Abstract
Objectives
To report and synthesize patterns of disease modifying agent (DMARD) use reported in observational studies of patients with established and early RA after the publication of ACR guidelines promoting universal DMARD use.
Methods
We searched PubMed for English-language full-length articles published between January 1, 2002, and October 1, 2012 that examined DMARD use. Data abstracted from articles included patient characteristics, country of study, time period studied, patient source, and treating physician type. Study quality was assessed using a modified Newcastle Ottawa Quality Assessment Scale.
Results
We reviewed 1287 abstracts; 98 full-length articles were selected for additional review, and 27 studies describing 28 cohorts of patients were included. Twelve studies described data from cohorts of patients with established RA, and DMARD use in this group of studies ranged from 73-100%. Five studies described data from patients sourced through administrative data demonstrated consistently lower DMARD use, ranging from 30-63%. Three studies conducted population-based surveys to define cases of RA where DMARD use ranged from 47-73%. Eight studies investigated patients with early RA. DMARD use among patients followed by rheumatologists ranged from 77-98% whereas DMARD use reported for patients seen by a mix of physicians was significantly lower (39-63%).
Conclusion
DMARD use in studies from RA cohorts or registries (in which patients were followed by rheumatologists) ranged from 73-100%, compared with 30-73% in studies from administrative data or population-based surveys (in which patients were not necessarily getting rheumatology subspecialty care).
In 2002, the American College of Rheumatology (ACR) endorsed rheumatoid arthritis (RA) treatment guidelines supporting the use of disease modifying anti-rheumatic drugs (DMARDs) in every patient with active RA at the earliest stage of disease, ideally within three months of disease onset, unless contraindications exist.1,2 These guidelines were based on results from clinical trials demonstrating that DMARDs slow the progression of RA by decreasing inflammation and reducing articular erosions, and observational studies showing that the use of these medications improves functional status and health-related quality of life.3,4 A set of process measures for RA developed through the Arthritis Foundation Quality Indicators Project included similar recommendations, and in 2005, the National Committee for Quality Assurance (NCQA) introduced a DMARD performance measure into the Healthcare Effectiveness Data and Information Set (HEDIS), making it the first nationally-applied quality measure to address care for patients with RA.5
Despite the compelling evidence favoring DMARD use, studies describing real-world DMARD prescribing patterns are limited. The most recent review of this literature was published in 2008, and described the evolution of treatment for RA from the 1980s onward, highlighting the rising use of methotrexate within clinical cohorts and registries world-wide.6 There has been no synthesis of recent studies since the advent of robust methods for defining cohorts of RA patients using administrative data. In this study, we performed a systematic review focused on observational studies reporting DMARD utilization since 2002 in order to understand the range of DMARD use in various settings around the world, after the guidelines promoting universal DMARD use were in place.
METHODS
Study Selection
We searched PubMed for English-language full-length articles published between January 1, 2002, and October 1, 2012 that examined DMARD use. DMARDs included non-biologic drugs (including methotrexate, sulfasalazine, hydroxychloroquine, and others); biologic drugs (including infliximab, etanercept, adalimumab, abatacept, and others). Glucocorticoids and non-steroidal antiflammatory drugs were not included in the DMARD category. The search started in 2002 since this was the year of publication of the new ACR guidelines advocating universal DMARD use for patients with active RA. Search terms included rheumatoid arthritis, anti-rheumatic agents and the MeSH terms physician practice patterns, management, or treatment (see Appendix 1). Reference lists from articles meeting study criteria were also reviewed for potential studies not identified by our initial search criteria.
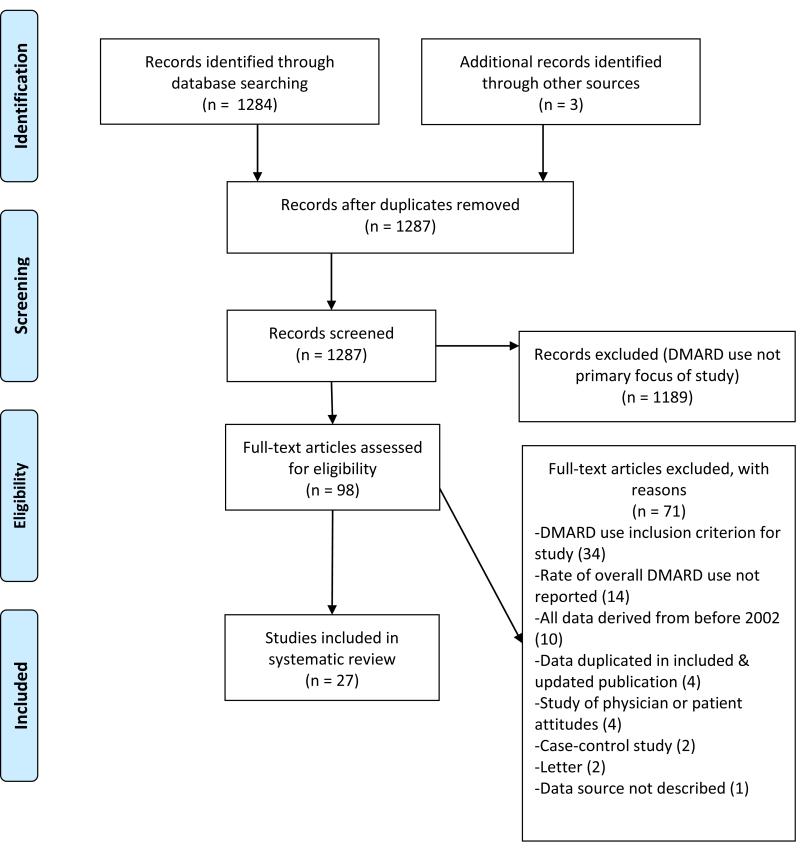
After the initial PubMed search, two authors assessed the abstracts of all retrieved articles (GS, JY) and selected articles relevant to this study for full-text review. We included cohort or cross-sectional studies that reported the proportion of RA patients using any (non-biologic or biologic) DMARD. Articles were excluded if they were review articles, clinical trials, or case-control studies, or if they included only data collected prior to the year 2002 (Figure 1). We also excluded studies that (1) had DMARD use as an inclusion criterion for patients in the study, (2) in which the proportion of patients receiving DMARDs was not reported, (3) describing data duplicated in a subsequent publication on the same cohort included in this review, (4) exclusively describing physician or patient attitudes, and (5) a study that did not explicitlyspecify its patient source.
Figure 1.
Flow diagram of articles retrieved from literature search and used in analysis.
Data Abstraction and Synthesis
All the remaining articles were assessed in detail using a structured abstraction form. This assessment included country of study, time period studied, patient source, and treating physician type, if specified (e.g., rheumatologist). Where possible, data were collected for biologic DMARD use or combination therapy. If data was stratified by year within a single study, we abstracted data for the most recent year(s) reported. Information necessary to assess study quality using a modified Newcastle Ottawa Quality Assessment Scale was also extracted (see Appendix 2).7,8
Included studies were categorized by data type, (RA registries; health care utilization information from insurance data (“administrative data”); or population-based surveys). We assessed disease duration of the included patients (early RA versus established RA, as described by the study authors). We did not attempt to formally summarize the results across studies using meta-analytic techniques because of substantial heterogeneity in study design. Proportions of patients using DMARDs as reported in each study are summarized using a forest plot for ease of interpretation.9
RESULTS
We reviewed 1287 abstracts and 98 full-length articles were selected for additional review (see Figure 1). After applying exclusion criteria, 27 studies describing 28 cohorts of patients were included. All of the included studies were written with the explicit purpose of describing DMARD “use,” “practices,” “patterns,” “initiation,” “trends,” or “quality of care.” Eleven studies were performed in Europe, 10 used data from the United States, and 7 were performed in other countries (see Table 1). Eight studies reported on DMARD use in RA patients with early RA as described by the study authors (disease duration < 3 years). Patients were derived from RA cohorts in 15 studies, administrative data in 9 studies, and population-based surveys in 3 studies. One study included data on 2 distinct cohorts, one with established RA and one with early RA, and is therefore listed twice.10 The quality of the 27 studies ranged from moderate to high based on a modified Newcastle Ottawa Quality Assessment Scale.
Table 1. Characteristics of included studies, sorted alphabetically.
| Reference | Location | Time period | Patient source description | Data type | N (denominator) |
RA definition | Follow-up time (mean, years) |
Quality rating (range 0-5) |
|---|---|---|---|---|---|---|---|---|
| Bonafede 2012 | US | 2004-2008 | Commercial database (privately insured) | Admin | 26,911 | 2 RA-coded visits | 1 | 5 |
| de Thurah 2010 | Denmark | 1996-2006 | Nationwide register | Admin | 1,516 | 1 RA-coded visit | 2 | 5 |
| Della Rossa 2010 | Italy | 2006-2007 | Population-wide physician & patient survey | Clinical | 34 | ACR criteria | CS | 5 |
| DeWitt 2009 | US | 2006 | RA database (ARAMIS) | Clinical | 679 | RA or suspected RA | CS | 3 |
| Edwards 2005 | UK | 1987-2002 | Nationwide register | Admin | 34,364 | 2 RA-coded visits | 7 | 5 |
| Gonzalez-Alvaro 2008 | Spain | 2000-2004 | 34 clinics (EMECAR) | Clinical | 789 | ACR criteria | 4 | 5 |
| Goycochea-Robles 2007 | Mexi co | 2002-2003 | Multiple clinics; 58 physicians | Clinical | 1,096 | ACR criteria | CS | 4 |
| Grijalva 2008 | US | 2004 | State-wide low-income health insurance program | Admin | 5,600 | 2 RA-coded visits | 1 | 5 |
| Jamal 2011 | Canada | 2003-2006 | 15 rheumatologists | Clinical | 204 | RA or suspected RA | CS | 4 |
| Kahn 2007 | US | 1999-2003 | University clinic | Clinical | 568 | RA or suspected RA | 1 | 5 |
| Khanna 2007 | US | 2003 | State-wide low-income health insurance program | Admin | 1,157 | 1 RA-coded visit | CS | 5 |
| Kiely 2009 | UK | 2002-2007 | 19 centers (ERAN) | Clinical | 691 | RA or suspected RA | 0.5 | 5 |
| Lukas 2009 | France | 2002-2005 | Early RA database; 14 centers (ESPOIR) | Clinical | 775 | RA or suspected RA | 0.3 | 5 |
| Montag 2011 | Australia | 2007-2008 | 2 community clinics | Clinical | 1,059 | RA or suspected RA | CS | 4 |
| Rantalaiho 2010 | Finland | 2006-2007 | Nationwide register | Admin | 3,628 | 1 RA-coded visit | 0.3 | 5 |
| Schmajuk 2007 | US | 1996-2003 | State-wide low-income health insurance program | Admin | 5,864 | 3 RA-coded visits | 1 | 5 |
| Schmajuk 2011 | US | 2005-2008 | Nationwide Medicare managed care plans | Admin | 93,143 | 2 RA-coded visits | 1 | 5 |
| Soderlin 2010 | Sweden | 2005 | RA registry (Malmo) | Clinical | 1,049 | ACR criteria | CS | 4 |
| Sokka 2008(a) | US | 2000-2004 | University clinic | Clinical | 103 | RA or suspected RA | 5 | 5 |
| Sokka 2008(b)* | Finland | 2000-2004 | Hospital clinic | Clinical | 497 | RA or suspected RA | 2 | 5 |
| Solomon 2012 | US | 1996-2007 | National Ambulatory Care Medical Survey | Clinical | 859 | 1 RA-coded visit | CS | 5 |
| Sung 2012 | Korea | 2008-2010 | Nationwide register | Clinical | 4,721 | ACR criteria | CS | 5 |
| Teh 2008 | Malaysia | 2006-2007 | Hospital clinic | Clinical | 154 | ACR criteria | CS | 4 |
| Westhoff 2009 | Germany | 2008 | Population-wide survey | Clinical | 41 | ACR criteria | CS | 4 |
| Widdifield 2010 | Canada | 1997-2006 | Province-wide physician billing data | Admin | 24,942 | 2 RA-coded visits | 1 | 5 |
| Yamanaka 2007 | Japan | 2006 | National registry (IORRA) | Clinical | 4,933 | ACR criteria | 0.5 | 4 |
| Yelin 2005 | US | 1999-2002 | Northern California RA Panel | Clinical | 438 | ACR criteria | 1 | 4 |
| Ziegler 2010 | Germany | 2007 | Nationwide register | Clinical | 3,323 | ACR criteria | 1 | 5 |
Location: US: United States; UK:United Kingdom;
Data type: Admin: Administrative data; Survey: Population-based survey; Clinical: Patients evaluated by a physician as part of a RA registry;
Follow-up: CS: cross-sectional
Numbers in italics are approximated from study data
Sokka 2008 appears twice because 2 distinct cohorts were described
Studies describing DMARD use in established RA
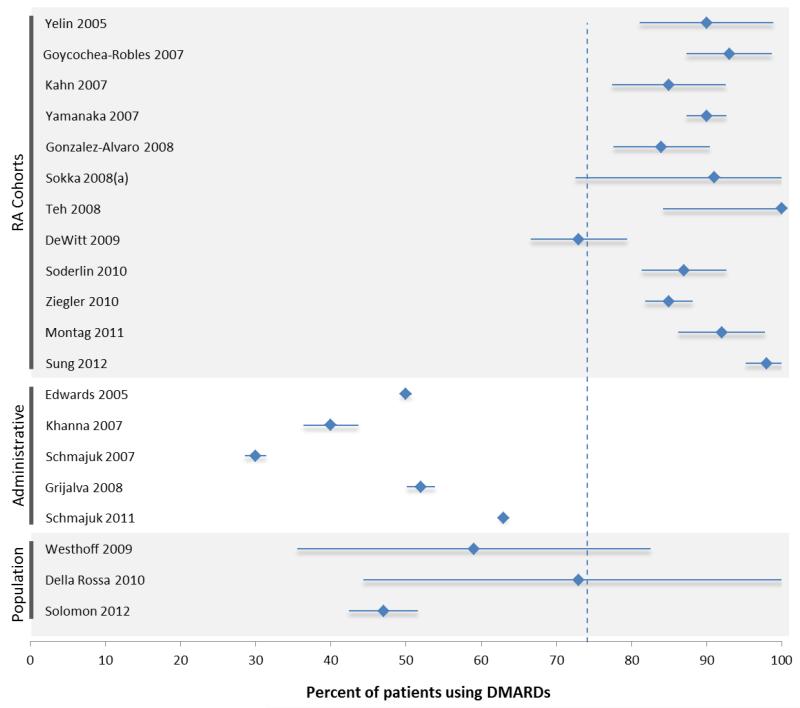
Twelve studies described data from cohorts of patients with established (not early) RA (see Table 2).10-21 Patients in these studies were mostly female, Caucasian, and in the 6th or 7th decade of life. Disease duration varied between studies from a mean of 5 years to a mean of over 20 years. Patients seen as part of RA cohorts were all treated by rheumatologists (see Table 2, physician type). DMARD use in this group of studies ranged from 73-100% (see Figure 2A). Use of biologic DMARDs ranged from 6-41%.
Table 2. Characteristics of patients within included studies with established RA, stratified by data source.
| Reference | Female (%) |
Caucasian (%) |
Age (years) |
Disease duration (years) |
RF+ (%) |
DAS score (mean (SD)) |
HAQ score (mean (SD)) |
Physician type |
DMARD use (%) |
Combination DMARDs |
Biologic DMARDs |
Most common DMARD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies of Registries or Clinics | ||||||||||||
| Yelin 2005 | 83 | 83 | 62 | 20 | - | - | 1.1 (-) | Rheum | 90 | - | 32 | MTX |
| Goycochea-Robles 2007 | 85 | - | 48 | 10 | - | 3.9 (1.3) | 1.2 (0.8) | Rheum | 93 | 41 | 6 | MTX |
| Kahn 2007 | 78 | 83 | 54 | 10 | - | - | - | Rheum | 85 | - | 28 | - |
| Yamanaka 2007 | 84 | - | 59 | 12 | - | 3.6 (-) | 0.8 (-) | Rheum | 90 | - | 3 | MTX |
| Gonzalez-Alvaro 2008 | 72 | - | 61 | 13 | 75 | 4.1 (1.4) | 1.2 (0.9) | Rheum | 84 | 24 | 16 | MTX |
| Sokka 2008(a) | 71 | - | 54 | 6 | 70 | - | 0.6 (0.5, 0.7)*¥ | Rheum | 91 | 12 | 6 | MTX |
| Teh 2008 | 84 | 0 | 53 | 5 | 66 | 4.3 (1.3) | - | Rheum | 100 | 36 | 0 | MTX |
| DeWitt 2009 | 78 | 87 | 65 | 24 | - | - | 1.4 (-) | Rheum | 73 | - | 41 | - |
| Soderlin 2010 | 74 | - | 63 | 14 | - | - | 0.75 (0.25–1.25)* | Rheum | 87 | - | 20 | MTX |
| Ziegler 2010 | 76 | - | 62 | 11 | - | 3.4 (1.3) | - | Rheum | 85 | 23 | 16 | MTX |
| Montag 2011 | 69 | - | 60 | - | - | - | Rheum | 92 | 26 | 15 | MTX | |
| Sung 2012 | 85 | - | 54 | 8 | 87 | 3.8 (1.4) | 0.7 (0.7) | Rheum | 98 | 67 | 6 | MTX |
|
| ||||||||||||
| Studies Using Administrative Data | ||||||||||||
|
| ||||||||||||
| Edwards 2005 | 71 | - | 58 | 9 | - | - | - | Mixed | 50 | - | - | SSZ |
| Khanna 2007 | 77 | 95 | 47 | - | - | - | - | Mixed | 40 | - | 12 | - |
| Schmajuk 2007 | 88 | 94 | >65 | - | - | - | - | Mixed | 30 | - | 6 | MTX |
| Grijalva 2008 | 78 | 74 | 57 | - | - | - | - | Mixed | 52 | - | 16 | MTX |
| Schmajuk 2011 | 75 | 82 | 74 | - | - | - | - | Mixed | 63 | - | - | - |
|
| ||||||||||||
|
Studies Using Population-Based
Surveys |
||||||||||||
|
| ||||||||||||
| Westhoff 2009 | - | - | - | 9 | 41 | 2.9 (1.0) | - | Mixed | 59 | - | - | - |
| Della Rossa 2010 | 68 | - | 66 | 14 | - | 4.0 (-) | 1.2 (-) | Mixed | 73 § | 5 | - | MTX |
| Solomon 2012 | 76 | 82 | >45 | - | - | - | Mixed | 47 | - | 15 | - | |
- : not reported
DMARD: disease modifying drug
RF+: rheumatoid factor positive; DAS: disease activity score; HAQ: Health Activity Questionnaire;
Rheum: Rheumatologists; Mixed: Not restricted to rheumatologists; MTX: methotrexate; SSZ: sulfasalazine Numbers in italics are approximated from study data
mean(SD) not available; median (IQR) reported instead
MHAQ reported instead of HAQ
personal communication ADR
Figure 2.
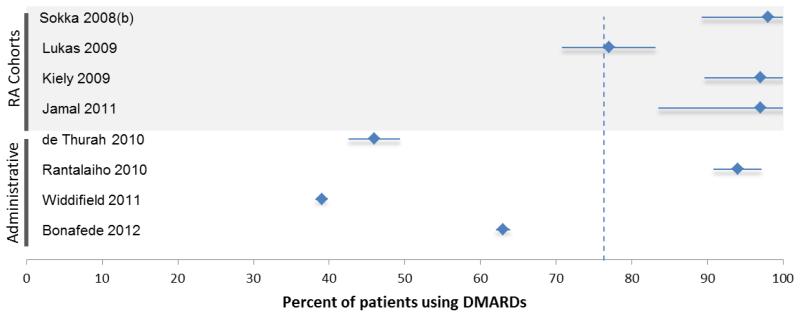
Graphical representation of the percent of patients using DMARDs reported in each study of patients with (A) established and (B) early rheumatoid arthritis. The x-axis ranges from 0-100 percent. The vertical dotted line represents the mean percent DMARD use in the studies within the figure: The mean for established RA studies was 74%; the mean for early RA studies was 76%. The studies have been sorted according to patient source. Figure 2A shows RA cohorts or registries in the top panel, administrative studies in the middle panel, and population-based surveys in the bottom panel. Figure 2B shows RA cohorts or registries in the top panel and administrative studies in the bottom panel. Each study listed shows a diamond and a horizontal “error bar” line – the diamond represents the reported percent of patients in each study who are taking DMARDs; the error bar lines indicate the 95% confidence intervals, which are inversely proportional to the sample size of the study (i.e., small studies have wide error bars; large studies have narrow error bars).
The percent of patients using DMARDs reported in each study of patients with established RA, stratified by data source.
The percent of patients using DMARDs reported in each study of patients with early RA, stratified by data source.
Five studies described data from patients sourced through administrative data.22-26 As expected, these studies were unable to report on factors such as disease duration, seropositivity, disability, or disease activity. Studies based on administrative sources selected patients based on their administrative diagnoses. As such, these patients may or may not have been followed by a rheumatologist during the course of clinical care (specifically, these patients may have been designated as having RA by a primary care or other type of provider); we have therefore designated the physician type as “mixed” (see Table 2). This is distinct from studies based on registries, in which patients were by necessity seen and treated by a rheumatologist. Overall DMARD use among patients in these studies was consistently lower compared with patients in RA cohorts and ranged from 30-63%.
Three studies conducted population-based surveys to define cases of RA.27,28,29 Again, these patients were followed by a mixed group of providers (Table 2, physician type). DMARD use ranged from 47-73%. A survey of Italian primary care physicians selected a sample of patients based on the Tuscany (Italy) register of primary care physicians: each GP was asked to complete a questionnaire regarding their patients with RA and to send it to the study center, where patients were examined and the diagnosis was confirmed (or overruled). Thirty-four patients were identified that had RA confirmed by ACR criteria (prevalence of RA was thus estimated at 0.5%), and DMARD use was determined by self-report. A three-stage population-based survey from Germany used a 20-item postal questionnaire of musculoskeletal symptoms and diagnoses followed by a more detailed questionnaire for patients who had possible RA.28 Respondents who reported diagnosis of RA, care by a rheumatologist, or met criteria by the modified ACR decision tree underwent a clinical examination. Investigators confirmed RA by ACR criteria in 41 respondents. DMARD use was ascertained at the time of the interview. Finally, a study of the U.S. National Ambulatory Care Medical Survey (NAMCS) reported on 859 patients who were designated as having RA by their treating physician. DMARD utilization was based on the medications listed by the physician.
Studies describing DMARD use in early RA
Eight studies investigated patients with early RA (see Figure 2B). Of these, 4 used disease cohorts and 4 used administrative data to define early RA patients (see Table 3).10,30-36 Patients in these studies were mostly female, 40-60 years of age, and had a disease duration of 3 years or less. Five of the 8 studies reported DMARD use in early RA patients followed by rheumatologists at the time of diagnosis (see Table 3, physician type). Although a Finnish study used a nationwide register to identify RA patients, a prerequisite for inclusion into the cohort was an RA diagnosis by a rheumatologist. DMARD use among patients in these studies was high, ranging from 77-98%. DMARD use as reported by the 3 studies of patients seen by a mix of physicians was significantly lower (39-63%) compared with the group followed by rheumatologists.
Table 3. Characteristics of patients within included studies with early RA, stratified by data source.
| Reference | Female (%) |
Caucasian (%) | Age (years) |
Disease duration (years) |
RF+ (%) |
DAS score (mean (SD)) |
HAQ score (mean (SD)) |
Physician type |
DMARD use (%) |
Combination DMARDs |
Biologic DMARDs |
Most common DMARD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies of Registries or Clinics | ||||||||||||
| Sokka 2008(b) | 69 | - | 58 | 0.5 | 51 | - | - | Rheum | 98 | 66 | 0 | SSZ |
| Lukas 2009 | 77 | - | 48 | 0.3 | 42 | 5.1 (1.3) | 1.0 (0.7) | Rheum | 77 | 6 | - | MTX |
| Kiely 2009 | 67 | - | 55 | 1 | 58 | 3.8 (2.6–4.9)* | 1.0 (0.3–1.6)* | Rheum | 97 | 9 | 0 | MTX |
| Jamal 2011 | 80 | 66 | 55 | 2.3 | 61 | - | - | Rheum | 97 | 22 | 0 | MTX |
|
| ||||||||||||
| Studies Using Administrative Data | ||||||||||||
|
| ||||||||||||
| de Thurah 2010 | 70 | - | 60 | <1 | - | - | - | Mixed | 46 | - | - | MTX |
| Rantalaiho 2010 | 68 | - | 56 | 0.3 | 63 | - | - | Rheum | 94 | 55 | 1 | MTX |
| Widdifield 2010 | 68 | - | 75 | <1 | - | - | - | Mixed | 39 | - | - | HCQ |
| Bonafede 2012 | 72 | - | 60 | <1 | 36 | - | - | Mixed | 63 | - | 23 | - |
- : not reported
DMARD: disease modifying agent
RF+: rheumatoid factor positive; DAS: disease activity score; HAQ: Health Activity Questionnaire;
Rheum: Rheumatologists; Mixed: Not restricted to rheumatologists; MTX: methotrexate; SSZ: sulfasalazine Numbers in italics are approximated from study data
mean(SD) not available; median (IQR) reported instead
DISCUSSION
We performed a systematic review of the literature published since 2002 reporting on the use of DMARDs among patients with RA. We found consistent patterns in DMARD use stratified by specialty care: the prevalence of DMARD use in studies from RA cohorts or registries (in which patients were followed by rheumatologists), ranged from 73-100%, compared with 30-73% in studies from administrative data or population-based surveys (in which patients were not necessarily receiving rheumatology subspecialty care).
There are at least 2 possible explanations for the differences in DMARD use detected based on subspecialty care. One possibility is that the differences are based on the accuracy of the RA diagnoses for patients in each type of study (misclassification). When using administrative data, 1 or 2 encounters coded for RA often serve as a proxy for an RA diagnosis. One recent study addressing the accuracy of this definition and found a definition using 2 claims coded for RA has a positive predictive value (PPV) of 55% compared to the gold-standard of RA diagnosis by a rheumatologist.37 (Within the Veterans Affairs38 health care system the PPV for 2 RA codes may be even lower.) Requiring 2 RA claims plus the use of a DMARD increases the PPV to 86%, but this definition would not be appropriate for studies assessing DMARD use. Since diagnoses provided by rheumatologists have a higher PPV for RA,39 misclassification may magnify the difference in DMARD use between patients who see rheumatologists versus those who do not (i.e., more patients not seen by rheumatologists do not have true RA, and therefore do not require DMARDs).
However, the differences in DMARD use detected based on subspecialty care may not be an artifact: a second possible explanation is that there is a true difference in the treatment patterns of rheumatologists vs. non-rheumatologist providers. Evidence to support this explanation can be found in 2 administrative studies that directly compared DMARD use in a subgroup of patients with known rheumatology contact to the overall population; both studies reported that patients with rheumatology contact were at least twice as likely to use a DMARD compared to those without.24,35 Two population-based surveys had similar results: a study from Germany showed that patients who met ACR criteria for RA but did not see a rheumatologist regularly were half as likely to be using DMARDs compared to those with no specialty care.28 A study using NAMCS data likewise found that a visit to a rheumatologist was the most significant correlate of DMARD prescribing, with a relative risk of 2.3 (95% CI 1.9-2.9) for DMARD prescription compared to patients seeing a non-rheumatologist.29 In combination, these data strongly suggest that the low DMARD use seen in administrative database studies are at least in part explained by lack of access to regular rheumatology care.
We found uniformly high DMARD use for patients seeing a rheumatologist, with one study reporting 100% of patients using DMARDs and the remainder reporting DMARD use in the 73-98% range. The study from the German Biologic Registry showed that 11% of patients had quiescent disease or relative contraindications to all available DMARDs.19 One possible implication of the data is that optimal performance on the RA quality measure assessing DMARD use is less than 100% and perhaps, after accounting for patient preferences, comorbidities, and contraindications, closer to 85 or 90%. When used for the purposes of evaluating the quality of care provided by an individual physician, small differences in performance on the RA DMARD measure among physicians at the top of the performance range likely represent differences in patient case-mix instead of meaningful gaps in the quality of the care provided. Based on the evidence reviewed here, programs relying on the RA DMARD measure to rank or tier physicians should consider defining a reasonable range for “high quality” performance, perhaps between 85 and 100%.
As it stands, a RA DMARD quality measure may be most useful as a measure of accountability at larger aggregates of the health care system, such as the health plan level or regional level. In our recent analysis of NCQA’s HEDIS RA DMARD measure among Medicare managed care plans, we found that performance on the RA DMARD measure varied widely by health plan with use ranging from 16-87% even after adjusting for case-mix; performance in different geographic regions ranged from 52-71% after adjustment for patient characteristics.26 In countries with single-payer health care systems, the RA DMARD measure can also be used identify patient populations at risk for suboptimal treatment, and therefore allow targeting of resources for quality improvement efforts.34
We found no clear patterns in DMARD use with regard to study country. This may be because reported DMARD use in each study is subject to differences based on the organization of the healthcare system in each country. Even within the group of studies using administrative data, results may not be directly comparable across countries since administrative studies in Europe are all-inclusive of the population whereas administrative studies in the US are often restricted to patients receiving public assistance. Moreover, we did not observe an obvious rise in DMARD use over time after 2002. A prior survey of studies on DMARD use from the 1980s through the early 2000s highlighted 10 studies from clinical registries in the late 1990s with DMARD use ranging from 52-94%.6 None of these studies were population-based, and presumably all patients were seen by rheumatologists. However, compared to studies included in this review, this suggests that at least among patients with access to subspecialty care, DMARD use may have increased over the past 10-20 years. Because very few administrative studies were performed prior to the year 2000, it is difficult to assess the effect of the 2002 ACR guidelines on patients outside of clinical cohorts or registries.
In summary, we reviewed the existing literature on DMARD use among patients with rheumatoid arthritis since 2002. Most studies showed that patients seen by rheumatologists were frequently treated with a DMARD, whereas studies of patients who were not necessarily receiving regular specialty care reported lower DMARD use. Quality measures that assess DMARD use may be more meaningful at the population level than the individual physician level and reflect access to rheumatologists.
Significance and Innovation.
Studies of DMARD utilization to date have reported on patients from registries or administratively-derived cohorts separately; we synthesize the literature from all studies addressing DMARD use since the promulgation of guidelines recommending universal DMARDs for patients with RA
Clear patterns emerge when studies are grouped according to patients’ contact with a rheumatologist: patients followed by rheumatologists are consistenly more likely to use DMARDs compared with patients seen by a group of unselected physicians
Support
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2TR000143 (GS), K23 AR060259 (JY), and R01 AR 056215 (DHS) as well as the Rosalind Russell Foundation (UCSF). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. GS and JY have no financial disclosures; DHS receives salary support through research grants from Amgen, Lilly, and CORRONA not related to this paper. He also serves in unpaid roles on two Pfizer sponsored trials.
Appendix 1. Search strategy
| Search | Add to builder |
Query | Items found |
|---|---|---|---|
| #1 | Add | Search (“arthritis, rheumatoid”[MeSH Terms] AND “antirheumatic agents”[MeSH Terms]) NOT (Randomized Controlled Trial[ptyp] OR Review[ptyp]) AND (physician practice patterns[MeSH] OR treatment[MeSH] OR management[MeSH] OR use OR utilization OR pattern OR patterns OR trend OR trends OR receipt OR quality) Filters: Publication date from 2002/01/01 to 2012/12/31; Humans; English; Adult: 19+ years |
1284 |
Appendix 2. Modified Newcastle-Ottawa Quality Assessment Scale
Possible points = 5; 3 for Selection domain; 2 for Outcome domain
Selection Domain (possible points = 1 per question for a starred response)
- Representativeness of the exposed cohort
- truly representative of the average rheumatoid arthritis patient in the community*
- somewhat representative of the average rheumatoid arthritis patient in the community *
- selected group of users eg nurses, volunteers
- no description of the derivation of the cohort
- Selection of the non exposed cohort
- drawn from the same community as the exposed cohort *
- drawn from a different source
- no description of the derivation of the non exposed cohort
- Ascertainment of exposure (rheumatoid arthritis diagnosis)
- secure record (eg surgical records) *
- structured interview *
- written self report
- no description
Outcome Domain (possible points = 1 per question for a starred response)
- Assessment of outcome
- independent blind assessment/physician exam and/or patient interview *
- record linkage *
- self report
- no description
- Adequacy of follow up of cohorts
- complete follow up - all subjects accounted for *
- subjects lost to follow up unlikely to introduce bias – (small number lost with > 90% follow up, or description provided of those lost) *
- follow up rate < 90% and no description of those lost
- no statement
Footnotes
The authors report no financial interests which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
REFERENCES
- 1.American College of Rheumatology Ad Hoc Committee on Clinical Guidelines Guidelines for the management of RA: 2002 update. Arthritis Rheum. 2002;46:328. [PubMed] [Google Scholar]
- 2.Pisetsky DS, St Clair EW. Progress in the Treatment of RA. JAMA. 2001;286:2787–90. doi: 10.1001/jama.286.22.2787. [DOI] [PubMed] [Google Scholar]
- 3.Pincus T, O’Dell JR, Kremer JM. Combination therapy with multiple disease-modifying antirheumatic drugs in RA: a preventive strategy. Ann Intern Med. 1999;131:768–774. doi: 10.7326/0003-4819-131-10-199911160-00009. [DOI] [PubMed] [Google Scholar]
- 4.Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2008;14:234–54. [PubMed] [Google Scholar]
- 5.MacLean CH, Saag KG, Solomon DH, Morton SC, Sampsel S, Klippel JH. Measuring quality in arthritis care: methods for developing the Arthritis Foundation’s quality indicator set. Arthritis Rheum. 2004;51:193–202. doi: 10.1002/art.20248. [DOI] [PubMed] [Google Scholar]
- 6.Sokka T, Envalds M, Pincus T. Treatment of rheumatoid arthritis: a global perspective on the use of antirheumatic drugs. Mod Rheumatol. 2008;18:228–39. doi: 10.1007/s10165-008-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells G, Shea BJ, O’Connell D, Peterson J, Welch V. [Accessed Sept 19, 2012];The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011 [Google Scholar]
- 8.Hootman J, Driban J, Sitler MR, Harris KP, Cattano NM. Reliability and validity of three quality rating instruments for systematic reviews of observational studies. Res. Synth. Method. 2011;2:110–118. doi: 10.1002/jrsm.41. [DOI] [PubMed] [Google Scholar]
- 9.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokka T, Pincus T. Ascendancy of weekly low-dose methotrexate in usual care of rheumatoid arthritis from 1980 to 2004 at two sites in Finland and the United States. Rheumatology (Oxford) 2008;47:1543–7. doi: 10.1093/rheumatology/ken316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yelin EH, Trupin LS, Katz PP. Impact of managed care on the use of biologic agents for rheumatoid arthritis. Arthritis and Rheum. 2005;53:423–30. doi: 10.1002/art.21178. [DOI] [PubMed] [Google Scholar]
- 12.Goycochea-Robles MV, Salinas CA, Guzmán-Vázquez S, Cardiel-Ríos MH. Prescription rheumatology practices among Mexican specialists. Arch Med Res. 2007;38:354–9. doi: 10.1016/j.arcmed.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Kahn KL, Maclean CH, Wong AL, Rubenstein LZ, Liu H, Fitzpatrick DM, et al. Assessment of American College of Rheumatology quality criteria for rheumatoid arthritis in a pre-quality criteria patient cohort. Arthritis Rheum. 2007;57:707–15. doi: 10.1002/art.22781. [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka H, Inoue E, Singh G, Tanaka E, Nakajima A, Taniguchi A, et al. Improvement of disease activity of rheumatoid arthritis patients from 2000 to 2006 in a large observational cohort study IORRA in Japan. Mod Rheumatol. 2007;17:283–9. doi: 10.1007/s10165-007-0587-6. [DOI] [PubMed] [Google Scholar]
- 15.González-Alvaro I, Descalzo MA, Carmona L. Estudio de la Morbilidad y Expresión Clínica de la Artritis Reumatoide Study Group. Trends towards an improved disease state in rheumatoid arthritis over time: influence of new therapies and changes in management approach: analysis of the EMECAR cohort. Arthritis Res Ther. 2008;10:R138. doi: 10.1186/ar2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teh CL, Wong JS. The pattern and clinical manifestations of rheumatoid arthritis in Sarawak General Hospital. Clin Rheumatol. 2008;27:1437–40. doi: 10.1007/s10067-008-0945-6. [DOI] [PubMed] [Google Scholar]
- 17.DeWitt EM, Lin L, Glick HA, Anstrom KJ, Schulman KA, Reed SD. Pattern and predictors of the initiation of biologic agents for the treatment of rheumatoid arthritis in the United States: an analysis using a large observational data bank. Clin Ther. 2009;31:1871–1880. doi: 10.1016/j.clinthera.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Söderlin MK, Lindroth Y, Turesson C, Jacobsson LT. A more active treatment has profound effects on the health status of rheumatoid arthritis (RA) patients: results from a population-based RA register in Malmö, Sweden, 1997-2005. Scand J Rheumatol. 2010;39:206–11. doi: 10.3109/03009740903313621. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler S, Huscher D, Karberg K, Krause A, Wassenberg S, Zink A. Trends in treatment and outcomes of rheumatoid arthritis in Germany 1997-2007: results from the National Database of the German Collaborative Arthritis Centres. Ann Rheum Dis. 2010;69:1803–8. doi: 10.1136/ard.2009.122101. [DOI] [PubMed] [Google Scholar]
- 20.Montag K, Gingold M, Boers A, Littlejohn G. Disease-modifying anti-rheumatic drug usage, prescribing patterns and disease activity in rheumatoid arthritis patients in community-based practice. Internal medicine journal. 2011;41:450–5. doi: 10.1111/j.1445-5994.2010.02240.x. [DOI] [PubMed] [Google Scholar]
- 21.Sung YK, Cho SK, Choi CB, Park SY, Shim J, Ahn JK, et al. Korean Observational Study Network for Arthritis (KORONA): establishment of a prospective multicenter cohort for rheumatoid arthritis in South Korea. Seminars in arthritis and rheumatism. 2012;41:745–51. doi: 10.1016/j.semarthrit.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Edwards CJ, Arden NK, Fisher D, Saperia JC, Reading I, Van Staa TP, et al. The changing use of disease-modifying anti-rheumatic drugs in individuals with rheumatoid arthritis from the United Kingdom General Practice Research Database. Rheumatology (Oxford) 2005;44:1394–8. doi: 10.1093/rheumatology/kei024. [DOI] [PubMed] [Google Scholar]
- 23.Khanna R, Smith MJ. Utilization and costs of medical services and prescription medications for rheumatoid arthritis among recipients covered by a state Medicaid program: a retrospective, cross-sectional, descriptive, database analysis. Clin Ther. 2007;29:2456–67. doi: 10.1016/j.clinthera.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57:928–34. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 25.Grijalva CG, Chung CP, Stein CM, Mitchel EF, Jr, Griffin MR. Changing patterns of medication use in patients with rheumatoid arthritis in a Medicaid population. Rheumatology (Oxford) 2008;47:1061–4. doi: 10.1093/rheumatology/ken193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmajuk G, Trivedi AN, Solomon DH, Yelin E, Trupin L, Chakravarty EF, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011;305:480–6. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della Rossa A, Neri R, Talarico R, Doveri M, Consensi A, Salvadori S, et al. Diagnosis and referral of rheumatoid arthritis by primary care physician: results of a pilot study on the city of Pisa, Italy. Clin Rheumatol. 2010;29:71–81. doi: 10.1007/s10067-009-1285-x. [DOI] [PubMed] [Google Scholar]
- 28.Westhoff G, Schneider M, Raspe H, Zeidler H, Runge C, Volmer T, et al. Advance and unmet need of health care for patients with rheumatoid arthritis in the German population--results from the German Rheumatoid Arthritis Population Survey (GRAPS) Rheumatology (Oxford) 2009;48:650–7. doi: 10.1093/rheumatology/kep045. [DOI] [PubMed] [Google Scholar]
- 29.Solomon DH, Ayanian JZ, Yelin E, Shaykevich T, Brookhart MA, Katz JN. Use of disease-modifying medications for rheumatoid arthritis by race and ethnicity in the National Ambulatory Medical Care Survey. Arthritis Care Res (Hoboken) 2012;64:184–9. doi: 10.1002/acr.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukas C, Guillemin F, Landewé R, van der Heijde D, Logeart I, Fautrel B, et al. Factors determining a DMARD initiation in early inflammatory arthritis patients. The ESPOIR cohort study. Clin Exp Rheumatol. 2009;27:84–91. [PubMed] [Google Scholar]
- 31.Kiely P, Williams R, Walsh D, Young A. Early Rheumatoid Arthritis Network. Contemporary patterns of care and disease activity outcome in early rheumatoid arthritis: the ERAN cohort. Rheumatology (Oxford) 2009;48:57–60. doi: 10.1093/rheumatology/ken406. [DOI] [PubMed] [Google Scholar]
- 32.Jamal S, Alibhai SM, Badley EM, Bombardier C. Time to treatment for new patients with rheumatoid arthritis in a major metropolitan city. J Rheum. 2011;38:1282–8. doi: 10.3899/jrheum.101315. [DOI] [PubMed] [Google Scholar]
- 33.de Thurah A, Norgaard M, Johansen M, Stengaard-Pedersen K. Time to methotrexate treatment in patients with rheumatoid arthritis referred to hospital. Scand J Rheumatol. 2010;39:19–25. doi: 10.3109/03009740903185987. [DOI] [PubMed] [Google Scholar]
- 34.Rantalaiho V, Kautiainen H, Virta L, Korpela M, Möttönen T, Puolakka K. Trends in treatment strategies and the usage of different disease-modifying anti-rheumatic drugs in early rheumatoid arthritis in Finland. Results from a nationwide register in 2000-2007. Scand J Rheumatol. 2011;40:16–21. doi: 10.3109/03009742.2010.486768. [DOI] [PubMed] [Google Scholar]
- 35.Widdifield J, Bernatsky S, Paterson JM, Thorne JC, Cividino A, Pope J, et al. Quality care in seniors with new-onset rheumatoid arthritis: a Canadian perspective. Arthritis Care Res (Hoboken) 2011;63:53–7. doi: 10.1002/acr.20304. [DOI] [PubMed] [Google Scholar]
- 36.Bonafede MM, Fox KM, Johnson BH, Watson C, Gandra SR. Factors associated with the initiation of disease-modifying antirheumatic drugs in newly diagnosed rheumatoid arthritis: a retrospective claims database study. Clinical therapeutics. 2012;34:457–67. doi: 10.1016/j.clinthera.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng B, Aslam F, Petersen NJ, Yu HJ, Suarez-Almazor ME. Identification of rheumatoid arthritis patients using an administrative database: A Veterans affairs study. Arthritis Care Res (Hoboken) 2012;64:1490–6. doi: 10.1002/acr.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz JN, Barrett J, Liang MH, Bacon AM, Kaplan H, Kieval RI, et al. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum. 1997;40:1594–600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]