Abstract
Schizophrenia is a substantially heritable disorder associated with disrupted neural transmission, as well as dysfunction of brain systems involved in higher cognitive processes. Among the several putative candidate genes for schizophrenia, the gene encoding dystrobrevin-binding-protein-1 (aka dysbindin) is associated with cognitive impairments, including memory and attention deficits, in both schizophrenia patients and non-schizophrenic individuals. The mechanism underlying these deficits is thought to be based in changes in glutamatergic and dopaminergic function within corticostriatal networks, circuitry known to be critical for schizophrenia. Recent support for this hypothesis derives from the study of mice with a null mutation in the dysbindin gene that exhibit memory dysfunction and abnormalities in excitatory neurotransmission in prefrontal and hippocampal networks. At a cellular level, dysbindin is thought to mediate pre-synaptic glutamatergic transmission. Here, we investigated whether loss of dysbindin expression also affects postsynaptic NMDA receptor function. We show that decreases in dysbindin are associated with specific decreases in NMDA-evoked currents in prefrontal pyramidal neurons, as well as decreases in expression of the obligatory NMDA receptor subunit (NR1). Furthermore, the degree of NR1 expression directly correlates with performance on a spatial working memory task, providing a mechanistic explanation for cognitive changes previously associated with dysbindin expression. These data show a significant down-regulation of NMDA receptors due to dysbindin deficiency and illuminate molecular mechanisms mediating the association between dysbindin insufficiency and cognitive impairments associated with schizophrenia, encouraging study of the dysbindin/NR1 expression association in humans with and at risk for the disease.
Keywords: dysbindin, DTNBP1, NMDA, glutamate, schizophrenia, working memory
Schizophrenia is highly heritable (1; 2), and liability likely involves both common genetic variants of small effect and rare structural variants of larger effect. Both linkage (3; 4) and association studies (5–7) have implicated dystrobrevin binding protein-1 (dysbindin or DTNBP1) as a promising susceptibility gene for this debilitating disorder and show that, biologically, risk haplotypes for dysbindin confer low protein expression. Accordingly, schizophrenia patients show reduced dysbindin mRNA or protein in prefrontal cortex (PFC) (8–10) and hippocampus (9; 11).
Both dysbindin variation (12; 13) and its chromosomal locus (chromosome 6p) (14; 15) have been associated with cognitive impairments characteristic of schizophrenia. Dysbindin has also been associated with spatial working memory (WM) in both healthy individuals (16), and patients with schizophrenia (17). WM is a key endophenotype for schizophrenia, and is considered to be a core deficit of the disorder (18; 19). Moreover, WM impairments in schizophrenia are associated with PFC dysfunction (20), while dysbindin variation associates with measures of PFC function in healthy subjects (21). Finally, genetic insufficiency in dysbindin expression in mice is associated with poor WM performance and functional changes within the PFC (22) that may correspond to aspects of prefrontal dysfunction in schizophrenia patients.
Dysbindin is expressed in axon terminals of glutamatergic pyramidal neurons (11) and influences glutamatergic function, likely through interactions with vesicular trafficking proteins. Dysbindin over-expression is associated with increased glutamate release and correspondingly higher extracellular glutamate (23), with dysbindin reductions leading to decreases in glutamate release (22; 23) via multiple mechanisms (22; 24). Such alterations of glutamatergic transmission intersect with the prominent glutamate hypothesis for schizophrenia which specifies a role for glutamate N-methyl-D-aspartate (NMDA) receptors in either causing glutamatergic dysfunction or mediating cognitive and behavioral sequelae (25–27).
Given the hypothesized role of NMDA-dependent glutamate transmission in schizophrenia, whether dysbindin also influences NMDA receptor function is of great importance. Therefore, we investigated the relationship between glutamate receptor dynamics and memory performance in a mouse model carrying a large genomic deletion exclusively within the dysbindin gene (28). We first assessed NMDA and AMPA receptor function in PFC pyramidal neurons in vitro using whole-cell recordings, finding a specific decrease in NMDA-evoked currents. Molecular quantitative analyses (RT-PCR) of the mandatory NMDA receptor subunit NR1 further confirmed that the functional alterations uncovered by electrophysiological recordings were transcriptional, as well. Finally, we tested the relationship of these changes with cognitive function in null mutants using a spatial WM task and found strong support for the previously reported association of dysbindin and schizophrenia with WM function. This adds a new dimension to existing work by confirming the mechanistic relationship between transcriptional regulation of NMDA receptor function and spatial WM deficits in dysbindin mutant mice and thereby illuminating molecular mechanisms mediating the association between dysbindin insufficiency and cognitive impairments associated with schizophrenia.
Materials and Methods
Animals
Animals
Studies were performed on dysbindin mutant mice (“sandy” mice) that have been backcrossed to the C57Bl background (Jackson Laboratories). All animals were generated by crossing heterozygous breeders, permitting direct comparisons between homozygous mutants, heterozygotes and littermate wild-type controls. Genotypes were determined by polymerase chain reaction. The wt product [472 bp] was amplified with the following primers: TGAGCCATTAGGAGATAAGAGCA and AGCTCCACCTGCTGAACATT; the dys− product [274 bp] was amplified with the following primers: TCCTTGCTTCGTTCTCTGCT and CTTGCCAGCCTTCGTATTGT). The fragments were separated on a 3% agarose gel.
Group-housed male mice were used in both the behavioral and electrophysiological studies. The mice in the behavioral experiments were 60–100 days of age during experimentation and sacrificed for the gene expression study upon completion of the behavioral task, while the mice used in the recording studies were 45–60 days of age. All experimental protocols were approved by the Chancellor’s Animal Research Committee at UCLA or the Medical University of South Carolina Institutional Animal Care and Use Committee.
Electrophysiology
Electrophysiological Recordings
Brain slices were prepared from male wt/wt, dys−/wt and dys−/dys− mice. Subjects were anesthetized with the inhalant isoflurane (Abbott Laboratories). The brain was rapidly removed and coronal slices were cut at 300 μm thickness in ice-cold high-sucrose solution containing (in mM): sucrose, 200; KCl, 1.9; Na2HPO4, 1.2; NaHCO3, 33; MgCl2, 6; CaCl2, 0.5; glucose, 10; ascorbic acid, 0.4. Slices were incubated at 33°C for at least 1 h before recordings; the incubation medium was an artificial cerebrospinal fluid solution containing (in mM): NaCl, 125; KCl, 2.5; NaH2PO4,1.25; NaHCO3, 25; MgCl, 4, CaCl, 1, d-glucose, 10; sucrose, 15; ascorbic acid, 0.4, continuously aerated with 5%CO2/95%O2. After incubation, slices were transferred to a submerged chamber and superfused with oxygenated artificial cerebrospinal fluid (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 2.0 CaCl2, 1.3 MgCl2, 10 glucose and 0.4 ascorbic acid, at room temperature. Recordings were made using a Multiclamp 700B amplifier (Axon Instruments, CA), connected to a computer running Windows XP and Axograph X software and later analyzed off-line. All recordings were obtained from pyramidal neurons in layers V or VI of the prelimbic or infralimbic cortex, identified using infrared-differential interference contrast optics and video-microscopy.
Voltage clamp
For voltage-clamp recordings, electrodes (3–7 MΩ resistance in situ) were filled with a solution containing (in mM): 135 CsCl, 10 HEPES, 2 MgCl2, 1 EGTA, 4 NaCl, 2 Na-ATP, 0.3 tris-GTP, 1 QX-314, 10 phosphocreatine; 285 mOsmols. Series resistances (10–20 MΩ) and input resistances were continually monitored throughout the experiment via a −1 mV (100 ms) hyperpolarizing pulse. All of the voltage-clamp experiments were performed in the presence of 100 μM picrotoxin. Peak AMPA and NMDA currents were evoked by application of either AMPA (1 μM) or NMDA (15 μM) to the bath for 30 seconds, the peak elicited current was measured and compared across genotypes. To calculate the AMPAR : NMDAR ratio, the average of 20 EPSCs at +40 mV was calculated before and after application of AP5 (50 μm) for 5 min to block the NMDA component of the response. NMDA current amplitude was calculated by subtracting the average amplitude with AP5 (AMPA EPSC) from the amplitude measured in the absence of AP5 (NMDA EPSC); the peak of the AMPA EPSC was divided by the peak of the NMDA EPSC to give the AMPA:NMDA ratio
Statistical Analysis
Parametric analyses of variance (one-way ANOVA) were used to examine main effects (e.g., genotype) and interactions with repeated measures, where appropriate. Significant main effects or interactions were followed up with post hoc tests (Fisher PLSD). All figures present data as mean ± SEM.
RT-PCR
Tissue from prefrontal cortex of 44 ‘sandy’ mice (19 wild-types: wt/wt, 15 heterozygous: dys−/wt, and 11 mutants: dys−/dys−) was excised and incubated in RNAlater solution (Ambion, Austin, TX) overnight at 4°C to allow thorough penetration of solution. Excised tissue samples were then transferred to −20°C for storage.
RNA extraction
Samples were homogenized in TriReagent (Sigma-Aldrich, St. Louis, MO) and total RNA was subsequently isolated using a standard phenol/chloroform method. Yield and purity was determined by absorbance at 230nm, 260nm and 280nm. Isolated RNA was treated with a DNAse step for removal of contaminating genomic DNA using the RNAqueous 4PCR kit (Applied Biosystems, Foster City, CA).
Real-Time quantitative RT-PCR
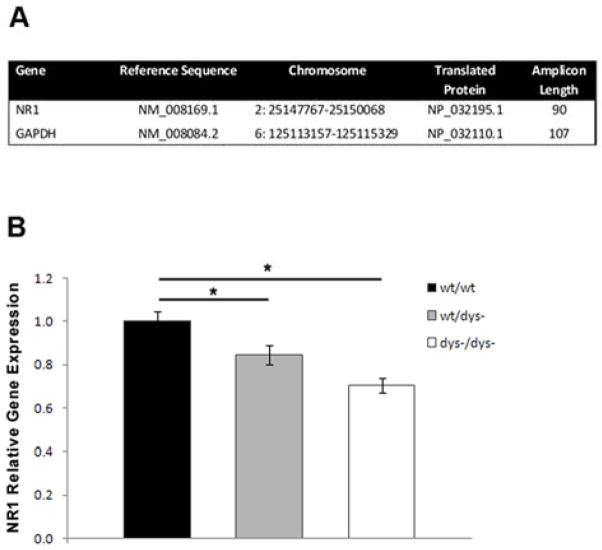
Quantification of gene expression was performed using a Taqman Gene Expression Assay for NR1 (Mm00433800_m1) as well as a Taqman Endogenous Control assay for GAPDH (Mm99999915_g1) used as a housekeeping (normalizing) gene (Figure 2A). Validation experiments were done to confirm that the efficiency of the target and endogenous control amplification was approximately equal (ΔCt < 0.1) as well as to confirm that PCR efficiencies for each target were 100% (±10%). Levels of mRNA expression were measured using a StepOne ABI instrument with a 48-well format (Applied Biosystems). Samples were amplified using a one-step protocol. All RNA samples in a plate, including controls, were amplified against a calibrator pool made up of all control samples used in the gene expression analysis. Each reaction, including a no template control, was done in triplicate. PCR thermal cycling conditions consisted of: 48ºC for 15 minutes, 95 ºC for 10 minutes, 40 cycles of 95 ºC for 15 seconds and 60 ºC for 1 minute. Briefly, each 20μl reaction mix consisted of 8.5μl of RNA, 1μl of Taqman Assay, 10μl of Taqman RNA-to-Ct 1-Step Master Mix and 0.5μl of RT Enzyme (Applied Biosystems). RT-PCR data was obtained with the StepOne Real-Time PCR system software (version 2.0.2, Applied Biosystems). Relative NR1 gene expression was quantified using the ΔΔCt Comparative method.
Figure 2.
Gene Expression Analyses. A: Details of assay targets used in RT-PCR to probe NR1 gene expression in prefrontal cortex of mice. B: NR1 gene expression levels in the prefrontal cortex across genotypes. RT-PCR showed a significant decrease of NR1 mRNA levels in both heterozygous and mutant animals compared to wildtypes.
Statistical Analysis
A one-way ANOVA was carried out to examine NR1 gene expression levels across genotypes. Statistics reported include mean ± SEM. Post hoc comparisons were performed when applicable. Individual amplification data relative to the pool from each one of the wt/wt samples were used in the analyses as the control group. SPSS 15.0 for Windows (SPSS Inc., Chicago, Ill) was used for all statistical tests. Statistical significance was set at p<0.05.
Working Memory
Behavioral Testing Procedures
Adult mice were trained and tested using a delayed non-match-to-position (DNMTP) test (22; 29; 30) Testing was performed in small aluminum and Plexiglas operant conditioning chambers (Med-Associates Inc., St Albans VT), fitted with a horizontal array of five nose-poke apertures on one-side of the box and a 14-mg pellet delivery magazine on the opposite side. This DNMTP task emphasizes retrospective encoding and maintenance of spatial information in a manner analogous to spatial delayed response tests used to measure WM in rats, monkeys and humans.
Each trial in the delayed non-match to sample task consists of both a sample phase and a choice phase. The inter-trial interval and time-out periods were 5 s and 3 s, respectively. In the sample phase, one randomly selected nose-poke aperture was illuminated for up to 15 s. A response into the illuminated aperture (correct sample-phase response) caused the aperture light to extinguish and the magazine light to be illuminated. The choice phase was initiated when a nose-poke in the lit magazine was detected. During the sample phase, a response in an unlit nose-poke aperture during stimulus presentation (incorrect sample-phase response) or failure to respond to the illuminated aperture during the 15 s presentation period (sample-phase omission) resulted in immediate termination of the trial and a time out period. During the choice phase, two apertures were illuminated for up to 15s: the sample aperture as well as another, randomly selected aperture (the non-match location). A response in any aperture other than that of the non-match location (incorrect choice-phase response) or failure to make a response while the apertures were illuminated (choice-phase omission) resulted in a time-out. Responses to the non-match location (correct response) triggered magazine illumination and pellet delivery.
During the first 30 days of training, there was no imposed delay, allowing the mice to acquire the task under relatively low memory load conditions. After this initial training, probe sessions were administered in which minimum delay periods were imposed between a correct sample-phase response and the first response into the magazine that initiated the choice phase. The imposed delays were either 0.5, 5 or 10 s and will be interpolated in pseudorandom order and at equivalent frequencies across the session. The tasks end after 75 trials are completed or 60 min passes, whichever comes first. In addition to the standard trials, 10% of trials consisted of a sensorimotor control condition, in which only the new light (the non-match location) was illuminated during the choice phase. This condition ensures that in the absence of the distraction of multiple lights, the mouse is able to avoid perseverating and select a new location.
Statistical Analyses
Performance by Load
Incorrect choices during the sample phase, overall performance in the choice phase, and performance in the sensorimotor control condition were calculated and analyzed by one-way analyses of variance, with genotype as the factor. For all conditions, the data from testing days 6–15 was pooled to increase the number of trials. Accuracy of responding during the choice phase across delay conditions was analyzed by repeated measures ANOVA with genotype as the factor and delay length as the repeated measure. Performance in the sensorimotor control condition and in the sample phase accuracy was tested using one-way ANOVA across the three genotypes. Post-hoc independent samples t-tests were performed to decompose significant effects.
Regression analysis
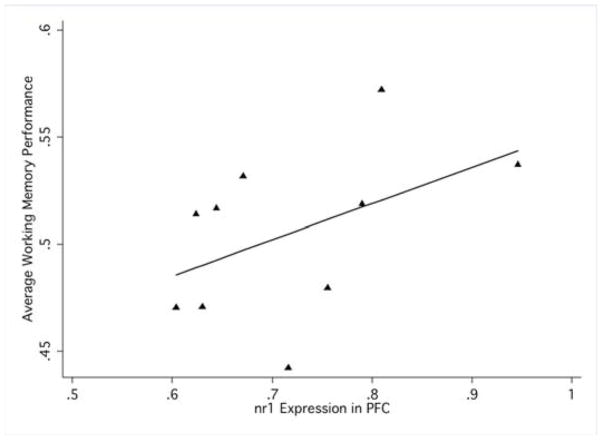
In the dys−/dys− mice that had both WM and nr1 expression data (n=10), a robust regression was performed in Stata (v8), with nr1 expression level in the prefrontal cortex predicting WM performance (overall percent correct across all delays). Data were tested for outliers; however, no studentized residuals were high enough (>2.5) to warrant exclusion.
Results
Previous studies have indicated that pre-synaptic glutamate release probability and quantal size are decreased in dysbindin null mutant mice (24); however, no studies to date have addressed whether alterations in post-synaptic receptor function may also be a consequence of loss of function of the gene. To address this point, we performed whole-cell voltage-clamp recordings from pyramidal neurons in layers V or VI of the prelimbic or infralimbic cortex. Whole cell patch clamp recordings showed that the amplitude of the currents evoked by NMDA application were significantly lower in both dys−/dys− (n=7) and dys−/wt+ (n=10) mice, as compared to wt/wt (n=6) littermate controls (Figure 1A; main effect of genotype: F[2,21]= 2.9, p= 0.04; one-way ANOVA). On the other hand, α-amino-3-hydroxyl-5-methyl-4-isoxazole (AMPA)-evoked currents were similar across genotype (dys−/dys−, n=7; dys−/dys+, n=10; wt/wt n=7; Figure 1B; F[2,21]= 0.3, p=0.41; one-way ANOVA). This finding is supported by additional experiments (data not shown) where the ratio of AMPA:NMDA current was significantly increased in dys−/dys− (1.6 ± 0.2, n=8) compared to wt/wt mice (1.2 ± 0.16; p=0.04, n=8; two-tailed t-test), while the contrast between wt/wt and dys−/wt (n=8) reached the trend level (p=0.08; two-tailed t-test). Our findings suggest that dysbindin deletion has significant and selective effects on NMDA receptor function in prefrontal cortex.
Figure 1.
Electrophysiological measurement of pharmacologically-evoked glutamate currents. A: Peak NMDA current amplitude (pA) for wt/wt (black bar), dys−/wt (grey bar), and dys−/dys− (white bar). B: Peak AMPA current (pA) measurements in wt/wt (black bar), dys−/wt (grey bar), and dys−/dys− (white bar). (**) denotes p<0.05 Representative traces from each experimental group are shown in insets below histograms, (●) shows exact time point of drug (NMDA 15 uM or AMPA 1 uM) application.
To explore the mechanistic basis of the change in NMDA receptor currents, we sought to determine whether transcriptional alterations in the expression of the obligatory subunit of the NMDA receptor complex, NR1, were associated with mutation of the dysbindin gene. A significant effect of genotype was found as indicated by a one-way ANOVA (n=44; F[2,42]= 10.14, p<0.001), confirming an association between regulation of NR1 expression and dysbindin (Figure 2B). Post hoc analyses further revealed significantly lower expression of NR1 in the prefrontal cortex of dys−/wt (n=15; 0.84 ± 0.043) mice compared to wt/wt littermates (n=19; 1.0 ± 0.048, p=0.039). A more pronounced decrease of NR1 mRNA levels was found in dys−/dys− mice (n=11; 0.70 ± 0.034, p<0.001) compared to controls.. These data show a significant downregulation of NMDA receptors due to dysbindin deficiency, thereby clarifying one aspect of the mechanism by which NMDA receptor function is compromised in mutants.
The mice used for the expression analysis had all previously been assessed using a DNMTP task (29; 30) that tests the ability of subjects to remember the spatial location of target stimuli across a series of brief delay periods in a manner analogous to spatial WM and which relies upon the prefrontal cortex. As previously demonstrated (22), genetic loss of dysbindin function was associated with an impairment in memory-guided performance as indicated by one-way ANOVA (n=37; F[2,34]=3.75, p=0.03), which cannot be attributed to an overall failure to perform the task as incorrect responses during the sample phase did not significantly differ (Figure 3A,B). Post-hoc t-tests revealed significant effects between wt/wt (n=10) and dys−/dys− (n=11; t19=2.51, p=0.02) and between wt/wt and dys−/wt (n=16; t24=2.40, p=0.03) but not wt/wt and dys−/wt (t25)= −0.24, p=0.82). When mice were tested for their ability to simply respond to a single visual target in the sensorimotor control condition, a one-way ANOVA indicated there was no significant effects of genotype (F[2,35]=2.23, p=0.11) (Figure 3C).
Figure 3.
Behavioral Analyses: A. Overall performance in the DNMTP task across genotypes showed a significant difference between groups. B. Incorrect responses during the sample phase of DNMTP task across genotype showed no significant difference. C. Accuracy in the sensorimotor control condition of the DNMTP task across genotype showed no significant difference, indicating that basic, non-WM functions associated with task performance are intact.
To assess whether changes in NR1 expression data had functional implications, the relationship of WM performance was assessed in animals that had data on both measures. A robust regression assessing the degree to which NR1 expression predicted response accuracy in the WM task was conducted. In dys−/dys− animals with intact data on each measure (n=10), there was a significant, positive linear relationship between NR1 expression and measures of WM (F[1,8]=5.74, p=0.044; r-squared=0.22), suggesting a common determinant for NMDA receptor regulation and cognition and elevating NMDA receptor hypofunction in prefrontal cortex as a proximal mechanism by which WM circuits fail in mutants (Figure 4).
Figure 4.
Relationship of gene expression and behavior: Robust regression indicates that NR1 relative gene expression positively predicts average WM performance on a DNMTP task in null mutant mice.
Discussion
Mutation of the dysbindin gene causally leads to impairments in WM measured with a DNMTP task. Because this impairment appears to depend upon the need for memory-guided performance (i.e., mutants are not impaired in sensorimotor control conditions or tasks), these deficits are likely to be due to impairments at a cognitive level (e.g. encoding, maintenance, retrieval). We have previously reported a decrease in DNMTP performance in dysbindin mutant mice on a different genetic (DBA) background (22); the present results demonstrate that the effect of dysbindin disruption on WM is not background specific. Moreover, in the current analysis we find cognitive deficits in mice carrying only one null mutant allele (evidence for haploinsufficiency), which is an important extension of our previous result as heterozygous mice may represent a more accurate model for dysbindin changes in schizophrenia than do full mutants.
Mechanistically, we show that in dysbindin mutant mice, pre- and post-synaptic aspects of excitatory glutamatergic transmission are significantly compromised and that transcriptional down-regulation of the NMDA receptor subunit NR1 may be implicated in the decreased NMDA-dependent glutamate signaling. Though a decrease in NR1 protein has yet to be confirmed, the change in mRNA and receptor function provides convergent evidence for a failure of normal NMDA-mediated excitatory transmission in the prefrontal cortex. This relationship extends to cognition because of the strong association between NR1 expression and WM performance, measured in the same subjects; this finding extends beyond previous reports reporting cognitive deficits associated with dysbindin mutation by providing further support for an important, hypothesized role for NMDA receptors in these deficits. These results strongly place dysbindin, and its influence on risk for schizophrenia and its endophenotypes, within the context of the NMDA receptor hypofunction model, long thought to represent a common mechanistic determinant of behavioral and cognitive dysfunction in the disorder (26; 31; 32)
Recent studies have linked natural variation in the gene encoding dysbindin to several of the cognitive deficits commonly observed in schizophrenia, including impaired WM (17; 22; 33). WM deficits have several important implications for schizophrenia patients- they may be a limiting factor for more complex cognitive functions (34), they could potentially account for symptomatology such as delusions, disorganization, and thought disorder (20), and they are correlated with functional outcome (35). WM impairments may function as a central deficit; therefore, understanding the underpinnings of these deficits is imperative. There is evidence that WM deficits in patients with schizophrenia is heritable (18), and the relationship between neural activity in prefrontal cortex and WM performance may be heritable as well (36). Though the specific genetic mechanisms responsible for this transmission are unknown, our data support the concept that variation in the dysbindin gene may be causally responsible for some of this relationship (22); other genes, including DISC1, likely contribute, as well.
For additional progress to be made, it is critical to understand the cellular- and circuit-level mechanisms by which these genes impact behavioral function. Though recent studies have reported decreased glutamate release in hippocampus and prefrontal cortex of dysbindin mutant mice (22; 24), none have addressed whether there is a postsynaptic change in glutamate receptors in these animals. Our data show a selective reduction in NMDA-evoked currents, a finding which is also supported by an increase in the AMPA:NMDA ratio. This reduction in NMDA number or function in prefrontal cortex could negatively impact cognitive ability via multiple mechanisms. Reduction in NMDA receptor conductance may result in a decrease in the recurrent activity of prefrontal cortical networks that is thought to be the cellular correlate of WM (37). Additionally, a reduction in NMDA activity may also dysregulate intrinsic inhibitory neurons of the prefrontal cortex, leading to reduced specificity and control over pyramidal network activation states related to WM.
As a susceptibility gene for schizophrenia, dysbindin insufficiency has been shown to have a severe impact on the glutamatergic system through mechanisms that are not fully understood (23; 24; 38). Our novel finding of a decrease in cortical NR1 mRNA expression of animals carrying the null mutation is important in that it identifies a specific molecular marker that is dysregulated due to dysbindin insufficiency. This finding is broadly consistent with reports of decreased NR1 in the PFC of postmortem schizophrenia brains (39; 40). Although other post mortem studies have found NR1 expression changes in the opposite direction (41; 42), these discrepant findings may stem from the age of the samples; generally speaking, studies involving tissues from younger patients (without life-long antipsychotic drug treatment and with fewer chronic illnesses) have identified reduced NR1 expression, similar to what we observe in dysbindin mutant mice. As the obligatory subunit of the NMDA receptor complex, this finding certainly begs attention to the potential functional effects this change may have on circuit and systems function in cortical regions. We showed here that down-regulation of NR1 is accompanied with a decreased in NMDA currents suggesting that the changes seen at a transcriptional level may have an important impact on synaptic transmission. How changes in NR1 gene expression result in alterations of synaptic transmission is not clear, but we can speculate the presence of an abnormality (at least at a regional level) in NMDA receptor cell membrane expression that could result in a compromised glutamatergic system characteristic of the disorder. In support of this, it has been reported that the NMDA receptor is dynamically affected by changes in expression and that the NR1 subunit might have a primary role in the trafficking of NMDA receptors to synaptic sites (43).
Furthermore, by correlating the WM and gene expression data within subjects, we were able to show that WM performance directly relates to the levels of expression of the NR1 subunit. Together with the electrophysiological results, this provides a clear hypothesis for how cognitive deficits arise after deletion of dysbindin expression, as well as a potential mechanism for their genetic transmission. Understanding this mechanism provides a target for pharmacological studies aimed at improving cognitive function in schizophrenia. For instance, this provides further evidence that enhancement of glutamatergic transmission, possibly by allosteric activation of the NMDA receptor complex, may be a key for treating cognitive deficits in schizophrenia.
By understanding the neurobiological functions for each of the proteins encoded by the genes involved in schizophrenia risk and the consequence of the functional mutations that associate with schizophrenia phenotypes, it is hoped that a convergent theory of cellular and network dysfunction in schizophrenia can be elucidated. In principle, it may be possible to determine the common biochemical and cellular pathways affected by the otherwise diverse set of schizophrenia risk genes in order to determine how multiple pathways converge to set the stage for the various phenotypic aspects of the disorder. Mechanistically, the data here provide a broader molecular explanation of the relationship between dysbindin expression and schizophrenia. Through decreased expression of dysbindin in as of yet unknown sub-cellular locations, activity-dependent release of glutamate from pre-synaptic terminals is inhibited 20–21 and specific transcriptional changes in NMDA-mediated transmission occur. These phenomena are potentially of exceptional importance because they demonstrate that the influence of dysbindin on schizophrenia risk may be exerted through a set of NMDA-dependent cellular and network processes already heuristically linked to the disorder (27). This result is critical because it further elevates the notion that there may be common neuronal adaptations amongst otherwise heterogeneous genetic and pharmacological models for schizophrenia that will represent a common biological pathway to schizophrenia. The search for these molecular convergences across models may offer the opportunity for unprecedented discoveries regarding the pathophysiology of psychotic disorders.
Acknowledgments
This work was supported by PHS Grants UL1-DE19580, PL1-NS062410MH-83269, K12-GM081265, and T32-NS048004:03, and a NARSAD Distinguished Investigator Award.
References
- 1.Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch Gen Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Cardno AG, Gottesman Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12–17. [PubMed] [Google Scholar]
- 3.Straub RE, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F, et al. A potential vulnerability locus for schizophrenia on chromosome 6p24-22: evidence for genetic heterogeneity. Nat Genet. 1995;11:287–293. doi: 10.1038/ng1195-287. [DOI] [PubMed] [Google Scholar]
- 4.Schwab SG, Knapp M, Mondabon S, Hallmayer J, Borrmann-Hassenbach M, Albus M, et al. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet. 2003;72:185–190. doi: 10.1086/345463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straub RE, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, et al. Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and follow-up of selected regions in all families provides evidence for multiple susceptibility genes. Mol Psychiatry. 2002;7:542–559. doi: 10.1038/sj.mp.4001051. [DOI] [PubMed] [Google Scholar]
- 6.Tang JX, Zhou J, Fan JB, Li XW, Shi YY, Gu NF, et al. Family-based association study of DTNBP1 in 6p22.3 and schizophrenia. Mol Psychiatry. 2003;8:717–718. doi: 10.1038/sj.mp.4001287. [DOI] [PubMed] [Google Scholar]
- 7.Kirov G, Ivanov D, Williams NM, Preece A, Nikolov I, Milev R, et al. Strong evidence for association between the dystrobrevin binding protein 1 gene (DTNBP1) and schizophrenia in 488 parent-offspring trios from Bulgaria. Biol Psychiatry. 2004;55:971–975. doi: 10.1016/j.biopsych.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG, et al. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet. 2009;18:3851–3863. doi: 10.1093/hmg/ddp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 10.Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res. 2008;98:105–110. doi: 10.1016/j.schres.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, et al. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15:1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- 13.Zinkstok JR, de Wilde O, van Amelsvoort TA, Tanck MW, Baas F, Linszen DH. Association between the DTNBP1 gene and intelligence: a case-control study in young patients with schizophrenia and related disorders and unaffected siblings. Behav Brain Funct. 2007;3:19. doi: 10.1186/1744-9081-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posthuma D, Luciano M, Geus EJ, Wright MJ, Slagboom PE, Montgomery GW, et al. A genomewide scan for intelligence identifies quantitative trait loci on 2q and 6p. Am J Hum Genet. 2005;77:318–326. doi: 10.1086/432647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallmayer JF, Kalaydjieva L, Badcock J, Dragovic M, Howell S, Michie PT, et al. Genetic evidence for a distinct subtype of schizophrenia characterized by pervasive cognitive deficit. Am J Hum Genet. 2005;77:468–476. doi: 10.1086/432816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf C, Jackson M, Kissling C, Thorne J, Linden D. Dysbindin-1 genotype effects on emotional working memory. Mol Psychiatry. doi: 10.1038/mp.2009.129. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donohoe G, Morris DW, Clarke S, McGhee KA, Schwaiger S, Nangle JM, et al. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: a preliminary study. Neuropsychologia. 2007;45:454–458. doi: 10.1016/j.neuropsychologia.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lonnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- 19.Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 21.Fallgatter AJ, Herrmann MJ, Hohoff C, Ehlis AC, Jarczok TA, Freitag CM, Deckert J. DTNBP1 (dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology. 2006;31:2002–2010. doi: 10.1038/sj.npp.1301003. [DOI] [PubMed] [Google Scholar]
- 22.Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. Dysbindin Modulates Prefrontal Cortical Glutamatergic Circuits and Working Memory Function in Mice. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13:2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- 24.Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181:791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 26.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 27.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’Brien EP, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrs W, Kuperman J, Avedian T, Roth RH, Jentsch JD. Alpha-2 adrenoceptor activation inhibits phencyclidine-induced deficits of spatial working memory in rats. Neuropsychopharmacology. 2005;30:1500–1510. doi: 10.1038/sj.npp.1300700. [DOI] [PubMed] [Google Scholar]
- 30.Aarde SM, Jentsch JD. Haploinsufficiency of the arginine-vasopressin gene is associated with poor spatial working memory performance in rats. Horm Behav. 2006;49:501–508. doi: 10.1016/j.yhbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- 32.Jentsch JD, Elsworth JD, Taylor JR, Redmond DE, Jr, Roth RH. Dysregulation of mesoprefrontal dopamine neurons induced by acute and repeated phencyclidine administration in the nonhuman primate: implications for schizophrenia. Adv Pharmacol. 1998;42:810–814. doi: 10.1016/s1054-3589(08)60870-4. [DOI] [PubMed] [Google Scholar]
- 33.Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R, Malhotra AK. DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophr Res. 2007;89:169–172. doi: 10.1016/j.schres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- 35.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 36.Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 38.Talbot K, Cho DS, Ong WY, Benson MA, Han LY, Kazi HA, et al. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet. 2006;15:3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- 39.Sokolov BP. Expression of NMDAR1, GluR1, GluR7, and KA1 glutamate receptor mRNAs is decreased in frontal cortex of “neuroleptic-free” schizophrenics: evidence on reversible up-regulation by typical neuroleptics. J Neurochem. 1998;71:2454–2464. doi: 10.1046/j.1471-4159.1998.71062454.x. [DOI] [PubMed] [Google Scholar]
- 40.Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- 41.Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;158:1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- 43.Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]