at 42.4%. Five patients (23.8%) were classified as having interstitial lung disease by CT scan and DETECT criteria as previously described. The majority of patients had PH and/or PH-ILD, however 7 patients did not have a diagnosis of PAH based on criterion of mean PAP ≥ 25 mmHg.
Table 1.
Baseline clinical characteristics (n=21)
| Variable | |
|---|---|
| Age, years | 57.1 ± 9.5 |
| Female, % | 17 (81.0) |
| Caucasian % | 19 (90.5) |
| Smoker, past or current, % | 9 (42.8) |
| Hypertension, % | 7 (33.3) |
| Diabetes mellitus, % | 0 (0) |
| Coronary artery disease, % | 1 (4.8) |
| Body mass index, kg/m2 | 28.8 ± 4.4 |
| B-type natriuretic peptide, pg/mL | 254.9 ± 500.0 |
| Creatinine, mg/dL | 1.1 ± 0.6 |
| Interstitial lung disease, % | 5 (23.8) |
| Forced expiratory volume in 1 sec (FEV1), % predicted | 75.4 ± 18.9 |
| Forced vital capacity (FVC), % predicted | 75.1 ± 19.5 |
| FEV1/FVC | 1.0 ± 0.09 |
| Total lung capacity, % predicted | 73.2 ± 20.3 |
| Diffusion capacity, % predicted | 42.4 ± 11.3 |
| Forced vital capacity/Diffusion capacity ratio | 1.9 ± 0.6 |
| Total lung capacity/Diffusion capacity ratio | 1.8 ± 0.7 |
*Values are n (%) for categorical variables or mean ± standard deviation for continuous variables
Cardiac catheterization and echocardiographic parameters
As shown in Table 2, mean RA pressure for the cohort was 8.2 ± 5.8 mmHg. PCWP was uniformly within normal range (9.6 ± 3.9 mmHg), and cardiac output was preserved. RV and PA systolic pressures were elevated without a gradient step-up, thus excluding any RV outflow or pulmonic valve obstruction. Mean PVR by right heart catheterization was > 2 Wood units (WU) in half of the study population (mean = 4.5 ± 3.7 WU). Echocardiographic parameters including tissue Doppler and TAPSE data are displayed in Table 2. Two subjects had undetectable TR jet profiles and were excluded from the analyses. All participants exhibited LV ejection fraction ≥ 50%. Mean PASP by echocardiographic measurement was elevated (47 ± 21 mmHg) and essentially identical to values obtained by invasive measures. Mean basal RV systolic performance as measured by tricuspid s’ velocity (12.1 ± 3.4 cm/s) and TAPSE (22.7 ± 5.4 mm) were within the normal range based on ASE guidelines (6).
Table 2.
Hemodynamic and echocardiographic parameters (n=21)
| Variable | |
|---|---|
| Hemodynamic parameters | |
| RA pressure, mm Hg | 8.2 ± 5.8 |
| RV systolic pressure, mm Hg | 46.9 ± 19.4 |
| RV diastolic pressure, mm Hg* | 4.5 ± 5.0 |
| Pulmonary capillary wedge pressure, mm Hg | 9.6 ± 3.9 |
| Pulmonary artery systolic pressure, mm Hg | 47.0 ± 20.2 |
| Pulmonary artery diastolic pressure, mm Hg | 18.3 ± 8.4 |
| Pulmonary artery mean pressure, mm Hg | 30.1 ± 12.5 |
| Pulmonary vascular resistance, WU/cm5 | 4.5 ± 3.7 |
| Cardiac output (Fick equation), L/min* | 5.2 ± 1.2 |
| Echocardiographic parameters | |
| LA, mm | 36.9 ± 5.5 |
| RA area, cm2 | 17.0 ± 7.0 |
| LA volume/BSA, ml/m2 | 28.1 ± 7.6 |
| RV basal diameter, mm | 31.7 ± 6.4 |
| RV end-diastolic area, cm2 | 19.8 ± 8.4 |
| RV fractional area change, % | 42.9 ± 13.1 |
| Peak tricuspid regurgitation velocity, m/s | 3.1 ± 0.7 |
| Pulmonary artery systolic pressure, mm Hg† | 47.0 ± 21.0 |
| Medial e’ velocity, cm/s | 7.6 ± 2.1 |
| Medial a’ velocity, cm/s | 10.0 ± 2.1 |
| Medial s’ velocity, cm/s | 8.8 ± 1.3 |
| Mitral E wave velocity, cm/s | 79.9 ± 23.3 |
| Mitral A wave velocity, cm/s | 89.6 ± 30.6 |
| E/A | 0.9 ± 0.3 |
| RV tissue Doppler MPI | 0.63 ± 0.2 |
| E/e’ (medial annulus) | 11.6 ± 5.9 |
| Tricuspid annular plane systolic excursion, mm | 22.7 ± 5.4 |
| Tricuspid e’ velocity, cm/s | 9.8 ± 3.8 |
| Tricuspid a’ velocity, cm/s | 13.7 ± 3.5 |
| Tricuspid s’ velocity, cm/s | 12.1 ± 3.4 |
| Peak tricuspid regurgitation velocity/VTI RV outflow tract | 0.2 ± 0.1 |
| Pulmonary vascular resistance, WU‡ | 2.3 ± 1.1 |
| RV fractional area change, % | 42.9 ± 13.1 |
Echocardiographic pulmonary artery systolic pressure = [4 × (peak tricuspid regurgitation velocity)2 + right atrial pressure]
Echocardiographically derived pulmonary vascular resistance (Woods units) = (peak tricuspid regurgitation velocity/VTI RV outflow tract ×10) + 0.16
Abbreviations: RA = right atrium, RV = right ventricle, WU = Woods units, LA = left atrium, MPI = myocardial performance index
Correlation of catheterization and echocardiographic hemodynamics
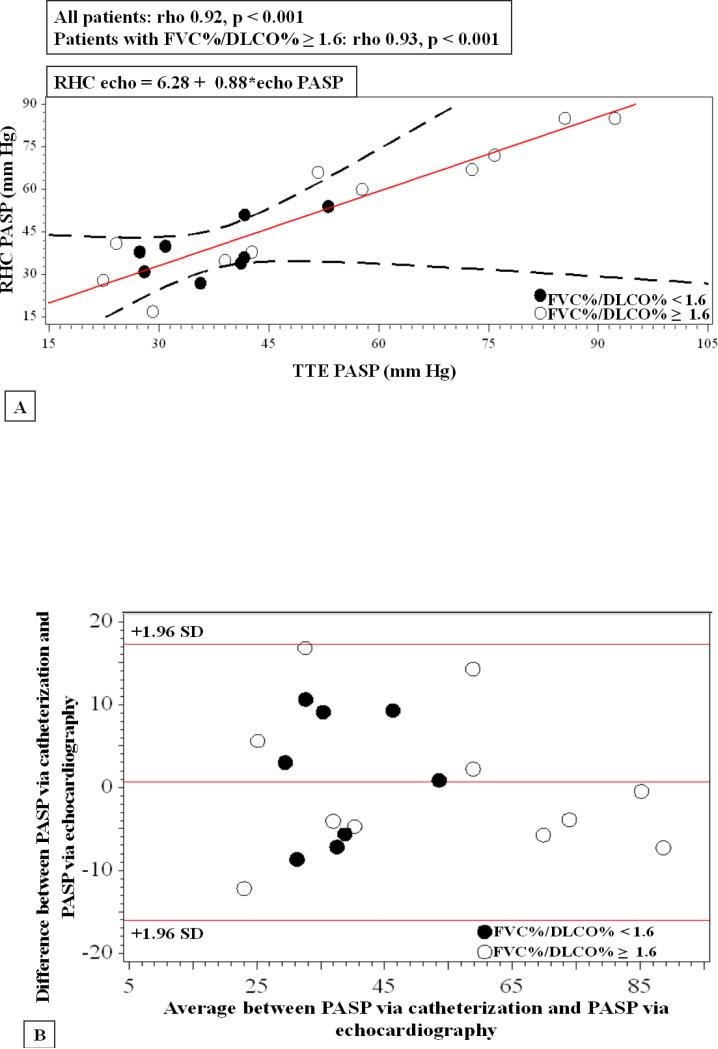
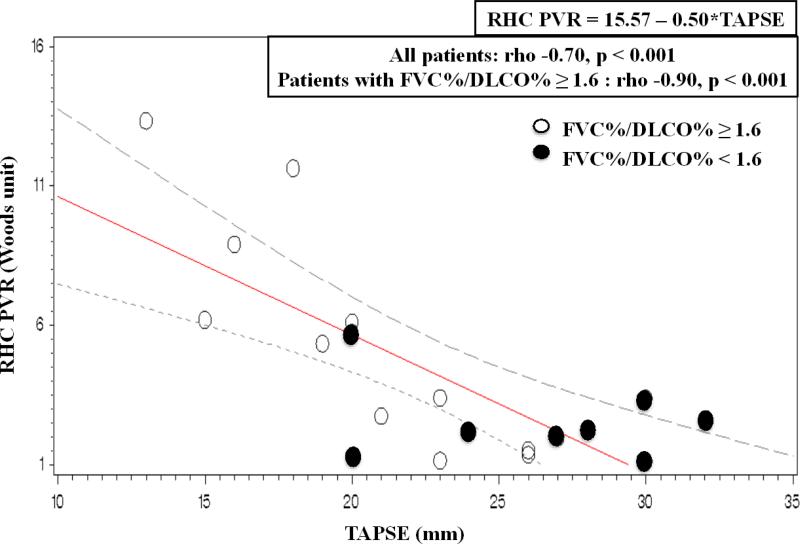
As displayed in Table 3, several echocardiographic measures correlated significantly with invasive hemodynamics with strongest correlation for PASP (rho = 0.92, p <0.001). The relationship between echo-derived PASP and RHC PASP is displayed in Figure 1, Panels A and B. Several newer right-heart echocardiographic variables were also closely associated with right heart catheterization measurements. TAPSE, a measure of systolic descent of the base of the RV free wall, correlated significantly with PASP, mean PAP, and PVR. The association was particularly strong for PVR (rho = -0.90, p<0.001) when analyses were performed in the subset of individuals with predominant PAH as stratified by FVC%/DLCO% ≥ 1.6 (Figure 2). An additional method of RV systolic function quantification using tricuspid s’ velocity also correlated significantly with invasive hemodynamic measures of PASP, mean PAP, and PVR (Table 3). Again, both PASP and PVR associations with tricuspid s’ velocity strengthened when stratified by FVC%/DLCO% ratio ≥ 1.6. RV end-diastolic area was only modestly related to PVRcath (rho = 0.46, p = 0.04), however when stratified by FVC%/DLCO% ≥ 1.6, the correlation strengthened (rho = 0.77, p = 0.004).
Table 3.
Correlations between Echocardiography and Right Heart Catheterization Parameters (n=21)
| Echocardiography | Catheterization | Rho | P-value |
|---|---|---|---|
| TAPSE | PASP | −0.67 | 0.002 |
| TAPSE | Mean PAP | −0.63 | 0.004 |
| TAPSE | PVR | −0.70 | <0.001 |
| TAPSE* | PVR* | −0.90* | <0.001* |
| Tricuspid s’ velocity | PASP | −0.66 | 0.003 |
| Tricuspid s’ velocity | Mean PAP | −0.67 | 0.002 |
| Tricuspid s’ velocity* | PASP* | −0.70 | 0.03 |
| Tricuspid s’ velocity | PVR | −0.68 | 0.002 |
| Tricuspid s’ velocity* | PVR* | −0.74* | 0.01* |
| Tricuspid e’ velocity | PASP | −0.64 | 0.006 |
| Tricuspid e’ velocity | Mean PAP | −0.55 | 0.02 |
| Tricuspid e’ velocity | PVR | −0.68 | 0.003 |
| Tricuspid a’ velocity | PASP | −0.49 | 0.04 |
| Tricuspid a’ velocity | Mean PAP | −0.47 | 0.05 |
| Tricuspid a’ velocity | PVR | −0.40 | 0.10 |
| TRV/VTIRVOT | PASP | 0.79 | <0.001 |
| TRV/VTIRVOT | Mean PAP | 0.79 | <0.001 |
| TRV/VTIRVOT | PVR | 0.75 | <0.001 |
| TRV/VTIRVOT* | PVR* | 0.87* | 0.003* |
| PASP | PASP | 0.92 | <0.001 |
| PASP | Mean PAP | 0.92 | <0.001 |
| PASP | PVR | 0.82 | <0.001 |
| E/e’ (medial annulus) | PCWP | 0.34 | 0.16 |
| RV area | PVR | 0.46 | 0.04 |
| RV area* | PVR* | 0.77* | 0.004* |
| RV FAC | PASP | −0.73 | 0.001 |
| RV FAC | Mean PAP | −0.71 | 0.001 |
| RV FAC | PVR | −0.78 | <0.001 |
| RV FAC* | PVR* | −0.77 | 0.005 |
In subjects with FVC%/DLCO% ≥ 1.6
Figure 1. Relationship between RHC and echo-derived PASP.
Panel A. Linear regression demonstrates significant correlation in this study between cath and echo PASP for all subjects (rho 0.92, p < 0.001). TTE= Transthoracic echocardiogram Panel B. Bland-Altman plot for echo-derived PASP and RHC PASP.
Figure 2. Relationship between RHC PVR and echo TAPSE measures.
Linear regression demonstrates significant correlation between TAPSE and RHC PVR for all subjects (rho −0.70, p < 0.001). The correlation strengthens when stratified by FVC%/DLCO% ≥ 1.6, (open circle symbol, rho −0.90, p < 0.001). Closed circle symbol represents FVC%/DLCO% < 1.6.
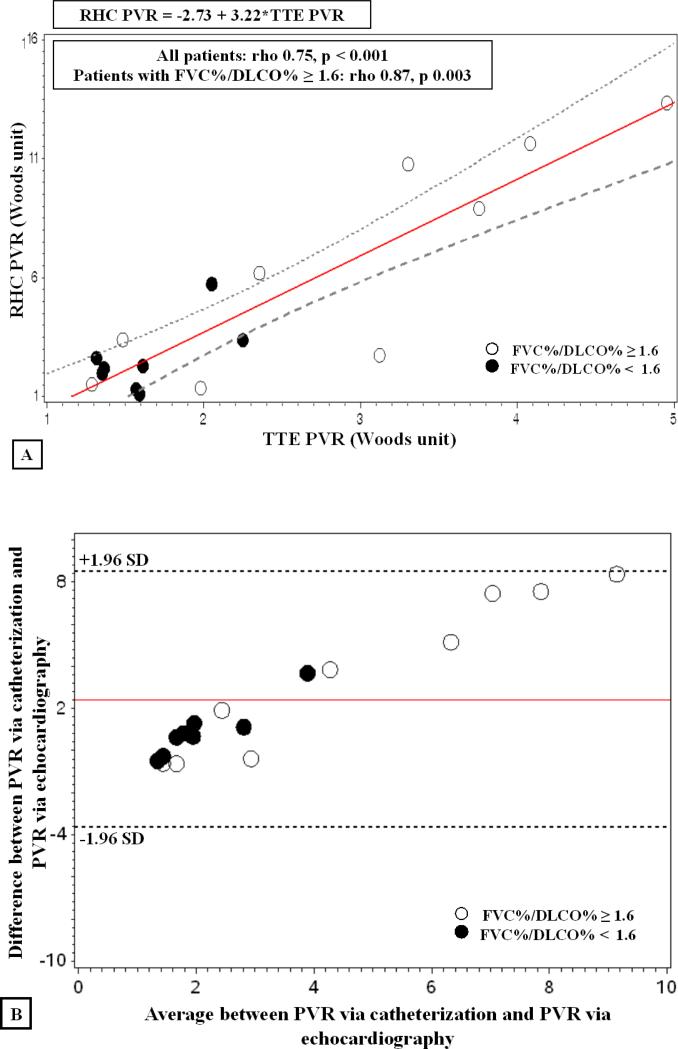
Catheterization and echocardiography-derived measurements of PVR correlated significantly (rho = 0.75, p <0.001). Consistent with other analyses, when stratified by FVC%/DLCO% ≥ 1.6, the association markedly strengthened (rho = 0.87, p =0.003) as shown in Figure 3, Panel A. Bland-Altman analysis for echo- and catheterization-derived PVR showed good agreement as displayed in Figure 3, Panel B. Additionally, as shown in Table 3, we demonstrated an inverse relationship between RV FAC and PVRcath (r= −0.78, p<0.001).
Figure 3. Relationship between RHC and echo-derived PVR.
Panel A. Linear regression demonstrates significant correlation between cath and echo PVR for all subjects (rho 0.75, p < 0.001). The correlation strengthens when stratified by FVC%/DLCO% ≥ 1.6, (open circle symbol, rho −0.87, p 0.003). Closed circle symbol represents FVC%/DLCO% < 1.6. Panel B. Bland-Altman plot for echo-derived PVR and RHC PVR.
DISCUSSION
In this present study, we carefully examined cardiopulmonary hemodynamics concurrently by cardiac catheterization and echocardiography in a scleroderma population with varying degrees of pulmonary vascular disease and demonstrated that several right heart measures including PASP, mPAP, and PVR strongly correlated with each other using both methodologies. Additionally, we demonstrated that novel measures of right-heart systolic function, specifically TAPSE, tricuspid s’ velocity, and RV FAC relate well to invasively measured PASP and PVR. Many of these relationships were stronger in the subset of individuals with a phenotype more suspect for PAH, as defined by FVC%/DLCO% ≥ 1.6.
Scleroderma-associated pulmonary hypertension (SSc-PAH) is a leading cause of scleroderma-related deaths (3) and elevated pulmonary pressures, particularly increased PVR, independently predict mortality in this population (2, 3, 20, 21). Hemodynamic data including PASP, mPAP, and PVR usually obtained via right heart catheterization are recommended for the definitive diagnosis and monitoring by ACCF/AHA Expert Consensus Document on Pulmonary Hypertension (7). Recent published data from the DETECT study places echocardiographic measures as an important clinical component of the evidence-based algorithm for the detection of PAH (16). In the present study, we provide evidence that clinically relevant right heart pressure measurements can be reasonably well estimated noninvasively using echocardiography. While previous studies have reported modest relationships in RHC-echo correlations in SSc-populations (5, 8, 9), the primary reasons for variability in findings may relate to technical issues such as temporal delays, sometimes days, between acquisition of Doppler and invasive measurements. In addition, inaccurate echo estimation and reliance on assumptions of RA pressure and/or utilization of technically poor quality TR Doppler jet profiles that underestimate effective regurgitant velocities may also have contributed to decreased accuracy. As PA systolic pressure remains one of the most used and emphasized parameters in clinical practice, we demonstrated that carefully performed echocardiograms can yield hemodynamic information comparable to invasively derived values. In particular, we found striking concordance in PASP determination by echo and catheterization. Our echo calculation of PVR was based on a derived formula from prior published data in 44 subjects of mixed etiology of pulmonary hypertension (14). While in our study PVRecho correlated significantly with PVRcath, accuracy was less than that observed for PA systolic pressure estimation. Frea and colleagues recently examined Doppler parameters in scleroderma patients without pulmonary hypertension and identified TRV/ VTIRVOT ratio, a surrogate of PVR, as one of the strongest predictors for incident PAH in their cohort (22). As such, despite some limitations, there is growing recognition that PVRecho provides clinical insight and further studies are warranted to refine the accuracy of this non-invasive variable specifically in SSc patients.
Another novel aspect of our study involved investigation of newer echo parameters of RV function in relation to RHC-derived hemodynamics. Tricuspid annular plane systolic excursion (TAPSE) represents a simple method that quantifies the distance of RV systolic annular apical displacement using M-mode and represents longitudinal RV systolic function. TAPSE is validated against RV ejection fraction and fractional area shortening, and recommended as a routine measure for the assessment of RV function (6). When evaluated in various PAH cohorts, impaired TAPSE is linked to reduced survival (10, 11). A recent study specifically examined this relationship in SSc-PAH patients demonstrating a negative association between RHC PVR and TAPSE consistent with our data, and TAPSE ≤ 1.7 cm conferred a 4-fold greater risk of death compared to patients with TAPSE > 1.7 cm (23). In our study, we demonstrated significant negative correlations between TAPSE and RHC measurements of PASP, mPAP, and PVR. The relationships were particularly striking in subjects with predominant PAH stratified by FVC%/DLCO% where PVR-TAPSE correlation reached rho = −0.9, P<0.001. As such, TAPSE not only provides prognostic information, but tracks closely with right heart hemodynamic overload, and may prove useful as a non-invasive marker of disease progression and risk-stratification in SSc-PAH patients.
In this context, we examined another newer echo method that quantifies RV systolic performance, tricuspid annular systolic (s’) velocity, that has evolved as a simple and reproducible technique which uses pulsed-wave tissue Doppler to measure peak longitudinal velocity excursion of the tricuspid annular and/or basal RV free wall. This measure of myocardial tissue velocity has been validated for the assessment of RV systolic function in patients with pulmonary hypertension and correlates well with TAPSE. In SSc patients without pulmonary hypertension, tricuspid annular s’ velocity was lower compared to age- and gender-matched controls and predicted RV dysfunction (24). In our study, tricuspid s’ velocity correlated significantly with PASP, mPAP, and PVR, suggesting that this parameter along with TAPSE provides information about extent of RV dysfunction in pulmonary hypertension. A limitation of tricuspid annular Doppler relates to its beam angle dependency and measurements must be acquired parallel to annular motion for accuracy. Lastly, we found RV FAC, another measure of RV systolic function, negatively correlated with RHC assessment. One small study in the scleroderma literature evaluated the prognostic value of FAC and found it did not predict PAH development (22).
Scleroderma comprises two primary pulmonary vascular disease phenotypes, ILD with subsequent development of PAH or isolated PAH without significant ILD (more frequently associated with limited cutaneous SSc). Recent data suggest that scleroderma with pulmonary hypertension resulting from ILD is associated with greater mortality risk as compared to individuals with isolated PAH (20, 25). Disease pathophysiology in scleroderma may vary depending on etiology of pulmonary hypertension with differential impact on the pulmonary vasculature and right heart with concomitant pulmonary parenchymal involvement. In our study, correlative findings strengthened when specifically examined in subjects with predominant PAH based on FVC%/DLCO% ≥ 1.6, a novel observation that has not been previously reported in echocardiographic or catheterization studies in scleroderma. Both FVC and DLCO% decrease to a similar extent in ILD, however in SSc-PAH, DLCO% has been found to be disproportionately reduced compared to FVC, thus leading to higher FVC%/ DLCO% ratios (3, 17). DLCO% has been shown in several studies to predict PAH in scleroderma , thus stratification by FVC%/DLCO% likely increases specificity for disease subsets that may have distinct pathophysiologies. While our subset sample size was small, the correlations were robust and warrant further investigation in larger clinical investigations to validate and gain further understanding of the impact of scleroderma subtypes on cardiopulmonary function.
LIMITATIONS
Our present study has several limitations. First, the investigation involved a relatively small number of subjects in a single-center tertiary referral hospital. The small number of participants limited our ability to perform multivariate analysis and rigorous statistical modeling with adjustment for multiple comparisons, thus our findings are in part exploratory and hypothesis generating. In addition, subjects in our investigation were part of the DETECT study and owing to inclusion criteria may not be representative of the broader disease spectrum of SSc. However, a small sample size may be expected since scleroderma is a rare disease. We nevertheless demonstrated strong correlations between RHC- and echo-measured variables in this group of individuals suggesting biological connection of the described relationships. Second, we did not assess RV strain as an additional evolving measure of RV systolic function, as it was beyond the technical capability of the image acquisition system. Third, pulmonary hypertension severity was generally in the moderate range, and further studies would be required to validate findings in more advanced disease. The findings are counterbalanced by the careful nature of our investigation, including single blinded operators for both RHC and echo procedures.
CONCLUSIONS
In conclusion, our findings suggest that echocardiography may be clinically useful in identifying and monitoring pulmonary hypertension and RV function non-invasively in scleroderma patients. The utilization of echocardiography can aid in screening and monitoring of PAH for progression and possibly treatment effects in these patients, but at this time would not replace RHC for the definitive diagnosis of PAH which remains the gold standard for precise and accurate assessment of pulmonary hemodynamics. Stratification by FVC%/DLCO% strengthened our findings prompting recognition that the cardiopulmonary impact of specific scleroderma disease subtypes warrants further investigation, as does the specific role of echocardiography in the screening and monitoring of scleroderma patients with and without pulmonary hypertension.
SIGNIFICANCE AND INNOVATIONS.
The present study evaluates the assessment of pulmonary hypertension in patients with systemic sclerosis using non-invasive techniques with transthoracic echocardiography including tricuspid annular plane systolic excursion (TAPSE), tricuspid annular systolic (s’) velocity, and RV fractional area change (FAC). These correlated significantly with multiple hemodynamic measurements obtained via right heart catheterization.
The relationship between echocardiographic parameters and right heart catheterization measurements were stratified by pulmonary function test parameters, specifically FVC%/DLCO% as an approach used to identify patients with isolated pulmonary arterial hypertension versus pulmonary hypertension secondary to interstitial lung disease that strengthened correlations.
A method to estimate pulmonary vascular resistance by echocardiography correlated robustly with assessments of pulmonary vascular resistance by right heart catheterization, particularly when stratified by FVC%/DLCO% ≥ 1.6. This provides a potential non-invasive approach to following pulmonary vascular resistance in patients with systemic sclerosis at risk or with pulmonary hypertension to potentially guide management.
Acknowledgments
Funding: Actelion Pharmaceuticals was the primary sponsor of the DETECT study. Funding for this study at our local institution, Boston Medical Center, included National Institutes of Health RFA-AR-11-002; NIAMS-CORT under HWF and RWS.
Footnotes
There are no conflicts of interests.
REFERENCES
- 1.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140(1):37–50. [PubMed] [Google Scholar]
- 2.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62(11):1088–93. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowal-Bielecka O, Avouac J, Pittrow D, Huscher D, Behrens F, Denton CP, et al. Echocardiography as an outcome measure in scleroderma-related pulmonary arterial hypertension: a systematic literature analysis by the EPOSS group. J Rheumatol. 2010;37(1):105–15. doi: 10.3899/jrheum.090661. [DOI] [PubMed] [Google Scholar]
- 5.Mukerjee D, St George D, Knight C, Davar J, Wells AU, Du Bois RM, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford) 2004;43(4):461–6. doi: 10.1093/rheumatology/keh067. [DOI] [PubMed] [Google Scholar]
- 6.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86-8. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Denton CP, Cailes JB, Phillips GD, Wells AU, Black CM, Bois RM. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol. 1997;36(2):239–43. doi: 10.1093/rheumatology/36.2.239. [DOI] [PubMed] [Google Scholar]
- 9.Hsu VM, Moreyra AE, Wilson AC, Shinnar M, Shindler DM, Wilson JE, et al. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol. 2008;35(3):458–65. [PubMed] [Google Scholar]
- 10.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 11.Ghio S, Klersy C, Magrini G, D'Armini AM, Scelsi L, Raineri C, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140(3):272–8. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 12.Picard MH, Adams D, Bierig SM, Dent JM, Douglas PS, Gillam LD, et al. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr. 2011;24(1):1–10. doi: 10.1016/j.echo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41(6):1021–7. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 15.Braunwald E. Braunwald's heart disease: a textbook of cardiovascular medicine. Elsevier Saunders; Philadelphia (PA): 2012. pp. 383–405. [Google Scholar]
- 16.Coghlan JG, Denton CP, Grunig E, Bonderman D, Distler O, Khanna D, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2013 May 18; doi: 10.1136/annrheumdis-2013-203301. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steen VD, Graham G, Conte C, Owens G, Medsger TA., Jr Isolated diffusing capacity reduction in systemic sclerosis. Arthritis Rheum. 1992;35(7):765–70. doi: 10.1002/art.1780350709. [DOI] [PubMed] [Google Scholar]
- 18.Fischer A, Bull TM, Steen VD. Practical approach to screening for scleroderma-associated pulmonary arterial hypertension. Arthritis Care Res (Hoboken) 2012;64(3):303–10. doi: 10.1002/acr.20693. [DOI] [PubMed] [Google Scholar]
- 19.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48(2):516–22. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 20.Mathai SC, Hummers LK, Champion HC, Wigley FM, Zaiman A, Hassoun PM, et al. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum. 2009;60(2):569–77. doi: 10.1002/art.24267. [DOI] [PubMed] [Google Scholar]
- 21.Trad S, Amoura Z, Beigelman C, Haroche J, Costedoat N, Boutin le TH, et al. Pulmonary arterial hypertension is a major mortality factor in diffuse systemic sclerosis, independent of interstitial lung disease. Arthritis Rheum. 2006;54(1):184–91. doi: 10.1002/art.21538. [DOI] [PubMed] [Google Scholar]
- 22.Frea S, Capriolo M, Marra WG, Cannillo M, Fusaro E, Libertucci D, et al. Echo Doppler predictors of pulmonary artery hypertension in patients with systemic sclerosis. Echocardiography. 2011;28(8):860–9. doi: 10.1111/j.1540-8175.2011.01467.x. [DOI] [PubMed] [Google Scholar]
- 23.Mathai SC, Sibley CT, Forfia PR, Mudd JO, Fisher MR, Tedford RJ, et al. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis-associated pulmonary arterial hypertension. J Rheumatol. 2011;38(11):2410–8. doi: 10.3899/jrheum.110512. [DOI] [PubMed] [Google Scholar]
- 24.Saxena N, Rajagopalan N, Edelman K, Lopez-Candales A. Tricuspid annular systolic velocity: a useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography. 2006;23(9):750–5. doi: 10.1111/j.1540-8175.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- 25.Launay D, Humbert M, Berezne A, Cottin V, Allanore Y, Couderc LJ, et al. Clinical characteristics and survival in systemic sclerosis-related pulmonary hypertension associated with interstitial lung disease. Chest. 2011;140(4):1016–24. doi: 10.1378/chest.10-2473. [DOI] [PubMed] [Google Scholar]