Abstract
We evaluated a multiplex-PCR to differentiate Mycobacterium bovis from M. tuberculosis Complex (MTC) by one step amplification based on simultaneous detection of pncA 169C > G change in M. bovis and the IS6110 present in MTC species. Our findings showed the proposed multiplex-PCR is a very useful tool for complementation in differentiating M. bovis from other cultured MTC species.
Keywords: bovine tuberculosis, tuberculosis, PCR-multiplex, diagnosis
Mycobacterium tuberculosis, which belongs to Mycobacterium tuberculosis Complex (MTC) together with M. bovis, M. bovis BCG, M. africanun, M. microti, M.canettii and M. pinnipedii, is the most common etiological agent of human tuberculosis (TB). However M. bovis may also be a relevant human pathogen in countries with high prevalence of bovine TB (OIE, 2009). Immuno-suppressing diseases, especially caused by Human Immunodeficiency Virus (HIV), is a risk factor for the development of human TB by M. bovis (Thoen et al., 2006; Cícero et al., 2009). Since M. bovis-caused human TB is clinically, pathologically and radiologically undistinguishable from M. tuberculosis-caused TB (De La Rua-Domenech, 2006), the fast differentiation of the two species has a high epidemiologic importance (Leite et al., 2003). Among molecular methods for detecting species belonging to the MTC, several markers have been employed. The IS6110 is highly used since this marker is found in several copies in the MTC genome (Eisenach et al., 1990; Ogusku et al., 2004). The pncA gene, which encodes the pyrazinamidase enzyme (PZAase) that converts the pro-drug pyrazinamide (PZA) to pyrazinoic acid (POA), has a single point mutation at nucleotide 169C > G (appears to be unique to M. bovis) is one of the markers that is commonly used to distinguish M. bovis from others MTC species (De Los Monteros et al., 1998; Kidane et al., 2002). In the current study we evaluated a rapid test to differentiate M. bovis from other MTC species by one step amplification based on simultaneous detection of pncA 169C > G change in M. bovis and the IS6110 present in MTC species. DNA from M. tuberculosis H37Rv (ATCC 27294), M. bovis AN5, M. avium (Central Laboratory - LACEN/PR), M. smegmatis (LACEN/PR), M. kansasii (LACEN/PR), M. fortuitum (LACEN/PR), M. szulgai (LACEN/PR), M. massilienses (LACEN/PR), M. abcessus (LACEN/PR), M. chelonae (LACEN/PR) reference strains, one M. bovis BCG, 17 M. bovis, isolated from bovine lymph nodes and indentified by biochemical and molecular methods in a previous work (Cardoso et al., 2009), 30 M. tuberculosis clinical isolates indentified by biochemical and molecular methods in a previous work (Noguti et al., 2010) were used. All M. bovis clinical isolates were previously characterized by spoligotyping (data not shown) and all M. tuberculosis by spoligotyping and MIRU (Noguti et al., 2010). Amplification was carried out with specific primers for M. tuberculosis complex TB1 (5′-CCTGCGAGCGTAGGCGTCGG-3′) and TB2 (5′-CTCGTCCAGCGCCGCTTCGG-3′) (Eisenach et al., 1990) which amplify a fragment of 123 base pairs (bp) of the IS6110 sequence and with specific primers for M. bovis pncATB-1.2 (5′-ATGCGGGCGT TGATCATCGTC-3′) and pncAMB-2 (5′-CGGTGTGC CGGAGAAGCCG-3′) (De Los Monteros et al., 1998; Bannalikar et al., 2006) which amplify a fragment of 186 bp of pncA gene. DNA extracts (1 μL) from all mycobacterial reference strains and clinical isolates, were amplified by multiplex-PCR using 24 μL reaction mixture containing 0.2 pmol/μL TB1 and TB2 primers (Invitrogen - Integrated DNA Technologies, Inc. Coralville, USA), 0.4 pmol/μL pncATB-1.2 and pncAMB-2 primers (Invitrogen - Integrated DNA Technologies, Inc. Coralville, USA) and PCR Master Mix (Promega Corporation, Madison, Wisconsin, USA), according to manufacturer’s instructions. Amplification was carried out in a Perkin-Elmer Gene Amp PCR System 2400 thermalcycler (Waltham, Massachusetts, USA) with an initial cycle of 5 min at 94 °C; 35 cycles of 1 min at 94 °C, 1 min at 68 °C, 1 min at 72 °C and a final extension at 72 °C for 10 min. M. tuberculosis H37Rv (1 ng) and M. bovis (1 ng) as positive and water as negative controls were included in all assays. PCR products were separated by electrophoresis on 2% agarose gel (Biotools/M&B Laboratories, S. A., Uniscience do Brasil, São Paulo, Brazil) at 100 V for 1 h in TBE. Gel were stained with ethidium bromide 0.5 μg/μL, visualized under an ultraviolet light Macrovue (Pharmacia Bioscience, Upsala, Sweden), and photodocumented digitally by Power Shot S215 (Cannon, NY, USA). A 100 bp DNA standard (Invitrogen Life Technologies, São Paulo, Brazil) was used as a DNA size marker.
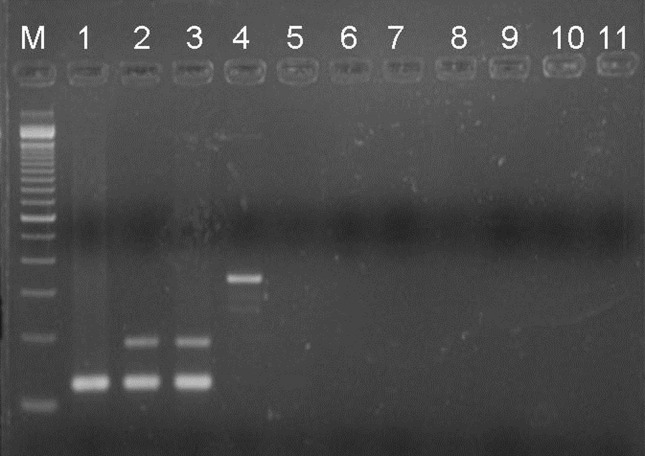
The multiplex-PCR showed to be specific to differentiate M. bovis from other MTC species by amplification of two DNA fragments sized 123 and 186 bp and a 123 bp DNA fragment respectively. No PCR product of specific sizes to M. tuberculosis and M. bovis were observed in amplification of DNA from nontuberculous mycobacteria reference strains except for M. avium that showed a DNA fragment sized more than 300 bp (Figure 1). Also, all M. tuberculosis and M. bovis clinical isolates (confirmed by spoligotyping) showed a single (123 bp) and two (123 and 186 pb) DNA fragments respectively, by multiplex-PCR.
Figure 1.
Specificity of multiplex-PCR for differentiate Mycobacterium bovis from M. tuberculosis Complex (MTC). Electrophoresis on 2% agarose gel. Line M: DNA molecular weight marker (100 bp); Line 1: M. tuberculosis H37Rv; Line 2: M. bovis AN5; Line 3: M. bovis BCG; Line 4: M. avium; Line 5: M. kansasii; Line 6: M. abcessus; Line 7: M. chelonae; Line 8: M. massiliense; Line 9: M. szulgai; Line 10: M. fortuitum; Line 11: negative control.
The multiplex-PCR combining TB1/TB2 (Eisenach et al., 1990) and pncATB-1.2/pncAMB-2 (De Los Monteros et al., 1998) primers, proposed in this study, could not differentiate among the six species of MTC, but it showed to be specific in differentiating M. bovis from the others. Exception was for M. bovis BCG, which could not be differentiated from wild M. bovis (clinical isolates), once the first one tested has the 169C > G mutation too.
The correct differentiation of M. bovis triggers to an appropriate therapy, knowledge on TB epidemiology and control of bovine infection (De Los Monteros et al., 1998; Bannalikar et al., 2006; De La Rua-Domenech et al., 2006). In fact, the proposed multiplex-PCR seems to be a very useful tool for complementation in differentiating M. bovis from others MTC species, which are slow-growing mycobacteria, where a small number of bacilli in cultures hinder identification by biochemical tests. Additionally, biochemical identification methods are not able to provide satisfactory results and the multiplex-PCR also has the advantage of being a fast method with results provided in one day. As consequence, a specific, fast and low cost diagnosis method is highly relevant to differentiate M. bovis isolates from the M. tuberculosis complex (Bannalikar et al., 2006).
The multiplex-PCR showed to be an important tool for differentiation of M. bovis from M. tuberculosis cultured isolates tested. Hopefully, the proposed multiplex-PCR should be tested with the other species from the MTC to set up its potential in differentiating them as the multiplex-PCR proposed by Yeboah-Manu et al. (2001). The multiplex-PCR added of a internal control should also be studied in clinical specimens as additional diagnostic tool mainly in regions where bovine and human TB coexist for the monitoring infection by M. bovis in human beings.
Acknowledgments
This study was supported in part by a grant from the Coordination for the Capacitation of Higher Education Personnel (CAPES), Brazil.
References
- Bannalikar AS, Verma R. Detection of Mycobacterium avium and Mycobacterium tuberculosis from human sputum cultures by PCR-RFLP analysis of hsp65 gene and pncA PCR. Indian J Med Res. 2006;123:165–172. [PubMed] [Google Scholar]
- Cardoso MA, Cardoso RF, Hirata RDC, Hirata MH, Leite CQF, Santos ACB, Siqueira VLD, Okano W, Rocha NS, Lonardoni MVC. Direct Detection of Mycobacterium bovis in Bovine Lymph Nodes by PCR. Zoonoses Public Health. 2009;56:465–470. doi: 10.1111/j.1863-2378.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- Cícero R, Olivera H, Hernández-Solis A, Ramírez-Casanova E, Escobar-Gutiérrez A. Frequency of Mycobacterium bovis as an etiologic agent in extrapulmonary tuberculosis in HIV-positive and -negative Mexican patients. Eur J Clin Microbiol Infect Dis. 2009;28:455–460. doi: 10.1007/s10096-008-0649-5. [DOI] [PubMed] [Google Scholar]
- De La Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: Incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis. 2006;86:77–109. doi: 10.1016/j.tube.2005.05.002. [DOI] [PubMed] [Google Scholar]
- De Los Monteros LEE, Galán JC, Gutiérrez SS, Marín JFG, Matín C, Dominguez L, Rafael L, Baquero F, Gómez-Mampaso E, Blásquez J. Allele-specific PCR method based on pncA and oxyR for distinguishing Mycobacterium bovis from Mycobacterium tuberculosis: Intraspecific M. bovis pncA sequence polymorphism. J Clin Microbiol. 1998;36:239–242. doi: 10.1128/jcm.36.1.239-242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach KD, Cave MD, Bates JH, Crawford JT. Polymerase chain reaction of a repetitive DNA sequence specific to Mycobacterium tuberculosis. J Infect Dis. 1990;161:977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Kidane D, Olobo JO, Habte A, Negesse Y, Aseffa A, Abate G, Yassin MA, Betreda K, Harboe M. Identification of the Causative Organism of Tuberculous Lymphadenitis in Ethiopia by PCR. J Clin Microbiol. 2002;40:4230–4234. doi: 10.1128/JCM.40.11.4230-4234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite CQF, Anno IS, Leite SRA, Roxo E, Morlock GP, Cooksey RC. Isolation and identification of Mycobacteria from livestock specimens and milk obtained in Brazil. Mem Inst Oswaldo Cruz. 2003;98:319–323. doi: 10.1590/s0074-02762003000300005. [DOI] [PubMed] [Google Scholar]
- Noguti EN, Leite CQF, Malaspina AC, Santos ACB, Hirata RDC, Hirata MH, Mamizuka EM, Cardoso RF. Genotyping of Mycobacterium tuberculosis isolates from a low-endemic setting in northwestern state of Paraná in southern Brazil. Mem Inst Oswaldo Cruz. 2010;105(6):779–785. doi: 10.1590/s0074-02762010000600008. [DOI] [PubMed] [Google Scholar]
- Ogusku MM, Salem JI. Analysis of different primers used in the PCR method for the diagnosis of tuberculosis in the state of Amazonas. J Bras Pneumol. 2004;30:433–439. [Google Scholar]
- OIE. [Acessed 17 Nov 2009];Bovine Tuberculosis. 2009 Avaliable at: http://www.cfsph.iastate.edu/Factsheets/pdfs/bovine_tuberculosis.pdf.
- Thoen C, Lobue P, Kantor I. The importance of Mycobacterium bovis as a zoonosis. Vet Microbiol. 2006;112:339–345. doi: 10.1016/j.vetmic.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Yeboah-Manu D, Yates MD, Wilson SM. Application of a Simple Multiplex PCR to Aid in Routine Work of the Mycobacterium Reference Laboratory. J Clin Microbiol. 2001;39:4166–4168. doi: 10.1128/JCM.39.11.4166-4168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]