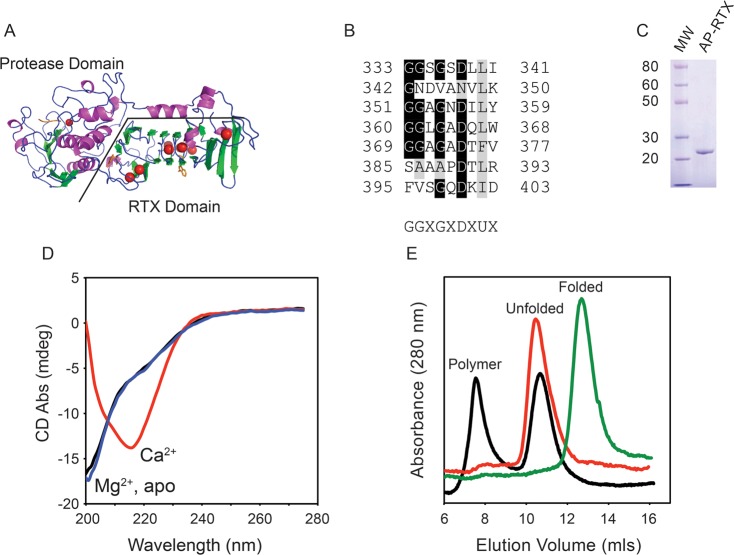
Figure 1.
Calcium-regulated conformation of the RTX domain. The calcium-dependent conformational changes of the alkaline protease domain from P. aeruginosa were evaluated in vitro. (A) Cartoon of the determined structure of alkaline protease. The N-terminal protease domain and a C-terminal, β-helix RTX domain are indicated. Calcium ions are shown as red spheres. (B) Individual RTX nonapeptide repeats from the Pseudomonas alkaline protease after their alignment. The nonapetide consensus sequence, G-G-X-G-X-(D/N)-X-U-X, is shown below, where X is any amino acid and U is a hydrophobic amino acid. (C) Representative Coomassie blue-stained SDS–PAGE gel of the purified RTX protein showing a single predominant species consistent with an unfolded monomer of RTX. (D) Representative CD spectra of the RTX protein refolded in the presence (red) and absence (black) of Ca2+ and in the presence of Mg2+ (blue). (E) Representative chromatogram from analytical gel filtration of the Ca2+-free (red) and Ca2+-bound (green) RTX protein immediately after refolding. Data for the RTX protein in the absence of Ca2+ after incubation for 24 h are colored black. The elution peaks for the folded, unfolded, and polymer states are labeled.