Abstract
Objective
To test the hypothesis that a breakfast meal with high carbohydrate/ low fat results in an earlier increase in postprandial glucose and insulin, a greater decrease below baseline in postprandial glucose, and an earlier return of appetite, compared to a low carbohydrate/high fat meal.
Design
Overweight but otherwise healthy adults (n=64) were maintained on one of two eucaloric diets: high carbohydrate/low fat (HC/LF; 55:27:18% kcals from carbohydrate: fat: protein) versus low carbohydrate/high fat (LC/HF; 43:39:18% kcals from carbohydrate: fat: protein). After 4 weeks of acclimation to the diets, participants underwent a meal test during which circulating glucose and insulin and self-reported hunger and fullness, were measured before and after consumption of breakfast from their assigned diets.
Results
The LC/HF meal resulted in a later time at the highest and lowest recorded glucose, higher glucose concentrations at 3 and 4 hours post-meal, and lower insulin incremental area under the curve. Participants consuming the LC/HF meal reported lower appetite 3 and 4 hours following the meal, a response that was associated with the timing of the highest and lowest recorded glucose.
Conclusions
Modest increases in meal carbohydrate content at the expense of fat content may facilitate weight gain over the long-term by contributing to an earlier rise and fall of postprandial glucose concentrations and an earlier return of appetite.
Keywords: Satiety, food intake, insulin, diet intervention
Excess energy intake has contributed to the obesity epidemic. During the last 30 years adults and children in the United States (US) have increased the frequency with which they consume food (Popkin & Duffey, 2010). This change has paralleled an increase in the proportion of carbohydrate and a reduction in the proportion of fat in the typical US diet (Briefel & Johnson, 2004; Marriott, Cole, & Lee, 2009). It is possible that meals with higher carbohydrate content or more refined sugars result in greater hunger or an earlier return of hunger (Fajcsak, Gabor, Kovacs, & Martos, 2008), and consequently contribute to excess intake and greater weight gain, but results have not been consistent (Clegg & Shafat, 2010; Raben, Agerholm-Larsen, Flint, Holst, & Astrup, 2003; Rolls, Roe, & Meengs, 2004).
Meals with higher carbohydrate content, especially those with more simple carbohydrates, render a greater acute rise and fall in circulating glucose (Brand-Miller, Stockmann, Atkinson, Petocz, & Denyer, 2009; Coulston, Liu, & Reaven, 1983; Galgani, Aguirre, & Díaz, 2006; Hertzler & Kim, 2003; Mayer, 1953; Nilsson, Ostman, Granfeldt, & Björck, 2008; Reynolds, Stockmann, Atkinson, Denyer, & Brand-Miller, 2009; Wolever, Yang, Zeng, Atkinson, & Brand-Miller, 2006), increase cerebral blood flow to areas of the brain responsible for reward and cravings (Lennerz et al., 2013), and influence the return of hunger and subsequent caloric intake (Page et al., 2011). Due to tight homeostatic control of circulating glucose, elevations above basal invoke a number of processes designed to reduce glucose, including mass action effects, insulin-stimulated disposal, and insulin inhibition of hepatic glucose production. These combined effects contribute to a subsequent drop in circulating glucose, potentially below basal concentrations (Bray, 1996). Although this reduction in glucose concentration was initially thought to signal hunger (Mayer, 1953), our current understanding is that the association between glucose and hunger is more complex, with dynamic changes in glucose potentially being of more importance than absolute concentrations. In the current study we will examine which of the multiple parameters describing postprandial glycemia are associated with differences in the return of appetite following a high carbohydrate/low fat (HC/LF) versus a low carbohydrate/high fat (LC/HF) meal.
Insulin plays an important role in regulating circulating glucose, and consequently, it too exhibits a dynamic postprandial response to the macronutrient composition of the meal. Insulin concentrations are higher following a high carbohydrate meal as compared to a lower carbohydrate meal (Barkoukis et al., 2007; Galgani et al., 2006; Reynolds et al., 2009). Although insulin promotes satiety in rodents through direct action on the brain, its glucose-lowering effects in humans may contribute to an apparent inverse association between peripheral insulin concentrations and satiety. Consistent with this, a positive association between insulin and hunger, independent of glucose concentrations, has been shown in humans (Rodin, Wack, Ferrannini, & DeFronzo, 1985). Other studies examining the role of insulin in the regulation of hunger and fullness however, have yielded equivocal results (Flint et al., 2007; Holt, Brand Miller, & Petocz, 1996; Holt & Miller, 1995; Rodin et al., 1985). Whether differences in circulating insulin, independent of glucose, following meals varying in carbohydrate and fat content contribute to differences in self-reported appetite is not known.
The overall objective of this study was to examine the effect of eucaloric HC/LF versus LC/HF breakfast meals on postprandial glucose, insulin, and self-reported appetite, among individuals maintained on these respective diets. We hypothesized that postprandial glucose and insulin concentrations would be more stable following the LC/HF meal, with excursions above and below basal of lesser magnitude, contributing to prolonged fullness following the LC/HF meal compared to the HC/LF meal. Specifically, we hypothesized that the HC/LF meal would result in an earlier increase in postprandial glucose and insulin, a greater subsequent decrease below baseline in postprandial glucose, and an earlier return of appetite, compared to a breakfast meal from a LC/HF diet. A secondary objective was to identify elements of the postprandial glucose and insulin profiles (e.g. time until highest and lowest recorded glucose, concentration at peak and nadir, rate of change, etc) that were associated with self-reported appetite following the HC/LF versus LC/HF meal.
Materials and Methods
Participants
Participants were healthy, overweight males and females aged 21–50 years. Exclusion criteria were type 1 or type 2 diabetes, polycystic ovary disease, BMI <26.5 or weight >300 pounds, weight change >5 pounds in last 6 months, regular exercise >2 hours per week, pregnancy, current breastfeeding, cholesterol medications, any diagnosed disorders of glucose or lipid metabolism, use of medication that could affect body composition or glucose metabolism (including oral contraceptives and blood pressure medications), current use of tobacco, use of illegal drugs in last 6 months, history of hypoglycemic episodes, major food allergies or food dislikes, women with inconsistent or absent monthly menstrual cycles, and a medical history that counter-indicated inclusion in the study. Participants were evaluated for glucose tolerance using a 2-h oral glucose tolerance test, and only those who had 2-h glucose in the normal or mildly impaired range (≤155 mg/dL) were eligible for the study. Participants were informed of the experimental design, and oral and written consent were obtained. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB).
Procedure
After enrollment into the study, participants were assigned to one of two diets (described below), on which they remained for the duration of the study (8 weeks). Details of the overall intervention have previously been published (Goss, Goree, et al., 2013). In brief, all food was provided by the General Clinical Research Center (GCRC) and participants presented to the GCRC each weekday morning to be weighed, to eat breakfast from their assigned diet, and to collect their food for the remainder of the day. On Fridays, sufficient food for the entire weekend was provided. Energy needs were estimated by the GCRC Research Dietitian using the Harris Benedict equations for males and females (with an activity factor of 1.5 for males and 1.35 for females). Energy provided to each individual was adjusted if necessary in order to maintain a stable body weight. For the purposes of this sub-study, a breakfast meal test was administered to each participant following 4 weeks of the eucaloric diet intervention, in order to examine their postprandial glucose and insulin response, along with the return of appetite following breakfast from their usual diet.
Diets
The HC/LF diet consisted of 55% calories from carbohydrates, 18% calories from protein, and 27% calories from fat (with <10% saturated fat). The LC/HF diet consisted of 43% calories from carbohydrates, 18% calories from protein, and 39% calories from fat (with <13% saturated fat). The average glycemic load was ≥75 points/1000 calories for the HC/LF diet and ≤45 points/1000 calories for the LC/HF diet, as analyzed by Minnesota Nutrition Data System for Research (NDSR) using glucose as reference. An 8-day menu rotation was used for each participant.
Breakfast Meal Test
Participants presented to the GCRC for their usual breakfast following an overnight fast. The food consumed during the test was the specific breakfast meal provided for that day for each participant, according to their group assignment (i.e. HC/LF or LC/HF). Examples of breakfast meal items for the HC/LF group included cereals such as Rice Krispies, Frosted Flakes, Special K, and pancakes or waffles with syrup. Examples of breakfast items for the LC/HF group included oatmeal, rye bread, English muffins, and Pillsbury Toaster Scramblers®. Blood samples were collected prior to and during the breakfast meal test via a flexible intravenous catheter which was placed in the antecubital space of the left arm. At time “zero”, participants began to consume breakfast and were required to do so within 20 minutes. Blood samples were collected at baseline (2 samples), and at times 15, 60, 90, 120, 180, and 240 minutes. Serum were separated and stored at −85° C until assay. Samples were analyzed for insulin and glucose.
Appetite
Perceived hunger and fullness were assessed by self-report (adapted from (Flint, Raben, Blundell, & Astrup, 2000; Stock et al., 2005)) during the breakfast meal test. Participants responded to each of the two questions prior to breakfast and at 15, 60, 90, 120, 180, and 240 minutes after each meal by marking a visual analog scale (VAS) ranging from zero mm (lowest) to 100 mm (highest).
Hormone analysis
Concentrations of glucose and insulin were analyzed in the Core Laboratory of the GCRC, Diabetes Research and Training Center (DRTC), and Nutrition Obesity Research Center (NORC). Glucose was measured in 3 µL sera using a glucose oxidase method (Stanbio Sirrus analyzer; Stanbio Laboratory, Boerne, TX). This analysis had a mean intra-assay coefficient of variation (CV) of 1.21%, and a mean inter-assay CV of 3.065%. Insulin was assayed in 50 µL aliquots with immunofluorescence (TOSOH AIA-II analyzer, TOSOH Corporation, South San Francisco, CA; mean inter-assay CV 4.42%; mean intra-assay CV 1.49%).
Statistical analysis
To assess the overall group difference in glucose and insulin response to the meal test, incremental area under the curve (iAUC) for glucose and insulin was calculated using the trapezoidal method, and independent groups’ t-tests were performed. T-tests were also used to compare other characteristics of the postprandial glucose and insulin curves such as highest and lowest recorded glucose and insulin (i.e. peak and nadir), time (minutes) at recorded highest and lowest glucose and insulin, and the rate of decline in glucose (calculated as the slope of time versus glucose concentration from recorded peak to nadir for each individual), after adjustment for fasting concentrations as appropriate. Given that the purpose of this study was to specifically focus on the return of appetite following the meal, independent groups t-tests were used to compare glucose, insulin, hunger, and fullness ratings at three and four hours after the breakfast meals.
Given the large number of variables used to characterize the shape of the postprandial glucose and insulin curves, and the collinearity between them, it was not appropriate to examine the association of each one with hunger and fullness at 3 and 4 hours after the meal. Therefore, we used exploratory factor analysis to reduce the variables into fewer “factors” that could explain variance in the return of appetite following the meal. Factor analysis involves a three-step process. First, a correlation matrix is created that includes all variables used in the analysis. Second, variables with shared correlations explaining the greatest amount of variance will be extracted as the first factor, with further factors extracted when other variables with shared correlations explain portions of the remaining variance. For the purposes of this study, variables with a loading of ≥0.7 were considered to be associated with a common factor, with higher loading reflecting a greater association between the variable and the factor. The final step in the factor analysis process is to use a mathematical rotation to achieve separation of the variables that load onto each factor (i.e. obtain a high factor loading on one factor but a low factor loading on another). In effect, this step is achieved by rotating the X–Y axis in order to fit the correlation line for each factor close to the variables that loaded onto that factor and separate from the variables loaded onto a second factor. The most commonly used method is varimax rotation which ensures the axes remain perpendicular to each other, and this was the type of rotation used in the current study. Factors that were identified in this process were inspected to ensure theoretical validity.
Exploratory multiple linear regression modeling was then used to examine whether any of these factors explained the between-group difference in self-reported hunger at 3 and 4 hours post-meal. Each was loaded into separate models predicting hunger at 3 or 4 hours from diet group, after adjusting for fasting hunger.
All insulin concentrations were log10 transformed prior to analyses. Insulin data from 3 participants were excluded from analyses due to significant sample hemolysis and consequent artificially low insulin values. Data presented are means ± SD unless otherwise indicated, and all analyses were conducted using the Statistical Package for the Social Sciences, version 18 (SPSS; SPSS Inc., Chicago, IL).
Results
A total of 64 participants completed this portion of the study (LC/HF N=35; HC/LF N=29). Demographic characteristics of the sample have been described previously (Goss, Goree, et al., 2013), and the average body weight of individuals in each group did not differ on the day of the breakfast meal test. By design, total energy and protein content of the breakfast meals did not differ (Table 1), but CHO, fructose, and glycemic load were higher, and fat content lower, in breakfast meals from the HC/LF menu (all P < 0.001). There was no difference in fiber, calcium, and potassium content of the meals, but there was more sodium in the LC/HF meal (P<0.001).
Table 1.
Content of breakfast meals (mean ± SD).
| Variable | LC/HF | HC/LF |
|---|---|---|
| Kcals | 570 ± 141 | 548 ± 87 |
| % of daily kcals | 20.2 ± 5.6 | 20.2 ± 3.6 |
| % kcals as protein | 16.4 ± 3.8 | 16.6 ± 5.5 |
| % kcals as CHO | 47.2 ± 10.5*** | 62.3 ± 8.8 |
| % kcals as fat | 38.1 ± 12.3*** | 23.8 ± 8.8 |
| % kcals as SFA | 14.6 ± 5.4*** | 8.3 ± 4.6 |
| % kcals as PUFA | 5.5 ± 2.4 | 4.6 ±1.7 |
| % kcals as MUFA | 15.0 ± 5.3*** | 9.1 ± 4.1 |
| Glycemic load | 29.2 ± 3.9*** | 47.9 ± 9.2 |
| Fructose (g) | 4.7 ± 44*** | 10.0 ± 5.2 |
| Glucose (g) | 3.4 ± 2.8*** | 12.7 ± 6.4 |
| Fiber (g) | 4.3 ± 2.3 | 5.4 ± 4.1 |
| Calcium (mg) | 528.2 ± 151.1 | 490.4 ± 148.9 |
| Sodium (mg) | 1026.3 ± 3114*** | 694.6 ± 211.6 |
| Potassium (mg) | 840.6 ± 182.5 | 777.8 ± 306.5 |
P < 0.001
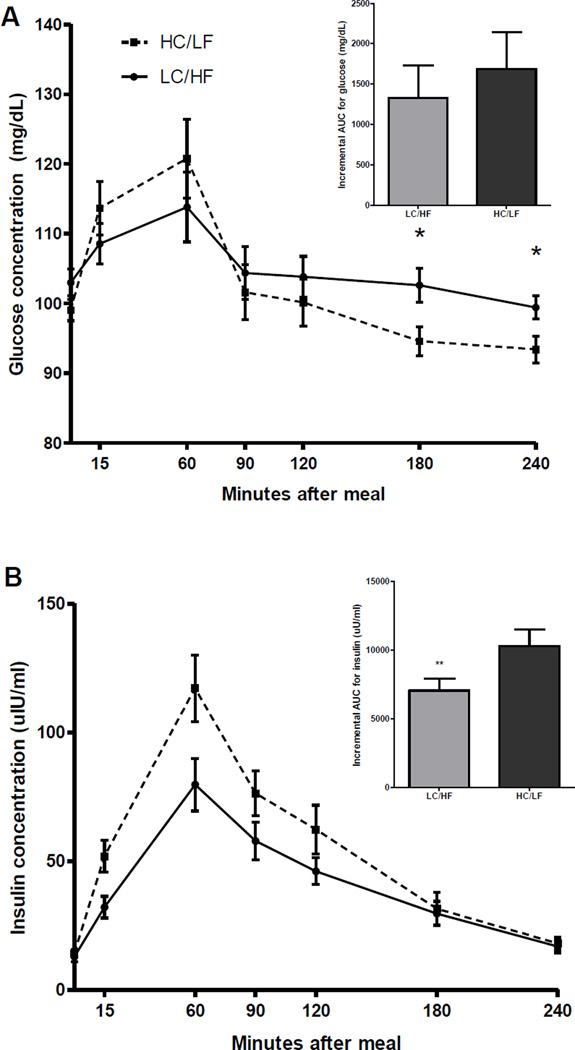
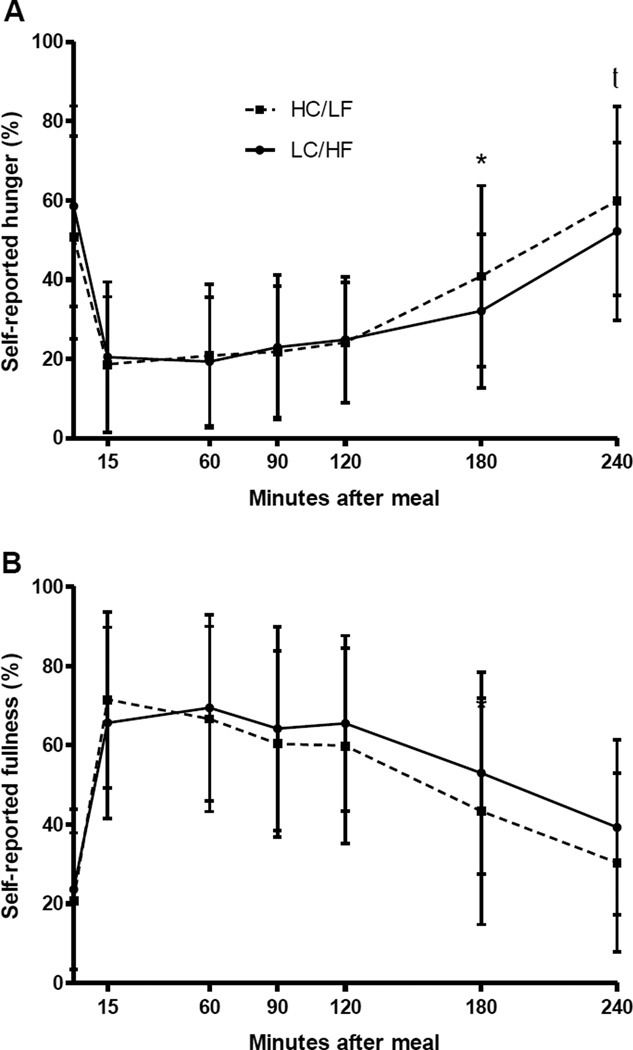
Figure 1 shows the overall glucose (A) and insulin (B) response following the meal. As shown, iAUC for insulin was lower for those fed the LC/HF breakfast as compared to the HC/LF breakfast (P<0.01), and glucose concentrations at 3 and 4 hours following the meal were also lower for those in the LC/HF group (P<0.05). Further descriptors of the shape of the postprandial glucose and insulin curves are presented in Table 2. Although the magnitude of the highest and lowest recorded glucose concentration did not differ between the groups, they did occur earlier following the HC/LF breakfast. Consistent with the results of the iAUC analysis, the highest recorded insulin concentration was lower for those fed the LC/HF meal compared to those fed the HC/LF meal. Hunger and fullness ratings across the whole meal are shown in Figure 2. After adjustment for fasting hunger, the HC/LF group reported more hunger at 3 (P<0.05) and 4 (P=0.07) hours following the meal, implying that consumption of a LC/HF meal delayed the return of hunger. Although there was not a statistical difference in perceived fullness following the meal, the trend for greater fullness (P=0.20) in the LC/HF group was consistent with their reduced hunger at the same time points.
Figure 1.
The incremental AUC for glucose (A) did not differ between the groups, but glucose concentrations were higher at 3 and 4 hours following the LC/HF meal (*P<0.05). The incremental AUC for insulin (B) was higher following the HC/LF breakfast versus the LC/HF breakfast (P<0.05; data shown are mean ± SEM).
Table 2.
Independent groups t-tests for main outcome variables by diet group (mean ± SD)
| LC/HF | HC/LF | |
|---|---|---|
| Fasting glucose (mg/dL) | 103.00 ± 11.33 | 99.05 ± 8.08 |
| Fasting insulin (uU/mL) | 12.87 ± 10.46 | 14.16 ± 7.45 |
| Time of highest recorded glucose (min) | 60.88 ± 39.93** | 38.28 ± 22.88 |
| Time of lowest recorded glucose (min) | 142.94 ± 69.31† | 115.86 ± 48.59 |
| Time of highest recorded insulin (min) | 68.18 ± 27.83 | 58.33 ± 20.10 |
| Time of lowest recorded insulin (min) | 232.50 ± 25.27† | 210.00 ± 51.61 |
| Highest recorded glucose (mg/dL) | 123.44 ± 24.09 | 129.03 ± 27.06 |
| Lowest recorded glucose (mg/dL) | 89.59 ± 18.40 | 88.93 ± 15.47 |
| Highest recorded insulin (uU/mL) | 88.40 ± 57.97* | 124.17 ± 63.32 |
| Lowest recorded insulin (uU/mL) | 18.46 ± 17.11 | 18.99 ± 13.55 |
| Decline of glucose from highest to lowest (mg/dL) | 33.85 ± 26.81 | 40.10 ± 23.53 |
| Rate of glucose decline ((glucose decline (mg/dL))/min) | 0.46 ± 0.32 | 0.61 ± 0.44 |
| 3 hr glucose (mg/dL) | 102.63 ± 14.24* | 94.59 ± 10.99 |
| 4 hr glucose (mg/dL) | 99.43 ± 9.74* | 93.41 ± 10.44 |
| 3hr insulin (uU/mL) | 29.69 ± 27.41 | 31.55 ± 32.80 |
| 4hr insulin (uU/mL) | 16.95 ± 15.07 | 18.09 ± 13.37 |
P<0.05;
P<0.01;
P<0.001;
0.05<P<0.10
Figure 2.
Self-reported hunger (A) and fullness (B) at each time point during the meal test (mean ± SEM). *P<0.05; t0.05<P<0.10 (adjusted for baseline).
Results of the exploratory factor analysis are shown in Table 3. Five factors with an eigenvalue >1.0 were identified, implying that each of these factors explained a meaningful amount of variance. All variables listed in Table 3, with the exception of the time at highest and lowest recorded insulin, and the rate of glucose decline (all of which did not load to any factor using our criteria), loaded onto only 1 distinct factor at the level of >0.70. The distinction between factors was clear, with no item loading at >0.50 on any second factor.
Table 3.
Factor loadings on varimax rotated solution of factor analysis.
| Scale name and items | Factor loading |
|---|---|
| Factor 1: Insulin concentrations | |
| Fasting | 0.89 |
| 3 hr | 0.80 |
| 4 hr | 0.89 |
| Peak concentration | 0.78 |
| Nadir concentration | 0.90 |
| Incremental AUC | 0.82 |
| Factor 2: Acute glucose excursion | |
| Peak concentration | 0.90 |
| Incremental AUC | 0.72 |
| Decline from peak to nadir | 0.83 |
| Factor 3: Glucose concentrations | |
| Fasting | 0.75 |
| 3 hr | 0.79 |
| 4 hr | 0.85 |
| Factor 4: Timing of highest and lowest recorded glucose | |
| Time at glucose peak | 0.73 |
| Time at glucose nadir | 0.80 |
| Factor 5: Lowest recorded glucose concentration | |
| Nadir concentration | 0.91 |
When the factors identified by the exploratory factor analysis were entered into the exploratory multiple linear regression models to determine whether they contributed to the association between meal type and hunger at 3 and 4 hours after the meal, it was found that only the factor related to the timing of the highest and lowest recorded glucose (factor 4) was independently related to hunger (Table 4). Furthermore, the addition of this factor abolished the association of meal type with hunger, suggesting that the earlier time at highest and lowest recorded glucose concentration was associated with the more rapid return of appetite after consumption of a HC/LF meal.
Table 4.
Linear regression models for hunger 3 and 4 hrs after breakfast meal. Data shown are standardized betas unless otherwise indicated.
| Hunger 3 hrs post-meal1 | Hunger 4 hrs post-meal1 | |
|---|---|---|
| Model 1: Base model with diet | 0.281***3 | 0.258***3 |
| Diet2 | −0.286* | −0.204† |
|
Model 2
: Base model plus times at which glucose peak & nadir occurred (Factor 4). |
0.285***3 | 0.308***3 |
| Diet2 | −0.095 | 0.010 |
| Factor 4 | −0.330* | 0.348* |
P<0.05;
P<0.01;
P<0.001;
0.05<P<0.10
Models are adjusted for baseline hunger.
Diet 1 = STD; Diet 2 = redCHO
Adjusted R2 for the model.
Discussion
This study was designed to examine whether consumption of a HC/LF breakfast meal compared to a LC/HF meal was associated with an earlier return of hunger, and whether specific characteristics of the insulin and glucose profiles identified using exploratory factor analysis were associated with perceptions of hunger. Results indicated that individuals who consumed the HC/LF meal had more self-reported hunger at 3 and 4 hours post meal, and that this greater hunger was explained primarily by the timing of the highest and lowest recorded glucose, with glucose and insulin concentrations contributing marginally. These results suggest that increases in carbohydrate content of a meal, with a concomitant decrease in fat content, reduces the time until hunger returns following the meal, and that this effect is mediated primarily by the earlier rise and fall in postprandial glucose concentrations.
The primary novel finding in this study was that a pattern of circulating glucose concentrations characterized by earlier postprandial glucose rise and fall following the HC/LF meal explained the greater self-reported hunger in this group at 3 and 4 hours following the test meal. Differences in post meal satiety are believed to be related to glucose and insulin profiles, but the specific characteristic of the profiles that explain differences in post meal satiety have not previously been shown. In this study, the HC/LF meal relative to the LC/HF meal was associated with earlier rise and fall of glucose. Further, the HC/LF meal was associated with lower absolute glucose concentrations at 3 and 4 hours, which declined below baseline. While both timing and concentration of glucose were significantly associated with perceived hunger, timing emerged as the primary predictor. In contrast, neither absolute insulin concentrations nor the timing of changes in serum insulin was associated with self-reported hunger, although serum insulin was higher following the HC/LF meal and undoubtedly participated in the glucose dynamic. Thus, our data suggest that the timing of the increase and decrease in blood glucose following a meal may be particularly relevant to perceptions of hunger.
Studies relating glucose concentrations per se (distinct from temporal aspects) to hunger and satiety have yielded equivocal results. Flint and colleagues found that although postprandial glucose concentration was unrelated to self-reported hunger and appetite, it was positively associated with subsequent energy intake at the next meal (Flint et al., 2006). Anderson and colleagues also found that postprandial glucose was inversely associated with subsequent energy intake (Anderson, Catherine, Woodend, & Wolever, 2002). Insulin may affect appetite as well; however the effect of insulin may be modulated by body weight, with a positive association being apparent only among normal weight adults (Flint et al., 2007). In the current study involving overweight adults, factors derived from glucose and insulin concentrations per se were only minimally related to hunger 3 and 4 hours following the meal, and did not explain the difference in hunger between the meal types provided. While the focus of the current study was on identifying glucose and insulin parameters associated with hunger 3 and 4 hours after the meal, we acknowledge that it is possible that glucose and insulin concentrations might have contributed to hunger and appetite, irrespective of meal type, more proximally to the meal.
Although the focus here was on the effect of a HC/LF versus LC/HF meal on hunger and appetite between meals, it should also be acknowledged that long-term maintenance on a HC/LF diet may have metabolic effects aside from hunger and potentially, increased meal frequency that may impair weight maintenance. In a previous controlled feeding study, we found that individuals maintained on the HC/LF diet did not lose as much fat as those on a LC/HF diet (Goss, Chandler-Laney, et al., 2013). Diets with lower carbohydrate content have been associated with greater lipolysis, lower lipogenesis, reduced fatty acid concentrations, and diminished storage of adipose tissue; all of which would facilitate weight maintenance (Forsythe et al., 2008; Gögebakan et al., 2011; Ludwig et al., 1999; Samaha et al., 2003). Consequently, under free-living conditions, maintenance on a HC/LF diet may impair body weight regulation via impaired appetite regulation and via metabolic changes that promote weight gain.
Strengths of this study include the rigorous assessment of multiple parameters describing the dynamic changes in postprandial glucose and insulin following meals differing by carbohydrate and fat composition. Furthermore, the use of exploratory factor analysis to identify common factors representing different aspects of the postprandial glucose and insulin profiles presented a novel approach with which to begin to understand which components of the glucose/insulin response are important in perceptions of hunger. This study was limited however, by the modest sample size and by the fact that, due to the nature of the diets used, we were unable to tease apart the effects of total carbohydrate content from total fat content, or from glycemic load or type of carbohydrate consumed.
Conclusions
To conclude, in this sample of overweight but otherwise healthy adults, a moderately HC/LF breakfast meal resulted in higher postprandial insulin, a more rapid acute rise and fall in peak glucose concentration, lower glucose concentrations hours following the meal, and more hunger at 3 and 4 hours after meal consumption. Importantly, the earlier rise and fall in circulating glucose, rather than the concentration of glucose per se, explained the earlier return of hunger following the HC/LF meal. This pattern describes a potential mechanism to explain why increases in meal frequency have occurred in the US concomitantly with increased carbohydrate and reduced fat content of diets. Future research needs to examine more closely the importance of type of carbohydrate and fat consumed on the timing of the glucose peak and subsequent second-meal intake, as an objective measure of appetite, and should examine whether long-term maintenance on a LC/HF diet will persistently reduce hunger and facilitate weight loss.
Acknowledgements
We thank Suzanne Choquette and Betty Darnell for bionutrition support, and Maryellen Williams and Cindy Zeng for conducting the laboratory analyses.
This study was supported by the National Institutes of Health R01DK67538, F32DK082028, P30DK56336, P60DK079626, and UL1RR025777.
Footnotes
There are no actual or potential conflicts of interest. There are no financial gains to disclose.
Chandler-Laney & Gower designed the study; Chandler-Laney, Goree, Ellis, and Casazza collected the data; Chandler-Laney, Goree and Gower analyzed the data; Chandler-Laney, Morrison, and Gower wrote the paper; Chandler-Laney and Gower had primary responsibility for final content. All authors read and approve the final manuscript.
References
- Anderson G, Catherine N, Woodend D, Wolever T. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. American Journal of Clinical Nutrition. 2002;76(5):1023–1030. doi: 10.1093/ajcn/76.5.1023. [DOI] [PubMed] [Google Scholar]
- Barkoukis H, Marchetti C, Nolan B, Sistrun S, Krishnan R, Kirwan J. A high glycemic meal suppresses the postprandial leptin response in normal healthy adults. Annuals of Nutrition and Metabolism. 2007;51(6):512–518. doi: 10.1159/000112309. [DOI] [PubMed] [Google Scholar]
- Brand-Miller J, Stockmann K, Atkinson F, Petocz P, Denyer G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a database of more than 1,000 foods. American Journal of Clinical Nutrition. 2009;89(1):97–105. doi: 10.3945/ajcn.2008.26354. [DOI] [PubMed] [Google Scholar]
- Bray GA. Static theories in a dynamic world: a glucodynamic theory of food intake. Obesity Research. 1996;4(5):489–492. doi: 10.1002/j.1550-8528.1996.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Briefel R, Johnson C. Secular trends in dietary intake in the United States. Annual Review of Nutrition. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- Clegg M, Shafat A. Energy and macronutrient composition of breakfast affect gastric emptying of lunch and subsequent food intake, satiety and satiation. Appetite. 2010;54(3):517–523. doi: 10.1016/j.appet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Coulston A, Liu G, Reaven G. Plasma glucose, insulin and lipid responses to high-carbohydrate low-fat diets in normal humans. Metabolism: Clinical and Experimental. 1983;32(1):52–56. doi: 10.1016/0026-0495(83)90155-5. [DOI] [PubMed] [Google Scholar]
- Fajcsak Z, Gabor A, Kovacs V, Martos E. The effects of 6-week low glycemic load diet based on low glycemic index foods in overweight/obese children--pilot study. Journal of the American College of Nutrition. 2008;27(1):12–21. doi: 10.1080/07315724.2008.10719670. [DOI] [PubMed] [Google Scholar]
- Flint A, Gregersen NT, Gluud LL, Møller BK, Raben A, Tetens I, Astrup A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. American Journal of Clinical Nutrition. 2007;98(1):17–25. doi: 10.1017/S000711450768297X. [DOI] [PubMed] [Google Scholar]
- Flint A, Møller B, Raben A, Sloth B, Pedersen D, Tetens I, Astrup A. Glycemic and insulinemic responses as determinants of appetite in humans. American Journal of Clinical Nutrition. 2006;84(6):1365–1373. doi: 10.1093/ajcn/84.6.1365. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell J, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International Journal of Obesity and Related Metabolic Disorders. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Forsythe C, Phinney S, Fernandez M, Quann E, Wood R, Bibus D, Volek J. Comparison of Low Fat and Low Carbohydrate Diets on Circulating Fatty Acid Composition and Markers of Inflammation. Lipids. 2008;43(1):65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- Galgani J, Aguirre C, Díaz E. Acute effect of meal glycemic index and glycemic load on blood glucose and insulin responses in humans. Journal of Nutrition. 2006;5:22. doi: 10.1186/1475-2891-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögebakan Ö, Kohl A, Osterhoff MA, van Baak MA, Jebb SA, Papadaki A, Pfeiffer AFH. Effects of Weight Loss and Long-Term Weight Maintenance With Diets Varying in Protein and Glycemic Index on Cardiovascular Risk Factors: The Diet, Obesity, and Genes (DiOGenes) Study: A Randomized, Controlled Trial. Circulation. 2011;124(25):2829–2838. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- Goss AM, Chandler-Laney P, Ovalle F, Goree LL, Azziz R, Desmond R, Gower B. Effects of a eucaloric reduced carbhoydrate diet on body composition and fat distribution in women with PCOS; Paper presented at the Endocrine Reviews; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Goree LL, Ellis AC, Chandler-Laney PC, Casazza K, Lockhart ME, Gower BA. Effects of diet macronutrient composition on body composition and fat distribution during weight maintenance and weight loss. Obesity (Silver Spring) 2013;21(6):1139–1142. doi: 10.1002/oby.20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzler S, Kim Y. Glycemic and insulinemic responses to energy bars of differing macronutrient composition in healthy adults. Medical Science Monitor. 2003;9(2):CR84–CR90. [PubMed] [Google Scholar]
- Holt S, Brand Miller J, Petocz P. Interrelationships among postprandial satiety, glucose and insulin responses and changes in subsequent food intake. European Journal of Clinical Nutrition. 1996;50(12):788–797. [PubMed] [Google Scholar]
- Holt S, Miller J. Increased insulin responses to ingested foods are associated with lessened satiety. Appetite. 1995;24(1):43–54. doi: 10.1016/s0195-6663(95)80005-0. [DOI] [PubMed] [Google Scholar]
- Lennerz BS, Alsop DC, Holsen LM, Stern E, Rojas R, Ebbeling CB, Ludwig DS. Effects of dietary glycemic index on brain regions related to reward and craving in men. American Journal of Clinical Nutrition. 2013 doi: 10.3945/ajcn.113.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig D, Majzoub J, Al-Zahrani A, Dallal G, Blanco I, Roberts S. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103(3):E26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- Marriott B, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. Journal of Nutrition. 2009;139(6):1228S–1235S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- Mayer J. Glucostatic mechanism of regulation of food intake. New England Journal of Medicine. 1953;249(1):13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Ostman E, Granfeldt Y, Björck I. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. American Journal of Clinical Nutrition. 2008;87(3):645–654. doi: 10.1093/ajcn/87.3.645. [DOI] [PubMed] [Google Scholar]
- Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Sinha R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. Journal of Clinical Investigation. 2011;121(10):4161–4169. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin B, Duffey K. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States. American Journal of Clinical Nutrition. 2010;91(5):1342–1347. doi: 10.3945/ajcn.2009.28962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben A, Agerholm-Larsen L, Flint A, Holst JJ, Astrup A. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. American Journal of Clinical Nutrition. 2003;77(1):91–100. doi: 10.1093/ajcn/77.1.91. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Stockmann K, Atkinson F, Denyer G, Brand-Miller J. Effect of the glycemic index of carbohydrates on day-long (10 h) profiles of plasma glucose, insulin, cholecystokinin and ghrelin. European Journal of Clinical Nutrition. 2009;63(7):872–878. doi: 10.1038/ejcn.2008.52. [DOI] [PubMed] [Google Scholar]
- Rodin J, Wack J, Ferrannini E, DeFronzo R. Effect of insulin and glucose on feeding behavior. Metabolism: Clinical and Experimental. 1985;34(9):826–831. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Roe LS, Meengs JS. Salad and satiety: energy density and portion size of a first-course salad affect energy intake at lunch. Journal of the American Dietetic Association. 2004;104(10):1570–1576. doi: 10.1016/j.jada.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Stern L. A Low-Carbohydrate as Compared with a Low-Fat Diet in Severe Obesity. New England Journal of Medicine. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR, Chanoine JP. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. Journal of Clinical Endocrinology and Metabolism. 2005;90(4):2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- Wolever T, Yang M, Zeng X, Atkinson F, Brand-Miller J. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. American Journal of Clinical Nutrition. 2006;83(6):1306–1312. doi: 10.1093/ajcn/83.6.1306. [DOI] [PubMed] [Google Scholar]