Abstract
Bisphenol A (BPA) is an environmental endocrine disrupter. Excess exposure to BPA may increase susceptibility to many metabolic disorders, but it is unclear whether BPA exposure has any adverse effects on the development of atherosclerosis. To determine whether there are such effects, we investigated the response of Watanabe heritable hyperlipidemic (WHHL) rabbits to 400-µg/kg BPA per day, administered orally by gavage, over the course of 12 weeks and compared aortic and coronary atherosclerosis in these rabbits to the vehicle group using histological and morphometric methods. In addition, serum BPA, cytokines levels and plasma lipids as well as pathologic changes in liver, adipose and heart were analyzed. Moreover, we treated human umbilical cord vein endothelial cells (HUVECs) and rabbit aortic smooth muscle cells (SMCs) with different doses of BPA to investigate the underlying molecular mechanisms involved in BPA action(s). BPA treatment did not change the plasma lipids and body weights of the WHHL rabbits; however, the gross atherosclerotic lesion area in the aortic arch was increased by 57% compared to the vehicle group. Histological and immunohistochemical analyses revealed marked increases in advanced lesions (37%) accompanied by smooth muscle cells (60%) but no significant changes in the numbers of macrophages. With regard to coronary atherosclerosis, incidents of coronary stenosis increased by 11% and smooth muscle cells increased by 73% compared to the vehicle group. Furthermore, BPA-treated WHHL rabbits showed increased adipose accumulation and hepatic and myocardial injuries accompanied by up-regulation of endoplasmic reticulum (ER) stress and inflammatory and lipid metabolism markers in livers. Treatment with BPA also induced the expression of ER stress and inflammation related genes in cultured HUVECs. These results demonstrate for the first time that BPA exposure may increase susceptibility to atherosclerosis in WHHL rabbits.
Introduction
Bisphenol A (BPA) is one of the world's most produced chemicals and is widely used as a key monomer for the production of polycarbonate plastic and epoxy resins [1]. These two commercial materials have been commonly used in the manufacture of various consumer goods, industrial products, and medical devices [2], [3]. Given the prevalence of BPA in our environment and daily lives, it can be detected in serum, urine, breast milk and saliva in the majority of populations in different countries [1], [2], [4]. Thus, concern has grown regarding whether BPA exposure can cause health problems in humans [3], as underscored by recent cross-sectional and longitudinal studies that show that urinary or serum levels of BPA are positively associated with various cardiovascular diseases (CVD). An epidemiological study analyzed data from the 2003–2004 National Health and Nutrition Examination Survey (NHANES) and was the first to report positive associations between higher urinary BPA concentrations and increased risks of cardiovascular diseases, including angina, coronary heart disease and heart attacks [5]. Other studies also found significant positive associations, which were independent of traditional CVD risk factors, between urinary BPA levels and coronary heart diseases using data from the 2003–2006 NHANES database and the European Prospective Investigation of Cancer in Norfolk, UK, in the 1990s [6], [7]. Recent epidemiological studies have also shown that either urinary or serum BPA levels were positively associated with coronary artery stenosis [8], carotid atherosclerosis [9], and peripheral arterial disease [10], suggesting that BPA exposure may be an emerging risk factor for the development of atherosclerosis. However, this latter hypothesis has not been verified experimentally using appropriate animal models. This is an important issue because it is not clear whether BPA exposure is causal for the development of atherosclerosis. In fact, the toxicological mechanisms of BPA in terms of atherosclerosis remain largely unknown [11]. Several studies have shown that BPA exposure increases atherosclerosis in mice [12], [13] and alters cardiac functions in both mice and rats [14]–[16].
Although these rodent studies are informative, it is not known whether these results can be extrapolated to humans because rodents are quite different from humans in terms of lipid metabolism, glucose metabolism, cardiovascular systems, and responses to inflammatory stimuli [17]–[19]. Furthermore, regional distributions of fat depots, their cellular compositions (e.g., brown vs. white fat, infiltration by macrophages), and regulations of resistin, agouti protein, adipsin, and adrenergic receptors are dissimilar between rodents and humans [20]. In this regard, there are advantages to studying lipid metabolism and atherosclerosis in rabbits rather than mice [17]. In addition, rabbits are phylogenetically closer to humans than are rodents [17]. Like humans but unlike rodents, rabbits are LDL-mammals and have plasma-cholesteryl-ester-transfer proteins, which are important regulators for lipid metabolism [17], [18]. Furthermore, the phenotypes and pathogenesis of atherosclerotic lesions in rabbits are similar to those observed in human atherosclerosis [17]. WHHL rabbits are genetically deficient in low-density lipoprotein receptors due to spontaneous 4-amino-acid deletions in the cysteine-rich ligand-binding domains in exon-4 positions of LDL receptors [21], [22]. Even when fed chow diets, which have been extensively used for the study of hypercholesterolemia, atherosclerosis, and insulin resistance, WHHL rabbits are characterized by hyperlipidemia and spontaneous atherosclerosis in both aorta and coronary arteries [23]–[25].
To examine whether BPA exposure plays any role in the development of atherosclerosis, we administered BPA daily at a dose of 400 µg/kg body weight (BW) to WHHL rabbits for 12 weeks. We found that BPA treatment enhanced atherosclerosis both in the aortic arch and coronary arteries. To the best of our knowledge, this is the first report using non-rodent models to investigate BPA exposure and atherosclerosis in the setting of hyperlipidemia.
Materials and Methods
Animals
Fourteen-week-old, post pubertal male WHHL rabbits provided by Kobe University (Kobe, Japan) were randomly divided into 2 groups (designated as vehicle and BPA-exposure groups, n = 6 for each group) and raised in a facility with constant temperature (25±2°C) and a 12∶12-hr light:dark cycle controlled strictly by an automatic device. Stainless-steel cages, automatic waterers and food containers were used in this study. All animals could access water ad libitum and were fed 100 g of a restricted standard chow diet (CR-3, CLEA Japan, Inc., Tokyo) every day. Daily food intake and weekly body weight were monitored strictly.
BPA (97% pure) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was first dissolved in ethanol and then diluted in corn oil (Wako Chemical Industries Co., Osaka, Japan). BPA solution (400 µg/kg for the exposure group) was administered by gavage every morning for 12 weeks. The vehicle group was administered the same volume of corn oil without BPA. The polypropylene tubes which are regarded as BPA free [26] were used for all procedures. A dose of 400 µg/kg was chosen for the following two reasons: (1) a previous study as well as preliminary experiments in our laboratory showed that monkeys, mice and Japanese white rabbits exposed to this dose exhibited average unconjugated 24-hr serum BPA levels that could be within the range observed in the general human population [27] and (2) based on the similarity of BPA pharmacokinetics between humans, monkeys and mice, we estimated the maximal daily human exposure to BPA via multiple routes to be approximately 500 µg/kg/day [27]–[29].
Before the experiment, we investigated serum BPA kinetics in WHHL rabbits within 24 hours of oral BPA administration. For this purpose, blood was collected at 0 (pre-feeding), 0.5, 1, 2, 4, and 24 hours after the rabbits were administered 400 µg/kg BPA by gavage. For BPA residual accumulation analysis, blood samples were collected biweekly from middle-ear arteries before gavage administration. All animal experiments were performed with the approval of the Animal Care Committee of the University of Yamanashi and complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health [30].
Measurements of serum BPA levels
Blood samples were allowed to stand at room temperature for 15 min to enable clotting, and then serum was obtained after centrifugation at 3,000 rpm for 20 min at 4°C. Serum was kept at −80°C until use. Serum BPA levels were measured by detecting unconjugated BPA using a Shimadzu Prominence LC-20A series high-performance liquid chromatography (HPLC) (Shimadzu, Kyoto, Japan) in conjunction with tandem mass spectrometry (MS/MS) (Applied Biosystems, Foster City, CA, USA) according to a previously described method [31]. The mean extraction recovery of BPA through the entire analytical procedure ranged from 81% to 113%. The BPA detection limits of this method were 0.02∼0.27 ng/mL. The details of this analytical procedure are shown in Technical Description S1. Serum BPA pharmacokinetic parameters, including the maximum attained value (Cmax), the terminal-phase half-life time (t½), and the average of the area under the curve (AUC) for 24 hr after gavage (Average AUC0–24) were calculated according to methods described elsewhere [27].
Analysis of blood biochemical parameters
Blood was collected from rabbits after 16 hr of fasting. The plasma levels of total cholesterol (TC), HDL-cholesterol (HDL-C), triglycerides (TG), and LDL-cholesterol (LDL-C) were measured using Wako assay kits (Wako Pure Chemical Industries, Osaka, Japan) according to established methods described in previous studies [30]. The plasma glucose concentrations were also measured using Wako glucose assay kits following the manufacturer’s instructions.
Analysis of serum cytokines levels
Blood was allowed to stand at room temperature for 15 min to clot and then serum was obtained after centrifugation at 3,000 rpm for 20 min at 4°C. The serum cytokines levels of TNF-α and IL-6 were analyzed using rabbit TNF-α and IL-6 ELISA kits (Cloud-Clone Corp. and USCN, Houston, USA) according to the manufacturer’s instructions. The details of procedures are shown in Technical Description S1.
Analysis of aortic atherosclerosis
At the end of the experiments, all rabbits were euthanized by injection of an overdose of sodium pentobarbital solution (100 mg/kg). Incidents of aortic atherosclerosis were quantified according to a previously described method [32]. The aortic trees were isolated and fixed in 10% buffered formalin. For analysis of the gross sizes of atherosclerotic lesions, whole aortas were stained with Sudan IV and photographed using a digital camera, after which sudanophilic areas were measured using an image analysis system (WinROOF ver. 7.0, Mitani Corporation, Tokyo, Japan). For histological analysis, the aortic arch was cut into 10 sections (3-µm-thick) and stained with hematoxylin and eosin (H&E) and Elastica van Gieson (EVG). To visualize the cellular components in the lesions, serial paraffin sections of each arch were immunohistochemically stained with monoclonal mouse antibodies (mAbs) against either macrophages (RAM11, Dako, working dilution 400×) or α-smooth muscle actin (HHF35, Dako, working dilution 200×) for smooth muscle cells (SMCs) [30], [32]. Positively immunostained areas of macrophages (Mφ) and SMCs in the lesions were quantified using an image analysis system and were expressed as percentages.
Analysis of coronary atherosclerosis
To assess coronary atherosclerosis, the left coronary artery roots were cut into 4 slices (3-µm-thick) in 50-µm intervals, and a total of 40 slices were sectioned serially. To analyze the lesions of coronary arteries, 10 serial sections were stained with H&E, and the lesions were observed under a light microscope, quantified using an image analysis system and expressed as the stenosis (%) of the lumen area [lesion area/(total lumen area)×100%] based on the method described previously [32]. To visualize the cellular components in the lesions, each of 10 serial sections was immunohistochemically stained with mAbs against Mφ and SMCs, as described above.
Pathological and morphometric analysis
Liver, heart, and adipose tissue (including subcutaneous and visceral regions) were collected and weighed when wet. In each case, the heart and the small pieces of liver and adipose tissue were fixed in 10% buffered formalin. The heart was sectioned into 5 blocks, and 3 sections were cut from each block at 500-µm intervals and then stained with H&E for analysis. For liver and adipose tissues, 3-µm-thick sections were cut serially and stained with H&E for histological analyses. Morphometric analysis of adipose tissue was conducted using a method described previously [30]. In brief, more than 300 adipocytes of each section were selected randomly, and cellular diameters were measured using an image analysis system (WinROOF ver. 7.0). Each block of heart was observed under a light microscope using H&E-stained sections, and the lesion areas in the cardiac muscle were quantified via the image analysis system.
Real-time RT-PCR analysis
A piece of liver was quickly frozen in liquid nitrogen, and total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Hepatic expression of genes related to ER stress, lipid metabolism, and inflammation [ER stress, including C/EBP homologous protein (Chop), binding immunoglobulin protein (Bip), and calreticulin (CALR); lipid metabolism, including peroxisome proliferator-activated receptor alpha (PPAR-α), liver X receptor alpha (LXR-α), and microsomal triglyceride transfer protein (MTTP); inflammation, including interleukin-1 beta (IL-1β), plasminogen activator inhibitor-1(PAI-1), and interleukin-6 (IL-6)] were analyzed using real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) using SYBR Premix Ex TaqTM kits (Takara, Tokyo, Japan) following the manufacturer’s instructions. Rabbit glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard for relative quantification, and the relative amount of mRNA was calculated using the 2−ΔΔCT method [33]. The primers used in this experiment are shown in Table S1.
Cell-culture experiments
To investigate the possible molecular mechanisms induced by BPA, we also performed cultured cell studies using human umbilical-cord-vein endothelial cells (HUVECs) (Cell Bank, Chinese Academy of Sciences, Shanghai, China) and rabbit aortic smooth muscle cells (SMCs). The details of the cell experiments are contained in Technical Description S1. Total RNA was extracted and qRT-PCR was performed as reported previously [33]. We examined the effects of BPA treatment on the gene expression of Bip, monocyte chemotactic protein-1 (MCP-1), nuclear factor-kappa B (NF-κB), tumor necrosis factor alpha (TNF-α), vascular cell-adhesion molecule-1 (VCAM-1), and vascular endothelial growth factor A (VEGF-A) on the HUVECs at 24 hr. The procedure and the primer sequences are shown in Technical Description S1 and Table S1. Meanwhile, the proliferation of SMCs caused by BPA treatment for 24 hr was also assessed using cell counting kit-8 (Dojindo Molecular Technologies, Inc., Rockville, USA) following the manufacturer’s instructions. The details of procedures are also shown in Technical Description S1.
Statistical Analysis
Statistical analysis was performed using SPSS 16.0 for Windows software (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test was used for nonparametric analysis of the positive areas of SMCs and macrophages (Mφ) in the aortic lesions. The Student’s t-test was used to compare the results of other assays. In each case, statistical significance was indicated by p<0.05.
Results
Serum levels of BPA
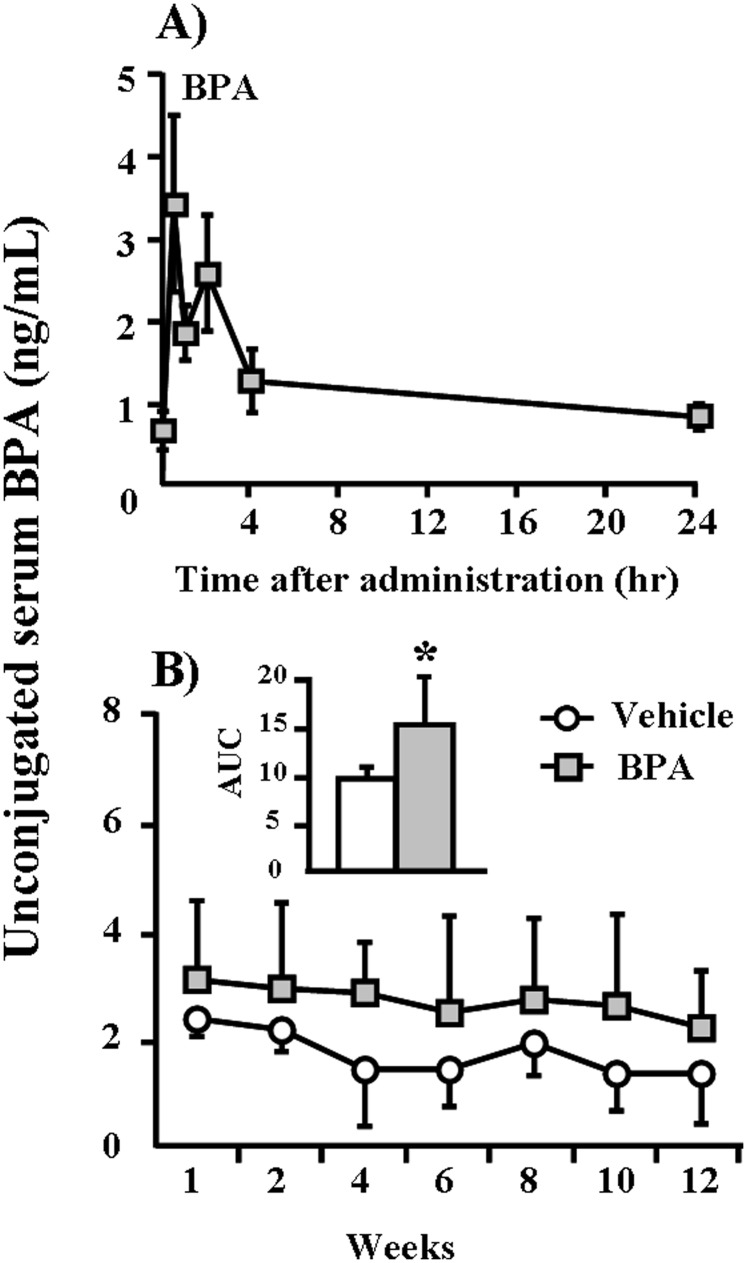
Because no data were available in the published literature, we first investigated the kinetics of serum BPA in WHHL rabbits after one session of BPA feeding. After gavage feeding of 400 µg/kg BPA, serum levels of BPA peaked within 1 hr and declined to normal levels at 24 hr (Figure 1A), suggesting that BPA can be rapidly absorbed and catabolized in rabbits. We also compared the serum BPA kinetics of WHHL rabbits with those of other species reported in the literature after BPA (400 µg/kg) exposure [27], [34]. The Cmax of unconjugated BPA in the serum of WHHL rabbits reached 3.41 ng/mL at 0.5 hr and declined sharply close to the initial level of 0.83 ng/mL at 24 hr (Figure 1A). The average AUC value of unconjugated BPA from 0 to 24 hr (1.22 ng/mL) in WHHL rabbits was slightly higher than values obtained in adult monkeys and mice (Table 1).
Figure 1. Kinetic changes and accumulation of serum BPA concentrations in WHHL rabbits.
(A) WHHL rabbits were fed BPA (400 µg/kg) by gavage, and serum levels of BPA were measured over the next 24 hr as described in the Materials and Methods section. Serum levels of BPA are expressed as the means ± SE. For each group, n = 6. (B) Serum levels of BPA were measured biweekly over the course of 12 weeks as described in the Materials and Methods section. Serum levels of BPA are expressed as the means ± SD, n = 6 for each group. Total exposure volumes during experiments are calculated as the areas under the curves (AUC’s) (shown in the insert). *p<0.05 vs. vehicle.
Table 1. Serum pharmacokinetic parameters for unconjugated BPA in WHHL rabbits compared with monkey and mice.
| Species | Gender | Age | BPA (µg/kg) | Cmax (ng/mL)a | Terminalt½ (hr)b | AverageAUC0–24 (ng/mL)c | References |
| WHHL rabbit | Male | Adult | 400 | 3.41 | 18.44 | 1.22 | This study |
| CD-1mice | Female | Adult | 400 | 3.28 | 33.64 | 0.70 | [27] |
| Rhesusmonkey | Female | Adult | 400 | 3.95 | 8.88 | 0.52 | [27] |
| CD-1mice | Female | Neonatal | 395 | 14.82 | 9.35 | 2.78 | [34] |
Cmax: maximum attained value; bTerminal t½: the terminal phase half-life;
AUC0–24: the area under the curve at t = 24 hr after gavage.
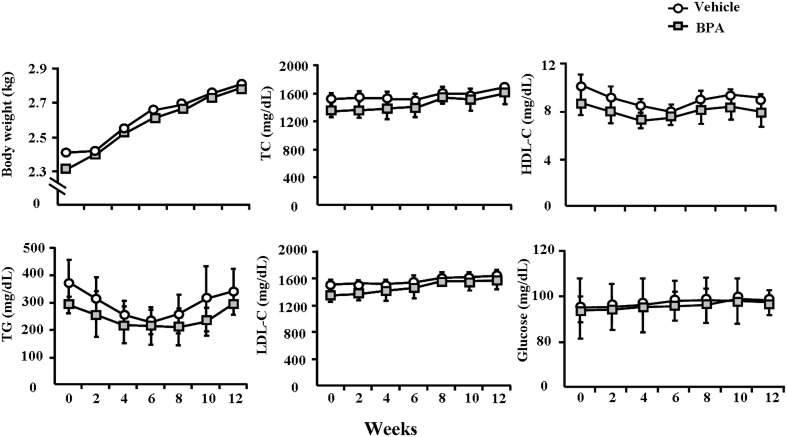
On the basis of serum BPA kinetics in WHHL rabbits shown above, we subsequently measured BPA serum levels at different weeks and examined whether any residual BPA accumulated in the serum when BPA was given daily for 12 weeks. As shown in Figure 1B, the serum BPA levels of vehicle WHHL rabbits were approximately 1.3∼2.3 ng/ml (similar to those of normal humans (0.5∼2.0 ng/mL) [1]. However, BPA administration led to a slight increase in serum BPA in WHHL rabbits (2.2∼3.1 ng/mL) during 12 weeks. Although there were no statistically significant differences at each time point between BPA-treated and vehicle groups, the total amounts of BPA expressed by AUC were significantly higher (p = 0.02) in the BPA-exposure group compared to the vehicle group. BPA exposure did not significantly affect body weight and plasma lipid and glucose levels in the BPA-exposure group compared to the vehicle group (Figure 2).
Figure 2. Body weights, serum lipids and glucose levels in WHHL rabbits over 12 weeks.
These indexes were monitored every 2 weeks. Data are expressed as the means ± SD, n = 6 for each group.
Serum cytokines levels
The serum levels of TNF-α and IL-6 were increased by 1.03-fold and 1.37-fold in the BPA group compared to the vehicle group at 12 weeks (Figure 3).
Figure 3. ELISA analysis of serum cytokines levels in WHHL rabbits.
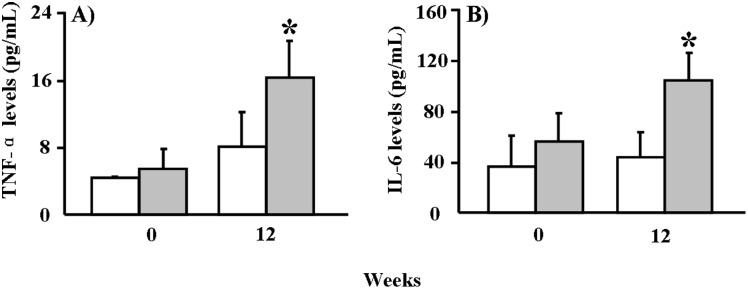
(A) TNF-α serum concentrations at 0 and 12 weeks. (B) IL-6 serum concentrations at 0 and 12 weeks. Data are expressed as the means ± SD, n = 6 for each group. *p<0.05 vs. vehicle.
Aortic and coronary atherosclerosis
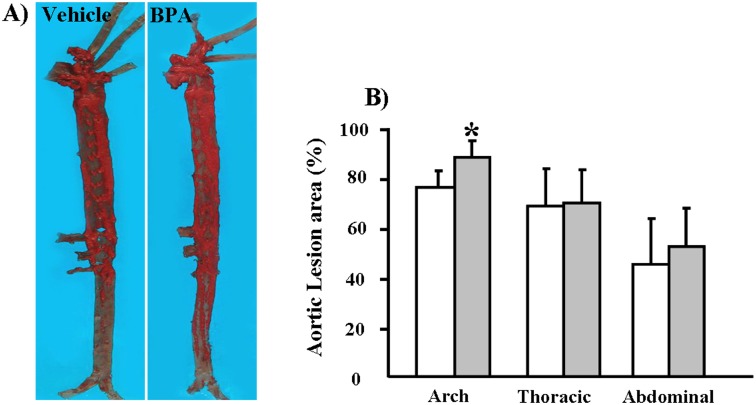
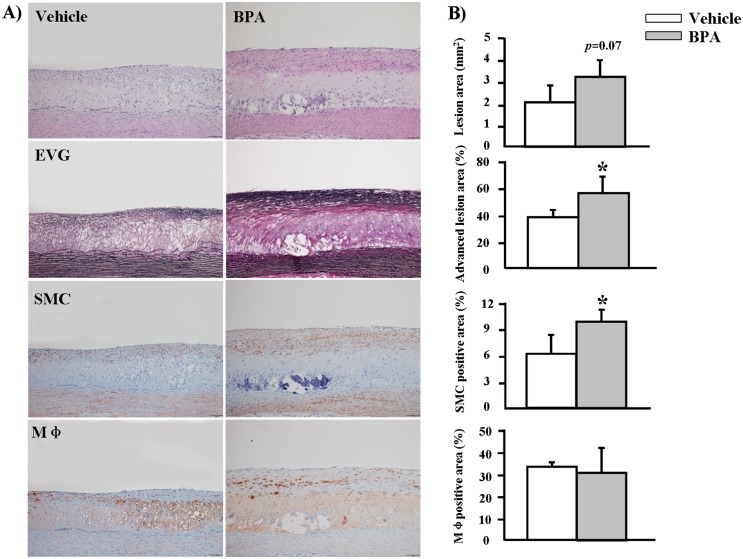
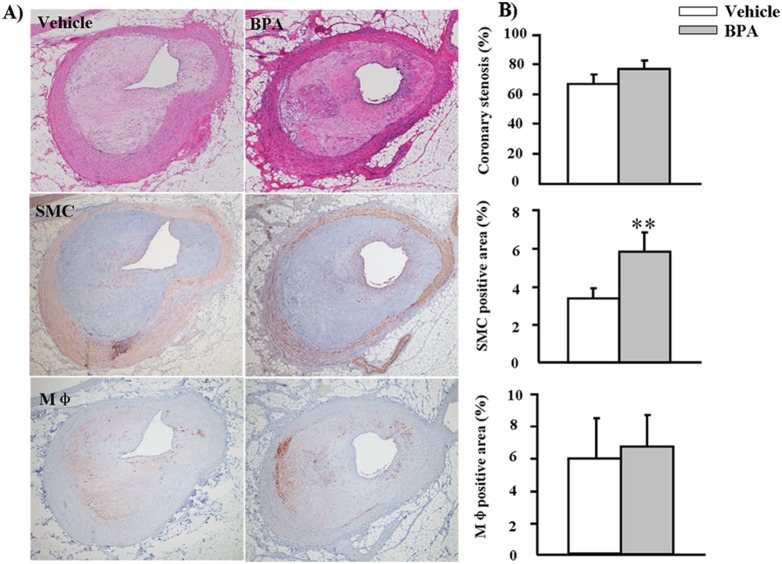
We found that, compared to the vehicle group, the gross lesion area in the BPA group was significantly increased by 17% (p = 0.04) in the aortic arch and that thoracic and abdominal aortic lesions were increased by 1.7% and 15%, respectively, but the latter effects were not statistically significant (p>0.05) (Figure 4). We quantified the microscopic lesion areas of the aortic arch and found a 57% increase in the lesions of WHHL rabbits (p = 0.07) compared to the vehicle group (Figure 5). Immunohistochemical staining showed that the increased lesion areas in the BPA-exposure group were characterized by marked increases in smooth muscle cells (60%, p = 0.03), whereas Mφ numbers did not differ significantly between the two groups (Figure 5). Furthermore, advanced lesion areas (advanced lesions, referring to those lesions that contain a fibrotic cap and a lipid or necrotic core with or without calcification) [35] were increased by 37% (p = 0.04) compared to the vehicle group. In addition to effects in the aorta, evidence of coronary stenosis increased by 11% (p>0.05) in the BPA-exposure groups compared to the vehicle group. Likewise, smooth muscle cells increased by 73% (p = 0.002) in the lesions; however, Mφ numbers displayed no obvious difference between the two groups (Figure 6).
Figure 4. Quantitative analysis of aortic atherosclerosis in WHHL rabbits at 12 weeks.
(A) Representative micrographs of the aortic trees stained with Sudan IV. (B) The sudanophilic en-face lesion areas in different parts of aortic trees were determined using an image analysis system as described in the Materials and Methods section. Data are expressed as the means ± SD, n = 6 for each group. *p<0.05 vs. vehicle.
Figure 5. Microscopic analysis of atherosclerosis in the aortic arches of WHHL rabbits at 12 weeks.
(A) Representative micrographs stained with H&E and EVG for intimal lesion analysis are shown in the top two panels. Representative micrographs stained with HHF35 and RAM11 mAbs for immunohistochemical analysis of SMCs and macrophages are shown in the lower two panels. (B) Intimal lesions, advanced lesions, SMCs and Mφ-positive areas of the sections were quantified using an image analysis system as described in the Materials and Methods section. Data are expressed as the means ± SD, n = 6 for each group. *p<0.05 vs. vehicle. Original magnification: 10×.
Figure 6. Microscopic analysis of atherosclerosis in the left coronary arteries of WHHL rabbits at 12 weeks.
(A) Representative micrographs stained with H&E for coronary stenosis analysis are shown in the top panel. Representative micrographs stained with HHF35 and RAM11 mAbs for immunohistochemical analysis of SMCs and Mφ are shown in the middle and lower panels. (B) Coronary stenosis, SMCs and Mφ-positive areas of the sections were quantified using an image analysis system as described in the Materials and Methods section. Data are expressed as the means ± SD, n = 6 for each group. **p<0.01 vs. vehicle. Original magnification: 4×.
Pathological examinations of Liver
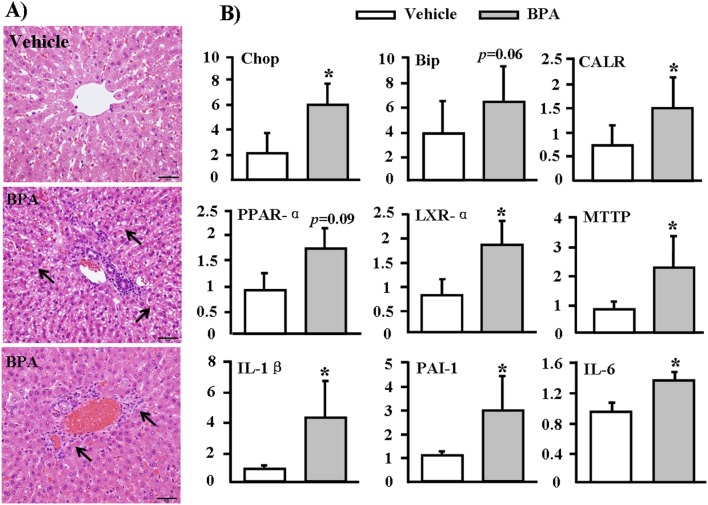
Liver histology results revealed focal hepatic injuries in the BPA-exposed groups, including mild steatosis (Figure 7A, middle) and inflammatory cell infiltration (Figure 7A, bottom). To investigate whether BPA affected hepatic gene expression, we performed real-time RT-PCR analysis, focusing on 3 pathways that may be involved in BPA-induced hepatic toxicity. We found that all ER stress genes (Chop, Bip, and CALR) were up-regulated in the livers of BPA-treated WHHL rabbits compared with those in the vehicle group (Figure 7B, top). Furthermore, 6 genes that mediate lipid metabolism and inflammation (PPAR-α, LXR-α, MTTP, IL-1β, PAI-1 and IL-6) were also up-regulated in livers from the BPA group (Figure 7B, middle and bottom).
Figure 7. Pathological changes and mRNA expression in the livers of WHHL rabbits at 12 weeks.
(A) Representative micrographs stained with H&E showed steatosis (middle) and inflammatory cells infiltration (lower) in the BPA-exposure group. Arrows highlight steatosis and inflammatory cells. (B) RT-PCR analysis of mRNA expressions in the livers of WHHL rabbits after BPA exposure. The mRNA expression levels of genes related to the ER pathway, lipid metabolism, and liver inflammation in WHHL rabbits treated with 400 µg/kg BPA for 12 weeks were analyzed using real-time RT-PCR. Expression levels are expressed relative to the data obtained in the vehicle group. Data are expressed as the means ± SD, n = 6 for each group. *p<0.05 vs. vehicles.
Pathological examinations of Adipose tissue
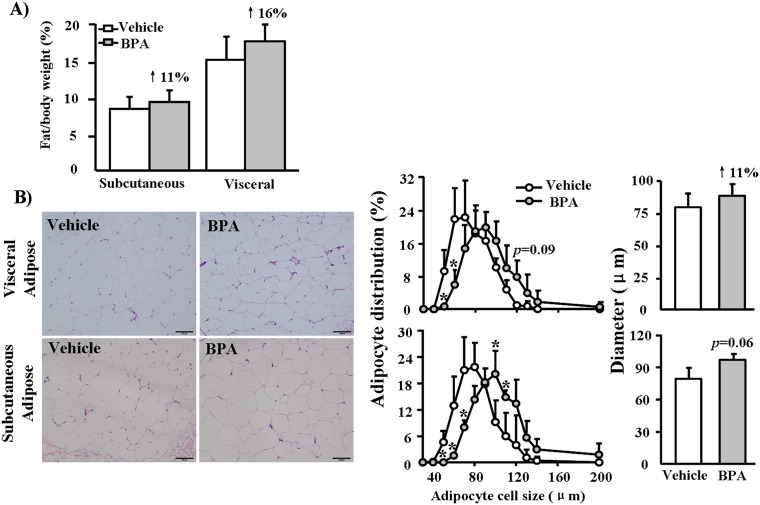
We found that total weights of subcutaneous and visceral adipose tissues were increased by 11% and 16%, respectively, in the BPA-exposure groups compared to the vehicle group, but these differences were not statistically significant (Figure 8A). Morphometric analysis showed that adipocyte sizes in both visceral and subcutaneous regions of the BPA-exposure groups shifted toward a large-cell population (meaning that large-sized cells predominated) compared to the vehicle group and that the average diameter was also slightly larger in the BPA-exposure groups (Figure 8B).
Figure 8. Pathological analysis of adipose in WHHL rabbits at 12 weeks.
(A) The total mass of adipose tissue was measured and expressed as the ratio of adipose tissue weight to body weight. (B) Representative micrographs of adipose tissue (H&E-stained specimens), morphometric analysis of adipocyte cell size distribution and mean diameters in the vehicle and BPA-exposure. (400 µg/kg) groups. Data are expressed as the means ± SD, n = 6 for each group. *p<0.05 vs. vehicles.
Pathological examinations of Heart
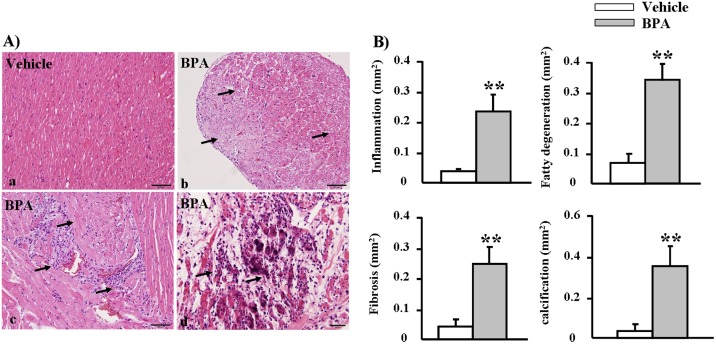
In histological and morphometric analyses, the hearts from BPA-exposure groups displayed cardiac damage accompanied by fatty degeneration, mononuclear cell infiltration and fibrosis. In some areas, myocardiocytes showed focal necrosis associated with calcium deposition or calcification (Figure 9). These findings indicate that chronic BPA exposure may directly lead to myocardial damage.
Figure 9. Pathological analyses of WHHL rabbit hearts at 12 weeks.
(A) Heart specimens stained with H&E show disarrayed myocardiocytes along with (b) fatty degeneration (c) inflammatory cell infiltration, fibrosis, and (d) calcification in the BPA-exposure group. (B) The lesion areas in the vehicle and BPA-exposure groups were quantified using an image analysis system. Data are expressed as the means ± SD, n = 6 for each group. **p<0.01 vs. vehicles.
Cultured cell experiments
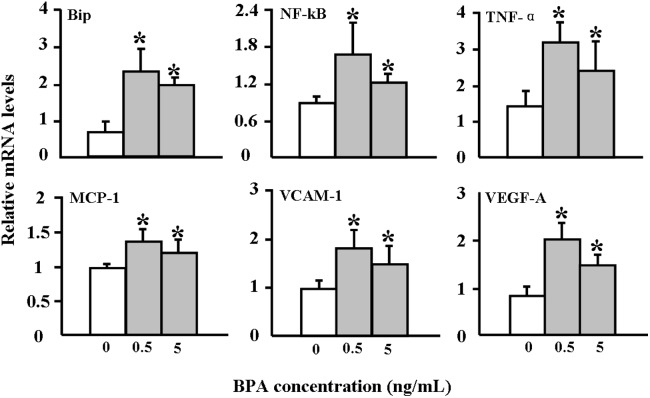
The finding that BPA exposure enhances atherosclerosis in WHHL rabbits prompted us to examine the possible mechanisms involved. We investigated the effects of BPA on HUVECs and rabbit aortic SMCs in vitro. BPA exposure did not affect cellular viability of HUVECs under such conditions (data not shown); however, incubation with BPA (0.5 and 5 ng/mL) for 24 hr up-regulated the expression of ER stress (Bip) and inflammatory reactions (MCP-1, NF-κB, TNFα, VCAM-1, and VEGF-A) related genes (Figure 10). Moreover, the SMC number was slightly increased after 0.5 and 5 ng/mL BPA exposure for 24 hr (Figure 11).
Figure 10. Effects of BPA incubation on ER stress and inflammatory responses in HUVEC cells.
Cultured HUVECs were incubated with either vehicle or BPA (0.5 and 5 ng/mL) for 24 hr, after which total RNA was extracted for RT-PCR analysis. The analysis was performed three times, and each assay was performed in triplicate. Representative data are shown as the means ± SD. *p<0.05 vs. vehicles.
Figure 11. Rabbit aortic smooth muscle cells (SMCs) proliferation.
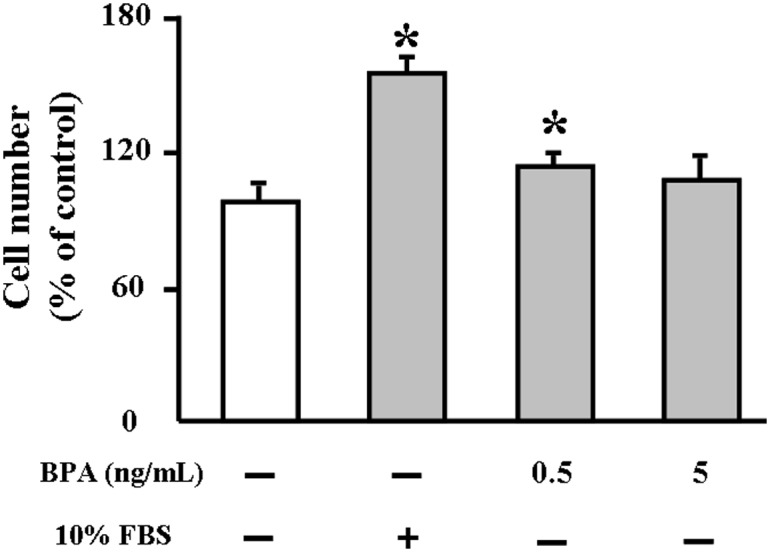
SMCs were treated with either vehicle or BPA (0.5 and 5 ng/mL) for 24 h and 10% fetal bovine serum was used as a positive control. Cell proliferation was determined by CCK-8 assay. Five replicates were performed in each assay. Data are represented as % of control. *p<0.05 vs. vehicles.
Discussion
The present study demonstrates for the first time that treatment with BPA (400 µg/kg body weight) for 12 weeks led to the enhancement of aortic and coronary atherosclerosis in WHHL rabbits. BPA-treated WHHL rabbits exhibited more advanced lesions and increased numbers of smooth muscle cells. BPA could be rapidly absorbed and eliminated in WHHL rabbits within 24 hr, resulting in serum BPA levels in WHHL rabbits that were not only comparable with the typical range found in humans but were also consistent with findings in rhesus monkeys and CD-1 mice [1], [27], suggesting that the serum levels of BPA could not predict the actual levels and toxic effects of BPA exposure in WHHL rabbits, even with large intakes of BPA from diet [35]. Although we controlled the background BPA contamination strictly and were unable to detect BPA in the laboratory blank samples (below the detection limits), there are still some BPA residuals in serum similar to those of normal humans in the vehicle group, possibly derived from other experimental materials or procedures such as oral gavage and blood drawing. This may also suggest that BPA exposure are unavoidable from various routes in the laboratory environment, which is an elusive laboratory challenge and require to be minimized as much as possible in the future study [36]. However, it is notable that the detectable levels of BPA similar to those of normal healthy humans in the vehicle group showed negligible effects on the WHHL rabbits, which is consistent with that in the general population [37]. On the other hand, the Cmax of BPA (3.41 ng/mL) reached in the serum of WHHL rabbits at 0.5 hr after BPA exposure, which is close to that in the old people with atherosclerosis (3.76 ng/mL), may be associated with the enhancement of atherosclerosis [9]. Such serum BPA levels did not significantly affect body weights and plasma lipid levels in WHHL rabbits, suggesting that BPA affects the arterial wall directly rather than by mediating plasma lipids as reported in mice [13]. These observations are consistent with reports that treatment of HUVECs with similar amounts of BPA induced up-regulation of ER stress and inflammation, both of which are involved in the pathogenesis of atherosclerosis [38].
Although the mechanisms responsible for increased SMCs in lesions of BPA-treated WHHL rabbits are unknown, we speculate that BPA may modulate the proliferation of SMCs. BPA treatment led to a mild increase of SMCs in vitro (Figure 11), suggesting that BPA effect on SMC growth may be indirect, possibly through mediation of growth factors derived from endothelial cells [39]. Previous studies have shown that BPA can influence cellular transport mechanisms by mediating Ca2+ channels in some cell types [40], [41]. In cultured human coronary SMCs, BPA has been shown to activate Maxi-K ion channels, which play critical roles in regulating smooth-muscle excitability [42]. Nevertheless, it is not clear whether the BPA-induced SMCs would affect the plaque stability because the lesions are also characterized by having much more lipid-rich and necrotic cores (Figure 5). Meanwhile, BPA exposure affected the functions of endothelial cells, inducing the up-regulation of surface adhesion molecules (VCAM-1), which can increase permeability, enhance leukocyte adhesion, and promote monocyte emigration, contributing to the development of atherosclerosis [43]. Moreover, BPA exposure could also induce various inflammation markers in the endothelial cells and increase the proinflammatory cytokines levels (eg, TNF-α and IL-6) in the serum, all of which may cause the apoptosis of intimal smooth muscle cell and breakdown of matrix [43]. Therefore, it will be necessary to investigate whether BPA can affect the rupture of atherosclerotic plaques.
In addition to BPA’s direct effects on the arterial wall, enhanced atherosclerosis in BPA-treated WHHL rabbits may be attributed to other pathological actions induced by BPA. For example, we found that BPA exposure increased adiposity and induced hypertrophy in adipocytes in both subcutaneous and visceral adipose tissue but that body weight remained unchanged. It has been reported that adipose tissue is the primary target of BPA exposure [44] and that perinatal or postnatal exposure to BPA induces adipocyte hypertrophy in mice and rats that is accompanied by lipogenic gene up-regulation [45], [46]. BPA, a lipophilic compound, can accumulate in adipose tissue, and detectable levels have been found in 50% of breast adipose tissue samples from women [47]. Thus, BPA accumulation in adipose tissue may constitute a secondary source of internal BPA exposure [48].
Moreover, BPA can affect adipocyte differentiation in cultured 3T3-L1 pre-adipocytes, leading to abnormal lipid metabolism [49]. Furthermore, BPA exposure can affect some factors, such as adipokines and cytokines, which are secreted from adipose tissue and can influence the functions of endothelial cells, SMCs, and Mφ in vessel walls [50], [51]. This may constitute another mechanism for BPA-induced enhancement of atherosclerosis [52].
In addition to its effects on adipose tissue, BPA exposure could also impose toxic effects on the liver, including steatosis and inflammatory cell infiltration, but plasma hepatic-marker levels were not increased (data not shown). BPA is rapidly catabolized in the liver within 24 hr, and so the accumulation of high levels of BPA in the liver is certainly detrimental to hepatocyte functions. The pathophysiological significance of BPA-induced amplification of genes related to ER stress, inflammation and lipid metabolism in the liver remains unknown; however, abnormal liver functions accompanied by increased adiposity could certainly contribute significantly to the development of atherosclerosis [50], [53], [54].
An important observation in this study is cardiac damage in BPA-treated WHHL rabbits. Although they require verification, several possible mechanisms may be at play. First, BPA can directly change cardiac functions, as demonstrated by reports that BPA infusion causes ventricular arrhythmias in isolated rat hearts [14], [55]. Second, BPA-induced cardiac damage may be attributed to ischemic effects, as evidenced by BPA-enhanced coronary atherosclerosis in WHHL rabbits. It remains to be seen whether BPA can induce spasms in coronary arteries as a result of BPA-induced up-regulation of ER stress and inflammatory response genes, as observed in cultured endothelial cells in the current study.
Conclusions
BPA is rapidly catabolized in WHHL rabbits within 24 hr, and serum levels of residual BPA were not significantly increased, suggesting that serum levels of BPA cannot predict the actual levels and toxic effects of BPA exposure [56]. Nevertheless, BPA exposure led to increased atherosclerosis in aortic and coronary atherosclerosis. Although the molecular mechanisms are still not completely understood, increased adiposity and impaired liver and cardiac functions induced by BPA treatment may be involved in the enhancement of atherosclerosis. Taken together, our results suggest that BPA exposure may increase susceptibility to atherosclerosis in the setting of hyperlipidemia.
Supporting Information
Primer sequences for quantitative real-time PCR in vivo and in vitro studies.
(PDF)
Technical description of methods.
(PDF)
Acknowledgments
We thank Sun Xia, Yi Lin, Qiansheng Huang, and Liangpo Liu for their help with project planning and measurements of serum BPA.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research was supported by (1) National Natural Science Foundation of China (21207127, 21277137, 21477124, and 41390240), (http://www.nsfc.gov.cn/): CF, SD. (2) Grants-in-aid for scientific research from Ministry of Education, Culture, Sports and Technology, Japan (KAKENHI 22390068), (http://www.mext.go.jp/): CF, JF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, et al. (2010) Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 118: 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calafat AM, Weuve J, Ye X, Jia LT, Hu H, et al. (2009) Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 117: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubin BS (2011) Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem 127: 27–34. [DOI] [PubMed] [Google Scholar]
- 4. Buttke DE, Sircar K, Martin C (2012) Exposures to endocrine-disrupting chemicals and age of menarche in adolescent girls in NHANES (2003–2008). Environ Health Perspect 120: 1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, et al. (2008) Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. J Am Med Assoc 300: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 6. Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS (2010) Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PloS one 5: e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, et al. (2012) Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125: 1482–1490. [DOI] [PubMed] [Google Scholar]
- 8. Melzer D, Gates P, Osborn NJ, Henley WE, Cipelli R, et al. (2012) Urinary bisphenol A concentration and angiography-defined coronary artery stenosis. PloS one 7: e43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lind PM, Lind L (2011) Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis 218: 207–213. [DOI] [PubMed] [Google Scholar]
- 10. Shankar A, Teppala S, Sabanayagam C (2012) Bisphenol A and peripheral arterial disease: Results from the NHANES. Environ Health Perspect 120: 1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lubick N (2010) Cardiovascular health: exploring a potential link between BPA and heart disease. Environ Health Perspect 118: A116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sui Y, Park SH, Helsley RN, Sunkara M, Gonzalez FJ, et al. (2014) Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J Am Heart Assoc 3: e000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim MJ, Moon MK, Kang GH, Lee KJ, Choi SH, et al. (2014) Chronic exposure to bisphenol A can accelerate atherosclerosis in high-fat-fed apolipoprotein E knockout mice. Cardiovas toxicol 14: 120–128. [DOI] [PubMed] [Google Scholar]
- 14. Yan S, Chen Y, Dong M, Song W, Belcher SM, et al. (2011) Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PloS one 6: e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel BB, Raad M, Sebag IA, Chalifour LE (2013) Lifelong exposure to bisphenol A alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci 133: 174–185. [DOI] [PubMed] [Google Scholar]
- 16. Posnack NG, Jaimes R 3rd, Asfour H, Swift LM, Wengrowski AM, et al. (2014) Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ Health Perspect 122: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan J, Watanabe T (2003) Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther 99: 261–282. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi T, Ito T, Shiomi M (2011) Roles of the WHHL rabbit in translational research on hypercholesterolemia and cardiovascular diseases. J Biomed Biotechnol 2011: 406473 doi:10.1155/2011/406473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, et al. (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, et al. (2008) Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect 116: 1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe Y (1980) Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit): incidence and development of atherosclerosis and xanthoma. Atherosclerosis 36: 261–268. [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto T, Bishop RW, Brown MS, Goldstein JL, Russell DW (1986) Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science 232: 1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koike T, Liang J, Wang X, Ichikawa T, Shiomi M, et al. (2004) Overexpression of lipoprotein lipase in transgenic Watanabe heritable hyperlipidemic rabbits improves hyperlipidemia and obesity. J Biol Chem 279: 7521–7529. [DOI] [PubMed] [Google Scholar]
- 24. Koike T, Liang J, Wang X, Ichikawa T, Shiomi M, et al. (2005) Enhanced aortic atherosclerosis in transgenic Watanabe heritable hyperlipidemic rabbits expressing lipoprotein lipase. Cardiovas Res 65: 524–534. [DOI] [PubMed] [Google Scholar]
- 25. Shiomi M, Fan J (2008) Unstable coronary plaques and cardiac events in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits: questions and quandaries. Curr Opin Lipidol 19: 631–636. [DOI] [PubMed] [Google Scholar]
- 26. Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, et al. (2003) Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect 111: 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, et al. (2011) Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ Health Perspect 119: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G (2010) Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol A. Environ Health Perspect. 118: 1051–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24: 139–177. [DOI] [PubMed] [Google Scholar]
- 30. Waqar AB, Koike T, Yu Y, Inoue T, Aoki T, et al. (2010) High-fat diet without excess calories induces metabolic disorders and enhances atherosclerosis in rabbits. Atherosclerosis 213: 148–155. [DOI] [PubMed] [Google Scholar]
- 31. Liu L, Xia T, Zhang X, Barr DB, Alamdar A, et al. (2014) Biomonitoring of infant exposure to phenolic endocrine disruptors using urine expressed from disposable gel diapers. Anal Bioanal Chem 406: 5049–5054. [DOI] [PubMed] [Google Scholar]
- 32. Koike T, Kitajima S, Yu Y, Nishijima K, Zhang J, et al. (2009) Human C-reactive protein does not promote atherosclerosis in transgenic rabbits. Circulation 120: 2088–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin Y, Sun X, Qiu L, Wei J, Huang Q, et al. (2013) Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis 4: e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor JA, Welshons WV, vom Saal FS (2008) No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod toxicol 25: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li S, Liang J, Niimi M, Bilal WA, Kang D, et al. (2014) Probucol suppresses macrophage infiltration and MMP expression in atherosclerotic plaques of WHHL rabbits. J Atheroscler Thromb 21: 648–658. [DOI] [PubMed] [Google Scholar]
- 36. Ye X, Zhou X, Hennings R, Kramer J, Calafat AM (2013) Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environ Health Perspect 121: 283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hengstler J, Foth H, Gebel T, Kramer P-J, Lilienblum W, et al. (2011) Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol. 41: 263–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hotamisligil GS (2010) Endoplasmic reticulum stress and atherosclerosis. Nat med 16: 396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Libby P, Ridker PM, Maseri A (2002) Inflammation and atherosclerosis. Circulation 105: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 40. Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, et al. (2005) Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect 113: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walsh DE, Dockery P, Doolan CM (2005) Estrogen receptor independent rapid non-genomic effects of environmental estrogens on [Ca2+] in human breast cancer cells. Mol Cell Endocrinol 230: 23–30. [DOI] [PubMed] [Google Scholar]
- 42. Asano S, Tune JD, Dick GM (2010) Bisphenol A activates Maxi-K (K(Ca)1.1) channels in coronary smooth muscle. Brit J Pharmacol 160: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbins SL, Kumar V, Abbas AK, Aster JC (2012) Robbins basic pathology (9th ed). In: Richard NM, editors. Blood vessels. Philadelphia: Elsevier Health Sciences. 338–344.
- 44. Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, et al. (2012) Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 55: 395–407. [DOI] [PubMed] [Google Scholar]
- 45. Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H (2007) Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb 14: 245–252. [DOI] [PubMed] [Google Scholar]
- 46. Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, et al. (2009) Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 117: 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandez M, Arrebola J, Taoufiki J, Navalón A, Ballesteros O, et al. (2007) Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod toxicol 24: 259–264. [DOI] [PubMed] [Google Scholar]
- 48. La Merrill M, Emond C, Kim MJ, Antignac J-P, Le Bizec B, et al. (2013) Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect 121: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K (2005) Bisphenol A accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci 84: 319–327. [DOI] [PubMed] [Google Scholar]
- 50. Fantuzzi G, Mazzone T (2007) Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol 27: 996–1003. [DOI] [PubMed] [Google Scholar]
- 51. Phrakonkham P, Viengchareun S, Belloir C, Lombès M, Artur Y, et al. (2008) Dietary xenoestrogens differentially impair 3T3-L1 preadipocyte differentiation and persistently affect leptin synthesis. J Steroid Biochem Mol Biol 110: 95–103. [DOI] [PubMed] [Google Scholar]
- 52. Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA (2012) The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol 354: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Targher G, Marra F, Marchesini G (2008) Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 51: 1947–1953. [DOI] [PubMed] [Google Scholar]
- 54. Guleria A, Duseja A, Kalra N, Das A, Dhiman R, et al. (2013) Patients with non-alcoholic fatty liver disease (NAFLD) have an increased risk of atherosclerosis and cardiovascular disease. Trop. Gastroenterol 34: 74–82. [DOI] [PubMed] [Google Scholar]
- 55. Yan S, Song W, Chen Y, Hong K, Rubinstein J, et al. (2013) Low-dose bisphenol A and estrogen increase ventricular arrhythmias following ischemia-reperfusion in female rat hearts. Food Chem Toxicol 56: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brent RL (2013) Urine chemical content nay be a false measure of environmental exposure. Pediatrics 132: e747–e748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for quantitative real-time PCR in vivo and in vitro studies.
(PDF)
Technical description of methods.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.