Abstract
Small molecules that increase the oxygen affinity of human hemoglobin may reduce sickling of red blood cells in patients with sickle cell disease. We screened 38 700 compounds using small molecule microarrays and identified 427 molecules that bind to hemoglobin. We developed a high-throughput assay for evaluating the ability of the 427 small molecules to modulate the oxygen affinity of hemoglobin. We identified a novel allosteric effector of hemoglobin, di(5-(2,3-dihydro-1,4-benzodioxin-2-yl)-4H-1,2,4-triazol-3-yl)disulfide (TD-1). TD-1 induced a greater increase in oxygen affinity of human hemoglobin in solution and in red blood cells than did 5-hydroxymethyl-2-furfural (5-HMF), N-ethylmaleimide (NEM), or diformamidine disulfide. The three-dimensional structure of hemoglobin complexed with TD-1 revealed that monomeric units of TD-1 bound covalently to β-Cys93 and β-Cys112, as well as noncovalently to the central water cavity of the hemoglobin tetramer. The binding of TD-1 to hemoglobin stabilized the relaxed state (R3-state) of hemoglobin. TD-1 increased the oxygen affinity of sickle hemoglobin and inhibited in vitro hypoxia-induced sickling of red blood cells in patients with sickle cell disease without causing hemolysis. Our study indicates that TD-1 represents a novel lead molecule for the treatment of patients with sickle cell disease.
Sickle cell disease (SCD) is an inherited disorder that affects millions of people throughout the world and an estimated 70 000–100 000 people in the United States.101 Under hypoxic conditions, deoxygenated sickle hemoglobin (deoxyHbS) polymerizes and distorts sickle cell red blood cells (SS RBCs) into a rigid and sickled shape, causing occlusion and thrombosis in small blood vessels.1−3 To treat SCD, various compounds have been developed, including molecules that increase the concentration of fetal hemoglobin (HbF),2,3 inhibit polymerization of HbS,1,4 promote SS RBC hydration or prevent dehydration of SS RBCs,5 and prevent adhesion of SS RBCs to capillary endothelium.6 Used clinically, hydroxyurea treats SCD by increasing the concentration of HbF in SS RBCs, because HbF does not enter in HbS polymers.2,3
Increasing the oxygen affinity of HbS represents a potential therapeutic strategy for SCD, because increased oxygen affinity leads to a decrease in the fraction of deoxyHbS.1,4,7 A variety of allosteric effectors have been identified that increase the oxygen affinity of hemoglobin including aromatic aldehydes,8−10 isothiocyanates,11 acyl salicylates derivatives,12,13 methyl acetyl phosphate,14 disulfides,15 and maleimides.16 These molecules bind to hemoglobin and preferentially stabilize the relaxed or R-state of hemoglobin relative to the tense or T-state,17−19 shifting the R to T state equilibrium toward the R-state, which has a higher oxygen affinity.10,18,20 For example, the aromatic aldehyde, 5-hydroxymethyl-2-furfural (5-HMF), increases the oxygen affinity of hemoglobin and is undergoing clinical studies as a treatment for SCD.8,10
For an allosteric effector to be a safe and effective treatment for SCD, the compound should be highly specific for hemoglobin and moderately increase the oxygen affinity without causing hemolysis at efficacious doses. To date, no rational design has been reported to confer all of these properties to small molecules. We therefore sought to identify novel allosteric effectors from a compound library with diverse chemical scaffolds.21
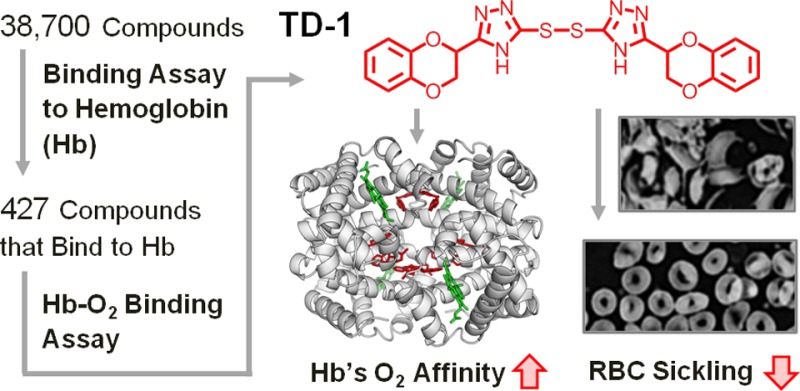
We report the identification of a novel allosteric effector for hemoglobin that was discovered by sequential high-throughput screening of small molecules to ascertain their abilities, first, to bind to hemoglobin using small molecule microarrays (SMMs)22 and, subsequently, to modulate the oxygen affinity of hemoglobin. Using X-ray crystallography, we revealed the three-dimensional structure of the hemoglobin-effector complex and identified the hemoglobin-binding sites of the allosteric effector, thus providing a rational explanation for its allosteric properties. Moreover, we demonstrated that the allosteric effector increases the oxygen affinity of HbS and inhibits in vitro sickling of SS RBCs under hypoxic conditions without causing hemolysis.
Results and Discussion
Identification of Compounds that Bind to Hemoglobin Using Small Molecule Microarrays
Compounds that selectively bind to hemoglobin were sought by screening 38 700 compounds that were affixed to glass slides as SMMs.23 The screened compounds included known bioactive species and chemicals from the Broad Institute of Harvard and MIT diversity-oriented synthesis library.21 The SMMs were incubated in an aqueous solution of hemoglobin and then rinsed. Compounds that bind to hemoglobin were detected using a primary antibody directed against hemoglobin followed by a dye-labeled antibody directed against the primary antibody. The assay was validated by confirming the binding of hemoglobin to 2,3-diphosphoglycerate (2,3-DPG), a naturally occurring allosteric effector,24 which was fixed on the microarray (Supporting Information Figure S1). We identified 427 small molecules that bind to hemoglobin, resulting in a hit rate of about 1% (Supporting Information Table S1).
Identification of Allosteric Effectors of Hemoglobin Using a Novel High-Throughput Hemoglobin Oxygen Binding Assay
To identify compounds that alter the oxygen affinity of hemoglobin from among the 427 hemoglobin binders, we developed a novel high-throughput hemoglobin oxygen binding assay (Supporting Information Figure S2). This assay is based on the spectrophotometric evaluation of the fraction of oxygenated hemoglobin (oxyHb%) and oxidized hemoglobin (metHb%)25,26 in 384-well plates. The assay was validated by measuring oxyHb% and metHb% in the presence or absence of two compounds known to modulate the oxygen dissociation curve (ODC) of purified human hemoglobin, myo-inositol hexaphosphate (IHP) and N-ethylmaleimide (NEM), at a ligand to hemoglobin tetramer ratio of 6:1 (mol/mol). The IHP-induced decrease and NEM-induced increase of the oxyHb% were consistent with the known ability of these compounds to shift the ODC to the right27 or the left,16 respectively, without increasing the metHb level. The high-throughput assay that we developed for evaluating the oxygen affinity of hemoglobin enabled a large number of measurements to be conducted simultaneously with only small quantities of test compounds (3 nmol per well). We identified six compounds (compounds 1–6) that alter oxyHb% without increasing metHb% (Figure 1A, Supporting Information Table S2, and S3).
Figure 1.
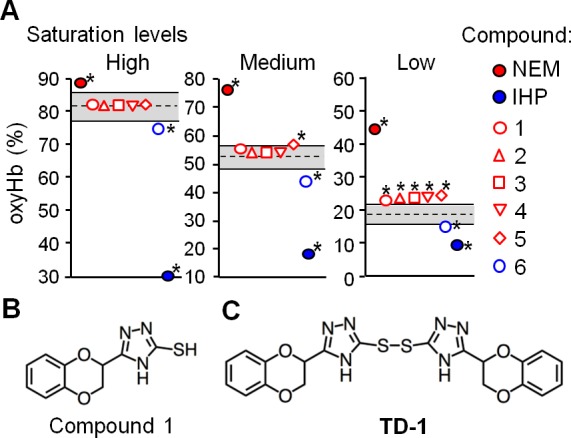
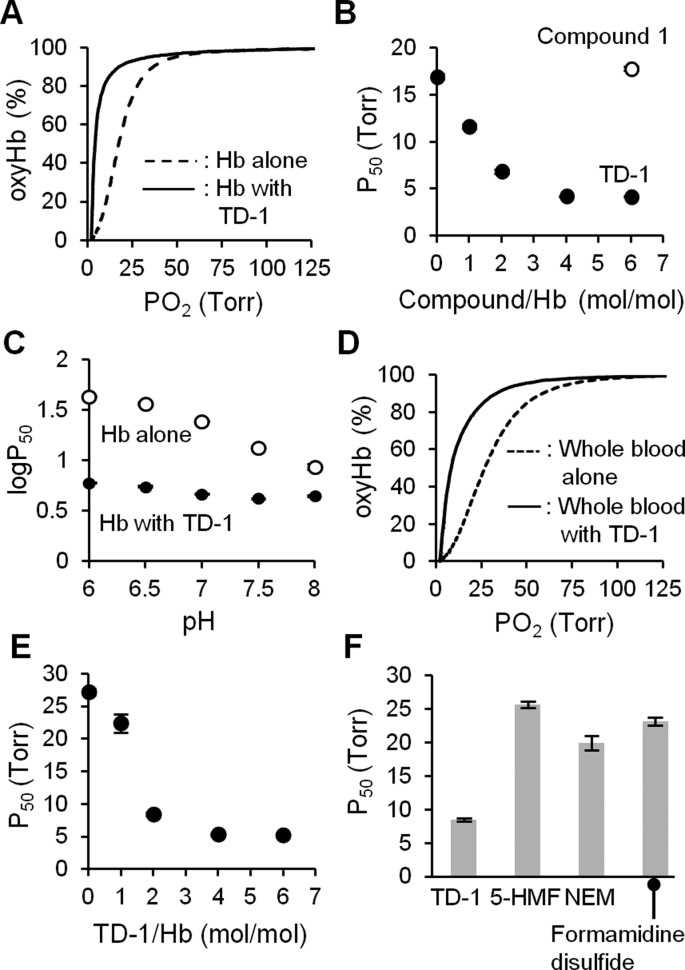
Identification of small molecules that alter the oxygen affinity of normal human hemoglobin. (A) The fraction of hemoglobin that binds oxygen (oxyHb%) was evaluated in the absence of added compounds (oxyHb%ctrl) or in their presence (oxyHb%cpd). The dashed line indicates the mean value of oxyHb%ctrl under each condition (high, medium, and low oxyHb saturation conditions) and the gray area bounded by solid lines indicates the range of the mean value ± 2.5 standard deviations of oxyHb%ctrl. Each symbol represents the mean value of oxyHb%cpd measured in quadruplicate. Incubation of hemoglobin with NEM and IHP increased and decreased the oxyHb%, respectively. Asterisks indicate compounds that the mean value of oxyHb%cpd was out of the gray area under each condition. (B) The chemical structure of compound 1. (C) The chemical structure of TD-1 (disulfide form of compound 1).
To characterize the allosteric effects of the six compounds, ODCs of hemoglobin (20 μM tetramer) were measured in the presence and absence of each compound at 37 °C, and the P50 (the partial oxygen pressure at which oxyHb% is 50%) was determined as a measure of oxygen affinity. In the absence of the compounds, the P50 of hemoglobin was 17 ± 0.3 Torr (data mean value ± s.d.). When the ODC was measured using a stock solution of compound 1 prepared in DMSO two or more months prior to mixing with hemoglobin, the P50 was 18 ± 0.1 (120 μM compound 1) and 10 ± 0.5 Torr (2 mM compound 1, P < 0.001 vs in the absence of compound 1). The decrease of P50 by compound 1 was in agreement with the result of the high-throughput assay showing an increase in oxyHb%.
Identification of the Disulfide Form of Compound 1 (TD-1) that Markedly Increases Hemoglobin Oxygen Affinity
Compound 1 contains a triazole ring and a benzodioxine group (Figure 1B). It is the only thiol among the 427 compounds that binds to hemoglobin. It is known that thiols can be oxidized to disulfides, and disulfides have been reported to increase the oxygen affinity of hemoglobin.15 We hypothesized that the disulfide form of compound 1, triazole disulfide (TD-1, Figure 1C), rather than compound 1, itself, increased oxyHb% in the high-throughput assay and decreased P50 in the ODC measurement. To test this hypothesis, the ODC of hemoglobin was measured after mixing with freshly prepared DMSO solutions of compound 1 or TD-1. When hemoglobin (20 μM tetramer) was mixed with compound 1 (120 μM) at 37 °C, and the ODC was measured after 10 min of incubation, the P50 was 17 ± 0.1 Torr. In contrast, incubation of hemoglobin with TD-1 markedly shifted the ODC to the left, reflecting a marked reduction of the P50 from 17 ± 0.3 to 4.2 ± 0.1 Torr (P < 0.001; Figure 2A). Moreover, the impact of TD-1 on the ODC was dose-dependent with efficacy observed even when the molar ratio of compound to hemoglobin was 1:1 (P50 12 ± 0.3 Torr; P < 0.001 vs without TD-1; Figure 2B). Incubation of hemoglobin with TD-1 reduced the Bohr effect (the increase of the P50 of hemoglobin induced by decreasing the pH).28 (Δlog P50)/ΔpH was −0.08 ± 0.02 vs −0.37 ± 0.04, with and without TD-1 (at a 6:1 molar ratio of TD-1 to hemoglobin), respectively (P < 0.001, Figure 2C).
Figure 2.
TD-1 increases the oxygen affinity of normal human hemoglobin (Hb) and whole blood. (A) The ODC of hemoglobin (Hb, 20 μM as tetramer) without and with TD-1 (120 μM in DPBS, pH 7.4, with 5 vol% DMSO) at 37 °C. (B) The P50 of hemoglobin mixed without and with Compound 1 (120 μM) or TD-1 (20, 40, 80, and 120 μM). (C) Relationship between pH and P50 of hemoglobin (20 μM) with and without addition of TD-1 (120 μM) in 0.1 M phosphate buffer (pH 6-8) with 5 vol% DMSO at 37 °C. (D) The ODC of whole blood without or with TD-1 (40 μM, 2:1 molar ratio to Hb tetramer) in Hemox solution with 0.8 vol% DMSO at 37 °C. (E) The P50 of whole blood mixed with TD-1 (20, 40, 80, and 120 μM). Each symbol represents the mean value of P50 measured in triplicate. (F) The P50 of whole blood mixed with TD-1 (40 μM), 5-HMF, formamidine disulfide, and NEM (all 120 μM). Each bar represents the mean value of P50 measured in triplicate. Error bars represent standard deviation.
To test whether TD-1 could reduce the P50 of hemoglobin in RBCs, we incubated whole blood (hemoglobin 0.13 g dL–1) from healthy human donors with TD-1 (40 μM; 2:1 molar ratio of TD-1 to hemoglobin) for 10 min at 37 °C, and the ODC was measured. TD-1 decreased the P50 from 27 ± 0.5 to 8.5 ± 0.3 Torr (p < 0.001, Figure 2D). The impact of TD-1 on the ODC was dose-dependent with efficacy observed even when the ratio of compound to hemoglobin was 1:1 (P50 = 22 ± 1.4 Torr; P < 0.001 vs without TD-1; Figure 2E). TD-1 (40 μM) reduced the P50 of whole blood greater than did other small molecules previously reported to increase the oxygen affinity of hemoglobin (Figure 2F). For example, at a 6:1 molar ratio of compound to hemoglobin, 5-HMF did not change the P50 (P50 = 26 ± 0.5 Torr), and NEM (P50 = 20 ± 1.1 Torr; P < 0.001 vs 40 μM TD-1) and diformamidine disulfide (P50 = 23 ± 0.6 Torr; P < 0.001 vs 40 μM TD-1) had only modest effects. Taken together, these results indicate that TD-1 potently augments the oxygen affinity of hemoglobin in blood.
Three-Dimensional Structure of the Hemoglobin Complexed with TD-1 Revealed by X-ray Crystallography
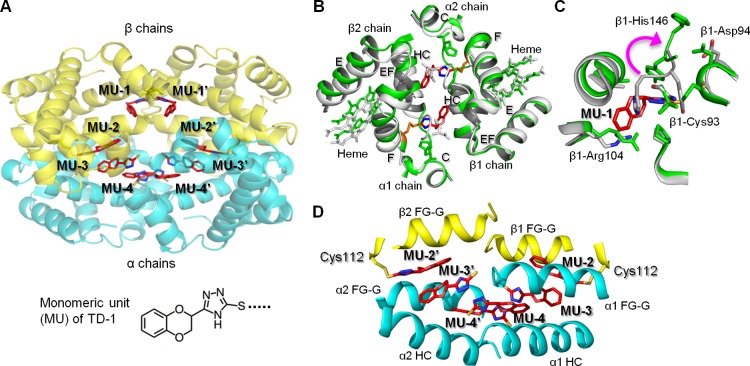
To investigate how TD-1 increases the oxygen affinity of hemoglobin, carboxyhemoglobin (COHb) was cocrystallized with a 10-fold molar excess of TD-1. We used COHb in the crystallographic studies since COHb is much more stable than oxyHb, which has the propensity to oxidize to metHb under ambient conditions. We obtained needle crystals of hemoglobin in the R3 state, one of the R state conformers.10,17,29 Detailed crystallographic and structural analysis parameters are reported in Supporting Information Table S4. The tetrameric structure of the R3 state revealed that eight monomeric units (MUs) of TD-1 were bound to hemoglobin (Figure 3A). Two MUs were located on the surface of hemoglobin forming a disulfide bond with β-Cys93 (MU-1/MU-1′; Figure 3B and Supporting Information Figure S3). The covalent binding of a MU to β-Cys93 resulted in significant tertiary and quaternary structural changes in hemoglobin as compared to the R3 structure in the absence of TD-1 (PDB code: 1YZI; Figure 3B and C). These changes include movement of the EF corner, F helix, and heme toward the interdimer interface and a narrower β-cleft and central water cavity that should lead to further stabilization of the R-state, as previously reported29 (Figure 3B). Similar structural movements are also observed when purified deoxyHb binds oxygen or CO (transition from T to the classical R state or from T to R3 state).17 Two other MUs formed disulfide bonds with the two β-Cys112 residues (MU-2/MU-2′) located in the central water cavity. The remaining MU molecules (MU-3/MU-3′ and MU-4/MU-4′) were located in the water cavity interacting noncovalently with amino acid residues lining the water cavity and extensively with each other, as well as with MU-2/MU-2′ further stabilizing the R-state (Figure 3D and Supporting Information Figure S7).
Figure 3.
The relaxed state (R3-state) of hemoglobin is stabilized by TD-1 via covalent and non-covalent bonding. (A) Structure of R3-state hemoglobin with TD-1 as monomeric units (MUs, red sticks). α-chains of hemoglobin are blue, and β-chains are yellow. Heme is not shown for clarity. (B) Binding of MU-1/MU-1′ (red sticks) to β-Cys93 (orange sticks) induces large tertiary and quaternary structural perturbations. Hemoglobin in the absence of MUs (R3 state, PDB code 1YZI) is shown in white/gray and hemoglobin in the presence of MUs (PDB code 4NI0) is shown in green. (C) Superposition of COHb without MUs (white ribbons) and COHb-MU complex (green ribbons). MU-1 is shown as red sticks. A magenta arrow represents the movement of β-His146 associated with MU-1/MU-1′ binding of Cys93. (D) Interactions of MU-2/MU-2′, MU-3/MU-3′, and MU-4/MU-4′ in the water cavity help to tie the four hemoglobin subunits together.
The high reactivity of TD-1 for covalent binding to hemoglobin is likely due to its triazole ring, which stabilizes the MU dissociated from TD-1.30,31 We observed covalent modification of hemoglobin by MU at β-Cys93 and β-Cys112 but not at α-Cys104. There are six cysteine amino acids in hemoglobin (α-Cys104, β-Cys93, and β-Cys112, and their symmetry-related pairs). β-Cys93 is exposed at the surface of hemoglobin in the oxygenated state, and β-Cys112 is located in the central water cavity, while α-Cys104 is buried in the protein’s interior. β-Cys93 is known to react with reagents such as disulfides,15,31 maleimide,32 nitric oxide,33 p-hydroxymercuribenzoate (PMB),34,35 and methyl bromide.36 α-Cys104 and β-Cys112 have been considered relatively non–reactive, since these residues are believed to be inaccessible.34 However, both PMB35 and methyl bromide36 can react with all the cysteine residues of hemoglobin. These results indicate that compounds that access the central cavity can react with the thiol of β-Cys112 even though β-Cys112 is not exposed on the surface of hemoglobin.
Stabilization of R-State (R3-state) Hemoglobin by TD-1
The crystal structure of hemoglobin bound to TD-1 suggests two mechanisms by which TD-1 can increase the oxygen affinity of hemoglobin. First, covalent binding of MU to β-Cys93 displaces the position of β-His146, sterically preventing the salt-bridge interaction between β-His146 and β-Asp94 in the T-state. Prevention of this salt-bridge interaction both destabilizes the T-state and stabilizes the R-state with a concomitant increase in oxygen affinity and impairment of the Bohr effect.37,38 A second mechanism by which TD-1 alters the oxygen affinity of hemoglobin is attributable to the binding of the six MUs in the central water cavity. Each of the six MUs makes hydrogen bond and/or hydrophobic interactions with the hemoglobin subunits and at least one other MU. These interactions help to tie all four hemoglobin subunits together, stabilizing the R state and restricting the subunit rotation that is required to transition to the T state.
TD-1 increased the oxygen affinity of hemoglobin in a manner different from other allosteric effectors, 5-HMF, and the bifunctional acylating agent bis(3,5-dibromosalicyl)fumarate. Two 5-HMF molecules form a Schiff-base interaction with the two N-terminal α-Val1 nitrogen atoms at the α-cleft of hemoglobin in the R2-state, stabilizing the relaxed state to increase hemoglobin oxygen affinity.9,39 Bis(3,5-dibromosalicyl)fumarate spans the 2,3-DPG binding site of hemoglobin40 by cross-linking β1-Lys82 to β2-Lys82, and as expected in the presence of 2,3-DPG, the oxygen affinity of the cross-linked hemoglobin is increased due to blockade of the 2,3-DPG site.13 The locations of all eight MUs of TD-1 are different from the 2,3-DPG binding site. 2,3-DPG binds at the β-cleft on the dyad axis, and preferentially stabilizes the T state of hemoglobin relative to the R state.40
Inhibition of Hypoxic Sickling of Red Blood Cells by TD-1
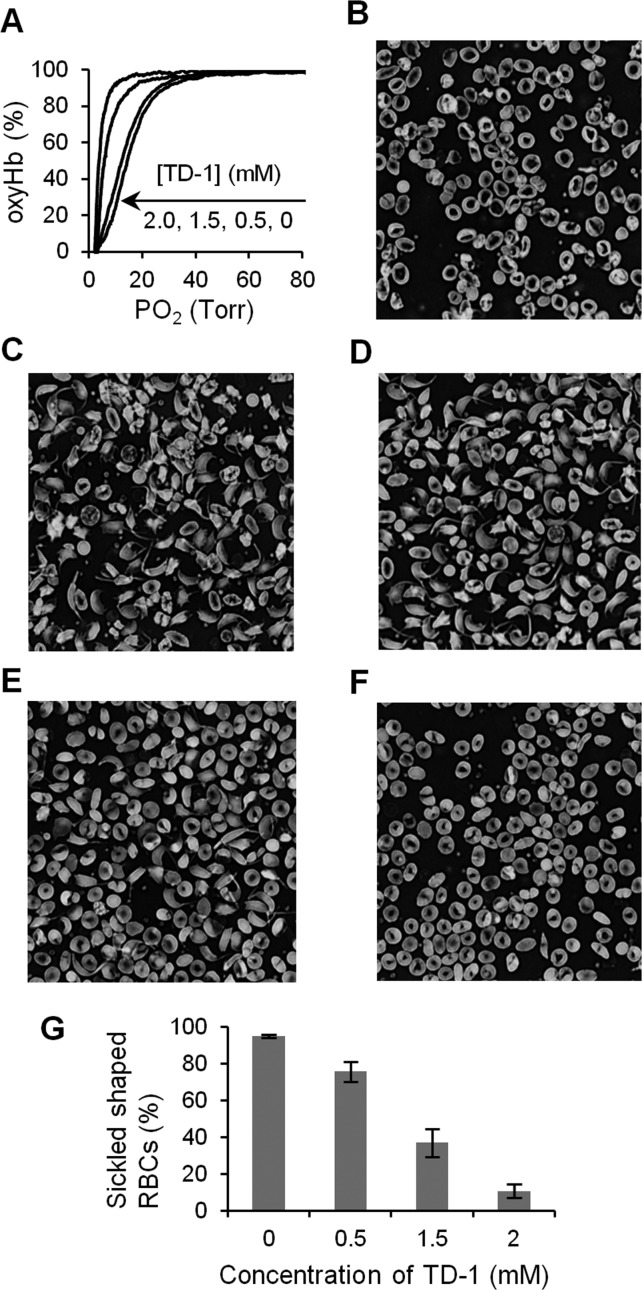
The pathophysiology of SCD is primarily driven by the polymerization of deoxyHbS. Since TD-1 increased the oxygen affinity of human adult hemoglobin (HbA), we investigated the ability of TD-1 to increase the oxygen affinity of HbS. When TD-1 was mixed with hemolysates of SS RBCs and the ODC was measured, the oxygen affinity increased in a dose-dependent manner (Figure 4A). We also investigated the ability of TD-1 to inhibit sickling of SS RBCs under experimental hypoxic conditions. SS RBCs were incubated in the presence and absence of three different concentrations (0.5, 1.5, and 2 mM) of TD-1 under hypoxic conditions (a gas mixture of 4% oxygen and 96% of nitrogen at 37 °C) for 3 h. In the absence of TD-1, deoxygenation of SS RBCs induced RBC transition from discocytes (Figure 4B) to sickled shapes (Figure 4C). Incubation of SS RBCs (hematocrit∼ 20%) with TD-1 prior to exposure to hypoxia reduced the percentage of sickled cells without causing hemolysis in a dose-dependent manner (Figure 4D–G). The percentage of SS RBCs was markedly reduced from 95 ± 0.3% (without addition of TD-1, mean value ± s.d.) to 11 ± 4% in the presence of TD-1 (2 mM, P < 0.001 differs vs SS RBCs incubated without TD-1). Taken together, these results demonstrate that TD-1 inhibits the sickling of human SS RBCs, likely by increasing the oxygen affinity of HbS.
Figure 4.
Anti-sickling effect of TD-1. (A) Representative ODCs of the hemolysates of SS RBCs without or with TD-1 at 25 °C. The ODC shifted to the left in a dose-dependent manner of TD-1. P50 of hemolysates from SS RBCs (hematocrit ∼20%) treated without or with 0.5, 1.5, and 2.0 mM of TD-1 were 13 ± 0.5 (data mean value ± s.d.), 12 ± 0.8, 7.2 ± 0.5, and 5.1 ± 1.1 Torr (P < 0.001, vs without TD-1), respectively. (B) Morphology of SS RBCs (hematocrit ∼20%) incubated under normoxic conditions revealed primarily discocytes with some irreversibly sickled cells. (C) Morphology of SS RBCs incubated with 4% oxygen at 37 °C for 3 hours revealed sickling of RBCs. SS RBCs were mixed with 0.5 mM (D), 1.5 mM (E), and 2 mM (F) of TD-1 before incubation with 4% oxygen at 37 °C for 3 hours. (G) Treatment with TD-1 reduced the SS RBC sickling induced by hypoxia in a dose-dependent manner. Error bars represent standard deviation.
The concentration of TD-1 (2 mM) required to reduce sickling of hypoxic SS RBCs by nearly 90% was less than that reported for 5-HMF (5 mM).8 Moreover, it is conceivable that because of its covalent interactions with hemoglobin, TD-1 may have a more sustained effect on oxygen affinity than do other allosteric effectors that do not form covalent bonds with hemoglobin. As a relevant corollary, our findings indicate that the binding of TD-1 to hemoglobin in RBCs is not significantly blocked by plasma molecules including albumin when more than a 1:1 molar ratio of TD-1 to hemoglobin is added to blood. If the beneficial effects of TD-1 on SS RBCs in vitro can be extended to animal models and, eventually, demonstration in clinical trials, our observations suggest that TD-1 may be an important new treatment for SCD.
Conclusions
We identified 427 compounds that bind to human hemoglobin using small molecule microarrays. We developed a high-throughput assay to evaluate the ability of hemoglobin-binding molecules to alter hemoglobin’s oxygen affinity. We identified TD-1, a molecule that potently increases the oxygen affinity of both purified human hemoglobin and hemoglobin within RBCs. X-ray crystallography revealed that binding of TD-1 to hemoglobin induced stabilization of the R-state. Remarkably, TD-1 potently inhibited hypoxia-induced sickling of SS RBCs without causing hemolysis. Taken together, our findings suggest that TD-1 represents a novel lead molecule for the treatment of patients with sickle cell disease.
Methods
Materials
A compound library containing 38 700 compounds was obtained from the Broad Institute of MIT and Harvard compound library21 and used for the SMM screening and in the hemoglobin oxygen assay. 2,3-DPG, IHP, NEM, and 5-HMF were purchased from Sigma-Aldrich. 5-(2,3-dihydro-1,4-benzodioxin-2-yl)-4H-1,2,4-triazole-3-thiol (compound 1) was purchased from Chembridge Corporation. Di(5-(2,3-dihydro-1,4-benzodioxin-2-yl)-4H-1,2,4-triazol-3-yl)disulfide (TD-1) was synthesized by Shanghai ChemPartner. Formamidine disulfide was purchased from Alfa Aesar.
The uses of the RBCs and whole blood for the reported studies were reviewed and approved by the Institutional Review Board (IRB) of Partners Human Research Committee. The use of blood from SCD patients for antisickling assays was reviewed and approved by the IRB at the Children’s Hospital of Philadelphia. Preparation of hemoglobin (HbA) is described in Supporting Information.
Identification of Compounds That Bind to Hemoglobin Using SMMs
Detailed methods about the preparation of SMMs and identification of compounds that bind to hemoglobin are described in Supporting Information.
Identification of Small Molecules That Alter the Oxygen Affinity of Hemoglobin
The ability of small molecules to alter the oxygen affinity of hemoglobin was determined by measuring the fraction of oxygenated hemoglobin (oxyHb%) and oxidized hemoglobin (metHb%) using spectrophotometry under three (high, medium, and low oxyHb saturation) conditions. Hemoglobin was mixed with test compounds, and the mixture was added to 240 central wells of 384-well plates (50 μL/well). The concentrations of hemoglobin, compound, and dimethyl sulfoxide (DMSO) were 10 μM, 60 μM, and 5 vol % in Dulbecco’s phosphate buffered saline (DPBS), respectively. A detailed scheme of the sample preparation is reported in Supporting Information and presented in Figure S2A.
The assay plates, a plate reader (MultiSkan GO, Thermo Fisher Scientific), a plate shaker, a thermometer, a humidity monitor, and a calibrated oxygen pressure meter were placed in an inflatable polyethylene chamber (AtmosBag, Sigma) (Supporting Information Figure S2B). The chamber was purged with a precise mixture of nitrogen and air, which was then continuously provided to control the fraction of oxygen gas in the chamber (FO2). Gas cylinders of nitrogen gas and air were connected to a gas mixer to adjust the flow rate of nitrogen gas and air to the chamber. Humidity was maintained (70–85%) in the chamber via a humidifier connected to the gas inlet. The temperature in the chamber was 25–27 °C.
For the “high oxyHb saturation” condition, the assay plates were incubated without shaking at FO2 0.012 for 12 h. The visible absorption spectra of the samples were measured from 500 to 700 nm at 2 nm steps using the plate reader. After the measurement of the absorption spectra, the plates were shaken for 1 h at FO2 0.012, and the spectra were remeasured to obtain the “medium oxyHb saturation” condition. The FO2 was then reduced to 0.005, and the plates were shaken for 1 h, and the spectra were again measured to obtain the “low oxyHb saturation” condition. To determine oxyHb% of each sample, the measured absorption spectra were analyzed by least-squared fitting to determine oxyHb% and metHb% using Solver (Excel 2007, Microsoft).
During development of the hemoglobin-oxygen binding assay, we controlled the uneven distribution of oxygenation values in 240 central wells by an adjustment factor determined for each well. This adjustment factor was determined by measuring absorption spectra of hemoglobin samples without test compounds in the 240 central wells of four plates at high, medium, and low oxyHb saturation conditions. For example, if the mean value of oxyHb% of all the 960 wells is 50%, and the mean value of oxyHb% in a well at position 3C is 47%, the adjustment factor for the well at position 3C is 50% – 47% = +3%. We found that this adjustment factor eliminated some false positives from the selection of allosteric effectors. To evaluate the ability of compounds to alter hemoglobin oxygen affinity, the value of oxyHb% was defined as the sum of oxyHb% calculated by combining the spectral deconvolution and the adjustment factor.
We selected the compounds that alter oxyHb% without increasing metHb% based on the value of the Z score for oxyHb% and metHb%. The Z score for oxyHb% (Zoxy) was calculated for each compound according to eq 1.
| 1 |
In this equation, oxyHb%cpd is the oxyHb% of hemoglobin incubated with a unique compound, and oxyHb%ctrl is the oxyHb% of hemoglobin without any added compounds. The Z score for metHb (Zmet) was also calculated according to eq 2.
| 2 |
In this equation, metHb%cpd is the metHb% of hemoglobin incubated with a unique compound, and metHb%ctrl is the metHb% of hemoglobin without any added compound. We designated compounds as able to alter oxyHb% without increasing metHb formation (“hit” compounds), if they satisfied Condition 1 (both Zoxy > 2.5 reflecting an increase of oxyHb% and Zmet < 2.5) or Condition 2 (both Zoxy < −2.5 reflecting a decrease of oxyHb% and Zmet < 2.5) in at least one of the three oxygen saturation conditions.
Measurement of the Oxygen Dissociation Curve (ODC)
The ODCs of hemoglobin and whole blood were measured with a HEMOX analyzer (TCS Scientific Corporation). Detailed methods are described in Supporting Information. P50 was determined from three independent ODC measurements.
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software). To compare P50 values at different concentrations of compound 1 and TD-1, a one-way analysis of covariance (ANOVA) with Bonferroni’s post tests was applied. The one way ANOVA with Bonferroni’s post tests was also applied to compare P50 values of whole blood incubated in the absence or presence of TD-1, 5-HMF, NEM, and formamidine disulfide. The slope of (Δlog P50)/ΔpH was evaluated by analysis of covariance (ANCOVA). P < 0.05 was considered statistically significant.
Crystallization of Hemoglobin with TD-1 and Data Collection
To obtain the hemoglobin structure in complex with TD-1, a freshly prepared solution of TD-1 in DMSO was incubated with oxyHb for 1 h at 37 °C at a hemoglobin tetramer-compound molar ratio of 1:10. The mixture was saturated with carbon monoxide (CO) to generate carboxyhemoglobin (COHb). Crystallization was carried out with a solution of 20–30 mg mL–1 COHb, in a mixture of 3.0–3.4 M sodium monobasic phosphate and potassium phosphate dibasic, at pH values ranging from 6.4 to 7.6 using vacutainer tubes.29,41 One or two drops of toluene were added to the solution in each tube to facilitate crystallization. CO was bubbled into the tubes, and then sealed. X-ray-quality crystals grew in 2–3 days, with almost all the crystals as long rectangular needles, which are typical of quaternary R3-state crystals.29 In addition to the needle crystals, a few of the crystallization setups contained trigonal bipyrimidal (octahedral) crystals: the classical quaternary R-state crystals.29 Prior to study by X-ray diffraction, the crystals were washed in a cryoprotectant solution containing 50 μL mother liquor and 10–15 μL glycerol.
Diffraction data of all crystals were collected at 100 K with a Rigaku IV ++ image plate detector using a Cu Kα X-rays (λ = 1.54 Å) from a MicroMax-007 source fitted with Varimax Confocal optics (Rigaku). The data sets were processed with the d*trek software (Rigaku) and the CCP4 suite of programs.42
Determination of the Structure of COHb in Complex with TD-1
COHb in complex with TD-1 crystallized in the space group P4122 with approximate unit-cell constants of a = 62 Å, b = 62 Å, and c = 176 Å with one dimer (α1β1) per asymmetric unit, and expectedly isomorphous to the previously published high-salt R3 state COHb crystal.29
The isomorphous R3 state α1β1 dimeric COHb structure (PDB code 1YZI) was used as a starting coordinate to refine the structure of COHb in complex with TD-1 using software packages CNS and Phenix.43,44 Model building and correction were carried out using COOT.45 Initial difference map of the R3 structure identified four monomeric units (MUs) of the divalent TD-1 (Supporting Information Figure S3A and S3D) and three toluene molecules (used in the crystallization experiment), which were modeled in the structure and refined (Supporting Information Figure S3B and S3E). The final crystallographic R-factor and R-free were 22.8 and 25.9, respectively. The X-ray data is summarized in Supporting Information Table S4. Structural figures in Figure 3 and Supporting Information Figures S3 and S7 were prepared with PyMol46 and ChemBioDraw Ultra (PerkinElmer).
Evaluation of Antisickling Effects of TD-1
The antisickling effect of TD-1 was evaluated according to the previously described procedure.8,47 Detailed methods are described in Supporting Information.
The percentage of sickled cells was obtained from four independent experiments for each condition. The percentage of sickle cells with and without TD-1 (2 mM) was compared using two-tailed, unpaired Student’s t tests (GraphPad Prism 5 software). P < 0.05 was considered statistically significant.
Acknowledgments
The authors wish to acknowledge J. Winkler, PhD (University of Pennsylvania) for helpful discussions and advice, T. Asakura, MD PhD (The Children’s Hospital of Philadelphia) for providing resources to evaluate in vitro sickling of SS RBCs under hypoxic conditions, S. Johnston, PhD, and J. Athanasopoulos, PhD (The Broad Institute), for evaluating the binding of TD-1 to blood. This work was supported by grants from the National Institutes of Health/NIH Heart, Lung, and Blood Institute (NIH/NHLBI) (HL103186, O.A.) and from the Foundation LeDucq (K.D.B.). Structural biology resources were provided, in part, by NIH award CA16059 to the VCU Massey Cancer Center (M.K.S.).
Supporting Information Available
Detailed methods. This material is available free of charge via the Internet at http://pubs.acs.org.
Accession Codes
The atomic coordinates and structure factor files have been submitted to the Protein Data Bank under an accession code 4NI0 for COHb with TD-1 (as R3-state with monomeric units of TD-1).
The authors declare the following competing financial interest(s): Massachusetts General Hospital has filed a patent related to TD-1.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- http://www.nhlbi.nih.gov/health/health-topics/topics/sca/atrisk.html (accessed Dec 7, 2013).
- Eaton W. A.; Hofrichter J. (1990) Sickle cell hemoglobin polymerization. Adv. Protein Chem. 40, 63–279. [DOI] [PubMed] [Google Scholar]
- Bunn H. F. (1997) Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 337, 762–769. [DOI] [PubMed] [Google Scholar]
- Rees D. C.; Williams T. N.; Gladwin M. T. (2010) Sickle-cell disease. Lancet 376, 2018–2031. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R.; Hofrichter J.; Eaton W. A. (1978) Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature 275, 238–240. [DOI] [PubMed] [Google Scholar]
- Bennekou P.; de Franceschi L.; Pedersen O.; Lian L.; Asakura T.; Evans G.; Brugnara C.; Christophersen P. (2001) Treatment with NS3623, a novel Cl-conductance blocker, ameliorates erythrocyte dehydration in transgenic SAD mice: A possible new therapeutic approach for sickle cell disease. Blood 97, 1451–1457. [DOI] [PubMed] [Google Scholar]
- Stuart J.; Mojiminiyi F. B.; Stone P. C.; Culliford S. J.; Ellory J. C. (1994) Additive in vitro effects of anti-sickling drugs. Br. J. Hamaetol. 86, 820–823. [DOI] [PubMed] [Google Scholar]
- Safo M. K.; Kato G. J. (2014) Therapeutic strategies to alter the oxygen affinity of sickle hemoglobin. Hematol. Oncol. Clin. North Am. 28, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulmalik O.; Safo M. K.; Chen Q.; Yang J.; Brugnara C.; Ohene-Frempong K.; Abraham D. J.; Asakura T. (2005) 5-Hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br. J. Hamaetol. 128, 552–561. [DOI] [PubMed] [Google Scholar]
- Safo M. K.; Abdulmalik O.; Danso-Danquah R.; Burnett J. C.; Nokuri S.; Joshi G. S.; Musayev F. N.; Asakura T.; Abraham D. J. (2004) Structural basis for the potent antisickling effect of a novel class of five-membered heterocyclic aldehydic compounds. J. Med. Chem. 47, 4665–4676. [DOI] [PubMed] [Google Scholar]
- Safo M. K., and Bruno S. (2011) Allosteric Effectors of Hemoglobin: Past, Present, and Future, pp 285–300, John Wiley & Sons, Ltd., New York. [Google Scholar]
- Park S.; Hayes B. L.; Marankan F.; Mulhearn D. C.; Wanna L.; Mesecar A. D.; Santarsiero B. D.; Johnson M. E.; Venton D. L. (2003) Regioselective covalent modification of hemoglobin in search of antisickling agents. J. Med. Chem. 46, 936–953. [DOI] [PubMed] [Google Scholar]
- Klotz I. M.; Tam J. W. O. (1973) Acetylation of Sickle Cell Hemoglobin by Aspirin. Proc. Natl. Acad. Sci. U.S.A. 70, 1313–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder J. A.; Walder R. Y.; Arnone A. (1980) Development of antisickling compounds that chemically modify hemoglobin S specifically within the 2,3-diphosphoglycerate binding site. J. Mol. Biol. 141, 195–216. [DOI] [PubMed] [Google Scholar]
- Ueno H.; Yatco E.; Benjamin L. J.; Manning J. M. (1992) Effects of methyl acetyl phosphate, a covalent antisickling agent, on the density profiles of sickle erythrocytes. J. Clin. Lab. Med. 120, 152–158. [PubMed] [Google Scholar]
- Garel M.; Beuzard Y.; Thillet J.; Domenget C.; Martin J.; Galacteros F.; Rosa J. (1982) Binding of 21 thiol reagents to human-hemoglobin in solution and intact-cells. Eur. J. Biochem. 123, 513–519. [DOI] [PubMed] [Google Scholar]
- Benesch R.; Benesch R. (1961) Chemistry of Bohr effect 1. Reaction of N-ethyl maleimide with oxygen-linked acid groups of hemoglobin. J. Biol. Chem. 236, 405–410. [Google Scholar]
- Safo M. K.; Ahmed M. H.; Ghatge M. S.; Boyiri T. (2011) Hemoglobin-ligand binding: Understanding Hb function and allostery on atomic level. Biochim. Biophys. Acta 1814, 797–809. [DOI] [PubMed] [Google Scholar]
- Eaton W. A.; Henry E. R.; Hofrichter J.; Bettati S.; Viappiani C.; Mozzarelli A. (2007) Evolution of allosteric models for hemoglobin. IUBMB Life 59, 586–599. [DOI] [PubMed] [Google Scholar]
- Yonetani T.; Park S. I.; Tsuneshige A.; Imai K.; Kanaori K. (2002) Global allostery model of hemoglobin. Modulation of O2 affinity, cooperativity, and Bohr effect by heterotropic allosteric effectors. J. Biol. Chem. 277, 34508–34520. [DOI] [PubMed] [Google Scholar]
- Yonetani T.; Kanaori K. (2013) How does hemoglobin generate such diverse functionality of physiological relevance?. Biochim. Biophys. Acta 1834, 1873–1884. [DOI] [PubMed] [Google Scholar]
- Seiler K. P.; George G. A.; Happ M. P.; Bodycombe N. E.; Carrinski H. A.; Norton S.; Brudz S.; Sullivan J. P.; Muhlich J.; Serrano M.; Ferraiolo P.; Tolliday N. J.; Schreiber S. L.; Clemons P. A. (2008) ChemBank: A small-molecule screening and cheminformatics resource database. Nucleic Acids Res. 36, D351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. M.; Weiwer M.; Koehler A. N. (2012) Unbiased binding assays for discovering small-molecule probes and drugs. Bioorg. Med. Chem. 20, 1979–1989. [DOI] [PubMed] [Google Scholar]
- Casalena D. E.; Wassaf D.; Koehler A. N. (2012) Ligand discovery using small-molecule microarrays. Methods Mol. Biol. 803, 249–263. [DOI] [PubMed] [Google Scholar]
- Benesch R.; Benesch R. (1967) Effect of organic phosphates from human erythrocyte on allosteric properties of hemoglobin. Biochem. Biophys. Res. Commun. 26, 162–167. [DOI] [PubMed] [Google Scholar]
- Ronda L.; Bruno S.; Faggiano S.; Bettati S.; Mozzarelli A. (2008) Oxygen binding to heme proteins in solution, encapsulated in silica gels, and in the crystalline state. Methods Enzymol. 437, 311–328. [DOI] [PubMed] [Google Scholar]
- Rivetti C.; Mozzarelli A.; Rossi G. L.; Henry E. R.; Eaton W. A. (1993) Oxygen binding by single crystals of hemoglobin. Biochemistry 32, 2888–2906. [DOI] [PubMed] [Google Scholar]
- Benesch R.; Benesch R. E.; Yu C. I. (1968) Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 59, 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. (1960) The nature and significance of the Bohr effect in mammalian hemoglobins. J. Gen. Physiol. 43, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo M. K.; Abraham D. J. (2005) The enigma of the liganded hemoglobin end state: A novel quaternary structure of human carbonmonoxy hemoglobin. Biochemistry 44, 8347–8359. [DOI] [PubMed] [Google Scholar]
- Gosselet M.; Mahieu J.-P.; Sebille B. (1988) Reactivity of aromatic and heterocyclic disulphides with thiol group of bovine serum albumin. Int. J.Biol. Macromol. 10, 241–247. [Google Scholar]
- Mahieu J. P.; Gosselet N. M.; Sebille B.; Garel M. C.; Beuzard Y. (1993) Reactivity of 42 disulfides with thiol group of human haemoglobin and human serum albumin. Int. J. Biol. Macromol. 15, 233–240. [DOI] [PubMed] [Google Scholar]
- Knee K. M.; Roden C. K.; Flory M. R.; Mukerji I. (2007) The role of β93 Cys in the inhibition of Hb S fiber formation. Biophys. Chem. 127, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S.; Jia L.; Eu J. P.; McMahon T. J.; Demchenko I. T.; Bonaventura J.; Gernert K.; Piantadosi C. A. (1997) Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276, 2034–2037. [DOI] [PubMed] [Google Scholar]
- Chiancone E.; Currell D. L.; Vecchini P.; Antonini E.; Wyman J. (1970) Kinetics of the reaction of the “masked” and “free” sulfhydryl groups of human hemoglobin with p-mercuribenzoate. J. Biol. Chem. 245, 4105–4111. [PubMed] [Google Scholar]
- Kan H. I.; Chen I. Y.; Zulfajri M.; Wang C. C. (2013) Subunit disassembly pathway of human hemoglobin revealing the site-specific role of its cysteine residues. J. Phys. Chem. B 117, 9831–9839. [DOI] [PubMed] [Google Scholar]
- Ferranti P.; Sannolo N.; Mamone G.; Fiume I.; Carbone V.; Tornqvist M.; Bergman A.; Malorni A. (1996) Structural characterization by mass spectrometry of hemoglobin adducts formed after in vivo exposure to methyl bromide. Carcinogenesis 17, 2661–2671. [DOI] [PubMed] [Google Scholar]
- Mozzarelli A.; Rivetti C.; Rossi G. L.; Henry E. R.; Eaton W. A. (1991) Crystals of haemoglobin with the T quaternary structure bind oxygen noncooperatively with no Bohr effect. Nature 351, 416–419. [DOI] [PubMed] [Google Scholar]
- Perutz M. F.; Wilkinson A. J.; Paoli M.; Dodson G. G. (1998) The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 27, 1–34. [DOI] [PubMed] [Google Scholar]
- Abdulmalik O.; Ghatge M. S.; Musayev F. N.; Parikh A.; Chen Q.; Yang J.; Nnamani I.; Danso-Danquah R.; Eseonu D. N.; Asakura T.; Abraham D. J.; Venitz J.; Safo M. K. (2011) Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin. Acta Crystallogr., Sect. D: Biol. Crystallogr. 67, 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone A. (1972) X-ray diffraction study of binding 2,3-diphosphoglycerate to human deoxyhemoglobin. Nature 237, 146–149. [DOI] [PubMed] [Google Scholar]
- Safo M. K.; Abraham D. J. (2003) X-ray crystallography of hemoglobins. Methods Mol. Med. 82, 1–19. [DOI] [PubMed] [Google Scholar]
- Winn M. D.; Ballard C. C.; Cowtan K. D.; Dodson E. J.; Emsley P.; Evans P. R.; Keegan R. M.; Krissinel E. B.; Leslie A. G.; McCoy A.; McNicholas S. J.; Murshudov G. N.; Pannu N. S.; Potterton E. A.; Powell H. R.; Read R. J.; Vagin A.; Wilson K. S. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr., Sect. D: Biol. Crystallogr. 67, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger A. T.; Adams P. D.; Clore G. M.; DeLano W. L.; Gros P.; Grosse-Kunstleve R. W.; Jiang J. S.; Kuszewski J.; Nilges M.; Pannu N. S.; Read R. J.; Rice L. M.; Simonson T.; Warren G. L. (1998) Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr., Sect. D: Biol. Crystallogr. 54, 905–921. [DOI] [PubMed] [Google Scholar]
- Adams P. D.; Grosse-Kunstleve R. W.; Hung L. W.; Ioerger T. R.; McCoy A. J.; Moriarty N. W.; Read R. J.; Sacchettini J. C.; Sauter N. K.; Terwilliger T. C. (2002) PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr., Sect. D: Biol. Crystallogr. 58, 1948–1954. [DOI] [PubMed] [Google Scholar]
- Emsley P.; Lohkamp B.; Scott W. G.; Cowtan K. (2010) Features and development of Coot. Acta Crystallogr., Sect. D: Biol. Crystallogr. 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific, San Carlos, CA. [Google Scholar]
- Hijiya N.; Horiuchi K.; Asakura T. (1991) Morphology of sickle cells produced in solutions of varying osmolarities. J. Clin. Lab. Med. 117, 60–66. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.