Abstract
An undifferentiated embryonal sarcoma of the liver is a rare malignant tumour. We highlight the diagnostic dilemma and differential diagnosis of a case involving a large cystic liver lesion in a young adult. A 20-year-old man presented with a large liver lesion to the Universiti Kebangsaan Malaysia Medical Center, Kuala Lumpur, Malaysia, in February 2012. The initial clinico-radiological presentations were suggestive of a liver abscess. A total tumour resection was performed and the final histopathological results of the resected specimen indicated an undifferentiated embryonal sarcoma of the liver. The ultrasound and computed tomographic images of the tumour were reviewed and found to be contradictory in appearance, as the tumour seemed predominantly solid in the ultrasound image and predominantly cystic in the computed tomographic image. Familiarisation with the imaging appearance of this tumour and a high index of suspicion is therefore crucial in making a successful diagnosis.
Keywords: Sarcoma, Liver Abscess, Ultrasound Imaging, X-Ray Computed Tomography, Case Report, Malaysia
Undifferentiated embryonal sarcoma (UES) of the liver is a rare malignant tumour with a primitive mesenchymal phenotype. It usually occurs in older children, with the peak age of presentation occurring in individuals between 6 and 10 years old.1,2 Despite its rarity, UES is an important but often overlooked differential diagnosis for a large cystic liver lesion in young adults. The diagnosis relies on a postoperative pathological analysis and immunohistochemical results. Familiarisation with the imaging findings is useful for a preoperative diagnosis and to avoid delay in commencing appropriate management. This paper presents a case of a UES of the liver mimicking a liver abscess in its initial clinico-radiological presentation.
Case Report
A 20-year-old immunocompetent man was admitted to the Universiti Kebangsaan Malaysia Medical Center in Kuala Lumpur, Malaysia, in February 2012. He presented with a two-week history of pain in the right hypochondriac region, intermittent high-grade fever, nausea and vomiting. He had no history of hepatitis B or C viral infections and denied any recent history of travelling. A physical examination revealed gross hepatomegaly.
A routine liver function test was unremarkable, with the white blood cell count in the upper limits of normal (9.4 × 109/L). The erythrocyte sedimentation rate, C-reactive protein and carbohydrate-associated antigen (CA)-19.9 were elevated, at 118 mm/hour, 6.4 mg/dL and 808 U/mL, respectively. The rest of the tumour markers, including the carcinoembryonic antigen and alpha-fetoprotein (AFP) markers, were within normal limits. All of the blood cultures were negative. Virology screening tests for the hepatitis viruses and a serology test for echinococcosis were also negative.
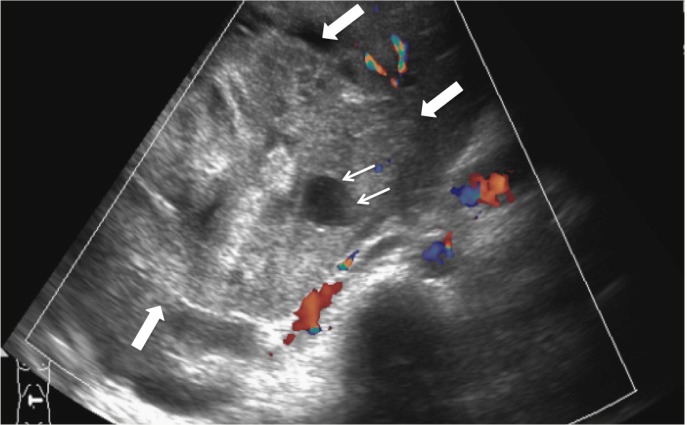
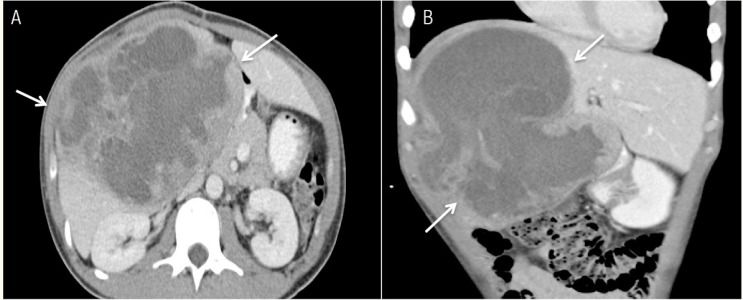
Ultrasonography (US) of the hepatobiliary system revealed a large, well-defined heterogeneous lesion in the right lobe of the liver [Figure 1]. A few anechoic locules were also noted within the lesion. There was no evidence of cirrhosis of the underlying liver parenchyma. A subsequent contrast-enhanced abdominal computed tomography (CT) scan performed on the same day showed a predominantly cystic lesion, with thick and irregular wall and internal septations. The cystic component had a Hounsfield unit (HU) of 18–30 HU, which was in keeping with complex fluid [Figures 2A & B]. In view of the patient’s age, fever, raised inflammatory markers and the cystic liver lesion observed via CT imaging, a preliminary diagnosis of a liver abscess was made. The solid components seen on the US images were thought to be unliquefied parts of the abscess.
Figure 1:
Ultrasound image of the liver showing a heterogenous hypovascular solid mass (thick arrows) with a small anechoic locule (thin arrows).
Figure 2 A & B:
Abdominal contrast-enhanced computed tomography images in both (A) axial and (B) coronal views, showing a large lesion (arrows) of predominantly cystic appearance with irregular wall and internal septations in the right lobe of the liver.
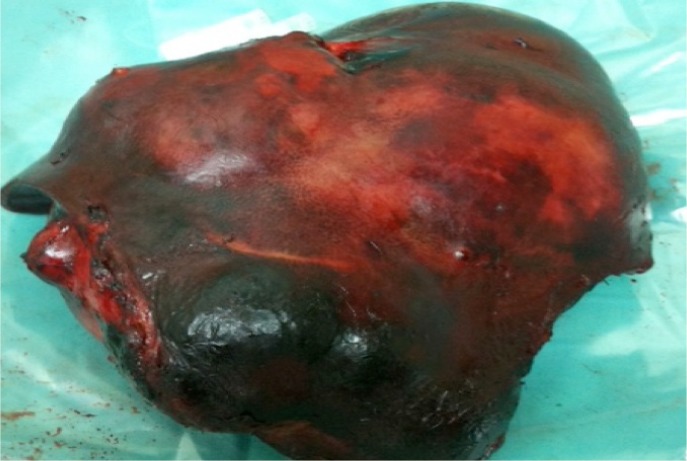
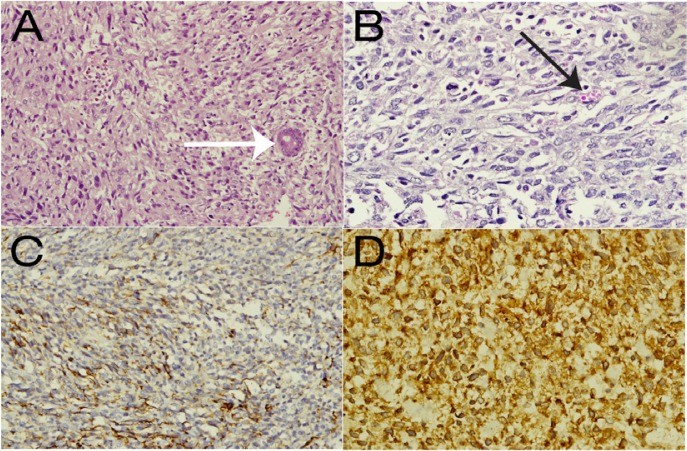
A US-guided pigtail drainage procedure was planned; however, aspiration from the initial puncture revealed minimal blood with no pus. Following this, a percutaneous liver biopsy of the lesion was performed instead. The histopathological results of the specimen were compatible with a non-specific malignant tumour. Subsequently, the patient underwent an extended right hepatectomy. Intraoperatively, a huge right lobe liver mass was revealed [Figure 3]. Cut sections of the resected tumour demonstrated extensive central necrosis and haemorrhage. Microscopically, the tumour was separated from the surrounding liver parenchyma by a dense sclerotic band. The tumour was composed of pleomorphic cells with spindle and satellite shapes, which were distributed in myxoid stroma [Figure 4A]. Some cells contained intracytoplasmic eosinophilic globules [Figure 4B], which were periodic acid-Schiff-positive and diastase labile. The tumour cells stained positively for desmin [Figure 4C], myogenic differentiation 1 protein [Figure 4D] and cluster of differentiation 10. The surgical margin was clear.
Figure 3:
Photo of the resected right liver lobe containing the liver mass.
Figure 4 A–D:
Images of the histological specimen. Haematoxylin and eosin stains showed (A) a sheet of round to spindle-shaped malignant cells with an entrapped bile duct (white arrow) at ×20 magnification and (B) the typical presence of cytoplasmic eosinophilic hyaline globules (black arrow) at ×40 magnification. Immunohistochemistry showed positivity for (C) desmin and (D) myogenic differentiation antigen 1 at ×40 magnification.
Postoperatively, the patient developed intra-abdominal sepsis and ascending colon bowel necrosis, requiring relaparotomy and a right hemicolectomy. The bowel necrosis was due to blood pressure-related ischaemia due to the prolonged retraction during the initial surgery. The patient was discharged 15 days after the operation. A follow-up CT scan performed six months later showed a large tumour recurrence in the residual left liver lobe. His prognosis was not favourable and no surgery was attempted due to the small residual liver volume. As a result, palliative chemotherapy using doxorubicin was the only option for treatment. Ideally, a liver transplantation should be considered in cases such as this; however, a living donor liver transplant programme is not yet in place for the adult population in Malaysia.
Discussion
A UES of the liver was first described in 1978.3 Although it is commonly seen in children, many cases have been reported among adult patients. There is a preponderance of the tumour to involve the right lobe of the liver.4 The tumour is usually large at presentation, often exceeding 10 cm, and can be as large as 30 cm. The macroscopic examination usually shows a well-demarcated soft mass that frequently has gelatinous, cystic, haemorrhagic and necrotic foci. Microscopically, the tumour is typically composed of spindle, oval or stellate cells, with ill-defined borders that are embedded in an abundant myxoid stroma.5
While there are no specific clinical features at presentation, tumour-related symptoms include abdominal mass, pain and weight loss. Fever is not unusual and has been reported in previous cases without any superimposed infections.1,6,7 The fever is likely related to the haemorrhage and necrosis found in the majority of these tumours, as exemplified in the present case, which may lead to an erroneous preliminary diagnosis of an underlying infective process. Usually, laboratory studies are non-specific and the tumour markers are not increased.8 This is contrary to the present case, where the CA-19.9 was noted to be significantly high.
Abdominal radiographs of patients with UES are typically normal. However, the sarcoma is large enough to be detected by US, CT or magnetic resonance imaging (MRI). These imaging techniques are useful to assess the extension of the tumour, including information regarding any associated vascular invasion, biliary obstruction or hilar adenopathies. While US is useful in demonstrating the solid nature of the tumour, CT and MRI findings can resemble those of cystic lesions. The predominant cystic appearance is related to the high water content of the abundant myxoid stroma within the tumour.9 Hence, findings of a large hepatic lesion with a seemingly cystic appearance on CT or MRI images and a paradoxically solid appearance on US images are highly suggestive of this tumour.9,10 Similar findings were observed in the present case but were only noted to be of significance in retrospect. The features depicted on the CT and the US images were later perceived to be non-specific. While a positron emission tomography scan is possible in cases of UES, its findings would also prove to be non-specific as the uptake of fluorodeoxyglucose would occur mostly in the periphery of the tumour.2,11 Cases of UES may be mistaken for a benign inflammatory pathology such as a hydatid cyst or pyogenic abscess.12
Most cases of embryonal sarcomas mimic hydatid cysts. The clinical symptoms and radiological images often overlap between these two diagnoses. Various reports have discussed this diagnostic dilemma,7,9,10 however the prevalence of hydatid disease in Malaysia is uncommon, especially in urban areas.13 Furthermore, Malaysia is considered a non-endemic area for echinococcus.13 In the current patient, there were no visible daughter lesions or peripheral calcifications, which are the hallmarks of a hydatid cyst.5 In addition, no splenic lesions were detected.5
Other differential diagnoses for a large, solitary, predominantly cystic liver lesion observed on a CT included a necrotic hepatocellular carcinoma, pyogenic abscess, chronic haematoma and a cavernous haemangioma. A pyogenic abscess or hydatid cyst were initially suspected in view of the pyrexia and raised inflammatory markers;2,3,7,10 however, cultures and serology tests were negative for echinococcus. Hepatocellular carcinoma was less likely considering the patient’s age, normal AFP level and the lack of underlying liver cirrhosis or viral hepatitis infection. There was also no history of trauma to support a diagnosis of chronic haematoma.
A UES is a rare mesenchymal tumour and usually a diagnosis of exclusion and histopathology are the determining factors in its diagnosis. Diagnoses such as a ruptured hydatid cyst, primary liver cancer or abscesses are more common than UES. In the current case, as the patient was of a young age and presented with both fever and pain, a pyogenic abscess was considered to be the most likely working diagnosis.
While the prognosis for a UES of the liver is poor, cases of long-term survival have been reported after complete surgical resection with adjuvant chemotherapy.14,15 Early and prompt diagnosis and therapy are therefore crucial. The prognosis is not related to the size and degree of differentiation of the UES, but to the degree of invasion and metastasis.2
Conclusion
A UES of the liver can be difficult to diagnose and both the clinical presentation and imaging appearance of this sarcoma may be misleading. Any discordance between CT and US images regarding the appearance of a large liver lesion, i.e. if the liver appears solid via US but predominantly cystic with CT imaging, should raise the possibility of UES as the diagnosis with a high index of suspicion.
References
- 1.Noguchi K, Yokoo H, Nakanishi K, Kakisaka T, Tsuruga Y, Kamachi H, et al. A long-term survival case of adult undifferentiated embryonal sarcoma of liver. World J Surg Oncol. 2012;10:65. doi: 10.1186/1477-7819-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao J, Fei L, Li S, Cui K, Zhang J, Yu F, et al. Undifferentiated embryonal sarcoma of the liver in a child: A case report and review of the literature. Oncol Lett. 2013;5:739–42. doi: 10.3892/ol.2012.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: Report of 31 cases. Cancer. 1978;42:336–48. doi: 10.1002/1097-0142(197807)42:1<336::AIDCNCR2820420151>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Chen JH, Lee CH, Wei CK, Chang SM, Yin WY. Undifferentiated embryonal sarcoma of the liver with focal osteoid picture: A case report. Asian J Surg. 2013;36:174–8. doi: 10.1016/j.asjsur.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Buetow PC, Buck JL, Pantongrag-Brown L, Marshall WH, Ros PR, Levine MS, et al. Undifferentiated (embryonal) sarcoma of the liver: Pathologic basis of imaging findings in 28 cases. Radiology. 1997;203:779–83. doi: 10.1148/radiology.203.3.9169704. [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Tireno B, Slanetz PJ. Undifferentiated embryonal sarcoma of the liver. AJR Am J Roentgenol. 2008;190:W261–2. doi: 10.2214/AJR.07.3058. [DOI] [PubMed] [Google Scholar]
- 7.Kalra N, Vyas S, Jyoti Das P, Kochhar R, Srinivasan R, Khandelwal N. Undifferentiated embryonal sarcoma of liver in an adult masquerading as complicated hydatid cyst. Ann Hepatol. 2011;10:81–3. [PubMed] [Google Scholar]
- 8.Tokunaga Y, Ryo J, Hoppou T, Kitaoka A, Tokuka A, Osumi K, et al. Hepatic undifferentiated (embryonal) sarcoma in an adult: A case report and review of the literature. Eur J Gastroenterol Hepatol. 2000;12:1247–51. doi: 10.1097/00042737-200012110-00014. [DOI] [PubMed] [Google Scholar]
- 9.Crider MH, Hoggard E, Manivel JC. Undifferentiated (embryonal) sarcoma of the liver. Radiographics. 2009;29:1665–8. doi: 10.1148/rg.296085237. [DOI] [PubMed] [Google Scholar]
- 10.Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: Differential CT and MR imaging features. Radiographics. 2001;21:895–910. doi: 10.1148/radiographics.21.4.g01jl16895. [DOI] [PubMed] [Google Scholar]
- 11.Lee MK, Kwon CG, Hwang KH, Choe W, Kim JE, Tchah H, et al. F-18 FDG PET/CT findings in a case of undifferentiated embryonal sarcoma of the liver with lung and adrenal gland metastasis in a child. Clin Nucl Med. 2009;34:107–8. doi: 10.1097/RLU.0b013e318192c36b. [DOI] [PubMed] [Google Scholar]
- 12.Kwon ON, Kim SH, Shin JY, Shin HW. Undifferentiated embryonal sarcoma of the liver in an adult: The verification of the high growth rate in the tumor. J Korean Soc Radiol. 2013;69:457–60. doi: 10.3348/jksr.2013.69.6.457. [DOI] [Google Scholar]
- 13.Kutty MK, Krishnan M, Nambiar B. A case of pulmonary hydatid disease. Med J Malaya. 1970;24:302–5. [PubMed] [Google Scholar]
- 14.Kim DY, Kim KH, Jung SE, Lee SC, Park KW, Kim WK. Undifferentiated (embryonal) sarcoma of the liver: Combination treatment by surgery and chemotherapy. J Pediatr Surg. 2002;37:1419–23. doi: 10.1053/jpsu.2002.35404. [DOI] [PubMed] [Google Scholar]
- 15.Baron PW, Majlessipour F, Bedros AA, Zuppan CW, Ben-Youssef R, Yanni G, et al. Undifferentiated embryonal sarcoma of the liver successfully treated with chemotherapy and liver resection. J Gastrointest Surg. 2007;11:73–5. doi: 10.1007/s11605-006-0044-4. [DOI] [PubMed] [Google Scholar]