Abstract
Over the past 2 decades the measurement of ground reaction forces (GRF) has been extensively used in dogs and cats to gain insights on normal locomotion, discrepancies under pathologic conditions, and biomechanical changes following surgical procedures. Ground reaction forces have become a well-established outcome measure of pain-related functional impairment in animals affected by experimental and naturally occurring osteoarthritis. This paper comprehensively reviews the nature of GRF and presents arguments regarding its measurement in osteoarthritis research.
Résumé
Mesure cinétique de la démarche du chien et du chat en contexte de recherche sur l’arthrose. Au cours des deux dernières décennies, la mesure des forces de réaction au sol (FRS) a été largement utilisée chez les chiens et les chats afin de mieux comprendre la locomotion normale, les anomalies en conditions pathologiques et les changements biomécaniques suivant une procédure chirurgicale. Les FRS au sol sont devenues un critère d’évaluation bien connu de la limitation fonctionnelle liée à la douleur chez l’animal atteint d’arthrose expérimentale et naturelle. Le présent manuscrit dresse un aperçu de la nature des FRS et présente les arguments qui supportent son usage dans un contexte de recherche sur l’arthrose.
(Traduit par les auteurs)
Introduction
Animals move from one point to another by means of sequential and coordinate motion of jointed appendages called limbs. Every time a limb interacts with the ground, the animal’s body is subjected to ground reaction forces (GRF) in response to muscular and inertial forces the limb exerts (1).
Ground reaction forces combined with the downward effect of gravity are external forces acting on a moving body (2). The study of GRF involves the field of kinetics which addresses forces associated with movement (3). Over the past 2 decades the measurement of GRF has increased in dogs and cats, particularly to gain insight on normal locomotion and discrepancies under pathologic conditions (4). In companion animals, GRF are commonly measured using a force platform, accounting for a large fraction of the publications in this field (4). When coupled to kinematic analyses, the measurement of the GRF also provides input for a complete description of the entire mechanical processes of locomotion (5).
Osteoarthritis is a highly prevalent musculoskeletal condition which involves structural changes and disability of the affected joint (6). Ground reaction forces have become well-established outcome measures of functional impairment in dogs and cats with osteoarthritis. This comprehensive review describes the nature of the GRF and derivatives and presents the fundamentals regarding their measurement in dogs and cats with experimental and naturally occurring osteoarthritis. The authors propose the use of GRF as an outcome measure which reflects pain-related functional impairment in the context of osteoarthritis.
Nature of ground reaction forces
Weight and force
There is a clear distinction between mass and weight. The mass of an object, denoted as m and expressed in kg, refers to the amount of matter contained in it. The weight of an object is the force directed downward in the vertical axis (Fvertical) in response to the gravitational acceleration of the body’s center of mass (COM) (Equation 1) (3).
| (Equation 1) |
where: g0 is the standard gravitational acceleration on earth.
Weighing an animal accurately isn’t easy as the reading changes according to the animal’s movement. When the mass of the body is constant, fluctuation in the measurement of the body weight involves additional acceleration (downward or upward) of the whole body. For a moving object, the net Fvertical corresponds to the weight of the body and additional force due to inertia (Equation 2) (1).
| (Equation 2) |
where: avertical is the acceleration (m/s2) of the COM in the vertical axis.
The direction of the additional acceleration (downward or upward) governs the net Fvertical which could therefore be higher or lower than the weight of the moving animal.
Action and reaction forces
Each time an object hits the ground or another object, 2 forces are in opposition: the action and the reaction forces. For each action, there is a reaction equal in magnitude and opposite in direction (3). For a body in contact with the ground, the downward Fvertical generates the same reaction back on the body. This reaction force is referred to as the GRF. When vertical displacement of the COM occurs, the effect of inertial force has to be considered (Equation 3).
| (Equation 3) |
Ground reaction force patterns
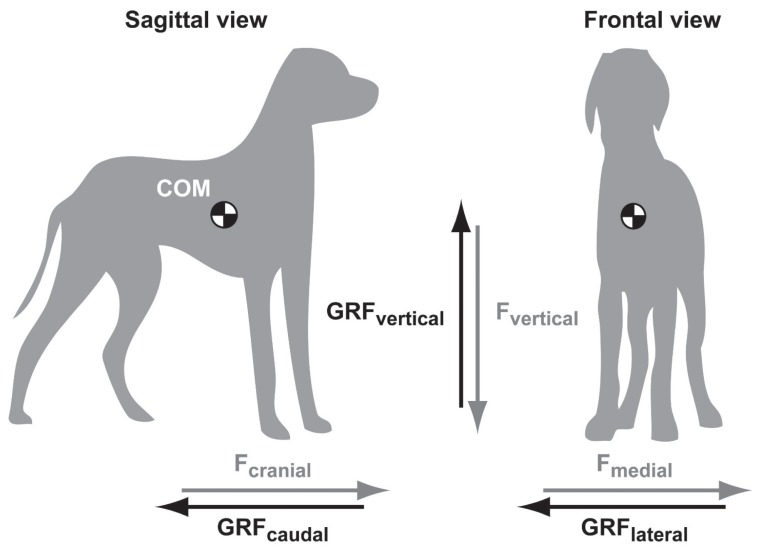
The gait of a quadruped is defined as a manner of moving which can differ according to sequence and rhythm of footfalls and to the number of support limb(s) in each stage of 1 cycle of footfalls (7). Whatever the type of gait involved, a series of rhythmic, alternating movements of the body results in the forward progression of the COM along a horizontal trajectory (8). The linear movement of the COM is disrupted by the alternation of footfalls. In humans, as well as in dogs and cats, the COM executes a sinusoidal displacement in the 3 orthogonal planes from which the direction and the magnitude of the net acceleration govern the presence of GRF in causal relationships (2). For an object having complex shape and different densities such as an animal’s body, direct measurement or calculation is required to precisely determine limb inertial parameters and thereby the COM. In dogs, although the COM is in close relationship with the length of the neck (7), the common position of the COM is illustrated in Figure 1 as previously described (9).
Figure 1.
Sagittal and frontal views of a dog in a standing position. The three orthogonal force (F) vectors are paired with their oppositely directed ground reaction forces (GRF). The center of mass (COM) is located according to previously published scheme (9) and is only indicative of its exact position. The Fmedial refers to the right limbs.
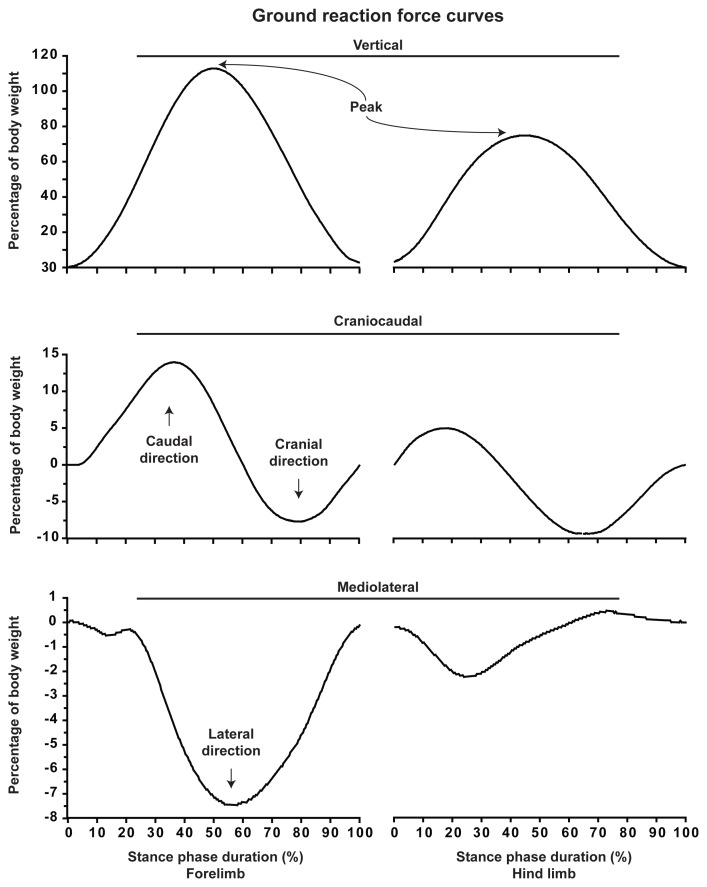
Movements of the COM give rise to Fcraniocaudal, Fmediolateral, and Fvertical (Figure 1). As a result, GRFcraniocaudal, GRFmediolateral, and GRFvertical are produced during the stance phase. Typical GRF versus time curves are illustrated in Figure 2. The GRF vectors are expressed herein as positive when directed upward, cranially, and medially.
Figure 2.
Typical curves of the ground reaction forces. Measurements are from a 27-kg golden retriever dog crossing the force plate at a trotting gait velocity (2.0 m/s). Ground reaction forces are considered positive when directed upward, cranially, and medially. The stance phase is expressed for the forelimb and hind limb to ease their distinction. Peaks denote the points of maximal values of the fore and hind limb GRFvertical.
Vertical ground reaction force
At the initiation of the stance phase, the GRFvertical begins to be measured as the mass of the dog in motion is gradually supported by the limb (Figure 2). Soon after, a maximal point is attained which is the highest product of the mass of the body and the net vertical acceleration of the COM. This point is referred as the peak of the GRFvertical (i.e., peak vertical force). The GRFvertical then decreases until toe-off which defines the end of the stance phase. In some situations the pelvic limb begins the stance phase when the thoracic limb is still in contact with the ground. This overlap explains why the GRFvertical doesn’t fall to zero when the thoracic limb has left the ground.
For a dog standing still on 4 limbs, the body weight is expected to be supported at 30% by each thoracic limb and at 20% by each pelvic limb (10). The COM being closer to the thorax contributes to this imbalance in pelvic-to-thorax weight distribution when standing (10). As illustrated in Figure 2, the GRFvertical of the thoracic limb reaches a maximal point (i.e., 113% of body weight) which is in accordance with the literature (11) (see Equation 3). Hence, the body absorbs a high level of forces in the vertical axis reaching more than 3 times the one observed at the stance (i.e., 30% versus 113% of body weight).
Craniocaudal and mediolateral ground reaction forces
The pattern of the GRFcraniocaudal involves successive caudal and cranial components (Figure 2). The GRFcaudal is in fact generated as a reaction to the force applied in the direction of movement (i.e., cranially or forward). Hence, as a result of the Fcranial, a GRF is generated and directed caudally (i.e., backward) which decelerates the dog’s motion. During the second half of the stance phase, the GRFcranial propels the dog forward.
A third force vector is also depicted during the gait of dog. Figure 2 illustrates the GRFmediolateral, which involves medial and lateral components. Although the pattern of GRFmediolateral grossly mimics those reported in the literature (12), there is no clear waveform established for GRFmediolateral in dogs and cats due to inconsistent results (10,12).
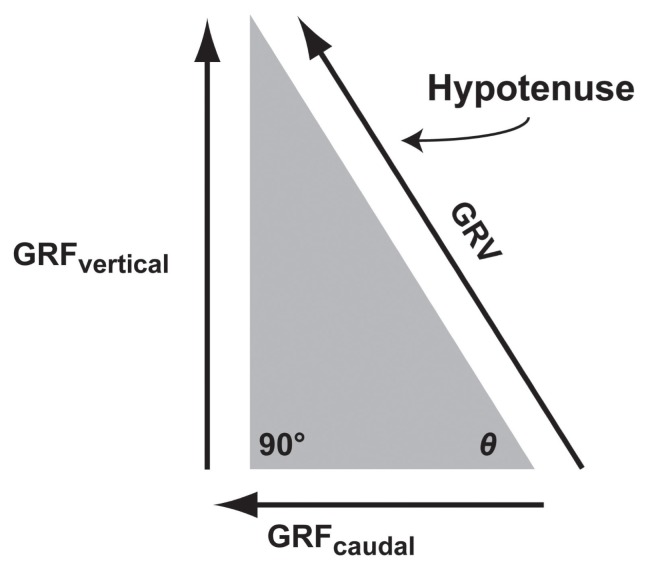
Ground reaction vector
The 3 GRF components are directed in opposite directions by 90° (Figure 1). The resultant GRF, which is the net effect of the 3 orthogonal GRF components, is called the ground reaction vector (GRV). In the sagittal plane, a right-angled triangle is formed by the GRFcraniocaudal (adjacent side) and the GRFvertical (opposite side) of the angle θ (Figure 3) (1). The hypotenuse, which defines the magnitude and orientation of the GRV, the GRF in the sagittal plane can be resolved according to the Pythagorean theorem (Equations 4 and 5) (1).
Figure 3.
Ground reaction vector. The concept of sagittal GRF ground reaction vector (GRV) is illustrated using a right angled triangle according to the Pythagorean theorem. In the sagittal plane, vertical ground reaction force (GRFvertical), and GRFcraniocaudal (caudal component) are orthogonal, giving the magnitude of the GRV as the square root of GRFvertical2 + GRFcraniocaudal2. The angle θ refers to the direction of the GRV obtained by the tangential−1 of GRFvertical/GRFcraniocaudal.
| (Equation 4) |
| (Equation 5) |
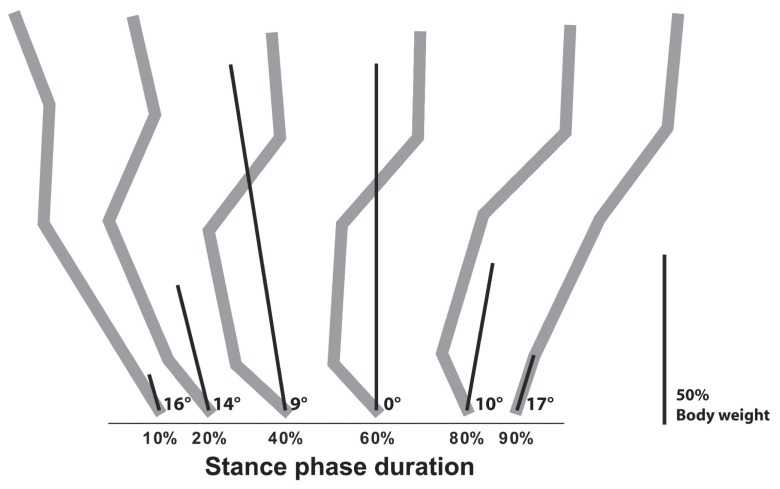
The sagittal GRV derived from the measures of a typical dog are illustrated at selected time points in Figure 4. As shown, the direction and the magnitude of the forelimb sagittal GRF GRV evolve over time. At 60% of the stance phase, the sagittal GRF GRV is perpendicular to the GRFcraniocaudal while being directed cranially thereafter. This force vector diagram can be used to determine where the path of the sagittal GRF GRV travels according to anatomical structures or joints at specific points of the stance phase (i.e., caudal to the brachialis muscle, through the carpal joint). In cats, the GRF vector was used to investigate walking strategy and muscle activity under different conditions (13). The GRV can also serve to calculate moment of force, which is the tendency of a force to rotate an object. A moment of force is denoted as M and expressed in newton-meters (Nm) (Equation 6) (3).
Figure 4.
Progression of the ground reaction vector. Illustration of the progression of the ground reaction vector (GRV) for a 27-kg dog crossing the force plate at a trotting gait velocity (2.0 m/s). The measures are for the forelimb. The degrees indicate the direction of the GRV at specific points of the stance phase. The length of the lines indicates the magnitude of the GRV. The direction and magnitude of the GRV are calculated according to the ground reaction force value presented in Figure 2. In the background, forelimb bone position is detailed in gray as previously illustrated (7) and is only indicative of its exact position. The length of the bar indicates 50% of body weight.
| (Equation 6) |
where: d is the perpendicular distance from the center of rotation to the force vector.
Center of force
The center of force (COF) is a coordinate pair (x,y) located within the surface of any parts of the body which are in contact with the ground. The COF corresponds to the average location where all the Fvertical act. When an animal is moving, the distribution of the Fvertical within the paw is modified and the COF changes accordingly. The COF can also be depicted as a trajectory according to its displacement during the stance phase. In humans, the path of the COF moves in a curvilinear fashion from heel to toes (14). In the dog, little is known about the COF trajectory. However, the Fvertical appears to show a distribution pattern among the pads in dogs (15). The COF can serve to determine limb positioning, and serve to precisely define where the GRV takes its origin (1,16).
Impulse, linear momentum, and power
The area under any force versus time curve represents the impulse denoted as I, which is expressed in N × s or in kg × m/s. Impulse is the time integral of a force (Equation 7) (3).
| (Equation 7) |
where: t is the time (s).
The linear momentum of a moving object is the product of the mass and velocity and is denoted as p, which is expressed in N × s or in kg × m/s (Equation 8) (3).
| (Equation 8) |
where: v is the velocity (m/s).
According to the impulse-momentum relationship, the impulse for a given time interval represents the change in linear momentum (Equation 9) (17).
| (Equation 9) |
It is common to report the impulse of the measured GRFvertical and GRFcraniocaudal which reflects the transfer of momentum from a moving limb to the ground. As illustrated in Figure 2, the forelimb undergoes craniocaudal transfer of linear momentum which is higher in the caudal direction. Conversely, the hind limb has a net linear momentum in the cranial direction. The overall net momentum (i.e., fore and hind limbs) has to be null in the craniocaudal axis to maintain a constant gait velocity, otherwise the dog undergoes acceleration or deceleration (16).
During gait, muscles generate or absorb power due to their ability to perform concentric or eccentric activities (3). Power, denoted as P and expressed in watts (W) or in Nm/s is the product of the force and its velocity (Equation 10) (1).
| (Equation 10) |
Using Equation 9, the net power output of a limb can be determined by multiplying the sum of the GRF (i.e., GRFcraniocaudal, GRFmediolateral and GRFvertical) by the velocity of the animal (18).
Ground reaction force measurement devices
Force transducers are typically designed to measure the strain in a material under load. An electrical output proportional to the applied force is generated and amplified. The most common type of device used to measure the GRF exerted by the body during locomotion is the force platform which consists of a steel plate with force transducers at each corner.
The force platform allows the measurement of GRF using strain gauges or piezoelectric crystals as a sensing element (3). As GRF have components in the 3 orthogonal planes, the force platform should have sensing elements arranged to capture all oriented strains for a complete measurement of the GRF generated during the locomotion of the animal. Force platforms can be used alone (10) or combined to allow the simultaneous measurement of the GRF among the 4 limbs (19). Suitable and reproducible measurement of the GRFvertical is also possible when integrated into a treadmill (20).
There is another type of device which allows the measurement of the GRFvertical as well as the surface of the body in contact with the ground (https://www.dropbox.com/s/002iu3l98jzssj8/Kinetic%20gait%20analysis.wmv?dl=0). This involves the concept of pressure, defined as a force expressed per unit area (3). Using sensitive elements which base their properties on the presence of conductive material and pressure-sensitive ink, it is possible to obtain the force/pressure-distribution pattern of a given paw during the stance phase. When integrated into a portable walkway (several feet long and a few millimeters thick), the plantar force/pressure measurement system allows the acquisition of GRFvertical from simultaneous and consecutive footfalls over several strides, which is particularly relevant for small- to medium-sized quadrupeds (21,22). This device restricts their measurement to the GRFvertical. A secondary role for the GRFcraniocaudal and GRFmediolateral is therefore assumed when using this device.
Ground reaction force acquisition setting
A typical force platform requires a long walkway (6 to 10 m) with the force platform positioned near the center to favor free motion and to avoid intuitive braking at the end of the runaway. The force platform can be either embedded in the floor or mounted flush with the surface of an elevated (5 to 8 cm) walkway. The walkway must be carefully made to avoid any vibration or echo, which can alter the signal (back noise) and disturb the animal. Effort must be addressed to avoid visual recognition or texture difference between the surfaces of the floor and the force platform.
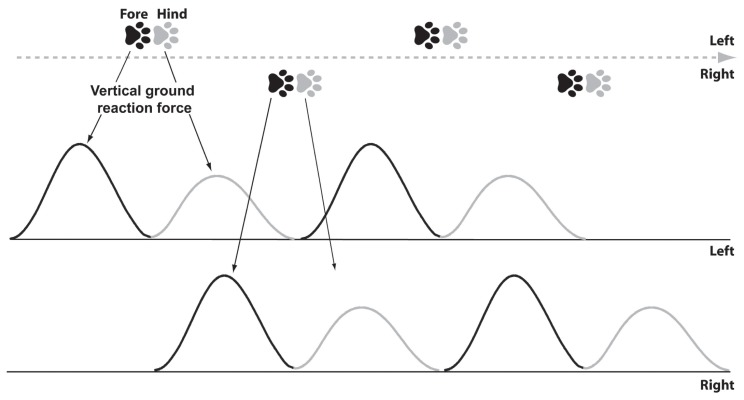
At the trot, a typical acceptable trial involves a single thoracic limb on the force plate followed by its pelvic counterpart (Figure 5). The limb must be in full contact with the surface of the force platform. Visual inspection or a posteriori video monitoring ensures quality of the trial and limb distinction. After unsuccessful attempts to obtain the desired limb on the force plate, the handler may have to modify the starting position. In numerous published investigations, 5 valid trials are acquired to record the GRF before being averaged or used as per trial measurements.
Figure 5.
Patterns of footfalls. Illustration of patterns of footfalls from a 23-kg dog crossing a force/pressure measurement system at a trotting gait velocity (2.0 m/s). The corresponding typical curves of the vertical ground reaction forces are also illustrated. For this dog, a cycle of footfalls has a 0.5-second duration and 0.5-meter long. Both fore and hind limb footfalls usually overlap, which is not illustrated here for clarity purposes.
The use of a common force platform is generally restricted to large quadrupeds (> 20 kg). In smaller dogs and in cats, the presence of more than a paw in contact with the force platform compromises the measurement by a summation process. Specially designed devices are required to record GRF in such animals (13,23,24). The general reluctance of cats to be handled with a leash also contributes to the paucity of force platform data in this species. Researchers use a plantar force/pressure measurement system in freely moving or thoroughly conditioned cats (21) (https://www.dropbox.com/s/002iu3l98jzssj8/Kinetic%20gait%20analysis.wmv?dl=0).1
The repeatability of the GRF measurement is of paramount importance. The peak of the GRFvertical has low dispersion in dogs (25) and thoroughly conditioned cats using a plantar force/pressure measurement system (26). Critical factors that need to be kept constant when measuring GRF are the velocity and acceleration of the whole body (27) which can be monitored using a set of 3 to 5 photoelectric cells. In the plantar force/pressure measurement system, the velocity of the animal is computed using time and distance indices.
To further improve the quality of the GRF measurement, the animals must be morphologically identical; however, the in-vivo reality brings high mass and anatomical size heterogeneities. According to the theory of dynamic similarity, cursorial quadrupeds move in a dynamically similar fashion. Therefore, GRF measures can be compared between different species as diverse as dogs, camels, and rhinoceroses (28). However, care should be taken to ensure that animals move at the same relative “body size-normalized” velocity (11), which is achieved by multiplying linear dimensions, time intervals, and GRF measures by different constants (29,30). Accordingly, it is common to normalize the peak of the GRF to the body weight of the animal and later express this measure in percentage of body weight. This normalization technique improves the homogeneity of the peak of the GRFvertical measured between animals while remaining subjected to intrinsic variation in animals (dog mainly) showing atypical body conformations (11).
Ground reaction force measurement in experimental model of osteoarthritis
Joint inflammatory pain
Osteoarthritis involves pain, functional limitation, and structural changes to the cartilage and other joint structures. This disease is highly prevalent in companion animals (31). The hallmark clinical manifestation of osteoarthritis is joint pain which occurs through inflammation (32) and a potentially associated peripheral and/or central nociceptive sensitization (33). Crippling joint pain has the potential to alter normal function, particularly locomotion. The phenomenon is referred to as pain-induced functional impairment and is one of the clinical signs of osteoarthritis (34).
Joint pain causes alteration in the normal use of the limb as demonstrated following injection of noxious substances into the synovial space of the stifle (35). The inflammation-related pain in this model is reflected to the musculoskeletal system by reluctance to support the body weight and inertial force, compromising standing and leading to a change in the gait pattern.
The transient decrease in GRFvertical induced in this model is used as an indicator of pain-related functional impairment to evaluate the potential of compounds having analgesic claims in dogs and cats (36). An accelerated restoration of function toward initial levels is then expected from the analgesia provided.
Structural changes of osteoarthritis
Structural osteoarthritis changes can be induced by surgical procedures such as meniscal lesions, varus and valgus osteotomy, myectomy, patellectomy, cartilage scarification, and transarticular impacts (37). In dogs and cats, a well-known surgical model of osteoarthritis is the cranial cruciate ligament transection (CCLT) of the stifle joint. The instability caused by sectioning of this passive primary stabilizer induces deleterious mechanical alterations, particularly excessive rotational and translational movements (38). Abnormal loading of specific regions of the joint, along with the release of catabolic and inflammatory mediators, disrupts the normal subchondral bone remodeling process, damages the cartilage, and inflames synovial tissues in a manner similar to human post-traumatic osteoarthritis (23,38,39).
Early after CCLT, there is an acute inflammatory process and a severe decrease in GRFvertical followed by gradual improvement over time (40,41). Up to 4 y after surgery, the GRF remain altered in dogs (42), while recovering to near pre-surgical level in about 1 year in cats (24). In addition to impairment of the surgically altered stifle, abnormalities also occur in the controlateral limb as denoted by an increase in GRFvertical. The redistribution of body weight and inertial forces to the controlateral limb was reported to be under the control of sensory feedback from the unstable joint to protect against a worsening of structural changes (43). This transfer, however, invalidates the use of the controlateral limb as a normal control in this model (44).
The GRFvertical which was shown to be a reliable measure in dogs undergoing CCLT (42) was used as an indicator of pain-related functional impairment in this model (45). Hence, beside the conventional tests for benefits associated with structural joint changes of osteoarthritis, investigators can gain insights on another feature of the disease process using GRF measurement; the level of disability associated with the use of the limb. This was recently confirmed by our group (46) demonstrating a structure — function (pain) relationship in the canine CCLT model, similar to the one observed in humans. Structure was assessed noninvasively by magnetic resonance imaging while the GRF measurement allowed a serial evaluation of pain-related functional impairment. This could explain the impressive translational predictability recorded from this model with products subsequently tested in humans (46). Other experimental models of osteoarthritis have been investigated using GRF to validate alteration in kinetics, including a postponed disruption of the CCL by monopolar radiofrequency (47).
Ground reaction forces measurement in naturally occurring osteoarthritis
Force platforms provide objective, repeatable, and clinically meaningful information about the functional abilities in dogs and cats affected by naturally occurring osteoarthritis (21,48). The GRFvertical has been shown to be abnormally lower in these species when radiographic signs of osteoarthritis are present (21,25,48). However, the relationship between severity of the structural changes of osteoarthritis, as assessed on radiographs, and the presence of abnormal GRF measurement is poor (49) and therefore not well understood. Moreover, the level of disability seems to differ according to the joint in which the structural changes of osteoarthritis are present. Dogs affected by osteoarthritis at the stifle have a more severe decrease in the GRFvertical compared with those having osteoarthritis of the hip joint (25).
The pain generated by osteoarthritis has the potential to alter the normal function of the limb, a phenomenon referred to as functional allodynia. The peak of the GRFvertical showed sensitivity and responsiveness for therapeutic approaches purported to alleviate the pain-related functional impairment in dogs and cats affected by osteoarthritis. Therefore, drug research and development often rely on randomized, placebo-controlled clinical trials with sequential GRF measurements to support pain alleviating properties of therapeutic compounds under investigation. In most cases, improvement is translated as an increment over the initial condition, as previously noted following non-steroidal anti-inflammatory drugs (50–53), complementary and alternative medicine (50,54,55), and veterinary therapeutic diets (56). Clinical improvement is further indicated when the change in GRF exceeds the one observed with a negative control (placebo) or when similar to a positive control such as a homologated treatment (55). The improvement in GRF measurement is not systematic, and the range of efficacy could reflect the nature of the tested product adapted (or not) to the inflammatory or neuropathic component of pain (46).
Other factors that influence pain-related functional impairment in the presence of osteoarthritis need to be taken into account. Recently, it was shown that changes in the body mass interfere with the measurement of the GRFvertical in dogs. Hence, when an increase in body mass occurred, dogs did not experience a proportional increase in the peak of the GRFvertical (57) suggesting a change in the gait pattern in the presence of osteoarthritis. In line with this finding, the level of impairment improved in obese osteoarthritic dogs when a decrease in body mass occurred (58). The impact of exercise in osteoarthritic dogs and cats (26) was also evaluated using GRF measurement. While daily leash walks improve the abnormal level of GRFvertical (59), care should be directed to avoid intensive exercise which exacerbates limb impairment (60).
In conclusion, the musculoskeletal systems of quadrupeds such as dogs and cats are able to modify the gait pattern in response to painful stimulation of the joint. In both experimental and clinical contexts of osteoarthritis, measurement of GRF allows quantification of the functional impairment. The measurement of GRF is therefore an outcome measure in osteoarthritis research, which reflects pain-related functional impairment.
Some issues need to be resolved. The first and by far the most critical one is identification of the structural changes that intervene in the pain-related functional impairment. The role of cartilage thinning and focal lesion, bone and meniscus alteration, and osteophyte growth as assessed using sensitive methods needs to be put in relationship with the GRF. Some work has been initiated on this issue (46). Whether or not the impairment is exclusively due to pain or involves biomechanical alterations in the normal dynamics and lubrication of the joint is unknown and remains of particular interest for osteoarthritis research.
The measurement of GRF leads to several raw parameters and derivatives. Precautions must be taken to determine a valid (or clinically significant) primary outcome measure. The peak of the GRFvertical is often selected to discern treatment efficacy in osteoarthritis. Measurement error (noise) and minimal detectable change for this endpoint have been established in osteoarthritic dogs (46), but not osteoarthritic cats. Such information is critical to discern that a change is meaningful and not a difference that might be reasonably expected from the measurement error. Without this information, investigators are unable to distinguish a placebo (or a nocebo) effect from normal variation. How changes in GRF measurement in dogs and cats affected by osteoarthritis evolve in response to sustained daily life activity is also relevant and has to be determined to improve the quality of data and conclusions from randomized controlled trials. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Video of the recording of vertical ground reaction force in a thoroughly conditioned cat using a plantar force/pressure measurement system. This system allows the acquisition of GRFvertical from simultaneous and consecutive footfalls over several strides, which is particularly relevant for small- to medium-sized quadrupeds.
References
- 1.Kirtley C. Clinical Gait Analysis: Theory and Practice. London, England: Elsevier Churchill Livingston; 2006. pp. 5–199. [Google Scholar]
- 2.DeLisa JA, Scientific USVHA, Section TP. Gait Analysis in the Science of Rehabilitation. Baltimore, Maryland: Diane Publishing; 1998. pp. 50–68. [Google Scholar]
- 3.Robertson DGE, Caldwell GE, Hamil J, Kamen G, Whittlesey SN. Research Methods in Biomechanics. Champaign, Illinois: Human Kinetics; 2004. pp. 73–102. [Google Scholar]
- 4.Gillette RL, Angle TC. Recent developments in canine locomotor analysis: A review. Vet J. 2008;178:165–176. doi: 10.1016/j.tvjl.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Ragetly CA, Griffon DJ, Mostafa AA, Thomas JE, Hsiao-Wecksler ET. Inverse dynamics analysis of the pelvic limbs in Labrador Retrievers with and without cranial cruciate ligament disease. Vet Surg. 2010;39:513–522. doi: 10.1111/j.1532-950X.2010.00680.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnston SA. Overview of pain in the lame patient. Vet Clin North Am Small Anim Pract. 2001;31:39–53. doi: 10.1016/s0195-5616(01)50037-6. [DOI] [PubMed] [Google Scholar]
- 7.Brown CM, Dalzell B. Dog Locomotion and Gait Analysis. Wheat Ridge, Colorado: Hoflin Publishing; 1986. pp. 1–154. [Google Scholar]
- 8.Marasovic T, Cecic M, Zanchi V. Analysis and interpretation of ground reaction forces in normal gait. WTOS. 2009;8:1105–1114. [Google Scholar]
- 9.Lee DV, Bertram JE, Todhunter RJ. Acceleration and balance in trotting dogs. J Exp Biol. 1999;202:3565–3573. doi: 10.1242/jeb.202.24.3565. [DOI] [PubMed] [Google Scholar]
- 10.Budsberg SC, Verstraete MC, Soutas-Little RW. Force plate analysis of the walking gait in healthy dogs. Am J Vet Res. 1987;48:915–918. [PubMed] [Google Scholar]
- 11.Voss K, Wiestner T, Galeandro L, Hassig M, Montavon PM. Effect of dog breed and body conformation on vertical ground reaction forces, impulses, and stance times. Vet Comp Orthop Traumatol. 2011;24:106–112. doi: 10.3415/VCOT-10-06-0098. [DOI] [PubMed] [Google Scholar]
- 12.Rumph PF, Lander JE, Kincaid SA, Baird DK, Kammermann JR, Visco DM. Ground reaction force profiles from force platform gait analyses of clinically normal mesomorphic dogs at the trot. Am J Vet Res. 1994;55:756–761. [PubMed] [Google Scholar]
- 13.Kaya M, Jinha A, Leonard TR, Herzog W. Multi-functionality of the cat medical gastrocnemius during locomotion. J Biomech. 2005;38:1291–1301. doi: 10.1016/j.jbiomech.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Jamshidi N, Rostami M, Najarian S, Menhaj MB, Saadatnia M, Salami F. Differences in center of pressure trajectory between normal and steppage gait. J Res Med Sci. 2010;15:33–40. [PMC free article] [PubMed] [Google Scholar]
- 15.Besancon MF, Conzemius MG, Evans RB, Ritter MJ. Distribution of vertical forces in the pads of Greyhounds and Labrador Retrievers during walking. Am J Vet Res. 2004;65:1497–1501. doi: 10.2460/ajvr.2004.65.1497. [DOI] [PubMed] [Google Scholar]
- 16.Lee DV, Stakebake EF, Walter RM, Carrier DR. Effects of mass distribution on the mechanics of level trotting in dogs. J Exp Biol. 2004;207:1715–1728. doi: 10.1242/jeb.00947. [DOI] [PubMed] [Google Scholar]
- 17.Seliktar R, Yekutiel M, Bar A. Gait consistency test based on the impulse-momentum theorem. Prosthet Orthot Int. 1979;3:91–98. doi: 10.3109/03093647909103089. [DOI] [PubMed] [Google Scholar]
- 18.Irschick DJ, Vanhooydonck B, Herrel A, Andronescu A. Effects of loading and size on maximum power output and gait characteristics in geckos. J Exp Biol. 2003;206:3923–3934. doi: 10.1242/jeb.00617. [DOI] [PubMed] [Google Scholar]
- 19.Bertram JE, Lee DV, Case HN, Todhunter RJ. Comparison of the trotting gaits of Labrador Retrievers and Greyhounds. Am J Vet Res. 2000;61:832–838. doi: 10.2460/ajvr.2000.61.832. [DOI] [PubMed] [Google Scholar]
- 20.Bockstahler BA, Skalicky M, Peham C, Muller M, Lorinson D. Reliability of ground reaction forces measured on a treadmill system in healthy dogs. Vet J. 2007;173:373–378. doi: 10.1016/j.tvjl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Guillot M, Moreau M, d’Anjou MA, Martel-Pelletier J, Pelletier JP, Troncy E. Evaluation of osteoarthritis in cats: Novel information from a pilot study. Vet Surg. 2012;41:328–335. doi: 10.1111/j.1532-950X.2012.00976.x. [DOI] [PubMed] [Google Scholar]
- 22.Moreau M, Rialland P, Pelletier JP, et al. Tiludronate treatment improves structural changes and symptoms of osteoarthritis in the canine anterior cruciate ligament model. Arthritis Res Ther. 2011;13:R98. doi: 10.1186/ar3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd SK, Muller R, Leonard T, Herzog W. Long-term periarticular bone adaptation in a feline knee injury model for post-traumatic experimental osteoarthritis. Osteoarthritis Cartilage. 2005;13:235–242. doi: 10.1016/j.joca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Suter E, Herzog W, Leonard TR, Nguyen H. One-year changes in hind limb kinematics, ground reaction forces and knee stability in an experimental model of osteoarthritis. J Biomech. 1998;31:511–517. doi: 10.1016/s0021-9290(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 25.Madore E, Huneault L, Moreau M, Dupuis J. Comparison of trot kinetics between dogs with stifle or hip arthrosis. Vet Comp Orthop Traumatol. 2007;20:102–107. doi: 10.1160/vcot-06-06-0052. [DOI] [PubMed] [Google Scholar]
- 26.Moreau M, Guillot M, Pelletier J-P, Martel-Pelletier J, Troncy É. Kinetic peak vertical force measurement in cats afflicted by coxarthritis: Data management and acquisition protocols. Res Vet Sci. 2013 doi: 10.1016/j.rvsc.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Riggs CM, DeCamp CE, Soutas-Little RW, Braden TD, Richter MA. Effects of subject velocity on force plate-measured ground reaction forces in healthy greyhounds at the trot. Am J Vet Res. 1993;54:1523–1526. [PubMed] [Google Scholar]
- 28.Alexander RM, Jayes AS. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J Zool. 1983;201:135–152. [Google Scholar]
- 29.Donelan JM, Kram R. The effect of reduced gravity on the kinematics of human walking: A test of the dynamic similarity hypothesis for locomotion. J Exp Biol. 1997;200:3193–3201. doi: 10.1242/jeb.200.24.3193. [DOI] [PubMed] [Google Scholar]
- 30.Duncan WJ. Physical similarity and dimensional analysis: An elementary treatise. London, England: Edward Arnold; 1953. pp. 1–156. [Google Scholar]
- 31.Johnston SA. Overview of pain in the lame patient. Vet Clin North Am Small Anim Pract. 2001;31:39–53. doi: 10.1016/s0195-5616(01)50037-6. [DOI] [PubMed] [Google Scholar]
- 32.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 33.Lascelles BD. Getting a sense of sensations. Vet J. 2013;197:115–117. doi: 10.1016/j.tvjl.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: Recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 35.McCarty DJ, Jr, Phelps P, Pyenson J. Crystal-induced inflammation in canine joints. I. An experimental model with quantification of the host response. J Exp Med. 1966;124:99–114. doi: 10.1084/jem.124.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll GL, Narbe R, Peterson K, Kerwin SC, Taylor L, DeBoer M. A pilot study: Sodium urate synovitis as an acute model of inflammatory response using objective and subjective criteria to evaluate arthritic pain in cats. J Vet Pharmacol Ther. 2008;31:456–465. doi: 10.1111/j.1365-2885.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- 37.Lampropoulou-Adamidou K, Lelovas P, Karadimas EV, et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2013 doi: 10.1007/s00590-013-1205-2. [DOI] [PubMed] [Google Scholar]
- 38.Tashman S, Anderst W, Kolowich P, Havstad S, Arnoczky S. Kinematics of the ACL-deficient canine knee during gait: Serial changes over two years. J Orthop Res. 2004;22:931–941. doi: 10.1016/j.orthres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Brandt KD, Dieppe P, Radin EL. Commentary: Is it useful to subset “primary” osteoarthritis? A critique based on evidence regarding the etiopathogenesis of osteoarthritis. Semin Arthritis Rheum. 2009;39:81–95. doi: 10.1016/j.semarthrit.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor BL, Visco DM, Heck DA, Myers SL, Brandt KD. Gait alterations in dogs after transection of the anterior cruciate ligament. Arthritis Rheum. 1989;32:1142–1147. doi: 10.1002/anr.1780320913. [DOI] [PubMed] [Google Scholar]
- 41.Trumble TN, Billinghurst RC, McIlwraith CW. Correlation of prostaglandin E2 concentrations in synovial fluid with ground reaction forces and clinical variables for pain or inflammation in dogs with osteoarthritis induced by transection of the cranial cruciate ligament. Am J Vet Res. 2004;65:1269–1275. doi: 10.2460/ajvr.2004.65.1269. [DOI] [PubMed] [Google Scholar]
- 42.Budsberg SC. Long-term temporal evaluation of ground reaction forces during development of experimentally induced osteoarthritis in dogs. Am J Vet Res. 2001;62:1207–1211. doi: 10.2460/ajvr.2001.62.1207. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor BL, Visco DM, Rogers PI, Mamlin LA, Brandt KD. Serial force plate analyses of dogs with unilateral knee instability, with or without interruption of the sensory input from the ipsilateral limb. Osteoarthritis Cartilage. 1999;7:567–573. doi: 10.1053/joca.1999.0261. [DOI] [PubMed] [Google Scholar]
- 44.Rumph PF, Kincaid SA, Visco DM, Baird DK, Kammermann JR, West MS. Redistribution of vertical ground reaction force in dogs with experimentally induced chronic hindlimb lameness. Vet Surg. 1995;24:384–389. doi: 10.1111/j.1532-950x.1995.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 45.Smith G, Jr, Myers SL, Brandt KD, Mickler EA, Albrecht ME. Effect of intraarticular hyaluronan injection on vertical ground reaction force and progression of osteoarthritis after anterior cruciate ligament transection. J Rheumatol. 2005;32:325–334. [PubMed] [Google Scholar]
- 46.Moreau M, Pelletier JP, Lussier B, et al. A posteriori comparison of natural and surgical destabilization models of canine osteoarthritis. Biomed Res Int. 2013:31. doi: 10.1155/2013/180453. ID:180453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez MJ, Kunz D, Vanderby R, Jr, Heisey D, Bogdanske J, Markel MD. A comparison of joint stability between anterior cruciate intact and deficient knees: A new canine model of anterior cruciate ligament disruption. J Orthop Res. 2003;21:224–230. doi: 10.1016/S0736-0266(02)00132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordquist B, Fischer J, Kim SY, et al. Effects of trial repetition, limb side, intraday and inter-week variation on vertical and craniocaudal ground reaction forces in clinically normal Labrador Retrievers. Vet Comp Orthop Traumatol. 2011;24:435–444. doi: 10.3415/VCOT-11-01-0015. [DOI] [PubMed] [Google Scholar]
- 49.Gordon WJ, Conzemius MG, Riedesel E, et al. The relationship between limb function and radiographic osteoarthrosis in dogs with stifle osteoarthrosis. Vet Surg. 2003;32:451–454. doi: 10.1053/jvet.2003.50051. [DOI] [PubMed] [Google Scholar]
- 50.Guillot M, Moreau M, Heit M, Martel-Pelletier J, Pelletier JP, Troncy E. Characterization of osteoarthritis in cats and meloxicam efficacy using objective chronic pain evaluation tools. Vet J. 2013 doi: 10.1016/j.tvjl.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Moreau M, Dupuis J, Bonneau NH, Desnoyers M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152:323–329. doi: 10.1136/vr.152.11.323. [DOI] [PubMed] [Google Scholar]
- 52.Moreau M, Lussier B, Doucet M, Vincent G, Martel-Pelletier J, Pelletier JP. Efficacy of licofelone in dogs with clinical osteoarthritis. Vet Rec. 2007;160:584–588. doi: 10.1136/vr.160.17.584. [DOI] [PubMed] [Google Scholar]
- 53.Vasseur PB, Johnson AL, Budsberg SC, et al. Randomized, controlled trial of the efficacy of carprofen, a nonsteroidal anti-inflammatory drug, in the treatment of osteoarthritis in dogs. J Am Vet Med Assoc. 1995;206:807–811. [PubMed] [Google Scholar]
- 54.Aragon CL, Hofmeister EH, Budsberg SC. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc. 2007;230:514–521. doi: 10.2460/javma.230.4.514. [DOI] [PubMed] [Google Scholar]
- 55.Hielm-Bjorkman A, Tulamo RM, Salonen H, Raekallio M. Evaluating complementary therapies for canine osteoarthritis — Part II: A homeopathic combination preparation (Zeel) Evid Based Complement Alternat Med. 2009;6:465–471. doi: 10.1093/ecam/nem143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreau M, Troncy E, Del Castillo JR, Bedard C, Gauvin D, Lussier B. Effects of feeding a high omega-3 fatty acids diet in dogs with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2012 doi: 10.1111/j.1439-0396.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 57.Moreau M, Troncy E, Bichot S, Lussier B. Influence of changes in body weight on peak vertical force in osteoarthritic dogs: A possible bias in study outcome. Vet Surg. 2010;39:43–47. doi: 10.1111/j.1532-950X.2009.00621.x. [DOI] [PubMed] [Google Scholar]
- 58.Marshall WG, Hazewinkel HA, Mullen D, De Meyer G, Baert K, Carmichael S. The effect of weight loss on lameness in obese dogs with osteoarthritis. Vet Res Commun. 2010;34:241–253. doi: 10.1007/s11259-010-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mlacnik E, Bockstahler BA, Muller M, Tetrick MA, Nap RC, Zentek J. Effects of caloric restriction and a moderate or intense physiotherapy program for treatment of lameness in overweight dogs with osteoarthritis. J Am Vet Med Assoc. 2006;229:1756–1760. doi: 10.2460/javma.229.11.1756. [DOI] [PubMed] [Google Scholar]
- 60.Beraud R, Moreau M, Lussier B. Effect of exercise on kinetic gait analysis of dogs afflicted by osteoarthritis. Vet Comp Orthop Traumatol. 2010;23:87–92. doi: 10.3415/VCOT-09-06-0068. [DOI] [PubMed] [Google Scholar]