Abstract
The neuronal dendritic tree is a key determinant of how neurons receive, compute, and transmit information. During early postnatal life, synaptic activity promotes dendrite elaboration. Spinal motor neurons utilize GluA1-containing AMPA (2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid) receptors (AMPA-R) to control this process. This form of developmental dendrite growth can occur independently of N-methyl-D-aspartate receptors (NMDA-R). This review focuses on the mechanism by which the GluA1 subunit of AMPA-R transforms synaptic activity into dendrite growth, and describes the essential role of the GluA1 binding partner SAP97 (synapse-associated protein of 97 kDa molecular weight) in this process. This work defines a new mechanism of activity-dependent development, which might be harnessed to stimulate the recovery of function following insult to the central nervous system.
Keywords: dendrite, activity dependent, AMPA, GluA1, growth, development
Introduction
Over the past several decades, it has become increasingly clear that nervous system development can be roughly divided into two periods: (1) a genetically driven, activity-independent phase that sets a rough wiring diagram, and (2) an activity-dependent phase that refines connectivity under the supervision of active synapses. During embryonic and very early postnatal life, spontaneous synaptic activity drives circuit maturation. Subsequently, environmentally evoked synaptic activity (experience-dependent development) plays the prominent role in shaping nervous system maturation. Together, these processes lead to the precisely patterned connectivity among neurons that underlies purposeful behavior.
Activity-dependent development
Activity-dependent development during pre- and postnatal life is an important mechanism for the specification of synaptic phenotype and connectivity. Here, we will review some of the key experiments undertaken in the visual system that highlight the cell biological processes underlying activity-dependent development. These introductory remarks provide the context for thinking about the role of activity-dependent processes in motor system development.
In the mammalian visual system, retinal ganglion cells (RGC) project to the lateral geniculate nucleus (LGN) of the thalamus. Thalamocortical (TC) connections from the LGN project to the visual cortex. In mature animals, right and left eye afferents are segregated from each other in both the LGN and visual cortex.1,2 For example, in adult cats and primates, TC afferents projecting to layer four of the visual cortex are organized into discrete right and left eye patches, referred to as ocular dominance columns (OCD).3 This circuitry arrangement subserves high acuity vision.4
In their classic work, Hubel and Weisel showed that this pattern of visual cortex innervation is not present at birth, as right and left eye afferents demonstrate extensive overlap.5 Most remarkably, they show that the segregation of right and left eye afferents is driven by visual experience.5 Monocular deprivation of one eye (by suturing the eyelid shut) during a discrete period in early postnatal life leads to a dramatic shift in TC innervation of the visual cortex.5 The afferents from the nondeprived eye innervate a larger territory of the visual cortex, while those from the deprived eye innervate a smaller territory.5 This experience-dependent shift in ocular dominance leaves a permanent imprint on visual system organization.5
How does environmentally evoked synaptic activity lead to changes in synaptic strength and connectivity? Substantial evidence supports the view that synapses will undergo strengthening and stabilization when the activity of pre- and postsynaptic elements is coincident. This model of synaptic plasticity was originally suggested in theoretical work by Daniel Hebb and has been most rigorously tested in long-term potentiation (LTP) paradigms.6 Many forms of LTP depend upon the activation of N-methyl-D-aspartate receptors (NMDA-Rs). NMDA-Rs are believed to be the coincidence detectors responsible for detecting the simultaneous activity of pre- and postsynaptic elements.7 The ionic mechanism underlying this process has been linked to the voltage-dependent block of NMDA-Rs by Mg2+.7 Patterned afferent input, sufficient to remove the Mg2+ block, allows NMDA-Rs to conduct Ca2+ influx, which inactivates several protein kinases, including Ca2+/calmodulin-dependent protein kinase II (CamKII).7 CamKII is necessary for the maintenance of LTP and phosphorylation of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid receptor (AMPA-R) subunits to increase their conductance.8 These and other calcium-activated processes drive the observed synaptic plasticity.8,9
In the visual system, activity-dependent processes drive large-scale alterations in the architecture of axons and dendrites. How do activity-dependent changes in synapses control the growth and distribution of axons and dendrites? Vaughn proposed the synaptotropic hypothesis: dendritic branches are formed near active synapses and synapse stablization consequently stabilizes dendrites.10 Beautiful in vivo work from the Haas lab implicates beta-neurexin (NRX) and neuroligin-1 (NLG1) in this process, in which NMDA-R–dependent synapse maturation was required for persistent NRX-NLG1 function in dendritogenesis.11 Blocking synaptogenesis thereby blocks dendrite outgrowth stabilization. The linkage of synaptic plasticity to neurite architecture is a fundamental principle in developmental neuroscience and provides an explanation for earlier observations that the size and complexity of the dendrite tree controls the qualitative and quantitative nature of the afferent input.12 In studies of rabbit ciliary ganglia, the number of ganglion cell primary dendrites is highly correlated with the number of innervating axons.13 This is not true of some of the cells in the neonate, where the initial set of inputs is confined to the cell body, allowing only one axon to survive.13 It is hypothesized that the complexity of some cells allows for a higher number of afferents to innervate the ganglion cell.13 Thus, a competition-based model of synapse formation holds for dendrite growth.
While many studies implicate AMPA-Rs in the control of dendrite growth, a consistent picture has yet to emerge. Blocking AMPAergic transmission in retinotectal neurons decreases synapse stabilization, and subsequent dendrite growth and stabilzation.14 Conversely, Casticas et al. showed that enhanced conductance of Ca2+-permeable AMPA-Rs inhibited neurite outgrowth in dissociated chick retinal neurons.15 Outside of the visual system, blockade of AMPA-Rs in chick motoneurons has also been seen to increase dendritic outgrowth in chick motoneurons, but only at certain time points in embryonic development.16 It is unclear what role NMDA-Rs played in these processes because blocking AMPA-Rs will prevent NMDA-R activation.
In the central nervous system (CNS), it is understood that activation of AMPA-Rs, sufficient to relieve the voltage-dependent magnesium block of NMDA-Rs, drives activity-dependent plasticity, synaptic stabilization, and patterned innervation.17 Less understood is the extent to which NMDA-R–independent mechanisms can drive activity-dependent developmental processes. Below we describe work in the spinal cord showing how AMPA-Rs assembled with the GluA1 subunit can promote activity-dependent development by an NMDA-R–independent process.
GluA1 promotes activity-dependent dendrite growth
Neonatal motor neurons express very high levels of GluA1 (both mRNA and protein). The properties of GluA1 can be modified by alternative splicing and editing at the glutamine/arginine Q/R site. The GluA1 expressed during this developmental period contains the flip alternatively spliced exon and is unedited in the Q/R site.18,19 Previous work has shown that neonatal motor neurons express Ca2+-permeable AMPA-Rs (as one would expect if they were enriched with GluA1(Q)).19 Taken together with the electrophysiological data, the work suggests that many AMPA-Rs are assembled with GluA1 homomers at this point in the development of motor neurons.
The unusually high level of GluA1 expression by neonatal motor neurons raises the possibility that AMPA-Rs assembled with GluA1 play a special role in activity-dependent motor system development. To examine this notion, we began by asking whether manipulation of GluA1 influenced spinal neuron dendritic architecture. Several approaches were taken. First, we found that knockdown of GluA1 expression inhibits dendrite growth. Conversely, overexpression of GluA1 in spinal neurons in vitro stimulates dendritic growth; this growth effect was blocked by the AMPA-R antagonist, CNQX (6-cyano-7-nitroquinoxaline-2, 3-dione).20 Second, we compared the effects of two types of overexpressed GluA1 in motor neurons in vivo. We used a version that robustly passes current (GluA1(Q)) and compared that with a version that passes very little current (GluA1(R)). Only overexpressed GluA1(Q) stimulated dendritic branching.21 These results suggest that the activity of AMPA-Rs assembled with GluA1 is a crucial step for dendrite growth and this effect is Ca2+ dependent. Subsequent in vitro work indicates that the degree of calcium permeability of AMPA-Rs assembled with GluA1 controls the dendritic growth process.21
One interpretation of the above results is that overexpression of GluA1 enhances neuronal depolarization, thereby promoting NMDA-R–mediated events. We think this is not true for a number of reasons. First, our in vivo observations were made in juvenile rodents at a time when motor neurons do not express NMDA-R.22 It is possible that prior in situ hybridization and immunohistological studies were insufficiently sensitive to detect NMDA-Rs in juvenile motor neurons. To address this possibility, we expressed GluA1(Q) in juvenile animals and simultaneously treated them with the NMDA-R antagonist, MK-801.23 We know that MK-801 was administered in an effective dose because LTP could not be evoked in these animals.23 Nonetheless, MK-801 did not block the pro-dendrite growth actions of overexpressed GluA1(Q).23 Second, we undertook in vitro pharmacological studies. Administration of MK-801 did not block the dendrite growth–promoting actions of GluA1(Q); in contrast, administration of the L-type calcium channel blocker nifedipine did block the GluA1(Q) effect.24 Taken together, these results suggest that GluA1 is sufficient to promote dendrite growth in an NMDA-R–independent manner.
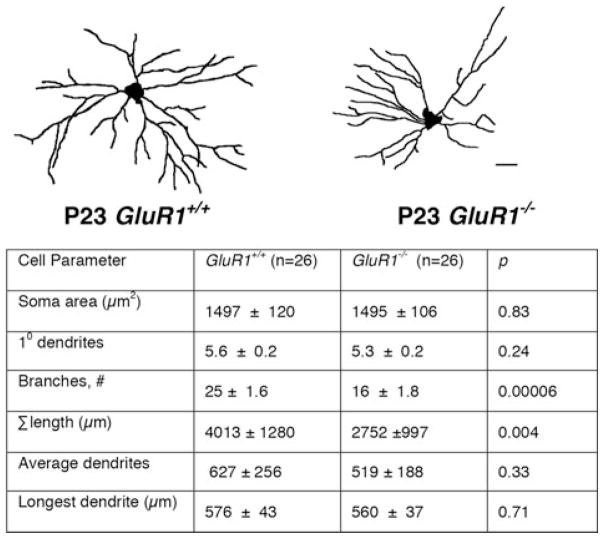
The work described previously primarily focuses on the effects of GluA1 on dendrite architecture in vitro. What about in vivo? To address this question, studies of the GluA1−/− mouse have been informative.20 Analysis of the dendritic tree reveals that motor neurons from GluA1−/− animals are smaller and less branched at P10 and P23 (see Fig. 1).20 This suggests that GluA1−/− motor neurons develop over a different trajectory than wild-type motor neurons. How does this decrease in the size and complexity of the motor neuron dendrite tree affect motor circuitry and behavior of the animal? To study the innervation of motor neurons within the segmental spinal cord, a recombinant pseudorabies virus engineered to express green fluorescence protein (PRV-GFP) was used. PRV-GFP labeling experiments revealed a distinct pattern of interneuronal connectivity in the spinal cord of GluA1−/− mice in comparison to wild-type mice. The greatest difference between genotypes was found in the number of contralaterally located interneurons, especially in Rexed’s lamina VIII.20 The stunted dendrite tree and change in interneuron connectivity correlated with a locomotor defect in the GluA1−/− animals. In comparison to wild-type counterparts, GluA1−/− mice showed poorer performance in grip strength, treadmill, and rotarod at P23 and adulthood.20 This suggests that GluA1 is important not only for dendrite growth, but also for patterning segmental spinal cord circuitry and motor behavior. Furthermore, changes in development during the postnatal period lead to deficits throughout adulthood.20
Figure 1.
Deletion of GluA1 from mice results in motor neurons with a smaller and simplified dendritic arbor (from Ref. 20). Spinal motor neurons of GluA1−/− mice compared to WT mice at P23. Mice at P23 have motor neurons with significantly fewer branches (the average WT dendritic tree has 25 ± 1.6 branches, compared to that of GluA1−/−, which has only 16 ± 1.8 branches (P < 0.0001)) and a smaller total tree size (the average dendritic tree of WT has a total length of 4013 ± 1280 μM, compared to 2750 ± 997 μM in the GluA1−/− mice (P < 0.05)). This effect is unique to the dendritic arbor, in that motor neuron soma size remains unchanged.
By what molecular mechanism does the activity of AMPA-Rs assembled with GluA1 control the morphology of motor neuron dendritic architecture? A series of experiments have indicated that the multidomain scaffolding protein, synapse-associated protein of 97 kDa molecular weight (SAP97), interacts with GluA1 and plays a key role in this process.25 The C-terminal seven amino acids of GluA1 physically interact with the second PDZ domain of SAP97. AMPA-R physiology and synaptic plasticity is entirely normal when the physical interaction between GluA1 and SAP97 is disrupted.26 This was demonstrated using mice in which the wild-type allele of GluA1 was replaced by a version of GluA1 lacking the C-terminal seven amino acids (GluA1Δ7 mice).26 Contrary to expectations, GluA1 also traffics normally to the cell surface in these mice, suggesting GluA1 chaperones SAP97 to synapses—not vice versa. This was demonstrated further using biochemical and imaging methodologies.25 However, the normal elaboration of motor neuron dendrites requires SAP97, which was demonstrated in vitro: knockdown of SAP97 decreases the total size of the dendritic tree and prevents the pro-dendrite growth effect of GluA1 overexpression.25 This was further confirmed in vivo: a smaller dendritic tree was found in the GluA1Δ7 mice (where SAP97 does not traffic to the cell surface) as well as mice with a conditional deletion of SAP97 from motor neurons.25 Finally, co-overexpression of GluA1 and SAP97 in vitro has a synergistic pro-dendrite growth effect, which depends solely on their colocalization at the plasma membrane, not on their physical association.25 This was demonstrated using a membrane-targeted version of SAP97 in conjunction with GluA1Δ7 or a membrane-targeted version of SAP97 with mutations in its PDZ2 domain that make it incapable of binding GluA1.25
Together, these experiments suggest that the endogenous GluA1–SAP97 complex is the necessary platform upon which GluA1-containg AMPA-R activity is translated into signals that stimulate dendritic growth and branching in the spinal cord.
Identifying downstream machinery and mechanisms
How do GluA1 and SAP97 work together to promote dendrite growth? One hypothesis is that GluA1 and SAP97 help form a complex during GluA1-containing AMPA-R activity to activate downstream effectors capable of stimulating dendritic growth. At this time, the molecular mechanism by which SAP97 and GluA1 promote dendritic growth and branching is unknown. We do, however, have evidence that the PDZ3 domain of SAP97 is crucial for these pro-growth effects of SAP97 and GluA1 (unpublished observations). Overexpression of SAP97 with a mutation in the PDZ3 domain no longer increases dendrite growth. Thus, it is logical to ask whether proteins that bind to the PDZ3 domain of SAP97 are part of the machinery to translate activity into growth. This is one current avenue for research, and key downstream players in the GluA1-mediated process are beginning to be identified.
Conclusions
Much research in the field of activity-dependent development has concentrated on the NMDA-subtype of glutamate receptors, although excellent work indicates that NMDA-R–independent forms of activity-dependent development exist. The mechanism by which AMPA-R assembled with GluA1 promote dendrite growth and branching in the spinal cord is one key example of a form of plasticity that is NMDA-R independent. In addition, one challenge in identifying the functions of many MAGUK proteins, such as PSD-95 or SAP97, has been their redundant functions in relation to synaptic plasticity.27,28 Remaining members of the membrane-associated guanylate kinases (MAGUK) family can compensate for loss of one MAGUK, but other MAGUK family members in the SAP97 conditional knockout do not compensate for the pro-growth effects of SAP97 on the dendrite tree. This suggests that this property of SAP97 is unique, and raises the possibility that proteins in the postsynaptic density involved in this process are unique as well.
Finally, understanding how dendrite growth is promoted during early postnatal life can provide insight into how one may intervene later in life to promote recovery following CNS insult. Part of the evidence supporting this idea lies in the fact that, following spinal cord injury, repetitive activation of specific neuronal pathways can result in a significant improvement in motor behavior via the increase in AMPA-ergic transmission, resulting in the stablization of dendrites and synapses.29,30 If we can better understand the mechanism underlying this phenomenon, we might be able to restore the features unique to early postnatal life, allowing for dendrite growth in patients following injury.
Acknowledgments
We wish to acknowledge the excellent work of Lei Zhang and Weiguo Zhou, which makes up a large portion of the work described here. This work was supported by the National Institutes of Health Grants R21NS060754 and R01NS052325 (to R.G.K.).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 2.Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 3.Shatz CJ. Emergence of order in visual system development. Proc Natl Acad Sci USA. 1996;93:602–608. doi: 10.1073/pnas.93.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey triate cortex. Phil Trans R Sec Lond. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 6.Hebb DO. The Organization of Behavior. John Wiley & Sons, Inc; New York: 1949. [Google Scholar]
- 7.Seeburg PH, Burnashev N, Kohr G, et al. The NMDA receptor channel: molecular design of a coincidence detector. Recent Progr Hormone Res. 1995;50:19–34. doi: 10.1016/b978-0-12-571150-0.50006-8. [DOI] [PubMed] [Google Scholar]
- 8.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Ann Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 9.Luthi A, Wikstrom MA, Palmer MJ, et al. Bidirectional modulation of AMPA receptor unitary conductance by synaptic activity. BMC Neurosci. 2004;5:44. doi: 10.1186/1471-2202-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- 11.Chen SX, Tari PK, She K, Haas K. Neurexin-neuroligin cell adhesion complexes contribute to synaptotropic dendritogenesis via growth stabilization mechanisms in vivo. Neuron. 2010;67:967–983. doi: 10.1016/j.neuron.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Oswald S. Principles of Cellular, Molecular, and Devlopmental Neuroscience. Springer-Verlag; New York: 1989. [Google Scholar]
- 13.Hume RI, Purves D. Geometry of neuonatal neurones and the regulation of synapse of elimination. Nature. 1981;293:469–471. doi: 10.1038/293469a0. [DOI] [PubMed] [Google Scholar]
- 14.Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in the intact brain. Proc Natl Acad Sci. 2006;103:12127–12131. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casticas M, Allcorn S, Mobbs P. Early activation of Ca(2+)-permeable AMPA receptors reduces neurite outgrowth in embryonic chick retinal neurons. J Neurobiol. 2001;49:200–211. doi: 10.1002/neu.1075. [DOI] [PubMed] [Google Scholar]
- 16.Ni X, Martin-Caraballo M. Differential effect of glutamate receptor blockade on dendritic outgrowth in chiken lumbar motoneurons. Neuropharmacology. 2010;58:593–604. doi: 10.1016/j.neuropharm.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Peng YR, He S, Marie H, et al. Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron. 2009;61:71–84. doi: 10.1016/j.neuron.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakowec MW, Yen L, Kalb RG. In situ hybridization analysis of AMPA receptor subunit gene expression in the developing rat spinal cord. Neuroscience. 1995;67:909–920. doi: 10.1016/0306-4522(95)00094-y. [DOI] [PubMed] [Google Scholar]
- 19.Jakowec MW, Fox AJ, Martin LJ, Kalb RG. Quantitative and qualitative changes in AMPA receptor expression during spinal cord development. Neuroscience. 1995;67:893–907. doi: 10.1016/0306-4522(95)00026-f. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Schessl J, Werner M, et al. Role of GluR1 in activity-dependent motor system development. J Neurosci. 2008;28:9953–9968. doi: 10.1523/JNEUROSCI.0880-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong GB, Werner M, Gazula VR, et al. Bidirectional control of motor neuron dendrite remodeling by the calcium permeability of AMPA receptors. Mol Cell Neurosci. 2006;32:299–314. doi: 10.1016/j.mcn.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Stegenga SL, Kalb RG. Developmental regulation of N-methyl-D-aspartate- and kainate-type glutamate receptor expression in the rat spinal cord. Neuroscience. 2001;105:499–507. doi: 10.1016/s0306-4522(01)00143-9. [DOI] [PubMed] [Google Scholar]
- 23.Inglis FM, Crockett R, Korada S, et al. The AMPA receptor subunit GluR1 regulates dendritic architecture of motor neurons. J Neurosci. 2002;22:8042–8051. doi: 10.1523/JNEUROSCI.22-18-08042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalb RG, Zhang L, Zhou W. The molecular basis of experience-dependent motor system development. Progr Motor Contr. 2013;782:23–38. doi: 10.1007/978-1-4614-5465-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou W, Zhang L, Guoxiang X, et al. GluR1 controls dendrite growth through its binding partner, SAP97. J Neurosci. 2008;28:10220–10233. doi: 10.1523/JNEUROSCI.3434-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CH, Takamiya K, Petralia RS, et al. Persistent hippocampal CA1 LTP in mice lacking the C-terminal PDZ ligand of GluR1. Nat Neurosci. 2005;8:985–987. doi: 10.1038/nn1432. [DOI] [PubMed] [Google Scholar]
- 27.Howard MA, Elias GM, Elias LA, et al. The role of SAP97 in synaptic glutamate receptor dynamics. Proc Natl Acad Sci. 2010;107:3805–3810. doi: 10.1073/pnas.0914422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schluter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Spooren AI, Janssen-Potten YJ, Snoek GJ, et al. Rehabilitation outcome of upper extremity skilled performance in persons with cervical spinal cord injuries. J Rehabil Med. 2008;40:637–644. doi: 10.2340/16501977-0231. [DOI] [PubMed] [Google Scholar]
- 30.Engesser-Cesar C, Anderson AJ, Basso DM, et al. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]