Abstract
Pyrethroid resistance is becoming a major problem for vector control programs, because at present, there are few suitable chemical substitutes for pyrethroids, as when used on bed nets the insecticide must have low mammalian toxicity as well as high activity to mosquitoes. Pyriproxyfen (PPF) is one of the most active chemicals among the juvenile hormone mimic (JHM) group. Sterilizing mosquitoes by using PPF could be a potential control measure for pyrethroid-resistant malaria vectors. We investigated the sterilizing effects of two types of PPF-impregnated bed nets – a 1% PPF-impregnated net and a 1% PPF +2% permethrin-impregnated net (Olyset Duo) – to pyrethroid-resistant wild population of Anopheles gambiae s.s. in western Kenya. High mortality of blood-fed mosquitos was observed 3 days post-collection, in the houses where PPF-impregnated nets were used, indicating the effect of PPF on the longevity of mosquitos that came in contact with the net. Reduction in the number of ovipositing females, number of eggs, and number of progeny per female were also observed in the houses in which both Olyset Duo and PPF-impregnated nets were used. This is the first field study showing the high sterilizing efficacy of PPF against wild pyrethroid-resistant An. gambiae s.s. population. In addition, we recognized the necessity of combined use of permethrin with PPF, in order to reduce the risk of mosquito bites and provide a level of personal protection. Further studies on wild pyrethroid-resistant mosquito populations such as An. arabiensis and An. funestus s.s. would provide more information on the practical use of the PPF-impregnated bed nets.
Introduction
After more than 30 years since the introduction of permethrin, synthetic pyrethroids have emerged as the newest class of insecticides in the vector control program [1]. On a global level, pyrethroids constitute approximately 81% of the spray utility, among which 68% is used for residual spraying and 24% for space spraying, and 100% of WHO-recommended insecticides for the treatment of long lasting mosquito nets (LNs) [2]. The resistance of vector mosquitoes is one of the main obstacles in effective vector control, because currently, there are no suitable substitutes for pyrethroids [1]. More than 90% of the current annual malaria incidence occurs in Africa. Anopheles gambiae Giles (An. gambiae s.s.), Anopheles arabiensis Patton and Anopheles funestus Giles (An. funestus s.s.) are the primary malaria vectors in sub-Saharan Africa. Recently, a causal relationship between the high coverage of LNs due to mass campaigns and the increase in the kdr frequency in An. gambiae s.s. have been reported [3], [4], [5]. In contrast, such kdr mutations do not seem to be common in An. arabiensis [6]–[8], but the metabolic resistance seems to be most common in this species [8], [9]. Fortunately, kdr mutations have not been reported in African malaria vectors except in these two species. Anopheles funestus s.s. is the major malaria vector in southern Africa. Re-emergence or increase of this species as well as the development of metabolic pyrethroid resistance in eastern and southern Africa have been reported in this species [9]–[15].
Juvenile hormone mimics (JHMs) developed from natural origins are one of the commercially successful chemicals in the insect growth regulators (IGRs) category, with a unique mode of action that is insect-specific, stage-specific, slow acting, and non-neurotoxic [16]. Methoprene [17] and pyriproxyfen [18] are the most successful JHMs. Such chemicals have been commonly used as larvicides, since most of the effects of JHMs are on the last instar larvae, which become deformed or die at the pupal stage [18]. Pyriproxyfen was reported to cause vacuolation, inhibited development of imaginal buds of Aedes aegypti (L) larvae, and histolysis such as disrupted mitochondria, abundant vacuoles and poorly structured cytoplasmic organelles [19]. Adult An. balabacensis Baisas, which survived 48 h of immersion in 0.005 ppb (one-eighth of LC50) of pyriproxyfen during the last larval instar, was found to show considerable reduction in sperm and egg production, as well as in blood feeding and mating activity [20].
JHMs also acted as a “sterilant” in adult insects. Methoprene was reported to affect ovarian development and adult longevity in Ae. aegypti [21], [22]. The number of eggs per female and hatchability decreased with the application of pyriproxyfen to female Ae. aegypti [23], [24], housefly (Musca domestica L.), and German cockroach (Blattella germanica L.) [25]. Aiku et al. [26] first demonstrated the use of pyriproxyfen-impregnated bed net as a sterilizing device for Anopheline mosquitoes. Ohashi et al. reported that the adult females of insecticide-susceptible An. gambiae strain were completely sterilized after exposure to 0.01% pyriproxyfen-treated nets (3.5 mg active ingredient [AI]/m2) before and after consuming blood. It was also seen that the adult longevity decreased by exposure to pyriproxyfen in a dose-dependent manner [27]. Similarly, Harris et al. reported that the laboratory colony of An. arabiensis, which blood-fed one day prior to pyriproxyfen exposure (3 mg [AI]/m2), produced no viable offspring, while other treatments (blood-fed 3 days before exposure, 1 day after exposure, and 3 days after exposure) had no significant effect [28].
The above findings suggested that sterilizing technique using pyriproxyfen could be one of the most effective measures for controlling pyrethroid-resistant malaria vectors. However, the field conditions in which people use bed nets and the wild population of malaria vectors come into contact with the bed nets voluntarily, have not been thoroughly studied. Here, we assessed the sterilizing effects of two types of pyriproxyfen-impregnated bed nets −1% pyriproxyfen-impregnated net and 1% pyriproxyfen +2% permethrin-impregnated net (Olyset Duo) – on a pyrethroid-resistant wild population of An. gambiae s.s. in western Kenya.
Materials and Methods
Bed nets
Three kinds of bed nets were provided by Sumitomo Chemical Co. Ltd. (Tokyo, Japan). The first one was impregnated with 1% pyriproxyfen (PPF), the second with 1% pyriproxyfen and 2% permethrin (Olyset Duo), and the third with 2% permethrin (Olyset Net). All bed nets were of the same size (130 cm [W]×180 cm [L]×150 cm [H]) and were made of polyethylene netting material (mesh 20 holes/cm2), with active ingredients incorporated into the polymer before monofilament yarn extrusion.
Study location
The study was conducted in Ragwe village, in the Suba south sub-county in Homabay county of Nyanza Province in Western Kenya. The Suba south sub-county has been identified as a high malaria transmission area in Kenya, with more than 50% of the population exposed to ≥40% Plasmodium falciparum parasite rate corrected to a standard age range of 2 years old to <10 years old [29]. The main malaria vectors in the area are An. gambiae s.s., An. arabiensis, and An. funestus s.s. Anopheles rivulorum Leeson, which belongs to the Funestus Group is a minor vector in that area [12]. These four species were recently reported to have developed multimodal pyrethroid resistance [9], [12]. Anopheles gambiae s.s. mainly dominated in Ragwe village [30].
Selection of houses and intervention with bed nets
Preliminary mosquito collection was done at the residential houses in Ragwe village and 15 houses, each with a relatively high mosquito density were selected. The houses had standard structures and sizes with eaves or gaps between the top of the wall and the roof, which was common in African houses. The number of people sleeping in bedrooms and living rooms, the number of bed nets (Olyset Net and Permanet) used before intervention, and the number of Olyset Net, Olyset Duo, and PPF-impregnated net after intervention are shown in Table 1.
Table 1. Information on the houses in which bed net interventions were conducted.
| House No. | No. of people sleepinga) | No. of bed nets used before intervention | No. of bed nets used after intervention | ||||
| Bedroom | Living room | Olyset Net | Permanet | Olyset Net (Oly) | Olyset Duo (Duo) | Pyriproxyfen (PPF) | |
| RAG002 | 2A | 3C | 0 | 3 | 2 | - | - |
| RAG004 | 2A | 1A+1C | 2 | 0 | - | 3 | - |
| RAG005 | 2A | 0 | 2 | 0 | - | - | 2 |
| RAG007 | 2A | 3C | 2 | 0 | 2 | - | - |
| RAG008 | 2A | 2C | 2 | 0 | - | 2 | - |
| RAG009 | 3A | 3C | 4 | 0 | - | - | 3 |
| RAG010 | 1A | 1C | 0 | 2 | 2 | - | - |
| RAG011 | 3A | 4C | 2 | 0 | - | 4 | - |
| RAG014 | 1A | 2C | 1 | 0 | - | - | 2 |
| RAG020 | 1A | 1A+1C | 0 | 2 | 1 | - | - |
| RAG023 | 1A | 1C | 1 | 0 | - | 2 | - |
| RAG027 | 2A | 1C | 2 | 0 | - | - | 2 |
| RAG028 | 2A | 4C | 2 | 0 | 2 | - | - |
| RAG029 | 1A | 6C | 1 | 0 | - | 1 | - |
| RAG030 | 2A | 0 | 1 | 1 | - | - | 2 |
A, adults (age>15 years); C, children (age ≤15 years).
New bed nets were supplied and hung in houses in sufficient numbers to cover all residents while sleeping. The distance between each house was 100–500 m. For each type bed net intervention, 5 houses were used. The house residents were informed about the study and their written consent was obtained before conducting the interventions on April 27 and 28, 2013.
Mosquito collection
Pre- and post-intervention mosquito collections were done in the morning (07∶00–09∶00) by three groups consisting of three staffs each, for ca. 20 min per house. Three clusters consisting of five houses each in the vicinity were arranged and three collection groups rotated the clusters daily. Mosquito collections were performed four times prior to intervention (April 22, 24, 25, and 26, 2013) and four times after intervention (April 29, 30, May 1, and 2, 2013). Anopheline mosquitoes resting on the walls, under furniture, or anywhere else in the house were collected using a battery-powered aspirator (C-cell aspirator, BioQuip Products, CA, USA). The nozzles and containers of the aspirators were changed for the collection in the houses with Olyset Duo and PPF-impregnated net to avoid contamination. The collected mosquitoes were examined microscopically in a laboratory to distinguish the Anopheles gambiae complex from other anopheline species, based on the identification keys of Gillies and Coetzee [31]. Individual species within An. gambiae s.l. were identified using the multiplex polymerase chain reaction (PCR) method described by Scott et al. [32].
Observation of egg production, hatchability, and longevity of blood-fed females
After the collection of mosquitoes, freshly blood-fed female mosquitoes were individually held in a 20-mL glass vial with a small amount of water (1–2 mL) and damp filter paper as an oviposition substrate until egg production or death occurred. The presence and the number of eggs oviposited were recorded daily and the whole egg batch was transferred to a paper cup (ca. 50 mL) with water to allow hatching. A daily record of egg batch hatching was conducted. It required 7–21 days to complete the observation, since egg hatching did not take place simultaneously. After collection of eggs and bodies, mosquitoes were identified by PCR as previously described. Ohashi et al. reported that the mean longevity of An. gambiae s.s. females exposed to the 0.1% PPF-treated nets were 5.6 days [27]. The mean longevity of the blood-fed females contacted 1% PPF-treated nets was thought to be less than 5.6 days. On the other hand, most blood-fed mosquitoes normally lay eggs in less than 4 days after blood meals and they sometimes die after oviposition. Therefore, in the present trial, the deaths of field-collected blood-fed females within 3 days were judged as the deaths caused by the effect of PPF and the comparison of the mortality between PPF-exposed mosquitoes (Olyset Duo and PPF-impregnated net) and unexposed ones (Olyset Net) was done.
Detection of Point Mutations in the Voltage-Gated Sodium Channel in An. gambiae s.s
PCR and direct DNA sequencing were performed to identify point mutations at 1014L in the field-collected mosquitoes according to the method by Kawada et al. [10], [11]. Direct DNA sequencing was performed using the 3730 DNA Analyzer (Applied Biosystems). The electropherogram of the targeted amino acid replacement was analyzed using MEGA 4.0 public domain software. The unique DNA haplotype sequences were deposited into the GenBank.
Data analysis
A generalized linear mixed model (GLMM) using the Poisson distribution was used to examine the number of mosquitoes collected before and after bed net intervention by using the package lme4 in R (http://www.R-project.org). The dates of collection and the houses were used as random effects. Comparisons of the total number of blood-fed and unfed females, the total number of dead and alive blood-fed females within 3 days after collection, and the total number of oviposited and non-oviposited blood-fed females before and after intervention of bed nets were performed with χ2 test using JMP 7.0J (SAS Institute Japan Inc., Tokyo, Japan). Kruskal–Wallis one-way analysis of variance was used to compare the number of oviposited eggs, hatchability, and the number of progenies before and after intervention.
Ethics Statement
The protocol for the study (Case No. 2522) was approved by the Scientific Steering Committee and the National Ethics Review Committee of the Kenya Medical Research Institute. All necessary permits were obtained for the described field studies. No mosquito collection was done without the approval of the head of the village and the owner and occupants of the collection house.
Results
Effect of Olyset Net, Olyset Duo, and PPF-impregnated net on the number of collected female An. gambiae s.s
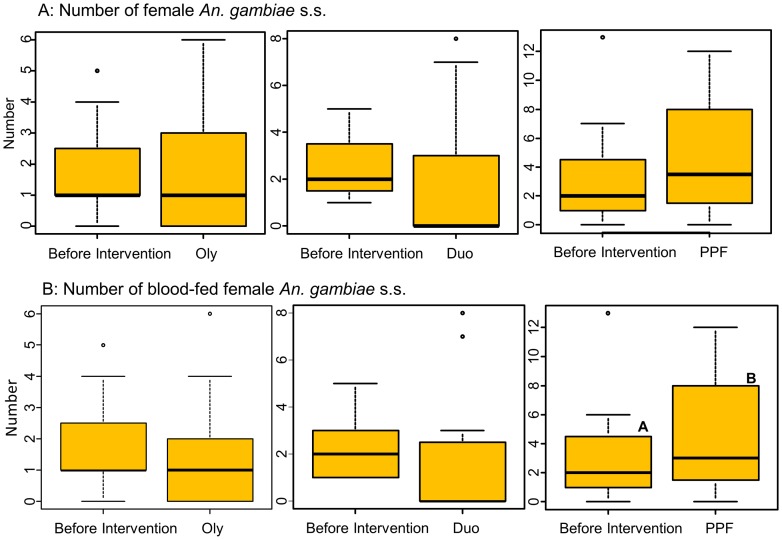
One hundred and forty-five female An. gambiae s.s. and 5 female An. arabiensis were collected before the intervention, while 153 An. gambiae s.s. and 6 An. arabiensis were collected afterward. The allelic frequency of the point mutation in the voltage-gated sodium channel (L1014S) in An. gambiae s.s. collected was 98.4% (n = 96, Accession AB776705, AB776706). Average numbers of An. gambiae s.s. females collected in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net were 1.90, 2.37, and 3.26 per house/day before intervention, and 1.56, 1.55, and 4.70 per house/day after intervention, respectively. Generalized linear mixed model for the number of collection revealed that net-by-intervention (before and after) interaction (P = 0.0047) was significant, while net (P = 0.083) and intervention (P = 0.740) were not significant. Significant difference was not observed in the number of mosquitoes collected in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net (P>0.1 for Olyset Net and Olyset Duo, and P = 0.092 for PPF-impregnated net; Figure 1). Average numbers of blood-fed An. gambiae s.s. females collected in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net were 1.85, 2.26, and 3.16 per house/day before intervention, and 1.33, 1.45, and 4.35 per house/day after intervention, respectively. Generalized linear mixed model for the number of collection revealed that both net (P<0.001) and net-by-intervention (before and after) interaction (P = 0.0089) were significant, while intervention (P = 0.891) was not significant. Decreased number of blood-fed females were observed in the houses with Olyset Net and Olyset Duo, although these differences were not significant (P>0.1), while a significant increase was observed in the houses with PPF-impregnated net (P<0.0001; Figure 1).
Figure 1. Box-and-whisker plots (medians: thick lines, with inter-quartile ranges) of the number of Anopheles gambiae s.s. females collected in the houses with Olyset Net (Oly), Olyset Duo (Duo), and PPF-impregnated net (PPF), before and after intervention.
A, Average number of female An. gambiae s.s. females collected/house/day; B, Average number of blood-fed An. gambiae s.s. females collected/house/day. Different letters indicate significant difference by GLMM analysis.
Effect of Olyset Net, Olyset Duo, and PPF-impregnated net on blood feeding, longevity of blood-fed females, and oviposition of An. gambiae s.s
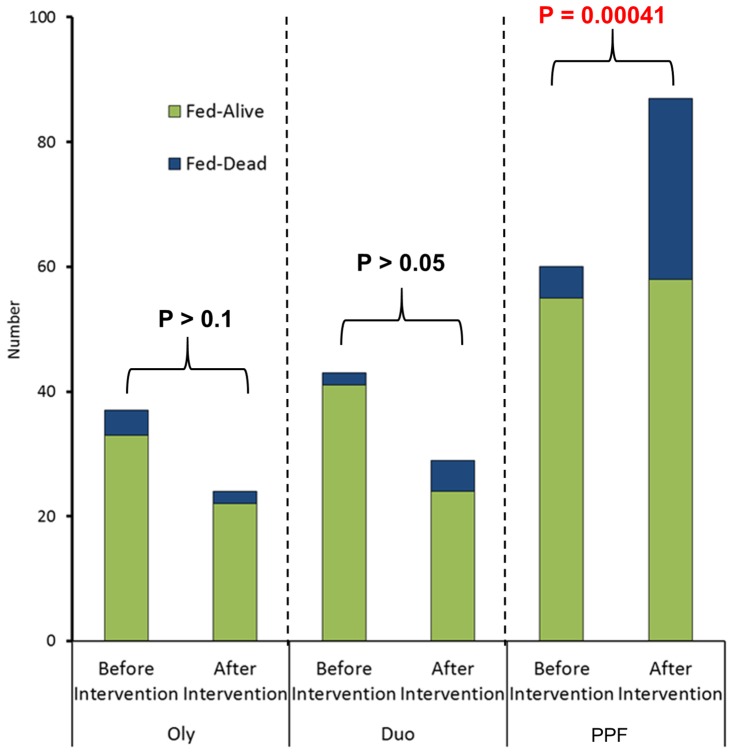
Mortality (3 days after collection) of the females in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net were 10.8%, 4.7%, and 8.3% before intervention, and 8.3%, 17.2%, and 33.3% after, respectively. The difference in the proportion of the total number of dead blood-fed females against the total number of live blood-fed females of An. gambiae s.s. before and after intervention of PPF-impregnated net was significant (P = 0.00041), while those in the houses with Olyset Net (P>0.1) and with Olyset Duo (P>0.05) were not significant (Figure 2).
Figure 2. Total number of dead (within 3 days after collection) (Fed-Dead) and alive (Fed-Alive) blood-fed females of Anopheles gambiae s.s. before and after intervention with Olyset Net (Oly), Olyset Duo (Duo), and PPF-impregnated net (PPF).
Figures indicate the P-value by χ2 test.
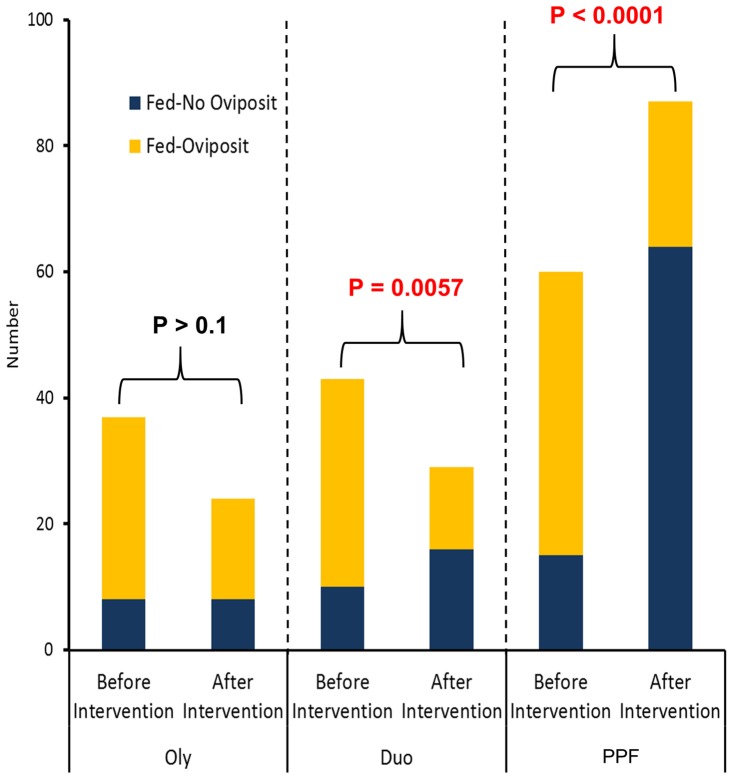
Percentage of the blood-fed females ovipositing in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net were 78.4%, 76.7%, and 75.0% before intervention, and 66.7%, 44.8%, and 26.4% after, respectively. Significant differences in the proportion of the total number of ovipositing blood-fed females vs. the total number of non-ovipositing blood-fed females of An. gambiae s.s. before and after intervention were observed in the houses with Olyset Duo (P = 0.0057) and PPF-impregnated net (P<0.0001), while that in the houses with Olyset Net was not significant (P>0.1) (Figure 3).
Figure 3. Total number of oviposited (Fed-Oviposit) and non-oviposited (Fed-No Oviposit) blood-fed females of Anopheles gambiae s.s. before and after intervention with Olyset Net (Oly), Olyset Duo (Duo), and PPF-impregnated net (PPF).
Figures indicate the P-value by χ2 test.
Effect of Olyset Net, Olyset Duo, and PPF-impregnated net on the fecundity of An. gambiae s.s
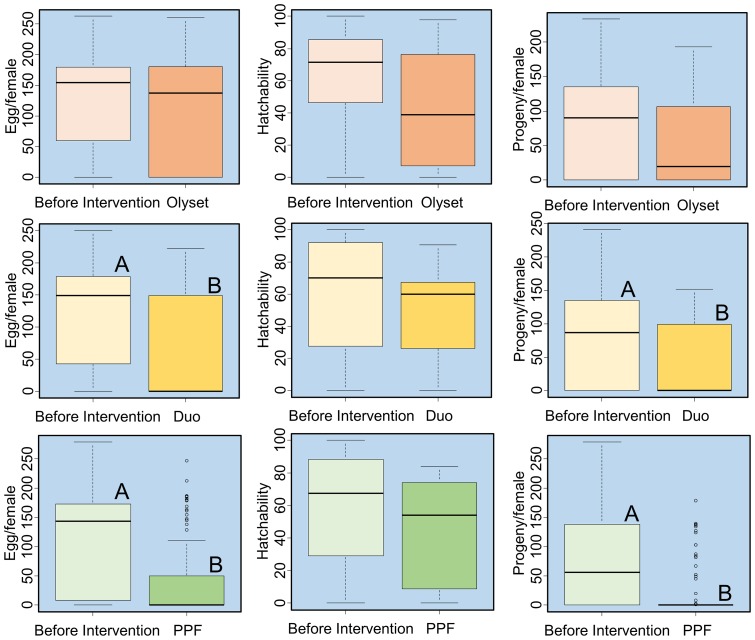
Average numbers of eggs oviposited before intervention in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net were 124.6, 126.9, and 118.1/female and those after intervention were 111.8, 66.1, and 39.5/female, respectively. The difference in the number of oviposited eggs among the pre-intervention and post-intervention houses was significant (P = 0.0008), and higher and significant reduction in the number of eggs were observed in the houses where Olyset Duo (P = 0.0083) and PPF-impregnated nets (P<0.0001) were used compared to that in the pre-intervention houses (Figure 4).
Figure 4. Box-and-whisker plots (medians: thick lines, with inter-quartile ranges) of the number of eggs oviposited, hatchability of eggs, and number of progenies produced by Anopheles gambiae s.s. females collected from houses with Olyset Net (Oly), Olyset Duo (Duo), and PPF-impregnated net (PPF), before and after intervention.
Different letters indicate significant difference by Kruskal-Wallis one-way analysis of variance.
Average hatchability of eggs oviposited by the blood-fed An. gambiae s.s. females collected before intervention in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net was 60.9%, 59.3%, and 57.9% and those in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net after intervention were 42.6%, 51.3%, and 42.9%, respectively. Hatchability in the pre-intervention houses and post-intervention houses was not significant (P = 0.852) (Figure 4).
Average numbers of progeny produced by the blood-fed An. gambiae s.s. females collected before intervention in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net was 79.9, 77.2, and 72.6/female and those in the houses with Olyset Net, Olyset Duo, and PPF-impregnated net after intervention were 54.7, 38.4, and 19.6/female, respectively. Difference in the number of progenies between the pre-intervention houses and post-intervention houses was significant (P = 0.0021), and considerably higher reduction was observed in the houses in which Olyset Duo (P = 0.0195) and PPF-impregnated nets (P<0.0001) were used compared to that in the pre-intervention houses (Figure 4).
Discussion
The An. gambiae s.s. population in the study area was pyrethroid-resistant governed by a point mutation of the voltage-gated sodium channel (L1014S) at high frequency, as previously reported [9], [33]. Additionally, a reduced repellency to pyrethroids has been reported in An. gambiae s.s. population in the study area as compared to the other malaria vectors, An. arabiensis and An. funestus s.s. [33]. This population attacked humans mainly at midnight irrespective of the use of LNs [34]. Therefore, we hypothesized that almost all blood-fed females had contact with the bed nets before or after the blood feeding, although the frequency of contact or total duration of contact was not measured in the present study.
An increase in the number of collected mosquitoes were observed in the houses in which PPF-impregnated nets were used. Further, some blood-fed mosquitoes were observed resting inside, as well as outside the PPF-impregnated bed nets, while no mosquitoes were collected from inside the permethrin-incorporated Olyset Net and Olyset Duo. This indicated that permethrin played a role in reducing the mosquito numbers inside the houses. No significant difference was noted in the number of blood-fed and unfed females of An. gambiae s.s. before and after intervention in the houses with Olyset Net, Olyset Duo, and PPF-impregnated nets were used. Since the present study was performed in houses where the residents had no restrictions on the sleeping times, some might have been outside the bed nets during midnight or might have slept under the bed nets in a manner that could have resulted in receiving mosquito bites.
Mortality (within 3 days after collection) of the blood-fed females in the houses with Olyset Duo and PPF-impregnated net increased from 4.7% to 17.2% and from 8.3% to 33.3%, respectively, after intervention. Significant differences were noted in the proportion of the number of dead blood-fed females against the number of live blood-fed females before and after intervention in the houses with PPF-impregnated nets. This increase in mortality clearly indicated the effect PPF had on the longevity of blood-fed females as previously reported [27]. Similarly, methoprene was reported to cause mortality to Ae. aegypti at 24 h following blood consumption [22]. Damages to internal tissues such as Malpighian tubules, midguts, and salivary gland cells have been observed in PPF-treated cat flea Ctenocephalides felis (Bouché), as reported by Meola et al. [35], and similar damages might have caused the deaths in the mosquitoes. Additionally, the percentage of the blood-fed females oviposited in the houses with Olyset Duo and PPF-impregnated nets reduced from 76.7% to 44.8% and from 75.0% to 26.4%, respectively, after intervention. Significant differences in the proportion of ovipositing, blood-fed females before and after intervention were observed in the houses with Olyset Duo and PPF-impregnated nets. The mortality rate in 3 days and the oviposition of the blood-fed females might be one of the means to determine the efficacy of PPF-impregnated bed nets in a field study, since the observation of egg production and hatchability requires time, special apparatus such as binoculars, is costly, and requires technical skill.
The number of oviposited eggs and progenies were significantly reduced in Olyset Duo and PPF-impregnated nets as compared to those before intervention. There was no difference in hatchability. In the current study, the same materials were used for Olyset Net and bed nets in which 1% PPF was impregnated. In the laboratory study using the laboratory-bred colony of An. gambiae s.s. (insecticide-susceptible ICIPE strain having 6.3% allelic L1014S mutation [9]), more than 90% reduction in offspring/blood-fed female was observed by the 3-min exposure to the same net materials (unpublished data). Ohashi et al. reported 100% reduction in fecundity of An. gambiae s.s. upon 3-min contact to the nets dipped in 0.01% isopropyl alcohol solution of PPF [27]. Kawada et al. reported that the minimum dosage of PPF, which causes sterility in female houseflies, was 5 µg per female [25]. On the other hand, 0.05–0.49 µg per female of PPF was thought to be effective in Ae. aegypti, since these amounts were detected in the mosquitoes that had reduced fecundity [24]. Even though the amount of PPF picked up by the mosquito after a short contact time with the PPF-impregnated net surface was not known, we believe it could be within the above range.
PPF has been reported to be effective against DDT, dieldrin, organophosphate, and carbamate-resistant anopheline mosquitoes as a larvicide, indicating the absence of cross-resistance of PPF to these insecticides [36]. However, only few studies have shown the relationship between pyrethroid-resistance and efficacy of PPF in mosquitoes. Kasai et al. suggested cross-resistance between etofenprox and insect growth regulators (IGRs) such as diflubenzuron and PPF in Culex pipiens pipiens, which were found in Japan [37]. There does not seem to be no other reports studying the above issue. Absence in cross resistance of PPF to other insecticides, especially pyrethroids, is essential for the selection of PPF as an active ingredient for LNs because of the aforementioned serious resistance problems in malaria vectors. The present study showing that the high sterilizing efficacy of PPF against pyrethroid-resistant An. gambiae s.s. might provide a solution to the above issue.
The current study also demonstrated the impact of permethrin and PPF when used in combination. The use of only PPF in a bed net might increase the risk of mosquito bites even though the mosquitoes that came in contact with the net might later have died (due to the impact of PPF on longevity) or have been sterilized. However, permethrin has an excito-repellent activity to mosquitoes that might reduce the contact chance of mosquitoes to bed net surface. Siegert et al. reported in a recent study that Olyset Net reduced the landing attempts of mosquitoes and elevated their flight frequency, resulting in low incidence of mortality [38]. Combined use of permethrin and PPF, therefore, might be antagonistic. The current study, however, demonstrated the effectiveness of permethrin incorporated PPF bed net (Olyset Duo) in reducing the number of ovipositing females and the number of eggs laid, and suppressing progeny through these mechanisms. The fact that a combination of a repellent pyrethroid and PPF was effective in sterilizing mosquitoes might be partly due to the reduced repellency of permethrin to An. gambiae s.s. in the study area [33]. Further, high anthropophily and midnight feeding habits [34] might have contributed positively to the effect of PPF. Recently, Ngufor et al. conducted the experimental hut trial of Olyset Duo against pyrethroid resistant laboratory colonies of An. gambiae s.s. [39]. The authors concluded that the pyrethroid-resistant mosquitoes which fail to be killed by permethrin could be sterilized if the net also contains PPF, resulting in greater reductions in the abundance of pyrethroid resistant malaria vectors.
The observation of oviposition and hatchability of mosquitoes requires skills, apparatus (binoculars and other equipment), and long term incubation of eggs, since it takes 1–3 weeks for the completion of egg hatching because egg hatching does not take place simultaneously [40]. We could not use large number of houses in the present study because of the limited manpower and apparatus. Larger scale field studies with sufficient number of skilled staffs and apparatus will be required for the further comprehension of the effectiveness of PPF. Further studies on the other wild pyrethroid-resistant mosquito populations such as An. arabiensis and An. funestus s.s. will provide a more convincing explanation to the above suggestions. Selection of PPF concentration, which would give the maximum efficacy while being cost-effective, as well as selection of the optimum concentration of permethrin or selection of another insecticide that can substitute for permethrin are issues of interest for future studies.
Acknowledgments
The protocol for the study was reviewed and approved by the Scientific Steering Committee (SSC) and the National Ethics Review Committee (ERC) of the Kenya Medical Research Institute (KEMRI). We are grateful to all the staff at the Thomas Odhiambo campus of the International Center of Insect Physiology and Ecology (ICIPE), for providing facilities and experimental insects. We also thank B. Awuor, F. Sonye, A. Oreng' Obielo, R. Muga, S. Omito, C. Opora, J. Oreng', M. Shikuku, D. Otieno, P. Oboyi, Springs of Hope, Mbita, Kenya, and C. Kimura, Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan, for providing technical support and assisting with the study, and J. Invest and J. Lucas, Sumitomo Chemical (UK), London, UK, for reviewing the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was funded as a joint research between Nagasaki University and Sumitomo Chemical Co. Ltd in creating the field and semi-field bioassay system for insecticide delivering technology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. KO is an employee of funder company, Sumitomo Chemical Co., Ltd. KO gave an advisory opinion at the intervention of the bed nets as one of the developer of Olyset Duo.
References
- 1. Nauen R (2007) Insecticide resistance in disease vectors of public health importance. Pest Manag Sci 63: 628–633. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg, Zaim M, Yadav RS, Soares A, Ameneshewa B, et al. (2012) Global trends in the use of insecticides for vector-borne disease control. Environmental Health Perspectives. http://dx.doi.org/10.1289/ehp.1104340. [DOI] [PMC free article] [PubMed]
- 3. Stump AD, Atieli FK, Vulule JM, Besansky NJ (2004) Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Med Trop Hyg 70: 591–596. [PubMed] [Google Scholar]
- 4. Mathias D, Ochomo EO, Atieli F, Ombok M, Bayoh MN, et al. (2011) Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malar J 10: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, et al. (2010) Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Himeidan YE, Chen H, Chandre F, Donnelly MJ, Yan G (2007) Short report: permethrin and DDT resistance in the malaria vector Anopheles arabiensis from eastern Sudan. Am J Trop Med Hyg 77: 1066–1068. [PubMed] [Google Scholar]
- 7. Kulkarni M, Rowland M, Alifrangis M, Mosha FW, Matowo J, et al. (2006) Occurrence of the leucine-to-phenylalanine knockdown resistance (kdr) mutation in Anopheles arabiensis populations in Tanzania, detected by a simplified high-throughput SSOP-ELISA method. Malar J 5: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawada H, Futami K, Komagata O, Kasai S, Tomita T, et al. (2011) Distribution of a knockdown resistance mutation (L1014S) in Anopheles gambiae s.s. and Anopheles arabiensis in Western and Southern Kenya. PLoS One 6: e24323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawada H, Dida GO, Ohashi K, Komagata O, Kasai S, et al. (2011) Multimodal pyrethroid resistance in malaria vectors, Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus s.s. in western Kenya. PLoS One 6: e22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wondji CS, Irving H, Morgan J, Lobo NF, Collins FH, et al. (2009) Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res 19: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, et al. (2000) Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol 14: 181–189. [DOI] [PubMed] [Google Scholar]
- 12. Kawada H, Dida GO, Sonye G, Njenga SM, Mwandawiro C, et al. (2012) Reconsideration of Anopheles rivulorum as a Vector of Plasmodium falciparum in Western Kenya: some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasit Vect 5: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, et al. (2014) Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar J. 13: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, et al. (2014) Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg. 90: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunt R, Edwardes M, Coetzee M (2010) Pyrethroid resistance in southern African Anopheles funestus extends to Likoma Island in Lake Malawi. Parasit Vectors. 3: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto J, Hirano M, Takimoto Y, Hatakoshi M (1993) Insect growth regulators for pest control, with emphasis on juvenile hormone analogs. Present status and future prospects. In: Duke, SO, Menn, JJ, Plimmer JR editors. Pest control with enhanced environmental safety (ACS symposium series 524). Washington, DC: American Chemical Society. 144–168.
- 17. Henrick CA, Staal GB, Siddall JB (1973) Alkyl-3,7,11-trimethyl-2,4-dodecadienoates, a new class of potent insect growth regulators with juvenile hormone activity. J Agr Food Chem 21: 354–359. [DOI] [PubMed] [Google Scholar]
- 18. Hirano M, Hatakoshi M, Kawada H, Takimoto Y (1998) Pyriproxyfen and other juvenile hormone analogues. Rev Toxicol 2: 357–394. [Google Scholar]
- 19. Syafruddin, Arakawa R, Kamimura K, Kawamoto F (1990) Histopathological effects of an insect growth regulator, 4-phenoxyphenyl (RS)-2-(2-pyridyloxy)propyl ether (pyriproxyfen), on the larvae of Aedes aegypti . Jpn J Sanit Zool 41: 15–22. [Google Scholar]
- 20. Iwanaga K, Kanda T (1988) The effects of a juvenile hormone active oxime ether compound on the metamorphosis and reproduction of an Anopheline vector, Anopheles balabacensis . Appl Entomol Zool 23: 186–193. [Google Scholar]
- 21. Judson CL, Lumen HZ (1976) Some effects of juvenile hormone and analogs on ovarian follicles of the mosquito Aedes aegypti (Diptera: Culicidae). J Med Entomol 13: 197–201. [DOI] [PubMed] [Google Scholar]
- 22. Klowden MJ, Chambers GM (1989) Ovarian development and adult mortality in Aedes aegypti treated with sucrose, juvenile hormone and methoprene. J Insect Physiol 35: 513–517. [Google Scholar]
- 23.Kawada H, Itoh T, Abe Y, Horio M (1993) Can mosquito be a carrier of larvicides? In: Proceedings of the 1st International Congress on Insect Pests in the Urban Environment, Cambridge, UK, 497.
- 24. Itoh T, Kawada H, Abe Y, Eshita Y, Rongsriyam Y, et al. (1994) Utilization of blood-fed females of Aedes aegypti as a vehicle for the transfer of the insect growth regulator pyriproxyfen to larval habitats. J Am Mosq Control Assoc 10: 344–347. [PubMed] [Google Scholar]
- 25. Kawada H, Senbo S, Abe Y (1992) Effects of pyriproxyfen on the reproduction of the housefly, Musca domestica, and the German cockroach, Blattella germanica . Jpn J Sanit Zool 43: 169–175. [Google Scholar]
- 26. Aiku AO, Yates A, Rowland M (2006) Laboratory evaluation of pyriproxyfen treated bednets on mosquito fertility and fecundity. A preliminary study. West Afr J Med 25: 22–6. [DOI] [PubMed] [Google Scholar]
- 27. Ohashi K, Nakada K, Ishiwatari T, Miyaguchi J, Shono Y, et al. (2012) Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae). J Med Entomol 49: 1052–1058. [DOI] [PubMed] [Google Scholar]
- 28. Harris C, Lwetoijera DW, Dongus S, Matowo NS, Lorenz LM, et al. (2013) Sterilising effects of pyriproxyfen on Anopheles arabiensis and its potential use in malaria control. Parasit Vect 6: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noor AM, Amin AA, Akhwale WS, Snow RW (2007) Increasing coverage and decreasing inequity in insecticide-treated bed net use among rural Kenyan children. PLoS Med 4: e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Futami K, Dida GO, Sonye G, Lutiali PA, Mwania MS, et al. (2014) Impacts of insecticide treated bed nets on Anopheles gambiae s.l. populations in Mbita district and Suba district, Western Kenya. Parasit Vect 7: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillies MT, Coetzee M (1987) A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region). South African Institute for Medical Research No. 55.
- 32. Scott JA, Brogdon WG, Collins FH (1993) Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg 49: 520–529. [DOI] [PubMed] [Google Scholar]
- 33. Kawada H, Ohashi K, Dida GO, Sonye G, Njenga SM, et al. (2014) Insecticidal and repellent activities of pyrethroids to the three major pyrethroid-resistant malaria vectors in western Kenya. Parasit Vect 7: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawada H, Ohashi K, Dida GO, Sonye G, Njenga SM, et al. (2014) Preventive effect of permethrin-impregnated long-lasting insecticidal nets on the blood feeding of three major pyrethroid-resistant malaria vectors in western Kenya. Parasit Vect 7: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meola R, Pullen S, Meola S (1996) Toxicity and histopathology of the growth regulator pyriproxyfen to adults and eggs of the cat flea (Siphonaptera: Pulicidae). J Med Entomol 33: 670–679. [DOI] [PubMed] [Google Scholar]
- 36. Kawada H, Shono Y, Itoh T, Abe Y (1993) Laboratory evaluation of an insect growth regulators against several species of Anopheline mosquitoes. Jpn J Sanit Zool 44: 349–353. [Google Scholar]
- 37. Kasai S, Shono T, Komagata O, Tsuda Y, Kobayashi M, et al. (2007) Insecticide resistance in potential vector mosquitoes for West Nile virus in Japan. J Med Entomol 44: 822–829. [DOI] [PubMed] [Google Scholar]
- 38. Siegert PY, Walker E, Miller JR (2009) Differential behavioral responses of Anopheles gambiae (Diptera: Culicidae) modulate mortality caused by pyrethroid-treated bednets. J Econ Entomol 102: 2061–2071. [DOI] [PubMed] [Google Scholar]
- 39. Ngufor C, N'Guessan R, Fagbohoun J, Odjo A, Malone D, et al. (2014) Olyset Duo (a pyriproxyfen and permethrin mixture net): An experimental hut trial against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus in Southern Benin. PLoS One 9: e93603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ebrahimi B, Shakibi S, Foster WA (2014) Delayed egg hatching of Anopheles gambiae (Diptera: Culicidae) pending water agitation. J Med Entomol 51: 580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.