Abstract
Background and Aims
The TERMINAL FLOWER 1 (TFL1) gene is pivotal in the control of inflorescence architecture in arabidopsis. Thus, tfl1 mutants flower early and have a very short inflorescence phase, while TFL1-overexpressing plants have extended vegetative and inflorescence phases, producing many coflorescences. TFL1 is expressed in the shoot meristems, never in the flowers. In the inflorescence apex, TFL1 keeps the floral genes LEAFY (LFY) and APETALA1 (AP1) restricted to the flower, while LFY and AP1 restrict TFL1 to the inflorescence meristem. In spite of the central role of TFL1 in inflorescence architecture, regulation of its expression is poorly understood. This study aims to expand the understanding of inflorescence development by identifying and studying novel TFL1 regulators.
Methods
Mutagenesis of an Arabidopsis thaliana line carrying a TFL1::GUS (β-glucuronidase) reporter construct was used to isolate a mutant with altered TFL1 expression. The mutated gene was identified by positional cloning. Expression of TFL1 and TFL1::GUS was analysed by real-time PCR and histochemical GUS detection. Double-mutant analysis was used to assess the contribution of TFL1 to the inflorescence mutant phenotype.
Key Results
A mutant with both an increased number of coflorescences and high and ectopic TFL1 expression was isolated. Cloning of the mutated gene showed that both phenotypes were caused by a mutation in the ARGONAUTE1 (AGO1) gene, which encodes a key component of the RNA silencing machinery. Analysis of another ago1 allele indicated that the proliferation of coflorescences and ectopic TFL1 expression phenotypes are not allele specific. The increased number of coflorescences is suppressed in ago1 tfl1 double mutants.
Conclusions
The results identify AGO1 as a repressor of TFL1 expression. Moreover, they reveal a novel role for AGO1 in inflorescence development, controlling the production of coflorescences. AGO1 seems to play this role through regulating TFL1 expression.
Keywords: Flower development, TERMINAL FLOWER 1, TFL1, ARGONAUTE1, AGO1, plant architecture, inflorescence architecture, flowering, Arabidopsis thaliana
INTRODUCTION
The architecture of the aerial part of a plant depends on the number, size, shape and position of its leaves, shoots and flowers (Benlloch et al., 2007). All these organs derive from the shoot apical meristem (SAM), a small group of stem cells located at the shoot apex (Bowman, 1994; Wolpert and Tickle, 2010). After germination, the SAM is a vegetative meristem that produces leaves and branches. When endogenous and environmental conditions are appropriate, transition to the flowering phase takes place and the SAM is transformed into an inflorescence meristem, which produces flowers (Amasino 2010; Huijser and Schmid, 2011; Andrés and Coupland, 2012).
In Arabidopsis thaliana (hereafter, arabidopsis), during the vegetative phase, the SAM produces leaves on its flanks without internode elongation, which leads to the formation of a rosette (Figs 1C and 5A, E). In contrast, internodes formed after the floral transition do elongate, leading to the formation of the main inflorescence, where two phases can be distinguished. During the first inflorescence phase (I1), the SAM produces cauline leaves on its flanks which subtend axillary lateral inflorescences, called coflorescences; in the second inflorescence phase (I2), the SAM produces flowers without subtending leaves (Ratcliffe et al., 1998) (Figs 1C and 5A, E). The arabidopsis inflorescence is an open raceme, where the flowers are formed only on the flanks of the inflorescence SAM, which has indeterminate growth and never develops into a flower (Alvarez et al., 1992; Weberling, 1992; Prusinkiewicz et al., 2007).
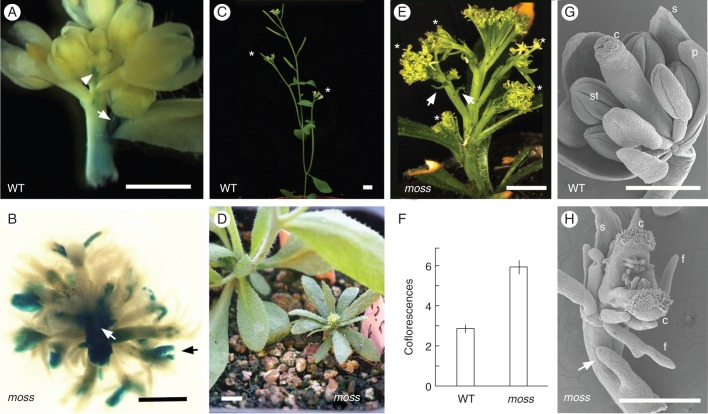
Fig. 1.
Phenotype of the moss mutant. (A) Expression pattern of TFL1::GUS in a bolting inflorescence apex of a wild-type (WT) parental plant. GUS signal is observed in the inflorescence meristem (arrowhead) and the inflorescence stem; a strong signal is seen in the stem of a developing coflorescence (arrow). GUS signal is not detected in flowers. (B) Expression pattern of TFL1::GUS in the main inflorescence of a moss plant. GUS signal is apparent in flowers (black arrow) and in the inflorescence stem (white arrow). (C) WT plant with two coflorescences (asterisks) in the main inflorescence stem. (D) A moss plant (right) beside a WT plant (left). (E) Inflorescence of a mature moss plant with five coflorescences (asterisks) in the main inflorescence stem. There is no elongation of the internodes between flowers. The presence of filamentous organs (arrows) in a coflorescence stem is observed. (F) Histogram showing the number of coflorescences in the main inflorescence stem of WT and moss plants. Values correspond to the average of 15 plants ± standard error. (G) Scanning electron microscopy (SEM) of a WT flower, showing the different floral organs. (H) SEM of a moss flower. A filamentous organ (arrow) is present in the pedicel; petals and stamens are replaced by filaments; and the carpels are unfused and ovules are exposed. Abbreviations: c, carpel; f, filament; p, petal; s, sepal; st, stamen. Scale bars: 5 mm in (A-E); 500 μm in (G) and (H).
Fig. 5.
Phenotype of ago1 tfl1 double mutants. (A) A Landsberg erecta (Ler) wild-type (WT) plant, with a main inflorescence with two coflorescences (asterisks). The tfl1-2 and moss mutants are in a Ler genetic background. (B) A tfl1-2 mutant plant. Instead of coflorescences, its main inflorescence has two axillary flowers (arrows). (C) Main inflorescence of a moss/ago1-103 mutant plant, with six coflorescences (asterisks). (D) A moss/ago1-103 tfl1-2 double mutant plant. Instead of coflorescences, its main inflorescence has one axillary flower (arrow). (E) A Columbia (Col) WT plant, with a main inflorescence with two coflorescences (asterisks). The genetic background of the tfl1-1 and ago1-26 mutants is Col. (F) A tfl1-1 mutant plant. Instead of coflorescences, its main inflorescence has two axillary flowers (arrows). (G) An ago1-26 mutant plant, with a main inflorescence with six coflorescences (asterisks). (H) An ago1-26 tfl1-1 double mutant plant. Instead of coflorescences, its main inflorescence has two axillary flowers (arrows). Scale bars: 5 mm.
This inflorescence architecture is based on the antagonistic action of two sets of genes that are expressed in distinct non-overlapping domains of the inflorescence apex. On the one hand, the main floral meristem identity genes LEAFY (LFY) and APETALA1 (AP1), which encode transcription factors, are expressed in the primordia formed at the flanks of the inflorescence SAM, conferring to these primordia the identity of floral meristems, as deduced from the fact that loss of function of these genes causes replacement of flowers by inflorescence-like structures (Mandel et al., 1992; Weigel et al., 1992; Blázquez et al., 2006). On the other hand, the shoot/inflorescence identity gene TERMINAL FLOWER 1 (TFL1) shows strong expression in the centre of the SAM of the main and lateral inflorescences (Bradley et al., 1997; Conti and Bradley, 2007) (Fig. 1A). Expression of TFL1 is required for the identity of these meristems, and loss of TFL1 function leads to the conversion of these inflorescence meristems into floral meristems, so that in tfl1 mutants the lateral coflorescences are replaced by axillary flowers and the main inflorescence shoot exhibits determinate growth, ending in a terminal flower (Shannon and Meeks-Wagner, 1991; Alvarez et al., 1992; Bradley et al., 1997). The complementary expression pattern of these two types of genes is, therefore, required for the architecture of arabidopsis inflorescence, where the SAM remains indeterminate and the flowers are formed at its flanks (Blázquez et al., 2006; Benlloch et al., 2007; Teo et al., 2013). This expression pattern is maintained by mutual repression between TFL1 and the floral meristem identity genes, and, in the absence of TFL1 function, in tfl1 mutants, LFY and AP1 expression invades the inflorescence meristems, correlating with the conversion of these meristems into floral meristems (Bradley et al., 1997; Liljegren et al., 1999; Ratcliffe et al., 1999; Ferrándiz et al., 2000).
TERMINAL FLOWER 1 not only controls determination of the inflorescence apex but it also regulates the length of the different developmental phases that the SAM goes through (Ratcliffe et al., 1998). In fact, TFL1 acts as a repressor of the transition to flowering, as shown by the fact that the vegetative phase in tfl1 mutants is shorter than in the wild type (Bradley et al., 1997; Ratcliffe et al., 1998). Conversely, constitutive expression of TFL1 in 35S::TFL1 plants causes late flowering and an enlargement of the I1 phase, with increased production of coflorescences (Ratcliffe et al., 1998). This role of TFL1 in controlling the transition to flowering correlates with its expression, at a low level, in the vegetative SAM (Bradley et al., 1997), and is thought to be mediated by its activity as a transcriptional co-repressor through its interaction with the bZIP-type transcription factor FD (Hanano and Goto, 2011). Possibly, TFL1 also repress flowering by interfering with the activity of FLOWERING LOCUS T (FT), a strong flowering promoter. FT is a homologous protein to TFL1, which also acts in a transcriptional complex with FD (Abe et al., 2005; Wigge et al., 2005), and TFL1 and FT have been suggested to compete for protein partners (Hanzawa et al., 2005; Ahn et al., 2006; Ho and Weigel, 2014).
In summary, the pattern of TFL1 expression seems pivotal to its function: it is weak in the vegetative SAM and upregulated with the floral transition, after which TFL1 is expressed strongly in the inflorescence SAM and also throughout the inflorescence stem (Bradley et al., 1997; Conti and Bradley, 2007). How this dynamic complex expression pattern is established and maintained is still poorly understood. Transcriptional repression of TFL1 in the floral meristem is the only well-known aspect of that question. On the one hand, multiple molecular genetic evidence indicates that the AP1 and LFY transcription factors act in the flower as repressors of TFL1 (Liljegren et al., 1999; Ratcliffe et al., 1999; Ferrándiz et al., 2000; Parcy et al., 2002). Recent support for this hypothesis has been provided by the demonstration of direct binding of AP1 and LFY to the 3′ region of the TFL1 gene (Kaufmann et al., 2010; Moyroud et al., 2011; Winter et al., 2011). In addition, a recent study has shown that SUPPRESSOR OF OVER-EXPRESSION OF CONSTANS 1 (SOC1), SHORT VEGETATIVE PHASE (SVP), AGAMOUS-LIKE 24 (AGL24) and SEPALLATA 4 (SEP4), from the MADS-box transcription factor family (like AP1), also contribute, acting redundantly and directly, to suppress TFL1 in the developing flower, and are essential for the repression of TFL1 by LFY and AP1 (Liu et al., 2013).
In addition to regulation through transcription factors, other transcriptional or post-transcriptional mechanisms might contribute to controlling the expression pattern of the TFL1 mRNA. One of these is RNA-mediated silencing, or RNA silencing, which is a central mechanism of gene regulation in eukaryotes (Carthew and Sontheimer, 2009). RNA silencing relies on ARGONAUTE (AGO) proteins, which constitute the core of the RNA-induced silencing complexes (RISCs), where they associate with distinct types of small RNAs that guide them to their targets through complementary base pairing (Baulcombe, 2004; Vaucheret, 2006; Bartel, 2009; Brodersen and Voinnet, 2009). In arabidopsis, AGO1 is the effector and pivotal component of RISCs that associate with microRNAs (miRNAs) and mediate post-transcriptional gene silencing (Vaucheret et al., 2004; Baumberger and Baulcombe, 2005; Qi et al., 2005). Characterization of ago1 mutants, which exhibit strong pleiotropic morphological phenotypes, shows that AGO1 contributes to the regulation of a wide variety of central developmental processes (Bohmert et al., 1998; Kidner and Martienssen, 2005a; Smith et al., 2009; Ji et al., 2011; Jover-Gil et al., 2012).
With the aim of identifying novel regulators of TFL1 expression, we carried out a genetic screening for mutants with altered TFL1 expression, making use of an arabidopsis reporter line containing a TFL1::GUS (β-glucuronidase) reporter transgene. We isolated a mutant with severe alterations in both TFL1 expression and inflorescence architecture, and found that a mutation in the AGO1 gene caused both phenotypes. Our results reveal a novel role for AGO1 in the control of inflorescence architecture, possibly through regulation of the expression of TFL1.
MATERIALS AND METHODS
Plant material and growth conditions
Arabidopsis thaliana plants were grown in a 1:1:1 mixture of sphagnum:perlite:vermiculite, at 21 °C under long-day photoperiods (16 h light), in growth cabinets, illuminated by cool-white fluorescent lamps (150 μE m−2 s−1). For the mutagenesis and the mutant screening, plants were grown in the greenhouse at 21 °C and long-day photoperiods, which were maintained with supplementary lighting [400 W Philips HDK/400 HPI (R) (N)].
The reporter Ler pBTG1 arabidopsis line used as the parent for the mutagenesis, and the moss/ago1-103, ago1-52 (Jover-Gil et al., 2012) and tfl1-2 (Alvarez et al., 1992) mutants were from the Landsberg erecta (Ler) genetic background. The ago1-26 (Morel et al., 2002) and tfl1-1 (Shannon and Meeks-Wagner, 1991) mutants were from the Columbia (Col) genetic background. ago1-26 was kindly provided by H. Vaucheret (INRA, Versailles, France).
The moss/ago103 mutant was backcrossed three times to Ler before being used for the experiments described here.
TFL1::GUS reporter constructs
The arabidopsis Ler pBTG1 line used as the parent for the mutagenesis was homozygous for the TFL1::GUS reporter construct pBTG1. All plants shown in Figs 2 and 4 were homozygous for the TFL1::GUS reporter construct pBTG6.
Fig. 2.
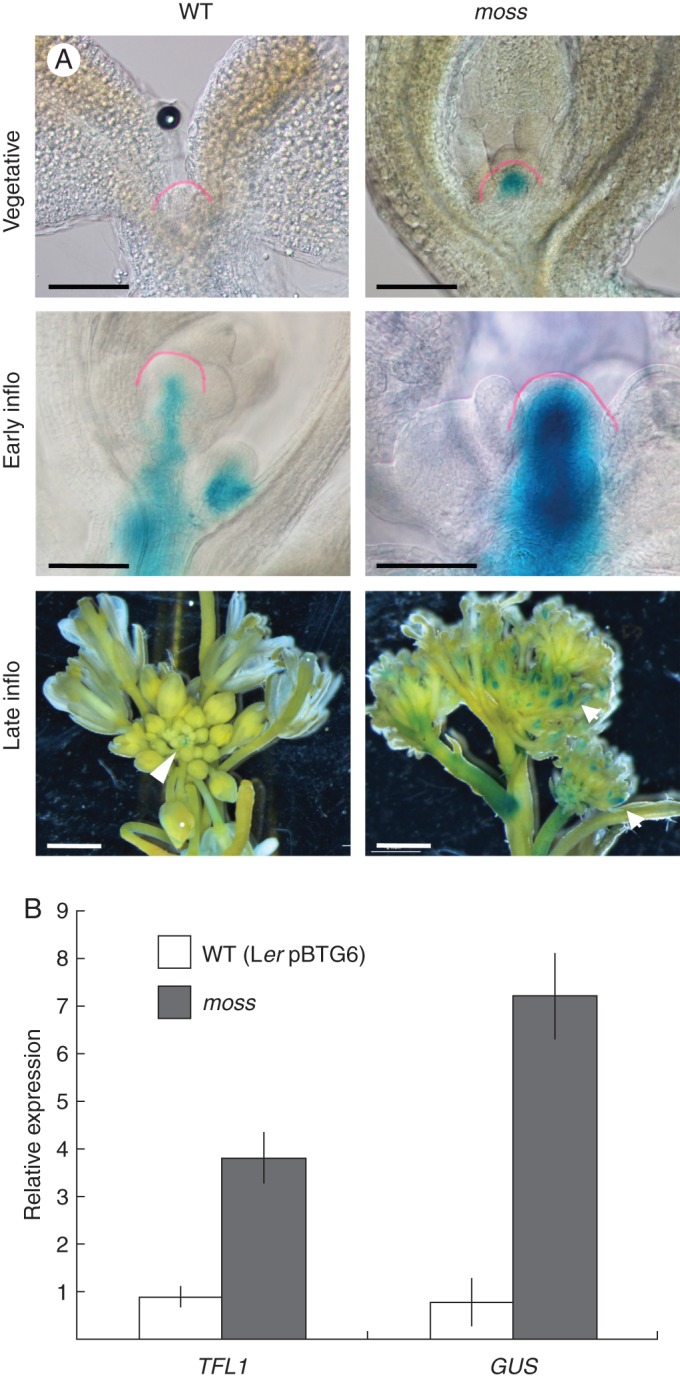
Effect of the moss mutation on the expression of TFL1. (A) Expression of TFL1::GUS in wild-type (WT) and moss shoot apices in different developmental stages. Apices from plants at equivalent developmental stages were compared. In late inflorescences, GUS signal in the WT apex is restricted to the inflorescence meristem (arrowhead), while in the moss apex signal is also observed in the flowers (arrows). The SAM in the vegetative and early inflorescence apices is outlined with a pink line. (B) mRNA levels of TFL1 and GUS in the inflorescence apices of WT (Ler pBTG6) and moss plants, determined by RT-qPCR. Samples were from main inflorescence apices from 40-day-old plants (= late inflorescence). The mRNA level of the UBIQUITIN 10 gene was used as reference. Values represent the means of three technical replicates ± standard error. Scale bars: 300 μm in vegetative and early inflorescence apices; 2 mm in late inflorescence apices.
Fig. 4.

Inflorescence phenotype of the ago1-26 mutant. (A) Main inflorescence of a Col wild-type (WT) plant, with three coflorescences (asterisks). (B) Main inflorescence of an ago1-26 mutant plant, with six coflorescences. (C) Expression of TFL1::GUS in the inflorescence apex of a Col WT plant. GUS signal is restricted to the inflorescence meristem (arrowhead). (D) Expression of TFL1::GUS in the inflorescence apex of an ago1-26 mutant plant. GUS signal is observed not only in the inflorescence meristem (arrowhead) but also in the flowers, in the floral organs and in the pedicels (arrows). Scale bars: 5 mm in (A) and (B); 1 mm in (C) and (D).
The pBTG1 construct was obtained by sub-cloning into the pBIN19 binary vector (Bevan, 1984) of a DNA fragment containing the GUS gene from the pBI121 vector (Jefferson et al., 1987) flanked by the complete 5′ intergenic region (2172 bp) and by a fragment of the 3′ intergenic region [2722 bp, starting downstream of the 3′-untranslated region (UTR)] of the Ler TFL1 gene (Supplementary Data Fig. S1). The 5′ and 3′ intergenic fragments in pBTG1 were amplified by PCR from a genomic clone from the TFL1 Ler gene, with the TFL15′intF and TFL15′intR primers, which add SalI and BamHI restriction sites, respectively, to the 5′ intergenic fragment, and with the TFL13′2.7F and TFL13′2.7R primers, which add SacI/XbaI and EcoRI/KpnI restriction sites, respectively, to the 2722 bp 3′ intergenic fragment (Supplementary Data Table S1). The pBTG6 construct was kindly provided by A. Serrano-Mislata and is identical to pBTG1 except that the TFL1 3′ intergenic region is a 4667 bp fragment with the complete 3′ intergenic region (starting after the stop codon of the TFL1 coding sequence; Supplementary Data Fig. S1; A. Serrano-Mislata and F. Madueño, IBMCP, Valencia, Spain, unpubl. res.). An equivalent TFL1 genomic construct, containing the same 5′ and 3′ intergenic regions as those in pBTG6, fully rescued the tfl1-1 mutant phenotype (Sohn et al., 2007; Kaufmann et al., 2010).
Mutagenesis, mutant screening and genetic analysis
Seeds from the Ler pBTG1 line were mutagenized with ethyl methanesulfonate (EMS; Weigel and Glazebrook, 2002), and 1850 M2 families, harvested from individual M1, plants were generated. For the mutant screening, 25 plants from each M2 family were grown in the greenhouse. After about 3 weeks of growth, the main inflorescence apices from these M2 plants were dissected and subjected to GUS staining. Plants with alterations in both the TFL1::GUS expression pattern and inflorescence architecture were retained for subsequent analysis.
Mapping and cloning of the moss/ago1-103 mutation
Low-resolution mapping of the moss mutation was performed as previously described (Ponce et al., 2006). In brief, the DNA of 50 F2 phenotypically mutant plants was individually extracted and used as a template to multiplex PCR co-amplify 32 simple sequence length polymorphism (SSLP) and insertion/deletion (In/Del) molecular markers using fluorescently labelled oligonucleotides as primers. For fine mapping, 421 F2 plants were used to assess iteratively linkage between moss and molecular markers designed according to the polymorphisms between Ler and Col-0 described at the Monsanto Arabidopsis Polymorphism Collection database (http://www.arabidopsis.org).
For sequencing of the moss allele, PCR products spanning the AGO1 transcriptional units were obtained using the oligonucleotide primers described in Jover-Gil et al. (2012) and as templates genomic DNA from Ler and from three different moss mutant plants. The sequences of the AGO1 gene obtained from the three moss plants were identical.
Genetic combinations
Homozygous single mutants were cross-fertilized, and double mutants were identified among the F2 segregants by novel phenotypes and confirmed by genotyping and/or by segregation of F3 progeny. Genotyping of the moss/ago1-103 mutation was performed with a derived cleaved amplified polymorphic sequence (dCAPS) marker with the primers dCAPmoss-Fw and dCAPmoss-Rv (see Supplementary Data Table S1), which, when followed by digestion with XbaI, generated a single fragment of 237 bp in the wild-type allele or two fragments of 210 and 27 bp in the mutant. Genotyping of the ago1-52 mutation (Jover-Gil et al., 2012) was performed by sequencing the genomic region amplified by primers AGOF8 and AGOR6 (Supplementary Data Table S1). Genotyping of the ago1-26 mutation (Morel et al., 2002) was performed by sequencing the genomic region amplified by primers AGOF1 and AGOR8 (Supplementary Data Table S1).
Histochemical detection detection of GUS activity
Tissue samples from Figs 1 and 4 were incubated for 8 h at 37 °C with medium astringency staining buffer (50 mm sodium phosphate, 2 mm ferrocyanide, 2 mm ferricyanide, 0·2 % Triton X-100 and 1 mm X-Gluc). Samples from Fig. 2 were incubated for 4 h at 37 °C with astringency staining buffer (50 mm sodium phosphate, 10 mm ferrocyanide, 10 mm ferricyanide, 0·2 % Triton X-100 and 1 mm X-Gluc). Following staining, plant material was incubated for 30 min in fixation solution [50 % ethanol (v/v), 10 % acetic acid (v/v), 5 % formaldehyde (w/v)], and cleared in chloralhydrate solution [72 % chloralhydrate (v/v) and 11 % glycerol (w/v)] All the plants used in the assays were homozygous for the TFL1::GUS reporter transgene.
Scanning electron microscopy (SEM)
Samples were vacuum infiltrated with 4 % formaldehyde (w/v) in 1× phosphate-buffered saline (PBS) for 10 min and fixed with fresh solution for 16 h at 4 °C. Samples were dehydrated in an ethanol series and critical point dried in liquid CO2 (Polaron E300 apparatus). Dried samples were mounted on stubs. Then, samples were coated with gold-palladium (4:1) in a Sputter Coater SCD005 (Baltec). Scanning electron microscopy was performed with a Jeol JSM-5410 microscope (10 kV).
Expression analyses
For quantitative PCR (RT-qPCR), 2000ng of total RNA, extracted with the RNeasy Plant Mini Kit (Qiagen), were treated with DNase (DNA-free kit, AmbioN) and used for cDNA synthesis, performed with the SuperScriptII cDNA synthesis kit (Invitrogen). The qPCR mix was prepared in a final volume of 20 μL containing 1200 ng of the cDNA, 1× Power SYBR Green master mix (Applied Biosystems) and 300 nm primers. Primers used to amplify the GUS and TFL1 cDNAs were: GUS-Fw and GUS-Rv for GUS, and TFL1-Fw and TFL1-Rv for TFL1 (Supplementary Data Table S1). Results were normalized to the expression of the UBQ10 gene (Czechowski et al., 2005), with the primers UBQ-Fw and UBQ-Rv (Supplementary Data Table S1). The PCRs were run and analysed using the ABI PRISM 7700 (Applied Biosystems) sequence detection system. The obtained data were treated according to Schmittgen and Livak (2008).
Sequence analysis
The search for miRNA target sites in the TFL1 mRNA sequence was carried out using different online databases (Griffiths-Jones, 2004; Rusinov et al., 2005; Griffiths-Jones et al., 2006, 2008; Kozomara and Griffiths-Jones, 2014).
RESULTS
Isolation of the arabidopis moss mutant
To identify regulators of the expression of the arabidopsis TFL1 gene, we screened an M2 mutant population derived from Ler pBTG1 M1 plants treated with EMS. The Ler pBTG1 line was generated by transformation of Ler with a TFL1::GUS reporter construct where the GUS gene is flanked by the complete 5′ intergenic region (approx. 2·2 kb) and approx. 2·7 kb of the 3′ intergenic region of TFL1 (Supplementary Data Fig. S1). In Ler pBTG1, GUS expression essentially reproduces the expression pattern of the endogenous TFL1 gene. In Ler pBTG1 bolting inflorescences, a strong GUS signal was detected in the apical inflorescence meristem, and in young coflorescence buds, but it was absent from flowers (Fig. 1A). Because TFL1 is a regulator of plant and inflorescence architecture, we selected plants exhibiting both an altered TFL1::GUS pattern and altered plant architecture.
In the screening, we identified an M2 family including mutant plants with a phenotype characterized by abnormal TFL1::GUS expression, which was strong in the inflorescence stem and ectopic in flowers, and also by a dramatically modified plant and inflorescence architecture (Fig. 1B, D, E). These plants were sterile, and in the progeny of some M2 fertile siblings from that family the phenotype segregated in a 1:3 proportion, indicating that this was due to recessive mutation(s) in a single locus. These mutant plants were very small (Fig 1D), with narrow leaves that apparently lacked their petioles (Fig. 1D, E) and a dwarf and compact inflorescence with minute, apparently filamentous, flowers (Fig. 1D, E). Due to their tiny size and the delicate, filamentous appearance of their inflorescences, we named this mutant moss.
The moss mutant has dramatic defects in the architecture of its inflorescence
We analysed in more detail the mutant inflorescence phenotype of moss plants (BC3 line) after being backcrossed three times to Ler. We observed that the dwarf phenotype of the moss plants was partly due to lack of elongation of the internodes between flowers. This caused a dramatic change in the architecture of its inflorescences, which were very compact and resembled an umbel rather than a raceme, the typical inflorescence of wild-type arabidopsis (Fig. 1C, E) (Weberling, 1992; Benlloch et al., 2007).
In the moss inflorescences, filamentous organs were seen in the inflorescence stem and in floral pedicels (Fig. 1E, H). Because they were green and frequently located at the base of the lateral inflorescences and of flowers, they might represent modified cauline leaves and bracts. Flowers of the moss mutant also exhibited severe alterations (Fig. 1G, H). The number of floral organs was reduced, petals and stamens were replaced by filamentous organs, and carpels were unfused; moss flowers were, consequently, sterile.
Finally, a conspicuous inflorescence phenotype in the moss plants was an increased number of coflorescences. The number of rosette leaves of moss mutant plants was essentially the same as in the wild-type plants, but the number of coflorescences produced by moss plants was about 2-fold more than that of the wild type (Table 1; Fig. 1E, F).
Table 1.
Plant architecture of mutants
| Genotype | Rosette leaves* | Cauline leaves† | Total leaves‡ | Coflorescences§ | Axillary flowers¶ | Terminal flower** |
|---|---|---|---|---|---|---|
| Ler | 6·73 ± 0·23 | 2·93 ± 0·12 | 9·67 ± 0·21 | 2·90 ± 0·12 | ND | – |
| moss | 6·27 ± 0·17 | 3·07 ± 0·38†† | 9·33 ± 0·46 | 5·91 ± 0·34 | ND | – |
| tfl1-2 | 5·60 ± 0·13 | 2·33 ± 0·19 | 7·93 ± 0·15 | 0·20 ± 0·14 | 2·13 ± 0·17 | + |
| moss tfl1-2 | 6·27 ± 0·45 | 0·73 ± 0·15 | 7·00 ± 0·50 | 0·13 ± 0·09 | 0·73 ± 0·15‡‡ | + |
| Col | 8·6 ± 016 | 2·4 ± 016 | 11·0 ± 021 | 2·4 ± 016 | ND | – |
| ago1-26 | 8·9 ± 028 | 7·4 ± 037 | 16·3 ± 0·6 | 7·4 ± 037 | ND | – |
| tfl1-1 | 5·80 ± 0·31 | 1·80 ± 0·15 | 7·60 ± 0·38 | 0·00 ± 0·00 | 1·80 ± 0·15 | + |
| ago1-26 tlf1-1 | 4·56 ± 0·29 | 3·67 ± 0·58 | 8·22 ± 0·83 | 0·56 ± 0·29 | 3·11 ± 0·61 | + |
Data are means ± standard error (n ≥ 12).
ND, not detected.
*Number of rosette leaves.
†Number of cauline leaves in the main inflorescence stem.
‡Number of rosette leaves + cauline leaves.
§Number of coflorescences in the main inflorescence stem.
¶Number of axillary flowers in the main inflorescence stem.
**Presence (+) or absence (−) of a terminal flower at the inflorescence apex.
††The number of cauline leaves in moss mutant genotypes might be underestimated, because, apparently, some of them are were transformed into filaments.
‡‡Because the number of cauline leaves in moss mutant genotypes might be underestimated, the number of axillary flowers in moss tfl1-2 might be underestimated.
The moss mutant has increased and ectopic expression of TFL1
As explained above, besides its defects in plant architecture, the moss mutant was selected because of its increased and ectopic expression of the TFL1::GUS reporter, which prompted us to study TFL1 expression in more detail in the moss mutant.
We analysed the expression of TFL1::GUS in the shoot apex during development (Fig. 2A). The analysis was performed with plants derived from the moss BC3 line that had lost the original pBTG1 reporter and where the reporter pBTG6 was introduced. The pBTG6 reporter construct is essentially identical to pBTG1 but contains the complete TFL1 3′ intergenic region (approx. 3·7 kb; Supplementary Data Fig. S1) and, thus, it reproduces the expression of the endogenous TFL1 gene more accurately. In moss pBTG6 plants, the expression of TFL1::GUS was stronger in the SAM both in the vegetative and in the inflorescence phases, becoming ectopic in the flowers of the mutant in the adult inflorescence (Fig. 2A).
We also analysed by RT-qPCR the expression of the endogenous TFL1 gene in adult inflorescence apices. The level of TFL1 mRNA was clearly higher in moss than in the wild type (Fig. 2B), indicating that the altered TFL1::GUS expression observed indeed reflects a stronger expression of the endogenous TFL1 gene in the mutant.
In F2 plants derived from backcrosses of moss to the parental wild type, all the plants with altered TFL1::GUS expression showed the characteristic moss plant architecture. This indicates that the plant architecture defects and the altered TFL1 expression are two aspects of the pleiotropic phenotype caused by the moss mutation and suggests that the gene mutated in moss regulates both TFL1 expression and plant architecture.
The moss phenotype is caused by a hypomorphic mutation in AGO1
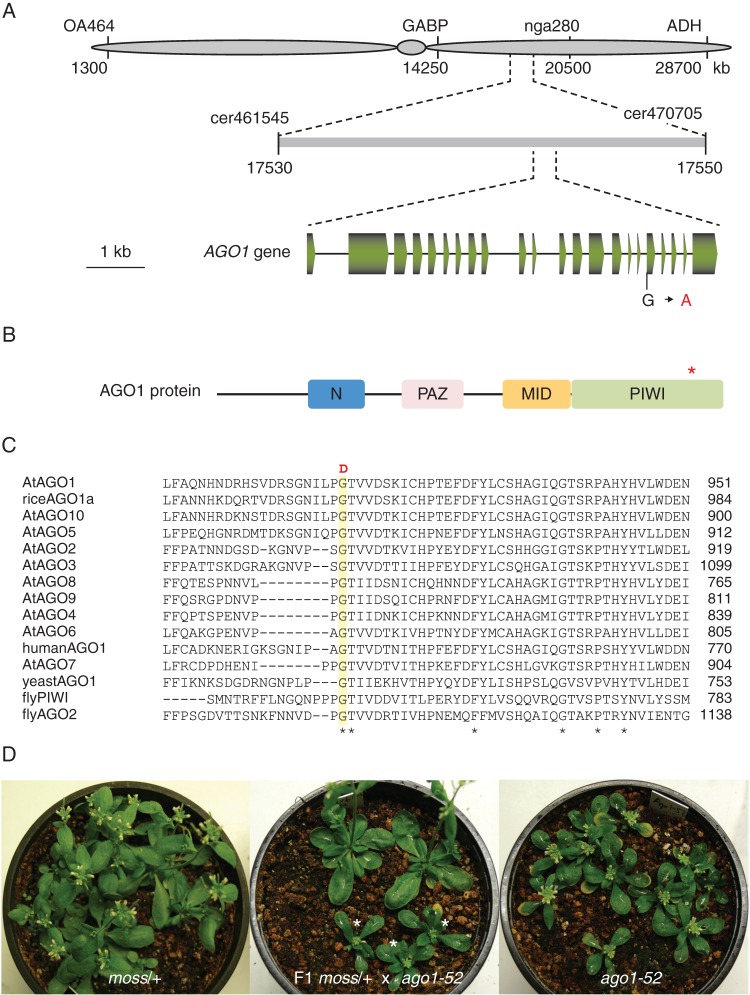
We followed a positional cloning approach to identify the gene responsible for the moss plant architecture mutant phenotype. The moss mutation was mapped to a 16 kb candidate region of chromosome 1 (Fig. 3A). This region encompass 26 annotated genes; one of these genes is AGO1, which encodes a pivotal component of the RNA silencing machinery (Vaucheret et al., 2004; Baumberger and Baulcombe, 2005; Qi et al., 2005). We observed that the inflorescence of the moss mutant resembled that of an ago1 fil yab triple mutant previously reported (Yang et al., 2006), which suggested that the moss mutation could map to the AGO1 gene. Sequencing of the AGO1 gene from the moss mutant identified a point mutation in the first nucleotide of the 18nth exon (G to A) that causes the replacement of a highly conserved glycine by aspartate in the PIWI domain of the AGO1 protein (Fig. 3B, C); the PIWI domain is required for the cleavage of target mRNAs (Cerutti et al., 2000; Liu et al., 2004; Song et al., 2004).
Fig. 3.
Cloning of the moss mutation. (A) Positional cloning of the moss mutation. Some of the markers used for linkage analysis are shown. (B) Schematic representation of the structure of the AGO1 protein, with indication of the N, PAZ, MID and PIWI domains. An asterisk indicates the position of the amino acid affected by the moss mutation. (C) Multiple alignment of the region of the arabidopsis AGO1 protein [AtAGO1 (O04379)] affected by the moss mutation with those of other AGO family members from Arabidopsis thaliana [AtAGO2 (Q9SHF3), AtAGO3 (Q9SHF2), AtAGO4 (Q9ZVD5), AtAGO5 (Q9ZVD5), AtAGO6 (O48771), AtAGO6 (Q9C793), AtAGO8 (Q3E984), AtAGO9 (Q84VQ0) and AtAGO10 (9XGW1)], Oryza sativa [riceAGO1a (Q6EU14)], Homo sapiens [humanAGO1 (Q9UL18)], Saccharomyces pombe [yeastAGO1 (O74957)] and Drosophila melanoganster [flyPIWI (Q9VKM1) and flyAGO2 (Q9VUQ5)]. Amino acid residues conserved in all the sequences are marked with an asterisk. The residue changed by the mutation in the moss mutant (G-D), shaded in yellow, is conserved in all the protein sequences. (D) moss/AGO1 heterozygotes (left), ago1-52/ago1-52 homozygotes (right) and their F1 progeny (centre), among which moss/ago1-52 heterozygotes segregated in a 1:1 (phenotypically mutant:wild-type) ratio. The latter was considered evidence of allelism.
To test whether this mutation in the AGO1 gene was responsible for the moss plant architecture phenotype, we performed an allelism test by crossing heterozygous moss/+ plants with plants homozygous for ago-52, a hypomorphic mutation causing a phenotype less severe than that of the moss mutant (Jover-Gil et al., 2012). One half of the resulting F1 progeny exhibited a wild-type phenotype (21 out of 45 F1 plants analysed), whereas the rest of the plants had a phenotype similar to that of the homozygous ago1-52 parent (Fig. 3D). This result demonstrates that the moss mutation and ago1-52 are allelic and that the architecture phenotype of moss plants is caused by the mutation in AGO1. Therefore, the moss mutant was called ago1-103 and hereafter we refer to moss as moss/ago1-103.
Reduction of AGO1 function causes ectopic expression of TFL1
To address whether the phenotype of increased ectopic expression of TFL1 observed in the moss/ago1-103 mutant was also due to the mutation in AGO1, we tested whether TFL1 expression was also altered in other ago1 mutants.
We studied ago1-26, another hypomorphic ago1 allele with a mutation causing the substitution of a conserved amino acid also in the PIWI domain (Morel et al., 202). The ago1-26 mutant exhibited a morphological phenotype that included: slight reduction in plant size; alteration in the shape of leaves, which had serrated blade margins and were narrow and apparently without petioles (Figs 4A, B and 5E, G); and flowers with reduced fertility, although with apparently normal organs. All these traits resembled those of moss/ago1-103, although they were milder in severity. In contrast to moss/ago1-103, in the inflorescences of ago1-26 mutant plants cauline leaves were not transformed into filaments, and flowers were separated by elongated internodes (Figs 4B and 5G). Nevertheless, ago1-26 plants had a larger number of coflorescences than its wild-type parent (Col) although it produced a similar number of rosette leaves (Table 1; Figs. 4A, B and 5E, G). This aspect of the ago1-26 inflorescence phenotype is similar to that of moss/ago1-103, which indicates that an increase in the number of coflorescences is indeed a characteristic trait of ago1 mutants.
To analyse the effect of the ago1-26 mutation on TFL1 expression, we introgressed the TFL1::GUS construct pBTG6 into ago1-26. As seen in moss/ago1-103, ectopic TFL1::GUS expression was observed in ago1-26 flowers (Fig. 4C, D).
Our observation that two independent ago1 mutants exhibit proliferation of coflorescences and ectopic TFL1 expression indicates that both traits are caused by the ago1 mutations and that AGO1 regulates both the expression of TFL1 and inflorescence architecture.
Proliferation of coflorescences in ago mutants is mediated by TFL1
It has been previously shown that high constitutive expression of TFL1 in 35S::TFL1 arabidopsis plants leads to a strong increase in the number of coflorescences (Ratcliffe et al., 1998). Therefore, the proliferation of coflorescences observed in the moss/ago1-103 and ago1-26 mutants might be due to their increased ectopic TFL1 expression. To assess this hypothesis, we generated moss/ago1-103 tfl1-2 and ago1-26 tfl1-1 double mutants, to test whether the lack of TFL1 function led to a reduction in the number of coflorescences in the ago1 mutants. Other aspects of the ago1 mutant phenotype, such as the floral defects or the lack of internode elongation between flowers (in moss/ago1-103), were not suppressed in the ago1 tfl1 double mutants.
The tfl1 mutations partly suppressed the inflorescence architecture observed in ago1 mutants; a drastic decrease in the number of vegetative leaves and coflorescences was observed in moss/ago1-103 tfl1-2 and ago1-26 tfl1-1 compared with their ago1 parents (Table 1; Fig. 5).
These results show that TFL1 mediates the increased production of coflorescences of ago1 mutants and indicate that AGO1 controls inflorescence architecture in part through the regulation of TFL1 expression.
DISCUSSION
In this paper we describe our work to identify regulators of TFL1 through a genetic screening for mutants with altered TFL1 expression. Our results show that AGO1 acts as a repressor of TFL1 expression and reveal a novel role for AGO1 as a regulator of inflorescence architecture. Our study provides evidence supporting that AGO1 contributes to the control of inflorescence architecture through its activity as a regulator of TFL1 expression.
Several studies involving the characterization of ago1 mutants have shown that AGO1 participates in the regulation of key developmental processes in arabidopsis (Jover-Gil et al., 2005; Kidner and Martienssen, 2005b; Zhang and Zhang, 2012). A large number of independent ago1 mutants have been described that show diverse phenotypes, ranging from hardly viable null alleles causing severe developmental defects (Bohmert et al., 1998; Kidner and Martienssen, 2004), to fully fertile hypomorphic alleles with only mild defects (Morel et al., 2002; Jover-Gil et al., 2012). Nevertheless, essentially all ago1 mutants show pleitropic phenotypes, which is consistent with the central role of AGO1 in miRNA-mediated gene silencing, a process that regulates the expression of a large number of genes (Vaucheret et al., 2004). Though moss/ago1-103 is a hypomorphic mutation that still allows development of a plant with a ‘complete’ inflorescence, the moss/ago1-103 plants show severe developmental defects. This is most probably due to the fact that the moss/ago1-103 mutation causes the substitution of a highly conserved amino acid in the C-terminal region of the AGO1 protein, possibly leading to a protein where the activity of the PIWI domain, required for the cleavage of mRNAs that are targeted by miRNAs (Cerutti et al., 2000; Liu et al., 2004; Song et al., 2004), is severely compromised.
AGO1 is involved in a wide variety of developmental processes. Thus, it participates in the control of general processes that affect the development of all aerial plant organs, such as organ polarity and the functioning of stem cells in the shoot meristems (Kidner et al., 2004), but it is also involved in other more specific processes. For instance, in leaf development, it plays a role in the growth of the lamina and in the venation patterning (Palatnik et al., 2003; Jover-Gil et al., 2012). In reproductive development, AGO1 has been shown to be involved in processes such as meristem identity and in the termination of floral stem cells (Kidner and Martienssen, 2005a; Ji et al., 2011). However, AGO1 does not seem to be involved in other important processes in reproductive development such as, for instance, the floral transition (measured as the number of rosette leaves), as indicated by the study of Kidner and Martienssen (2005a) and by our own results. In most cases, it has been shown that AGO1 affects these processes by regulating the expression of transcription factors that are key developmental regulators (Jover-Gil et al., 2005; Kidner and Martienssen, 2005b; Zhang and Zhang, 2012).
Our study reveals a novel role for AGO1 in reproductive development, controlling inflorescence architecture. Thus, both in moss/ago1-103 and in ago1-26 the first inflorescence phase (I1) is enlarged, with an increase in the number of coflorescences produced. Our results indicate that this phenotype, which resembles the effect of TFL1 overexpression in 35S::TFL1 transgenic plants, with a very enlarged I1 phase, is due to the misexpression of TFL1 in the ago1 mutants. Moreover, suppression of proliferation of coflorescences in ago1 tfl1 double mutants indicates that this proliferation requires the activity of TFL1 and supports that this role of AGO1 in the regulation of inflorescence architecture is based on its activity as a repressor of TFL1 expression.
Though moss/ago1-103 has an increased number of coflorescences, it does produce the same number of rosette leaves as the wild type. This is in contrast to what occurs in 35::TFL1 but it is what is observed in other ago1 mutants (ago1-26, this study; Kidner and Martienssen, 2004). The similar length of the rosette leaves phase in moss/ago1-103 and the wild type might be due to the fact that the level of TFL1 expression in that phase in moss/ago1-103 is quite moderate, while in the early inflorescence apex it is much higher. It does not seem surprising that in the rosette leaves phase the phenotype of moss/ago1-103 differs from that of 35::TFL1, where expression of TFL1 is strong and constitutive.
How does AGO1 repress TFL1 expression? One possibility would be that TFL1 expression was controlled by post-transcriptional gene silencing, the TFL1 transcript being a target of miRNA-guided degradation or translational arrest, mediated by AGO1. This is the case for several key developmental regulators such as some members of the TCP transcription factor family involved in leaf morphology (Palatnik et al., 2003) or the HD-ZIP III transcription factors involved in leaf polarity (Kidner and Martienssen, 2004). However, it seems unlikely that miRNA-mediated gene silencing is the cause of the misregulation of TFL1 expression in the ago1 mutants. Thus, though the TFL1 genomic fragments in pBTG1 (the reporter construct in the line used in the mutagenesis; Supplementary Data Fig. S1) only contain a very small part of the TFL1 transcribed sequence, the 5′UTR, the moss/ago1-103 mutant still showed high ectopic expression of the TFL1::GUS transgene from pBTG1 (Fig. 1B). The 5′UTR of TFL1 is only 45 nucleotides long (A. Serrano-Mislata and F. Madueño, IBMCP, Valencia, Spain, unpubl. res.), and in the analysis of that sequence we did not identify any predicted target site for miRNAs. Therefore, this strongly indicates that AGO1 regulation does not regulate TFL1 expression by directly acting on its transcript
Alternatively, AGO1 might be indirectly involved in the repression of TFL1, acting on other direct regulators of TFL1. The floral identity genes AP1 and LFY encode transcription factors that directly bind to sequences in the regulatory region of TFL1 (Kaufmann et al., 2010; Moyroud et al., 2011; Winter et al., 2011), and are required to repress its expression in the flower (Ratcliffe et al., 1999; Liljegren et al., 1999; Bowman et al., 1993; Ferrándiz et al., 2000; Parcy et al., 2002). The inflorescence phenotype of ago1 mutants somehow resembles that of loss-of-function alleles of AP1 and LFY, which also have an increased number of coflorescences, because the most basal flowers in their inflorescences are replaced by inflorescence-like structures (Schultz and Haugh, 1991; Mandel et al., 1992; Weigel et al., 1992; Bowman et al., 1993). As reported by Kidner and Martienssen (2005a), the expression of AP1 and LFY is downregulated in ago1 mutants. Therefore, a likely possibility is that the low levels of AP1 and LFY in ago1 mutants lead to the increased expression of TFL1, ectopic in flowers, and to the proliferation of coflorescences. Because AP1 and LFY do not seem to be direct targets of miRNA-mediated regulation either, it might possibly be necessary to look for factors upstream of AP1 and LFY to understand fully how AGO1 regulates TFL1 expression.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Hervé Vaucheret for the ago1-26 seeds, Antonio Serrano-Mislata for the pBTG6 construct, and Cristina Ferrándiz for critical reading of the manuscript. The collaboration of the IBMCP staff from the greenhouse, sequencing and microscopy facilities is also acknowledged. This work was supported by grants from the Spanish Ministerio de Ciencia e Innovación (BIO2009-10876 and CSD2007-00057), the Spanish Ministerio de Economía y Competitividad (BFU2012-38929) and the Generalitat Valenciana (ACOMP2012-101). P.F.N. was supported by a fellowship from the I3P program of CSIC.
LITERATURE CITED
- Abe M, Kobayashi Y, Yamamoto S, et al. A bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Miller D, Winter VJ, et al. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO Journal. 2006;25:605–614. doi: 10.1038/sj.emboj.7600950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Guli C, Yu X, Smyth D. TERMINAL FLOWER1: a gene affecting inflorescence development in Arabidopsis thaliana. The Plant Journal. 1992;2:103–116. [Google Scholar]
- Amasino R. Seasonal and developmental timing of flowering. The Plant Journal. 2010;61:1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- Andres F, Coupland G. The genetic basis of flowering responses to seasonal cues. Nature Reviews Genetics. 2012;13:627–639. doi: 10.1038/nrg3291. [DOI] [PubMed] [Google Scholar]
- Baulcombe DC. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Baulcombe DC. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits rnicroRNAs and short interfering RNAs. Proceedings of the National Academy of Sciences; USA. 2005. pp. 11928–11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlloch R, Berbel A, Serrano-Mislata A, Madueno F. Floral initiation and inflorescence architecture: a comparative view. Annals of Botany. 2007;100:659–676. doi: 10.1093/aob/mcm146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Research. 1994;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Ferrándiz C, Madueno F, Parcy F. How floral meristems are built. Plant Molecular Biology. 2006;60:855–870. doi: 10.1007/s11103-006-0013-z. [DOI] [PubMed] [Google Scholar]
- Bohmert K, Camus I, Bellini C, Bouchez D, Caboche M, Benning C. AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO Journal. 1998;17:170–180. doi: 10.1093/emboj/17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J, Alvarez J, Weigel D, Meyerowitz E. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development. 1993;119:721–743. [Google Scholar]
- Bowman J. Arabidopsis: an atlas of morphology and development. New York: Springer-Verlag; 1994. [Google Scholar]
- Bradley DJ, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence commitment and architecture in Arabidopsis. Science. 1997;275:80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- Brodersen, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nature Reviews Molecular Cell Biology. 2009;10:141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti L, Mian N, Bateman A. Domains in gene silencing and cell differentiation proteins: the novel PAZ domain and redefinition of the Piwi domain. Trends in Biochemical Sciences. 2000;25:481–482. doi: 10.1016/s0968-0004(00)01641-8. [DOI] [PubMed] [Google Scholar]
- Conti L, Bradley DJ. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. The Plant Cell. 2007;19:767–778. doi: 10.1105/tpc.106.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrándiz C, Gu Q, Martienssen RA, Yanofsky MF. Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development. 2000;127:725–734. doi: 10.1242/dev.127.4.725. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S. The microRNA Registry. Nucleic Acids Research. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Research. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Research. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano S, Goto K. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. The Plant Cell. 2011;23:3172–3184. doi: 10.1105/tpc.111.088641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzawa Y, Money T, Bradley D. A single amino acid converts a repressor to an activator of flowering. Proceedings of the National Academy of Sciences; USA. 2005. pp. 7748–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WW, Weigel D. Structural features determining flower-promoting activity of Arabidopsis FLOWERING LOCUS T. The Plant Cell. 2014;26:552–564. doi: 10.1105/tpc.113.115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser P, Schmid M. The control of developmental phase transitions in plants. Development. 2011;138:4117–4129. doi: 10.1242/dev.063511. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Liu X, Yan J, et al. ARGONAUTE10 and ARGONAUTE1 regulate the termination of floral stem cells through two microRNAs in arabidopsis. PLoS Genetics. 2011;7:e1001358. doi: 10.1371/journal.pgen.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover-Gil S, Candela H, Ponce MR. Plant microRNAs and development. International Journal of Developmental Biology. 2005;49:733–744. doi: 10.1387/ijdb.052015sj. [DOI] [PubMed] [Google Scholar]
- Jover-Gil S, Candela H, Robles P, et al. The microRNA pathway genes AGO1, HEN1 and HYL1 participate in leaf proximal–distal, venation and stomatal patterning in Arabidopsis. Plant and Cell Physiology. 2012;53:1322–1333. doi: 10.1093/pcp/pcs077. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Wellmer F, Muiño JM, et al. Orchestration of floral initiation by APETALA1. Science. 2010;328:85–89. doi: 10.1126/science.1185244. [DOI] [PubMed] [Google Scholar]
- Kidner CA, Martienssen RA. Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature. 2004;428:81–84. doi: 10.1038/nature02366. [DOI] [PubMed] [Google Scholar]
- Kidner CA, Martienssen RA. The role of ARGONAUTE1 (AGO1) in meristem formation and identity. Developmental Biology. 2005a;280:504–517. doi: 10.1016/j.ydbio.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Kidner CA, Martienssen RA. The developmental role of microRNA in plants. Current Opinion in Plant Biology. 2005b;8:38–44. doi: 10.1016/j.pbi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Research. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta G, Yanofsky MF. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. The Plant Cell. 1999;11:1007–1018. doi: 10.1105/tpc.11.6.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Teo ZWN, Bi Y, et al. A conserved genetic pathway determines inflorescence architecture in Arabidopsis and rice. Developmental Cell. 2013;24:612–622. doi: 10.1016/j.devcel.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature. 1992;360:273–277. doi: 10.1038/360273a0. [DOI] [PubMed] [Google Scholar]
- Morel J-B, Godon C, Mourrain P, et al. Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. The Plant Cell. 2002;14:629–639. doi: 10.1105/tpc.010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyroud E, Minguet EG, Ott F, et al. Prediction of regulatory interactions from genome sequences using a biophysical model for the Arabidopsis LEAFY transcription factor. The Plant Cell. 2011;23:1293–1306. doi: 10.1105/tpc.111.083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik J, Allen E, Wu X, et al. Control of leaf morphogenesis by microRNAs. Nature. 2003;425:257–263. doi: 10.1038/nature01958. [DOI] [PubMed] [Google Scholar]
- Parcy F, Bomblies K, Weigel D. Interaction of LEAFY, AGAMOUS and TERMINAL FLOWER1 in maintaining floral meristem identity in Arabidopsis. Development. 2002;129:2519–2527. doi: 10.1242/dev.129.10.2519. [DOI] [PubMed] [Google Scholar]
- Ponce MR, Robles P, Lozano FM, Brotóns MA, Micol JL. Low-resolution mapping of untagged mutations. Methods in Molecular Biology. 2006;323:105–113. doi: 10.1385/1-59745-003-0:105. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E. Evolution and development of inflorescence architectures. Science. 2007;316:1452–1456. doi: 10.1126/science.1140429. [DOI] [PubMed] [Google Scholar]
- Qi Y, Denli AM, Hannon GJ. Biochemical specialization within Arabidopsis RNA silencing pathways. Molecular Cell. 2005;19:421–428. doi: 10.1016/j.molcel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, et al. A common mechanism controls the life cycle and architecture of plants. Development. 1998;125:1609–1615. doi: 10.1242/dev.125.9.1609. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Bradley DJ, Coen E. Separation of shoot and floral identity in Arabidopsis. Development. 1999;126:1109–1120. doi: 10.1242/dev.126.6.1109. [DOI] [PubMed] [Google Scholar]
- Rusinov V, Baev V, Minkov IN, Tabler M. MicroInspector: a web tool for detection of miRNA binding sites in an RNA sequence. Nucleic Acids Research. 2005;33:696–700. doi: 10.1093/nar/gki364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schultz E, Haughn GW. LEAFY, a homeotic gene that regulates inflorescence development in Arabidopsis. The Plant Cell. 1991;3:771–781. doi: 10.1105/tpc.3.8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner D. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. The Plant Cell. 1991;3:877–892. doi: 10.1105/tpc.3.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner D. Genetic interactions that regulate inflorescence development in Arabidopsis. The Plant Cell. 1993;5:639–655. doi: 10.1105/tpc.5.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Willmann MR, Wu G, et al. Cyclophilin 40 is required for microRNA activity in Arabidopsis. Proceedings of the National Academy of Sciences, USA; 2009. pp. 5424–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EJ, Rojas-Pierce M, Pan S, et al. The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proceedings of the National Academy of Sciences, USA; 2007. pp. 18801–18806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-J, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- Teo ZWN, Song S, Wang Y-Q, Liu J, Yu H. New insights into the regulation of inflorescence architecture. Trends in Plant Science. 2013;19:158–165. doi: 10.1016/j.tplants.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Vaucheret H. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes and Development. 2006;20:759–771. doi: 10.1101/gad.1410506. [DOI] [PubMed] [Google Scholar]
- Vaucheret H, Vazquez F, Crété P, Bartel DP. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes and Development. 2004;18:1187–1197. doi: 10.1101/gad.1201404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weberling F. Morphology of flowers and inflorescences. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Weigel D, Glazebrook J. Arabidopsis: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- Weigel D, Alvarez JP, Smyth DR, Yanofsky MF, Meyerowitz EM. LEAFY controls floral meristem identity in Arabidopsis. Cell. 1992;69:843–859. doi: 10.1016/0092-8674(92)90295-n. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, et al. Integration of spatial and temporal information during floral induction in arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- Winter CM, Austin RS, Blanvillain-Baufumé S, et al. LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Developmental Cell. 2011;20:430–443. doi: 10.1016/j.devcel.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Wolpert L, Tickle C. Principles of development. Oxford: Oxford University Press; 2010. [Google Scholar]
- Yang L, Wang H, Xu Y, Huang H. Characterizations of a hypomorphic argonaute1 mutant reveal novel AGO1 functions in Arabidopsis lateral organ development. Plant Molecular Biology. 2006;61:63–78. doi: 10.1007/s11103-005-5992-7. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang X. Argonautes compete for miR165/166 to regulate shoot apical meristem development. Current Opinion in Plant Biology. 2012;15:1–7. doi: 10.1016/j.pbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.