Abstract
Background
Inconsistent results were reported in recent literature regarding the association between methylenetetrahydrofolate reductase (MTHFR) C677T/A1298C polymorphisms and the susceptibility of congenital heart disease (CHD). In this study, we performed a meta-analysis to investigate the associations by employing multiple analytical methods.
Methods
Literature search was performed and published articles were obtained from PubMed, Embase and CNKI databases based on the exclusion and inclusion criteria. Data were extracted from eligible studies and the crude odds ratios and their corresponding 95% confidence intervals (CIs) were calculated using random or fix effects model to evaluate the associations between the MTHFR C677T/A1298C polymorphisms and CHD development. Subgroup based analysis was performed by Hardy–Weinberg equilibrium, ethnicity, types of CHD, source of control and sample size.
Results
Twenty-four eligible studies were included in this meta-analysis. Significant association was found between fetal MTHFR C677T polymorphism and CHD development in all genetic models. The pooled ORs and 95% CIs in all genetic models indicated that MTHFR C677T polymorphism was significantly associated with CHD in Asian, but not Caucasian in subgroup analysis. The maternal MTHFR C677T polymorphism was not associated with CHD except for recessive model. Moreover, neither maternal nor fetal MTHFR A1298C polymorphism was associated with CHD.
Conclusion
The fetal MTHFR C677T polymorphism may increase the susceptibility to CHD. Fetal MTHFR C677T polymorphism was more likely to affect Asian fetus than Caucasian. The MTHFR A1298C polymorphism may not be a risk of congenital heart disease.
Keywords: Congenital heart disease, MTHFR, Polymorphism, Association, Meta-analysis, Folic acid
1. Introduction
Congenital heart disease (CHD) is one of most common congenital anomalies. CHD is a major cause of fetal loss and death in newborns less than one year of age all over the world. Approximately, CHD accounts for 28% of the major congenital anomalies (van der Linde et al., 2011). The generally accepted prevalence of CHD was about 8 per 1000 live births, which poses a serious challenge to healthcare (Bernier et al., 2010). Remarkable progresses have been achieved in CHD diagnosis and cardiac surgery during the past decades, resulting in an increased survival rate of neonates with CHD (Greutmann and Tobler, 2012). However, more patients with CHD have grown up who comprised of a special population: patients with grown-up congenital heart disease (GUCH) (Khairy et al., 2010; van der Linde et al., 2011). It was reported that the prevalence of patients with GUCH was estimated to be 4 per 1000 adults. Long-term medical care and related resource cost are needed for patients with GUCH, and rapidly increase healthcare burden.
Since more GUCH patients survive, more are now in childbearing age. Thus, it is very important to characterize the etiology of congenital heart disease, which has not been well understood yet. Several classic studies including the Baltimore–Washington Infant Study have indicated that the cause of CHD was multifactorial, and both genetic background and environmental factors may play important roles in the development of CHD (Richards and Garg, 2010; Shieh et al., 2012). Importantly, due to the advances in molecular techniques, accumulating evidences have suggested that genetic factors were dominant (Bruneau, 2008). It was known that a large proportion of CHDs were characterized with aneuploidy or abnormal chromosomal number (Blue et al., 2012; Pierpont et al., 2007). About 50% of children who were born with Trisomy 21 have atrial and ventricular septal defects or atrioventricular canal lesion. With completion of the Human Genome Project, associations between single gene mutations and CHD have also been extensively studied. It has been reported that the mutations in single genes including TBX5, JAG1, NKX2.5 and GATA4 have been associated with the development of CHD (Basson et al., 1997; Oda et al., 1997; Schott et al., 1998; Zhang et al., 2008).
The association between folic acid metabolism and the development of CHD has been explored recently. Maternal supplement of folic acid has been proved to reduce the incidence of CHD as well as other congenital heart disease (van Beynum et al., 2010). Single nucleotide polymorphisms of many genes involved in the folate pathway have been identified to affect the function of the genes or folic acid metabolism and thus increase the risk of CHD (Locke et al., 2010; Shaw et al., 2009). The flavin adenine dinucleotide-dependent enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR) catalyzes the reduction of methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is required for the remethylation of homocysteine to methionine (Ueland et al., 2001). Hyperhomocysteinemia was believed to be a high risk for the development of heart defects (Verkleij-Hagoort et al., 2006, 2007). Elevating the level of 5-methyltetrahydrofolate, a major circulating folic acid, prevented CHD by reducing maternal homocysteine plasma level (Lamers et al., 2004). Therefore, the polymorphisms of MTHFR may be closely related to the risk of CHD. It was reported that two MTHFR SNPs including MTHFR C677T (p.Ala222Val, ID: rs1801133) and MTHFR A1298C (p.Glu429Ala, rs1801131) were potentially associated with CHD (van Driel et al., 2008). The amino acid transition in MTHFR C677T (Ala-Val) has resulted in a thermolabile protein associated with reduced enzyme activity in vivo, which may increase plasma homocysteine level (Huhta and Hernandez-Robles, 2005). The MTHFR A1298 C has also been reported to moderately reduce MTHFR activity in vivo (Weisberg et al., 1998).
To date, a large number of studies regarding the associations between MTHFR gene polymorphisms and risk of CHD have been published. However, the results of these studies were confounding and inconsistent. Herein, we performed a meta-analysis of all published studies until January 2013 to investigate the association between the two SNPs (MTHFR 677CT and MTHFR 1298AC) and CHD patients and their mothers.
2. Materials and methods
2.1. Literature and search strategy
The PubMed, Embase, Web of knowledge and CNKI (China National Knowledge Infrastructure) database searches were performed to identify all the eligible papers. The search terms were used as the following: (MTHFR or methylenetetrahydrofolate reductase or folic acid) and (variant or polymorphism or SNP) and (congenital heart disease or heart defect or CHD or congenital anomalies). The publication languages were restricted to English and Chinese. Moreover, potentially relevant studies were evaluated by reviewing the titles and abstracts, and studies matching the criteria were carefully retrieved. If more than one study was published using the same data, only the study with a larger population was included. The literature search was updated on January, 31, 2013.
2.2. Inclusion criteria and data extraction
The eligible studies should meet the following inclusion criteria: (1) Investigation of association between the MTHFR polymorphisms (including C677T or A1298C or both) and congenital heart disease; (2) a case–control study; (3) providing sufficient data on genotype frequencies of the MTHFR C677T and/or A1298C polymorphisms and sufficient data for calculation of an odd ratio (OR) with 95% confidence interval (CI). The exclusion criteria were as follows: (1) reviews, case report, editorial or comment; (2) a duplicated study; (3) studies providing insufficient data or data in poor quality; and (4) studies without control. Based on the inclusion and exclusion criteria, data extraction from each study was performed by two authors (Wang, Hou) independently to ensure that the data extraction were accurate. The following information was extracted from each study: (1) name of the first author; (2) year of publication; (3) country of origin; (4) ethnicity of the study population; (5) source of controls (population based or hospital based); (6) sample size of case and controls; (7) types of congenital heart disease; (8) genotype distributions in cases and controls; and (9) whether population involved in the study was in Hardy–Weinberg equilibrium (HWE).
2.3. Statistical analysis
Meta-analysis was performed to evaluate the association between MTHFR polymorphisms and risk of developing CHD. Firstly, crude ORs with 95% CIs were calculated to assess the strength of the correlation between the MTHFR C677T/A1298C polymorphisms (including maternal and fetal) and risk of CHD. Pooled ORs and 95% CIs were calculated for the multiplicative, co-dominant, dominant, and recessive genetic models respectively. The significances of pooled ORs were analyzed by Z tests, and the criteria for statistically significant were p < 0.05. A Q test was conducted to determine the possible heterogeneity, and p < 0.10 or I > 50% indicated an obvious heterogeneity. Pooled ORs (95% CI) were calculated by random effects model (DerSimonian–Laird method) or fix effects model (Mantel–Haenszel method). Subgroup analysis was performed by ethnicity, types of CHD, source of controls and sample size (n < 100 vs. n > 100). Sensitivity analysis were performed to evaluate the stability of the results by removing one case–control study each time to assess the influence of the individual data on pooled ORs. Begg's funnel plot was generated to indicate the possible publication bias. Moreover, the Egger quantitative tests were also performed, and p < 0.05 was considered statistically significant. To obtain reliable data, two authors (Wang, Hou) have performed the statistical analysis independently by using the same data and the programs. Data analyses were performed using STATA version 12 (Stata Corporation, College Station, Texas, USA).
3. Results
3.1. Characteristics of the studies included
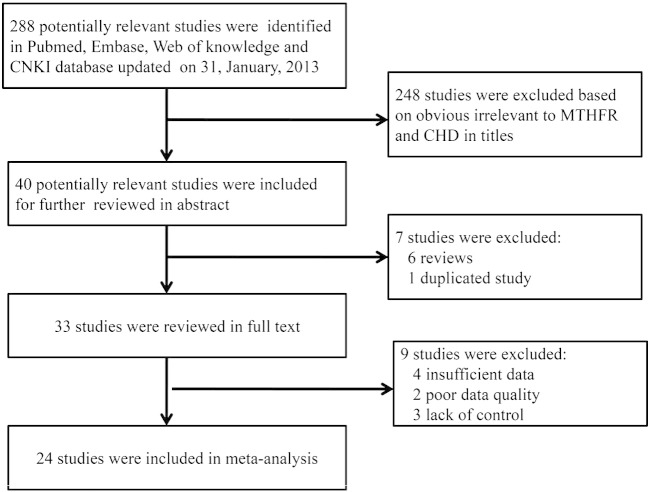
Totally, we have identified 288 potentially relevant studies by employing the search strategy described above. Based on obvious irrelevance to MTHFR and CHD in titles, 248 papers from the 288 potentially relevant papers were excluded. After reading the abstracts of the remaining 40 studies, 7 studies were further excluded, as 6 studies were reviews and one study was a duplicated study. To further polish target studies, the remaining studies were reviewed in full text. Of these, 9 studies were excluded, due to insufficient data, data with poor quality or papers without control. After careful screening, 24 eligible studies were finally included in this meta-analysis (Balderrabano-Saucedo et al., 2013; Bozovic et al., 2011; Galdieri et al., 2007; Garcia-Fragoso et al., 2010; Gong et al., 2009, 2012; Hobbs et al., 2010; Junker et al., 2001; Lee et al., 2005; Li et al., 2005, 2009a, 2009b; Liu et al., 2005; Peng et al., 2009; Sanchez-Urbina et al., 2012; Shaw et al., 2005; Storti et al., 2003; van Beynum et al., 2006; van Driel et al., 2008; Wang, 2006; Wintner et al., 2007; Xu et al., 2010; Yan and Li, 2003; Zhu et al., 2006). The search strategy and inclusion/exclusion of studies were shown in a flow chart (Fig. 1). Among these studies, fourteen studies investigated the maternal MTHFR C677T polymorphism with 1745 cases and 2044 controls and nineteen studies investigated the fetal MTHFR C677T polymorphism with 2697 cases and 3434 controls. In addition, there were 4 studies investigating maternal MTHFR A1298C polymorphism with 432 cases and 532 controls and 5 studies investigating fetal MTHFR A1298C polymorphism with 945 cases and 1074 controls. Concerning Hardy–Weinberg equilibrium, 3 studies were not conformed to HWE. In these papers, 9 studies included both maternal and fetal MTHFR polymorphisms and 5 studies included both MTHFR C677T and A1298C polymorphisms. Moreover, 11 studies were performed in Caucasian and 12 studies were performed in Asian. The general characteristics of the studies included were listed in Table 1. The genotype and allele distributions of maternal C677T and A1298C polymorphisms in all the studies included were shown in Table 2. For the fetal polymorphisms, the genotype and allele frequencies of C677T and A1298C were shown in Table 3.
Fig. 1.
Flow diagram of the study selection process.
Table 1.
Characteristics of the studies included on associations between MTHFR C677T/A1298C polymorphisms and congenital heart disease.
| First author | Year | Country | Ethnicity | Source of controls | Genotyping method | Types of CHD | Maternal or fetal | SNP sites |
HWE | |
|---|---|---|---|---|---|---|---|---|---|---|
| MTHFR 677CT | MTHFR 1298AC | |||||||||
| Balderrabano-Saucedo (Balderrabano-Saucedo et al., 2013) | 2013 | Mexico | Caucasian | HB | RFLP | All types | Maternal | Yes | Yes | |
| Božovic (Bozovic et al., 2011) | 2011 | Croatia | Caucasian | PB | RFLP | All types | Both | Yes | Yes | Yes |
| Hobbs (Hobbs et al., 2010) | 2010 | United States | Caucasian | PB | TaqMan | All types | Maternal | Yes | Yes | |
| García-Fragoso (Garcia-Fragoso et al., 2010) | 2010 | Puerto Rico | Caucasian | HB | RFLP | All types | Both | Yes | No | |
| Xu (Xu et al., 2010) | 2010 | Chinese | Asian | HB | RFLP | All types | Fetal | Yes | Yes | Yes |
| Li (Li et al., 2009b) | 2009 | Chinese | Asian | HB | RFLP | All types | Fetal | Yes | Yes | |
| van Driel (van Driel et al., 2008) | 2008 | Netherlands | Caucasian | PB | RFLP | All types | Both | Yes | Yes | Yes |
| Wintner (Wintner et al., 2007) | 2007 | Austria | Caucasian | HB | Microarray | All types | Maternal | Yes | Yes | |
| van Beynum (van Beynum et al., 2006) | 2006 | Netherlands | Caucasian | PB | RFLP | All types | Maternal | Yes | Yes | |
| Galdieri (Galdieri et al., 2007) | 2007 | Brazil | Caucasian | HB | RFLP | All types | Both | Yes | Yes | Yes |
| Zhu (Zhu et al., 2006) | 2006 | Chinese | Asian | NA | RFLP | ASD/PDA | Both | Yes | Yes | |
| Lee (Lee et al., 2005) | 2005 | Chinese | Asian | HB | DHPLC | All types | Fetal | Yes | Yes | |
| Shaw (Shaw et al., 2005) | 2005 | United States | Caucasian | PB | Hybridization | All types | Fetal | Yes | Yes | |
| Storti (Storti et al., 2003) | 2003 | Italy | Caucasian | HB | RFLP | CD | Both | Yes | Yes | Yes |
| Junker (Junker et al., 2001) | 2001 | Germany | Caucasian | NA | NA | All types | Fetal | Yes | Yes | |
| Sanchez-Urbina (Sanchez-Urbina et al., 2012) | 2012 | Mexico | Caucasian | PB | RFLP | All types | Both | Yes | No | |
| Yan (Yan and Li, 2003) | 2003 | Chinese | Asian | HB | RFLP | All types | Fetal | Yes | Yes | |
| Gong (Gong et al., 2012) | 2012 | Chinese | Asian | HB | MassArray | CD | Fetal | Yes | Yes | |
| Wang (Wang, 2006) | 2004 | Chinese | Asian | HB | RFLP | All types | Both | Yes | No | |
| Peng (Peng et al., 2009) | 2009 | Chinese | Asian | HB | DHPLC | All types | Maternal | Yes | Yes | |
| Li (Li et al., 2009a) | 2009 | Chinese | Asian | HB | RFLP | All types | Fetal | Yes | Yes | |
| Gong (Gong et al., 2009) | 2009 | Chinese | Asian | HB | RFLP | All types | Fetal | Yes | Yes | |
| Liu (Liu et al., 2005) | 2005 | Chinese | Asian | HB | RFLP | CD | Fetal | Yes | Yes | |
| Li (Li et al., 2005) | 2005 | Chinese | Asian | PB | RFLP | All types | Both | Yes | Yes | |
Abbreviations: HWE, Hardy–Weinberg equilibrium; NA, not available; RFLP, restriction fragment length polymorphism; DHPLC, denaturing high performance liquid chromatography; CD, conotruncal heart defects; ASD, atrial septal defect; PDA, patent ductus arteriosus; PB, population-based; HB, hospital-based.
Table 2.
Genotype and allele distributions of maternal MTHFR C677T/A1298C polymorphisms in case-control studies included.
| Study | Sample size case/control | Genotype distribution |
Allele distribution |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
A (case/control) | B (case/control) | ||||||||
| AA | AB | BB | AB + BB | AA | AB | BB | AB + BB | ||||
| MTHFR 677CT polymorphism | CC | CT | TT | CT + TT | CT | TT | CT + TT | C | T | CC | |
| Balderrabano-Saucedo | 31/62 | 7 (22.6%) | 12 (38.7%) | 12 (38.7%) | 24 (77.4%) | 24 (38.7%) | 31 (50%) | 7 (11.3%) | 38 (61.3%) | 26/79 | 36/45 |
| Božovic | 52/55 | 26 (50%) | 20 (38%) | 6 (12%) | 26 (50%) | 19 (35%) | 28 (51%) | 8 (14%) | 36 (65%) | 72/66 | 32/44 |
| Hobbs | 572/363 | 285 (51.5%) | 203 (36.7%) | 65 (11.8%) | 268 (48.5%) | 191 (53.7%) | 128 (36%) | 37 (10.4%) | 165 (46.4%) | 773/510 | 333/202 |
| García-Fragoso | 27/220 | 10 (37%) | 11 (41%) | 6 (22%) | 17 (63%) | 84 (38%) | 115 (52%) | 21 (10%) | 136 (62%) | 31/283 | 23/157 |
| van Driel | 230/251 | 91 (40%) | 117 (51%) | 22 (9%) | 139 (60%) | 111 (44%) | 104 (42%) | 36 (14%) | 140 (56%) | 299/326 | 161/176 |
| Wintner | 31/31 | 17 (54.84%) | 11 (35.48%) | 3 (9.68%) | 14 (45.16%) | 10 (32.26%) | 17 (54.84%) | 4 (12.9%) | 21 (67.74%) | 45/37 | 17/25 |
| Van Beynum | 158/261 | 72 (45.6%) | 68 (43%) | 18 (11.4%) | 86 (54.4%) | 131 (50.2%) | 107 (41%) | 23 (8.8%) | 130 (49.8%) | 212/369 | 104/153 |
| Galdieri | 47/26 | 27 (57.45%) | 15 (31.91%) | 5 (10.64%) | 20 (42.55%) | 10 (38.46%) | 15 (57.70%) | 1(3.84%) | 16 (61.54) | 69/17 | 25/17 |
| Zhu | 56/102 | 6 (10.71%) | 27 (48.21%) | 23 (41.08%) | 50 (89.29%) | 20 (19.61) | 57 (55.88%) | 25 (24.51%) | 82 (80.39%) | 39/97 | 73/107 |
| Storti | 103/200 | 27 (26%) | 53 (52%) | 23 (22%) | 76 (74%) | 52 (26%) | 108 (54%) | 40 (20%) | 148 (74%) | 107/212 | 99/188 |
| Sanchez-Urbina | 60/62 | 8 (13.3%) | 38 (63.3%) | 14 (23.3%) | 52 (86.6%) | 13 (21%) | 37 (59.7%) | 12 (19.3%) | 49 (79%) | 54/63 | 66/61 |
| Wang | 104/208 | 25 (24.04%) | 60 (57.69%) | 19 (18.27%) | 79 (76.39%) | 49 (23.56%) | 120 (57.69%) | 39 (18.75%) | 159 (76.44%) | 110/218 | 98/198 |
| Li | 183/102 | 32 (17.49%) | 90 (49.18%) | 61 (33.33%) | 151 (82.51%) | 20 (19.61%) | 57 (55.88%) | 25 (24.51%) | 82 (80.39%) | 154/97 | 212/107 |
| Peng | 91/101 | 32 (35.2%) | 48 (52.7%) | 11 (12.1%) | 59 (64.8%) | 46 (45.5%) | 44 (43.6%) | 11 (10.9%) | 55 (54.5%) | 112/136 | 70/66 |
| MTHFR 1298AC polymorphism | AA | AC | CC | AC + CC | AA | AC | CC | AC + CC | A | C | |
| Božovic | 52/55 | 21 (40%) | 27 (52%) | 4 (8%) | 31 (60%) | 27 (49%) | 27 (49%) | 1 (2%) | 28 (51%) | 69/81 | 35/29 |
| van Driel | 230/251 | 104 (45%) | 102 (45%) | 24 (10%) | 126 (55%) | 116 (46%) | 104 (42%) | 31 (12%) | 135 (54%) | 310/336 | 150/166 |
| Galdieri | 47/26 | 26 (55.32%) | 17 (36.17%) | 4 (8.51%) | 21 (44.68%) | 15 (57.7%) | 10 (38.46%) | 1 (3.84%) | 11 (42.3%) | 69/25 | 25/12 |
| Storti | 103/200 | 49 (48%) | 46 (45%) | 8 (7%) | 54 (52%) | 101 (50%) | 86 (43%) | 13 (7%) | 99 (50%) | 144/288 | 62/112 |
Table 3.
Genotype and allele distributions of fetal MTHFR C677T/A1298C polymorphisms in case–control studies included.
| Study | Sample size case/control | Genotype distribution |
Allele distribution |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
A (case/control) | B (case/control) | ||||||||
| AA | AB | BB | AB + BB | AA | AB | BB | AB + BB | ||||
| MTHFR 677CT polymorphism | CC | CT | TT | CT + TT | CC | CT | TT | CT + TT | C | T | |
| Božovic | 54/58 | 20 (37%) | 28 (52%) | 6 (11%) | 34 (63%) | 25 (43%) | 26 (45%) | 7 (12%) | 33 (57%) | 68/76 | 40/40 |
| García-Fragoso | 27/220 | 9 (33%) | 14 (52%) | 4 (15%) | 28 (67%) | 84 (38%) | 115 (52%) | 21 (10%) | 136 (62%) | 32/283 | 22/157 |
| Xu | 502/527 | 162 (32.2%) | 244 (48.6%) | 96 (19.1%) | 340 (67.7%) | 151 (28.7%) | 261 (49.5%) | 115 (21.8%) | 376 (71.3%) | 568/563 | 436/491 |
| Li | 104/208 | 16 (15.38%) | 42 (40.38%) | 46 (44.24%) | 88 (84.62%) | 55 (26.44%) | 114 (54.81%) | 39 (18.75%) | 153 (73.56%) | 74/224 | 134/192 |
| van Driel | 229/251 | 99 (43%) | 103 (45%) | 27 (12%) | 130 (57%) | 119 (47%) | 107 (43%) | 25 (10%) | 132 (53%) | 301/345 | 157/157 |
| Galdieri | 58/38 | 30 (51.72%) | 21 (36.21%) | 7 (12.07%) | 28 (48.28%) | 18 (47.37%) | 14 (36.84%) | 6 (15.79%) | 20 (52.63%) | 81/50 | 35/26 |
| Zhu | 56/103 | 7 (12.5%) | 22 (39.28%) | 27 (48.21%) | 49 (87.49%) | 22 (21.4%) | 57 (55.3%) | 24 (23.3%) | 81 (78.6%) | 36/101 | 76/105 |
| Lee | 213/195 | 110 (51.64%) | 89 (41.78%) | 14 (6.57%) | 103 (48.35%) | 114 (58.46%) | 68 (34.87%) | 13 (6.67%) | 81 (41.54%) | 309/296 | 117/94 |
| Shaw | 151/428 | 67 (44.37%) | 68 (45.03%) | 16 (10.6%) | 84 (55.63%) | 177 (41.36%) | 199 (46.5%) | 52 (12.14%) | 251 (58.64%) | 202/553 | 100303 |
| Storti | 103/200 | 28 (27%) | 55 (53%) | 20 (20%) | 75 (73%) | 52 (26%) | 108 (54%) | 40 (20%) | 148 (74%) | 111/212 | 95/188 |
| Junker | 114/228 | 51 (44.7%) | 42 (36.8%) | 21 (18.4%) | 63 (55.2%) | 129 (56.6%) | 78 (34.2%) | 21 (9.2%) | 99 (43.4%) | 144/336 | 84/120 |
| Sanchez-Urbina | 60/62 | 7 (11.7%) | 41 (68.3%) | 12 (20%) | 53 (88.3%) | 9 (14.5%) | 46 (74.2%) | 7 (11.3%) | 53 (85.5%) | 55/64 | 65/60 |
| Yan | 174/103 | 28 (16.1%) | 89 (51.14%) | 57 (32.76%) | 146 (83.9%) | 22 (21.36%) | 57 (55.34%) | 24 (23.3%) | 81 (78.64%) | 145/101 | 203/105 |
| Gong | 244/136 | 45 (18.4%) | 123 (50.4%) | 76 (31.1%) | 199 (81.5%) | 43 (31.6%) | 72 (52.9%) | 21 (15.4%) | 93 (68.3%) | 213/158 | 275/114 |
| Wang | 104/208 | 16 (15.38%) | 42 (40.38%) | 39 (18.75%) | 81 (59.13%) | 55 (26.44%) | 114 (54.81%) | 39 (18.75%) | 153 (73.56%) | 74/224 | 120/192 |
| Li | 144/168 | 26 (18.06%) | 52 (36.11%) | 66 (45.83%) | 118 (81.94%) | 49 (29.17%) | 84 (50%) | 35 (20.83%) | 119 (70.83%) | 104/182 | 184/154 |
| Gong | 80/80 | 10 (12.5%) | 41 (51.3%) | 29 (36.3%) | 70 (87.6%) | 17 (21.3%) | 40 (50%) | 23 (28.8%) | 63 (78.8%) | 61/74 | 99/86 |
| Liu | 97/118 | 19 (19.6%) | 54 (55.7%) | 24 (24.7%) | 78 (80.4%) | 33 (27.9%) | 69 (58.5%) | 16 (13.6%) | 85 (72.1%) | 92/135 | 102/101 |
| Li | 183/103 | 30 (16.4%) | 95 (51.91%) | 58 (31.69%) | 153 (83.6%) | 22 (21.36%) | 57 (55.34%) | 24 (23.3%) | 81 (78.64%) | 155/101 | 211/105 |
| MTHFR 1298AC polymorphism | AA | AC | CC | AC + CC | AA | AC | CC | AC + CC | A | C | |
| Božovic | 54/58 | 30 (55%) | 22 (41%) | 2 (4%) | 24 (45%) | 25 (43%) | 30 (52%) | 3 (5%) | 33 (57%) | 82/80 | 26/36 |
| Xu | 502/527 | 316 (62.9%) | 168 (33.5%) | 18 (3.6%) | 186 (37.1%) | 326 (61.9%) | 185 (35.1%) | 16 (3%) | 201 (38.1%) | 800/837 | 204/217 |
| van Driel | 229/251 | 112 (49%) | 90 (39%) | 27 (12%) | 117 (51%) | 97 (39%) | 129 (51%) | 25 (10%) | 154 (61%) | 314/323 | 144/179 |
| Galdieri | 57/38 | 35 (61.40%) | 21 (36.84%) | 1 (1.76%) | 22 (38.6%) | 19 (50%) | 16 (42.11%) | 3 (7.89%) | 19 (50%) | 91/54 | 23/22 |
| Storti | 103/200 | 45 (43%) | 47 (46%) | 11 (11%) | 58 (57%) | 101 (50%) | 86 (43%) | 13 (7%) | 99 (50%) | 137/288 | 69/112 |
3.2. Quantitative data analysis
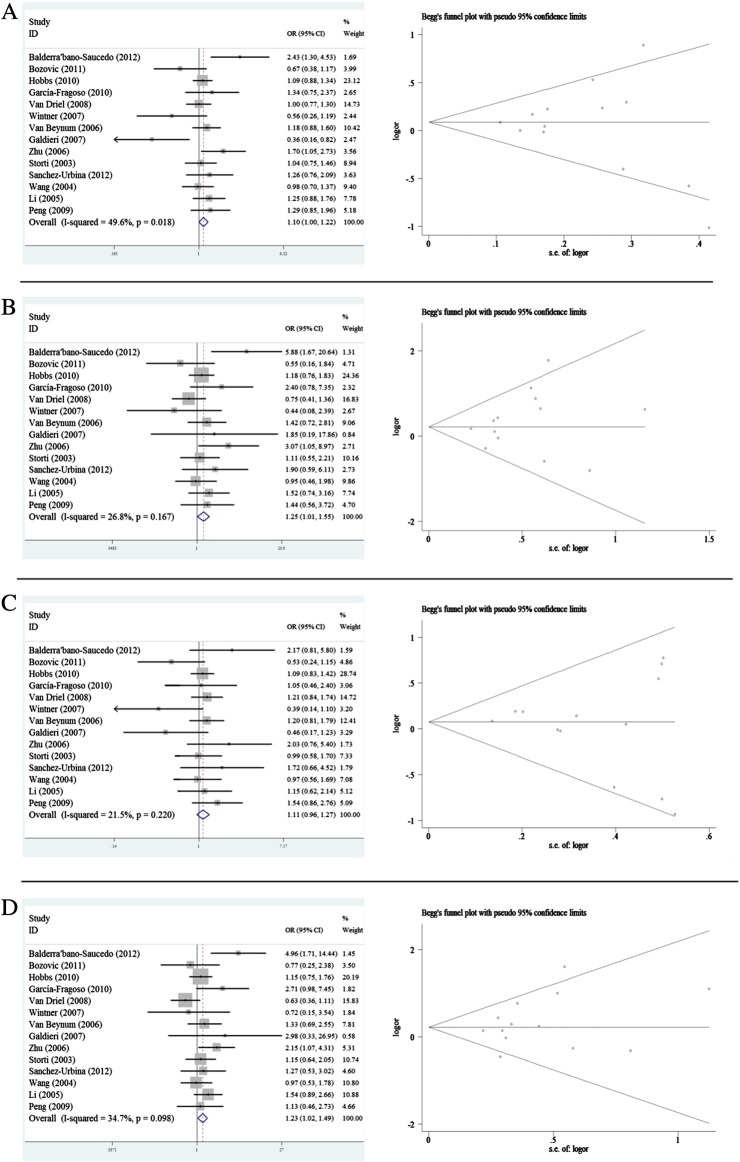
For the maternal MTHFR C677T polymorphism, the results indicated no statistically significant association between the polymorphism and the susceptibility to CHD in all genetic models except for recessive model and co-dominant model (T vs. C: OR = 1.103, 95% CI 0.999–1.218; TT vs. CC: OR = 1.254, 95% CI 1.012–1.553; TT + CT vs. CC: 1.105, 95% CI 0.958–1.275; TT vs. TC + CC: OR = 1.235, 95% CI 1.024–1.489) (Table 4 Fig. 2). In the subgroup analysis by ethnicity, no significant association was observed in Asian population in all genetic models except for recessive model (T vs. C: OR = 1.204, 95% CI 0.973–1.490; TT vs. CC: OR = 1.454, 95% CI 0.921–2.295; TT + CT vs. CC: OR = 1.170, 95% CI 0.802–1.707; TT vs. TC + CC: OR = 1.431, 95% CI 1.011–2.024) (Table 4). No association was detected in Caucasians in all genetic models. In the stratified analysis by types of CHD, there was a significant association between C667T and ASD/PDA, however, the results were not reliable because only one study was performed in ASD/PDA patients (Table 4). In the subgroup of source of control, association was only observed in the recessive model of hospital based control subgroup (TT vs. TC + CC: OR = 1.550, 95% CI 1.106–2.170) (Table 4). In the sample size subgroup analysis, there was no significant association between CHD and maternal C677T in all genetic models of large sample studies. However, we have observed a significant association in co-dominant model and recessive model with small sample studies (Table 4).
Table 4.
Pooled ORs and 95% CIs of the association between maternal MTHFR C677T polymorphism and CHD.
| Contrasts | No. of studies | Total case/control | T vs. C |
TT vs. CC |
TT + CT vs. CC |
TT vs. TC + CC |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | PH | OR | 95% CI | PH | OR | 95% CI | PH | OR | 95% CI | PH | |||
| All | 14 | 1745/2044 | 1.103 | 0.999–1.218 | 0.018 | 1.254 | 1.012–1.553 | 0.167 | 1.105 | 0.958–1.275 | 0.220 | 1.235 | 1.024–1.489 | 0.098 |
| Study in HWE | 12 | 1523/1515 | 1.098 | 0.984–1.224 | 0.007 | 1.247 | 0.986–1.578 | 0.104 | 1.092 | 0.935–1.275 | 0.130 | 1.244 | 1.012–1.528 | 0.076 |
| Ethnicity | ||||||||||||||
| Caucasian | 10 | 1311/1531 | 1.062 | 0.945–1.193 | 0.014 | 1.189 | 0.925–1.527 | 0.121 | 1.067 | 0.909–1.251 | 0.145 | 1.165 | 0.926–1.466 | 0.066 |
| Asian | 4 | 434/513 | 1.204 | 0.973–1.490 | 0.175 | 1.454 | 0.921–2.295 | 0.207 | 1.170 | 0.802–1.707 | 0.438 | 1.431 | 1.011–2.024 | 0.228 |
| Types of CHD | ||||||||||||||
| All types | 11 | 1586/1742 | 1.072 | 0.960–1.197 | 0.016 | 1.202 | 0.947–1.525 | 0.140 | 1.071 | 0.917–1.250 | 0.197 | 1.192 | 0.964–1.474 | 0.067 |
| CD | 1 | 103/200 | 1.043 | 0.745–1.461 | – | 1.107 | 0.554–2.213 | – | 0.989 | 0.576–1.699 | – | 1.150 | 0.645–2.052 | – |
| ASD/PDA | 1 | 56/102 | 1.697 | 1.054–2.731 | – | 3.067 | 1.048–8.974 | – | 2.033 | 0.765–5.403 | – | 2.147 | 1.068–4.314 | – |
| Source of controls | ||||||||||||||
| HB | 6 | 514/750 | 1.092 | 0.883–1.351 | 0.004 | 1.504 | 0.999–2.264 | 0.120 | 0.952 | 0.701–1.293 | 0.156 | 1.550 | 1.106–2.170 | 0.099 |
| PB | 6 | 1175/1192 | 1.060 | 0.936–1.201 | 0.573 | 1.078 | 0.823–1.412 | 0.500 | 1.090 | 0.914–1.300 | 0.135 | 1.028 | 0.803–1.318 | 0.500 |
| Sample size | ||||||||||||||
| Small | 6 | 1441/1486 | 1.081 | 0.963–1.214 | 0.884 | 1.110 | 0.865–1.425 | 0.672 | 1.115 | 0.947–1.314 | 0.977 | 1.090 | 0.874–1.361 | 0.333 |
| Large | 7 | 304/558 | 1.136 | 0.913–1.412 | 0.001 | 1.854 | 1.161–2.961 | 0.090 | 0.953 | 0.682–1.331 | 0.034 | 1.838 | 1.252–2.700 | 0.199 |
Abbreviations: OR, odds ratio; CI, confidence interval; PH, p value based on Q test for between-study heterogeneity; HWE, Hardy–Weinberg equilibrium; CD, conotruncal heart defects; ASD, atrial septal defect; PDA, patent ductus arteriosus; PB, population-based; HB, hospital-based.
Fig. 2.
Forest plot of meta-analysis of association between maternal MTHFR C677T polymorphism and CHD risk and funnel plot analysis on the detection of publication bias. (A) Meta-analysis in a random effects model for C vs. T (additive model); (B) meta-analysis in a random effects model for CC vs. TT (co-dominant model); (C) meta-analysis in a random effects model for TT + CT vs. CC (dominant model); (D) meta-analysis in a random effects model for TT vs. CC + CT (recessive model). Left panel: forest plot analysis, each study is shown by the point of estimating the OR and 95% CIs for corresponding ORs were shown by extending lines; right panel: funnel plot analysis, each point represents an individual study. LogOR, natural logarithm of OR, perpendicular line denotes the mean effect size.
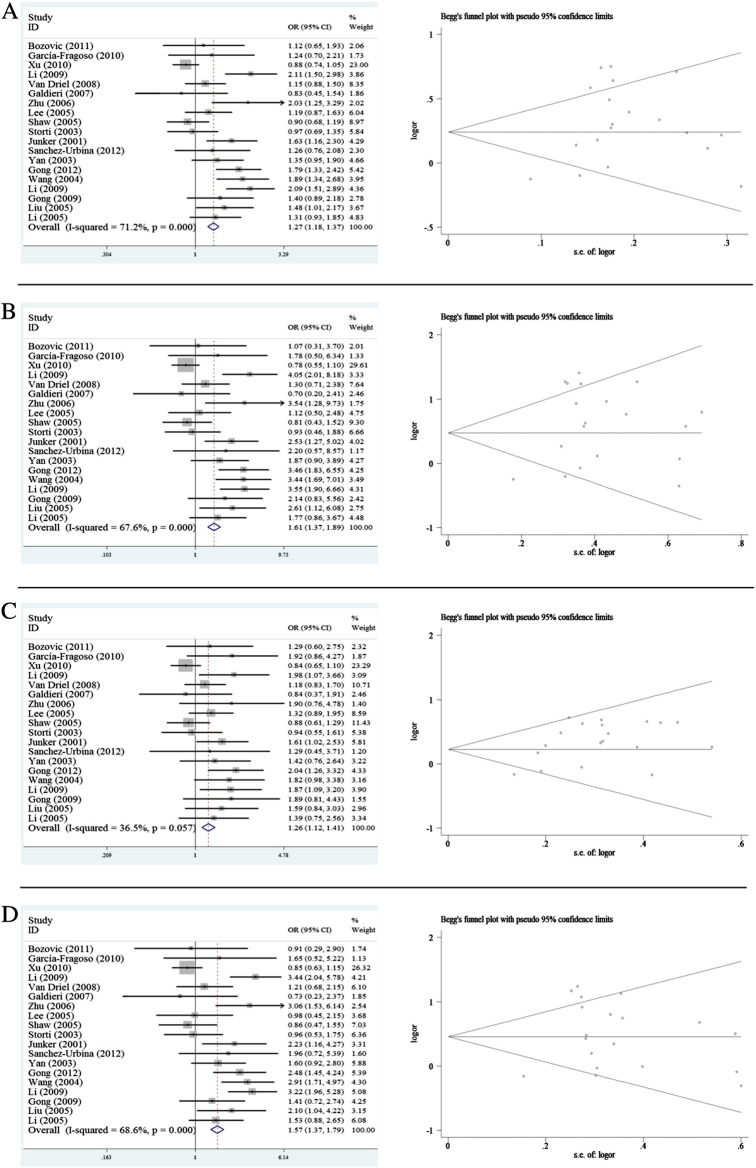
For the fetal MTHFR C667T polymorphism, the overall results suggested a significant association of polymorphism with CHD susceptibility (T vs. C: OR = 1.271, 95% CI 1.178–1.372; TT vs. CC: OR = 1.610, 95% CI 1.374–1.885; TT + CT vs. CC: OR = 1.258, 95% CI 1.120–1.414; TT vs. TC + CC: OR = 1.565, 95% CI 1.370–1.788) (Table 5, Fig. 3). In the subgroup by ethnicity, fetal MTHFR C677T was associated with CHD in Asian populations for all genetic models, however, no significant association was found in Caucasian (Table 5). In the stratified analysis by types of CHD, significant associations were detected between fetal MTHFR C677T and all types of CHD for all genetic models (Table 5). Similar significant association was also observed in CD and ASD/PDA, however, the positive result in CD was not reliable because it was derived from one study. Interestingly, a significant association was observed in hospital based control subgroup rather than in population based control subgroup. By considering sample size, significant results were also found in all genetic models in both small and large sample subgroups (Table 5).
Table 5.
Pooled ORs and 95% CIs of the association between fetal MTHFR C677T polymorphism and CHD.
| Contrasts | No. of studies | Total case/control | T vs. C |
TT vs. CC |
TT + CT vs. CC |
TT vs. TC + CC |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | PH | OR | 95% CI | PH | OR | 95% CI | PH | OR | 95% CI | PH | |||
| All | 19 | 2697/3434 | 1.271 | 1.178–1.372 | 0.000 | 1.610 | 1.374–1.885 | 0.000 | 1.258 | 1.120–1.414 | 0.057 | 1.565 | 1.370–1.788 | 0.000 |
| Study in HWE | 17 | 2657/3006 | 1.247 | 1.155–1.348 | 0.000 | 1.537 | 1.308–1.807 | 0.000 | 1.238 | 1.101–1.392 | 0.070 | 1.492 | 1.302–1.711 | 0.000 |
| Ethnicity | ||||||||||||||
| Caucasian | 8 | 796/1485 | 1.108 | 0.970–1.264 | 0.252 | 1.233 | 0.925–1.643 | 0.296 | 1.151 | 0.956–1.385 | 0.444 | 1.196 | 0.920–1.556 | 0.399 |
| Asian | 11 | 1901/1949 | 1.358 | 1.240–1.487 | 0.000 | 1.793 | 1.487–2.162 | 0.000 | 1.345 | 1.164–1.555 | 0.042 | 1.695 | 1.455–1.976 | 0.000 |
| Types of CHD | ||||||||||||||
| All types | 15 | 2197/2877 | 1.233 | 1.136–1.339 | 0.000 | 1.496 | 1.259–1.777 | 0.000 | 1.230 | 1.087–1.391 | 0.099 | 1.474 | 1.274–1.707 | 0.000 |
| CD | 3 | 444/454 | 1.391 | 1.148–1.686 | 0.026 | 2.053 | 1.371–3.075 | 0.021 | 1.471 | 1.074–2.015 | 0.106 | 1.751 | 1.249–2.455 | 0.059 |
| ASD/PDA | 1 | 56/103 | 2.031 | 1.255–3.287 | – | 3.536 | 1.284–9.735 | – | 1.901 | 0.757–4.778 | – | 3.065 | 1.529–6.142 | – |
| Source of controls | ||||||||||||||
| HB | 12 | 1850/2201 | 1.322 | 1.208–1.448 | 0.000 | 1.720 | 1.431–2.067 | 0.000 | 1.323 | 1.146–1.526 | 0.021 | 1.633 | 1.401–1.904 | 0.000 |
| PB | 5 | 677/902 | 1.101 | 0.945–1.283 | 0.509 | 1.225 | 0.871–1.723 | 0.488 | 1.101 | 0.881–1.376 | 0.688 | 1.215 | 0.902–1.638 | 0.542 |
| Sample size | ||||||||||||||
| Small | 7 | 432/679 | 1.332 | 1.134–1.565 | 0.537 | 1.877 | 1.294–2.722 | 0.570 | 1.501 | 1.147–1.964 | 0.875 | 1.655 | 1.224–2.237 | 0.390 |
| Large | 12 | 2265/2755 | 1.254 | 1.154–1.363 | 0.000 | 1.551 | 1.306–1.843 | 0.000 | 1.221 | 1.077–1.385 | 0.013 | 1.531 | 1.323–1.772 | 0.000 |
Abbreviations: OR, odds ratio; CI, confidence interval; PH, p value based on Q test for between-study heterogeneity; HWE, Hardy–Weinberg equilibrium; CD, conotruncal heart defects; ASD, atrial septal defect; PDA, patent ductus arteriosus; PB, population-based; HB, hospital-based
Fig. 3.
Forest plot of meta-analysis of association between fetal MTHFR C677T polymorphism and CHD risk and funnel plot analysis on the detection of publication bias. (A) Meta-analysis in a random effects model for C vs. T (additive model); (B) meta-analysis in a random effects model for CC vs. TT (co-dominant model); (C) meta-analysis in a random effects model for TT + CT vs. CC (dominant model); (D) meta-analysis in a random effects model for TT vs. CC + CT (recessive model). Left panel: forest plot analysis, each study is shown by the point of estimating the OR and 95% CIs for corresponding ORs were shown by extending lines; right panel: funnel plot analysis, each point represents an individual study. LogOR, natural logarithm of OR, perpendicular line denotes the mean effect size.
For MTHFR A1298C polymorphism, the results showed no significant association between this polymorphism and CHD risk in either maternal or fetal groups (maternal: T vs. A: OR = 1.043, 95% CI 0.855–1.271; CC vs. AA OR = 1.109, 95% CI 0.692–1.775; CC + AC vs. AA: OR = 1.108, 95% CI 0.856–1.435; CC vs. AC + AA: OR = 0.735, 95% CI 0.467–1.157; fetal: C vs. A OR = 0.938, 95% CI 0.812–1.083; CC vs. AA: OR = 1.058, 95% CI 0.719–1.558; CC + AC vs. AA: OR = 0.871, 95% CI 0.728–1.042; CC vs. AC + AA: OR = 1.184, 95% CI 0.815–1.721). In the subgroup analysis of either maternal or fetal polymorphisms, there was no statistically significant association in each subgroup by ethnicity, types of CHD, source of controls and sample size under all genetic models (Table 6).
Table 6.
Pooled ORs and 95% CIs of the association between maternal/fetal MTHFR A1298C polymorphism and CHD.
| Contrasts | No. of studies | Total case/control | C vs. A |
CC vs. AA |
CC + AC vs. AA |
CC vs. AC + AA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | PH | OR | 95% CI | PH | OR | 95% CI | PH | OR | 95% CI | PH | |||
| Maternal | ||||||||||||||
| All studies | 4 | 432/532 | 1.043 | 0.855–1.271 | 0.585 | 1.109 | 0.692–1.775 | 0.405 | 1.108 | 0.856–1.435 | 0.912 | 0.735 | 0.467–1.157 | 0.053 |
| All types of CHD | 3 | 329/332 | 1.018 | 0.805–1.287 | 0.407 | 1.062 | 0.617–1.825 | 0.250 | 1.102 | 0.810–1.499 | 0.769 | 0.615 | 0.366–1.034 | 0.063 |
| CD | 1 | 103/200 | 1.107 | 0.765–1.601 | – | 1.268 | 0.493–3.262 | – | 1.124 | 0.699–1.809 | – | 1.276 | 0.512–3.183 | – |
| HB | 2 | 150/226 | 1.040 | 0.741–1.458 | 0.407 | 1.405 | 0.594–3.323 | 0.634 | 1.120 | 0.731–1.716 | 0.970 | 1.412 | 0.613–3.253 | 0.627 |
| PB | 2 | 282/306 | 1.044 | 0.818–1.344 | 0.263 | 1.003 | 0.572–1.761 | 0.134 | 1.102 | 0.796–1.524 | 0.468 | 0.557 | 0.324–0.957 | 0.048 |
| Small | 2 | 99/81 | 1.150 | 0.712–1.856 | 0.224 | 3.476 | 0.700–17.271 | 0.625 | 1.289 | 0.708–2.350 | 0.684 | 3.266 | 0.674–15.825 | 0.682 |
| Large | 2 | 333/451 | 1.022 | 0.822–1.270 | 0.599 | 0.961 | 0.581–1.591 | 0.500 | 1.071 | 0.804–1.426 | 0.800 | 0.608 | 0.372–0.993 | 0.059 |
| Fetal | ||||||||||||||
| All studies | 5 | 945/1074 | 0.938 | 0.812–1.083 | 0.171 | 1.058 | 0.719–1.558 | 0.331 | 0.871 | 0.728–1.042 | 0.128 | 1.184 | 0.815–1.721 | 0.516 |
| All types of CHD | 4 | 842/874 | 0.884 | 0.756–1.034 | 0.422 | 0.924 | 0.600–1.421 | 0.460 | 0.813 | 0.670–0.987 | 0.281 | 1.085 | 0.715–1.646 | 0.496 |
| CD | 1 | 103/200 | 1.295 | 0.901–1.861 | – | 1.899 | 0.791–4.562 | – | 1.315 | 0.815–2.121 | – | 1.720 | 0.742–3.987 | – |
| HB | 3 | 662/765 | 1.017 | 0.851–1.216 | 0.145 | 1.221 | 0.725–2.057 | 0.168 | 0.991 | 0.799–1.229 | 0.275 | 1.216 | 0.729–2.027 | 0.233 |
| PB | 2 | 283/309 | 0.805 | 0.630–1.028 | 0.627 | 0.889 | 0.499–1.582 | 0.603 | 0.648 | 0.467–0.898 | 0.846 | 1.149 | 0.664–1.989 | 0.582 |
| Small | 2 | 111/96 | 0.667 | 0.428–1.041 | 0.781 | 0.351 | 0.084–1.460 | 0.461 | 0.616 | 0.354–1.073 | 0.949 | 0.427 | 0.105–1.730 | 0.416 |
| Large | 3 | 834/978 | 0.976 | 0.838–1.136 | 0.150 | 1.166 | 0.778–1.749 | 0.429 | 0.907 | 0.750–1.096 | 0.064 | 1.293 | 0.875–1.912 | 0.758 |
| Caucasian | 4 | 443/547 | 0.902 | 0.743–1.095 | 0.108 | 1.014 | 0.636–1.618 | 0.209 | 0.793 | 0.615–1.023 | 0.106 | 1.183 | 0.757–1.848 | 0.353 |
| Asian | 1 | 502/527 | 0.984 | 0.794–1.219 | – | 1.161 | 0.582–2.316 | – | 0.955 | 0.742–1.229 | – | 1.188 | 0.599–2.356 | – |
Abbreviations: OR, odds ratio; CI, confidence interval; PH, p value based on Q test for between-study heterogeneity; HWE, Hardy–Weinberg equilibrium; CD, conotruncal heart defects; PB, population-based; HB, hospital-based.
3.3. Source of heterogeneity
As shown in Table 4, heterogeneity between studies was significant (p < 0.10) under additive, and recessive genetic models for maternal MTHFR C677T. Moreover, evidence for heterogeneity between studies was also found in all genetic models for fetal MTHFR C677T. For the MTHFR A1298C polymorphism, significant heterogeneity was only found in recessive model of maternal polymorphism. No evidence for heterogeneity between studies was detected for maternal MTHFR C677T in the co-dominant and dominant models, for maternal and fetal MTHFR A1298C under all genetic models except for maternal recessive model.
3.4. Sensitivity analysis
In the sensitivity analysis, the overall association between the maternal MTHFR C677T genotype and CHD was not substantially changed by excluding one study at a time (data not shown). Similar results were also found in fetal MTHFR C677T, maternal and fetal MTHFR A1298C.
3.5. Potential publication bias
Except for dominant model of fetal MTHFR C677T and co-dominant model of maternal A1298C polymorphisms, no publication bias could be detected by employing Egger's test for studies on maternal MTHFR C677T polymorphism (T vs. C: p = 0.647; TT vs. CC: p = 0.324; TT + CT vs. CC p = 0.533; TT vs. TC + CC: p = 0.269); fetal MTHFR C677T polymorphism (T vs. C: p = 0.077; TT vs. CC: p = 0.110; TT vs. TC + CC: p = 0.057); maternal MTHFR A1298C polymorphism (C vs. A: p = 0.882; CC + AC vs. AA: p = 0.330; CC vs. AC + AA: p = 0.107); and fetal MTHFR A1298C polymorphism (C vs. A: p = 0.493; CC vs. AA: p = 0.576; CC + AC vs. AA: p = 0.576; CC vs. AC + AA: p = 0.576). The results of Egger's test suggested publication bias in dominant model of fetal MTHFR C677T polymorphism (TT + CT vs. CC: p = 0.006) and co-dominant model of maternal A1298C polymorphism (CC vs. AA: p = 0.049). The Begg's tests of corresponding genetic models in forest plots were shown in Figs. 2 and 3.
4. Discussion
To date, it is known that genetic and environmental risks may be the causes of congenital heart diseases. Importantly, numerous studies have suggested the role of folic acid metabolism in the CHD development (Ueland et al., 2001). MTHFR is a key enzyme in folic acid conversion process, and its activity may be related with a variety of diseases including CHD (Li et al., 2013; Long et al., 2012). It was reported that the C677T mutation of MTHFR could render the enzyme thermolabile with approximately 50% reduced activity and increased plasma homocysteine concentrations (Huhta and Hernandez-Robles, 2005). Therefore, the variants of the MTHFR gene may modulate the activity of MTHFR and may be an important determinant of CHD development. Several studies have reported the potential association between MTHFR polymorphisms (C677T and A1298C) and CHD, however, the results were not consistent (Balderrabano-Saucedo et al., 2013; Galdieri et al., 2007). Our current comprehensive meta-analysis could better evaluate the association between MTHFR C677T/A1298C and susceptibility of CHD. Two studies have performed meta-analysis in association between MTHFR C677T polymorphism and CHD two years ago (Nie et al., 2011; Yin et al., 2012). However, the MTHFR A1298C polymorphism was not analyzed in either of the two studies. Moreover, subgroup analysis based methods were not employed in previous studies. We have analyzed the association between MTHFR polymorphisms and CHD by multiple methods in all genetic models and included more recent studies. To our knowledge, this is the first meta-analysis on association between MTHFR polymorphisms and CHD including both C677T and A1298C.
For the MTHFR C677T polymorphism, most studies have indicated that maternal C677T was not a strong risk of CHD, however, some reports have suggested its potential role in CHD development. In our finding, no statistically significant difference was detected in genotype or allele frequencies of MTHFR C677T polymorphism in the mothers of CHD patients compared with controls. Only marginal association between maternal C677T polymorphism and CHD was found in recessive model. The finding was consistent with the previous studies involving both maternal and fetal C677T polymorphism. Particularly, we found a significant association between maternal C677T and CHD in recessive genetic models of Asian subgroup, however, similar result was not observed in Caucasian. This discrepancy of association between Asian and Caucasian groups may be attributed to the different genetic background and environmental factors.
Our results have indicated that fetal MTHFR C677T polymorphism was significantly associated with CHD in all genetic models. It was evident that fetal MTHFR C677T polymorphism was an important risk in the development of CHD. To explain the results, we speculated that decreased fetal MTHFR enzyme activity may result in a local hyperhomocystein environment, in which the heart could not develop normally (Lu et al., 2011). These evidences have supported the viewpoint that fetal MTHFR C677T polymorphism was more important than maternal MTHFR C677T polymorphism, and concentration of homocystein in fetus may influence heart development rather than maternal homocystein concentration. In addition, we found that fetal MTHFR C677T was significantly associated with CHD in Asian, while no statistically significant association was found in Caucasian population. Consistent with the result from recessive model of maternal analysis, the fetal MTHFR C677T was more likely to be associated with CHD in Asian than Caucasian. The results have validated the notion that MTHFR C667T may be in combination with other genetic background and environment factors to affect the fetal heart development. By considering the source of controls, the association between MTHFR C667T polymorphism and CHD was significant in hospital based control group, though, not significant in population based control. The confounding results from two subgroups categorized by source of control have indicated that hospital based and population based control was not homogenous in this study. We believe that the comparability between cases and controls contributes to the disagreement of these two subgroups.
For the MTHFR A1298C polymorphism, we found no statistically significant association between this polymorphism and CHD either in maternal or fetal analysis. Our finding has demonstrated that MTHFR A1298C may not be a risk of congenital heart disease development. However, some studies indicated that the interaction between MTHFR 1298 C allele and folic acid supplement increased the risk of having a child with CHD (van Driel et al., 2008). Considering that minimal eligible studies included in our meta-analysis, this result should be validated with more studied and large pooled samples in future. In subgroup analysis by ethnicity, only one study was performed in Asian population to investigate fetal A1298C polymorphism and four studies were performed in Caucasian population. Because of the importance of MTHFR polymorphisms in Asian CHD development, we suggest that more studies investigating association between A1298C polymorphism and CHD be performed in Asian population.
5. Potential study limitations
Although we made these findings in this meta-analysis, there were several limitations. First, our study was mainly based on unadjusted odd ratios, and the potential covariates including gender, age, vitamin supplement, smoking or other environmental factors, which might influence the final results, were unable to control. Second, significant heterogeneity in the study was presented in overall and subgroup analysis. We have investigated the study heterogeneity including geographic region, ethnicity, and source of control. However, none of them was identified as the potential source of heterogeneity between studies by meta-regression (data not shown). We estimated that other unknown confounding factors may help explain the between-study heterogeneity. Third, it was known that there were several subtypes of congenital heart diseases. However, only a few studies included in our meta-analysis have classified their cases by types of CHD. To analyze this issue, we need more studies involving CHD cases with clear subtypes.
6. Conclusion
Despite the limitations mentioned above, the present study has demonstrated that the fetal MTHFR C677T polymorphism is an important risk of developing congenital heart diseases. Our findings also suggest that MTHFR A1298C polymorphism does not increase the susceptibility to CHD. Interestingly, we found that fetal MTHFR C677T polymorphism more likely affects Asian fetus than Caucasian fetus in the development of CHD.
Conflicts of interest
There was no potential conflict of interest.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (31160230) and the Provincial Science and Technology, Yunnan, People's Republic of China (2010CD210, 2011FB166, 2009FXW003).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Balderrabano-Saucedo N.A., Sanchez-Urbina R., Sierra-Ramirez J.A., Garcia-Hernandez N., Sanchez-Boiso A., Klunder-Klunder M., Arenas-Aranda D., Bravo-Hernandez G., Noriega-Zapata P., Vizcaino-Alarcon A. Polymorphism 677C–> T MTHFR gene in Mexican mothers of children with complex congenital heart disease. Pediatr. Cardiol. 2013;34:46–51. doi: 10.1007/s00246-012-0380-y. [DOI] [PubMed] [Google Scholar]
- Basson C.T., Bachinsky D.R., Lin R.C., Levi T., Elkins J.A., Soults J., Grayzel D., Kroumpouzou E., Traill T.A., Leblanc-Straceski J., Renault B., Kucherlapati R., Seidman J.G., Seidman C.E. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt–Oram syndrome. Nat. Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- Bernier P.L., Stefanescu A., Samoukovic G., Tchervenkov C.I. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2010;13:26–34. doi: 10.1053/j.pcsu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Blue G.M., Kirk E.P., Sholler G.F., Harvey R.P., Winlaw D.S. Congenital heart disease: current knowledge about causes and inheritance. Med. J. Aust. 2012;197:155–159. doi: 10.5694/mja12.10811. [DOI] [PubMed] [Google Scholar]
- Bozovic I.B., Vranekovic J., Cizmarevic N.S., Mahulja-Stamenkovic V., Prpic I., Brajenovic-Milic B. MTHFR C677T and A1298C polymorphisms as a risk factor for congenital heart defects in Down syndrome. Pediatr. Int. 2011;53:546–550. doi: 10.1111/j.1442-200X.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- Bruneau B.G. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- Galdieri L.C., Arrieta S.R., Silva C.M., Pedra C.A., D'Almeida V. Homocysteine concentrations and molecular analysis in patients with congenital heart defects. Arch. Med. Res. 2007;38:212–218. doi: 10.1016/j.arcmed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Garcia-Fragoso L., Garcia-Garcia I., Leavitt G., Renta J., Ayala M.A., Cadilla C.L. MTHFR polymorphisms in Puerto Rican children with isolated congenital heart disease and their mothers. Int. J. Genet. Mol. Biol. 2010;2:43–47. [PMC free article] [PubMed] [Google Scholar]
- Gong T., Li F., Shi J., fen J., Tang X., Li X., Ren Y. Relationship between risk factors during pregnancy, maternal MTHFR gene 677C-T, plasma Hcy levels and congenital heart disease in children. Matern. Child Health Care China. 2009;24:5. [Google Scholar]
- Gong D., Gu H., Zhang Y., Gong J., Nie Y., Wang J., Zhang H., Liu R., Hu S. Methylenetetrahydrofolate reductase C677T and reduced folate carrier 80G > A polymorphisms are associated with an increased risk of conotruncal heart defects. Clin. Chem. Lab. Med. 2012;50:1455–1461. doi: 10.1515/cclm-2011-0759. [DOI] [PubMed] [Google Scholar]
- Greutmann M., Tobler D. Changing epidemiology and mortality in adult congenital heart disease: looking into the future. Future Cardiol. 2012;8:171–177. doi: 10.2217/fca.12.6. [DOI] [PubMed] [Google Scholar]
- Hobbs C.A., Cleves M.A., Karim M.A., Zhao W., MacLeod S.L. Maternal folate-related gene environment interactions and congenital heart defects. Obstet. Gynecol. 2010;116:316–322. doi: 10.1097/AOG.0b013e3181e80979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhta J.C., Hernandez-Robles J.A. Homocysteine, folate, and congenital heart defects. Fetal Pediatr. Pathol. 2005;24:71–79. doi: 10.1080/15227950591008240. [DOI] [PubMed] [Google Scholar]
- Junker R., Kotthoff S., Vielhaber H., Halimeh S., Kosch A., Koch H.G., Kassenbohmer R., Heineking B., Nowak-Gottl U. Infant methylenetetrahydrofolate reductase 677TT genotype is a risk factor for congenital heart disease. Cardiovasc. Res. 2001;51:251–254. doi: 10.1016/s0008-6363(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Khairy P., Ionescu-Ittu R., Mackie A.S., Abrahamowicz M., Pilote L., Marelli A.J. Changing mortality in congenital heart disease. J. Am. Coll. Cardiol. 2010;56:1149–1157. doi: 10.1016/j.jacc.2010.03.085. [DOI] [PubMed] [Google Scholar]
- Lamers Y., Prinz-Langenohl R., Moser R., Pietrzik K. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am. J. Clin. Nutr. 2004;79:473–478. doi: 10.1093/ajcn/79.3.473. [DOI] [PubMed] [Google Scholar]
- Lee C.N., Su Y.N., Cheng W.F., Lin M.T., Wang J.K., Wu M.H., Hsieh F.J. Association of the C677T methylenetetrahydrofolate reductase mutation with congenital heart diseases. Acta Obstet. Gynecol. Scand. 2005;84:1134–1140. doi: 10.1111/j.0001-6349.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Cheng J., Zhu W., Dao J., Yan L., Li M., Li S. Study of serum Hcy and polymorphism of Hcy metabolic enzymes in 192 families affected by congenital heart disease. J. Peking Univ. 2005;37:6. [PubMed] [Google Scholar]
- Li D., Jing X., Wang H. Correlations between MTHFR gene polymorphism, exposure to chemicals during pregnancy and congenital heart disease. Chin. J. Public Health. 2009;25:3. [Google Scholar]
- Li D., Jing X., Wang H., Ye W., Fan H. Study of correlationship between congenital heart disease and 5,10-methylenetetra hydrofolate reductase gene polymorphism or folacin intakes. Chin. J. Prev. Med. 2009;43:5. [PubMed] [Google Scholar]
- Li R., Wang R., Li Y., Li X., Feng Y., Jiang C. Association study on MTHFR polymorphisms and meningioma in northern China. Gene. 2013;516(2):291–293. doi: 10.1016/j.gene.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Liu F., Bai P., Chen S., Qiu W., Liu X., Zhang Y. Association between 5,10-methylenetetrahydrofolate reductase C677T polymorphisms and conotruncal heart defects in Chinese children. Chin. J. Contemp. Pediatr. 2005;7:4. [Google Scholar]
- Locke A.E., Dooley K.J., Tinker S.W., Cheong S.Y., Feingold E., Allen E.G., Freeman S.B., Torfs C.P., Cua C.L., Epstein M.P., Wu M.C., Lin X., Capone G., Sherman S.L., Bean L.J. Variation in folate pathway genes contributes to risk of congenital heart defects among individuals with Down syndrome. Genet. Epidemiol. 2010;34:613–623. doi: 10.1002/gepi.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., Yang X., Liu X., Yang P. Methylenetetrahydrofolate reductase (MTHFR) polymorphisms and susceptibility for cervical lesions: a meta-analysis. PLoS One. 2012;7:e52381. doi: 10.1371/journal.pone.0052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Wang H., Wang X. Relationship of hyperhomocysteinemia in pregnant rats and congenital heart defects in the newborn rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:68–73. doi: 10.3969/j.issn.1672-7347.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Nie Y., Gu H., Gong J., Wang J., Gong D., Cong X., Chen X., Hu S. Methylenetetrahydrofolate reductase C677T polymorphism and congenital heart disease: a meta-analysis. Clin. Chem. Lab. Med. 2011;49:2101–2108. doi: 10.1515/CCLM.2011.673. [DOI] [PubMed] [Google Scholar]
- Oda T., Elkahloun A.G., Pike B.L., Okajima K., Krantz I.D., Genin A., Piccoli D.A., Meltzer P.S., Spinner N.B., Collins F.S., Chandrasekharappa S.C. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat. Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- Peng T., Li X., Wang L. Effects of periconceptional folate intake and methylenetetrahydrofolate Reductase gene C677T polymorphism of pregnant woman on congenital heart disease in offspring. J. Environ. Health. 2009;26:4. [Google Scholar]
- Pierpont M.E., Basson C.T., Benson D.W., Jr., Gelb B.D., Giglia T.M., Goldmuntz E., McGee G., Sable C.A., Srivastava D., Webb C.L. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- Richards A.A., Garg V. Genetics of congenital heart disease. Curr. Cardiol. Rev. 2010;6:91–97. doi: 10.2174/157340310791162703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Urbina R., Galaviz-Hernandez C., Sierra-Ramirez J.A., Rangel-Villalobos H., Torres-Saldua R., Alva-Espinoza C., Ramirez-Duenas Mde L., Garcia-Cavazos R., Arambula-Meraz E. Methylenetetrahydrofolate reductase gene 677CT polymorphism and isolated congenital heart disease in a Mexican population. Rev. Esp. Cardiol. (Engl. Ed) 2012;65:158–163. doi: 10.1016/j.recesp.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Schott J.J., Benson D.W., Basson C.T., Pease W., Silberbach G.M., Moak J.P., Maron B.J., Seidman C.E., Seidman J.G. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- Shaw G.M., Iovannisci D.M., Yang W., Finnell R.H., Carmichael S.L., Cheng S., Lammer E.J. Risks of human conotruncal heart defects associated with 32 single nucleotide polymorphisms of selected cardiovascular disease-related genes. Am. J. Med. Genet. A. 2005;138:21–26. doi: 10.1002/ajmg.a.30924. [DOI] [PubMed] [Google Scholar]
- Shaw G.M., Lu W., Zhu H., Yang W., Briggs F.B., Carmichael S.L., Barcellos L.F., Lammer E.J., Finnell R.H. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med. Genet. 2009;10:49. doi: 10.1186/1471-2350-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh J.T., Bittles A.H., Hudgins L. Consanguinity and the risk of congenital heart disease. Am. J. Med. Genet. A. 2012;158A:1236–1241. doi: 10.1002/ajmg.a.35272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti S., Vittorini S., Lascone M.R., Sacchelli M., Collavoli A., Ripoli A., Cocchi G., Biagini A., Clerico A. Association between 5,10-methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and conotruncal heart defects. Clin. Chem. Lab. Med. 2003;41:276–280. doi: 10.1515/CCLM.2003.043. [DOI] [PubMed] [Google Scholar]
- Ueland P.M., Hustad S., Schneede J., Refsum H., Vollset S.E. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol. Sci. 2001;22:195–201. doi: 10.1016/s0165-6147(00)01675-8. [DOI] [PubMed] [Google Scholar]
- van Beynum I.M., Kapusta L., den Heijer M., Vermeulen S.H., Kouwenberg M., Daniels O., Blom H.J. Maternal MTHFR 677C > T is a risk factor for congenital heart defects: effect modification by periconceptional folate supplementation. Eur. Heart J. 2006;27:981–987. doi: 10.1093/eurheartj/ehi815. [DOI] [PubMed] [Google Scholar]
- van Beynum I.M., Kapusta L., Bakker M.K., den Heijer M., Blom H.J., de Walle H.E. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: a registry-based case-control study in the northern Netherlands. Eur. Heart J. 2010;31:464–471. doi: 10.1093/eurheartj/ehp479. [DOI] [PubMed] [Google Scholar]
- van der Linde D., Konings E.E., Slager M.A., Witsenburg M., Helbing W.A., Takkenberg J.J., Roos-Hesselink J.W. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- van Driel L.M., Verkleij-Hagoort A.C., de Jonge R., Uitterlinden A.G., Steegers E.A., van Duijn C.M., Steegers-Theunissen R.P. Two MTHFR polymorphisms, maternal B-vitamin intake, and CHDs. Birth Defects Res. A Clin. Mol. Teratol. 2008;82:474–481. doi: 10.1002/bdra.20463. [DOI] [PubMed] [Google Scholar]
- Verkleij-Hagoort A.C., Verlinde M., Ursem N.T., Lindemans J., Helbing W.A., Ottenkamp J., Siebel F.M., Gittenberger-de Groot A.C., de Jonge R., Bartelings M.M., Steegers E.A., Steegers-Theunissen R.P. Maternal hyperhomocysteinaemia is a risk factor for congenital heart disease. BJOG. 2006;113:1412–1418. doi: 10.1111/j.1471-0528.2006.01109.x. [DOI] [PubMed] [Google Scholar]
- Verkleij-Hagoort A., Bliek J., Sayed-Tabatabaei F., Ursem N., Steegers E., Steegers-Theunissen R. Hyperhomocysteinemia and MTHFR polymorphisms in association with orofacial clefts and congenital heart defects: a meta-analysis. Am. J. Med. Genet. A. 2007;143A:952–960. doi: 10.1002/ajmg.a.31684. [DOI] [PubMed] [Google Scholar]
- Wang H. 2006. The Study About Correlationship Between Congenital Heart Disease and MTHFR Gene Polymorphism and Environmental Factors. [Google Scholar]
- Weisberg I., Tran P., Christensen B., Sibani S., Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol. Genet. Metab. 1998;64:169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- Wintner S., Hafner E., Stonek F., Stuempflen I., Metzenbauer M., Philipp K. Association of congenital cardiac defects and the C677T methylenetetrahydrofolate reductase polymorphism. Prenat. Diagn. 2007;27:704–708. doi: 10.1002/pd.1761. [DOI] [PubMed] [Google Scholar]
- Xu J., Xu X., Xue L., Liu X., Gu H., Cao H., Qiu W., Hu Z., Shen H., Chen Y. MTHFR c.1793G > A polymorphism is associated with congenital cardiac disease in a Chinese population. Cardiol. Young. 2010;20:318–326. doi: 10.1017/S1047951110000247. [DOI] [PubMed] [Google Scholar]
- Yan L., Li Y. Association between MTHFR C677T polymorphism and congenital heart disease. J. Peking Univ. 2003;35:2. [Google Scholar]
- Yin M., Dong L., Zheng J., Zhang H., Liu J., Xu Z. Meta analysis of the association between MTHFR C677T polymorphism and the risk of congenital heart defects. Ann. Hum. Genet. 2012;76:9–16. doi: 10.1111/j.1469-1809.2011.00687.x. [DOI] [PubMed] [Google Scholar]
- Zhang W., Li X., Shen A., Jiao W., Guan X., Li Z. GATA4 mutations in 486 Chinese patients with congenital heart disease. Eur. J. Med. Genet. 2008;51:527–535. doi: 10.1016/j.ejmg.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Zhu W.L., Li Y., Yan L., Dao J., Li S. Maternal and offspring MTHFR gene C677T polymorphism as predictors of congenital atrial septal defect and patent ductus arteriosus. Mol. Hum. Reprod. 2006;12:51–54. doi: 10.1093/molehr/gah252. [DOI] [PubMed] [Google Scholar]