Abstract
Parkinson’s disease (PD) is characterized by the progressive degeneration of nigrostriatal dopaminergic neurons leading to motor deficits. The mechanisms underlying the preferential vulnerability of nigrostriatal dopaminergic neurons in PD remain poorly understood. Recent evidence supports a role for mitochondrial dysfunction and increased oxidative stress in PD pathogenesis. Genetic and pathological studies also point to alpha-synuclein as a critical factor in both familial and sporadic forms of the disease; alpha-synuclein pathology affects mitochondrial function but is widespread in PD brain, raising the question of its role in the greater vulnerability of nigrostriatal neurons in PD. We have examined mitochondrial function and oxidative damage in mice overexpressing human wild type alpha-synuclein broadly throughout the nervous system under the Thy1 promoter (Thy1-aSyn mice) between 4 and 8 months of age. Similar levels of alpha-synuclein accumulation in mitochondria were detected in the ventral midbrain, striatum and cortex of Thy1-aSyn mice. However, analysis of mitochondrial respiration using Seahorse XF analyzer showed defects in mitochondrial respiratory complexes I, II, IV and V specifically in the midbrain, and IV and V in the striatum, of Thy1-aSyn mice compared to wild type littermates; mitochondrial complex I activity assay by ELISA confirmed a 40% inhibition specifically in the ventral midbrain. Mitochondrial dysfunction can contribute to oxidative stress and we observed a 40% increase in 4-hydroxynenal and 2-fold increase in malondialdehyde levels, indicative of a high level of lipid peroxidation, specifically in ventral midbrain of Thy1-aSyn mice. The levels of peroxiredoxin 2, a neuronal antioxidant enzyme that is involved in removal of H2O2 and other toxic peroxides were decreased in the midbrain whereas its oxidized form increased 4-fold, suggesting that antioxidant defences were compromised in this region. In contrast, peroxiredoxin 2 increased in striatum and cortex, which may contribute to their protection in the presence of high levels of alpha-synuclein. Thus, in mice over-expressing alpha-synuclein, mitochondrial dysfunction occurred preferentially in nigrostriatal dopaminergic neurons many months before striatal dopamine loss occurs at 14 months of age. This may contribute to a higher level of oxidative stress that overwhelms antioxidant defence in these neurons, leading to their increased vulnerability in PD.
Keywords: Alpha synuclein, mitochondrial dysfunction, peroxiredoxin, lipid peroxidation, oxidative damage
INTRODUCTION
Mitochondrial dysfunction and oxidative stress have been implicated in the pathophysiology of PD for many years and recent data have revived interest in these mechanisms (Subramaniam and Chesselet, 2013). Both are linked in a vicious cycle to which the point of entry may vary depending of the cause of PD. For example, mutations in PINK 1 and parkin that cause early onset recessive familial forms of PD may primarily affect the clearance of defective mitochondria, causing oxidative stress as a secondary effect (Bueler, 2010, McCoy and Cookson, 2012). In contrast, environmental toxins such as paraquat, a redox cycler that produces oxidative stress, may directly damage mitochondria that are affected by high oxygen free radicals (Jones and Vale, 2000, Yumino et al., 2002, Mollace et al., 2003, Thiruchelvam et al., 2005). The dopaminergic neurons may be particularly vulnerable to either source of damage because their spontaneous firing properties leads to high calcium load which may alter mitochondrial function (Chan et al., 2007). This combines with their high level of dopamine, leading to the formation of quinones, increased reactive oxygen species, and oxidative stress (Berman and Hastings, 1999, Rabinovic et al., 2000, Gluck and Zeevalk, 2004, Chinta and Andersen, 2008, Gautam and Zeevalk, 2011). In support of this scenario, epidemiological studies have shown that calcium blockers that penetrate the blood brain barrier decrease PD risks (Ritz et al., 2010), while genetic polymorphisms in genes regulating dopamine homeostasis modulate PD risk, in particular in the presence of exogenous toxins (Ritz et al., 2009, McGuire et al., 2011, Wang et al., 2011). Thus, a combination of intrinsic factors may make the nigrostriatal dopaminergic neurons more vulnerable than other neurons to external triggers of pathology in PD.
Alpha-synuclein, a protein that accumulates widely in central and peripheral neurons of PD patients, is likely a major player in PD pathophysiology (Lansbury and Lashuel, 2006, Uversky, 2007, Cookson and van der Brug, 2008, Winklhofer et al., 2008). Indeed genetic evidence that mutations in the PD gene cause familial forms of PD indicates a causal role of alpha-synuclein in PD, while genome wide associations have validated a strong link between alpha-synuclein and PD (Spillantini and Goedert, 2000, Edwards et al., 2010). Like other proteins involved in PD, alpha-synuclein is present in mitochondria in PD brain and affects mitochondrial function in vitro and in vivo (Li et al., 2007, Devi et al., 2008, Nakamura et al., 2008, Shavali et al., 2008, Zhang et al., 2008). It is unclear, however, whether this effect of alpha-synuclein on mitochondria is regionally specific and could contribute to the greater vulnerability of nigrostriatal dopaminergic neurons in PD. To address this question, we turned to a mouse model of broad overexpression of alpha-synuclein under the Thy-1 promoter (Rockenstein et al., 2002, Chesselet et al., 2012). We measured alpha-synuclein levels in mitochondrial fractions isolated from different brain regions, mitochondrial function with the Seahorse apparatus, complex 1 activity, lipid peroxidation and peroxiredoxin 2 (Prdx2) levels in ventral midbrain (VM/SN), striatum, and cortex. We show that Thy1-aSyn mice exhibit impairments of mitochondrial respiratory complexes including complex I specifically in brain regions that contain the nigrostriatal dopaminergic neurons. These alterations were accompanied by regionally specific changes in levels of Prdx2 and of its oxidized form, and in levels of lipid peroxidation, suggesting that greater alterations in mitochondrial function by alpha-synuclein may contribute to the vulnerability of nigrostriatal dopaminergic neurons in PD.
Materials and Methods
Animals
Mice over-expressing human, full length, wild type alpha synuclein under the murine Thy1-promoter (Thy1-aSyn mice) were developed previously by the laboratory of Dr. E. Masliah at UCSD and were designated as ‘line 61’ (Rockenstein et al. 2002). Thy1-aSyn mice are maintained on a hybrid C57BL6/DBA2 background as described previously (Rockenstein et al., 2002, Chesselet et al., 2012). The genotypes of all Thy1-aSyn and wild-type (WT) mice were determined at 1 month of age and confirmed at the end of the experiment by polymerase chain reaction (PCR) amplification analysis of tail DNA. Male transgenic and their male wild type littermates from multiple litters were used in this study after performing a power analysis to determine the sample size using GB-STAT software. Animals were grouped not more than 4 in each cage and maintained on a reverse light/dark cycle. Food and water were available ad libitum. Animal care was accomplished in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals and all experimental procedures were approved by the UCLA Institutional Care and Use Committee.
Mice aged 4-months, 6-months or 8-months were euthanized by cervical dislocation and their brains were removed quickly. Immediately, brain regions including VM/SN, striatum and cortex (overlying the striatum) were dissected out using a coronal brain matrix on ice-cold glass plate. Fresh brain tissue from one hemisphere was used for mitochondrial isolation and the other hemisphere was stored at −80°C for other biochemical assays.
Isolation of mitochondria
Mitochondria from VM/SN, striatum and cortex were isolated immediately following the manufacturer’s protocol (Sigma; #MITOISO1). Briefly, fresh brain tissue was homogenized with 10 volumes of 1x extraction buffer (10 mM HEPES, pH 7.5, 200 mM mannitol, 70 mM sucrose and 1 mM EGTA) containing 2mg/ml albumin on ice at 4°C. The homogenate was centrifuged at 600 x g for 5 min. The supernatant was removed and centrifuged at 11,000 x g for 10 min. Then the pellet was resuspended in 10 volumes of extraction buffer. Resuspended homogenate was centrifuged at 600 x g for 5 min and the subsequent supernatant was centrifuged at 11,000 x g for 10 min. The pellet was suspended in 1x storage buffer (10 mM HEPES, pH 7.4, 250 mM sucrose, 1 mM ATP, 0.08 mM ADP, 5 mM sodium succinate, 2 mM potassium phosphate dibasic and 1 mM DTT) and assayed for protein concentration by the Bradford essay.
Western blotting
For Western blotting analysis, brain tissue was homogenized in 3 volumes of ice-cold modified radioimmunoprecipitation assay (RIPA) lysis buffer (50mM Tris HCl, pH 7.5, 1% NP-40, 0.25% sodium deoxycholate, 150mM NaCl and 1mM EDTA) containing protease inhibitors (Complete protease inhibitor tablets, Roche, USA; #11697498001) and phosphatase inhibitors (PhosphoSTOP, Roche, USA; #0-4906845001). The homogenates were centrifuged at 13,000 rpm for 15 min at 4°C and the supernatant was retained for Western blotting. Protein concentration was determined by the Bradford assay. Sample buffer (Invitrogen, NuPAGE LDS; NP0007) was added to the samples, heated at 70°C for 10 min, and 10–25μg of protein was separated on 10% Bis-Tris gel (Invitrogen, NuPAGE Novex; #NP0303BOX) using 1X SDS running buffer (Invitrogen, NuPAGE MOPS; #NP0001) at 120V for ~90 min. The resolved proteins were transferred to nitrocellulose membrane using Invitrogen X-cell II blot module apparatus at 200mA for 1.5h. Following transfer, protein binding sites were blocked with blocking buffer (StartingBlock T20, Thermo Scientific, USA; #37539) for 1h. Then the membrane was incubated with mouse anti-α-synuclein monoclonal antibody (BD transduction laboratories, USA; #610787; at 1:5000 dilution), mouse anti-porin monoclonal (Abcam, USA; #ab14734; at 1:20000 dilution), mouse anti-GAPDH (Millipore, USA; #MAB374; at 1:20000 dilution), rabbit anti-LAMP2 (Novus Biologicals, USA; #NBP1-95696; at 1:1000 dilution), mouse anti-Prdx2 antibody (Abcam, USA; #ab16738; at 1:2000 dilution), rabbit anti-Prdx-SO3 antibody (Abcam, USA; #ab16830; at 1:2000 dilution) or rabbit anti-beta-actin antibody (Abcam, USA; #ab75186; at 1:5000 dilution) in blocking buffer overnight at 4°C. After incubation with primary antibodies the membrane was washed with PBST (0.2% tween in 0.1M phosphate buffer saline) and incubated with goat anti-mouse (Millipore, USA; #AP127P; at 1:20,000 dilution) or goat anti-rabbit (Millipore, USA; #AP156P; at 1:10,000 dilution) horseradish peroxidase-conjugated antibodies in blocking buffer for 1h. The blots were then developed using SuperSignal West Dura (Thermo Scientific, USA; #34075) and exposed to Kodak biomax film (Sigma, USA; #Z373508). The resulting bands were scanned and quantified using ImageJ analysis software (NIH). The optical density values (OD) were normalized to loading control porin (mitochondria enriched fraction) or beta-actin (whole lysate) and graphically presented as fold difference or percentage fold difference compared to wild-type control after statistical analysis on absolute values.
Mitochondrial DNA content
Total genomic DNA from brain tissue was isolated by phenol/chloroform/isoamyl alcohol extraction. Mitochondrial and nuclear DNA were amplified by qPCR with 50 ng of DNA and primers in the D-Loop region (5′-aatctaccatcctccgtgaaacc; tcagtttagctacccccaagtttaa-3′) and Tert gene (5′-ctagctcatgtgtcaagaccctctt; gccagcacgtttctctcgtt-3′), respectively (Vergnes et al., 2011).
Measurement of respiration from isolated mitochondria
An XF24 analyzer (Seahorse Bioscience) was used to measure respiration from freshly isolated mitochondria (4 μg of mitochondrial protein per well). Two assays were used, as described (Rogers et al., 2011). In the coupling assay, basal respiration was driven by 10 mM succinate (complex II respiration) in presence of 2 μM rotenone. Four mM ADP, 2.5 μM oligomycin, 4 μM FCCP, and 1.5 μg/ml antimycin A were sequentially injected to measure state 3, state 4o, state 3u, and to inhibit respiration, respectively. In the electron flow assay, basal respiration was driven by 10 mM pyruvate and 2 mM malate (complex I respiration). Two μM rotenone, 10 mM succinate, 1.5 μg/ml antimycin A, and 0.1 mM TMPD were sequentially injected to inhibit complex I, measure complex II, inhibit complex III and measure complex IV respiration, respectively. Measurements were done on four Thy1-aSyn mice and four wild-type littermates in triplicates that were averaged for mouse.
Complex I activity assay
Mitochondrial OXPHOS complex I activity was determined by immunocapture ELISA following the manufacturer’s protocol (complex I enzyme activity assay kit, Abcam, USA; #ab109721). Briefly, 50 μg of protein from isolated mitochondrial samples, as described before, was incubated to the wells of microplate pre-coated with complex I capture antibody for 3h at room temperature. Then activity of the immunocaptured complex I enzyme from the samples was determined by measuring the oxidation of NADH to NAD+ and simultaneous reduction of a dye which leads to increased absorbance at 450 nm. Each sample was measured in duplicates and the activity was expressed as change in absorbance per minute per amount of sample loaded into the well.
4-hydroxynonenal assay
Brain tissue was homogenized in 5 volumes of 20 mM sodium phosphate buffer, pH 7.0, containing 1 mM DTT and protease inhibitor cocktail (Complete protease inhibitor tablets, Roche, USA). Homogenates were centrifuged at 13,000 rpm for 15 min at 4°C and the supernatant was used for the assay after determining the protein concentrations by Bradford assay. The assay for 4-HNE was performed as per manufacturer’s protocol (Oxiselect HNE-His adduct ELISA kit, Cell Biolabs Inc., USA; #STA-334). Briefly, HNE-BSA standards, reduced BSA standards and brain homogenate samples (20 μg/ml) were adsorbed on to a 96-well plate for 2h at 37°C. HNE-protein adducts present in the standards and samples were probed with an anti-HNE-His antibody. Then HRP-conjugated secondary antibody was added and the absorbance was measure at 450 nm. The levels HNE-protein adducts were determined by comparing with the standard curve of predetermined HNE-BSA standards. Each sample was measured in duplicates and the levels were expressed as amount of HNE-protein adducts per amount of sample loaded.
Malondialdehyde assay
Brain tissue homogenates were prepared as mentioned for Western blotting using modified RIPA buffer with protease inhibitors. MDA assay was performed as per manufacturer’s protocol (BIOXYTECH MDA-586, OxisResearch, USA; #21044). Each sample was measured in duplicates and levels were calculated comparing to MDA standard curve, and expressed as ratio compared to wild type control mice.
Statistics
Statistical analysis was performed using SigmaPlot software (version 12.0, Systat Software Inc.). Western blotting, complex I activity and lipid peroxidation assay data were analyzed using two-tailed Student’s t-test (when data were homoscedastic) or, as indicated in the text with Mann Whitney U-test (when data were heteroscedastic). These tests were used on each brain region separately since the experiments were performed independently in each region. Two way repeated measures ANOVA was used to compare the mitochondrial respiration using Seahorse analyzer. For this analysis each brain region was considered independent and the different assays (substrate additions) which were performed on the same mitochondrial preparation were considered as ‘repeated measures’. Post hoc tests were performed using Fisher’s least significant difference (LSD) to compare WT and Thy1-aSyn mice (single comparisons). Outliers in all the analysis were excluded using Grubb’s outlier test. The investigator was blinded to the groups when assessing the outcome of the experiments.
Results
Increased mitochondrial accumulation of α-synuclein in Thy1-aSyn mice
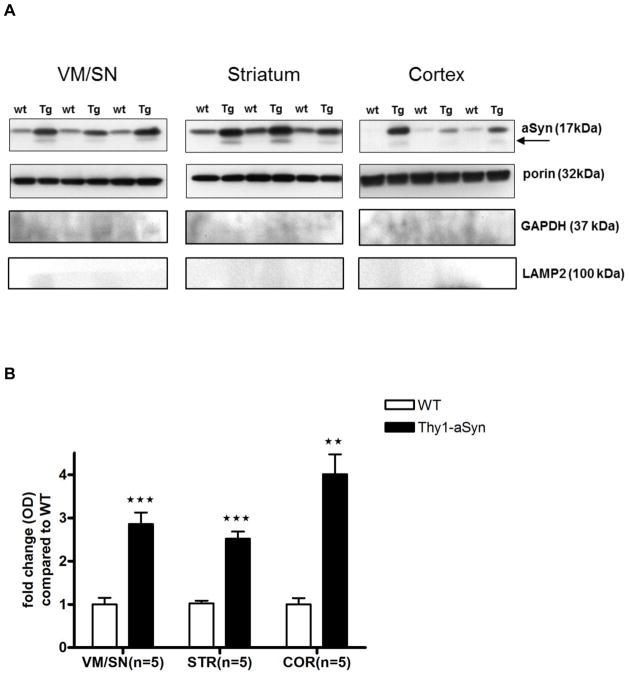
To verify that α-synuclein accumulates in mitochondria in our model, as previously described in whole lysates (Chesselet et al., 2012), we isolated mitochondria-enriched fractions from dissected brain regions and performed Western blots with an antibody that binds to both mouse and human α-synuclein. The α-synuclein monomer (17 kDa) accumulated to higher levels in mitochondria, similar to whole lysates, of 4 months old Thy1-aSyn mice compared to their wild-type littermates in all regions examined: VM/SN (3-fold; t8 = 6.047, p = 0.0003), striatum (2.5-fold; t8 = 8.547, p = 0.0001) and cortex (4-fold; t8 = 6.234, p = 0.0079; Mann Whitney U-test used due to unequal variances) (Fig. 1). In addition to the monomeric form, accumulation of a truncated form of α-synuclein (~12kDa) was observed in mitochondrial fractions of Thy1-aSyn mice but not in wild-type littermates, though this might be related to the low levels of α-synuclein in the wild-type mice. To ensure purity, the isolated mitochondria-enriched fractions were immunoblotted with cytosolic (GAPDH) and lysosomal (LAMP2) markers and the results showed no contamination (Fig. 1). Mitochondrial DNA density was measured in 8–9 months old mice by real time PCR and results show that mitochondrial DNA density was not altered in VM/SN, striatum or cortex of Thy1-aSyn mice compared to wild type mice (Fig. 2). Thus, Thy-1 aSyn mice show accumulation of alpha-synuclein in their mitochondria without marked regional differences, as expected from the broad distribution of the transgenic protein throughout the brain in this model (Chesselet et al., 2012).
Fig. 1.
Mitochondrial accumulation of aSyn were significantly higher in Thy1-aSyn mice (n=5) compared to wild type littermates (n=5) expressed as mean ± SEM. A. Western blots, arrow indicates truncated aSyn B. Densitometric analysis by ImageJ with porin as loading control. ***p<0.001, **p<0.01 by Student’s t-test or Mann Whitney U-test (used due to unequal variances in cortex) compared to respective wt control group.
Fig. 2.
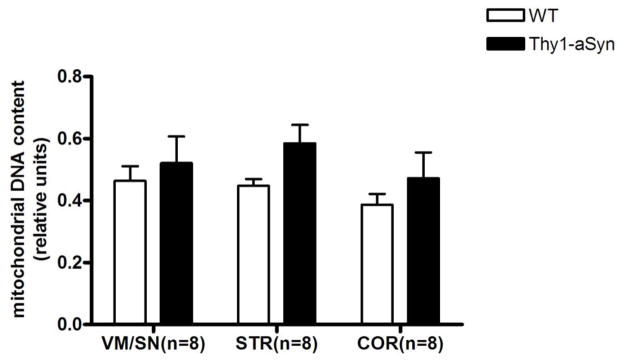
Relative mitochondrial DNA content was not altered in 8–9 months old Thy1-aSyn mice (n=8) compared to wild type littermates (n=8) expressed as mean ± SEM. Mitochondrial DNA was measured by real time PCR with primers in the D-loop region and normalized to nuclear DNA (Tert gene). Student’s t-test compared to respective wt control group.
Mitochondrial bioenergetics measurements show defects specific to striatum and VM/SN
We further examined mitochondrial bioenergetics by measuring oxygen consumption rate (OCR) in freshly isolated mitochondria from different brain regions of 6-month old mice with a Seahorse XF24 analyzer. We examined the degree of coupling between the electron transport chain (ETC) and the oxidative phosphorylation machinery using different substrates and injections by coupling assay, and we measured the sequential electron flow through different complexes of the ETC by an electron flow assay (Rogers et al., 2011).
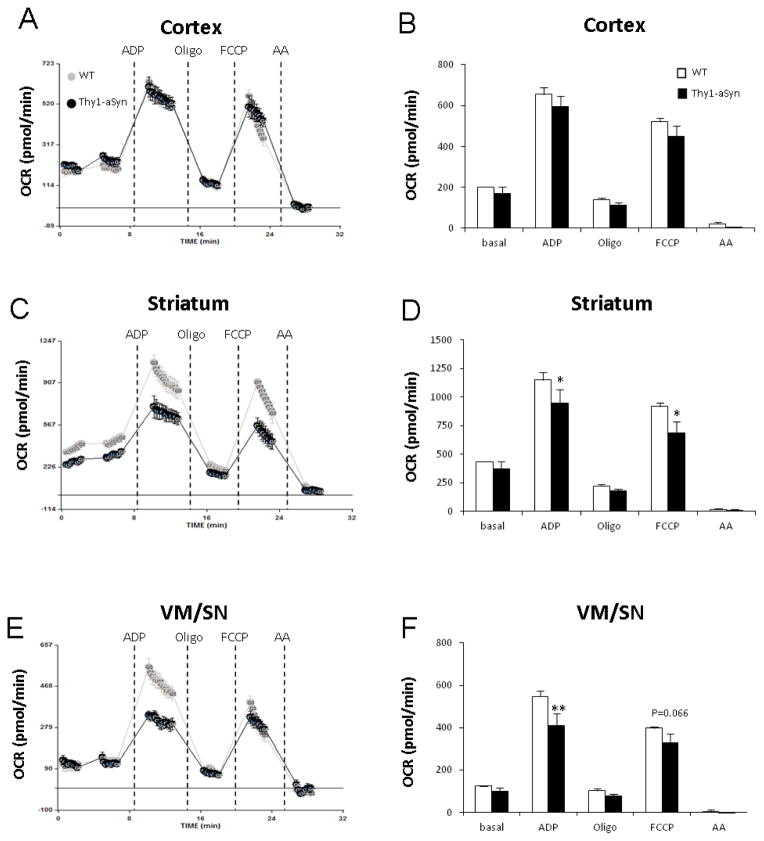
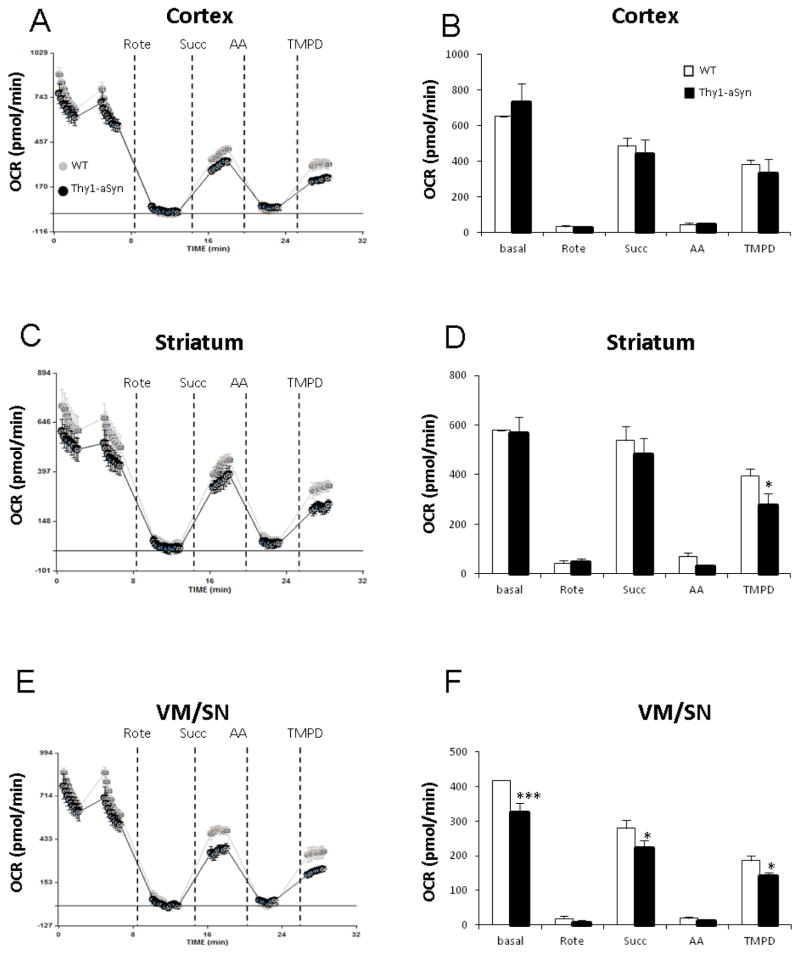
In the coupling assay, isolated mitochondria were incubated with succinate (complex II-driven respiration) and rotenone (complex I inhibitor). Two way Repeated Measures ANOVA for overall coupling assay measurements in VM/SN (F1,6 = 4.527, p = 0.077), striatum (F1,6 = 3.088, p = 0.129) and cortex (F1,6 = 1.971, p = 0.210) followed by post hoc all pairwise multiple comparison analysis revealed the following impairments. Injection of ADP revealed a decreased mitochondrial response in VM/SN (25%, p= 0.001) and striatum (18%, p= 0.025) of Thy1-aSyn mice suggesting impairment in complex V (ATP synthase) but no such reduction was found in cortex (p= 0.151). Further addition of FCCP (mitochondrial uncoupler) showed a 25% reduction in maximal respiration in the striatum (p= 0.013), and a similar trend in VM/SN (18%, p= 0.066), of Thy1-aSyn mice (Fig. 3). Basal respiration in the electron flow assay was driven by pyruvate and malate (complex I-driven respiration) in an uncoupled state (in the presence of FCCP). Two way Repeated Measures ANOVA for overall electron flow assay measurements in VM/SN (F1,6 = 11.279, p = 0.015), striatum (F1,6 = 1.225, p = 0.311) and cortex (F1,6 = 0.0064, p = 0.939) followed by post hoc all pairwise multiple comparison analysis revealed several mitochondrial ETC defects. In VM/SN of Thy1-aSyn mice, we observed a 22% reduction in basal respiration (p<0.001, complex I-defect), 20% reduction in respiration after succinate injection (p= 0.012, complex II-defect), and 22% decrease after TMPD injection (p= 0.047, complex IV-defect). In striatum of Thy1-aSyn mice, a 30% reduction (p=0.044) in respiration was recorded after TMPD injection. However, no significant difference was observed in cortex between WT and Thy1-aSyn mice in any ETC defect measured (Fig. 4). Altogether, these results suggest that mitochondria from Thy1-aSyn mice possess mitochondrial defects in several ETC complexes, specifically in the brain regions containing the cell bodies and terminals of the nigrostriatal dopaminergic neurons.
Fig. 3.
Mitochondrial respiration measurement on isolated mitochondria by coupling assay showed impairment in nigrostriatal tissue. A, B: cortex; C, D: striatum; E, F: VM/SN. Measurements were done on four Thy1-aSyn mice and four wild-type mice, averaged and expressed as mean ± SEM. *p<0.05, **p<0.01 compared to wild type group by Two-way RM-ANOVA, followed by Fisher’s LSD post hoc test.
Fig. 4.
Mitochondrial respiration measurement on isolated mitochondria by electron flow assay showed impairment in nigrostriatal tissue. A, B: cortex; C, D: striatum; E, F: VM/SN. Measurements were done on four Thy1-aSyn mice and four wild-type mice, averaged and expressed as mean ± SEM. *p<0.05, ***p<0.001 compared to wild type group by Two-way RM-ANOVA, followed by Fisher’s LSD post hoc test.
Inhibition of mitochondrial complex I activity is specific to VM/SN in Thy1-aSyn mice
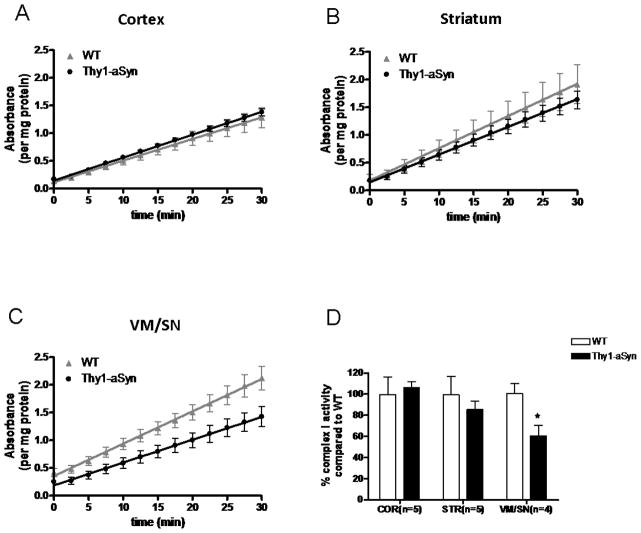
The respiration measurements using Seahorse analyzer suggested that in Thy1-aSyn mice, bioenergetics deficits are regionally limited and, in particular spare the cerebral cortex despite a high level of alpha-synuclein overexpression in this region (Chesselet et al., 2012), and accumulation of alpha-synuclein in cortical mitochondria (Fig. 1). To determine whether alpha-synuclein also altered complex I activity in Thy1-aSyn mice in a regionally specific manner, we measured enzyme activity of complex I in isolated mitochondria from VM/SN, striatum and cortex of 4-month old Thy1-aSyn mice by immunocapture ELISA. The results showed a 40% decrease in mitochondrial complex I activity in VM/SN (t6 = 2.888, p = 0.028). However, no changes in complex I activity was observed in striatum (t7 = 0.649, p = 0.537) and cortex (t8 = 0.3723, p = 0.719) suggesting that reduction in complex I activity is specific to VM/SN despite global overexpression and mitochondrial accumulation of α-synuclein in these mice (Fig. 5).
Fig. 5.
VM/SN specific reduction in complex I activity measured by ELISA. Change in complex I activity over 30 min time period in cortex (n=5) (A), striatum (n=5) (B) and VM/SN (n=4) (C). Percentage complex I activity (D) of Thy1-aSyn mice compared to wild type littermates expressed as mean ± SEM. *p<0.05 by Student’s t-test compared to respective wt control group.
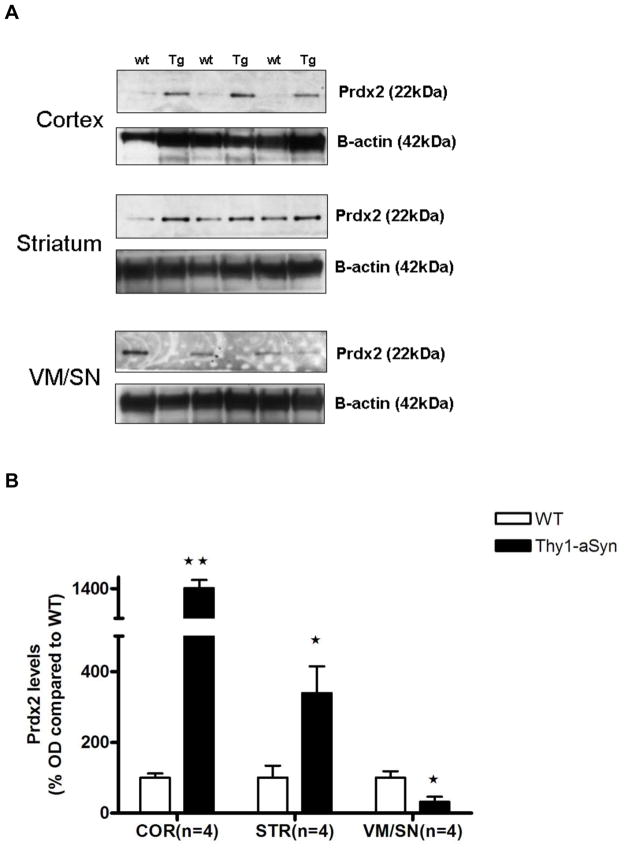
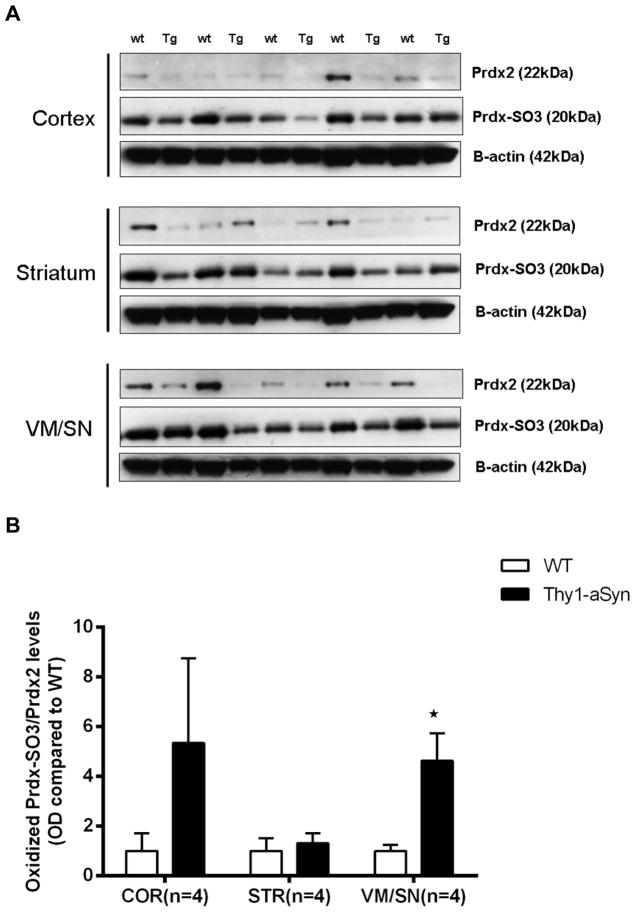
Low Prdx2 and elevated oxidized Prdx levels in VM/SN
Prdx2 is localized almost exclusively in neurons (Jin et al., 2005, Fang et al., 2007) and can thus be an indicator of neuronal oxidative stress. To measure the levels of neuronal oxidative stress we measured the protein levels of Prdx2 and its over-oxidized form (Prdx-SO3) in 8 months old Thy1-aSyn mice by Western blotting. The results show that Prdx2 levels were up-regulated in striatum (339.3%, t5 = 3.181, p=0.0245) and cortex (1420.8%, t5 = 4.636, p=0.0057) of Thy1-aSyn mice similar to PD patients which might represent a protective response to compensate high levels oxidative stress induced by over-expression of alpha-syuclein in the Thy1-aSyn mice. On the contrary, Prdx2 levels in VM/SN were decreased by 67.9% (t6 = 2.898, p=0.0274) in Thy1-aSyn mice compared to their wild type littermates (Fig. 6). A highly oxidative environment might cause an increased oxidation of the peroxiredoxins leading to loss of their protective function. Interestingly, a 4-fold increase in oxidized-Prdx was observed in VM/SN (t7 = 3.605, p=0.0159; Mann Whitney U-test used due to unequal variances) of Thy1-aSyn mice (Fig. 7). This increase in oxidized-Prdx was not found in striatum (t4 = 0.4896, p=0.6501) or in cortex (t5 = 1.467, p=0.2286; Mann Whitney U-test used due to unequal variances) where the levels of oxidized-Prdx were highly variable. Taken together, these results suggest that nigral cells have lower functional Prdx2 compared to cortex and striatum due its oxidative modification. This may result from a higher level of oxidative stress in these cells in the presence of excess alpha-synuclein and may result in increased lipid peroxidation leading to oxidative cellular damage.
Fig. 6.
Alterations in Prdx2 levels in 5–6 months old Thy1-aSyn mice (n=4) and wild type littermates (n=4) measured by Western blotting and expressed as mean ± SEM of percentage fold difference. A. Representative Western blots B. Densitometric analysis by ImageJ with beta-actin as loading control. *p<0.05, **p<0.01 by Student’s t-test compared to respective wt control group.
Fig. 7.
Elevated oxidized-Prdx-SO3 levels in VM/SN of 8 months old Thy1-aSyn mice (n=5) compared to wt littermates (n=5) measured by Western blotting and expressed as mean ± SEM. A. Western blots B. Densitometric analysis by ImageJ normalized to prdx2 with beta-actin as loading control. *p<0.05 by Student’s t-test or Mann Whitney U-test (used due to unequal variances in VM/SN) compared to respective wt control group.
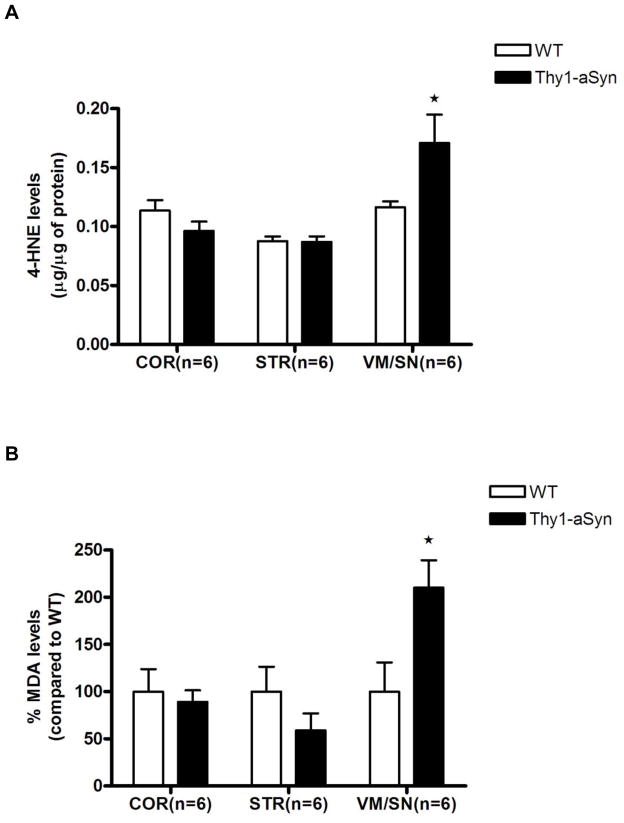
Elevation of lipid peroxidation markers in VM/SN
Primary lipid peroxidation products such as lipid peroxides are unstable and decompose to form more complex and reactive compounds including 4-hydroxynenal (4-HNE) and malondialdehyde (MDA). To assess the effects of reduced levels of functional Prdx2 in VM/SN of Thy1-aSyn mice on lipid peroxidation, we measured the levels of 4-HNE (6-month old mice) and MDA (8-month old mice) by ELISA and colorimetric assay, respectively. Confirming regional increases in lipid peroxidation, 4-HNE levels were increased 47% in VM/SN (t8 = 2.724, p=0.0381; Mann Whitney U-test used due to unequal variances) of Thy1-aSyn mice compared to its wild type littermates, without significant changes in striatum (t10 = 0.1326, p=0.8972) or cortex (t10 = 1.449, p=0.1781) (Fig. 8A). Similarly, a 210% increase in MDA levels was observed in VM/SN (t9 = 2.427, p=0.0382) of Thy1-aSyn mice but not in striatum (t8 = 1.289, p=0.2335) or cortex (t10 = 0.4426, p=0.6675) (Fig. 8B). These results indicate that the levels of lipid peroxidation were severely increased specifically in the VM/SN of Thy1-aSyn mice.
Fig. 8.
Increase in lipid peroxidation markers A. 4-hydroxynonenal, B. MDA levels in the VM/SN of Thy1-aSyn mice (n=6) compared to wild type littermates (n=6) expressed as mean ± SEM. *p <0.05 by Student’s t-test or Mann Whitney U-test (used due to unequal variances in HNE assay in VM/SN) compared to respective wt control group.
Discussion
In the present study we show early and regionally selective alterations in mitochondrial function, oxidative stress, and Prdx2 levels in a model of broad overexpression of human alpha-synuclein in mice. The Thy1-aSyn mice provide a useful model to identify regional effects that may underlie differential vulnerability of nigrostriatal dopaminergic neurons in PD because they overexpress human wild type alpha-synuclein to a moderate extent throughout the brain (Rockenstein et al., 2002, Chesselet et al., 2012). This was confirmed in the present study by the presence of similarly high levels of human and mouse alpha-synuclein in mitochondria isolated from different brain regions. Thus, the greater alterations in mitochondrial function uncovered in this study in striatum and substantia nigra are likely due to endogenous factors that render neurons in these regions less able to cope with alpha-synuclein induced mitochondrial dysfunction.
Our data of a regional decrease in complex I activity in the Thy1-aSyn mice are in agreement with previous reports in the postmortem substantia nigra of patients with idiopathic PD (Schapira et al., 1990, Hattori et al., 1991, Keeney et al., 2006) but reveal, for the first time, that alpha-synuclein alters mitochondrial function to a greater extend in the regions containing nigrostriatal dopaminergic neurons than in other brain regions, specifically the cerebral cortex. This suggests that the greater endogenous level of oxidative stress caused in nigrostriatal neurons by the presence of dopamine, coupled to the high level of calcium accumulating in these neurons due to their pace making activity (Chan et al., 2007) render their mitochondria more vulnerable to alpha-synuclein accumulation, which is also present in the brain of patients with sporadic PD (Devi et al., 2008).
Accumulation of alpha-synuclein in mitochondria of Thy1-aSyn mice confirms recent data in the same mouse model, including the presence of a truncated form of alpha-synuclein (Sarafian et al., 2013). Previous studies have found that alpha-synuclein can induce mitochondrial dysfunction in cell models. Specifically, either wild type or mutated α-synuclein has been reported to bind to inner mitochondrial membrane and interact with complex I resulting in decreased complex I activity and impaired mitochondrial function (Elkon et al., 2002, Li et al., 2007, Devi et al., 2008, Nakamura et al., 2008, Shavali et al., 2008, Zhang et al., 2008, Chinta et al., 2010). A similar effect was observed in vivo, in mice over-expressing alpha-synuclein specifically in dopaminergic neurons (Chinta et al., 2010) and a recent study shows that the levels of complex I enzyme is down regulated in anterior brain homogenates of transgenic mice overexpressing wild-type α-synuclein (Bender et al., 2013). In contrast, one recent work reports that wild type alpha-synuclein from both in vitro and in vivo (brain tissue from human and mice) models is not present in mitochondria but rather in mitochondria-associated endoplasmic reticulum (ER) membranes (MAM). In addition, pathogenic point mutations in human alpha-synuclein resulted in reduced MAM association, decreased MAM functions and increased fragmentation of mitochondria (Guardia-Laguarta et al., 2014). Overall these studies suggest that alpha-synuclein can bind to inner mitochondrial membrane and/or to mitochondria-associated endoplasmic reticulum (ER) membranes (MAM). However, these studies did not examine the possibility that alpha-synuclein effects may differ in brain regions that show differential vulnerability to PD. Recent in vitro data (Ryan et al., 2013) show that mutated A53T alpha-synuclein reduces mitochondrial spare respiratory capacity, measured with the Seahorse analyzer, in dopaminergic neurons induced from human cells, similar to our findings with expression of wild type alpha-synuclein in vivo. Evidence that mitochondrial dysfunction induced by environmental toxins caused nitrosative modifications of the Mef2-PGC1alpha pathway specifically in cells expressing A53T alpha-synuclein suggests a mechanism by which mutated alpha-synuclein may exacerbate the effects of mitochondrial dysfunction (Ryan et al., 2013). We show that in the ventral midbrain, the brain region that contains the cell bodies of dopaminergic neurons, the impairment in mitochondrial function is accompanied by increased oxidation of lipids and of peroxiredoxin, suggesting that antioxidant defenses can be impaired in the presence of the excess oxidative stress specifically in these regions. In contrast to ventral midbrain, Prdx2 levels were increased and no lipid peroxidation was observed in striatum and cortex, suggesting more efficient antioxidant defense in these two regions that do not show significant cell loss in PD. Prdx2, the neuronal antioxidant enzyme that protects from lipid peroxidation, is induced in brains of PD patients (Basso et al., 2004). Interestingly, a recent study showed that overexpression of Prdx2 in vitro and in vivo protects against 6-OHDA toxicity in dopaminergic neurons (Hu et al., 2011). Furthermore, Dawson and co-workers showed that mice lacking DJ-1, a model of recessive familial PD, had deficits in scavenging mitochondrial H2O2 and in fact described DJ-1 as an atypical peroxiredoxin-like peroxidase indicating the importance of Prdx enzymes in PD. Additionally, Prdx2 is highly expressed in murine brain cells that use oxygen at high rates (Ichimiya et al., 1997, Sarafian et al., 1999, Jin et al., 2005). Thus the differential changes in Prdx2 levels we have observed in ventral midbrain versus striatum and cortex may both be a consequence of a greater level of oxidative stress resulting from diminished mitochondrial function in this region and also contribute to the greater vulnerability of nigrostriatal dopaminergic neurons in PD. Even though the striatum contains axon terminals of nigrostriatal dopaminergic neurons, these constitute only a very small percentage of tissue and the bulk of Prdx2 in striatal homogenates is likely to represent enzyme present in the main striatal neurons, the medium sized GABAergic neurons that are preserved in PD.
Despite evidence that alpha-synuclein accumulates in mitochondria its main effects could be through regulation of nuclear genes involved in mitochondrial function, especially PGC1 alpha, through direct interaction with its promoter (Siddiqui et al., 2012). Indeed, over-expression of alpha-synuclein in vivo and in vitro decrease PGC1 alpha and PGC1 alpha-regulated genes. Importantly, PGC1 alpha is decreased in PD patients and increasing its levels in vitro protects cells against alpha-synuclein toxicity (Zheng et al., 2010). However, this mechanism is unlikely to play a primary role in the effects observed here because transcriptome data in laser captured nigrostriatal dopaminergic neurons of 8 months old Thy1-aSyn mice showed an increase in PGC1 alpha mRNA, suggesting a compensatory effect at that age (Mortazavi, Richter, and Chesselet, unpublished observations).
In conclusion, the present data indicate that despite a broad expression of alpha-synuclein throughout the brain, Thy1-aSyn mice show regional alterations in mitochondrial function and oxidative stress, supporting the notion that endogenous properties of these neurons contribute to their increased vulnerability in PD. In addition, our results show that Thy1-aSyn mice can serve as a useful model to test potential neuroprotective drugs that may prevent mitochondrial dysfunction.
Highlights.
Mice overexpressing wild type human alpha synuclein show reduced mitochondrial function from an early age
The reduction in mitochondrial function is present in ventral midbrain and striatum but not cortex
Neuronal Prdx2 is decreased and its oxidized form increased in midbrain only
Markers of lipid peroxidation are elevated in midbrain but not striatum or cortex
Acknowledgments
This work was supported by PHS grants NS-P50 NS38367 (UCLA Morris K. Udall Parkinson Disease Research Center of Excellence), ES-P01 ES016732 (Center for Gene-Environment in Parkinson’s disease), S10RR026744 (National Center for Research Resources), P01 HL028481 (National Institutes of Health) and gifts to the UCLA Center for the Study of Parkinson’s disease at UCLA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sudhakar Raja SUBRAMANIAM, Email: subramaniamsr@ucla.edu.
Laurent VERGNES, Email: lvergnes@ucla.edu.
Nicholas R. FRANICH, Email: n.franich@gmail.com.
Karen REUE, Email: reuek@ucla.edu.
References
- Basso M, Giraudo S, Corpillo D, Bergamasco B, Lopiano L, Fasano M. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteomics. 2004;4:3943–3952. doi: 10.1002/pmic.200400848. [DOI] [PubMed] [Google Scholar]
- Bender A, Desplats P, Spencer B, Rockenstein E, Adame A, Elstner M, Laub C, Mueller S, Koob AO, Mante M, Pham E, Klopstock T, Masliah E. TOM40 mediates mitochondrial dysfunction induced by alpha-synuclein accumulation in Parkinson’s disease. PloS one. 2013;8:e62277. doi: 10.1371/journal.pone.0062277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. Journal of neurochemistry. 1999;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- Bueler H. Mitochondrial dynamics, cell death and the pathogenesis of Parkinson’s disease. Apoptosis : an international journal on programmed cell death. 2010;15:1336–1353. doi: 10.1007/s10495-010-0465-0. [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9:297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Andersen JK. Redox imbalance in Parkinson’s disease. Biochimica et biophysica acta. 2008;1780:1362–1367. doi: 10.1016/j.bbagen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neuroscience letters. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR, van der Brug M. Cell systems and the toxic mechanism(s) of alpha-synuclein. Experimental neurology. 2008;209:5–11. doi: 10.1016/j.expneurol.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. The Journal of biological chemistry. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Annals of human genetics. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon H, Don J, Melamed E, Ziv I, Shirvan A, Offen D. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. Journal of molecular neuroscience : MN. 2002;18:229–238. doi: 10.1385/JMN:18:3:229. [DOI] [PubMed] [Google Scholar]
- Fang J, Nakamura T, Cho DH, Gu Z, Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam AH, Zeevalk GD. Characterization of reduced and oxidized dopamine and 3,4-dihydrophenylacetic acid, on brain mitochondrial electron transport chain activities. Biochimica et biophysica acta. 2011;1807:819–828. doi: 10.1016/j.bbabio.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck MR, Zeevalk GD. Inhibition of brain mitochondrial respiration by dopamine and its metabolites: implications for Parkinson’s disease and catecholamine-associated diseases. Journal of neurochemistry. 2004;91:788–795. doi: 10.1111/j.1471-4159.2004.02747.x. [DOI] [PubMed] [Google Scholar]
- Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S. alpha-Synuclein is localized to mitochondria-associated ER membranes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:249–259. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Tanaka M, Ozawa T, Mizuno Y. Immunohistochemical studies on complexes I, II, III, and IV of mitochondria in Parkinson’s disease. Annals of neurology. 1991;30:563–571. doi: 10.1002/ana.410300409. [DOI] [PubMed] [Google Scholar]
- Hu X, Weng Z, Chu CT, Zhang L, Cao G, Gao Y, Signore A, Zhu J, Hastings T, Greenamyre JT, Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya S, Davis JG, O’Rourke DM, Katsumata M, Greene MI. Murine thioredoxin peroxidase delays neuronal apoptosis and is expressed in areas of the brain most susceptible to hypoxic and ischemic injury. DNA and cell biology. 1997;16:311–321. doi: 10.1089/dna.1997.16.311. [DOI] [PubMed] [Google Scholar]
- Jin MH, Lee YH, Kim JM, Sun HN, Moon EY, Shong MH, Kim SU, Lee SH, Lee TH, Yu DY, Lee DS. Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neuroscience letters. 2005;381:252–257. doi: 10.1016/j.neulet.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. Journal of toxicology Clinical toxicology. 2000;38:123–128. doi: 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- Li WW, Yang R, Guo JC, Ren HM, Zha XL, Cheng JS, Cai DF. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007;18:1543–1546. doi: 10.1097/WNR.0b013e3282f03db4. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Cookson MR. Mitochondrial quality control and dynamics in Parkinson’s disease. Antioxidants & redox signaling. 2012;16:869–882. doi: 10.1089/ars.2011.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire V, Van Den Eeden SK, Tanner CM, Kamel F, Umbach DM, Marder K, Mayeux R, Ritz B, Ross GW, Petrovitch H, Topol B, Popat RA, Costello S, Manthripragada AD, Southwick A, Myers RM, Nelson LM. Association of DRD2 and DRD3 polymorphisms with Parkinson’s disease in a multiethnic consortium. Journal of the neurological sciences. 2011;307:22–29. doi: 10.1016/j.jns.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollace V, Iannone M, Muscoli C, Palma E, Granato T, Rispoli V, Nistico R, Rotiroti D, Salvemini D. The role of oxidative stress in paraquat-induced neurotoxicity in rats: protection by non peptidyl superoxide dismutase mimetic. Neuroscience letters. 2003;335:163–166. doi: 10.1016/s0304-3940(02)01168-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH. Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovic AD, Lewis DA, Hastings TG. Role of oxidative changes in the degeneration of dopamine terminals after injection of neurotoxic levels of dopamine. Neuroscience. 2000;101:67–76. doi: 10.1016/s0306-4522(00)00293-1. [DOI] [PubMed] [Google Scholar]
- Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Annals of neurology. 2010;67:600–606. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environmental health perspectives. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. Journal of neuroscience research. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA, Murphy AN. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PloS one. 2011;6:e21746. doi: 10.1371/journal.pone.0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, Lee B, Lopez K, Nutter A, Shan B, Molokanova E, Zhang Y, Han X, Nakamura T, Masliah E, Yates JR, 3rd, Nakanishi N, Andreyev AY, Okamoto SI, Jaenisch R, Ambasudhan R, Lipton SA. Isogenic Human iPSC Parkinson’s Model Shows Nitrosative Stress-Induced Dysfunction in MEF2-PGC1alpha Transcription. Cell. 2013 doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian TA, Ryan CM, Souda P, Masliah E, Kar UK, Vinters HV, Mathern GW, Faull KF, Whitelegge JP, Watson JB. Impairment of mitochondria in adult mouse brain overexpressing predominantly full-length, N-terminally acetylated human alpha-synuclein. PloS one. 2013;8:e63557. doi: 10.1371/journal.pone.0063557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian TA, Verity MA, Vinters HV, Shih CC, Shi L, Ji XD, Dong L, Shau H. Differential expression of peroxiredoxin subtypes in human brain cell types. Journal of neuroscience research. 1999;56:206–212. [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Journal of neurochemistry. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Shavali S, Brown-Borg HM, Ebadi M, Porter J. Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neuroscience letters. 2008;439:125–128. doi: 10.1016/j.neulet.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, Melov S, Andersen JK. Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson’s disease. Free radical biology & medicine. 2012;53:993–1003. doi: 10.1016/j.freeradbiomed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Annals of the New York Academy of Sciences. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Progress in neurobiology. 2013;106–107:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Buckley B, Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. The Journal of biological chemistry. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. Journal of neurochemistry. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Chin R, Young SG, Reue K. Heart-type fatty acid-binding protein is essential for efficient brown adipose tissue fatty acid oxidation and cold tolerance. The Journal of biological chemistry. 2011;286:380–390. doi: 10.1074/jbc.M110.184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. European journal of epidemiology. 2011;26:547–555. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. The EMBO journal. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumino K, Kawakami I, Tamura M, Hayashi T, Nakamura M. Paraquat- and diquat-induced oxygen radical generation and lipid peroxidation in rat brain microsomes. Journal of biochemistry. 2002;131:565–570. doi: 10.1093/oxfordjournals.jbchem.a003135. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Ueda K, Yu S, Yang H. Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody. Brain research. 2008;1244:40–52. doi: 10.1016/j.brainres.2008.08.067. [DOI] [PubMed] [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grunblatt E, Moran LB, Mandel SA, Riederer P, Miller RM, Federoff HJ, Wullner U, Papapetropoulos S, Youdim MB, Cantuti-Castelvetri I, Young AB, Vance JM, Davis RL, Hedreen JC, Adler CH, Beach TG, Graeber MB, Middleton FA, Rochet JC, Scherzer CR. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Science translational medicine. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]