Abstract
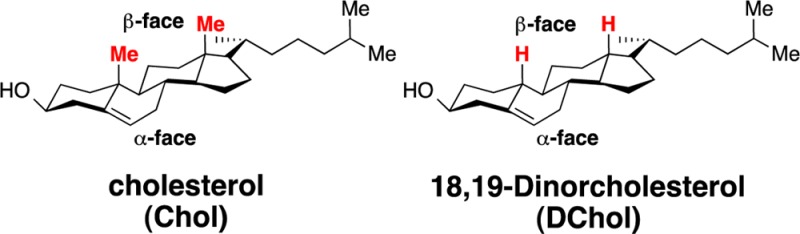
One of the long-standing issues surrounding cholesterol (Chol) relates to its two-faced character. In particular, the consequences of its having a rough β-face and a smooth α-face on its structural influence in cell membranes has remained elusive. In this study, direct comparisons have been made between cholesterol and a “smoothened” analog, DChol (i.e., 18,19-dinorcholesterol) using model membranes and a combination of nearest-neighbor recognition, differential scanning calorimetry, fluorescence, and monolayer measurements. Taken together, these results indicate that subtle differences exist between the interaction of these two sterols with the different states of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC). Chol has a greater condensing power than DChol, but only slightly so, i.e., on the order of a few tens of calories per mole.
Introduction
Eukaryotic cell membranes are rich in cholesterol.1 Because this sterol is thought to play a major role in determining the structure and function of these life-sustaining enclosures, it has been the subject of numerous investigations.2−10 One of the long-standing issues surrounding cholesterol relates to its two-faced character, where the presence of two methyl groups on its β-face creates a roughness that is distinct from its smooth α-face (Chart 1). Whether this combination of a rough and smooth face has any influence on the interactions between cholesterol and neighboring lipids has remained as a matter of debate.
Chart 1.
Previous experiments and molecular dynamics simulations suggest that the interactions of saturated phospholipid chains with the smooth face of Chol are stronger than with the rough face.11−13 However, recent atomic-scale molecular dynamics simulations that compared membranes made from Chol and DPPC with ones made from DChol (18,19-dinorcholesterol) plus DPPC have led to the hypothesis that the presence of these two different faces adds to cholesterol’s condensing power.14,15 Specifically, it was proposed that a lower degree of tilt by cholesterol allows it to lie in a more upright position and parallel to the acyl chains of neighboring phospholipids. Alternatively, one can imagine that DChol may have a greater condensing power because of better contact between the saturated acyl chains and two smooth faces, affording stronger van der Waals interactions.9,12−15
Owing to the recent synthesis of DChol, we have now been able to address this issue experimentally using a combination of nearest-neighbor recognition, fluorescence, and monolayer measurements (Scheme 1).16 We have also compared the action of Chol and DChol on the melting behavior of DPPC by the use of high-sensitivity differential scanning calorimetry (DSC).
Scheme 1.
Experimental Methods
All methods that were used in carrying out monolayer measurements at the air/water interface and fluorescence and nearest-neighbor recognition measurements in liposomal membranes (200 nm, prepared via extrusion) were similar to those previously reported.9 Differential scanning calorimetry (DSC) was performed in multilamellar (MLVs) and large unilamellar vesicles (LUVs, 0.1 μm size) as previously described.17
Results and Discussion
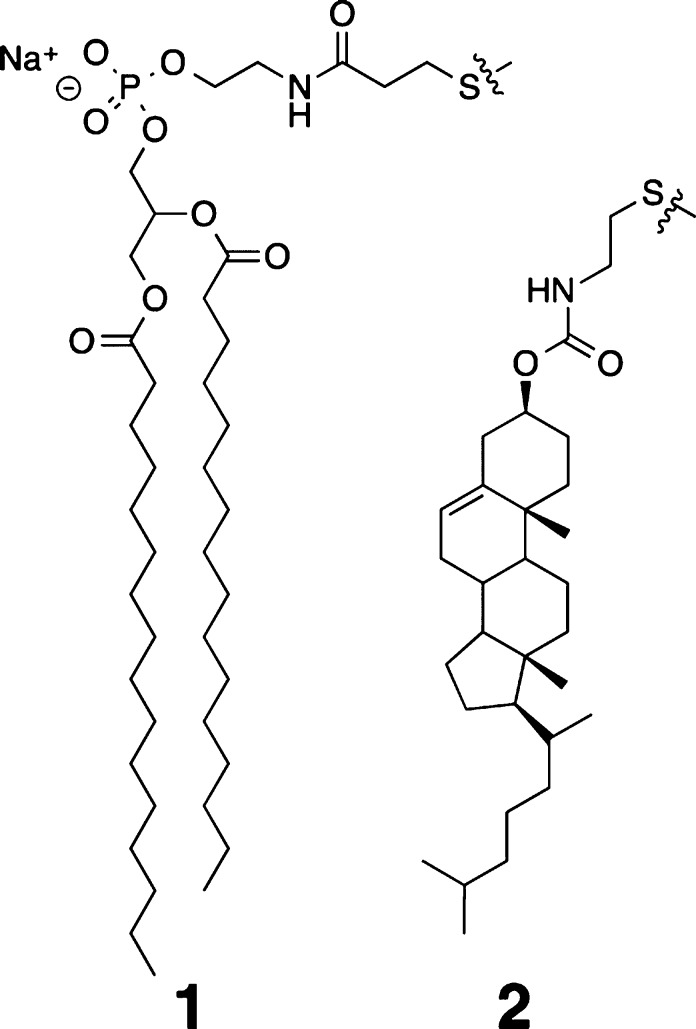
To compare the condensing power of Chol and DChol, we first carried out nearest-neighbor recognition (NNR) measurements using the exchangeable lipids, 1 and 2 (Chart 2). Such measurements reveal the thermodynamic tendency of these exchangeable monomers to become nearest neighbors and can be used to quantify the compactness of a lipid bilayer.18 Thus, we measured the nearest-neighbor preferences of 1 and 2 in host membranes made from DPPC plus Chol as well as ones made from DPPC and DChol. In each case, monomer exchange was carried out via thiolate–disulfide displacement reactions.19 Equilibrium constants, K, were calculated from the concentrations of the homodimers, {1-1} and {2-2}, and the heterodimer, {1-2}, that were present. Specifically, the equilibrium constant, K, is equal to {1-2}2/({1-1})({2-2}). If one takes statistical considerations into account, a nearest-neighbor interaction free energy, ω1-2, can then be calculated from ω1-2 = −1/2RT ln(K/4).20
Chart 2.
Using experimental procedures similar to those previously described, we made NNR measurements using sterol-rich liposomes derived from DPPC (Table 1). In the absence of added sterol, these DPPC-based membranes exist in the liquid-disordered state, and the mixing of 1 with 2 is random.21,22 In contrast, the incorporation of 40 mol % Chol leads to a strong preference for 1 and 2 becoming nearest neighbors.22 As is evident from Table 1, the substitution of Chol with DChol produces a similar condensing effect, where Chol is stronger by a few tens of calories per mole.
Table 1. Nearest-Neighbor Interactions of 1 with 2a.
| sterolb | mol % | K | ω1-2 (cal/mol) |
|---|---|---|---|
| Cholc | 0 | 3.9 ± 0.3 | 8 ± 21 |
| Cholc | 40 | 9.2 ± 0.2 | –260 ± 7 |
| Chol/DChol | 20/20 | 8.1 ± 0.7 | –220 ± 27 |
| DChol | 40 | 8.3 ± 0.7 | –230 ± 28 |
Measurements were made at 45 °C in host membranes derived from DPPC and the indicated sterol(s). In each case, 2.5 mol % 1 and 2.5 mol % 2 were present.
Chol refers to cholesterol.
Taken from ref (22).
As a further test of the relative condensing power of Chol and DChol, we examined their influence on the compactness of liposomal membranes made from DPPC using a fluorescence assay. Specifically, we incorporated phase-sensitive probe Laurdan into each membrane and measured its generalized polarization (GP) value as a function of temperature. Here, GP = (I440 – I490)/(I440 + I490), and I440 and I490 are the fluorescence emission intensities at the indicated wavelengths. As discussed elsewhere, GP values reflect the polarity surrounding the Laurdan molecule in a lipid bilayer and are very sensitive to changes in the phase of the membrane.23 To ensure that each of these dispersions was stable, a small amount (2.5 mol %) of 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DPPG) was included in the liposomes.
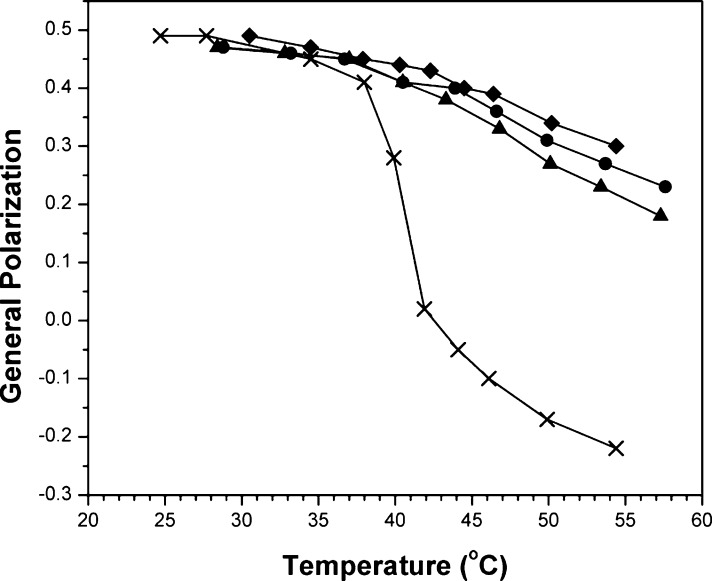
In the presence of a low concentration of cholesterol (2.5 mol %), a well-defined gel to liquid-crystalline phase transition is evident, having a melting temperature (Tm) of ca. 41 °C (Figure 1). When a high cholesterol concentration is included in the bilayer (i.e., 40 mol %), which forms the liquid-ordered phase over this entire temperature range, the GP values decrease modestly with increasing temperature.21 When cholesterol is fully replaced by DChol, a more pronounced decrease in the GP values is apparent, especially above 41 °C. Similar liposomes containing 20 mol % cholesterol and 20 mol % DChol produced an intermediate profile. These results are consistent with the conclusion that DChol is a weaker condensing agent than cholesterol and that this difference in condensing power is very small.
Figure 1.
Plot of general polarization vs temperature in liposomes made from DPPC/DPPG/Chol/DChol with the following molar percentages: (◆) 57.5/2.5/40/0, (●) 57.5/2.5/20/20, (▲) 57.5/2.5/0/40, and (×) 95/2.5/2.5/0. Data for (◆) and (×) have previously been reported.9 Error values lie within the data points themselves.
To test further for possible differences in the interaction between Chol and DChol with phospholipids, we measured their effects on the melting behavior of DPPC bilayers by DSC. Our principal findings are shown in Figure 2 and Table 2.
Figure 2.
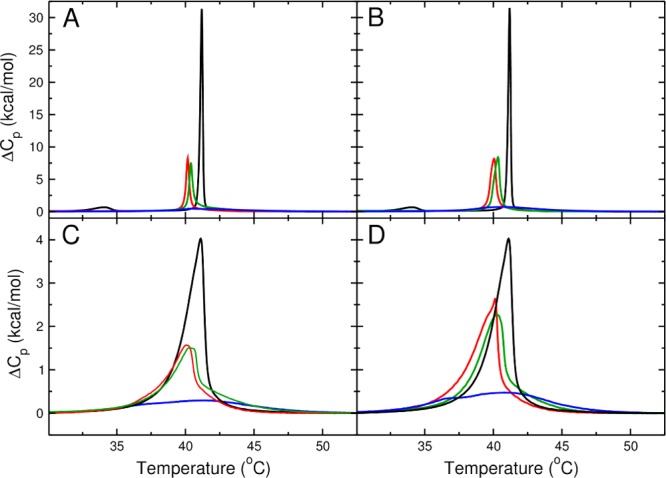
Excess heat capacity (ΔCp) curves determined by DSC. (A) DPPC/Chol MLV, (B) DPPC/DChol MLV, (C) DPPC/Chol LUV, and (D) DPPC/DChol LUV. Pure DPPC (black) and mixtures containing 5 (red), 10 (green), and 20 (blue) mol % sterol are shown. The curves are corrected for phospholipid concentration, determined by phosphate assay on the LUV, and corrected by baseline subtraction. Scan rate, 0.2 °C/min. The MLV curves are normalized by the heats determined in the LUV.
Table 2. Thermodynamic Data from DSC Analysis.
| lipid | vesicle | Tm (°C)a | ΔH (kcal/mol) |
|---|---|---|---|
| DPPC | LUV | 41.1 ± 0.2 | 8.8 ± 1.0 |
| MLV | 41.2 ± 0.1 | 8.7b | |
| DPPC/Chol 95:5 | LUV | 40.1 ± 0.2 | 5.3 ± 0.9 |
| MLV | 40.4 ± 0.1 | ||
| 90:10 | LUV | 40.4 ± 0.4 | 6.3 ± 1.0 |
| MLV | 40.4 ± 0.1 | ||
| 80:20 | LUV | 41.3 ± 0.4 | 3.0 ± 0.5 |
| MLV | 41.2 ± 0.1 | ||
| DPPC/DChol 95:5 | LUV | 40.1 ± 0.5 | 7.7 ± 0.2 |
| MLV | 40.4 ± 0.1 | ||
| 90:10 | LUV | 40.3 ± 0.5 | 7.5 ± 1.6 |
| MLV | 40.1 ± 0.4 | ||
| 80:20 | LUV | 40.8 ± 0.1 | 4.6 ± 0.3 |
| MLV | 40.6 ± 0.2 |
Tm is defined as the position of the maximum in the heat capacity curve. The values shown correspond to means and standard deviations (SD) of more than 20 determinations in DPPC and DPPC/Chol mixtures (except for DPPC/Chol (95:5), which had only 4 determinations) and 2 to 3 determinations for DPPC/DChol. These SD values do not explicitly take into account the errors associated with baseline corrections and phosphate analyses needed to calculate ΔH. We estimate that the inclusion of those sources of error results in a relative error of 10–15% in ΔH.
At low concentrations (5–10 mol %), Chol and DChol cause a slight freezing-point depression that is similar in magnitude (ca. 1 °C). This finding, by itself, suggests that both sterols have a similar preference for the liquid over the gel phase and that they have similar interactions with gel and liquid-disordered phospholipids. However, an inspection of the excess heat capacity (ΔCp) endotherms reveals that Chol affects the DPPC phase transition more than DChol, indicating that there are, in fact, some differences in sterol–phospholipid interactions. The values of the enthalpy change (ΔH) that are shown in Table 2 also support weaker DChol–DPPC interactions compared to Chol–DPPC interactions. Because of the limited quantity of DChol that was available for this study, which required a 27-step synthesis, only a few repeat experiments were possible. For this reason, the standard deviations listed for mixtures containing DChol should not be overinterpreted. Although we believe that the differences in ΔH between DPPC mixtures of Chol and DChol are real, they must be regarded as tentative at the present time.
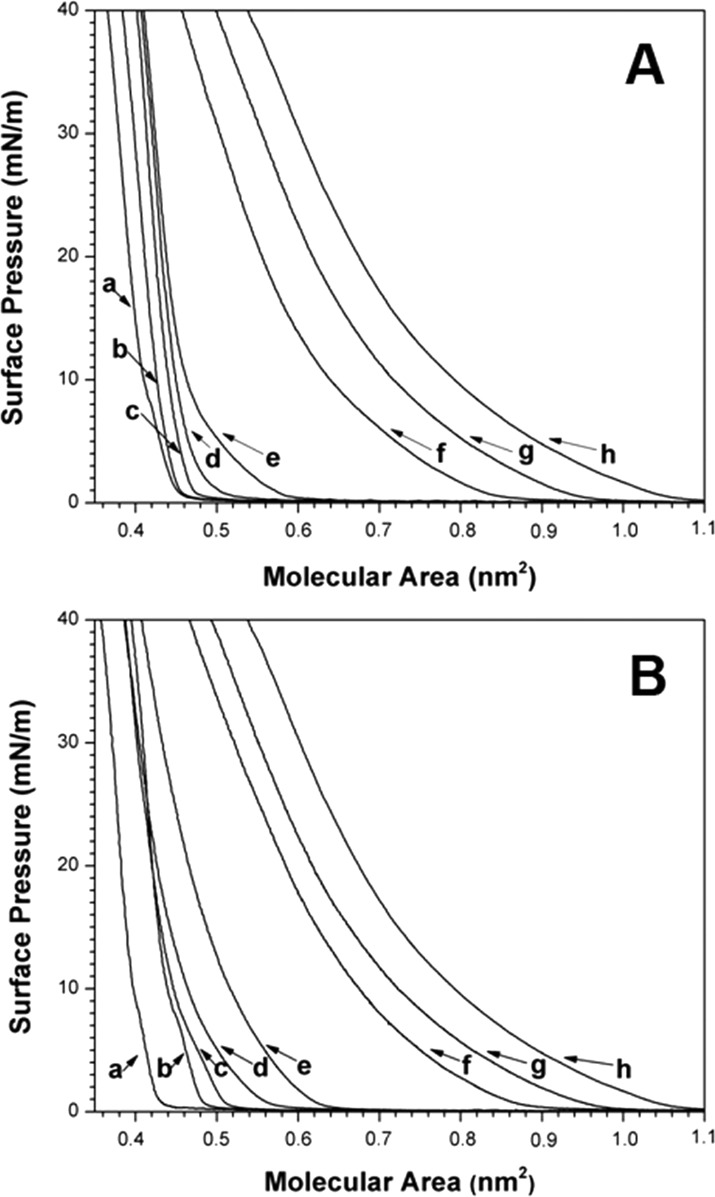
Finally, we have compared the condensing effects of DChol versus Chol via classic monolayer experiments.2 Figure 3 shows a series of surface pressure–area isotherms that were obtained for monolayers made from 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and cholesterol, and also DMPC and DChol, at the air/water interface as a function of the mole fraction of DMPC. For these measurements, DMPC was used instead of DPPC for convenience because of its lower gel to liquid-crystalline phase-transition temperature (Tm = 24 °C).1 Corresponding molecular area–additivity curves that were derived from these data at 10 mN/m, along with ideal additivity lines, are shown in Figure 4. Two features of the latter are noteworthy: (i) the maximum condensing effect by both sterols occurs when the sterol/phospholipid ratio is ∼1 and (ii) the condensing effect of these sterols is very similar, with cholesterol appearing to be slightly stronger than DChol.
Figure 3.
Surface pressure–area isotherms for (A) DMPC/DChol and (B) DMPC/Chol over a Tris buffer (pH 7.4) at 25 °C using the following mole fractions of DMPC: (a) 0.0, (b) 0.1, (c) 0.2, (d) 0.5, (e) 0.6, (f) 0.8, (g) 0.9, and (h) 1.0.
Figure 4.
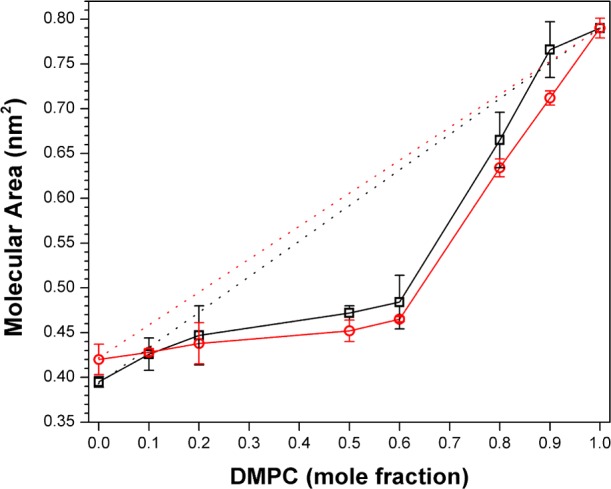
Molecular area–additivity curves for (□) DMPC/DChol and (○) DMPC/Chol with a surface pressure of 10 mN/m. Ideal additivities are shown by dotted lines. Error bars that are not visible lie within the symbols themselves.
Conclusions
The affinity that Chol and DChol have toward ordered phospholipid chains has been compared using a combination of nearest-neighbor recognition, calorimetry, fluorescence, and monolayer measurements. We have found that the two sterols have very similar interactions with the phospholipid but the condensing power of Chol is slightly stronger, i.e., its interaction with liquid-ordered DPPC is more favorable by a few tens of calories per mole. Within experimental error, DSC analysis has revealed no difference between the effects of Chol and DChol on the melting temperature of DPPC. However, the shape of the excess heat capacity (ΔCp) endotherms at low sterol concentrations is different. The decrease in ΔH that is induced by Chol appears to be slightly greater than that caused by DChol. Taken together, these results indicate that the combination of a rough and a smooth face found in cholesterol leads to slightly stronger interactions with ordered phospholipids as compared to DChol, which has two smooth faces. In other words, Chol is slightly better in inducing the liquid-ordered state than is DChol. Thus, our results are consistent with the hypothesis that the presence of a rough and a smooth face in Chol is important for sterol orientation in the bilayer, which in turn leads to improved interactions with the phospholipids.14,15 The hypothesis that the elimination of roughness in cholesterol’s β-face, by itself, will result in stronger interactions with phospholipids seems to be incorrect. Rather, it appears that sterol orientation and smoothness may be working together synergistically.9,12−15
Previous Monte Carlo simulations have shown that differences in nearest-neighbor interaction free energies on the order of tens of calories per mole can lead to significant changes in domain size distributions.20 On the basis of the differences that we have found between Chol and DChol, one can imagine that the combination of a rough and smooth face in cholesterol could play a role in defining the structure and function of lipid domains thought to exist in eukaryotic cell membranes.25−29
In eukaryotic membranes, most phospholipids contain a saturated acyl chain in the sn-1 position and an unsaturated chain with a cis double bond in the sn-2 position. It has been suggested that these “hybrid” lipids, having an ordered and a disordered chain, might be responsible for the breakdown of large rafts into nanodomains because they could act as 2D surfactants, placing themselves at the interface between cholesterol-rich, liquid-ordered domains and liquid-disordered regions. It is tempting to speculate that the rough and smooth faces of Chol might be playing a similar role. However, recent experimental evidence argues against this concept, at least in the case of hybrid lipids.30 Specifically, it has been shown that a hybrid lipid (POPC) behaves no differently from a low-melting, saturated phospholipid (DLPC) in affecting the size of liquid-ordered domains. Thus, hybrid lipids do not seem to be capable of promoting a 2D micellization of lipid rafts. Our results show that the difference in the interactions of Chol and DChol with DPPC is very small but significant. Although a comparison of the interactions of Chol and DChol with hybrid lipids remains to be carried out, we believe that their difference is likely to be of the same magnitude—on the order of tens of calories per mole. Whether such small differences in energy can significantly affect domain size distributions also awaits experimental verification.
In a broader context, cell membranes are also rich in proteins, accounting for ca. 50% of their total weight.1 Whether this two-faced character of cholesterol has any influence on the structure, lateral organization, and functioning of these proteins is a separate but important issue that remains to be clarified.
Acknowledgments
This work was funded by the National Science Foundation, CHE-1145500 (S.L.R), the National Institutes of Health, HL67773 (D.F.C) and 5 T32 HL00725 (L.M.-M.), and the North Carolina Biotechnology Center, 2009-IDG-1031 (P.F.A).
Supporting Information Available
Experimental procedures and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Gennis R. B.Biomembranes: Molecular Structure and Function; Springer-Verlag: New York, 1989. [Google Scholar]
- Leathes J. B. On the role of fats in vital phenomena. Lancet 1925, 208, 853–856. [Google Scholar]
- Demel R. A.; van Deenen L. L. M.; Pethica B. A. Monolayer interactions of phospholipids and cholesterol. Biochim. Biophys. Acta 1967, 135, 11–19. [Google Scholar]
- Stockton B. W.; Smith I. C. P. A deuterium nuclear magnetic resonance study of the condensing effect of cholesterol on egg phosphatidylcholine bilayer membranes. I. perdeuterated fatty acid probes. Chem. Phys. Lipids 1976, 17, 251–261. [DOI] [PubMed] [Google Scholar]
- Vist M.; Davis J. H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: deuterium nuclear magnetic resonance and differential scanning calorimetry. Biochemistry 1990, 29, 451–464. [DOI] [PubMed] [Google Scholar]
- Huang J.; Feigenson G. W. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 1999, 76, 2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A.; McConnell H. Condensed complexes in vesicles containing cholesterol and phospholipids. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 12662–12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung W.-C.; Lee M.-T.; Chen F.-Y.; Huang H. W. The condensing effect of cholesterol in lipid bilayers. Biophys. J. 2007, 92, 3960–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly T.; Wang M.; Regen S. L. The origin of cholesterol’s condensing effect. Langmuir 2011, 27, 2159–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt H. A.; Meyer T.; Nikolaus J.; Baek D. J.; Haralampiev I.; Thomas L.; Bittman R.; Muller P.; Herrmann A.; Huster D. Cholesterol’s aliiphatic side chain modulates membrane properties. Angew. Chem., Int. Ed. 2013, 52, 12848–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannock D.A.; Lewies R. N. A. H.; McElhaney R.N. Comparative calorimetric and spectroscopic studies of the effects of lanosterol and cholesterol on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes. Biophys. J. 2006, 91, 3327–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit S.A.; Jakobsson E.; Scott H.L. Simulation of the early stages of nano-domain formation in mixed bilayers of sphingomyelin, cholesterol, and dioleylphosphatidylcholine. Biophys. J. 2004, 87, 3312–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog T.; Pasenkiewicz-Gierula M. Non-polar interactions between cholesterol and phospholipids: a molecular dynamics simulation study. Biophys. Chem. 2004, 107, 151–164. [DOI] [PubMed] [Google Scholar]
- Rog T.; Pasenkiewiccz-Gierula M.; Vattulainen I.; Karttunen M. What happens if cholesterol is made smoother: importance of methyl substituents in cholesterol ring structure on phosphatidylcholine-sterol interaction. Biophys. J. 2007, 92, 3346–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyry S.; Rog T.; Karttunen M.; Vattulainen I. Significance of cholesterol methyl groups. J. Phys. Chem. B 2008, 112, 2922–2929. [DOI] [PubMed] [Google Scholar]
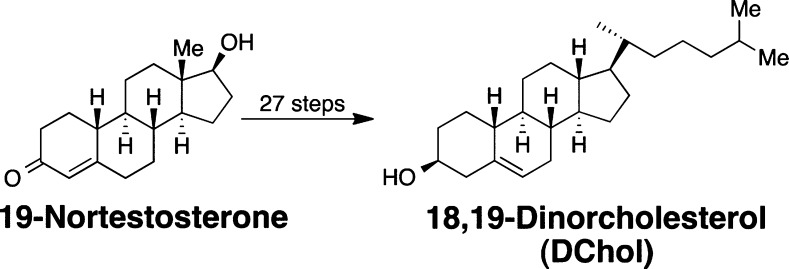
- Mydock-McGrane L.; Rath N. P.; Covey D. F. The synthesis of a “smoothened” cholesterol: 18,19-di-norcholesterol. J. Org. Chem. 2014, 79, 5636–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlovics J.A.; Wheaten S.A.; Almeida P.F. Phase separation and fluctuations in mixtures of a saturated and an unsaturated phospholipid. Biophys. J. 2012, 102, 2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H.; Zhang J.; Jing B.; Regen S. L A chemical sensor for the liquid-ordered phase. J. Am. Chem. Soc. 2005, 127, 8813–8816. [DOI] [PubMed] [Google Scholar]
- Bang E.-K.; Lista M.; l Sforazzini G.; Sakai N.; Matile S. Poly(disulfide)s. Chem. Sci. 2012, 3, 1752–1763. [Google Scholar]
- Almeida P. F. Thermodynamics of lipid interactions in complex bilayers. Biochim. Biophys. Acta 2009, 1788, 72–85. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B.; Thompson T. E. Cholesterol-induced phase immiscibility in membranes. Proc. Natl. Acad. Sci. U.S.A. 1991, 88, 8686–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkyilmaz S.; Almeida P. F.; Regen S. L. Effects of isoflurane, halothane and chloroform on the interactions and lateral organization of lipids in the liquid-ordered phase. Langmuir 2011, 27, 14380–14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parassi T.; DiStefano M.; Loiero M.; Ravagnan G.; Gratton E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys. J. 1994, 66, 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova V.P.; Heimburg T. Histogram method to obtain heat capacities in lipid monolayers, curved bilayers, and membranes containing peptides. Phys. Rev. 2001, 63, 041914. [DOI] [PubMed] [Google Scholar]
- Simons K.; Ikonen E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Brown D. A.; London E. Structure of detergent-resistent membrane domains: does phase separation occur in biological membranes?. Biochem. Biophys. Res. Commun. 1997, 240, 1–7. [DOI] [PubMed] [Google Scholar]
- Lingwood D.; Simons K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [DOI] [PubMed] [Google Scholar]
- Goh S. L.; Amazon J. J.; Feigenson G. W. Towards a better raft model: modulated phases in the four-component bilayer, DSPC/DOPC/POPC/CHOL. Biophys. J. 2013, 104, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomovitz R.; Maibaum L.; Schick M. Macroscopic phase separation, modulated phases, and microemulsions: a unified picture of rafts. Biophys. J. 2014, 106, 1979–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle F.A.; Doktorova M.; Goh S. L.; Standaert R.F; Katsaras J.; Feigenson G. W. Hybrid and nonhybrid lipids exert common effects on membrane raft size and morphology. J. Am. Chem. Soc. 2013, 135, 14932–14935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.