Abstract
In vitro and in vivo studies have shown conflicting results regarding heat shock protein (HSP) and leptin correlation. More importantly both HSP70 and leptin are correlated with C reactive proteins. The purpose of the present study was to study the correlation between serum HSP70 and leptin levels in patients with type 2 diabetes stratified according to gender. We performed a cross sectional study on the established groups of patients with type 2 diabetes defined as 1: patients with long standing diabetes for more than 3 years; and 2: patients with newly diagnosed diabetes within recent 6 months who were not on any glucose lowering treatment other than by dietary means alone and 3: healthy controls. Patients with long standing diabetes had higher HSP70, HbA1c and triglyceride than controls. Serum leptin levels were significantly lower in patients with newly diagnosed diabetes. Women with type 2 diabetes had higher leptin levels compared to men, both before and after treatment. We showed a positive correlation between leptin and HSP70 levels in women with type 2 diabetes. The correlation was strongest in women with newly diagnosed diabetes (r = 0.59) and was attenuated in women who were on treatment (r = 0.3). The significance of this correlation was only observed in women with type 2 diabetes. There was no correlation between leptin and HSP70 in men. The positive correlation between leptin and HSP is observed in chronic inflammation such as type 2 diabetes. It could be hypothesized that the observed correlation between serum HSP70 and leptin implies a higher state of chronic inflammation.
Keywords: Heat-shock protein 70, Leptin, Diabetes mellitus Type 2, Gender characteristics
Highlights
-
•
Leptin was positively correlated to HSP70 in women with newly diagnosed diabetes.
-
•
The correlation was highest in women with newly diagnosed diabetes and attenuated after treatment.
-
•
There was no correlation between leptin and HSP70 in diabetic men and healthy controls.
1. Introduction
Leptin, an adipocyte derived cytokine, primarily was known to be involved in the regulation of food intake and energy expenditure (Dardeno et al., 2010). Later on studies showed the role of leptin in the immune system modulation (Lord et al., 1998). The studies describing the role of leptin in diabetes are so conflicting and no one could classify it into either pro-inflammatory or anti-inflammatory groups (Lam and Lu, 2007; Weisberg et al., 2003; Xu et al., 2003). Similarly heat shock proteins (HSPs) are endogenous highly conserved molecules produced by various cells, including macrophage, monocyte and polymorphonuclears when exposed to high temperature, inflammatory cytokines and oxidative stress (Kalmar and Greensmith, 2009). The HSP genes are considered as vitagenes, which are involved in preserving cellular homeostasis during stressful conditions, which are induced by co-ordinating information flow along its convergent, divergent and multiply branched signaling pathways (Calabrese et al., 2010, 2011). In vitro and in vivo studies have shown conflicting results regarding HSP–leptin correlation. More importantly both HSP70 and leptin are correlated with C reactive protein (CRP) (Molvarec et al., 2009, 2011). The purpose of the present study was to study the correlation between serum HSP70 and leptin levels in patients with type 2 diabetes stratified according to gender.
2. Methods
We performed a cross sectional study on the established groups of patients with type 2 diabetes defined as 1: patients with long standing diabetes for more than 3 years; and 2: patients with newly diagnosed diabetes within recent 6 months who were not on any glucose lowering treatment other than by dietary means alone and 3: healthy controls. Patient recruitment was from the diabetes clinic of Vali-Asr Hospital, affiliated with Tehran University of Medical Sciences. Controls were healthy volunteers from the patients' concomitants or hospital staffs. Healthy controls were selected from those without any known disease including type 2 diabetes, hyperlipidemia, ischemic heart disease, and malignancy. Patients and controls were matched according to age, sex and body mass index (BMI). Diabetes was diagnosed according to the criteria of the American Diabetes Association (Seo et al., 2009). Exclusion criteria were smoking, pregnancy, creatinine > 1.5 mg/dl or GFR < 70 cc/min, glomerulonephritis, congestive heart failure, use of antioxidant, statins, hormone replacement therapy and hospital admission in previous 6 months. None of the participants had overt diabetic complications. Demographic and anthropometric data including age, sex, duration of diabetes, height, weight in light clothing, and blood pressure in sitting position were recorded. Blood pressure was re-measured twice after 5 min and averaged. The BMI (kg/m2) was calculated according to the Quetelet formula. The research was carried out according to the principles of the Declaration of Helsinki. All the patients received and signed written informed consent. The local ethics review committee of Tehran University of Medical Science approved the study protocol.
3. Blood samples
Blood samples were collected after 12 h of fasting, centrifuged and were kept at − 70 °C until analysis. Serum creatinine, fasting blood sugar (FBS), total cholesterol, triglycerides, high-density lipoprotein- (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and HbA1c were measured for all participants. Glucose measurements (intra-assay coefficient of variation [CV] 2.1%, inter-assay CV 2.6%) were carried out using the glucose oxidase method. Cholesterol, HDL, LDL and triglycerides were determined using direct enzymatic methods (Parsazmun, Karaj, Iran). Measurements of HSP70 were performed using a quantitative sandwich ELISA immunoassay (EKS-715, Stressgen, USA). The intra- and inter-assay CVs ranged between 4.5% and 7%. Serum leptin concentration was determined by an enzyme-linked immunosorbent assay (DRG Instruments GmbH, Germany) with an intra-assay CV of 5.9–6.9% and an inter-assay CV of 8.6–11.5%. The measurements for the patients with long standing diabetes and controls were performed separately. We standardized the values obtained for leptin for further comparison. CRP was assessed using a two-site, enzyme-linked immunosorbent assay (ELISA) (Diagnostic Biochem, London, Ontario, Canada). Sensitivity of the assay was 10 ng/l. Intra- and inter-assay CVs were 8% and 10% respectively.
4. Statistical analysis
The statistical package SPSS 17 for windows (Chicago, Illinois, USA) was used for analysis. Variables are presented as mean and standard error of mean (SEM). Paired samples t test was used to compare variables, before and after treatment. Independent samples t test was employed to compare men and women. Pearson's correlation test was employed to study the correlation between leptin, HSP70, CRP, BMI and waist circumference. Partial correlation coefficient was employed to study the correlation between the studied variables after adjustment for BMI.
5. Results
Primary characteristics of the groups of patients and controls are presented in Table 1. Patients with long standing diabetes had higher leptin, HSP70, HbA1c and triglyceride than controls. Serum leptin levels were significantly lower in patients with newly diagnosed diabetes, compared to other groups (Table 1). Women with newly diagnosed diabetes had higher leptin and CRP levels compared to men (Table 2). Table 2 presents the characteristics of patients with newly diagnosed diabetes, stratified according to gender.
Table 1.
Comparing demographic and biochemical variables among patients with long standing diabetes, patients with newly diagnosed diabetes and healthy controls.
| Controls (n = 41) |
Newly diagnosed diabetes (N = 44) |
Long standing diabetes (n = 44) |
P value | |
|---|---|---|---|---|
| Age (years) | 52.7 ± 1.72 | 52.0 ± 1.77 | 55.1 ± 1.45 | NS |
| BMI (kg/m2) | 26.8 ± 0.50 | 27.8 ± 0.72 | 27.4 ± 0.58 | NS |
| Waist circumference (cm) | 99.0 ± 1.23 | 97.2 ± 1.58 | 91.2 ± 1.49 | NS |
| Diastolic blood pressure (mm Hg) | 78.4 ± 1.16 | 87.5 ± 2.39 | 78.6 ± 1.48 | NS |
| Systolic blood pressure (mm Hg) | 125.2 ± 1.55 | 127.9 ± 3.54 | 125.7 ± 2.03 | NS |
| FBS (mg/dl) | 85.9 ± 1.26 | 181.9 ± 8.48 | 187.2 ± 10.14 | < 0.001 |
| HbA1c (%) | 4.8 ± 0.06⁎⁎⁎ | 8.3 ± 0.36 | 7.8 ± 0.28 | < 0.001 |
| Creatinine (mg/dl) | 0.93 ± 0.024 | 0.8 ± 0.033 | 0.89 ± 0.03 | NS |
| Triglyceride (mg/dl) | 135.0 ± 9.85⁎⁎ | 201.9 ± 14.8 | 185.1 ± 15.50⁎ | < 0.001 |
| Cholesterol (mg/dl) | 183.1 ± 5.0⁎⁎ | 206.4 ± 6.3 | 215.8 ± 5.56 | < 0.001 |
| LDL (mg/dl) | 92.7 ± 2.73⁎⁎ | 123.9 ± 6.03 | 113.9 ± 2.91 | < 0.01 |
| HDL (mg/dl) | 43.2 ± 2.22 | 44.7 ± 1.85 | 43.5 ± 1.05 | NS |
| Standardized leptin | 0.48 ± 0.05⁎⁎ | 0.27 ± 0.03 | 0.41 ± 0.03⁎⁎ | NS |
| HSP70 (ng/ml) | 0.60 ± 0.10 | 0.77 ± 0.03 | 0.92 ± 0.16 | < 0.05 |
| CRP (mg/ml) | 1.8 ± 0.21⁎⁎ | 3.6 ± 0.35 | 3.4 ± 0.27 | < 0.01 |
Variables are expressed as mean ± standard error of mean (SEM). The column of “P value” is presented when comparing patients with long standing diabetes vs. controls.
P < 0.05 when comparing patients with long standing diabetes or controls vs. patients with newly diagnosed diabetes.
P < 0.01 when comparing patients with long standing diabetes or controls vs. patients with newly diagnosed diabetes.
P < 0.001 when comparing patients with long standing diabetes or controls vs. patients with newly diagnosed diabetes.
Table 2.
Characteristics of patients with newly diagnosed diabetes according to gender.
| Men (n = 19) | Women (n = 25) | |
|---|---|---|
| Age (year) | 56 ± 2.6⁎ | 49 ± 2.1 |
| Waist circumference (cm) | 96 ± 1.7 | 98 ± 2.6 |
| Hip circumference (cm) | 103 ± 2.1 | 107 ± 2.7 |
| BMI (kg/m2) | 26 ± 0.54⁎⁎ | 29 ± 1.0 |
| Systolic blood pressure (mm Hg) | 133 ± 5.5 | 123 ± 4.6 |
| Diastolic blood pressure (mm Hg) | 137 ± 52.7 | 76 ± 3.1 |
| Diabetes duration (years) | 4 ± 1.9 | 1.9 ± 0.60 |
| Fasting blood sugar (mg/dl) | 186 ± 14.5 | 189.4 ± 10.5 |
| HbA1c (%) | 9 ± 0.65 | 8.2 ± 0.41 |
| Cholesterol (mg/dl) | 206 ± 11.9 | 212 ± 6.8 |
| HDL (mg/dl) | 43 ± 2.6 | 46 ± 2.8 |
| LDL (mg/dl) | 126 ± 10.8 | 124 ± 6.5 |
| Triglyceride (mg/dl) | 195 ± 26.7 | 209 ± 15.9 |
| HSP70 (ng/ml) | 0.76 ± 0.04 | 0.77 ± 0.05 |
| Leptin standardized | 0.19 ± 0.02 ⁎⁎⁎ | 0.58 ± 0.07 |
| CRP (mg/ml) | 2.6 ± 0.4⁎⁎ | 4.1 ± 0.4 |
Variables are expressed as mean ± standard error of mean (SEM).
P < 0.05 when comparing men with women.
P < 0.01 when comparing men with women.
P < 0.001 when comparing men with women.
6. Correlation
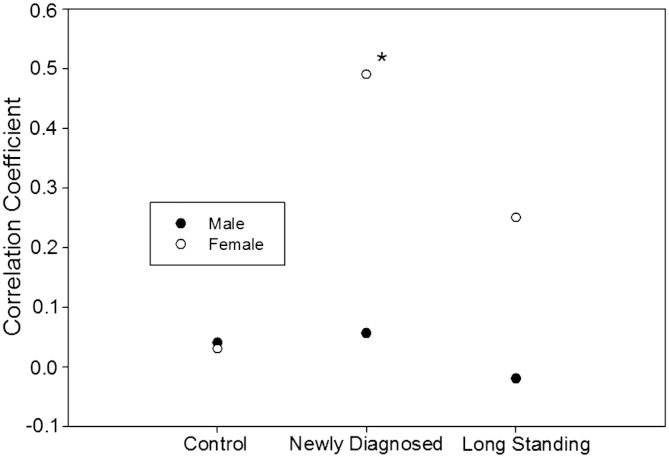
We studied the correlation between serum HSP70, leptin, BMI, and waist circumference (Table 3). While HSP70 was significantly correlated in women with newly diagnosed diabetes, there were not such correlations in men with newly diagnosed diabetes, men and women in long standing diabetes and controls. This correlation remained significant after adjustment for BMI (Table 3). Fig. 1 shows the correlation coefficient between leptin and HSP70 in the studied groups stratified according to gender. The correlation coefficient is not different between men and women in control group. The greatest difference exists between patients with newly diagnosed diabetes and patients with long standing diabetes (Fig. 1).
Table 3.
Presenting the correlation of leptin, CRP and Hsp70 in the studied groups, before and after adjustment for BMI.
| Before BMI adjustment |
After BMI adjustment |
|||||||
|---|---|---|---|---|---|---|---|---|
| CRP | Leptin | HSP70 | CRP | Leptin | HSP70 | |||
| Newly diagnosed diabetes (n = 44) |
CRP | Men | – | 0.38 | 0.52⁎ | – | 0.29 | 0.50⁎ |
| Women | – | 0.13 | 0.27 | – | 0.14 | 0.32 | ||
| Leptin | Men | 0.38 | – | 0.16 | 0.29 | – | 0.08 | |
| Women | 0.13 | – | 0.59⁎ | 0.14 | – | 0.44⁎ | ||
| HSP70 | Men | 0.52⁎ | 0.16 | – | 0.50⁎ | 0.08 | – | |
| Women | 0.27 | 0.59⁎ | – | 0.32 | 0.44⁎ | – | ||
| Waist circumference | Men | 0.25 | 0.46 | 0.16 | ||||
| Women | − 0.07 | 0.32 | 0.01 | |||||
| BMI | Men | 0.35 | 0.33 | 0.27 | ||||
| Women | 0.0 | 0.65⁎⁎ | 0.19 | |||||
| Long standing diabetes (n = 44) |
CRP | Men | – | 0.34 | − 0.01 | – | 0.02 | − 0.02 |
| Women | – | 0.63⁎⁎ | 0.42 | – | 0.64⁎⁎ | 0.42 | ||
| Leptin | Men | 0.34 | – | − 0.01 | 0.02 | – | − 0.04 | |
| Women | 0.63⁎⁎ | – | 0.31 | 0.64⁎⁎ | – | 0.29 | ||
| HSP70 | Men | − 0.01 | − 0.01 | – | − 0.02 | − 0.04 | – | |
| Women | 0.42 | 0.31 | – | 0.42 | 0.29 | – | ||
| Waist circumference | Men | 0.25 | 0.65⁎⁎ | − 0.23 | ||||
| Women | 0.25 | 0.42 | − 0.07 | |||||
| BMI | Men | 0.51⁎ | 0.62⁎⁎ | 0.02 | ||||
| Women | 0.01 | 0.21 | 0.03 | |||||
| Controls (n = 41) |
CRP | Men | – | 0.46⁎ | 0.11 | – | 0.36 | 0.23 |
| Women | – | 0.62⁎⁎ | 0.34 | – | 0.43⁎ | 0.31 | ||
| Leptin | Men | 0.46⁎ | – | − 0.04 | 0.36 | – | 0.07 | |
| Women | 0.62⁎⁎ | – | 0.03 | 0.43⁎ | – | − 0.07 | ||
| HSP70 | Men | 0.11 | − 0.04 | – | 0.23 | 0.07 | – | |
| Women | 0.34 | 0.03 | – | 0.31 | − 0.07 | – | ||
| Waist circumference | Men | 0.03 | 0.12 | 0.08 | ||||
| Women | 0.05 | 0.23 | 0.03 | |||||
| BMI | Men | 0.36 | 0.45 | − 0.23 | ||||
| Women | 0.53⁎⁎ | 0.64⁎⁎ | 0.14⁎⁎⁎ | |||||
P < 0.05.
P < 0.01.
P < 0.001.
Fig. 1.
Demonstrating the correlation coefficient between serum HSP70 and leptin levels in healthy controls, patients with newly diagnosed and patients with long standing diabetes; stratified according to gender. * P < 0.05.
7. Discussion
Here we showed the appearance of a positive correlation between leptin–HSP70 in women with type 2 diabetes. The correlation was highest in women with newly diagnosed diabetes and was attenuated in women who were on treatment. The significance of this correlation was only observed in women with type 2 diabetes. There was no correlation between leptin and HSP70 in men (both newly diagnosed and long standing disease).
There are contradictory results between leptin and HSP-70 correlation. Most of the studies which have shown a positive correlation are carried out in disease states. However, while some have shown a positive correlation, others have shown a negative one. In consistent with our findings Rong Quan and collaborators showed that leptin activates HSP70 expression in a dose dependent manner in MCF-7 breast cancer cell line (Xue et al., 2012). Similarly Bonior and collaborators showed that leptin administration stimulates the expression of HSP60 in pancreatic acinar and beta cell lines. Leptin protects pancreatic acinar cell line from the damage of acute pancreatitis (Bonior et al., 2006).
On the other hand, Figueiredo et al. showed that leptin administration would decrease food intake and HSP70 levels in the liver and hypothalamus of chickens but not in the muscle. The effect of leptin on HSP70 levels was independent of the inhibition of food intake (Figueiredo et al., 2007). In another study, geranylgeranylacetone (GGA) administration induced HSP70 expression in liver, which rendered physiologic protection against high fat diet in mice, while decreased leptin levels (Adachi et al., 2010). Sirotken et al. showed that leptin administration inhibited the transcription of HSP70 in ovarian follicular granulosa cells. Heat stress, but not malnutrition can promote depletion of HSP70 in ovarian cells, which is partly reversed by leptin (Sirotkin and Bauer, 2011).
Why there is a discrepancy in different studies reporting leptin–HSP70 correlation? We hypothesized that the answer is the duality of HSP70 behavior in different inflammatory or non-inflammatory environments (Nakhjavani et al., 2012). As stated, the positive correlation between leptin and HSP is observed in chronic inflammation such as type 2 diabetes (our findings) and breast cancer; while all other studies, which have shown a negative correlation, are carried out in the normal cells, without any chronic inflammatory state. We have previously shown the positive correlation of HSP70 with ADMA, a nitric oxide inhibitor, in chronic inflammatory states while it was negatively correlated to ADMA in normal physiology (Nakhjavani et al., 2012). This may explain the discrepancy of leptin–HSP70 correlation in different studies, as they have different inflammatory states.
One could argue that this correlation is only present in women, while we do not observe such a correlation in men. Both leptin and HSP70 have been shown to be higher in women (Nakhjavani et al., 2010a, 2010b; Nakhjavani et al., 2011; Rossi et al., 2012; Samara et al., 2010). Leptin plays a crucial role in modulating immune response. Earlier studies on leptin deficient mouse have shown marked reduced number of lymphocytes with impaired humoral response and thymic atrophy (Gruver and Sempowski, 2008; Mancuso et al., 2002). Lipoatrophy in HIV infected patients is assumed to be a result of reduced leptin in these patients (Papaevangelou et al., 2007). Serum leptin levels were significantly elevated in patients with multiple myeloma and chronic lymphatic leukemia (Pamuk et al., 2006). Serum leptin levels are increased in women with type 2 diabetes (Nakhjavani et al., 2010a, 2010b). Why is it so? The answer depends on one's view of gender difference in type 2 diabetes. One explanation was that a greater adipose tissue in women is the underlying reason (Rossi et al., 2012). On the other hand, studies have repeatedly shown that women with type 2 diabetes and metabolic syndrome have higher serum leptin levels, after adjustment for body fat composition (Meyers et al., 2008; Thomas et al., 2000). The other explanation could be that an increased serum leptin level is associated with accelerated immune cell activation in women with type 2 diabetes. Interestingly we have previously shown that women with long standing diabetes have higher serum HSP70 levels (Nakhjavani et al., 2010a, 2010b). Rodrigues-Krause and collaborators have suggested a role of BMI on extracellular HSP70 levels (Rodrigues-Krause et al., 2012). After adjustment for BMI, leptin–HSP70 correlation remained significant. Glucose lowering therapy increased leptin and did not decrease HSP70 levels. Moreover while intracellular HSP70 induction in response to pro-inflammatory stimuli can exert anti-inflammatory effects, extracellular HSPs may signal danger, activating immune cells (Atalay et al., 2009; Blasi, 2008). Since immune system is more active in women compared to men (De Leon-Nava and Morales-Montor, 2006; Grimaldi, 2006), one would argue that the observed correlation between serum HSP70 and leptin implies a higher state of chronic inflammation. In consistent with our findings Zulkit et al. showed that food restriction induces leptin and HSP70 protein expression in female broiler chicken (Zulkifli et al., 2002).
The principal limitation of the present study is its cross sectional nature which precludes the determination of the direction of causality, however we took advantage of a relatively large sample size and close similarity between groups in most of the potentially confounding variables. In conclusion we showed the appearance of leptin–HSP70 correlation in women with diabetes, which was attenuated after treatment.
Acknowledgments
This study is not financially supported by any institution.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Manouchehr Nakhjavani, Email: nakhjavanim@tums.ac.ir.
Afsaneh Morteza, Email: aafsaneh03@gmail.com.
Arash Aghajani Nargesi, Email: arash.aan@gmail.com.
Ebrahim Mostafavi, Email: eb_mostafavi@yahoo.com.
Alireza Esteghamati, Email: esteghamati@tums.ac.ir.
References
- Adachi H., Kondo T., Ogawa R., Sasaki K., Morino-Koga S., Sakakida M., Kawashima J., Motoshima H., Furukawa N., Tsuruzoe K., Miyamura N., Kai H., Araki E. An acylic polyisoprenoid derivative, geranylgeranylacetone protects against visceral adiposity and insulin resistance in high-fat-fed mice. Am. J. Physiol. Endocrinol. Metab. 2010;299:E764–E771. doi: 10.1152/ajpendo.00075.2010. [DOI] [PubMed] [Google Scholar]
- Atalay M., Oksala N., Lappalainen J., Laaksonen D.E., Sen C.K., Roy S. Heat shock proteins in diabetes and wound healing. Curr. Protein Pept. Sci. 2009;10:85–95. doi: 10.2174/138920309787315202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi C. The autoimmune origin of atherosclerosis. Atherosclerosis. 2008;201:17–32. doi: 10.1016/j.atherosclerosis.2008.05.025. (Epub 2008 May 23) [DOI] [PubMed] [Google Scholar]
- Bonior J., Jaworek J., Konturek S.J., Pawlik W.W. Leptin is the modulator of HSP60 gene expression in AR42J cells. J. Physiol. Pharmacol. 2006;57(Suppl. 7):135–143. [PubMed] [Google Scholar]
- Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V., Cornelius C., Cuzzocrea S., Iavicoli I., Rizzarelli E., Calabrese E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol. Aspects Med. 2011;32:279–304. doi: 10.1016/j.mam.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Dardeno T.A., Chou S.H., Moon H.S., Chamberland J.P., Fiorenza C.G., Mantzoros C.S. Leptin in human physiology and therapeutics. Front. Neuroendocrinol. 2010;31:377–393. doi: 10.1016/j.yfrne.2010.06.002. (Epub 2010 Jun 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon-Nava M.A., Morales-Montor J. Immune sexual dimorphism: can sex steroids affect the Th1/Th2 cytokine profile? Rev. Invest. Clin. 2006;58:161–169. [PubMed] [Google Scholar]
- Figueiredo D., Gertler A., Cabello G., Decuypere E., Buyse J., Dridi S. Leptin downregulates heat shock protein-70 (HSP-70) gene expression in chicken liver and hypothalamus. Cell Tissue Res. 2007;329:91–101. doi: 10.1007/s00441-007-0414-6. [DOI] [PubMed] [Google Scholar]
- Grimaldi C.M. Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive B cells. Curr. Opin. Rheumatol. 2006;18:456–461. doi: 10.1097/01.bor.0000240354.37927.dd. [DOI] [PubMed] [Google Scholar]
- Gruver A.L., Sempowski G.D. Cytokines, leptin, and stress-induced thymic atrophy. J. Leukoc. Biol. 2008;84:915–923. doi: 10.1189/jlb.0108025. (Epub 2008 May 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar B., Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliv. Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. (Epub 2009 Feb 25) [DOI] [PubMed] [Google Scholar]
- Lam Q.L., Lu L. Role of leptin in immunity. Cell. Mol. Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- Lord G.M., Matarese G., Howard J.K., Baker R.J., Bloom S.R., Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Mancuso P., Gottschalk A., Phare S.M., Peters-Golden M., Lukacs N.W., Huffnagle G.B. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J. Immunol. 2002;168:4018–4024. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- Meyers J.A., Liu A.Y., McTiernan A., Wener M.H., Wood B., Weigle D.S., Sorensen B., Chen-Levy Z., Yasui Y., Boynton A., Potter J.D., Ulrich C.M. Serum leptin concentrations and markers of immune function in overweight or obese postmenopausal women. J. Endocrinol. 2008;199:51–60. doi: 10.1677/JOE-07-0569. (Epub 2008 Jul 9) [DOI] [PubMed] [Google Scholar]
- Molvarec A., Rigo J., Jr., Lazar L., Balogh K., Mako V., Cervenak L., Mezes M., Prohaszka Z. Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones. 2009;14:151–159. doi: 10.1007/s12192-008-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvarec A., Szarka A., Walentin S., Beko G., Karadi I., Prohaszka Z., Rigo J., Jr. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod. Biol. Endocrinol. 2011;9:124. doi: 10.1186/1477-7827-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M., Esteghamati A., Tarafdari A.M., Nikzamir A., Ashraf H., Abbasi M. Association of plasma leptin levels and insulin resistance in diabetic women: a cross-sectional analysis in an Iranian population with different results in men and women. Gynecol. Endocrinol. 2010:17. doi: 10.3109/09513590.2010.487583. [DOI] [PubMed] [Google Scholar]
- Nakhjavani M., Morteza A., Meysamie A., Esteghamati A., Khalilzadeh O., Esfahanian F., Khajeali L., Feiz F. Serum heat shock protein 70 and oxidized LDL in patients with type 2 diabetes: does sex matter? Cell Stress Chaperones. 2010:26. doi: 10.1007/s12192-010-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M., Morteza A., Asgarani F., Mokhtari A., Esteghamati A., Khalilzadeh O., Rahbari G. Metformin restores the correlation between serum-oxidized LDL and leptin levels in type 2 diabetic patients. Redox Rep. 2011;16:193–200. doi: 10.1179/1351000211Y.0000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M., Morteza A., Asgarani F., Khalilzadeh O., Ghazizadeh Z., Bathaie S.Z., Esteghamati A. The dual behavior of heat shock protein 70 and asymmetric dimethylarginine in relation to serum CRP levels in type 2 diabetes. Gene. 2012;498:107–111. doi: 10.1016/j.gene.2012.01.085. [DOI] [PubMed] [Google Scholar]
- Pamuk G.E., Demir M., Harmandar F., Yesil Y., Turgut B., Vural O. Leptin and resistin levels in serum of patients with hematologic malignancies: correlation with clinical characteristics. Exp. Oncol. 2006;28:241–244. [PubMed] [Google Scholar]
- Papaevangelou V., Papassotiriou I., Vounatsou M., Chrousos G., Theodoridou M. Changes in leptin serum levels in HIV-infected children receiving highly active antiretroviral therapy. Scand. J. Clin. Lab. Invest. 2007;67:291–296. doi: 10.1080/00365510601045062. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Krause J., Krause M., O'Hagan C., De Vito G., Boreham C., Murphy C., Newsholme P., Colleran G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17:293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi I.A., Bochud M., Bovet P., Paccaud F., Waeber G., Vollenweider P., Taffe P. Sex difference and the role of leptin in the association between high-sensitivity C-reactive protein and adiposity in two different populations. Eur. J. Epidemiol. 2012;27:379–384. doi: 10.1007/s10654-012-9671-0. [DOI] [PubMed] [Google Scholar]
- Samara A., Herbeth B., Aubert R., Berrahmoune H., Fumeron F., Siest G., Visvikis-Siest S. Sex-dependent associations of leptin with metabolic syndrome-related variables: the Stanislas study. Obesity (Silver Spring) 2010;18:196–201. doi: 10.1038/oby.2009.156. [DOI] [PubMed] [Google Scholar]
- Seo S., Guo D.F., Bugge K., Morgan D.A., Rahmouni K., Sheffield V.C. Requirement of Bardet–Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin A.V., Bauer M. Heat shock proteins in porcine ovary: synthesis, accumulation and regulation by stress and hormones. Cell Stress Chaperones. 2011;16:379–387. doi: 10.1007/s12192-010-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Burguera B., Melton L.J., III, Atkinson E.J., O'Fallon W.M., Riggs B.L., Khosla S. Relationship of serum leptin levels with body composition and sex steroid and insulin levels in men and women. Metabolism. 2000;49:1278–1284. doi: 10.1053/meta.2000.9519. [DOI] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R.Q., Gu J.C., Yu W., Wang Y., Zhang Z.T., Ma X.M. Effects of exogenous human leptin on heat shock protein 70 expression in MCF-7 breast cancer cells and breast carcinoma of nude mice xenograft model. Chin. Med. J. (Engl) 2012;125:680–686. [PubMed] [Google Scholar]
- Zulkifli I., Che Norma M.T., Israf D.A., Omar A.R. The effect of early-age food restriction on heat shock protein 70 response in heat-stressed female broiler chickens. Br. Poult. Sci. 2002;43:141–145. doi: 10.1080/00071660120109953. [DOI] [PubMed] [Google Scholar]