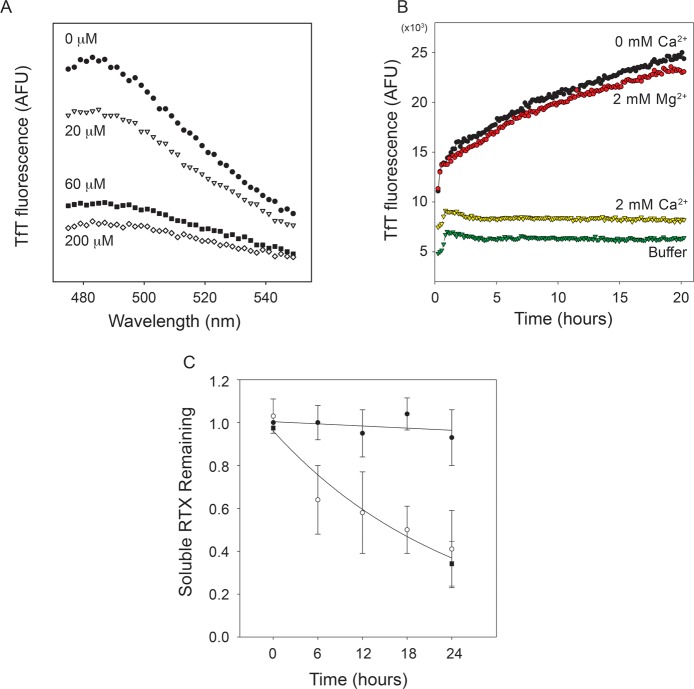
Figure 3.
Characterization of RTX polymers. The Ca2+-dependent aggregation of the RTX domain was evaluated using ThT fluorescence. (A) The purified RTX domain was refolded into buffers containing increasing concentrations of Ca2+, and emission spectra of ThT fluorescence were collected after overnight incubation. Increasing Ca2+ concentrations resulted in decreasing maximal emission intensities [0 (●), 20 (▽), 60 (■), and 200 μM Ca2+ (◇)]. Background fluorescence of buffer without RTX protein was subtracted from each of the emission spectra. (B) The kinetics of ThT fluorescence were monitored by emission at 482 nm as a function of time in the presence or absence of Ca2+ and Mg2+. RTX protein in the absence of Ca2+ showed an increase in ThT fluorescence (black circles), as did the protein incubated in Mg2+ (red circles). This increase in TfT fluorescence was not observed with the Ca2+-bound protein (yellow triangles) or in buffer controls (green triangles). (C) Analytical gel filtration was used to assess the soluble, monomeric RTX protein in solution through the time course of aggregation. The level of soluble RTX protein in the absence of Ca2+ decreased as a function of incubation time (○), as did the level of protein incubated in 2 mM Mg2+ (■). Calcium-bound (2 mM) RTX protein remained soluble and monomeric over the incubation times tested (●).